Abstract
The present work analyses different chitosan synthesized derivatives from the point of view of their chemical structure (Fourier transform infrared spectroscopy, deacetylation degree, elemental analysis and average molecular weight) as well as different physicochemical properties such as density, kinematic and dynamic viscosity, intrinsic viscosity and surface tension. The characterization of different solutions of these solutes has a high importance due to the large potential uses of these compounds. Chitosan has low solubility in water, and for this reason, the present work has synthesized soluble chitosan derivatives. Important differences between original chitosan and synthesized derivatives have been found in different properties.
El presente trabajo analiza distintos derivados sintetizados del quitosano desde el punto de vista de su estructura química (FTIR, grado de desacetilación, análisis elemental y peso molecular medio), así como de distintas propiedades físico-química como son la densidad, viscosidad cinemática y dinámica, viscosidad intrínseca y tensión superficial. La caracterización de las disoluciones acuosas de estos solutos tiene una elevada importancia debido al gran potencial en el uso de estos compuestos. El quitosano tiene una baja solubilidad en agua y por este motivo este trabajo ha sintetizado derivados solubles de quitosano. Se han encontrado diferencias importantes en algunas propiedades entre los resultados experimentales correspondientes al quitosano y a sus derivados.
Palabras clave:
Introduction
An important number of research studies and reviews have stated the potential use of chitosan in a great number of industrial processes, taking into account its worthy characteristics and the fact that it is the second abundant natural polymer in the world – the first one is cellulose (Jang, Kong, Jeong, Lee, & Nah, Citation2004). Several possible uses of this polymer are centred on different fields such as agriculture and the environment, the pharmaceutical industry, as well as food processes (Chen et al., Citation2008; Ho, Mi, Sung, & Kuo, Citation2009). Chitosan has shown a wide range of uses in the last few years due to its extraordinary characteristics: biocompatibility and biodegradability.
Despite all the interesting characteristics shown by chitosan to be used in numerous industrial processes, there is an important limitation related to its low solubility in water. Chitosan is an insoluble substance in aqueous solutions with pH values corresponding to neutral or alkaline characters (Cravotto, Tagliapietra, Robaldo, & Trotta, Citation2005). Then, the use of this substance is limited to processes using this polymer in organic acid solutions. With the aim of overcoming this difficulty, different studies have reached an enhancement of the chitosan solubility by means of chemical modifications in the molecule, mainly with the inclusion of other functional groups (Chung, Tsai, & Li, Citation2006; Sashiwa & Shigemasa, Citation1999). This kind of techniques uses the amino group present in the chitosan monomer because its group shows very interesting features to carry out synthesis reactions. The modified chitosan has shown some characteristics that allow important uses in several processes. To set an example, alkyl chitosans can be used in textile processes and the development of membranes; on the other hand, carboxyalkyl chitosans have been used for molecular sieves and viscosity builders (Majeti & Ravi, Citation2000).
The potential uses for chitosan aqueous solutions or derivatives imply the high importance of characterization studies of this kind of polymer aqueous solutions. Let us take as an example, the molecular weight of this polymer, playing an important role in the use of these substances for the pharmaceutical products development (Mao et al., Citation2004). On the other hand, the rheological characterization of polymer aqueous solutions is usually a necessary study previous to the use of this kind of solutions in different processes. The effect of viscosity must be analysed in the quality control or in the designed equipment processes, since a high viscosity (commonly observed in polymer solutions) can modify the industrial processes effectiveness (Yang, Chou, & Li, Citation2002). For this reason, when a chemical modification is produced in the polymer, performing a characterization about different physical–chemical properties is required, in order to analyse the chemical modification influence upon these properties. Then, the present work has carried out an important characterization of chitosan and chitosan derivatives upon different properties such as viscosity, rheological behaviour or surface tension, that have a high importance in the industrial uses of these liquid phases. Also, the surface tension is an interesting attribute to analyse when polymer aqueous solutions are used, since this kind of liquid systems commonly show a dynamic behaviour (Nahringbauer, Citation1995, Citation1997).
Materials and methods
Purification and synthesis
The commercial chitosan (Sigma-Aldrich, Madrid, Spain, >99%) was treated (Hatashi, Citation1993) with a sodium hydroxide (Panreac, Barcelona, Spain, >98%) aqueous solution (40%) for 4 h at a temperature of 95°C, and the refined chitosan was obtained after repeating the filtration and neutralization twice. Five grams of the refined chitosan was dissolved in 20 g of a 2% aqueous solution of acetic acid (Sigma-Aldrich, >99.7%) and precipitated by neutralization with a 2% sodium hydroxide (Panreac) aqueous solution under stirring. The regenerated chitosan was washed with water and then with methanol (Sigma-Aldrich, >99.8%) and thereafter immersed in dimethylformamide (Panreac, >99.8%) for 3 h and then pressed. Thus, a pre-treated chitosan was obtained.
Sulphonation of chitosan was performed using the methodology developed in previous studies (Gamzazade, Skyyar, Nasibov, Suskov, & Knirel, Citation1997), although with several modifications to improve the synthesis of this kind of chitosan (Suwan et al., Citation2009). The preparation of the sulphating reagent to produce the chitosan modification consisted of the drop addition of 4.5 mL chlorosulphonic acid (Sigma-Aldrich, 99%) with stirring to 30 mL of dimethylformamide (Panreac), which had been previously cooled to a temperature in the range of 0–4°C. The reaction mixture was then stirred without cooling until the solution reached room temperature. One gram of this mixture was added to the sulphonating reagent, and the reaction mixture was stirred overnight at room temperature. The polymer was neutralized with a sodium hydroxide (Panreac) aqueous solution (20%) and then precipitated in cold methanol (Sigma-Aldrich).
Carboxymethyl chitosan was synthesized by means of the procedure described in the literature (Chen, Tian, & Du, Citation2004; Miao et al., Citation2006). Purified chitosan (5 g) was suspended in 30 g of a sodium hydroxide (Panreac) aqueous solution (50%); it was swelled for 1 h at room temperature and kept at −20°C for 12 h for alkalization; and then thawed at room temperature. The alkali chitosan was suspended in isopropanol (Sigma-Aldrich, >99.8%) (150 mL) and the resulted slurry was stirred at a moderate speed. Subsequently, the monochloroacetic acid (Sigma-Aldrich, >99%) (20 g) was added in five equal portions every 5 min. The mixture was stirred for 4 h in a water bath at 50°C. Afterwards, the resultant solution was adjusted to pH 7 and filtered. Then, the resulting solution was concentrated and precipitated by pouring into acetone (Panreac, >99.5%). The white precipitate was thoroughly rinsed with methanol, vacuum dried at 50°C and stored in a desiccator.
Physicochemical characterization
Fourier transform infrared spectroscopy
The chitosan (C), chitosan sulphate (CS) and carboxymethyl chitosan (CC) powders were mixed with KBr (Panreac, 499%) in the ratio of 1:100 in mass and pressed into a pellet. The spectra were obtained with a Bruker (Germany) IFS-66v model. The IR spectra of different solids were recorded as KBr pellets in the range of 4000–400 cm−1.
Elemental analysis
The elemental analysis was performed in a Thermo Finningan (Italy) Flash model 1112 equipment that allows the quantification of carbon, hydrogen, nitrogen and sulphur content.
Deacetylation degree
A titration method has been employed to develop this analysis. An accurate excess of hydrogen chloride (Panreac, >99.5%) is added to a known amount of polymers and the remaining amount of hydrogen chloride is back titrated with a sodium hydroxide solution. The resultant titration curve shows two equivalence points: the first one corresponds to the excess hydrogen chloride, while the second corresponds to the protonated chitosans. Thus, the difference between them would correspond to the free deacetylated amino groups (Balazs & Sipos, Citation2007; Sweidan et al., Citation2011). For the two equivalence points (inflection points) of the titration curve, let us determine the volume of sodium hydroxide aqueous solution employed to reach these point. And on the basis of these values, the polymers deacetylation is calculated using Equation (1).
Density, viscosity, intrinsic viscosity and average molecular weight
The average molecular weight determination was achieved via calculating the intrinsic viscosity by means of Huggins and Kramer equations (Rao, Citation1999) (Equations (2) and (3)). The solutions were prepared in sodium chloride (Sigma-Aldrich, >99.5%) aqueous solution (0.1 M), since Mark–Houwink constants have been obtained from literature (El-Sherbiny & Smyth, Citation2010; Parada, Crespín, Miranda, & Katime, Citation2004; Vongchan, Sajomsang, Subyen, & Kongtawelert, Citation2002). These constants have been determined using this solvent to prevent the polyelectrolyte expansion in the solution while size exclusion chromatography was used to determine these constants.
The dynamic viscosity (η) was obtained from the product of the kinematic viscosity (ν) and the corresponding density (ρ) of the mixture, in terms of Equation (4) for each mixture composition.
Surface tension measurements
The surface tension was determined employing a Krüss (Hamburg, Germany) K-11 tensiometer and the Wilhelmy plate method. The plate employed was a commercial platinum one supplied by Krüss. This platinum plate was cleaned with water and acetone and flame-dried before each measurement. On the whole, each surface tension value reported was the average of 10 measurements except in the case of polymers, since they present a measure of dynamic surface tension. Before measuring the surface tension, the samples were stirred in a thermostated vessel that was closed to prevent evaporation. The measurement vessel was connected to a controlled thermostat-cryostat bath (Selecta Frigiterm, Barcelona, Spain).
Rheological behaviour
An Anton Paar DV-1P digital thermostated rotational viscometer with two coaxial cylinders was used to carry out the rheological measurements. This equipment allows the shear rate variation in a wide range by using different spindles.
Results and discussion
Supplementary shows the FTIR spectrum corresponding to chitosan with characteristic bands corresponding to this molecule (Balanta, Grande, & Zuluaga, Citation2010; Singh et al., Citation2009). At 3435 cm−1 and 3286 cm−1, the O‒H stretch and ‒NH2 groups appear, respectively. We can also appreciate the bands corresponding to the C‒H group at 2892 cm−1. The amide band corresponding to the C‒O stretch of the acetyl group appears at 1640 cm−1, and at 1567 cm−1, we can observe the amide band corresponding to the N‒H stretch. The FTIR spectrum corresponding to CS showed the appearance of characteristic S˭O and S‒O bond-stretching absorptions at 1235, 1068 and 807 cm−1, respectively, being in accordance with the sulphonation of chitosan (Suwan et al., Citation2009). The spectrum of CC shows the band at 1629 cm−1, which can be attributed to the carboxylation. Additionally, the formation of CC is also confirmed by the intensification of the band at 1073 corresponding to the C‒O‒C stretching. The band at 3428 cm−1 becomes wider and weaker, which suggests that the carboxylation occurred on some of both the amino and primary hydroxyl sites of the glucosamine units of the chitosan structure (Miao et al., Citation2006).
shows the experimental results for elemental analysis of polymers employed in this work (C, CS and CC). The values obtained for chitosan are similar to the ones already published by other authors (Ríos-Donato, Navarro-Mendoza, Ávila-Rodríguez, & Mendizábal-Mijares, Citation2006). Regarding the results obtained for the others polymers, and taking into account that the aim of including different functional groups is related to the enhancement of chitosan solubility in water, shows the changes in the elemental analysis. These changes were produced by the modification of polymers in relation to the original chitosan. The values for the chitosan sulphate (CS) show the inclusion of sulphur in the polymer structure, obtaining a similar incorporation to previous works that had used a similar synthesis procedure (Ríos-Donato et al., Citation2006). On the other hand, for carboxymethyl chitosan (CC), a reduction in the quantity of carbon and nitrogen atoms is observed, which could have been caused by the inclusion of oxygen in the polymer. On the basis of these experimental results, we can conclude that the inclusion of the functional groups is produced in both cases.
Table 1. Elemental analysis for different polymers employed in present work.
Tabla 1. Análisis elemental de los distintos polímeros empleados en el presente estudio.
In relation to the deacetylation degree, shows the different titration curves for each polymer, which allows the deacetylation degree calculation on the basis of the inflection points situation. The values of sodium hydroxide volumes corresponding to these points have been calculated using the first derivate criterion which is shown in for chitosan. shows the amino content of each polymer using the calculation procedure described in the experimental section.
Figure 1. Titration curves for deacetilation studies. (•) C; (▪) CS; (▴) CC. (□) first derivate for C.
Figura 1. Curvas de valoración correspondientes a los studios de desacetilación (•) C; (▪) CS; (▴) CC. (□) primera derivada para C.
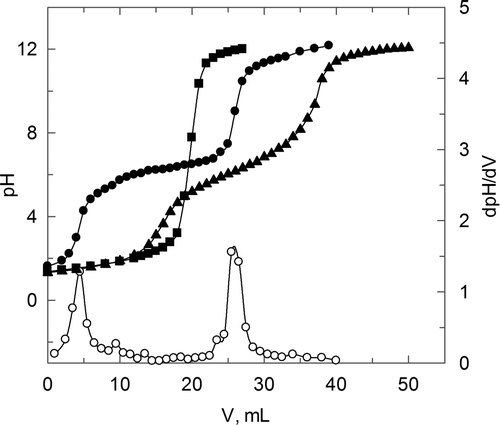
Table 2. Deacetylation degree of polymers used in present work.
Tabla 2. Grado de desacetilación de los polímeros usados en el presente estudio.
The experimental data corresponding to chitosan show that the first inflection point is reached when a pH change is produced from values near to initial until being near to 6. The second change in this variable is produced until pH = 12. This behaviour for the original chitosan is similar to the one observed for the carboxymethyl chitosan, although with important differences in relation to the corresponding behaviour for chitosan sulphate. In the last case, a second inflection point is not observed and the change is produced from the initial pH to a value close to 12. This behaviour indicates that the non-existence of free amino groups is concluded for CS. This result is in disagreement with previous studies (Gamzazade et al., Citation1997) that have used the same synthesis procedure to produce the substitution of the sulphate group in hydroxide and amino groups, but not with a 100% of substitution. On the other hand, other studies (Suwan et al., Citation2009) also show the inclusion of the sulphate group in both kinds of centres with a 100% of inclusion, which is in agreement with the results obtained in this work.
The behaviours commented in relation to the titration curves shown in allows us the calculation of the polymers deacetilation degree using Equation (1) (see material and methods section), which are shown in . For carboxymethyl chitosan (CC), the percentage of free amino groups remains constant regarding the original chitosan (C), which indicates that the carboxylic group is included in the hydroxilic centres (3 and 6 carbons) or in the acetylated amino group, being in agreement with previous studies (Miao et al., Citation2006). In relation to the chitosan sulphate, free amino groups do not exist.
The molecular weight of polymers employed in this study was unknown; therefore, in order to characterize these polymers, the average molecular weight has been calculated. To do this, it was necessary to determine the intrinsic viscosity obtained by the combined application of Huggins and Kramer equations (Equations (2) and (3)). A similar procedure has been employed by different authors (Chuah, Lin-Vien, & Soni, Citation2001; Ma & Pawlik, Citation2007) with suitable results.
Different concentrations of chitosan, chitosan sulphate and carboximethyl chitosan in sodium chloride aqueous solution (0.1 M) were prepared to obtain relative viscosities in a range of 1.2–1.6 to assure a good accuracy and linearity of extrapolation to a zero concentration.
The density of polymer aqueous solutions has been settled in order to determine the absolute viscosity from the kinematic viscosity values. The behaviour obtained indicates slight interactions among the polymer–solvent and polymer–polymer molecules and then a non-influence of polymer concentration has been observed for all the polymers concentration ranges used in this work.
Supplementary shows the experimental data obtained for the combined use of Huggins and Kramer equations for all the polymers, on the basis of calculated values of dynamic viscosity using kinematic viscosity and density data. For these equations, the intercept in these plots corresponds to the intrinsic viscosity for each polymer. The good quality of the experimental data is confirmed by the agreement of both linear regressions at a zero polymer concentration. The calculated data for intrinsic viscosity for each polymer are shown in . The intrinsic viscosity indicates the effective specific volume of one isolated polymer and, for this reason, this measurement must be developed by extrapolation at an infinite dilution. This value depends on the size and shape of the molecule, as well as on the interactions with solvent and work temperature.
Table 3. Intrinsic viscosity, Mark–Houwink constants and average molecular weight of chitosan (C), chitosan sulphate (CS) and carboxymethyl chitosan (CC).
Tabla 3. Viscosidad intrínseca, constantes de Mark-Houwink y peso molecular medios del quitosano (C), sulfato de quitosano (CS) y carboximetil quitosano (CC).
The average molecular weight was determined using the corresponding Mark-Houwink equation (Equation (6)) and the intrinsic viscosity experimental value. The Mark–Houwink constants for these polymers were obtained from literature (Maghami & Roberts, Citation1988; Nishimura & Tokura, Citation1987; Sovilj & Petrovi, Citation2007) and the value of these parameters and calculated value of the average molecular weight are included in .
The present work also studies the flow behaviour of different aqueous solutions corresponding to the polymers previously analysed, taking into account the exceptional importance of this physical property upon different industrial operations (Álvarez, Sanjutjo, Cancela, & Navaza, Citation2000; Jiao, Xueqiong, & Juntag, Citation1998; Sovilj & Petrovi, Citation2007). The rheological behaviour of aqueous solutions of chitosan (C), chitosan sulphate (CS) and carboxymethyl chitosan (CC) has been analysed on the basis of the influence of the shear rate applied to the samples upon apparent viscosity and shear stress. In relation to chitosan aqueous solutions, due to the low solubility of this polymer in aqueous solution, the solvent employed was an acetic acid aqueous solution (1% v/v). The influence of the shear rate upon the apparent viscosity is shown in , which shows a decrease in this variable when the shear rate increases. This behaviour is clearer at low values of shear rate until they reach a practically constant value in viscosity.
Figure 2. Influence of shear rate and polymer type upon flow behaviour. C p = 4 g · L−1. (▪) C, (•) CC, (□) CS.
Figura 2. Influencia del gradiente de velocidad y del tipo de polímero sobre el comportamiento de flujo. C p = 4 g · L−1. (▪) C, (•) CC, (□) CS.
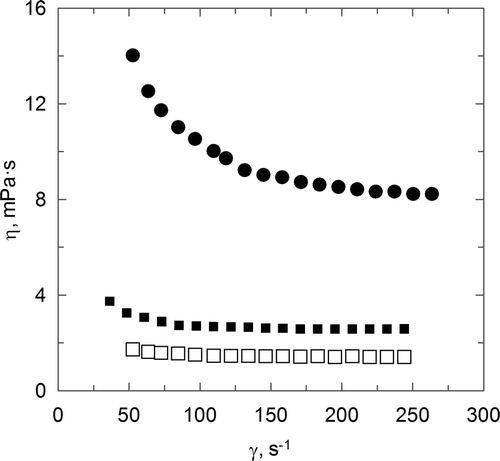
Aqueous solutions of chitosan sulphate show a low influence of shear rate and also a low value of viscosity. A similar behaviour was observed for all the concentrations employed in this study. This fact indicates that the effect of chitosan sulphate has no influence upon the rheological behaviour in the analysed composition range. On the other hand, and related to the influence of shear rate upon the apparent viscosity (), the low influence of the polymer concentration is observed again, but a slight increase in the value of viscosity with a polymer concentration is obtained. A non-influence of shear rate upon the apparent viscosity is observed, and this behaviour indicates that these aqueous solutions are included in Newtonian fluids. If we compare it to the previously described behaviour for chitosan aqueous solutions, lower values of viscosity are obtained and this behaviour could be related to a better spatial distribution, confirmed by the lower value in the intrinsic viscosity of this polymer, previously commented and also related to the reduction in the molecular weight.
The observed behaviours for carboxymethyl chitosan aqueous, mainly those related to the influence of the shear rate upon the apparent viscosity, a different behaviour from the previously one commented for chitosan sulphate is observed. Then, an increase in the shear rate produces an important decrease in the apparent viscosity. For this reason, the carboxymethyl chitosan aqueous solutions are included in the non-Newtonian and pseudoplastic fluids. On the other hand, shows the influence of CC concentration upon the flow behaviour. An increase in the polymer concentration produces an increase in the apparent viscosity value. A higher effect of the shear rate is also observed when the polymer concentration increases.
Figure 3. Effect of shear rate upon apparent viscosity for carboxymethyl chitosan chitosan aqueous solutions. (○) CCC = 0.4 g · L−1; (•) CCC = 1 g · L−1; (□) CCC = 2 g · L−1; (▪) CCC = 4 g · L−1.
Figura 3. Influencia del gradient de velocidad sobre la viscosidad aparente para disoluciones acuosas de carboximetil quitosano. (○) CCC = 0.4 g · L−1; (•) CCC = 1 g · L−1; (□) CCC = 2 g · L−1; (▪) CCC = 4 g · L−1.
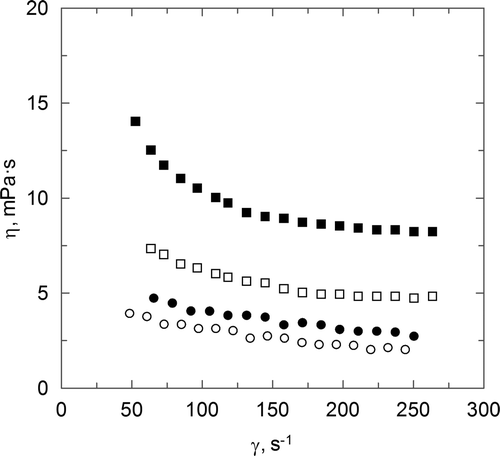
The behaviour observed for chitosan aqueous solutions (see ) shows a non-Newtonian flow included in the pseudoplastic fluids, since a decrease in the apparent viscosity value is observed when the shear rate increases. This behaviour is in agreement with previous studies (Cho, Heuzey, Beégin, & Carreau, Citation2006; Mucha, Citation1998) that have employed chitosan in aqueous solution. To confirm this behaviour, the power law (Equation (7)) has been used to fit the experimental data and, more specifically, the influence of the shear rate upon the shear stress.
Supplementary shows the power law behaviour in relation to the experimental data at a fix polymer concentration, which is considered to be suitable with low deviations. Supplementary Figure S3 also shows the value of the slope that corresponds to the behaviour index and takes a value less than 1 for CC aqueous solutions. The behaviour index values less than 1 has been obtained for all the chitosan and carboxymethyl chitosan aqueous solutions. This fact confirms the conclusion previously reached: chitosan aqueous solutions have a non-Newtonian and pseudoplastic behaviour. The corresponding flow behaviour index for aqueous solutions of chitosan sulphate takes values near to the unity, which confirms the previous results. And this index values include the CS aqueous solutions in the group of Newtonian fluids.
Other interesting physical property with high importance in different processes is the surface tension due to the special influence upon hydrodynamics, wetability, etc., mainly in solutions that involve the presence of amphiphilic solutes (i.e. surfactants) (Rosso, Huo, & Stenstrom, Citation2006; Rosu, Marlina, Kaya, & Schumpe, Citation2007).
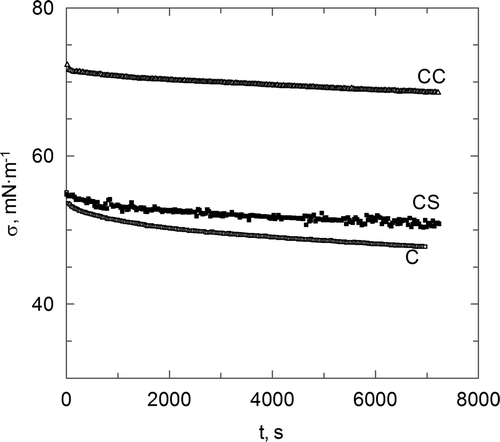
shows the surface tension of chitosan aqueous solutions and the first conclusion is that the value of this physical property shows a slow dynamic and, then, this property changes its value throughout the time until reaching a constant value. Different studies have obtained experimental results in agreement with these present data for different polymers aqueous solutions (Gau, Yu, & Zografi, Citation1993; Makri & Doxastakis, Citation2007; Nahringbauer, Citation1995).
Figure 4. Dynamics of surface tension chitosan aqueous solutions.
Figura 4. Tensión superficial dinámica de disoluciones acuosas de quitosano.
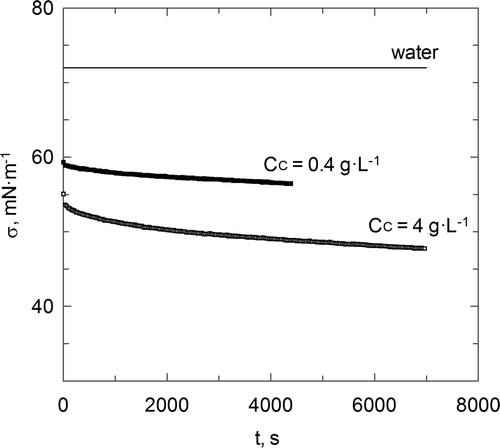
This surface tension dynamics is due to the complex behaviour of this kind of substances in aqueous solution and at the gas–liquid interface. Supplementary shows an example of the supposed behaviour at a gas–liquid interface of this kind of systems (Nahringbauer, Citation1997). Taking into account this behaviour, the conformational changes in the polymer chains produce modifications in the surface tension throughout the time. The same behaviour has been found for the other polymers used in this work (see Supplementary ), but there are certain differences regarding the surface tension magnitude for the carboxymethyl chitosan aqueous solutions in comparison with chitosan aqueous solutions and chitosan sulphate. Carboxymethyl chitosan aqueous solutions produce similar values than pure water.
On the other hand, shows the effect caused by the polymers concentration upon the surface tension value obtained at equilibrium. This figure confirms the behaviour previously described in Supplementary Figure S5 (for the highest polymer concentration). Aqueous solutions of carboxymethyl chitosan always take higher values for the surface tension than the corresponding ones for the other polymers, showing this way a similar behaviour.
Figure 5. Influence of polymers concentration upon surface tension.
Figura 5. Influencia de la concentración de cada polímero sobre la tensión superficial.
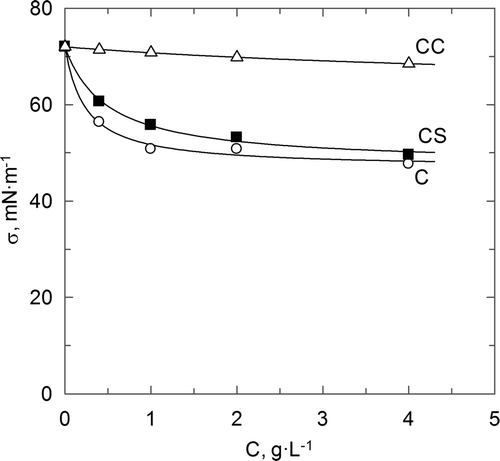
In all cases, when the polymer concentration increases in the liquid phase, a decrease in the value of the surface tension is observed, caused by the increase of the polymer presence at the gas–liquid interface. A similar behaviour in relation to the polymer concentration influence has been found by different researchers (Fainerman et al., Citation2006; Nahringbauer, Citation1997). Being more specific, for chitosan aqueous solutions and chitosan sulphate, a decrease in the surface tension value is produced when the polymer concentration increases until reaching a constant value of this property. This is due to the saturation of the interface with polymer molecules that do not allow the surface polymer concentration increasing.
Conclusions
The chemical modifications of chitosan to produce chitosan sulphate and carboxymethyl chitosan have been confirmed by FTIR and elemental analysis. The polymers deacetilation degree was also determined to analyse where the inclusion was produced. The chitosan derivatives were characterized on the basis of the average molecular weight using the intrinsic viscosity and Mark–Houwink constants. Chitosan sulphate showed an important decrease in molecular weight caused by the aggressive treatment with chlorosulphonic acid.
The influence of the polymer type and concentration upon different properties – density, kinematic viscosity, rheological behaviour and surface tension – has been studied. Carboxymethyl chitosan aqueous solutions showed the higher apparent viscosity values and with the most important deviation from the Newtonian behaviour. On the other hand, carboxymethyl chitosan showed a surface tension value near to the system in the absence of a polymer (pure water), while the presence of chitosan and chitosan sulphate caused an important decrease in the surface tension. All the samples showed a surface tension dynamic behaviour.
Supplementary Figure S1. FTIR spectra for chitosan, carboxymethyl chitosan and chitosan sulphate.
Figura adicional S1. Espectro de FTIR correspondiente al quitosano, carboximetil quitosano y sulfato de quitosano.
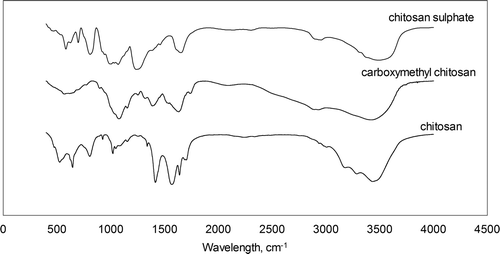
Supplementary Figure S2. Determination of intrinsic viscosity using Huggins and Kramer equations. (○) and (•), chitosan; (□) and (▪) carboxymethyl chitosan; (▴) and (▵) chitosan sulphate.
Figura adicional S2. Determinación de la viscosidad intrínseca mediante las ecuaciones de Huggins y Kramer. (○) y (•) quitosano, (□) y (▪) carboximetil quitosano. (▴) y (▵) sulfato de quitosano.
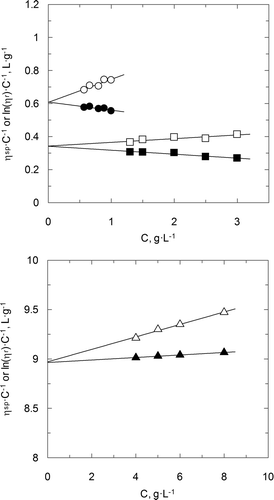
Supplementary Figure S3. Power law fit for carboxymethyl chitosan and chitosan sulphate aqueous solutions. C p = 2 g · L−1. (○) CC, (•) CS
Figura adicional S3. Ajuste a la ley de la potencia correspondiente a disoluciones acuosas de carboximetil quitosano y sulfato de quitosano. C p = 2 g · L−1. (○) CC, (•) CS.
Supplementary Figure S4. Hypothetical polymer conformation at the air–liquid interface (Nahringbauer, 1997).
Figura adicional S4. Hipotética conformación del polímero en la interface aire-líquido (Nahringbauer, 1997).
Supplementary Figure S5. Influence of time upon surface tension in aqueous solutions of chitosan (C), chitosan sulphate (CS) and carboxymethyl chitosan (CC). C p = 4 g · L−1.
Figura adicional S5. Influencia del tiempo sobre la tensión superficial en disoluciones acuosas de quitosano (C), sulfato de quitosano (CS) y carboximetil quitosano (CC). C p = 4 g · L−1.
Supplementary material
The supplementary material for this article is available at http://dx.doi.org/10.1080/19476337.2012.722565.
Supplementary Figures S1-S5
Download PDF (244.7 KB)Acknowledgements
Financial support for this work was provided by Ministerio de Ciencia e Innovación (project number: CTQ2010-18077). Diego Gómez-Díaz acknowledges to Ministerio de Ciencia e Innovación his “Ramón y Cajal” position.
References
- Álvarez , E. , Sanjurjo , B. , Cancela , A. and Navaza , J.M. 2000 . Mass transfer and influence of physical properties of solutions in a bubble column . Chemical Engineering Research and Design , 78 : 889 – 893 .
- Balanta , D. , Grande , C.D. and Zuluaga , F. 2010 . Extracción, identificación y caracterización de quitosano del micelio de aspergillus niger y sus aplicaciones como material bioadsorbente en el tratamiento de aguas . Revista Iberoamericana de Polímeros , 11 : 297 – 316 .
- Balazs , N. and Sipos , P. 2007 . Limitations of pH potentiometric titration for the determination of the degree of deacetylation of chitosan . Carbohydrate Research , 342 : 124 – 130 .
- Chen , L.Y. , Tian , Z.G. and Du , Y.M. 2004 . Synthesis and pH sensitivity of carboxymethyl chitosan-based polyampholyte hydrogels for protein carrier matrices . Biomaterials , 25 : 3725 – 3732 .
- Chen , P.H. , Kuo , T.Y. , Liu , F.H. , Hwang , Y.H. , Ho , M.H. , Wang , D.M. … and Hsieh , H.J. 2008 . Use of dicarboxylic acids to improve and diversify the material properties of porous chitosan membranes . Journal Agricultural and Food Chemistry , 56 : 9016 – 9021 .
- Cho , J. , Heuzey , M.-C. , Beégin , A. and Carreau , P.J. 2006 . Viscoelastic properties of chitosan solutions: Effect of concentration and ionic strength . Journal of Food Engineering , 74 : 500 – 515 .
- Chuah , H.H. , Lin-Vien , D. and Soni , U. 2001 . Poly(trimethylene terephthalate) molecular weight and Mark–Houwink equation . Polymer , 42 : 7137 – 7139 .
- Chung , Y.C. , Tsai , C.F. and Li , C.F. 2006 . Preparation and characterization of watersoluble chitosan produced by Maillard reaction . Fisheries Science , 72 : 1096 – 1103 .
- Cravotto , G. , Tagliapietra , S. , Robaldo , B. and Trotta , M. 2005 . Chemical modification of chitosan under high-intensity ultrasound . Ultrasonics Sonochemistry , 12 : 95 – 98 .
- El-Sherbiny , I.M. and Smyth , H.D.C. 2010 . Poly(ethylene glycol)–carboxymethyl chitosan-based pH-responsive hydrogels: Photo-induced synthesis, characterization, swelling, and in vitro evaluations potential drug carriers . Carbohydrate Research , 345 : 2004 – 2012 .
- Fainerman , V.B. , Lylyk , S.V. , Ferri , J.K. , Miller , R. , Watzke , H. , Leser , M.E. and Michel , M. 2006 . Adsorption kinetics of proteins at the solution/air interfaces with controlled bulk convection . Colloids and Surfaces A: Physicochemical and Engineering Aspects , 282–283 : 217 – 221 .
- Gamzazade , A. , Skyyar , A. , Nasibov , S. , Suskov , A. and Knirel , Y.A. 1997 . Structural features of sulfated chitosans . Carbohydrate Polymers , 34 : 113 – 116 .
- Gau , C.-S. , Yu , H. and Zografi , G. 1993 . Surface viscoelasticity of hydroxypropyl cellulose and hydroxyethyl cellulose monolayers at the air/water interface . Macromolecules , 26 : 2524 – 2529 .
- Hatashi , J. 1993 . U.S. Patent No. 5229504 , Washington , DC : U.S. Patent and Trademark Office .
- Ho , Y.C. , Mi , F.L. , Sung , H.W. and Kuo , P.L. 2009 . Heparin-functionalized chitosan–alginate scaffolds for controlled release of growth factor . International Journal of Pharmaceutics , 376 : 69 – 75 .
- Jang , M.K. , Kong , B.G. , Jeong , Y.I. , Lee , C.H. and Nah , J.W. 2004 . Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources . Journal of Polymer Science, Part A: Polymer Chemistry , 42 : 3423 – 3432 .
- Jiao , Z. , Xueqing , Z. and Juntag , Y. 1998 . O2 transfer to pseudoplastic fermentation broths in air-lift reactors with different inner designs . Biotechnology Techniques , 12 : 729 – 732 .
- Ma , X. and Pawlik , M. 2007 . Intrinsic viscosities and Huggins constants of guar gum in alkali metal chloride solutions . Carbohydrate Polymers , 70 : 15 – 24 .
- Maghami , G. and Roberts , G. 1988 . Evaluation of the viscometric constants for chitosan . Makromolecular Chemistry and Physics , 189 : 195 – 200 .
- Majeti , N.V. and Ravi , K. 2000 . A review of chitin and chitosan applications . Reactive & Functional Polymers , 46 : 1 – 27 .
- Makri , E.A. and Doxastakis , G.I. 2007 . Surface tension of phaseolus vulgaris and coccineus proteins and effect of polysaccharides on their foaming properties . Food Chemistry , 101 : 37 – 48 .
- Mao , S. , Shuai , X. , Unger , F. , Simon , M. , Dianzhou Bi , D. and Kissel , T. 2004 . The depolymerization of chitosan: Effects on physicochemical and biological properties . International Journal of Pharmaceutics , 281 : 45 – 54 .
- Miao , J. , Chen , G. , Gao , C. , Lin , C. , Wang , D. and Sun , M. 2006 . Preparation and characterization of N,O-carboxymethyl chitosan (NOCC)/polysulfone (PS) composite nanofiltration membranes . Journal of Membrane Science , 280 : 478 – 484 .
- Mucha , M. 1998 . Rheological properties of chitosan blends with poly(ethyleneoxide) and poly(vinyl alcohol) in solution . Reactive & Functional Polymers , 38 : 19 – 25 .
- Nahringbauer , I. 1995 . Dynamic surface tension of aqueous polymer solutions, I: Ethyl(hydroxyethyl)cellulose (BERMOCOLL cst-103 . Colloid Interface Science , 176 : 318 – 328 .
- Nahringbauer , I. 1997 . Polymer-surfactant interaction as revealed by the time dependence of surface tension. The EHEC/SDS/Water system . Langmuir , 13 : 2242 – 2249 .
- Nishimura , S.-I. and Tokura , S. 1987 . Preparation and antithrombogenic activities of heparinoid from 6-O-(carboxymethyl)chitin . International Journal of Biological Macromolecules , 9 : 225 – 232 .
- Parada , L.G. , Crespín , G.D. , Miranda , R. and Katime , I. 2004 . Caracterización de quitosano por viscosimetría capilar y valoración potenciométrica . Revista Iberoamericana de Polímeros , 5 : 1 – 16 .
- Rao , M.A. 1999 . Rheology of fluids and semisolid foods: Principles and applications , Gaithersburg , , USA : Aspen Publishers .
- Ríos-Donato , N. , Navarro-Mendoza , R. , Ávila-Rodríguez , M. and Mendizábal-Mijares , E. 2006 . Obtención de sulfato de quitosano y su aplicación en el proceso de coagulación-floculación de suspensiones coloidales aniónicas de caolinita . Revista Iberoamericana de Polímeros , 7 : 145 – 161 .
- Rosso , D. , Huo , D.L. and Stenstrom , M.K. 2006 . Effects of interfacial surfactant contamination on bubble gas transfer . Chemical Engineering Science , 61 : 5500 – 5514 .
- Rosu , M. , Marlina , A. , Kaya , A. and Schumpe , A. 2007 . Surfactant adsorption onto activated carbon and its effect on absorption with chemical reaction . Chemical Engineering Science , 62 : 7336 – 7343 .
- Sashiwa , H. and Shigemasa , Y. 1999 . Chemical modification of chitin and chitosan 2: Preparation and water soluble property of N-acylated or N-alkylated partially deacetylated chitins . Carbohydrate Polymers , 39 : 127 – 138 .
- Singh , J. , Dutta , P.K. , Dutta , J. , Hunt , A.J. , Macquarrie , D.J. and Clark , J.H. 2009 . Preparation and properties of highly soluble chitosan-l-glutamic acid aerogel derivative . Carbohydrate Polymers , 76 : 188 – 195 .
- Sovilj , V. and Petrovi , L. 2007 . Interaction and phase separation in the system HPMC/NaCMC/SDS . Colloids & Surfaces A: Physicochemical Engineering Aspects , 298 : 94 – 98 .
- Suwan , J. , Zhang , Z. , Li , B. , Vongchan , P. , Meepowpan , P. , Zhang , F. … and Linhardt , R.J. 2009 . Sulfonation of papain-treated chitosan and its mechanism for antiacoagulant activity . Carbohydrate Research , 344 : 1190 – 1196 .
- Sweidan , K. , Jaber , A.-M. , Al-jbour , N. , Obaidat , R. , Al-Remawi , M. and Badwan , A. 2011 . Further investigation on the degree of deacetylation of chitosan determined by potentiometric titration . Journal of Excipients and Food Chemicals , 2 : 16 – 25 .
- Vongchan , P. , Sajomsang , W. , Subyen , D. and Kongtawelert , P. 2002 . Anticoagulant activity of a sulfated chitosan . Carbohydrate Research , 337 : 1239 – 1242 .
- Yang , T.-C. , Chou , C.-C. and Li , C.-F. 2002 . Preparation, water solubility and rheological property of the N-alkylated mono or disaccharide chitosan derivatives . Food Research International , 35 : 707 – 713 .