Abstract
Silver Tequila 100% agave was aged in new oak barrels from four French regions. The evolution of physicochemical parameters regulated by Official Mexican Standard, as well as color, turbidity, total acidity and pH were assayed during 34 weeks of maturation. Analysis of variance (ANOVA) showed significant differences between barrel origin on furan-2-carboxaldehyde, dry extract, color and turbidity; and throughout maturation time on all evaluated parameters, except for total acidity, ethanol, and butan-2-ol. The principal component analysis (PCA) made it possible to separate three different groups corresponding to Silver (S), Aged (A), and tequilas during ripening transition (G), as well as to describe an evolution in two steps. Finally, general discriminant analysis (GDA) made it possible to classify correctly 69% of the samples according to barrel origin and 100% of the samples as G and A tequilas.
Tequila Blanco 100% de agave fue madurado en barricas nuevas de roble de 4 regiones francesas. La evolución de los parámetros físicoquímicos regulados por la Norma Oficial Mexicana, así como el color, turbidez, acidez total y pH fueron evaluados durante 34 semanas de maduración. El análisis de varianza mostró diferencias estadísticamente significativas entre el origen de las barricas para furan-2-carboxaldehído, extracto seco, color y turbidez; y a lo largo del tiempo de maduración para todos los parámetros evaluados excepto para acidez total, etanol y butan-2-ol. El análisis de componentes principales permitió separar tres grupos correspondientes a tequilas Blanco (S), Reposado (A), y tequilas en la transición de la maduración (G), así como describir una evolución en dos etapas. Finalmente, el análisis discriminante general permitió clasificar correctamente 69% de las muestras de acuerdo al origen de la barrica y el 100% de las muestras como tequilas G y A.
Introduction
Tequila is an alcoholic beverage obtained from fermentation and distillation of the juice of cooked heads of blue Agave (A. tequilana Weber). Two categories of this drink can be distinguished according to the Official Mexican Standard of Tequila (NOM-006-SCFI-2005, 2006). In “tequila 00% Agave” only pure agave juice is allowed to be fermented and distilled. When the product is produced by adding up to 49% (w/v) of sugar from other sources (mainly from sugar cane), it is called “Tequila.” The latter is also known as “Mixed Tequila.” This product can be bottled immediately after distilling or placed in barrels for maturation before bottling. Based on the characteristics acquired in processes subsequent to distillation, tequila is classified as “Blanco or Silver,” product bottled after distillation and alcohol content adjustment; “Joven or Gold,” product that could be smoothed by adding authorized products; “Reposado or Aged,” product matured for a minimum of 2 months in oak containers; “Añejo or Extra Aged,” product matured at least 1 year in 600 L maximum oak barrels; and “Extra Añejo or Ultra-Aged”, product matured at least 3 years in 600 L maximum oak barrels.
Maturation is a slow transformation that allows the tequila to acquire very peculiar characteristics of flavor, principally during its residence inside white oak containers (Quercus alba) or holm oak containers (Quercus ilex). Maturation time through the limits established by regulation is determined in function to the physicochemical and sensory characteristics that each company wishes to attribute to a given brand.
Studies in wine have shown that changes during maturation are related with “bouquet” characteristic of the product and they, principally, depend on maturation time (Escalona, Birkmyre, Piggott, & Paterson, Citation2002; Perez-Prieto, Lopez-Roca, Martinez-Cutillas, Pardo-Minguez, & Gomez-Plaza, 2003), species and geographic origin of wood used in the construction of the barrels (Ancín, Garde, Torrea, & Jimenez, 2004; Chatonnet & Dubourdieu, Citation1998; de Souza et al., Citation2007; Perez-Coello, Sanz, & Cabezudo, Citation1999), age of the barrel (Garde Cerdán, Rodríguez Mozaz, & Ancín Azpilicueta, 2002; Pérez-Prieto, López-Roca, Martínez-Cutillas, Pardo Mínguez, & Gómez-Plaza, Citation2002), toasting degree and barrel capacity (Cadahía, Fernández de Simón, & Jalocha, Citation2003; Chatonnet, Cutzach, Pons, & Dubourdieu, Citation1999; Perez-Prieto, Lopez-Roca, Martinez-Cutillas, Pardo-Minguez, & Gomez-Plaza, Citation2003), the storage conditions such as temperature, the disposition of the barrels, humidity, light, ventilation, as well as the beverage itself (Cedeño & Álvarez-Jacobs, 2003; Garde Cerdán, Torrea Goñi, & Ancín Azpilicueta, Citation2004; Pino, Pérez, & Nuñez, Citation1996).
Moreover, in distilled beverages, several investigations have been focused on the evaluation of different factors that might modify favorably or unfavorably the physicochemical characteristics and composition of some spirits such as sake (Isogai, Utsunomiya, Kanda, & Iwata, Citation2005), cachaça (de Souza et al., Citation2007), cider distillates (Mangas, Rodriguez, Moreno, & Blanco, Citation1996a), rum (Pino et al., Citation1996), and tequila (Muñoz-Muñoz, Grenier, Gutiérrez-Pulido, & Cervantes-Martínez, Citation2008), among others, during maturation. For tequila in particular, several studies have been directed to the use of different methodologies that have allowed separating and identifying a great number of volatile compounds in the stages of cooking, fermentation and distillation (Arellano, Pelayo, Ramirez, & Rodriguez, Citation2008; Carreon-Alvarez et al., Citation2011; Mancilla-Margalli & Lopez, Citation2002; Morán-Marroquín, Córdova, Valle-Rodríguez, Estarrón-Espinosa, & Díaz-Montaño, Citation2011; Prado-Ramírez et al., Citation2005). For commercial products, investigations have been made to determine the volatile composition in both Silver and matured tequilas (Benn & Peppard, Citation1996; Lachenmeier, Richling, Lopez, Frank, & Schreier, Citation2005; Lachenmeier, Sohnius, Attig, & Lopez, Citation2006; Lopez & Dufour, Citation1999; Martín del Campo et al., Citation2011; Peña-Alvarez, Capella, Juarez, & Labastida, Citation2006; Vallejo-Cordoba, Gonzalez-Cordova, & Estrada-Montoya, Citation2004).
In matured tequilas, diminishing of the concentration of higher alcohols and the increasing of acids, esters and aldehydes (Cedeño & Álvarez-Jacobs, 2003) has been observed, as well as the effect of the housing of the barrels on the physicochemical quality of tequila. However, what is uncertain is how the volatile compounds evolve during maturation and factors that have a significant impact on this phenomenon. Thus, the objective of this study was to evaluate changes on physicochemical parameters and volatile compounds regulated by the Official Mexican Standard for Tequila, throughout maturation using oak barrels from four different French regions under real cellar conditions in a Distillery, and to find out those differentiating compounds of tequilas according to their age as well as the origin of barrel used. French barrels were selected for the Tequila Distillery in order to produce a high sensorial quality product in accordance with their interest.
Materials and methods
Chemicals and reagents
All reagents and chromatographic standards were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA) ACS reagent and purity ≥98%, respectively. The ethanol solvent LiChrosolv® was purchased from Merck (Darmstadt, Germany), chromatographic grade.
Tequila samples
A single lot of Silver Tequila (100% agave, 40% ethanol v/v) from the Tequila, Jalisco, Mexico region was used. It was placed in new oak barrels made in four different French regions: Allier (Region I, RI), Limousin (Region II, RII), Tronçais (Region III, RIII), and Centre de la France (Region IV, RIV); four barrels with a capacity of 230–240 L made in each region at medium toast were used. All barrels were conditioned at the origin and made with the same cooperage technique (data not provided for the Distillery). The product was obtained directly from a Tequila Distillery and maturation carried out in its cellar (18 ± 5°C average temperature, 50 ± 5% Rel. Humidity, and darkness).
Sampling and analysis
Sampling was carried out every two weeks, over a period of 34 weeks (8.5 months). Before maturation began, a sample of Silver Tequila was taken as witness (T). For each sampling, a 1 L sample was obtained from each barrel, and then they were homogenized to give one lot of 4 L corresponding to each region. This homogenization was done since in Tequila Distilleries it is usual to mix the product from barrels filled with the same batch in order to have a homogeneous product. Samples were analyzed by triplicate and the mean of each analysis was reported.
Physicochemical analysis
Alcohol content expressed as % ethanol (v/v) at 20°C was measured with a densitometer DMA-48 (Anton Paar, Graz, Austria).
Dry extract was quantified by evaporation to dryness (NOM-006-SCFI-1994, 1997). Twenty-five milliliters of sample were placed in an evaporating dish (at constant mass) and evaporated using a hot plate at 40°C, then was placed in an oven at 100°C for at least 1 h to achieve a constant mass. Result was expressed as g/L.
Aldehydes were quantified chemically according to Mexican Standard (NMX-V-005-NORMEX-2005, 2005) by placing 100 mL of distilled tequila, 100 mL of distilled water, and 20 mL of 0.05 N sodium bisulfate solution in an Erlenmeyer flask and were allowed to stand for 30 min at room temperature in a dark place. Then, 25 mL of 0.05 N iodine solution was added and the excess of iodine was titrated with a 0.05 N sodium thiosulfate solution and some drops of 1% starch solution. Results were expressed as mg/100 mL of anhydrous ethanol.
Total acidity was determined by titrimetry (Bailly, Jerkovic, Marchand-Brynaert, & Collin, Citation2006). 0.5 mL of 5% phenolphthalein solution was added to 25 mL of distilled sample and was titrated with 0.1 N sodium hydroxide. Results were expressed as mg of acetic acid/100 mL of anhydrous ethanol.
Turbidity was measured with a turbidimeter HACH 2100 a model (Loveland, CO, USA) expressed as NTU (Nephelometric turbidity units). Color was analyzed directly; sample transmittance was measured at 520 nm with a colorimeter Milton Roy Spectronic 21D (Thermo Spectronic, Madison, WI, USA). pH was also evaluated directly with an Oakton 2500 series equipment (Vernon Hills, Il, USA).
Major volatile compounds in the tequila: higher alcohols (propan-1-ol, butan-2-ol, 2-methylpropan-1-ol, and 3-methylbutan-1-ol), methanol, esters (ethyl 2-hydroxypropanoate and ethyl acetate) and furan-2- carboxaldehyde were determined by gas chromatography-FID and quantified as mg/100 mL of anhydrous ethanol in accordance with the Mexican Standard (NMX-V-005-NORMEX-2005, 2005).
Chromatographic analysis
Gas chromatographic analyses were carried out by using a gas chromatograph HP 5890 Series II (Hewlett-Packard, Palo Alto, USA) equipped with a flame ionization detector. The compounds were separated in a HP-FFAP capillary column, 50 m × 0.20 mm ID × 0.30 μm thickness (Hewlett-Packard). The oven program was 55°C during 6.5 min, increasing 10°C/min until 165°C, then 30°C/min until 220°C and held for 5 min. Injector and detector temperatures were kept at 220°C and 240°C, respectively. A sample volume of 0.5 μL was automatically injected using nitrogen as carrier gas at 1.0 mL/min. Quantitative data were obtained by interpolation of peak areas in the calibration plots built by the analysis of 10 solutions containing known amounts of the analytes mentioned above, using pentan-2-ol as internal standard.
Statistical analysis
The data obtained were analyzed by using STATISTICA software (StatSoft, Tulsa, USA). Analysis of variance (ANOVA) and Fisher's multiple range test of minimal significant differences (LSD), allowed evidencing variables that showed significant differences among samples. Then, correlation analysis was applied to the data set in order to evaluate the relation among the evaluated parameters (p < 0.05). Next, principal component analysis (PCA) was carried out, in order to identify the most important parameters changing, due to maturation time and/or barrel type. Principal component analysis made it possible to evaluate the whole data set instead of individual parameters.
Finally, general discriminant analysis (GDA) was applied to the data set in order to evaluate the possibility to discriminate the tequila samples according to the barrel region (four regions, RI–RIV) and to the maturation time (17 samplings every 2 weeks, 2–34 weeks). This statistical tool generated discriminant rules or functions that made it possible to classify experimental units in two or more populations defined in a unique way. Additionally, this tool made it possible to reduce the amount of initial variables by selecting those having more impact in the discrimination. A forward stepwise method (p inclusion 0.05, p exclusion 0.05) was applied in order to minimize the model size. The selected variables were those with a significant (p < 0.05) F value.
Results and discussion
Physicochemical parameters evolution
depicts minimum and maximum values determined for the samples evaluated at the beginning and the end of ripening and their comparison with those parameters specified in the Official Mexican Standard of Tequila (NOM-006-SCFI-2005, 2006). All the physicochemical parameters evaluated herein showed an evolution throughout the maturation time (Supplementary (a–j) and ). As expected, each parameter evolved differently throughout aging. It can be observed that the determinations effectuated over the maturation period were within the limits specified by the regulation; however, it can also be observed that the only parameter that did not meet these specifications was the furan-2-carboxaldehyde which was higher compared to the maximum level allowed.
Figure 1. Principal Component Analysis (PCA) plots of the two first components PC1 and PC2. (a) Factorial map of the scores, and (b) factor loadings. S: Silver, G: Tequilas during ripening transition, and A: Aged Tequilas.
Figura 1. Análisis de Componentes Principales (PCA). Gráfico de los dos primeros componentes PC1 y PC2. (a) Mapa factorial de individuos, y (b) cargas de los factores. S: Blanco, G: Tequilas en transición a la maduración y A: Tequilas reposados.
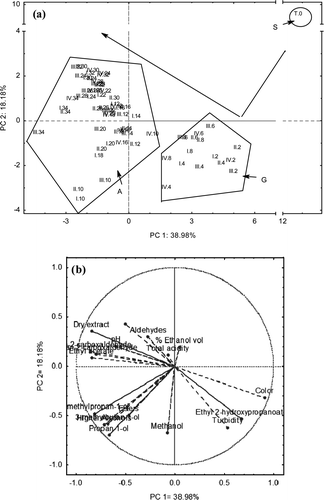
Table 1. Results of the physiochemical analysis of tequilas compared to the Official Mexican Standard of Tequila.
Tabla 1. Resultados de los análisis fisicoquímicos de tequilas comparados con la Legislación Mexicana del Tequila.
Table 2. Analysis of variance (ANOVA) and Fisher Least Significant Difference test (LSD) results of the chemical analysis of tequila as affected by maturation time and origin with a confidence interval of 95%.
Tabla 2. Resultados del análisis de varianza (ANOVA) y la prueba de distancias mínimas significativas de Fisher (LSD) para los análisis químicos de tequila. Efecto del tiempo de maduración y el origen de la barrica, con un intervalo de confianza del 95%.
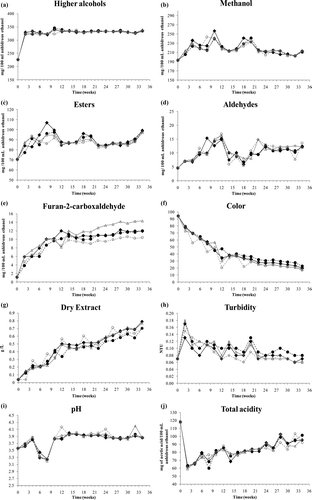
The concentration of higher alcohols (Supplementary a) showed an increase with significant differences (p < 0.000) between the witness (T) and the first sampling (0–2 weeks), as well as between the 8 and 10 weeks maturation (2 and 2.5 months), and remained steady thereafter (3 to 8.5 months) showing no significant differences (). No significant differences (p > 0.05) were observed between barrels (). This behavior is consistent with studies focused on rum, wine and cider distillates (Mangas, Rodríguez, Moreno, & Blanco, Citation1996b; Pino et al., Citation1996). However, it does not coincide with Cedeño and Álvarez-Jacobs (2003), who report that these volatile compounds diminish throughout tequila maturation. However, an increase in the concentration of higher alcohols in wines aged in new barrels of American oak has been observed by Ortega Heras, Rivero-Pérez, Pérez-Magariño, González-Huerta, and González-Sanjosé (Citation2008) and Câmara, Alves, and Marques (Citation2006). This fact has been correlated with the possible hydrolysis of some esters, or evaporation during maturation in barrels that also could contribute to this phenomenon.
On the other hand, methanol concentration increased significantly (p = 0.000) as shown in . It increased substantially until week 4, and then fluctuated until week 20 when its concentration dropped steadily until the end of maturation. This behavior was similar for all barrels (p = 0.318) except for those from the Allier region (Supplementary b, ) which showed a more dramatic fluctuation and a lower mean concentration. This could be due to the extraction of methanol generated during wood toasting by pyrolysis of lignin (Fessenden, Fessenden, & Logue, Citation1998).
Ester concentration presented significant differences (p = 0.000) during maturation as shown in . Esters showed drastic increase until week 4 and then fluctuated until the end of maturation for all aged liquors, where average concentration of 98 mg/100 mL is reached at week 34 (Supplementary c). Although no significant differences were found among barrels (p = 0.102), changes were more evident in liquors aged in the Allier barrel (). Previous research reports with rum and tequila evidenced a similar behavior (Cedeño & Álvarez-Jacobs, 2003; Pino et al., Citation1996). It is noteworthy to mention that the behavior throughout maturation of ethyl acetate and ethyl 2-hydroxypropanoate were different when evaluated separately, even if they both showed significant differences (p = 0.000). During the Silver Tequila maturation, ethyl 2-hydroxypropanoate decreases while ethyl acetate increases (). Both compounds showed no significant differences among barrels (p = 0.102 and p = 0.942, respectively) (). In a study related to rum maturation, Pino et al. (Citation1996) observed a similar behavior for ethyl acetate, concluding that this ester can be formed by reactions between ethanol and acetic acid in an acidic media. The same behavior is reported by Ralph (Citation2003) who researched American whiskeys.
The acetaldehyde content, referred as aldehydes according to the Official Mexican Standard of Tequila (2005), changed significantly (p = 0.000) during aging () although it presented an irregular behavior (Supplementary d). In the first twelve weeks of maturation, a considerable increase was observed. From there, a decrease was sustained up to week 20, after which it increased again maintaining a behavior fairly constant until the end of the sampling program (). No significant differences were observed among barrels (). Cedeño and Álvarez-Jacobs (2003) also observed an increased aldehyde concentration in matured tequilas. The aldehydes are formed principally during fermentation, however, they can also be obtained from oxidation reactions of ethanol (Liebmann & Scherl, Citation1949; Mosedale & Puech, Citation1998; Ralph, Citation2003). Liebmann and Scherl (1949) described an irregular aldehyde concentration in whiskeys ongoing to maturation. These authors found increased concentrations especially at the beginning of the maturation process and a linearly steady increase during the last stages of aging. Moreover, the acetaldehyde is an intermediary in the formation of other important compounds. The formation of acetic acid and the ethyl acetate by ethanol oxidation via acetaldehyde is an example of it (Morrison & Boyd, Citation1986; Mosedale & Puech, Citation1998; Ralph, Citation2003).
Furan-2-carboxaldehyde presented a significant (p = 0.000) and steady increase during the maturation period, which was most remarkable during the first twelve weeks (Supplementary e). Significant differences were also observed among barrel origin, mainly between Tronçais and Centre de la France regions, where average concentrations of 14.3 and 10.4 mg/100 mL of anhydrous alcohol were reached at the end of ripening, respectively. Moreover, tequilas matured in barrels of Allier and Limousin regions did not show significant differences throughout the 20–34 weeks maturation period. The increase of furan-2-carboxaldehyde content during maturation has been demonstrated and reported before (Fernandez de Simon, Cadahia, & Jalocha, Citation2003; Mangas, Rodriguez, Moreno, Suarez, & Blanco, Citation1996c; Mosedale & Ford, Citation1996; Mosedale & Puech, Citation1998; Perez-Prieto et al., Citation2003; Rabier & Moutounet, 1991). Mangas et al. (Citation1996c) observed an increase in furan-2-carboxaldehyde concentration throughout a 15 month maturation of cider distillates. Fernandez de Simon et al. (Citation2003) observed an increase of furan-2-carboxaldehyde content in wines aged 12 and 21 months in Limousin and Allier barrels (corresponding to RII and RI in our study, respectively), but did not find significant differences between these two barrel origins. Nevertheless, Mosedale and Ford (Citation1996) found significant differences in furan-2-carboxaldehyde content in wood from Limousin and Tronçais regions (corresponding to RII and RIII in our study, respectively). Perez-Prieto et al. (Citation2003) observed a rapid increase in furan-2-carboxaldehyde by at least four-fold the initial concentration in a wine matured during 6 months in American oak barrels of 220–500 L capacity used previously for aging Monastrell wine.
In our study, significant differences between these barrels were found (). In determinations of furan-2-carboxaldehyde performed as part of the quality control of aged and ultra-aged tequilas destined to market, it has been found that the value of furan-2-carboxaldehyde can reach a maximum of 4 mg/100 mL of anhydrous alcohol in periods of maturation of a year in American oak barrels. More elevated furan-2-carboxaldehyde values have been reported in matured agave distilled beverages (Munoz-Munoz et al., Citation2010; Muñoz Rodriquez, Wrobel, & Wrobel, 2005). Muñoz Rodriguez et al. (Citation2005) found furan-2-carboxaldehyde concentrations up to 23 μg/mL in 100% agave Silver and aged tequilas. In a tequila containing 40% ethanol (v/v), this concentration is equivalent to 5.75 mg/100 mL of anhydrous alcohol.
Furan-2-carboxaldehyde is mainly formed during the toasting of the barrels by thermal degradation of hemicelluloses present in wood. This compound is then extracted by ethanol and water during the contact of the alcoholic beverage with the inner walls of the barrel in the stage of maturation. The concentration of furan-2-carboxaldehyde extract depends largely on the alcohol content of drinks, the degree of wood toasting and contact time (Chatonnet, Boidron, & Pons, Citation1989; Garde Cerdán et al., Citation2004). Furan-2-carboxaldehyde concentration in tequila is often higher than in other spirits and wines since it is produced from the degradation of carbohydrates by Maillard reactions during the stage of cooking the agave heads (Mancilla-Margalli & Lopez, Citation2002) as well as during the distillation stage (Prado, Citation2004). In our study, furan-2-carboxaldehyde concentrations were observed 3.5-fold the maximum allowed by regulation after 34 weeks maturation. This may be due to the type of wood used and the fact that all the barrels were new.
According to the values of transmittance, a steady color increase was observed during the whole process (Supplementary f, ) reflected by a significant (p = 0.000) transmittance decrease. Significant color evolution was also observed among barrels (p = 0.001). The lower color increase was obtained in liquors matured in the Limousin barrel followed by counterparts stored in Allier barrels (). Mosedale and Ford (Citation1996) found significant differences in color in ethanolic (63% ethanol solutions) extracts obtained from wood of the Limousin (RII) and Tronçais (RIII) regions, differences were also found in the present study (). This behavior could be attributed either to degradation or dissolution of phenolic compounds (tannins) (Rabier & Moutounet, 1991).
Dry extract presented a significant (p = 0.000) gradual increase during the whole process of maturation (Supplementary g), but this behavior showed significant differences (p = 0.015) among barrels, where the tequila aged in Limousin barrels showed the lower mean values ().
Turbidity presented significant differences (p = 0.000) throughout maturation. Its value increased dramatically in the first 2 weeks of storage (Supplementary h), then a moderate decrease was observed until the end of maturation (). Significant differences were observed among barrels (). Turbidity could be due to the amount of matter extracted from the barrels during the maturation process and the afterwards observed decrement to precipitation owning to the repose time as has been reported before for other distilled beverages (Mosedale & Puech, Citation1998).
The increase in color, dry extract, and turbidity can be attributed to the extraction of materials from the wood (cellulose and hemicellulose); this phenomenon had a direct effect in the increase of the concentration of dry extract during maturation (Conner, Reid, & Jack, 2003; Mosedale & Puech, Citation1998).
There was a significant increase of pH (p > 0.000) during the 8.5 months of observation (Supplementary i, ), but no differences (p = 0.259) were found between barrels (). These results are different from those reported by Mosedale and Ford (Citation1996) who found significant differences in pH in wood from Limousin (RII) and Tronçais (RIII) regions after extracting with 63% ethanol solution. In studies performed in white and red wines, an increase has been reported in the titratable acidity and decrease of pH (Wilker & Gallander, Citation1988); however, in this study a correlation was not observed. It is important to mention that wine is not a distilled beverage, although in general, in alcoholic beverages the acetic acid is the most abundant acid, so the acidity is reported as concentration of acetic acid equivalents. In wine, acetic acid is generated during fermentation via acetaldehyde mainly by acetic bacteria (Etiévant Citation1991). In tequila and whisky, acetic acid is chiefly formed from the degradation of the acetyl group by degradation of hemicelluloses or by oxidation of ethanol (Morrison & Boyd, Citation1986; Mosedale & Puech, Citation1998; Ralph, Citation2003).
The most remarkable change in total acidity was observed between the witness and the first sampling (2 weeks), presenting a drastic decrease (Supplementary j) then increasing steadily until the end of maturation. Nevertheless, no significant differences were found for total acidity throughout maturation (p = 0.455), nor for barrels (p = 0.400). However, significant differences were found for the interaction between maturation time and barrels (p = 0.002) (data not shown). According to studies performed on other distilled liquors, it was expected that the acidity would increase gradually with maturation time (Cedeño & Álvarez-Jacobs, 2003). In this research, acidity did not increase gradually during the first two weeks of maturation, but did occur starting on the fourth week. Nevertheless, a rapid increase of this parameter during the first months of whiskey aging has been described before (Liebmann & Rosenblatt, Citation1943). Organic acids may form by direct hydrolysis; and due to leaching from the wood by acid or alkaline hydrolysis of hemicelluloses. On the other hand, acids participate in the formation of esters (Bakker, Picinelli, & Bridle, Citation1993; Escalona et al., Citation2002; Liebmann & Rosenblatt, Citation1943; Rabier & Moutounet, 1991; Ribereau-Gayon, Citation1978).
Correlation and principal component analysis (PCA)
The correlations between the parameters evaluated are depicted in . Most of the parameters showed significant correlations (p < 0.05), except for total acidity and butan-2-ol (data not shown), which showed no significant correlation with any other parameter. The % ethanol (v/v) was only correlated with methanol content (R =−0.31). As expected, aldehyde content had a positive correlation with furan-2-carboxaldehyde concentration (R = 0.39). Unsurprisingly, color showed a positive and negative correlation with turbidity (R = 0.68) and furan-2-carboxaldehyde (R =−0.77), respectively. Among the evaluated esters, ethyl acetate and ethyl 2-hydroxypropanoate showed a strong negative correlation (R =−0.75). Additionally, they showed opposite correlation with furan-2-carboxaldehyde (R = 0.56 and R =−0.56, respectively), dry extract (R = 0.76 and R =−0.72, respectively), color (R =−0.78 and R = 0.76, respectively), turbidity (R =−0.49 and R = 0.62, respectively), and pH (R = 0.54 and R =−0.55, respectively). The esters content (ethyl acetate + ethyl 2-hydroxypropanoate) did not show the same correlations compared to the individual esters. The individual higher alcohols and their sum showed significant correlations among them, as well as with furan-2-carboxaldehyde, dry extract, color, methanol, ethyl acetate, pH, and esters; however, they were not correlated with aldehydes (). These results are in agreement with previous studies in whiskeys (Liebmann & Scherl, Citation1949). Liebmann and Scherl (Citation1949) established a correlation between esters and solids (dry extract), as well as solids and color evolution throughout whisky maturation. They also described a correlation between total acids and esters and total acids and solids, but in our study no correlation was found.
Table 3. Correlation coefficients for the parameters analyzed throughout tequila maturation.a
Tabla 3. Coeficientes de correlación para los parámetros analizados a lo largo de la maduración del tequila.a
The PCA factors loadings plot and factorial map for the evaluated parameters defined by PC1 and PC2 are shown in (a and b). These components explained 39% and 18.2% of total variance, respectively. In the factorial map (a), three different groups of samples are separated according to the maturation time. The first group (S) includes only the witness sample or Silver Tequila and was separated by a combination of axe 1 and axe 2. The second group (G) included samples matured from 2 weeks to 8 weeks. These samples were separated by a combination of both axes. Finally, the third group (A) grouped samples from 10 weeks to 34 weeks maturation corresponding to Aged Tequilas. This group was described by a combination of both axes. Those groups followed a two-step evolution, the first one from week 0 to week 2 and the second one from week 2 to week 34. In the first step, a strong decrease of the factor coordinates of variables (weeks of maturation) is observed for both axes. In the second step, an increase is observed for both axes.
Most of the studied variables had a strong correlation with PC1 (R < 0.02) except for methanol, butan-2-ol, total acidity, aldehydes and % ethanol (v/v) on the factor loadings plot (b). Color (R = 0.91), ethyl 2-hydroxypropanoate (R = 0.68), and turbidity (R = 0.54) showed a strong positive correlation with this component while furan-2-carboxaldehyde (R =−0.86), ethyl acetate (R =−0.85), dry extract (R =−0.85), 2-methylpropan-1-ol (R =−0.81), higher alcohols (R =−0.72), 3-methylbutan-1-ol (R =−0.68), and propan-1-ol (R =−0.67), showed negative correlations. On the other hand, propan-1-ol, methanol, turbidity, higher alcohols, 3-methylbutan-1-ol, and ethyl 2-hydroxypropanoate, showed high negative correlations with PC2 (R < −0.50).
Discriminant analysis (GDA)
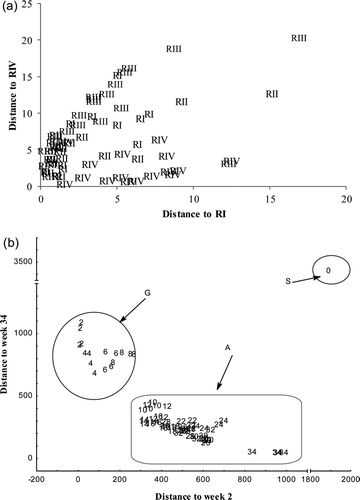
Forward stepwise analysis for barrel regions led to the selection of three parameters (p inclusion 0.05, p exclusion 0.05): color, turbidity and furan-2-carboxaldehyde. Only 69% of the samples were correctly classified according to barrel origin. Cooman graphics of Mahalanobis distances (a) did not show defined groups according to barrel origin. Samples of RIV (Centre de France) showed a higher number of samples classified correctly (82.4%). The lower level of correct classification was for RI (Allier) with 47% of correct classification. This could be due to the geographical proximity between the evaluated regions and cooperage practices since the discriminant parameters are related with these techniques (Conner et al., Citation2003; Mosedale & Puech, Citation1998).
Figure 2. Comman's graph for tequila samples classification. (a) According to barrel origin (RI: Allier, RII: Limousin, RIII: Tronçais, and RIV: Centre de la France), and (b) According to age (8–34 weeks).
Figura 2. Gráfica de Comman para la clasificación de muestras de tequila. (a) Según el origen de las barricas (RI: Allier, RII: Limousin, RIII: Tronçais y RIV: Centro de Francia), y (b) Según la edad (8–34 semanas).
Supplementary Figure 1. Evolution of some physicochemical parameters throughout maturation time of tequila stored in oak barrels of different origin. (—♦—) RI: Allier, (- -•- -) RII: Limousin, (—▵—) RIII: Tronçais, and (- -○- -) RIV: Centre de la France.
Figura adicional 1. Evolución de algunos parámetros fisicoquímicos durante la maduración de tequila almacenado en barricas de roble de origen diferente. (—♦—) RI: Allier, (- -•- -) RII: Limousin, (—▵—) RIII: Tronçais, and (- -○- -) Centro de Francia.
Forward stepwise GDA analysis according to maturation time (weeks) led to the selection of 12 parameters (p inclusion 0.05, p exclusion 0.05): color, pH, turbidity, furan-2-carboxaldehyde, ethyl acetate, ethyl 2-hydroxypropanoate, esters, aldehydes, as well as methanol, 2-methylpropan-1-ol, and 3-methylbutan-1-ol. The obtained model made it possible to classify correctly 97% of the samples. Misclassification was observed for samples matured for 24 and 26 weeks. In both cases, only 75% of samples were classified correctly. All the S (0 weeks) and G samples (2–8 weeks) were correctly classified (b). Nevertheless, some aged (A) samples (10–34 weeks) were misclassified. They were classified as any other matured tequila, but not as G tequila. When tequilas were separated in three groups: S (0 weeks), G (2–8 weeks) or Aged (10–34 weeks), 100% of these samples were correctly classified (b).
Different studies have demonstrated that cooperage, wood origin region, and maturation time, affect compounds found in matured alcoholic beverages (Bujake, Citation1992; Fernandez de Simon et al., Citation2003; Mosedale & Puech, Citation1998). Fernandez de Simon et al. (Citation2003) discriminated samples stored in American and European barrels specially based on furanic compounds using canonical discriminant analysis. But this analysis could not discriminate between samples stored in barrels made in Limousin and Allier French regions.
Discrimination according to maturation time is consistent with changes reported in whiskey by Bujake (Citation1992). The author reported a high correlation between maturation time and esters, aldehydes, total color, solids, ethyl acetate, esters, 2-methylpropan-1-ol, and 3-methylbutan-1-ol concentrations.
Conclusions
Analysis of variance made it possible to identify parameters that significantly varied due to barrel origin and maturation time. Principal component analysis made it possible to classify tequilas according to their maturation time into three well differentiated groups corresponding to Silver (initial distilled), tequilas during ripening transition (G, 2 to 8 weeks) and Aged (10 to 34 weeks) tequilas according to the Official Mexican Standard. Principal component analysis also made it possible to describe the maturation evolution by a combination of PC1 and PC2. These changes could be associated to the evolution of different physicochemical parameters. Finally, GDA of data made it possible to classify correctly 69% of tequilas according to the barrel origin and 97% according to maturation time (weeks). The model obtained for ripening time made it possible to classify correctly 100% of samples as G or Aged. A larger study is necessary to confirm these results with a higher number of barrels to evaluate differences among barrels from the same region, cooperage techniques and a longer maturation time.
Additionally, in this study only the main physicochemical parameters were evaluated. Nevertheless, further studies will be necessary to assess the effect of aging time and/or barrel origin in the minor aroma compounds and sensory properties of the aged products.
Supplementary material
The supplementary material for this article is available at http://dx.doi.org/10.1080/19476337.2012.727033.
Supplementary Figure 1. Evolution of some physicochemical parameters throughout maturation time of tequila stored in oak barrels of different origin.
Download PDF (915.3 KB)Acknowledgments
J.E. López-Ramírez would like to thank CONACYT for the given scholarship toward the realization of this work. The authors would like to thank to Tequila Herradura Inc., as well as PhD. M. Cedeño Cruz and PhD. J. Alvarez-Jacobs who kindly facilitated the samples, barrels and tequila's cellar for the study. Additionally, the authors would like to thank PhD. Sergio Román Othón Serna Saldivar, Professor of the Departamento de Biotecnología e Ingeniería de Alimentos, ITESM Campus Monterrey for the English language revision of the manuscript.
References
- Ancín , C. , Garde , T. , Torrea , D. and Jimenez , N. 2004 . Extraction of volatile compounds in model wine from different oak woods: effect of SO2 . Food Research International , 37 : 375 – 383 . doi: 10.1016/j.foodres.2004.01.007
- Arellano , M. , Pelayo , C. , Ramirez , J. and Rodriguez , I. 2008 . Characterization of kinetic parameters and the formation of volatile compounds during the tequila fermentation by wild yeasts isolated from agave juice . Journal of Industrial Microbiology & Biotechnology , 35 : 835 – 841 .
- Bailly , S. , Jerkovic , V. , Marchand-Brynaert , J. and Collin , S. 2006 . Aroma extraction dilution analysis of Sauternes wines. Key role of polyfunctional thiols . Journal of Agricultural and Food Chemistry , 54 : 7227 – 7234 .
- Bakker , J. , Picinelli , A. and Bridle , P. 1993 . Model wine solutions – Color and composition changes during aging . Vitis , 32 : 111 – 118 .
- Benn , S.M. and Peppard , T.L. 1996 . Characterization of tequila flavor by instrumental and sensory analysis . Journal of Agricultural and Food Chemistry , 44 : 557 – 566 .
- Bujake , J.E. 1992 . Beverage spirits, distilled, Kirk-Othmer Encyclopedia of Chemical Technology , (4th ed. , Hoboken, NJ : John Wiley & Sons, Inc .
- Cadahía , E. , Fernández de Simón , B. and Jalocha , J. 2003 . Volatile compounds in Spanish, French, and American oak woods after natural seasoning and toasting . Journal of Agricultural and Food Chemistry , 51 : 5923 – 5932 . doi: 10.1021/jf0302456
- Câmara , J.S. , Alves , M.A. and Marques , J.C. 2006 . Changes in volatile composition of Madeira wines during their oxidative ageing . Analytica Chimica Acta , 563 ( 1–2 ) : 188 – 197 . doi: 10.1016/j.aca.2005.10.031
- Carreon-Alvarez , A. , Herrera-Gonzalez , A. , Casillas , N. , Prado-Ramirez , R. , Estarron-Espinosa , M. and Soto , V. 2011 . Cu (II) removal from tequila using an ion-exchange resin . Food Chemistry , 127 : 1503 – 1509 .
- Cedeño , M. and Álvarez-Jacobs , J. 2003 . “ Production of tequila from agave: Historical influences and contemporary processes ” . In The alcohol textbook: A reference for the beverage, fuel and industrial alcohol industries , Edited by: Jaques , K.A. , Lyons & , T.P. and Kelsall , D.R. 225 – 246 . Nottingham , , UK : Nottingham University Press .
- Cerdán , T.G. , Rodríguez Mozaz , S. and Ancín Azpilicueta , C. 2002 . Volatile composition of aged wine in used barrels of French oak and of American oak . Food Research International , 35 : 603 – 610 . doi: 10.1016/S0963-9969(01)00151-X
- Chatonnet , P. , Boidron , J.N. and Pons , M. 1989 . Incidence du traitement thermique du bois de chêne sur sa composition chimique. 2e Partie: évolution de certains composés en fonction de l'intensité de brûlage . Connaiss. Vigne Vin , 23 : 223 – 250 .
- Chatonnet , P. , Cutzach , I. , Pons , M. and Dubourdieu , D. 1999 . Monitoring toasting intensity of barrels by chromatographic analysis of volatile compounds from toasted oak wood . Journal of Agricultural and Food Chemistry , 47 : 4310 – 4318 .
- Chatonnet , P. and Dubourdieu , D. 1998 . Comparative study of the characteristics of American white oak (Quercus alba) and European oak (Quercus petraea and Q. robur) for Production of barrels used in barrel aging of wines . American Journal of Enology and Viticulture , 49 ( 1 ) : 79 – 85 .
- Conner , J. , Reid , K. and Jack , F. 2003 . “ Maturation and blending ” . In Whisky: Technology, production and marketing , Edited by: Russell , I. 209 – 240 . London : Academic Press, Inc .
- de Souza , P.P. , Siebald , H.G.L. , Augusti , D.V. , Neto , W.B. , Amorim , V.M. and Catharino , R.R. 2007 . Electrospray ionization mass spectrometry fingerprinting of Brazilian artisan cachaca aged in different wood casks . Journal of Agricultural and Food Chemistry , 55 : 2094 – 2102 .
- Escalona , H. , Birkmyre , L. , Piggott , J.R. and Paterson , A. 2002 . Effect of maturation in small oak casks on the volatility of red wine aroma compounds . Analytica Chimica Acta , 458 : 45 – 54 .
- Etiévant , P.X. 1991 . “ Wine ” . In Volatile compounds in foods and beverages , Edited by: Maarse , H. 483 – 546 . New York, NY : Marcel Dekker Inc .
- Fernandez de Simon , B. , Cadahia , E. and Jalocha , J. 2003 . Volatile compounds in a Spanish Red wine aged in barrels made of Spanish, French, and American oak wood . Journal of Agricultural and Food Chemistry , 51 : 7671 – 7678 .
- Fessenden , R.J. , Fessenden , J.S. and Logue , M.W. 1998 . Organic chemistry , (6th ed.) , Pacific Grove : Brooks/Cole Pub Co .
- Garde Cerdán , T. , Torrea Goñi , D. and Ancín Azpilicueta , C. 2004 . Accumulation of volatile compounds during ageing of two red wines with different composition . Journal of Food Engineering , 65 : 349 – 356 .
- Isogai , A. , Utsunomiya , H. , Kanda , R. and Iwata , H. 2005 . Changes in the aroma compounds of sake during aging . Journal of Agricultural and Food Chemistry , 53 : 4118 – 4123 .
- Lachenmeier , D.W. , Richling , E. , Lopez , M.G. , Frank , W. and Schreier , P. 2005 . Multivariate analysis of FTIR and ion chromatographic data for the quality control of tequila . Journal of Agricultural and Food Chemistry , 53 : 2151 – 2157 .
- Lachenmeier , D.W. , Sohnius , E.M. , Attig , R. and Lopez , M.G. 2006 . Quantification of selected volatile constituents and anions in Mexican Agave spirits (Tequila, Mezcal, Sotol, Bacanora) . Journal of Agricultural and Food Chemistry , 54 : 3911 – 3915 .
- Liebmann , A.J. and Rosenblatt , M. 1943 . Changes in whisky while maturing . Industrial & Engineering Chemistry , 35 : 994 – 1002 .
- Liebmann , A.J. and Scherl , B. 1949 . Changes in whisky while maturing . Industrial & Engineering Chemistry , 41 : 534 – 543 .
- Lopez , M.G. and Dufour , J.P. 1999 . Charm analyses of Blanco, Reposado, and Anejo tequilas . Abstracts of Papers of the American Chemical Society , 218 79-AGFD
- Mancilla-Margalli , N.A. and Lopez , M.G. 2002 . Generation of Maillard compounds from inulin during the thermal processing of Agave tequilana Weber var. azul . Journal of Agricultural and Food Chemistry , 50 : 806 – 812 .
- Mangas , J. , Rodriguez , R. , Moreno , J. and Blanco , D. 1996a . Volatiles in distillates of Cider aged in American oak wood . Journal of Agricultural and Food Chemistry , 44 ( 1 ) : 268 – 273 .
- Mangas , J. , Rodríguez , R. , Moreno , J. and Blanco , D. 1996b . Changes in the major volatile compounds of cider distillates during maturation . Lebensmittel-Wissenschaft und-Technologie , 29 : 357 – 364 .
- Mangas , J. , Rodriguez , R. , Moreno , J. , Suarez , B. and Blanco , D. 1996c . Evolution of aromatic and furanic congeners in the maturation of cider brandy: A contribution to its characterization . Journal of Agricultural and Food Chemistry , 44 : 3303 – 3307 .
- Martín del Campo , S.T. , Gómez Hernández , H.E. , Gutiérrez , H. , Escalona , H. , Estarrón , M. and Cosío , Ramírez, R. 2011 . Volatile composition of tequila. Evaluation of three extraction methods . CyTA – Journal of Food , 9 : 152 – 159 .
- Morán-Marroquín , G.A. , Córdova , J. , Valle-Rodríguez , J.O. , Estarrón-Espinosa , M. and Díaz-Montaño , D.M. 2011 . Effect of dilution rate and nutrients addition on the fermentative capability and synthesis of aromatic compounds of two indigenous strains of Saccharomyces cerevisiae in continuous cultures fed with Agave tequilana juice . International Journal of Food Microbiology , 151 ( 1 ) : 87 – 92 .
- Morrison , R.T. and Boyd , R.N. 1986 . Química Orgánica (P. Fiedler, Trans , 2nd ed.) , Mexico : Addison-Wesley Iberoamericana .
- Mosedale , J.R. and Ford , A. 1996 . Variation of the flavour and extractives of European oak wood from two French forests . Journal of the Science of Food and Agriculture , 70 : 273 – 287 .
- Mosedale , J.R. and Puech , J.L. 1998 . Wood maturation of distilled beverages . Trends in Food Science & Technology , 9 : 95 – 101 .
- Munoz-Munoz , A.C. , Pichardo-Molina , J.L. , Ramos-Ortiz , G. , Barbosa-Garcia , O. , Maldonado , J.L. and Meneses-Nava , M.A. 2010 . Identification and quantification of furanic compounds in tequila and mezcal using spectroscopy and chemometric methods . Journal of the Brazilian Chemical Society , 21 : 1077 – 1087 .
- Muñoz-Muñoz , A.C. , Grenier , A.C. , Gutiérrez-Pulido , H. and Cervantes-Martínez , J. 2008 . Development and validation of a high performance liquid chromatography-diode array detection method for the determination of aging markers in tequila . Journal of Chromatography A , 1213 : 218 – 223 .
- Muñoz Rodriguez , D.M. , Wrobel , K. and Wrobel , K. 2005 . Determination of aldehydes in tequila by high-performance liquid chromatography with 2,4-dinitrophenylhydrazine derivatization . European Food Research and Technology , 221 : 798 – 802 .
- NMX-V-005-NORMEX-2005 . 2005 . Bebidas alcohólicas – Determinación de aldehídos, ésteres, metanol y alcoholes superiores – Métodos de ensayo (pp , 7 México : Diario Oficial de la Federación .
- NOM-006-SCFI-1994 . 1997 . Norma Oficial Mexicana. Bebidas alcohólicas-Tequila-Especificaciones , México : Diario Oficial de la Federación .
- NOM-006-SCFI-2005 . 2006 . Norma Oficial Mexicana. Bebidas alcohólicas-Tequila-Especificaciones , 6 – 23 . México : Diario Oficial de la Federación .
- Ortega Heras , M. , Rivero-Pérez , M.D. , Pérez-Magariño , S. , González-Huerta , C. and González-Sanjosé , M.L. 2008 . Changes in the volatile composition of red wines during aging in oak barrels due to microoxygenation treatment applied before malolactic fermentation . European Food Research and Technology , 226 : 1485 – 1493 .
- Peña-Alvarez , A. , Capella , S. , Juarez , R. and Labastida , C. 2006 . Determination of terpenes in tequila by solid phase microextraction-gas chromatography-mass spectrometry . Journal of Chromatography A , 1134 ( 1–2 ) : 291 – 297 .
- Perez-Coello , M.S. , Sanz , J. and Cabezudo , M.D. 1999 . Determination of volatile compounds in hydroalcoholic extracts of French and American oak wood . American Journal of Enology and Viticulture , 50 : 162 – 165 .
- Pérez-Prieto , L.J. , López-Roca , J.M. , Martínez-Cutillas , A. , Pardo Mínguez , F. and Gómez-Plaza , E. 2002 . Maturing wines in oak barrels. Effects of origin, volume, and age of the barrel on the wine volatile composition . Journal of Agricultural and Food Chemistry , 50 : 3272 – 3276 . doi: 10.1021/jf011505r
- Perez-Prieto , L.J. , Lopez-Roca , J.M. , Martinez-Cutillas , A. , Pardo-Minguez , F. and Gomez-Plaza , E. 2003 . Extraction and formation dynamic of oak-related volatile compounds from different volume barrels to wine and their behavior during bottle storage . Journal of Agricultural and Food Chemistry , 51 : 5444 – 5449 .
- Pino , J. , Pérez , R. and Nuñez , M. 1996 . Componentes Volátiles del Ron: Influencia del Tiempo de Añejamiento y Tipo de Barril . Tecnología de alimentos , 31 : 5 – 10 .
- Prado , R. 2004 . “ Destilación ” . In Ciencia y Tecnología del Tequila, Avances y Perspectivas , 1st ed. , Edited by: Gschaedler , A. 123 – 170 . Guadalajara : CIATEJ, AC .
- Prado-Ramírez , R. , Gonzáles-Alvarez , V. , Pelayo-Ortiz , C. , Casillas , N. , Estarrón , M. and Gómez-Hernández , H.E. 2005 . The role of distillation on the quality of tequila . International Journal of Food Science & Technology , 40 : 701 – 708 .
- Rabier , P. and Moutounet , M. 1991 . “ Evolution D´Extractibles de Bois de Chene Dans Une Eau-de-Vie de Vin Incidence du Thermotraitement des Barriques ” . In Les eaux-de-vie traditionnelles d'origine viticole , Edited by: Bertrand , A. 220 – 230 . Paris : Lavoisier - TEC & DOC .
- Ralph , R. 2003 . “ Production of American whiskies: Bourbon, corn, rye and Tennessee ” . In The alcohol textbook: A reference for the beverage, fuel and industrial alcohol industries , Edited by: Jaques , K.A. , Lyons & , T.P. and Kelsall , D.R. 211 – 223 . Nottingham , , UK : Nottingham University Press .
- Ribereau-Gayon , P. 1978 . “ Wine flavor ” . In Flavor of foods and beverages: Chemistry and technology , Edited by: Charalambous , G. and E. Inglett , G. 362 – 371 . New York : Academic Press .
- Vallejo-Cordoba , B. , Gonzalez-Cordova , A.F. and Estrada-Montoya , M.d.C. 2004 . Tequila volatile characterization and ethyl ester determination by solid phase microextraction gas chromatography/mass spectrometry analysis . Journal of Agricultural and Food Chemistry , 52 : 5567 – 5571 .
- Wilker , K.L. and Gallander , J.F. 1988 . Comparison of Seyval blanc wine aged in barrels and stainless steel tanks with oak chips . American Journal of Enology and Viticulture , 39 ( 1 ) : 38 – 43 .