Abstract
The objective of this study was to determine the best combination of process variables to produce nixtamalized corn flour (NCF) with the lowest level of lipid deterioration. The nixtamalization conditions used were calcium concentrations from 0.4 to 2.56 g Ca(OH)2/L in water and steeping times of 6.34 to 17.65 h. The cooking time was 40 min at 94°C. The cooked and steeped grains were washed and milled to obtain masa which was then dehydrated and milled until the NCF was obtained. Oil was extracted by means of hexane from each sample of processed corn. The level of least oil deterioration of nixtamalized samples was based on the following response variables: saponification, acidity, peroxides, iodine values and extinction coefficient measured at 270 nm (K270). The best nixtamalization conditions for obtaining the highest oil stability were found at Ca(OH)2 lower than 0.75% and steeping times less than 12 h.
El objetivo de este estudio fue determinar la mejor combinación de las variables de proceso para producir harina de maíz nixtamalizada (HMN) con el menor deterioro de los lípidos. Las condiciones de nixtamalización usadas fueron concentraciones de hidróxido de calcio en un rango de 0,4 a 2,56 g Ca(OH)2/L en agua y tiempos de reposo de 6,34 y 17,65 h. La temperatura de cocimiento fue de 94°C por 40 min. Los granos de maíz cocidos y reposados fueron lavados y molidos para obtener masa, la cual fue deshidratada y molida hasta obtener la HMN. A cada muestra de maíz procesado le fue extraído el aceite con hexano. El punto donde existió una menor oxidación de aceite fue basado en las siguientes variables de respuesta: valores de saponificación, de acidez, de peróxidos, de iodo y el K270. Las mejores condiciones de nixtamalización para obtener la mayor estabilidad del aceite fueron encontradas a concentraciones de Ca(OH)2 inferiores a 0,75% y tiempos de reposo menos de12 h.
Keywords:
Palabras clave:
Introduction
Maize is the staple crop in rural and urban areas of North and Central Latin American countries, such as Mexico and Guatemala, where it is consumed in the form of tortillas (Hernández-Uribe, Ramos-López, Yee-Madeira, & Bello-Pérez, 2010) that are common food in Central America. Worldwide, they are important ingredients in snacks or bread substitutes. A tortilla is thin, unleavened flattened bread made from finely ground maize and characterized by its flexible texture and easy handling (Scazzina, Del Rio, Serventi, Carini, & Vittadini, 2008). Corn tortillas are prepared using alkaline cooking of corn kernels by a process called nixtamalization (Vázquez-Carrillo, García-Lara, Salinas-Moreno, Bergvinson, & Palacios-Rojas 2011). The process involves the cooking of corn kernels in water (2 to 3 L of water/kg of maize) with calcium hydroxide (Ca(OH)2) at a concentration of 1% to 3% at temperatures close to boiling point for 35 to 70 min. Subsequently, there is a steeping stage with duration of 0 to 16 h. Afterwards, the alkaline solution (nejayote) is decanted, and the cooked and steeped corn kernels are washed to remove fragments of pericarp and residual calcium ions. Finally, the corn kernels are ground in a stone mill until dough is obtained from which tortillas (Pérez-Flores, Moreno-Martínez & Méndez-Albores, 2011) or snacks are made.
Several studies have reported changes in protein and starch content when nixtamalization is applied when converting corn to tortillas (Bressani & Scrimshaw, 1958; Gomez, Lee, J.K., McDonough, C.M., Waniska, R.D., & Rooney, 1992; Gómez-Aldapa, Martínez-Bustos, Figueroa & Ordorica, 1999; Martínez-Flores, Martínez-Bustos, Figueroa & González-Hernández, 2002; Méndez-Montealvo, García-Suárez, Paredes-López & Bello-Pérez, 2008; Ortega, Villegas, & Surinder, 1986; Vivas, Waniska, & Rooney, 1987). There are several studies with reference to changes in corn lipids during alkaline cooking (López-Duarte & Vidal-Quintanar, 2009; Martínez-Bustos et al., 2001; Martínez-Flores, Garnica-Romo, Romero & Yahuaca, 2006; Marquez-Castillo & Vidal-Quintanar, 2011; Vidal-Quintanar, Love, Love, White, & Johnson, 2003). However, further research is necessary on the chemical changes in lipids during the corn nixtamalization. This is important because nixtamalized corn can be useful source of essential fatty acids. In general, linoleic acid, which is an essential omega–6 fatty acid, comprises 61.9% of corn oil (Weber, 1999).
Oil auto-oxidation consists of three stages: (i) the formation of free radicals; (ii) their propagation; and (iii) termination. Free radicals can react with unsaturated fatty acids to form more free radicals. Atmospheric oxygen can then react with the lipid free radicals to form hydroperoxides (Knothe & Dunn, 2003). These latter compounds are known as primary products of oxidation. As the oxidation process continues, hydroperoxides can be degraded to aldehydes, ketones, lactones, alcohols and acids (Lam & Proctor, 2003; Porter, Caldwell & Mills, 1995). These compounds are known as secondary oxidation products. During nixtamalization, unsaturated fatty acids can be oxidized as a result of exposure to air, light, temperature and metals. Martínez-Flores et al. (2006) found that alkaline cooking causes some changes in corn oil. However, this study was carried out considering only the cooking stage of the corn; that is, the authors did not evaluate the effect of steep time during nixtamalization. Vidal-Quintanar et al. (2003) and López-Duarte and Vidal-Quintanar (2009) studied the effect of storage time on the shelf life of nixtamalized corn flour (NCF) in terms of oxidative changes in the oil contained in the stored flour.
The most common methods used to evaluate the degree of oxidation in oils subjected to a particular process are frequently related to the indirect measurement of primary (saponification, acid and iodine and peroxide values (PVs) and the extinction coefficient measured at 232 nm [K232]) and secondary (peroxide and anisidine values and the extinction coefficient measured at 270 nm [K270]) oxidation compounds.
Response surface methodology (RSM) is a group of mathematical techniques for modeling and analyzing problems in which a response of interest to the investigator is influenced by several variables, and the objective is to optimize this response. One such RSM technique is central composite design (CCD), which makes possible the quantitative description of the relationship between the dependent and the independent variables of a process (Castaño-Tostado & Domínguez-Domínguez, 2010). The RSM is useful in finding the best processing condition in which lipids suffer least oxidation. The aim of this study in particular was to determine the effect of alkaline thermal processing conditions and suggest the optimum level in which oil has the highest oxidative stability.
Materials and methods
For this study, we used commercial corn kernels known as “La Barca” with hard endosperm, which were acquired in a local market in the city of Morelia, Michoacán, Mexico. The commercial product “Nixtacal” was used as a source of food grade calcium hydroxide (Grupo Calidra, D.F., México).
Nixtamal preparation
Corn kernels (10 kg) were boiled in distilled water (20 L) for 40 min at different concentrations of Ca(OH)2 according to the afore-mentioned experiment setup (). The cooked corn was steeped for several hours (see ). The residual liquid (nejayote) was removed, and cooked corn kernels were washed twice with distilled water to extract excess Ca(OH)2 and fragments of pericarp. The cooked and washed corn grains are called “nixtamal”. The nixtamal was ground to obtain dough, which was then dried in a “flash”-type dryer at 260°C for 3 s (CINVESTAV-IPN, Querétaro, Mexico) and stored in a vacuum at 4°C until the lipids were extracted.
Table 1. Experimental design based on independent variables (Ca(OH)2concentration and steeping time) and response variables (saponification, acidity, peroxide and iodine values and K270).
Tabla 1. Diseño experimental basado en variables independientes (Ca(OH2) concentración y tiempo de reposo) y variables de respuesta (valores de saponificación, acidez, peróxido y iodo).
Lipid extraction
The dehydrated dough from each treated material (200 g) was mixed with 500 mL hexane and placed in dark jars for 24 h. Samples were vigorously shaken for 4 h to extract the oil. These extracts were filtered, concentrated in a rotary evaporator (Yamato, model BM 100, Santa Clara, CA, USA) and stored at 4°C in dark jars. Parameters of oil quality, i.e. saponification (SV), peroxide (PV), acidity (AV) and iodine (IV), were determined for each oil samples according to the methods of the Association of Official Analytical Chemists (AOAC, 1990). The oil extracted from unprocessed corn “La Barca” was used as a control.
Extinction coefficient measured at 270 nm (K270)
The K270 measured on samples of nixtamalized corn oil was calculated from the absorption values measured at 270 nm in the ultraviolet region using a Jenway spectrophotometer model 6405 (Beacon Road, Stone, Staffordshire ST15 0SA, UK). The calculation was based on the equation Kλ = Dλ/C, where Kλ is the extinction coefficient for each specific wavelength, Dλ is the absorption and C is the oil concentration in g/100 ml (Paz-Antolín & Molero-Meneses, 2000).
Experimental design and statistical analysis
A CCD was applied to find the best combination of variables in the nixtamalization process that could optimize the production of instant corn flour, based on the presence of minimal changes in lipid oxidation. The CCD used in this study was based on a 2k factorial treatment with 2k additional axial points and centre points nc. The coordinates of the axial points of the axes of the coded factor were (±α, 0, 0, … , 0), (0, ±α, 0, 0 …, 0), and (0, 0, 0, … , ±α), and the central points were (0, 0, 0, … , 0). A CCD can have different properties, depending on the selection of α in the axial points, such as, orthogonality, rotability and uniformity. The property of rotability applied in this case was obtained by establishing α = (2k)1/4. The value of α for a design with two factors was α = 1.414. The model includes four factorial points, five replicates at the centre point and four axial points. The dependent variables were the concentration of Ca(OH)2 (X1 = 0.75%, 1.5% and 2.25%) and the steeping time of cooked grains in the alkaline solution (X2 = 8, 12 and 16 h). The response variables were SV, AV, PV, IV and K270. An approximate second-order mathematical model was assumed to describe the relationship between each response variable (SV, AV, PV, IV and K270) and the independent variables X1 and X2 as shown in Equation (1):
where Y1 is the response variable; X1 is the concentration of Ca(OH)2; X2 is the steeping time and β0, β1, β2, β12, β11 and β22 are the regression coefficients. Statistical analyses were performed using Minitab 15 and Statistica 7 software.
Results and discussion
During alkaline cooking, water and calcium ions diffuse into the corn kernel structures. The calcium hydroxide can directly saponify the free fatty acids (FFAs), forming calcium salts of aliphatic acids, or because of hydrolysis of the ester bond of the fatty acids contained in the mono-, di- and triglycerides, forming saponified fatty acids and glycerol, as shown in Equations (2) and (3).
(3)
The SV, PV, AV, IV and K270 of oils from nixtamalized corn kernels are shown in .
Saponification value
The saponification value (SV) is expressed by the number of milligrams of potassium hydroxide (KOH) required to saponify 1 g of oil (Knothe, 2002). The FFAs and the fatty acids found inside the structure of mono-, di- and triglycerides are susceptible to saponification, an irreversible reaction which results in the formation of the metal salt of the acid that can no longer react with the hydroxyl group to regenerate ester.
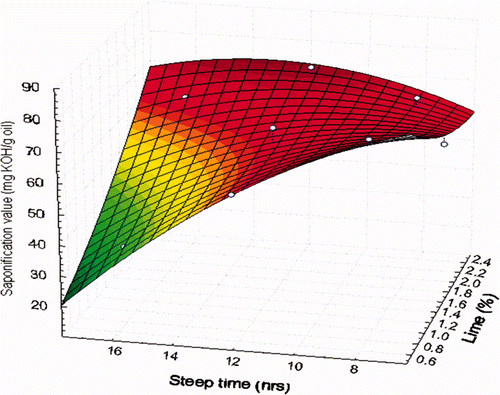
In this study, the SV obtained from the oils extracted from nixtamalized corns ranged from 35.70 to 75.80 mg KOH/g oil (). The SV of oils extracted from NCF decline on average to a value of 60.76 mg KOH/g oil, representing a 20.82% decrease from the initial value of the control unprocessed corn oil (76.74 mg KOH/g oil). It is noteworthy that a large amount of FFAs in the oil leads to a high SV. The opposite occurs when the SV analysis is carried out on a lipid with few FFAs, producing a low SV. In our study, the low SV in the nixtamalization-processed oils compared to the control oil could be due to calcium ions from the Ca(OH)2 used for corn nixtamalization reacting with the carboxyl groups of FFAs, thus significantly decreasing the binding sites of potassium ions present when SV is measured.
The maximum SV (75.80 mg KOH/g oil) was observed at a concentration of 0.75% Ca(OH)2 and 8 h of steeping time (), which is similar to the SV of the control corn oil (76.64 mg KOH/g oil). The minimum SV (35.70 mg KOH/g oil) was observed at a concentration of 0.75% Ca(OH)2 and steeping time of 16 h. This suggests that the free calcium ions coming from the alkaline solution during the cooking of the corn kernels may be reacting with the carboxyl groups of the FFAs of the corn oil, further intensifying the reaction; with increased steeping time, there must have been a greater diffusion of calcium into the germ, which reacted with the FFAs, thereby reducing the SV. According to Rendón-Villalobos et al. (2009), when corn kernels are cooked during nixtamalization, the triglycerides are degraded to FFAs, and some may bind to calcium ions, thus reducing the SV.
Figure 1. The saponification values of oil samples from nixtamalized corn.
Figura 1. Valores de saponificación de muestras de aceite de maíz nixtamalizado.
Regression analysis shows that the SV significantly depended on Ca(OH)2 concentration and steeping time (p ≤ 0.001) and on the interaction of Ca(OH)2 concentration with steeping time (p ≤ 0.05). The predictive model explains the 90.7% of the total variation in the SV (p ≤ 0.001) ().
Table 2. Regression coefficients and variance analysis for the response variables.
Tabla 2. Coeficientes de regresión y análisis de varianza para las respuestas variables.
Acidity value
The acidity value (AV) is one of the quality indices most frequently used during extraction, storage and sale of vegetable oils. FFAs can be produced from enzymatic hydrolysis during the extraction process of edible vegetable oils or can be formed from auto-oxidation, which causes the breakdown of the ester bond of the mono-, di- and triglycerides to their corresponding FFAs (Chaiyasit, Elias, McClements & Decker, 2007). Therefore, the AV is a measure of the hydrolysis of the ester bond of the mono-, di- and triglycerides molecules, to free the fatty acids (Paradiso, Gomes, Nasti, Caponio, & Summo, 2010; Thomaidis & Georgiou, 1999) in edible vegetable oils.
In this study, the AV was affected by the concentration of Ca(OH)2 (p ≤ 0.05) and the interaction of Ca(OH)2 concentration and steeping time (p ≤ 0.05). The predictive model explained 81.77% (p ≤ 0.05) of the total variation in the AV (). In long steeping times and high concentrations of Ca(OH)2, the AV decreased (). During nixtamalization, the percentage of FFAs depends on processing conditions (steeping time and concentration of Ca(OH)2). It is likely that during thermal-alkaline cooking applied to the corn kernels, two simultaneous processes occurred. In the first, fatty acids were released through the hydrolysis of triglyceride ester bonds. In the second, calcium hydroxide present in the alkaline suspension in the cooking led to the formation of saponifiable compounds, as reported by Winkler-Moser and Breyer (2011), and therefore would not have had a high FFA content; at the same time, the AV remained low.
Figure 2. The acid values of oil samples from nixtamalized corn.
Figura 2. Valores de acidez de muestras de aceite de maíz nixtamalizado.
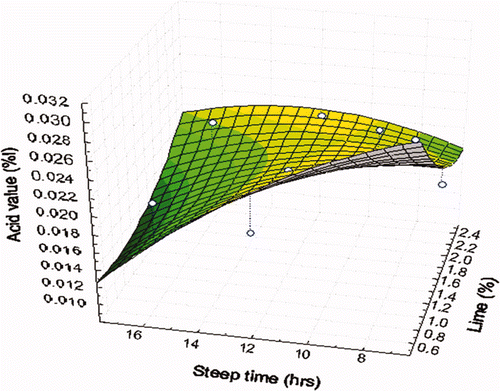
It should be noted that the average AV obtained in the different nixtamalized oil treatments (0.019%) was similar to corn oil controls (0.02%).
Peroxide value
During oil auto-oxidation, the triglyceride structure is modified, producing a large number of compounds. Oils containing a high concentration of polyunsaturated fatty acids (linoleic and linolenic acids) can easily oxidise (Thomaidis & Georgiou, 1999), to produce primary oxidising compounds, such as peroxides and hydroperoxides that are typically evaluated by means of the PV (Guillen & Goicoechea, 2009).
Peroxides are highly unstable structures; once the auto-oxidation starts, it does not stop until all present free radicals are inactivated (Méndez & Falqué, 2007). During oil oxidation, the PV is high due to the generation of peroxides and hydroperoxides, which can then be transformed into a wide variety of products, such as aldehydes, ketones and lactones, which are considered to be secondary oxidation products. Consequently, the PV begins to decrease during the different stages of oil oxidation.
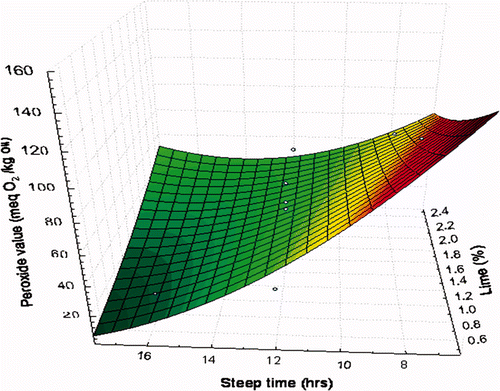
In this study, the PV obtained from the oils extracted from NCFs ranged from 18.40 to 120.20 meq O2/kg oil (). The PV of oils samples rose on average to 51.53 meq O2/kg oil, representing an increase of 214% over the initial PV of the unprocessed corn oil control (16.40 meq O2/kg oil). In this study, the PV depended significantly on the steeping time (p ≤ 0.001). Guillén and Cabo (2002) reported a PV of 250 meq O2/kg oil in sunflower oil during the primary stage of oxidation. Therefore, it may be suggested that in our study, the primary oxidation stage was not as significant as that reported by Guillén and Cabo (2002).
The predictive model explains 84.3% of the total variation (p ≤ 0.001) (). The maximum PV (120.20 meq O2/kg oil) was observed at a concentration of Ca(OH)2 of 0.75% with a steeping time of 8 h; the minimum PV (18.40 meq O2/kg oil) was observed at a concentration of 0.75% Ca(OH)2 with a steeping time of 16 h. In general, high PVs were observed at low steeping times and low concentrations of Ca(OH)2 () suggesting that as the primary stage of oil oxidation started and the steep time increased, peroxides were converted to secondary oxidation products, and thus the PV decreased. Studies by Martínez-Flores et al. (2006) indicated that the PV remained relatively constant at a concentration of Ca(OH)2 of 1.0% in nixtamalized corn oils. However, they found that the PV decreased when the concentration of Ca(OH)2 increased from 1.5 to 3.0%. Vidal-Quintanar et al. (2003) reported a rapid PV increase in stored corn dough, followed by a decrease (50%) because the peroxides and hydroperoxides were converted to secondary oxidation products. López-Duarte and Vidal-Quintanar (2009) indicated that the oil contained in the nixtamalized dough was oxidised rapidly on par with increased storage temperature.
Iodine value
The degree of unsaturation of a triglyceride can be expressed as the iodine value (IV), which is defined as the number of iodine grams absorbed by 100 g of fat. This definition explores the ability of a carbon–carbon double bond to participate in the formation of halogens (Gooch, 2001; Knothe, 2002).
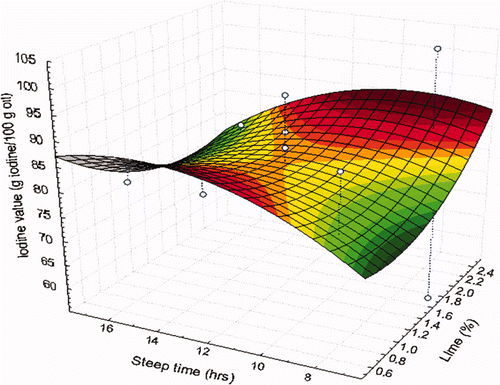
In this study, the IV obtained from the oils extracted from NCF ranged from 58.37 to 102.03 g iodine/100 g oil (). The IV of nixtamalized oils declined on average to a value of 81.89 g iodine/100 g oil, representing a reduction of 16.84% over the initial value of the unprocessed corn oil control (98.47 g iodine/100 g oil). This indicates that there was a decrease in the degree of unsaturation in the fatty acids present in thermal-alkaline processed corn oil. The decrease of IV coincided with increased PV, in which the double bonds were altered to form free radicals and hydroperoxides and therefore had fewer unsaturated lipids with a concomitant decrease of IV.
The IV tended to decrease when intermediate steeping times (10 h) were combined with high concentrations of Ca(OH)2 (1.50 to 2.25%) ( ). The results of this particular investigation are consistent with those obtained by Martínez-Flores et al. (2006), who reported that the IV decreased when alkaline cooking was performed with a concentration of at least 2.5% of Ca(OH)2.
Determination of K270
UV spectroscopy, specifically the absorbance at wavelengths between 232 nm and 274 nm, enables the degree of deterioration of an oil to be investigated (Paz-Antolín & Molero-Meneses, 2000). This is because the primary oxidation compounds (peroxides and hydroperoxides containing conjugated dienes) absorb at nearly 232 nm, whereas the secondary oxidation products (aldehydes, ketones and acids) absorb at wavelengths of 262, 268, 270 and 274 nm. Guzmán, Baeten, Fernández-Pierna & García-Mesa (2011) and Muik, Lendl, Molina-Díaz, and Ayora-Cañada (2005) indicated that absorption at 270 nm and respective extinction coefficient (K270) are related to the presence of carbonyl compounds (secondary oxidation stage) and conjugated dienes and trienes.
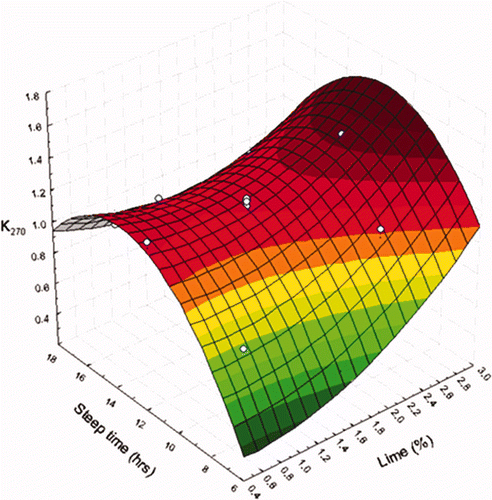
In the present study, the range of values for K270 was from 0.48 to 1.27 (). The model developed to predict the minimum value of K270 in oils from NCFs explained 93.15% of the total variation (p ≤ 0.001), showing that K270 depended significantly (p ≤ 0.05) on Ca(OH)2 concentration, steeping time and its square value ().
We observed that, K270 increased as did steeping time, reaching a maximum at 12 h. This was followed by a decrease without reaching the initial minimum value (Figure 5). Steeping time plays an important role in nixtamalization, as this stage allows a higher penetration of the alkaline solution into the grain, given that the germ is one of the most susceptible structures due to its proximity to the pedicel. This leads to an interaction between the oil contained in the germ and the alkaline solution, promoting the oxidation of fatty acids. Furthermore, there was an upward tendency in K270 from 0.3 to 1.1 as the concentration of Ca(OH)2 increased from 0.5 to 3.0%.
Relationship between the response variable K270 and PV
The value of K270 (secondary oxidation products) has a direct relationship with the PV (primary oxidation products). Secondary oxidation compounds originate from the degradation of the primary oxidation compounds so that, in this study, there was an inverse relationship between the two; i.e. as hydroperoxides decreased, secondary compounds increased. The conditions of maize nixtamalization process can provoke, to a greater or lesser extent, the oxidation of fatty acids in oil, with three possible resulting scenarios: (i) a high concentration of hydroperoxides and low concentration of secondary oxidation compounds; (ii) a balance between the concentration of primary and secondary oxidation compounds; or (iii) a low concentration of hydroperoxides and high concentration of secondary compounds.
Thus, at the highest PV registered during the shortest steeping time (6 h), the value of K270 was low, and inversely at the lowest PV during long steeping times (>12 h), the K270 was highest.
The Pearson's correlation analysis () showed a positive relationship (p ≤ 0.05) between AV–PV, SV–PV and SV–AV. This analysis indicates a linear relationship between two variables, i.e. if one tends to increase and also the other, then the correlation coefficient is positive. Conversely, if one variable increases and the other decreases, the correlation coefficient is negative (Gopinath, Puhan & Nagarajan, 2009). AV shows a positive relation with PV (0.878412) because when there is a higher amount of FFAs, the predisposition to form peroxides increases. There is also a positive correlation between SV and PV (0.699015) and between VS and VA (0.652862). In addition, we also observed a negative relationship between K270 and PV (−0.54005), because as PV (primary oxidation products) decreased, K270 (secondary oxidation products) increased.
Table 3. Pearson's correlation analysis of saponification, acidity, peroxide and iodine values and K270 in nixtamalized corn oils.
Tabla 3. Análisis de correlación de Pearson de los valores de saponificación, acidez, peróxido y iodo y de K270 en aceites de maíz nixtamalizados.
Conclusion
The physicochemical parameters analysed in the different nixtamalized oil samples suggest that the process known as thermal-alkaline nixtamalization induces oxidative deterioration of the samples, particularly during prolonged steeping times. The PV and K270 were the response variables that provided the strongest evidence for oil stability which is affected by the thermal-alkaline process. Both values were greater when longer steeping times and higher concentrations of Ca(OH)2 were used during the heat-alkaline process. Therefore, it is inferred that the use of Ca(OH)2 at concentrations lower than or equal to 0.75% and steeping times shorter than 12 h are indicated in order to achieve a greater stability in corn oil when the thermal-alkaline nixtamalization process is applied.
Acknowledgments
Berenice Yahuaca Juárez is grateful for the PhD scholarship granted her by PROMEP, Mexico, reference number UMSNH-214. The authors acknowledge the financial support provided by the Coordinación de la Investigación Científica, UMSNH (Project 26.1, 2009).
References
- AOAC . 1990 . Official methods of analysis (15th ed.) , Arlington, TX : Association of the Official Analytical Chemists .
- Bressani , R and Scrimshaw , N.S. 1958 . Lime-heat effects on corn nutrients: Effect of lime treatment on “in vitro” availability of essential amino acids and solubility of protein fractions in corn . Journal of Agriculture and Food Chemistry , 6 : 774 – 778 .
- Castaño-Tostado , E and Domínguez-Domínguez , J. 2010 . Diseño de Experimentos. Estrategias y Análisis en Ciencia y Tecnología , Querétaro, Qro., México : Universidad Autónoma de Querétaro .
- Chaiyasit , W , Elias , R.J , McClements , D.J and Decker , E.A. 2007 . Role of physical structures in bulk oils on lipid oxidation . Critical Reviews in Food Science and Nutrition , 47 : 299 – 317 .
- Gomez , M.H , Lee , J.K , McDonough , C.M , Waniska , R.D and Rooney , L.W. 1992 . Corn starch changes during tortilla and tortilla chip processing . Cereal Chemistry , 69 : 275 – 279 .
- Gómez-Aldapa , C , Martínez-Bustos , F , Figueroa , J.D.C and Ordorica , F.C.A. 1999 . A comparison of the quality of whole corn tortillas made from instant corn flours by traditional or extrusion processing . International Journal of Food Science and Technology , 34 : 391 – 399 .
- Gooch , E.E. 2001 . Determination of the iodine value of selected oils: An experiment combining FTIR spectroscopy with iodometric titrations . TheChemical Educator , 6 : 7 – 9 .
- Gopinath , A , Puhan , S and Nagarajan , G. 2009 . Theoretical modelling of iodine value and saponification value of biodiesel fuels from their fatty acid composition . Renewable Energy , 34 : 1806 – 1811 .
- Guillén , M.D and Cabo , N. 2002 . Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils . Food Chemistry , 77 : 503 – 510 .
- Guillen , M.D and Goicoechea , E. 2009 . Oxidation of corn oil at room temperature: Primary and secondary oxidation products and determination of their concentration in the oil liquid matrix from 1H nuclear magnetic resonance data . Food Chemistry , 116 : 183 – 192 .
- Guzmán , E , Baeten , V , Fernández-Pierna , J.A and García-Mesa , J.A. 2011 . Application of low-resolution Raman spectroscopy for the analysis of oxidized olive oil . Food Control , 22 : 2036 – 2040 .
- Hernández-Uribe , J.P , Ramos-López , G , Yee-Madeira , H and Bello-Pérez , L.A. 2010 . Physicochemical, rheological and structural characteristics of starch in maize tortillas . Plant Foods for Human Nutrition , 65 : 152 – 157 .
- Knothe , G. 2002 . Review: Structure indices in FA chemistry. How relevant is the iodine value? . Journal of the American Oil Chemists´ Society , 79 : 847 – 854 .
- Knothe , G and Dunn , R.O. 2003 . Dependence of oil stability index of fatty compounds on their structure and concentration and presence of metals . Journal of the American Oil Chemists’ Society , 80 : 1021 – 1026 .
- Lam , H.S and Proctor , A. 2003 . Lipid hydrolysis and oxidation on the surface of milled rice . Journal of the American Oil Chemists Society , 80 : 563 – 567 .
- López-Duarte , A.L and Vidal-Quintanar , R.L. 2009 . Oxidation of linoleic acid as a marker for shelf life of corn flour . Food Chemistry , 114 : 478 – 483 .
- Márquez-Castillo, A. and Vidal-Quintanar, R.L. (2011). Improvements in the shelf life of commercial corn dry masa flour (CMF) by reducing lipid oxidation. Journal of Food Science, 76, C236–C241. doi: 10.1111/j.1750-3841.2010.01983.x
- Martínez-Bustos , F , Martínez-Flores , H.E , San Martín-Martínez , E , Sánchez-Sinencio , F , Chang , Y.K , Barrera-Arellano , D and Rios , E. 2001 . Effect of the components of maize on the quality of masa and tortillas during the traditional nixtamalization process . Journal of the Science of Food and Agriculture , 81 : 1455 – 1462 .
- Martínez-Flores , H.E , Garnica-Romo , M.G , Romero , V.J.U and Yahuaca , J.B. 2006 . Evaluating the quality of lipids during alkaline cooking of corn . Journal of Food Lipids , 13 : 177 – 185 .
- Martínez-Flores , H.E , Martínez-Bustos , F , Figueroa , J.D.C and González-Hernández , J. 2002 . Studies and biological assays in corn tortillas made from fresh masa prepared by extrusion and nixtamalization processes . Journal of Food Science , 67 : 1196 – 1199 .
- Méndez , A.I. and Falqué , E. 2007 . Effect of storage time and container type on the quality of extra-virgin olive oil . Food Control , 18 : 521 – 529 .
- Méndez-Montealvo , G , García-Suárez , F.J , Paredes-López , O and Bello-Pérez , L.A. 2008 . Effect of nixtamalization on morphological and rheological characteristics of maize starch . Journal of Cereal Science , 48 : 420 – 425 .
- Muik , B , Lendl , B , Molina-Díaz , A and Ayora-Cañada , M.J. 2005 . Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy . Chemistry and Physics of Lipids , 134 : 173 – 182 .
- Ortega , E.I , Villegas , E and Surinder , K.V. 1986 . A comparative study of protein changes in normal and quality protein maize during tortilla making . Cereal Chemistry , 63 : 446 – 451 .
- Paradiso , V.M , Gomes , T , Nasti , R , Caponio , F and Summo , C. 2010 . Effects of free fatty acids on the oxidative processes in purified olive oil . Food Research International , 43 : 1389 – 1394 .
- Paz-Antolín , I and Molero-Meneses , M. 2000 . Aplicación de la espectrofotometría UV-visible al estudio de la estabilidad térmica de aceites vegetales comestibles . Grasas y Aceites , 51 : 424 – 428 .
- Pérez-Flores , G.C , Moreno-Martínez , E and Méndez-Albores , A. 2011 . Effect of microwave heating during alkaline-cooking of aflatoxin contaminated maize . Journal of Food Science , 6 : T48 – T52 .
- Porter , N.A , Caldwell , S.E and Mills , K.A. 1995 . Mechanisms of free radical oxidation of unsaturated lipids . Lipids , 30 : 277 – 290 .
- Rendón-Villalobos , J.R , Bello-Pérez , L.A , Agama-Acevedo , E , Islas-Hernández , J.J , Osorio-Díaz , P and Tovar , J. 2009 . Composition and characteristics of oil extracted from flaxseed-added corn tortilla . Food Chemistry , 117 : 83 – 87 .
- Scazzina , F , Del , Rio, D , Serventi , L , Carini , E and Vittadini , E. 2008 . Development of nutritionally enhanced tortillas . Food Biophysics , 3 : 235 – 240 .
- Thomaidis , N.S and Georgiou , C.A. 1999 . Edible oil analysis by flow injection . Laboratory Automation & Information Management , 34 : 101 – 114 .
- Vázquez-Carrillo , G , García-Lara , S , Salinas-Moreno , Y , Bergvinson , D.J and Palacios-Rojas , N. 2011 . Grain and tortilla quality in Landraces and improved maize grown in the Highlands of Mexico . Plant Foods for Human Nutrition , 66 : 203 – 208 .
- Vidal-Quintanar , R.L , Love , M.H , Love , J.A , White , P.J and Johnson , L.A. 2003 . Lipid-autoxidation-limited shelf life of nixtamalized instant corn masa . Journal of Food Lipids , 10 : 153 – 163 .
- Vivas , E.N , Waniska , R.D and Rooney , L.W. 1987 . Effect of tortilla production on proteins in sorghum and maize . Cereal Chemistry , 64 : 384 – 389 .
- Weber , E.J. 1999 . “ Lipids in kernel ” . In Corn: Chemistry and technology , Edited by: Watson& , A.S and Ramstad , E.P . 316 – 317 . Minneapolis, MN : American Association of Cereal Chemists .
- Winkler-Moser , J.K and Breyer , L. 2011 . Composition and oxidative stability of crude oil extracts of corn germ and distillers grains . Industrial Crops and Products , 33 : 572 – 578 .