Abstract
The continuing and worldwide growth of pressure processing technologies to pasteurize and sterilize foods justifies the need to study the effects on functional compounds and abiotic contaminants as affected by high-pressure processing (HPP) and pressure-assisted thermal processing (PATP). Substantially more research will be required to determine the complex effects of the food matrix on chemical reactions leading to losses of nutrients and functional components, production of toxic compounds, and to modifications of toxic residues of chemicals used in food production or coming from food contact materials. In PATP treatments, pressure can also increase, decrease or have no effect on the thermal degradation rate of these substances. HPP has no major negative and often beneficial effects on the retention of nutrients and functional components. However, information on PATP effects is very limited and additional research will be required before implementing this promising new technology.
El crecimiento mundial de las tecnologías basadas en alta presión hidrostática (APH) y de procesado térmico asistido por presión (PTAP) empleadas para pasterizar y esterilizar alimentos justifica la necesidad de estudiar los efectos que provocan en componentes funcionales y en contaminantes abióticos. Se necesita mucha investigación para conocer los efectos de la presurización y del tipo de alimento sobre las reacciones químicas que provocan pérdida de componentes nutritivos y funcionales y sobre aquellas que forman compuestos tóxicos o modifican residuos tóxicos de sustancias químicas empleadas para producir alimentos o procedentes de materiales en contacto con ellos. En el tratamiento PATP, el aumento de presión puede incrementar, disminuir o no afectar la velocidad de la degradación térmica de componentes del alimento. En general, los tratamientos APH no tienen efectos negativos y suelen ser beneficiosos en cuanto a retención de componentes nutritivos y funcionales. Sin embargo, la información sobre los efectos PATP es muy limitada, requiriéndose investigación adicional para poder implementar de forma segura esta tecnología innovadora.
Palabras clave:
- procesado por alta presión hidrostática (APH)
- procesado térmico asistido por alta presión (PTAP)
- antioxidantes
- vitaminas
- polifenoles
- contaminantes no bióticos
- acrilamida
- hidrocarburos policíclicos aromáticos (PAHs)
- aminas heterocíclicas aromáticas (HCAs)
- cloropropanoles
- materiales plásticos de envasado alimentario
- pesticidas
Introduction
High-pressure processing (HPP) technology has been developed as an alternative to thermal processes with the aim of obtaining microbiologically safe food products while avoiding undesirable changes in the sensory, physicochemical, and nutritional properties of foods (Bermúdez-Aguirre & Barbosa-Cánovas, Citation2011; Campus, Citation2010; Mújica-Paz, Valdez-Fragoso, Tonello Samson, Welti-Chanes, & Torres, Citation2011; Palou, López-Malo, & Welti-Chanes, 2002; Tellez Luis, Ramirez, Pérez Lamela, Vazquez, & Simal Gándara, 2001; Torres & Velazquez, Citation2005; Torres, Sanz, Otero, Pérez Lamela, & Saldaña, 2009a; Welti-Chanes, San Martín-González, & Barbosa-Cánovas, 2006). Most commercial HPP treatments are in the 400–700 MPa range andare applied at refrigerated to moderate temperature (under ∼50°C). Under these conditions, HPP is considered a nonthermal method and has become oneof the innovative food processing technologies most accepted by consumers (Cardello, Citation2003; Cardello, Schutz, & Lesher, Citation2007; Evans & Cox, Citation2006). A recent development, not yet commercialized but with an application already approved by the US Food & Drug Administration (www.nafwa.org/blog/, accessed 5 March 2009), is the use of pressure treatments at higher temperatures, a method known as pressure assisted thermal processing (PATP) (Bermúdez-Aguirre & Barbosa-Cánovas, Citation2011; Mújica-Paz et al., Citation2011; Torres, Sanz, Otero, Pérez Lamela, & Saldaña, 2009b; Valdez-Fragoso, Mújica-Paz, Welti-Chanes, & Torres, Citation2011). Regarding HPP effects on food composition, research has shown a higher retention of nutrients and functional compounds including no changes in antioxidant capacity when compared with other preservation processes such as thermal treatments (Oey, Lille, van Loey, & Hendrickx, Citation2008; Oey, van der Plancken, van Loey, & Hendrickx, Citation2008); however, there are still very few studies reporting PATP effects on foods (Ramirez, Saraiva, Pérez Lamela, & Torres, Citation2009). In addition, there is a renewed interest on the impact on human health of substances formed during food heating including the formation of acrylamide, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic aromatic amines (HCAs) among others (Eisenbrand et al., Citation2007; Kanekanian, Citation2010). A continuing goal of food processors and regulatory agencies is to ensure that foods have no residues of abiotic contaminants from pesticide applications or from interactions between plastic materials and foodstuffs. The risks of these compounds, and of derivatives formed under PATP conditions and during product distribution and storage, have not been determined. Although many publications have shown that HPP at refrigeration and room temperature preserves food freshness and has minimum effects on food composition (Mújica-Paz et al., Citation2011; Pérez Lamela & Torres, Citation2008; Shellhammer, Aleman, McDaniel, & Torres, Citation2003; Torres et al., Citation2009a), the effect of PATP treatments on chemical changes in foods is largely unknown (Ramirez et al., Citation2009). The assessment of this technology and any other novel process should include studies on potential chemical risks (Segovia Bravo et al., Citation2011) and losses of important nutrients such as vitamins or functional compounds such as polyphenols with desirable antioxidant activity.
Studies of chemical reactions under PATP conditions should include a kinetic analysis allowing the determination of temperature and pressure effects on chemical reaction rates (Segovia Bravo et al., Citation2011). Reactions can be accelerated or inhibited by pressure. Most importantly, if a thermal degradation reaction producing a toxic compound is too slow to produce detectable amounts at conventional pressures in the relatively short time of food processing, the reaction rate could increase with pressure. Such chemical reactions in foods could become an important toxic risk under PATP conditions. On the other hand, reactions forming detectable amounts of toxic compounds in foods treated by conventional thermal processing could be inhibited by pressure. In this case, PATP treatments would reduce the toxic risk of such foods. However, at present it is not possible to predict if reactions will be accelerated or inhibited by pressure, a determination that requires experimental work for each reaction and each food matrix of interest. Finally, no new reaction mechanisms have been found necessary to interpret chemical changes under PATP conditions. For example, studies in model food systems (Laing, Schlueter, & Labuza, Citation1978) at atmospheric pressure, and up to 600 MPa in buffer solutions (Oey, Verlinde, Hendrickx, & van Loey, Citation2006) and in orange juice (Polydera, Stoforos, & Taoukis, Citation2003) have shown that ascorbic acid losses follow first order kinetics.
High-pressure processing principles
In pressure processing, foods are placed in vessels filled with a fluid, generally water mixed with either vegetal or mineral oil for equipment lubrication and corrosion prevention purposes. The surrounding liquid exerts hydrostatic pressure on the food which is transmitted into the food almost instantaneously and uniformly, independently of the composition, size and shape of the food product and pressure vessel (Torres et al., Citation2009a). Due to adiabatic food compression, the temperature increases about 3°C per 100 MPa depending on the food compressibility value (Rasanayagam et al., Citation2003), pressure transmitting medium used, and initial food and medium temperature (Hogan, Kelly, & Sun, 2005; Rasanayagam et al., Citation2003). Once the desired pressure is reached, no additional energy is consumed. The nearly instant and uniform application of pressure across thefood facilitates scaling processes from laboratory toindustrial scale (Torres & Velazquez, Citation2005), an important commercialization advantage of this novel processing technology. This ease of scale-up is not true in PATP, as the scale-up requires complex calculations of heat transfer and temperature changes caused by compression and decompression of the food and the pressurized fluid which have thermophysical properties changing with pressure and temperature.
A second thermodynamic consideration governing HPP processes is the Le Chatelier–Braun principle stating that under equilibrium conditions, chemical reactions, phase transitions or conformational changes involving a volume reduction will be favored by pressure, while in the opposite case, the change will be inhibited (Ramirez et al., Citation2009; Welti-Chanes et al., Citation2006). In systems under equilibrium, the modification of a variable such as pressure will shift the equilibrium point in the direction reducing its effect (e.g. according to the partial molar volumes of reactants and products). However, in food processing, the rate of chemical reaction is generally more important than the equilibrium point because processing times are too short to reach the latter. This consideration, required for the correct interpretation of pressure effects during food processing, cannot be ignored (Valdez-Fragoso et al., Citation2011). Under equilibrium conditions, the effect of the pressure p on the relation between the reaction molar volume change ΔV, defined as the difference between the partial volume of products and reactants, and the equilibrium constant for the reaction, K, isgoverned by the following expression (Torres, Chotyakul, Velazquez, Saraiva, & Pérez Lamela, 2010; Torres et al., Citation2009b):
where ΔV is the reaction molar volume change ΔV (m3mol−1), R is the universal gas constant (8.31 × 10−6 MPa m3 K−1 mol−1), T is the absolute temperature (K), K is the reaction equilibrium constant, and p is pressure (MPa). A correct application of Le Chatelier–Braun principle is the prediction of the temporary pH shift induced by pressure (Paredes-Sabja, Gonzalez, Sarker, & Torres, Citation2007). Although, pH returns to its original value when the pressure is reduced, the pressure-induced pH-shift could have an effect on chemical reactions and on the inactivation of enzymes and microorganisms while foods are at high pressure. Samaranayake and Sudhir (Citation2010) reported anexperimental procedure to measure pH under high pressure (up to 785 MPa at 25°C). Hopefully, this will lead to prediction models of the pH shift in foods.
The preservation of nutritional quality in HPP-treated foods reflects the lack of pressure effects on covalent bonds up to 1000–2000 MPa, i.e. values exceeding the 700 MPa level used commercially (Bárcenas, Altamirano-Fortoul, & Rosell, Citation2010; Mozhaev, Heremans, Frank, Masson, & Balny, Citation1994). However, pressure affects the weaker bonds (Masson, Tonello, & Balny, Citation2001; Welti-Chanes et al., Citation2006) causing the inactivation of microorganisms and of enzymes responsible for food spoilage. The high temperatures used in PATP will affect covalent bonds requiring a determination of the kinetics of the resulting chemical changes to allow the optimization of process conditions. PATP effects on chemical reaction kinetics can be investigated by expressing the change in concentration (c) with respect to time (t) as follows (Valdez-Fragoso et al., Citation2011):
where k is the reaction rate constant at a given pressureand temperature while n is the reaction order. Integration of Equation (2) yields the following expressions:
The expression with the best correlation coefficient (R
2) is used to determine the pressure and temperature effects on the reaction rate constant k. In all chemical reactions, there is a transient state in the path from reactants to products defined as the active state. Reaching this transient state requires a temperature-independent energy increase of the reactants defined as the Arrhenius activation energy (Ea
). This value can be calculated using the Arrhenius expression (
EquationEquation 6) in its linearized form (
EquationEquation 7
).
where ko
is a constant. A quantity derived from the pressure dependence of the chemical rate constant k (
EquationEquation 8) is the activation volume, Va
, defined as the difference between the partial molar volume of the active state and that of the reactants (McNaught & Wilkinson, Citation1997). This property should not be confused with the reaction molar volume change ΔV (m3 mol−1), previously defined (
EquationEquation 1
). Because the active state is transient and its lifetime is too short for direct experimental quantification, values of Va
are estimated by evaluating the effect of pressure p at constant temperature T on the chemical reaction rateconstant k (Mussa & Ramaswamy, Citation1997) and obtained by linear regression of ln k versus pressure p (Equation 9).
The greater the magnitude of Va (positive or negative) the higher the sensitivity of a chemical reaction to pressure while reactions with Va = 0 are pressure independent (Mussa & Ramaswamy, Citation1997; Valdez-Fragoso et al., Citation2011). The corresponding pressure effects on Ea values are a decrease, no change or an increase if Va < 0, = 0, or >0, respectively. Mostimportantly, if a thermal degradation reaction producing a toxic compound is too slow to produce detectable amounts at conventional pressures in the relatively short time of food processing, the reaction rate will increase dramatically with pressure if it has a large negative Va value. However, reactions forming detectable amounts of toxic compounds under conventional thermal processing conditions will be inhibited by pressure if they are characterized by positive Va values reducing the toxic risk of such foods. At present, it is not possible to predict if a reaction is characterized by positive or negative Va values. This critical value in the assessment of PATP effects on food quality and safety requires experimental work for each chemical reaction in the food matrix of interest. Unfortunately, experimental work reporting Va values remains extremely limited making it very difficult to optimize the retention of nutrients (Ramirez et al., Citation2009) and assess the potential acceleration or inhibition of the formation toxic substances (Segovia Bravo et al., Citation2011). This limitation is an important constraint to the commercialization of PATP technologies, particularly in countries following the European Union novel food law model. PATP is affected by these regulations because this technology was not used before May 15, 1997 (Anonymous, Citation2002).
Pressure processing effects on low concentration compounds in foods
Research on pressure processing effects on the loss of nutrients (e.g. vitamins) and functional compounds (e.g. polyphenols), inhibition or acceleration of toxic compounds (e.g. acrylamide) formed during high temperature processing, concentration and fate of the undesirable residues originating from the migration of substances from food contact materials (e.g. plasticizers) or from chemicals used in food production (e.g. pesticides), is just beginning.
Pressure processing effects on desirable compounds
Functional compounds are substances that have preventive health effects or can enhance physiological performance. They are found in plants, animals, or produced by microorganisms and are consumed as partof a food, or added to foods in a purified or concentrated form. Nutrients are considered functional compounds if they have health benefits beyond their role in normal growth and physiological maintenance. Functional foods are those containing or formulated with functional compounds (Lockwood, Citation2007; Wildman, Citation2001a, Citation2001b).
Supplementary Table 1. Pressure processing effects on functional compounds in foods and model systems.
Tabla adicional 1. Efectos del procesado por altas presiones sobre compuestos funcionales en alimentos y sistemas modelo.
Pressure processing effects on vitamins
According to their solubility, vitamins are classified as fat-soluble and water soluble compounds. The fat-soluble vitamins (A, D, E and K) can be stored in the body and thus they do not need to be consumed on a daily basis. The water-soluble vitamins, C and the B group (thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folate, and cobalamin) must be consumed daily (Pressman & Buff, 2000). Conventional processes have detrimental effects on vitamins, particularly on vitamin C, and thus HPP research has focused on its retention.
Vitamin C
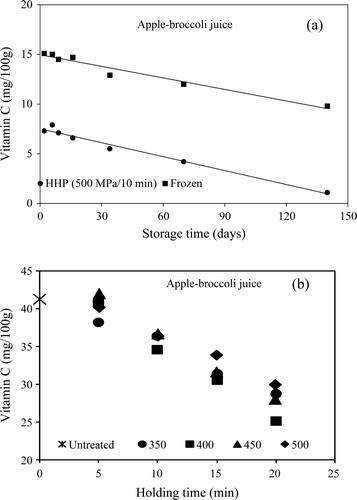
Vitamin C, present mainly as l-ascorbic and dehydroascorbic acid, is found primarily in fruits and vegetables, particularly in citrus fruits, chile, tomatoes, potatoes and greens (Eitenmiller & Landen, Citation1999). It can be classified as a functional compound due to health benefits beyond strengthening the immune system and being a cofactor for two enzymes necessary for the production of collagen and carnitine, a component of heart muscle, skeletal tissue, liver and other tissues. Vitamin C, acting as an antioxidant, may prevent oxidative damage to lipids, DNA and proteins, which has been linked to the development of chronic degenerative diseases such as cardiovascular disease, cancer and cataracts (Carr & Frei, Citation1999). The stability of vitamin C is higher in the pH 4–6 range decreasing as pH approaches its pK1 (4.04). Its degradation rate depends also on oxygen availability, presence of other antioxidants, thermal processing conditions, presence of transition metals, oxidizing lipid effects, presence of reducing substances, light, and ascorbic acid oxidase activity. l-ascorbic acid is a characteristic reductone and thus non-enzymatic Maillard browning reactions can decrease vitamin C content in foods (Eitenmiller & Landen, Citation1999). Due toits sensitivity to oxygen and temperature, this vitamin is used as an indicator in the development ofconventional (Barba, Esteve, & Frigola, Citation2010; Krebbers, Matser, Koets, Bartels, & van den Berg, 2002) and novel food preservation processes. Many studies on the retention of vitamins when using pressure processing technologies have focused on vitamin C in orange juice. Vitamin C degradation follows first order reaction kinetics with respect to treatment time, and also during storage after the HPP treatment (Houska et al., Citation2006). Some authors have found that at low pressure, vitamin C retention is inversely proportional to the pressure level, but at higher values the trend is reversed (Hsu, Tan, & Chi, Citation2008; Patras, Brunton, da Pieve, & Butler, 2009a; Patras, Brunton, da Pieve, Butler, & Downey, 2009b). Houska et al. (Citation2006) found that during the first 70 d of storage the vitamin C content in a broccoli and apple juice mixture decreased 2.2 ± 0.3 times faster for the HPP treated product (500 MPa for 10 min) than forthe frozen control. The same authors showed that holding time decreased vitamin C retention, reaching a 30% loss for a 20 min HPP treatment; however, pressure level had a minor effect. A decrease of ∼30% in ascorbic acid and total vitamin content was reported for HPP-treated (300–500 MPa for 10 min at 25°C) tomato juice with no significant effect of pressure level; and, after 28 d storage at 25°C, changes in concentration of ascorbic acid and total vitamin C were negligible (Hsu, Citation2008; Hsu et al., Citation2008). A study conducted by Sánchez-Moreno et al. (Citation2005) suggest that HPP treatment (400 MPa for 1 min at 40°C) of orange juice may oxidize l-ascorbic acid to dehydroascorbic acid. These authors found l-ascorbic acid content decreased 79% after the HPP treatment with no change in total vitamin C content during refrigerated storage. In other studies, pressure-treated green bell peppers showed a decrease of about 15–20% of ascorbic acid content, while red peppers showed an increase of about 10–20% (Castro et al., Citation2008) and yellow peepers an increase of 11 to 48% (Castro, Saraiva, Domingues, & Delgadillo, Citation2011).
Supplementary Figure 1. Vitamin C degradation kinetics. (a) Comparison during storage of frozen and high-pressure processed (HPP) samples. (b) Effect of high pressure holding time and pressure level (adapted from Houska et al., 2006).
Figura adicional 1. Cinética de degradación de la vitamina C. a) Comparación durante el almacenamiento de muestras congeladas y tratadas por alta presión hidrostática (APH). b) Efecto del tiempo de presurización y nivel de alta presión hidrostática. (Adaptado de Houska et al., 2006).
Folate
The term folate designates microbial- and plant-synthesized compounds based on a pteridine ring (acid N-[(6-pteridinil) methyl]-p-amino benzoic acid) conjugated with one or more units of l-glutamic acid. Although folic acid is not found in nature, it is more stable and thus it is preferably used in fortified foods and drug formulations. The metabolically active form of folic acid is the coenzyme tetrahydrofolate which has a pteridine ring and several glutamic acid residues. Folic acid can help prevent cervical cancer and perhaps other types of cancer. Lack of folic acid causes homocysteine accumulation in the blood and damage to arteries leading to cardiovascular disease. Along with pyridoxine and cobalamin, folic acid participates in the elimination of homocysteine from the body (de Vriese, Verbeke, Schrijvers, & Lameire, Citation2002). Loss of folate occurs through oxidative cleavage of the C–9N–10 bond following first order kinetics. Reducing agents such as vitamin C protect folate during thermal processing. Folate losses are higher in aerobic environments increasing with light exposure and the presence of metals (e.g. Fe2+) and sodium nitrite. In the pH 5–12 range and in the absence of light, folic acid is relatively stable up to 100°C. The number of glutamate residues attached to folate does not influence stability. Regarding the effects of pressure on folate, studies done by Verlinde, Indrawati, Hendrickx, and van Loey (Citation2008) have shown that the folate content of broccoli to be largely influenced by treatment conditions, 48 to 78% losses were observed after 100 to 600 MPa 25 min treatments at 25 to 45°C.
Vitamin E, tocotrienols and tocopherols
Vitamin E is the term used for fat-soluble 6-hydroxychroman compounds exhibiting the biological activity of α-tocopherol. Vitamin E is a natural antioxidant for tissues containing unsaturated fatty acids. The vitamin E family includes α-, β-, γ-, and δ-tocopherol characterized by a saturated side chain with three isoprenoid units, and the corresponding unsaturated α-, β-, γ-, and δ-tocotrienol with double bonds at the 3,7, and 11 position of the isoprenoid side chain. Tocopherols and tocotrienols vary structurally depending on the number and location of methyl groups on the chromanol ring. Vitamin E is an important antioxidant, and together with vitamin C, protects low-density lipoproteins (LDL) from oxidation reactions known to initiate atherosclerosis (Eitenmiller & Landen, Citation1999). The vitamin E antioxidant activity is significantly affected by light, heat, alkali pH, lipoxidase reactions, metals such as iron and copper, and free radicals. In the absence ofoxygen, tocopherols and tocotrienols are stable to heat and alkali. Regarding the effects of pressure on these compounds, a recent study showed that 5 min treatments at 400 to 600 MPa do not decrease significantly the concentration of γ-, δ- and α-tocopherol in human milk (Moltó-Puigmartí, Permanyer, Castellote, & López-Sabater, Citation2011).
Vitamin A and carotenoids
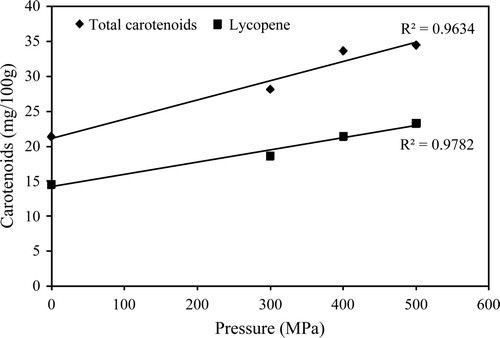
Vitamin A can be defined as isoprenoid compounds with the biological activity of all-trans retinol (Eitenmiller & Landen, Citation1999), a β-ionone ring with a side chain of 3 isoprenoid units linked at the 6 position of the ring. The conjugated double bound system includes 5,6-β-ionone ring carbons and the isoprenoid side chain. The carotenoids α-, β- and γ-carotene and β-criptoxanthin are considered vitamin A precursors because they have at least one non-hydroxylated β-ionone ring with a C11 polyene chain. Lycopene, presenting a linear structure, is the simplest carotenoid. Modifications in its structure lead to all other carotenoids found in nature. Carotenoids found frequently in fruits are lycopene, β-carotene, α-cryptoxanthin, β-cryptoxanthin, zeaxanthin, violaxanthin, and lutein. Most carotenoids in ripe fruits are esterified with fatty acids but can be found free in some fruits and vegetables (Rodriguez-Amaya, Citation2001). In addition to their provitamin A activity, carotenoids have shown beneficial effects on the initiation, progression and proliferation of cancer; reduction of cardiovascular disease, and prevention of macular degeneration (Faulks & Southon, 2001). Vitamin A is sensitive to oxygen, light, and acid pH. Sánchez-Moreno et al. (Citation2005) showed that treating orange juice at 400 MPa for 1 min at 40°C increases its vitamin A content by 38.7% suggesting a pressure increase of vitamin A extractability, or that some precursors are converted to vitamin A. However, these probable causes for the vitamin A content increase have not been evaluated experimentally. Carotenoids are susceptible to isomerization and oxidation during processing and storage resulting in the loss of color and biological activity and the formation of volatile compound affecting sensory properties. Oxidation depends on the presence of oxygen, metals, enzymes, unsaturated lipids, prooxidants, antioxidants, light exposure, type and physical state of carotenoids, treatment severity, packaging material, and storage conditions. Thermal treatments promote trans-cis isomerization (Rodriguez-Amaya, Citation2001). While some authors report significant losses in total carotenoid content (Barba et al., Citation2010; Patras et al., 2009a, 2009b), others have reported no significant changes immediately after the HPP treatment or during storage (Carreño, Gurrea, Sampedro, & Carbonell, Citation2011; Esteve, Barba, Palop, & Frigola, Citation2009; Fernández-García, Butz, Bognàr, & Tauscher, Citation2001; Houska et al., Citation2006; McInerney, Seccafien, Stewart, & Bird, Citation2007). In orange juice treated at 400 MPa for 5 min, Esteve et al. (Citation2009) found that changes in total carotenoid content were insignificant when compared with fresh product; however, after 1 week storage at 4 and 10°C, about 10% of carotenoids were degraded, and after 6 weeks the degradation was about 75%; however, these losses were lower than those for thermally treated products. Hsu et al. (Citation2008) reported that 300 to 500 MPa treatments for 10 min at 25°C increased the extractability of total carotenoids and lycopene of tomato juice. De Ancos et al. (Citation2002) showed that treating orange juice at 100 to 350 MPa for 5 min at 30°C increased total carotenoids by 20 to 43%, and α-, β-carotene, α- and β-criptoxanthin by 60, 50, 63 and 42%, respectively. Sánchez-Moreno et al. (Citation2005) showed that a 400 MPa treatment of orange juice for 1 min at 40°C increased α-, β-criptoxanthin, zeaxanthin, lutein, α-carotene, β-carotene, and total carotenoids by 45.8, 43.2, 44.5, 75.4, 33.8, 30.2, and 53.9%, respectively. Varma, Karwe and Lee (Citation2010) observed in tomato puree treated at 320–620 MPa for 3 min, an increase of about 35 and 50% in the cis-lycopene and all-trans isomers, respectively, compared with the untreated control suggesting that HPP causes conformational changes of this carotenoid. Qiu et al. (Citation2006) reported in tomato puree treated at 100 to 600 MPa for 12 min at 20±1°C, total lycopene increased slightly without a significant pressure level effect, except that at 500 MPa an increase of 21% was obtained. No explanation for the increase at this particular pressure was provided. No significant changes in the percentage of 13-cis isomer lycopene were observed after HPP treatment compared with the untreated sample. In general, lycopene loss and conformational changes during storage appear to follow first order kinetics.
Supplementary Figure 2. Effect of high-pressure processing (HPP) at 300 to 500 MPa for 10 min at 25°C on the total carotenoids and lycopene content of tomato juice (adapted from Hsu, 2008).
Figura adicional 2. Efecto del procesado por alta presión hidrostática (APH) sobre el contenido total de carotenoides y licopeno en zumo de tomate (Adaptado de Hsu, 2008).
Pressure processing effects on phenolic compounds
Phenolic compounds, classified as flavonoids and non-flavonoids, influence the taste, flavor and appearance of foods, and because of their health-promoting properties (Tomás-Barberán & Espín, Citation2001) can be considered functional compounds. Non-flavonoids include phenolic acids (benzoic and hydroxycinnamic acids), stilbenes, and gallotannins (Cheynier, Citation2005; Tapas, Sakarkar, & Kabde, Citation2008).Flavonoids include anthocyanins, flavonols, flavanols, flavones, flavanones, isoflavones and proanthocyanidins (Tripoli, La Guadia, Giammanco, Di Majo, & Diammanco, Citation2007). Flavonoids are found as glycosides, aglycones and methylated derivatives (Tapas et al., Citation2008). The conjugation of flavonoids with sugars is most common. Composition of flavonoids in fruits and vegetables is varied but some flavonoids are restricted to specific foods such as flavanones found in citrus fruits only (Gattuso, Barreca, Gargiulli, Leuzzi, & Caristi, Citation2007). Flavonoids exhibit antioxidant, anti-inflammatory, antiviral, antimicrobial and antiallergenic activities; they also inhibit human platelet aggregation and can chelate metals (Tapas et al., Citation2008). Epidemiological studies have shown an inverse relationship between dietary flavonoid intake and incidence of cardiovascular diseases and cancer (Hertog, Hollman, & van de Putte, Citation1993). Some flavonoids such as quercetin have shown antidiabetic effects (Tapas et al., Citation2008). Phenolic compounds are highly unstable yielding various reaction products when fruits are damaged and during their processing and storage (Cheynier, Citation2005). Losses between 75 to 80% of the quercetin content of onion and tomatoes were observed after boiling for 15 min, 65% after microwave oven cooking, and 30% after frying (Crozier, Lean, McDonald, & Black, Citation1997). Studies on HPP effects on phenolic compounds show that in most cases, pressure increases the concentration of phenolic compounds. Ferrari, Maresca and Ciccarone (Citation2010) reported increases of 41% in the polyphenol content of pomegranate juice after 400 MPa for 10 min at 50°C while conventional thermal treatments at the same temperature/time showed no effect. At pressures higher than 400 MPa, or for longer treatment times, the polyphenol content decreased or remained unaffected. Xi et al. (Citation2009) showed that for 1 to 10 min treatments increased the extraction yield of polyphenols by 15, 18, 23, 26, 30, and 30% at pressures of 100, 200, 300, 400, 500, and 600 MPa, respectively, with no significant effect of treatment time. In orange juice, HPP increased the extraction of flavonoids, and their concentration did not change after 10 d at 4°C (Sanchez Moreno, Plaza, de Ancos, & Cano, Citation2003). Sánchez-Moreno et al. (Citation2005) reported in orange juice increases of 20.2, 39.9, and 34.6% in total flavanones, naringin and hesperetin, respectively, after 400 MPa for 1 min at 40°C. Losses of some phenolics have been reported also. Lambert et al. (Citation1999) found that cinnamic acid decreased by 29 and 20% at 200 and 500 MPa, respectively, when compared with the control, but at 800 MPa cinnamic acid increased by 415%, i.e. from 113 to 582 μg/kg. Some researchers have reported that HPP has minimal influence on the anthocyanin content of fruit juices (Tiwari, O'Donnell, & Cullen, Citation2009). Corrales, Fernández García, Butz and Tauscher (Citation2009) studied the effect of 0.1 and 200 to 600 MPa for 30 min at 20°C to 90°C on anthocyanin compounds. The highest concentration of total anthocyanin monoglucosides was obtained at 200 MPa while 600 MPa yielded more acylated anthocyanin glucosides. The amount of anthocyanins extracted with HPP combined with temperature was 1.2 to 1.9 times higher than with thermal treatments at 0.1 MPa at the same temperature.
Pressure processing effects on antioxidant activity
One of the mechanisms by which functional compounds exert their beneficial effects in human health has been related to their antioxidant activity. Phenolics in fruits and vegetables, as well as vitamin C, are said to be effective antioxidants. It has been shown that vitamin C contributes in 100% to the total antioxidant activity of Florida orange juice (Gardner, White, McPhail, & Duthie, Citation2000). Vitamin C scavenges free radicals such as O2−, OH−, peroxy radicals and singlet oxygen, protecting the intracellular and extracellular structures (Francis, Citation2000; Gardner et al., Citation2000). Carotenoids prevent potentially damaging radical production due to their polyene structure (Faulks & Southon, 2001). The antioxidant activity is the most important bioactivity of functional compounds studied in high-pressure technologies. The effect of HPP and PATP on this property has shown contradictory results. While some authors suggest that HPP increases antioxidant activity (Corrales et al., Citation2009), others have found the opposite effect (Barba et al., Citation2010) or reported no effects (Sanchez Moreno et al., Citation2003).
Pressure processing effects on undesirable abiotic contaminants in foods
An increasing demand for processed foods with higher quality, safety, and convenience, particularly in advanced economy countries, and reflecting new social consumer habits makes it desirable and financially feasible to implement innovative food processing technologies. However, the potential risks of toxic substances formed in the food, or originated from abiotic substances caused, or increased by novel treatments such as HPP, are not fully known. There are many examples of undesirable and even toxic compounds formed during high-temperature processing and home preparation of foods including carcinogenic and mutagenic compounds such as acrylamide, heterocyclic amines (HCAs), polycyclic aromatic hydrocarbons (PAHs), and chloropropanols (Studer, Blank, & Stadler, Citation2004). Food processors must also demonstrate that their products do not contain detectable amounts of toxic compounds coming from packaging materials or present as phytosanitary applications residues. Packaging polymers may contain also high levels of trace elements affecting food quality (Dayel, Horayess, Hefni, & Durahim, Citation2009). The greatest exposure to pesticides comes from residues in food (Kraybill, Citation1969) but these are reduced by processing and food preparation processes (Kaushik, Satya, & Naik, Citation2009; Keikotlhaile, Spanoghe, & Steurbaut, Citation2010). Furthermore, toxic substances found in foods may be transformed into various toxic metabolites as in the case of some PAHs (Fournier, Feidt, Dziurla, Grandclaudon, & Jondreville, Citation2010), pesticides (Ahmed, Citation2001; Kan & Meijer, Citation2007), and packaging components (Chen, Chen, Tang, & Mao, Citation2008). This makes it very difficult to identify all toxic substances of consumer risk.
Supplementary Table 2. Potentially toxic compounds formed during food processing.
Tabla adicional 2. Compuestos potencialmente tóxicos generados durante el procesado de alimentos.
Acrylamide
Content of acrylamide (CH2=CHCONH2, CAS Registry Number 79–0601) in foods ranges from <1 to 8000 μg/kg (Anonymous, Citation2005b) and is formed during heat treatments as a result of the Maillard reaction between free amino acids and reducing sugars (Mottram, Wedzicha, & Dodson, Citation2002). It can be found in potato products (crisps, chips, French fries), bakery products (bread, biscuits, crackers), cereals (roasted grains, popcorn, barley), drinks (beer, roasted coffee, tea), pasta (noodles), poultry, fish, seafood, nuts and baby foods (Anonymous, Citation2005b). Important levels of acrylamide residues in starch-based foods were detected first in 2002 by Swedish authorities (www.mindfully.org/Food/Acrylamide-Heat-Processed-Foods26apr02.htm, accessed 11 November 2010) and had a major international impact since this compound is considered probably carcinogenic to humans by theInternational Agency for Research on Cancer (Weisshaar, Citation2004a). Under conventional thermal processing, acrylamide formation requires a minimum of ∼100°C while temperatures in excess of 120°C yield significantly higher amounts (Pedrenski, Citation2007), a consideration particularly important in fried, baked and grilled potato products which have been found to be a significant source of dietary acrylamide. The concentration of free asparagine and free reducing sugar are reaction-limiting factors (Mottram et al., Citation2002; Stadler, Blank, & Varga, Citation2002; Weisshaar, Citation2004b; Yaylayan, Wnorowski, & Perez Locas, Citation2003; Zhang & Zhang, Citation2007; Zyzak et al., Citation2003). Reaction steps in the acrylamide formation pathway characterized by large negative Va values will be accelerated. Moreover, the pressure-induced pH shift during PATP treatments could also affect reaction rates. Similar situations couldbe true for the formation at high temperature of other toxic compounds (Segovia Bravo et al., Citation2011). The combined effects of temperature and pressure on acrylamide formation has been studied (Hill, Ledward, & Ames, Citation1996; Isaacs & Coulson, Citation1996; Jaeger, Janositz, & Knorr, Citation2010; Moreno, Molina, Olano, & López-Fandiño, Citation2003; Schwarzenbolz, Klostermeyer, & Henle, Citation2000, Citation2002) but although PATP conditions affect reaction rates, i.e. increasing or decreasing if Va values are negative or positive, respectively, none of these studies have included determination of Va values. Some studies have evaluated PATP effects on end Maillard reaction products responsible of flavor or browning (Deters, Hofmann, & Schieberle, 2003; Heberle, Schieberle, & Hofmann, 2003) while others have determined the increase or decrease on the formation of intermediate or final products (Isaacs & Coulson, Citation1996; Moreno et al., Citation2003; Schwarzenbolz et al., Citation2000, Citation2002). Although, these findings had raised further concerns about acrylamide formation, a very recent work based on model systems has shown thatPATP decreases the formation of acrylamide (deVleeschouwer, van der Plancken, van Loey, & Hendrickx, 2011). However, confirmation of this favorable finding in foods and determination of Va values are still pending.
Chloropropanols and chloropropanol fatty esters
The presence of fatty acid esters of 3-monochloro-1,2-propanediol (3-MCPD esters) has been studied in foods and food ingredients, particularly in refined vegetable oils (Seefelder & Schilter, Citation2011). These compounds were identified at the end of 1970s from model solutions containing hydrochloric acid and lipids. Crews et al. (Citation2003) reported that acid hydrolysis produce a number of chloropropanols in acid-hydrolyzed vegetable products (Velisek et al., Citation1978). Chloropropanols are formed in protein hydrolysates by the reaction of hydrochloric acid with residual lipids associated with the proteinaceous materials used in their production (Collier, Cromie, & Davies, Citation1991). They were described as intermediate products in the formation pathway of MCPDs and dichloropropanols (DCPs) (Davidek, Velíšek, Kubelka, Janíĉek, & Ŝimicová, Citation1980). A recent review has reported that 3-MCPD esters are formed during processing togetherwith a number of other structurally related and toxicologically relevant chemicals such as 2-monochloro-1,3-propanol esters (2-MCPD esters) and glycidyl esters (Schilter, Scholz, & Seefelder, Citation2011). The safety significance of these substances is difficult to appreciate because of insufficient data (Seefelder & Schilter, Citation2011). 3-MCPD is classified as a non-genotoxic threshold carcinogen with a provisional maximum tolerable daily intake of 2 μg kg body weight−1 d−1 (Eisenbrand & Habermeyer, Citation2010). Maximum limits for 3-MCPD have been set for acid hydrolyzed vegetable protein (acid HVP) and soy sauce in regulations of the European Union and by Codex Alimentarius, ranging from 0.02 to 1.0 mg kg−1 (Anonymous, Citation2001, Citation2005a). In the literature reviewed, related to foods processed by HPP and PATP, no studies on pressure effects on the formation or levels of chloropropanols and its esters were found.
Aromatic toxic food compounds (PAHs and HCAs)
PAHs are a group of compounds comprised of two or more fused aromatic rings. Due to their carcinogenic activity, PAHs have been included in the European Union (EU) and the US Environmental Protection Agency (EPA) priority lists of toxic risks. Diet is the largest source of human exposure to these contaminants (88–98%) (Tepe, Daferera, Sokmen, Sokmen, & Polissiou, Citation2005). Their presence in foods depends strongly on the cooking method with grilling and smoking of meat, fish and other meats as important sources of PAH formation in foods (Farhadian, Jinap, Abasa, & Sakara, Citation2010; García Falcón, González Amigo, Lage Yusty, & Simal Lozano, Citation1999; García Falcón, González Amigo, Lage Yusty, López de Alda Villaizán, & Simal Lozano, 1996; García-Falcón & Simal-Gándara, Citation2005; García-Falcón, Cancho-Grande, & Simal-Gándara, Citation2005; Ishizaki, Saito, Hanioka, Narimatsu, & Kataoka, Citation2010). In these foods, the production of PAHs increases linearly in the 400 to 1000°C range. At these high temperatures, organic compounds are fragmented producing large a number of relatively stable PAHs (Jägerstad & Skog, Citation2005). However, their production under extreme pressure conditions, 600–800 MPa, combined with the application of lower temperatures (80–120°C) has not been studied. At this time, the kinetics of these chemical reactions at the high pressure and elevated temperature of PATP treatments remains unknown (Segovia Bravo et al., Citation2011). Another group of toxic compounds formed during food heating are HCAs characterized by two or three rings with an exocyclic amino group attached to one of the rings (Nagao, Honda, Seino, Yahagia, & Sugimura, Citation1977). The formation of HCAs appears to bethe result of the condensation via the Maillard reactionof free amino acids, creatine, creatinine, monosaccharides, disaccharides and dipeptides, all of which may act as precursors during high temperature cooking (Jägerstad & Skog, Citation2005; Jagerstad, Skog, Arvidsson, & Solyakov, 1998; Pais, Salmon, Knize, & Felton, Citation1999). The formation of HCAs has been reported at temperatures between 125–300°C (Jagerstad et al., 1998); therefore, its formation risk in PATP-treated foods is extremely high (Segovia Bravo et al., Citation2011).
Supplementary Table 3. Polycyclic aromatic hydrocarbons (PAHs) of toxic concern in foods (adaped from Segovia Bravo et al., 2011).
Tabla adicional 3. Hidrocarburos Policíclicos Aromáticos (HPAs): compuestos tóxicos considerados un riesgo potencial en alimentos procesados (adaptado de Segovia Bravo et al., 2011).
Pressure processing effects on substances from food packaging materials
Polymers combined with crosslinking agents, additives, solvents, catalysts and other compounds are used in single and multiple layers, or combined with other materials, to form the packaging solutions (Piringer & Baner, Citation2000) used for pressure-treated foods. Since most foods are pressure-treated after packaging, it is necessary to study food and package interactions withthe HPP/PATP process including effects of the pressure transmitting fluid (Devlieghere, Vermeiren, & Debevere, Citation2004; Ozen & Floros, Citation2001). In the case of PATP-treatments, the package has to retain physical integrity and chemical composition at high temperature and pressure. Most studies on food packaging materials used for HPP-treated foods have focused on the modification of physical and mechanical properties such as tensile strength, delamination, wrinkling, elongation at failure point, film thickness and melting point temperature (Galotto et al., Citation2008; Lambert et al., Citation2000). There is a need for further research on HPP effects on mass transfer processes between food, packaging films, and storage environment (Pereira & Vicente, Citation2010). These mass transfer processes can be grouped into permeation (mass transfer across the packaging material in both directions), sorption or scalping (food constituents passing into the packaging material from the food), and migration (packaging constituent passing into the food). HPP/PATP-packaging-food-environment interactions are affected by the polymer type (single or multilayer structures), food composition (fat and water content, pH, etc.), processing conditions, pressurizing fluid, and subsequent storage conditions. The analysis of these interactions is complex since packaging materials contain multiple components with specific composition sometimes unknown to the food processor. The possibility of packaging components, and packaging degradation substances formed during high temperature and pressure processing, transferring into foods where they can experience further chemical changes has to be investigated. For example, in the case of Bisphenol A and Novolac epoxy resins used to coat food cans, past research included determinations of multiple resin derivatives formed during their application to cans and from food interactions under the storage conditions used (Paseiro Losada, Pérez Lamela, López Fabal, Sanmartín Fenollera, & Simal Lozano, Citation1997; Sendón García, Paseiro Losada, & Pérez Lamela, 2003). Research published on modifications of mass transfer affecting the barrier properties of plastic packaging materials by pressure processing technologies include studies on moisture (Le-Bail, Hamadami, & Bahuaud, Citation2006), oxygen, and carbon dioxide permeability (Caner, Hernandez, & Harte, Citation2004); sorption of volatile compounds (Caner, Hernandez, Pascall, Balasubramaniam, & Harte, Citation2004) and migration phenomena (Galotto et al., Citation2010). Although these interactions are well-studied for plastic food packaging materials used in foods treated by conventional technologies (e.g. Sajilata, Savitha, Singhal, & Kanetkar, Citation2007), information on HPP/PATP effects on package-food-environment interactions is severely limiting (Segovia Bravo et al., Citation2011).
Pressure processing effects on pesticide residues
Pesticides are compounds used to control pests, increase shelf-life and retain quality. Legislations controlling their production, marketing and use (e.g. Anonymous, Citation1983) defined them as substances or a mixture of substances used to control harmful agents for plants or prevent their action; facilitate or regulate vegetal production but not including compounds used as nutrients or soil fertilization; preserve vegetal products including wood; destroy undesirable vegetal organisms; destroy part of vegetable material; prevent undesirable growth of plants; turn inoffensive a harmful organism; and, destroy or prevent the action of harmful organisms. Strict legal requirements specify the limit of maximum residual (LMR) level allowed in foods to be consumed. Since one of the most common routes of consumer pesticide exposure is food consumption (Keikotlhaile, Spanoghe, & Steurbaut Citation2010), a pesticide treatment must control the pest factor while minimizing adverse effects on the commodity quality and safety (Follett & Neven, Citation2006). Food processing causes significant reductions in the amount of pesticide residues. In the case of fruits and vegetables (Keikotlhaile et al., Citation2010), these processes include washing, blanching, peeling, pureeing, cooking, canning, roasting, frying, drying, milling, fermentation, thermal treatments, freezing, and boiling. As in the case of packaging materials in contact with foods, pesticide formulations contain multiple compounds that could also pass into foods and by multiple chemical reactions generate various byproducts depending on food composition, processing factors, and storage conditions. Evaporation, co-distillation and/or thermal degradation have been shown to modify pesticides inbaked foods (Sharma, Satya, Kumar, & Tewary, Citation2005); grapes (Athanasopoulos, Pappas, Kyriakidis, & Thanos, Citation2005), cherries (Fahey, Nelson, & Ballee, Citation1970), tomato products (Kontou, Tsipi, & Tzia, Citation2004), and apricot (Cabras et al., Citation1998). The toxicity of degradation compounds has to be studied because these derivatives may exhibit higher or lower toxicity than the components in the untreated pesticide formulation. Again, HPP and PATP effects have not been determined on these pesticide formulations. Therefore, the fate of residual pesticide formulations in foods subjected to these pressure processing technologies remains unknown.
Conclusions
A kinetic approach is recommended to study the effect of pressure processing technologies, particularly when implementing pressure-assisted thermal processing (PATP), on the concentration of minor desirable chemicals such as nutrients and functional ingredients, toxic compounds formed by chemical reactions of food components, and on toxic compounds transferred from food contact materials or from pesticide applications. These compounds can be further transformed by chemical reactions in the food. This kinetic approach should include the determination of the pressure-induced pH shift and its effect on the various steps in the pathway of a chemical reaction, and most importantly the estimation of Va values for these reaction steps. Unfortunately probes to measure the pH shift are still in the development process, and information on Va values is extremely limited. These knowledge gaps must be overcome to ensure the production of HPP/PATP foods of high quality and safety.
In general, HPP causes no significant losses of functional compounds in foods, and often HPP has been to induce much lower losses than conventional thermal processes. Vitamin C, carotenoids and folate are among the most studied compounds but Va values, particularly when using PATP treatments, are generally unavailable. Additional research is required on important compounds such as vitamin E. Polyphenols seems to be favored by HPP treatments and in some cases HPP may increase their availability. Studies performed on antioxidant activity are few and contradictory. This may reflect the diverse methods used to quantify antioxidant activity in different foods.
Several toxic compounds of abiotic origin can be present or formed in foods processed by pressure-processing technologies. Some of them result from thermal processing such as acrylamide, PAHs, HCAs and chloropropanols esters, while others come from production processes as residues of plastic packaging and other food contact materials, and still others are residues from pesticide applications. While HPP treatments have been shown to have some beneficial effects on packaging properties, the literature on the effects of pressure-processing technologies on these compounds is still incomplete, particularly for PATP treated foods. Also research is needed to find potential reactions and degradation products of these compounds, and when consumed one needs to determine the potential toxicity of their metabolites.
Acknowledgments
General support to the University of Vigo research group was provided from the European Regional Development Fund (ERDF). Nattaporn Chotyakul and Mirian Pateiro Moure acknowledge Xunta de Galicia for their contracts sponsorship through the Research Project funded by the INCITE program of the Galician Council of Innovation and Industry (Ref. 09TAL019383PR). Authors Zamantha Escobedo-Avellaneda and Jorge Welti-Chanes acknowledge the financial support from Tecnológico de Monterrey (Research Chair Funds CAT-200), and CONACYT-SEP (Research Project 101700 and Scholarship Program).
References
- Ahmed , F. E. 2001 . Analyses of pesticides and their metabolites in foods and drinks . Trends in Analytical Chemistry , 20 : 649 – 661 .
- Anonymous. 1983 . “ Technical-sanitary regulation to produce, market and use pesticides ” . In Real Decreto 3349/1983, 30th November. State Official Bulletin BOE Number 20, 24/01/84
- Anonymous. 2001 . European Commission. Setting maximum levels for certain contaminants in foodstuffs: Commission Regulation (EC) No. 466/2001 . Official Journal of the European Communities , L77 : 1 – 13 .
- Anonymous. 2002 . Discussion paper: Implementation of Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients , Brussels, BE : Directorate General Health and Consumer Protection (SANCO D4), European Commission .
- Anonymous. 2005a . Discussion Paper on Chloropropanols. Codex Committee on Food Additives and Contaminants 05/37/32 The Hague, The Netherlands
- Anonymous. 2005b . “ Summary and conclusions of the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) ” . Geneva, Switzerland
- Athanasopoulos , P. E. , Pappas , C. , Kyriakidis , N. V. and Thanos , A. 2005 . Degradation of methamidophos on soultanina grapes on the vines and during refrigerated storage . Food Chemistry , 91 : 235 – 240 .
- Aubourg, S.P., Vázquez, M., Torres, J.A., Lavilla, M., Saraiva, J., & Gallardo, J.M. (2011) Aplicación de la tecnología de altas presiones hidrostáticas en la mejora de la calidad de especies pelágicas grasas congeladas. Ruta Pesquera, 78 (Noviembre), 76, 78
- Barba , F. J. , Esteve , M. J. and Frigola , A. 2010 . Ascorbic acid is the only bioactive that is better preserved by high hydrostatic pressure than by thermal treatment of a vegetable beverage . Journal of Agricultural and Food Chemistry , 58 : 10070 – 10075 .
- Bárcenas , M. E. , Altamirano-Fortoul , R. and Rosell , C. M. 2010 . Effect of high pressure processing on wheat dough and bread characteristics . Lebensmittel Wissenschaft und Milchwirtschaft , 43 : 12 – 19 .
- Bermúdez-Aguirre , D. and Barbosa-Cánovas , G. V. 2011 . An update on high hydrostatic pressure, from the laboratory to industrial applications . Food Engineering Reviews , 3 ( 1 ) : 44 – 61 . doi:10.1007/s12393-010-9030-4
- Cabras , P. , Angioni , A. , Garau , V. L. , Melis , M. , Pirisi , F. M. , Cabitza , F. and Cubeddu , M. 1998 . Pesticide residues onfield-sprayed apricots and in apricot drying processes . Journal of Agricultural and Food Chemistry , 46 : 2306 – 2308 .
- Campus , M. 2010 . High pressure processing of meat, meat products and seafood . Food Engineering Reviews , 2 : 256 – 273 .
- Caner , C. , Hernandez , R. J. and Harte , B. R. 2004 . High-pressure processing effects on the mechanical, barrier and mass transfer properties of food packaging flexible structures: A critical review . Packaging Technology and Science , 17 ( 1 ) : 23 – 29 .
- Caner , C. , Hernandez , R. J. , Pascall , M. , Balasubramaniam , V. M. and Harte , B. R. 2004 . The effect of high-pressure food processing on the sorption behaviour of selected packaging materials . Technology and Science , 17 : 139 – 153 .
- Cardello , A. V. 2003 . Consumer concerns and expectations about novel food processing technologies: Effects on product liking . Appetite , 40 : 217 – 233 . doi:10.1016/S0195-6663(03)00008-4
- Cardello , A. V. , Schutz , H. G. and Lesher , L. L. 2007 . Consumer perceptions of foods processed by innovative and emerging technologies: A conjoint analytic study . Innovative Food and Emerging Technologies , 8 : 73 – 83 . doi:10.1016/j.ifset.2006.07.002
- Carr , A. C. and Frei , B. 1999 . Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans . American Journal of Clinical Nutrition , 69 : 1086 – 1107 .
- Carreño , J. M. , Gurrea , M. C. , Sampedro , F. and Carbonell , J. V. 2011 . Effect of high hydrostatic pressure and high-pressure homogenisation on Lactobacillus plantarum inactivation kinetics and quality parameters of mandarin juice . European Food Research and Technology , 232 : 265 – 274 .
- Castro , S. M. , Saraiva , J. A. , Domingues , F. M.J. and Delgadillo , I. 2011 . Effect of mild pressure treatments and thermal blanching on yellow bell peppers (Capsicum annuum L.) . Lebensmittel Wissenschaft und Technologie , 44 : 363 – 369 .
- Castro , S. M. , Saraiva , J. A. , Lopes-da-Silva , J. A. , Delgadillo , I. , van Loey , A. , Smout , C. and Hendrickx , M. 2008 . Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.) . Food Chemistry , 107 : 1436 – 1449 .
- Chen , J. S. , Chen , C. L. , Tang , C. L. and Mao , I. F. 2008 . The internal exposure of Taiwanese to phthalate. An evidence of intensive use of plastic materials . Environment International , 34 : 79 – 85 .
- Cheynier , V. 2005 . Polyphenols in foods are more complex than often thought . The American Journal of Clinical Nutrition , 81 : 223S – 229S .
- Collier , P. D. , Cromie , D. D.O. and Davies , A. P. 1991 . Mechanism of formation of chloropropanols present in protein hydrolysates . Journal of the American Oil Chemists Society , 68 : 785 – 790 .
- Corrales , M. , Fernández García , A. , Butz , P. and Tauscher , B. 2009 . Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure . Journal of Food Engineering , 90 : 415 – 421 .
- Crews , C. , Hasnip , S. , Chapman , S. , Hough , P. , Potter , N. , Todd , J. and … Matthews , W. 2003 . Survey of chloropropanols in soy sauces and related products purchased inthe UK in 2000 and 2002 . Food Additives and Contaminants , 20 : 916 – 922 .
- Crozier , A. , Lean , M. E.J. , McDonald , M. S. and Black , C. 1997 . Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery . Journal of Agricultural and Food Chemistry , 45 : 590 – 595 .
- Davidek , J. , Velíšek , J. , Kubelka , V. , Janíĉek , G. and Ŝimicová , Z. 1980 . Glycerol chlorohydrins and their esters as products of the hydrolysis of tripalmitin, tristearin and triolein with hydrochloric acid . Zeitschrift fuer Lebensmittel-Untersuchung und-Forschung , 171 : 14 – 17 .
- Dayel , A. O. , Horayess , A. O. , Hefni , J. and Durahim , A. A. 2009 . Trace elements in packaging polymers . Research Journal of Chemistry and Environment , 13 ( 1 ) : 92 – 98 .
- de Ancos , B. , Sgroppo , S. , Plaza , L. and Cano , M. P. 2002 . Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment . Journal of the Science of Food and Agriculture , 82 : 790 – 796 .
- de Vleeschouwer , K. , van der Plancken , I. , van Loey , A. and Hendrickx , M. E. 2011 . The effect of high pressure-high temperature processing conditions on acrylamide formation and other Maillard reaction compounds . Journal of Agricultural and Food Chemistry , 58 : 11740 – 11748 . doi:10.1021/jf102697b
- de Vriese , A.S. , Verbeke , F. , Schrijvers , B. F. and Lameire , N. H. 2002 . Is folate a promising agent in the prevention and treatment of cardiovascular disease in patients with renal failure? . Kidney International , 61 : 1199 – 1209 .
- Deters , F. , Hofmann , T. and Schieberle , P. 2003 . “ Influence of high hydrostatic pressure on the formation of key Maillard-type flavour compounds from d-glucose and l-proline ” . In Advances in high pressure bioscience and technology , Edited by: Winter , R. Vol. 2 , 347 – 350 . Berlin : Springer Verlag .
- Devlieghere , F. , Vermeiren , L. and Debevere , J. 2004 . New preservation technologies: Possibilities and limitations . International Dairy Journal , 14 : 273 – 285 .
- Eisenbrand , G. , Engel , K. H. , Werner , W. , Hartwig , A. , Knorr , D. , Knusden , L. and … Vieths , S. 2007 . Thermal processing of food: Potential health benefits and risks , Weinheim : Wiley-VCH Verlag GmbH & Co. KGaA .
- Eisenbrand , G. and Habermeyer , M. 2010 . Where are thedangers lurking? Toxicological assessment of acrylamide and 3-monochloropropane-1,2-diol . Aktuel Ernahrungsmed , 35 : S22
- Eitenmiller , R. R. and Landen , W. O. 1999 . Vitamin analysis for the health and food sciences , Boca Raton FL : CRC Press Inc .
- Esteve , M. J. , Barba , F. J. , Palop , S. and Frigola , A. 2009 . The effects of non-thermal processing on carotenoids in orange juice . Czech Journal of Food Sciences , 27 : S304
- Evans , G. and Cox , D. N. 2006 . Australian consumers’ antecedents of attitudes towards foods produced by novel technologies . British Food Journal , 108 : 916 – 930 .
- Fahey , J. E. , Nelson , P. E. and Ballee , D. L. 1970 . Removal of Gardona from fruit by commercial preparative methods . Journal of Agricultural and Food Chemistry , 18 : 866 – 868 .
- Farhadian , A. , Jinap , S. , Abasa , F. and Sakara , Z. I. 2010 . Determination of polycyclic aromatic hydrocarbons in grilled meat . Food Control , 21 : 606 – 610 .
- Faulks , M. and Southon , S. 2001 . “ Carotenoids, metabolism and disease ” . In Handbook of nutraceuticals and functional foods , Edited by: Wildman , R.E.C. 143 – 156 . Boca Ratón FL : CRC Pres, Inc .
- Fernández-García , A. , Butz , P. , Bognàr , A. and Tauscher , B. 2001 . Antioxidative capacity, nutrient content and sensory quality of orange juice and an orange-lemon-carrot juice product after high pressure treatment and storage in different packaging . European Food Research and Technology , 213 : 290 – 296 .
- Ferrari , G. , Maresca , P. and Ciccarone , R. 2010 . The application of high hydrostatic pressure for the stabilization of functional foods: Pomegranate juice . Journal of Food Engineering , 100 : 245 – 253 .
- Follett , P. A. and Neven , L. G. 2006 . Current trends in quarantine entomology . Annual Review of Entomology , 51 : 359 – 385 .
- Fournier , A. , Feidt , C. , Dziurla , M. A. , Grandclaudon , C. and Jondreville , C. 2010 . Transfer kinetics to egg yolk andmodeling residue recovered in yolk of readily metabolized molecules: Polycyclic aromatic hydrocarbons orally administered to laying hens . Chemosphere , 78 : 1004 – 1010 .
- Francis , F.J. (Ed.). 2000 . Wiley encyclopedia of food science and technology , Vol. 4 , Hoboken NJ : John Wiley & Sons, Inc .
- Galotto , M. J. , Ulloa , P. A. , Escobar , R. , Guarda , A. , Gavara , R. and Miltz , J. 2010 . Effect of high-pressure food processing on the mass transfer properties of selected packaging materials . Packaging Technology and Science , 23 : 253 – 266 .
- Galotto , M. J. , Ulloa , P. A. , Hernández , D. , Fernández-Martín , F. , Gavara , R. and Guarda , A. 2008 . Mechanical and thermal behaviour of flexible food packaging polymeric films materials under high pressure/temperature treatments . Packaging Technology and Science , 21 : 297 – 308 .
- García-Falcón , M. S. , Cancho-Grande , B. and Simal-Gándara , J. 2005 . Minimal clean-up and rapid determination of polycyclic aromatic hydrocarbons in instant coffee . Food Chemistry , 90 : 643 – 647 .
- García-Falcón , M.S. , González Amigo , S. , Lage Yusty , M.A. , López de Alda Villaizán , M.J. and Simal Lozano , J. 1996 . Enrichment of benzo[a]pyrene in smoked foodproducts and determination by high-performance liquid chromatography fluorescence detection . Journal of Chromatography A , 753 : 207 – 215 .
- García Falcón , M.S. , González Amigo , S. , Lage Yusty , M.A. and Simal Lozano , J. 1999 . Determination of benzo[a]pyrene in some Spanish commercial smoked products by HPLC-FL . Food Additives and Contaminants , 16 ( 1 ) : 9 – 14 .
- García-Falcón , M. S. and Simal-Gándara , J. 2005 . Polycyclic aromatic hydrocarbons in smoke from different woods and their transfer during traditional smoking into chorizo sausages with collagen and tripe casings . Food Additives and Contaminants , 22 ( 1 ) : 1 – 8 .
- Gardner , P. T. , White , T. A.C. , McPhail , D. B. and Duthie , G. G. 2000 . The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices . Food Chemistry , 68 : 471 – 474 .
- Gattuso , G. , Barreca , D. , Gargiulli , C. , Leuzzi , U. and Caristi , C. 2007 . Flavonoid composition of citrus juices . Molecules , 12 : 1641 – 1673 .
- Heberle , I. , Schieberle , P. and Hofmann , T. 2003 . “ Influence of high hydrostatic pressure on the formation of non-enzymatic browning products formed in Maillard-type reactions ” . In Advances in high pressure bioscience and technology , Edited by: Winter , R. Vol. 2 , 341 – 345 . Berlin : Springer Verlag .
- Hertog , M. G.L. , Hollman , P. C.H. and van de Putte , B. 1993 . Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices . Journal of Agricultural and Food Chemistry , 41 : 1242 – 1246 .
- Hill , V. M. , Ledward , D. A. and Ames , J. M. 1996 . Influence of high hydrostatic pressure and pH on the rate of Maillard browning in a glucose-lysine system . Journal of Agricultural and Food Chemistry , 44 : 594 – 598 .
- Hogan , E. , Kelly , A. L. and Sun , D. 2005 . “ High pressure processing of foods: And overview ” . In Emerging technologies for food processing , Edited by: Sun , D. 3 – 32 . London UK : Academic Press/Elsevier .
- Houska , M. , Strohalm , J. , Kocurova , K. , Totusek , J. , Lefnerova , D. , Triska , J. and … Paulickova , I. 2006 . High pressure and foods – Fruit/vegetable juices (Special Section: CHISA 2004, 379-471) . Journal of Food Engineering , 77 : 379 – 471 .
- Hsu , K.-C. 2008 . Evaluation of processing qualities of tomato juice induced by thermal and pressure processing . LWT – Food Science and Technology , 41 : 450 – 459 .
- Hsu , K.-C. , Tan , F.-J. and Chi , H.-Y. 2008 . Evaluation ofmicrobial inactivation and physicochemical properties of pressurized tomato juice during refrigerated storage . LWT – Food Science and Technology , 41 : 367 – 375 .
- Isaacs , N. and Coulson , M. 1996 . Effect of pressure on processes modelling the Maillard reaction . Journal of Physical and Organic Chemistry , 9 : 639 – 644 .
- Ishizaki , A. , Saito , K. , Hanioka , N. , Narimatsu , S. and Kataoka , H. 2010 . Determination of polycyclic aromatic hydrocarbons in food samples by automated on-line in-tube solid-phase microextraction coupled with high-performance liquid chromatography-fluorescence detection . Journal of Chromatography A , 1217 : 5555 – 5563 .
- Jaeger , H. , Janositz , A. and Knorr , D. 2010 . The Maillard reaction and its control during food processing. The potential of emerging technologies . Pathologie Biologie , 58 : 207 – 213 .
- Jägerstad , M. and Skog , K. 2005 . Genotoxicity of heat-processed foods . Mutation Research , 574 : 156 – 172 .
- Jägerstad , M. , Skog , K. , Arvidsson , P. and Solyakov , A. 1998 . Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods . Zeitschrift fur Lebensmittel-Untersuchung und-Forschung , 207 : 419 – 427 .
- Kan , C. A. and Meijer , G. A.L. 2007 . The risk of contamination of food with toxic substances present in animal feed . Animal Feed Science and Technology , 133 : 84 – 108 .
- Kanekanian , A. 2010 . Book review: Thermal processing of food: Potential health benefits and risks – Symposium proceedings . International Journal of Dairy Technology , 63 ( 1 ) : 145
- Kaushik , G. , Satya , S. and Naik , S. N. 2009 . Food processing a tool to pesticide residue dissipation – A review . Food Research International , 42 : 26 – 40 .
- Keikotlhaile , B. M. , Spanoghe , P. and Steurbaut , W. 2010 . Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach . Food and Chemical Toxicology , 48 : 1 – 6 .
- Kontou , S. , Tsipi , D. and Tzia , C. 2004 . Stability of the dithiocarbamate pesticide maneb in tomato homogenates during cold storage and thermal processing . Food Additives and Contaminants , 21 : 1083 – 1089 .
- Kraybill , H. F. 1969 . Significance of pesticide residues in foods in relation to total environmental stress . Canadian Medical Association Journal , 100 : 204 – 215 .
- Krebbers , B. , Matser , A. , Koets , M. , Bartels , P. V. and van , den Berg, R. 2002 . High pressure-temperature processing as an alternative for preserving basil . High Pressure Research , 22 : 711 – 714 .
- Laing , B. M. , Schlueter , D. L. and Labuza , T. P. 1978 . Degradation kinetics of absorbic acid at high temperature and wateractivity . Journal of Food Science , 43 : 1440 – 1443 .
- Lambert , Y. , Demazeau , G. , Largeteau , A. and Bouvier , J.-M. 1999 . Changes in aromatic volatile composition of strawberry after high pressure treatment . Food Chemistry , 67 ( 1 ) : 7 – 16 .
- Lambert , Y. , Demazeau , G. , Largeteau , A. , Bouvier , J. M. , Laborde-Croubit , S. and Cabannes , M. 2000 . Packaging for high-pressure treatments in the food industry . Packaging Technology and Science , 13 : 63 – 71 .
- Le-Bail , A. , Hamadami , N. and Bahuaud , S. 2006 . Effect of high pressure processing on the mechanical and barrier properties of selected packagings . Packaging Technology and Science , 19 : 237 – 243 .
- Lockwood , B. 2007 . Nutraceuticals , 1 – 18 . London, UK : Pharmaceutical Press .
- Masson , P. , Tonello , C. and Balny , C. 2001 . High-pressure biotechnology in medicine and pharmaceutical science . Journal of Biomedicine and Biotechnology , 1 : 85 – 88 .
- McInerney , J. K. , Seccafien , C. A. , Stewart , C. M. and Bird , A. R. 2007 . Effects of high pressure processing on antioxidant activity, and total carotenoid content and availability, in vegetables . Innovative food science & emerging technologies , 8 : 543 – 548 .
- McNaught , A. D. and Wilkinson , A. 1997 . Compendium of chemical terminology: IUPAC recommendations , 2nd ed. , Ames IA : Blackwell Science .
- Moltó-Puigmartí , C. , Permanyer , M. , Castellote , A. I. and López-Sabater , M. C. 2011 . Effects of pasteurisation and high-pressure processing on vitamin C, tocopherols and fatty acids in mature human milk . Food Chemistry , 124 : 697 – 702 .
- Moreno , F. J. , Molina , E. , Olano , A. and López-Fandiño , R. 2003 . High-pressure effects on maillard reaction between glucose and lysine . Journal of Agricultural and Food Chemistry , 51 : 394 – 400 .
- Mottram , D. S. , Wedzicha , B. L. and Dodson , A. T. 2002 . Acrylamide is formed in the Maillard reaction . Nature , 419 : 448 – 449 .
- Mozhaev , V. V. , Heremans , K. , Frank , J. , Masson , P. and Balny , C. 1994 . Exploiting the effects of high hydrostatic pressure in biotechnological applications . Trends in Biotechnology , 12 : 493 – 501 .
- Mújica-Paz , H. , Valdez-Fragoso , A. , Tonello Samson , C. , Welti-Chanes , J. and Torres , J. A. 2011 . High-pressure processing technologies for the pasteurization and sterilization of foods . Food and Bioprocess Technology , 4 : 969 – 985 . doi:10.1007/s11947-011-0543-5
- Mussa , D. M. and Ramaswamy , H. S. 1997 . Ultra high pressure pasteurization of milk: Kinetics of microbial destruction and changes in physico-chemical characteristics . Lebensmittel Wissenschaft und Technologie , 30 : 551 – 557 .
- Nagao , M. , Honda , M. , Seino , Y. , Yahagia , T. and Sugimura , T. 1977 . Mutagenicities of smoke condensates and thecharred surface of fish and meat . Cancer Letters , 2 : 221 – 226 .
- Oey , I. , Lille , M. , van Loey , A. and Hendrickx , M. 2008 . Effectof high pressure processing on colour, texture and flavour of fruit and vegetable-based food products: A review . Trends in Food Science & Technology , 19 : 320 – 328 .
- Oey , I. , van , der Plancken, I. , van Loey , A. and Hendrickx , M. 2008 . Does high pressure processing influence nutritional aspects of plant based food systems? . Trends in Food Science & Technology , 19 : 300 – 308 .
- Oey , I. , Verlinde , P. , Hendrickx , M. E. and van Loey , A. 2006 . Temperature and pressure stability of l-ascorbic acid and/or [6s] 5-methyltetrahydrofolic acid: A kinetic study . European Food Research and Technology , 223 : 71 – 77 .
- Ozen , B. F. and Floros , J. D. 2001 . Effects of emerging food processing techniques on the packaging materials . Trends in Food Science and Technology , 12 : 60 – 67 .
- Pais , P. , Salmon , C. P. , Knize , M. and Felton , J. S. 1999 . Formation of mutagenic/carcinogenic heterocyclic amines in dry-heating model systems, meats, and meat drippings . Journal of Food Chemistry , 47 : 1098 – 1108 .
- Palou , E. , López-Malo , A. and Welti-Chanes , J. 2002 . “ Innovative fruit preservation methods using high pressure ” . In Engineering and food for the 21st century , Edited by: Welti-Chanes , J. , Barbosa-Cánovas , G. V. and Aguilera , J. M. 715 – 725 . Boca Ratón FL : CRC Pres, Inc .
- Paredes-Sabja , D. , Gonzalez , M. , Sarker , M. R. and Torres , J. A. 2007 . Combined effects of hydrostatic pressure, temperature, and pH on the inactivation of spores of Clostridium perfringens Type A and Clostridium sporogenes in buffer solutions . Journal of Food Science , 72 : M202 – M206 .
- Paseiro Losada , P. , Pérez Lamela , C. , López Fabal , F. , Sanmartín Fenollera , P. and Simal Lozano , J. 1997 . Two RP-HPLC sensitive methods to quantify and identify badge and its hydrolysis products. Part 1: European Union aqueous food simulants . Journal of Agricultural and Food Chemistry , 45 : 3493 – 3500 .
- Patras , A. , Brunton , N. P. , da Pieve , S. and Butler , F. 2009 . Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées . Innovative Food Science and Emerging Technologies , 10 : 308 – 313 .
- Patras , A. , Brunton , N. P. , da Pieve , S. , Butler , F. and Downey , G. 2009 . Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purees . Innovative Food Science and Emerging Technologies , 10 ( 1 ) : 16 – 22 .
- Pedrenski , F. 2007 . The canon of potato science: Acrylamide . Potato Research , 50 : 411 – 413 . doi:10.1007
- Pereira , R. N. and Vicente , A. A. 2010 . Environmental impact of novel thermal and non-thermal technologies in food processing . Food Research International , 43 : 1936 – 1943 .
- Pérez Lamela , C. and Torres , J. A. 2008 . Pressure processing of foods: Microbial inactivation and chemical changes in pressure-assisted thermal processing (PATP) – Part 2 . Agro FOOD Industry Hi-Tech , 19 : 34 – 36 .
- Piringer , O. G. and Baner , A.L. (Eds.). 2000 . Plastic packaging materials for food , Weinheim Germany : John Wiley and Sons .
- Polydera , A. C. , Stoforos , N. G. and Taoukis , P. S. 2003 . Comparative shelf life study and vitamin C loss kinetics in pasteurized and high pressure processed reconstituted orange juice . Journal of Food Engineering , 60 : 21 – 29 .
- Qiu , W. , Jiang , H. , Wang , H. and Gao , Y. 2006 . Effect of high hydrostatic pressure on lycopene stability . Food Chemistry , 97 : 516 – 523 .
- Ramirez , R. , Saraiva , J. A. , Pérez Lamela , C. and Torres , J. A. 2009 . Reaction kinetics analysis of chemical changes inpressure-assisted thermal processing, PATP . Food Engineering Reviews , 1 ( 1 ) : 16 – 30 .
- Rasanayagam , V. , Balasubramaniam , V. M. , Ting , E. Y. , Sizer , C. E. , Bush , C. and Anderson , C. 2003 . Compression heating of selected fatty food materials during high-pressure processing . Journal of Food Science , 68 ( 1 ) : 254 – 259 .
- Rodriguez-Amaya , D. B. 2001 . A guide to carotenoid analysis in foods , Washington DC : ILSI Press, Inc .
- Sajilata , M. G. , Savitha , K. , Singhal , R. S. and Kanetkar , V. R. 2007 . Scalping of flavors in packaged foods . Comprehensive Reviews in Food Science and Food Safety , 6 : 17 – 35 .
- Samaranayake , C. P. and Sudhir , K. S. 2010 . In situ measurement of pH under high pressure . Journal of Physics and Chemistry B , 114 : 13326 – 13332 .
- Sanchez Moreno , C. , Plaza , L. , de Ancos , B. and Cano , M. P. 2003 . Effect of high-pressure processing on health-promoting attributes of freshly squeezed orange juice (Citrus sinensisL.) during chilled storage . European Food Research and Technology , 216 ( 1 ) : 18 – 22 .
- Sánchez-Moreno , C. , Plaza , L. , Elez Martinez , P. , de Ancos , B. , Martin Belloso , O. and Cano , M. P. 2005 . Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing . Journal of Agricultural and Food Chemistry , 53 : 4403 – 4409 .
- Schilter , B. , Scholz , G. and Seefelder , W. 2011 . Fatty acid esters of chloropropanols and related compounds in food: Toxicological aspects . European Journal of Lipid Science and Technology , 113 : 309 – 313 .
- Schwarzenbolz , U. , Klostermeyer , H. and Henle , T. 2000 . Maillard-type reactions under high hydrostatic pressure: Formation of pentosidine . European Food Research and Technology , 211 : 208 – 210 .
- Schwarzenbolz , U. , Klostermeyer , H. and Henle , T. 2002 . Maillard reaction under high hydrostatic pressure: Studies on the formation of protein-bound amino acidderivatives . International Congress Series , 1245 : 223 – 227 .
- Seefelder , W. and Schilter , B. 2011 . Structural diversity of dietary fatty acid esters of chloropropanols and related substances . European Journal of Lipid Science and Technology , 113 : 319 – 322 .
- Segovia Bravo , K. , Ramírez , R. , Durst , R. , Escobedo-Avellaneda , Z.J. , Welti-Chanes , J. , Sanz , P.D. and Torres , J.A. 2011 . Formation risk of toxic compounds in pressure-assisted thermally processed foods . Journal of Food Science , : In press
- Sendón , García, R. , Paseiro Losada , P. and Pérez Lamela , C. 2003 . Determination of compounds from epoxy resins in food simulants by HPLC-fluorescence . Chromatographia , 58 : 337 – 342 .
- Sharma , J. , Satya , S. , Kumar , V. and Tewary , D. K. 2005 . Dissipation of pesticides during bread-making . Chemical Health & Safety , 12 ( 1 ) : 17 – 22 .
- Shellhammer , T. H. , Aleman , G. D. , McDaniel , M. R. and Torres , J. A. A comparison of the sensory and chemical properties of orange and apple juices treated with and without high pressure . Paper presented at the IFT Annual Meeting . Chicago IL
- Stadler , R. H. , Blank , I. and Varga , N. 2002 . Acrylamide from Maillard reaction products . Nature , 419 : 449 – 450 .
- Studer , A. , Blank , I. and Stadler , R. H. Thermal processing contaminants in foodstuffs and potential strategies of control . Czech Journal of Food Sciences, 22 (Special Issue for Proceedings of Chemical Reaction in Food V, Prague, Czechoslavakia) . pp. 1 – 10 .
- Tapas , A. R. , Sakarkar , D. M. and Kabde , R. B. 2008 . Flavonoids as nutraceuticals: A review . Tropical Journal of Pharmaceutical Research , 7 : 1089 – 1099 .
- Tellez Luis , S.J. , Ramirez , J. A. , Pérez Lamela , C. , Vazquez , M. and Simal , Gándara, J. 2001 . Application of high hydrostatic pressure in the food preservation . Ciencia y Tecnologia Alimentaria , 3 : 66 – 80 .
- Tepe , B. , Daferera , D. , Sokmen , A. , Sokmen , M. and Polissiou , M. 2005 . Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) . Food Chemistry , 90 : 333 – 340 . doi:10.1016/j.foodchem.2003.09.013
- Tiwari , B. K. , O'Donnell , C.P. and Cullen , P. J. 2009 . Effect of non thermal processing technologies on the anthocyanin content of fruit juices . Trends in Food Science & Technology , 20 : 137 – 145 .
- Tomás-Barberán , F. A. and Espín , J. C. 2001 . Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables . Journal of the Science of Food and Agriculture , 81 : 853 – 876 .
- Torres , J. A. , Chotyakul , N. , Velazquez , G. , Saraiva , J. A. and Pérez Lamela , C. (2010, October 6–8). Integration ofstatistics and food process engineering: Assessing theuncertainty of thermal processing and shelf-life estimations . Paper presented at the VI Congreso Españolde Ingeniería de Alimentos, Logroño, La Rioja, España .
- Torres , J. A. , Sanz , P. D. , Otero , L. , Pérez Lamela , C. and Saldaña , M. D.A. Engineering principles to improve food quality and safety by high pressure processing . Processing effects on safety and quality of foods . Edited by: Ortega-Rivas , E. pp. 379 – 414 . Boca Raton FL : CRC Taylor & Francis, Inc .
- Torres , J. A. , Sanz , P. D. , Otero , L. , Pérez Lamela , C. and Saldaña , M. D.A. Temperature distribution and chemical reactions in foods treated by pressure-assisted thermal processing . Processing effects on safety and quality of foods . Edited by: Ortega-Rivas , E. pp. 415 – 440 . Boca Raton FL : CRC Taylor & Francis, Inc .
- Torres , J. A. and Velazquez , G. 2005 . Commercial opportunities and research challenges in the high pressure processing of foods . Journal of Food Engineering , 67 ( 1–2 ) : 95 – 112 .
- Tripoli , E. , La Guadia , M. , Giammanco , S. , Di Majo , D. and Diammanco , M. 2007 . Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review . Food Chemistry , 104 : 466 – 479 .
- Valdez-Fragoso , A. , Mújica-Paz , H. , Welti-Chanes , J. and Torres , J. A. 2011 . Reaction kinetics at high pressure and temperature: Effects on milk flavor volatiles and on chemical compounds with nutritional and safety importance in several foods . Food and Bioprocess Technology , 4 : 986 – 995 . doi:10.1007/s11947-010-0489-z
- Varma , S. , Karwe , M. V. and Lee , T.-C. 2010 . Effect of high hydrostatic pressure processing on lycopene isomers . International Journal of Food Engineering , 6 : 1 – 20 .
- Velisek , J. , Davidek , J. , Hajslova , J. , Kubelka , V. G. , Janice , K. and Mankova , B. 1978 . Chlorohydrins in protein hydrolysates . Zeitschrift für Lebensmittel-Untersuchung und-Forschung , 167 : 241 – 244 .
- Verlinde , P. , Indrawati , O. , Hendrickx , M. E. and van Loey , A. 2008 . High-pressure treatments induce folate polyglutamate profile changes in intact broccoli (Brassica oleraceae L. cv. Italica) tissue . Food Chemistry , 111 ( 1 ) : 220 – 229 .
- Weisshaar , R. 2004a . Acrylamide in heated potato products. Analytics and formation routes . European Journal of Lipid Science and Technology , 106 : 786 – 792 .
- Weisshaar , R. 2004b . Acrylamide in bakery products – Results from model experiments . Deutsche Lebensmittel Rundschau , 100 : 92 – 97 .
- Welti-Chanes , J. , San , Martín-González, F. and Barbosa-Cánovas , G. V. 2006 . “ Water and biological structures at high pressure ” . In Water properties of food, pharmaceutical, and biological materials , Edited by: Buera , P. , Welti-Chanes , J. , Llilford , P. and Corti , H. 205 – 232 . Boca Raton, FL : CRC Press, Inc .
- Wildman , R. E.C. 2001a . “ Classifying nutraceuticals ” . In Handbook of nutraceuticals and functional foods , Edited by: Wildman , R. E.C. 13 – 30 . Boca Raton, FL : CRC Press Inc .
- Wildman , R. E.C. 2001b . “ Nutraceuticals ” . In Handbook of nutraceuticals and functional foods , Edited by: Wildman , R. E.C. 1 – 12 . Boca Raton FL : CRC Press Inc .
- Xi , J. , Shen , D. , Zhao , S. , Lu , B. , Li , Y. and Zhang , R. 2009 . Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction . International Journal of Pharmaceutics , 382 ( 1–2 ) : 139 – 143 .
- Yaylayan , V. , Wnorowski , A. and Perez Locas , C. 2003 . Why asparagine needs carbohydrates to generate acrylamide . Journal of Agricultural and Food Chemistry , 51 : 1753 – 1757 .
- Zhang , Y. and Zhang , Y. 2007 . “ Formation and reduction of acrylamide in Maillard reaction: A review based on the current state of knowledge ” . In Critical Reviews in Food Science and Nutrition
- Zyzak , D. V. , Sanders , R. A. , Stojanovic , M. , Tallmadge , D. H. , Eberhart , B. L. , Ewald , D. K. and … Villagran , M.D. 2003 . Acrylamide formation mechanism in heated foods . Journal of Agricultural and Food Chemistry , 51 : 4782 – 4787 .