Abstract
The aim was to study thermo-physical and antimicrobial properties of gelatin-chitosan films by gelatin origin (bovine and salmon), chitosan concentration and physical state (glassy or rubbery). Thermo-physical properties (pH, Bloom grade, color, isotherms, glass transition temperature, moisture uptake rate, molecular mobility and film solubility) and antimicrobial effect against Escherichia coli, Salmonella thyphimurium and Listeria monocitogenes were evaluated. The results showed that the presence of chitosan increased the water content of both gelatin films and improved the physical performance of both films, especially for salmon gelatin, without a significant (p > 0.05) change of color. Antimicrobial activity was effective against all bacteria depending on chitosan concentration and glassy or rubbery state, with the highest effect in glassy state. In conclusion, the evaluated films could be a potential application as bioactive edible films for fresh foods.
El objetivo fue estudiar si las propiedades termofísicas y antimicrobianas de películas basadas en gelatina-quitosano son influenciadas por el origen de la gelatina (bovino o salmon), la concentración de quitosano y el estado físico (vítreo o gomoso) de la misma. Se evaluaron las propiedades físicas (pH, °Bloom, color, isotermas, velocidad de captación de agua, movilidad molecular y solubilidad) y térmicas (temperatura de transición vítrea) junto con el efecto antimicrobiano contra Escherichia coli, Salmonella thyphimurium and Listeria monocitogenes. Los resultados mostraron diferencias por el origen de la gelatina y la presencia de quitosano, donde la presencia de este último generó un aumento el contenido de agua de las películas, no cambió significativamente el color (p > 0,05) y mejoró su solubilidad, especialmente para gelatina de salmón. La actividad antimicrobiana fue efectiva contra todas las bacterias evaluadas, la cual dependió de la concentración de quitosano y del estado de la película, siendo más efectiva en estado vítreo. En conclusión, la película evaluada puede tener una potencial aplicación como película comestible bioactiva para alimentos frescos.
Introduction
Films and coatings based on gelatin have been an interesting research field in recent years as extenders of the shelf-life of fresh foods and being at the same time environmentally friendly materials. Also the commercial viability of the utilization of fish industry by-product has promoted the use of gelatin from various sources. The functionality of coatings including active compounds such as antimicrobials and antioxidants has been extensively studied (Greener & Fennema, Citation2002; Pereda, Ponce, Marcovich, Ruseckaite, & Martucci, Citation2011). Previous work had been focused on developing films with improved mechanical and water barrier properties to protect food from drying and exposure to light, by combination of gelatin with others biopolymers, such as lipids, bioactive peptides, proteins and plasticizers (Ahmad, Benjakul, Prodpran, & Agustini, Citation2012; Gómez-Estaca, Montero, Fernández-Martín, & Gómez-Guillén, Citation2009; Rivero, García, & Pinotti, Citation2010).
The physical properties of gelatin are strongly related to their structure (Yang & Wang, Citation2009), which is mainly stabilized by intra- and inter-chain hydrogen bonding of an almost continuous repeating of the Gly–X–Y sequence, were X is mostly proline and Y is mostly hydroxiproline (Haug, Draget, & Smidsrød, Citation2004). These amino acids are present in higher concentration in most warm-blooded animals compared to gelatins from cold water fish, obtaining higher temperatures of gelation and fusion (∼ 30°C) compared to gelatins from cold water fish (∼ 8°C) (Díaz, López, Matiacevich, Osorio, & Enrione, Citation2011; Karim & Bhat, Citation2008). Interestingly, the increase in demand for gelatin from non-mammals sources (Halal and Kosher population) has promoted the use of other sources such as salmon gelatin (SG) which is available as by-product from the fish industry (about 5% of whole fish), making its production advantageous compared to other sources.
In order to obtain a bioactive film with antimicrobial properties, antimicrobial compounds such as chitosan could be added to these edible films (Celis, Azocar, Enrione, Paez, & Matiacevich, Citation2012; Gómez-Estaca, López de Lacey, López-Caballero, Gómez-Guillén, & Montero, Citation2010; Pereda et al., 2011). Chitosan is a natural linear polysaccharide (Li, Wang, Chen, Huangfu, & Xie, Citation2008), with advantages for food applications, which includes non-toxic, biodegradable and antimicrobial properties (Dutta, Tripathi, Mehrotra, & Dutta, Citation2009) due to the available reactive amino and hydroxyl groups (Dutta, Dutta, & Tripathi, Citation2004). The hydrogen bonds strongly stabilize their inter- and intra-molecular structure but becoming soluble at low pH (6.5) (Fan, Hu, & Shen, Citation2009). Their antimicrobial activity has been demonstrated against Gram-positive and Gram-negative bacteria, filamentous fungi and yeasts (Kong, Chen, Xing, & Park, Citation2010). This activity is associated with the availability of R–NH3 + groups that interact with the microbial cell membrane (Abugoch, Tapia, Villamán, Yazdani-Pedram, & Díaz-Dosque, Citation2011; Pereda et al., 2011).
Composite films of gelatin-chitosan have been reported to have improved mechanical and transport properties compared with those of a single component film (Pereda et al., 2011; Rivero, García, & Pinotti, Citation2009). Interactions between both polymers by polyelectrolyte complexes through electrostatic interaction between the amino group of chitosan and the negatively charged side-chain groups in gelatin have been previously reported (Yin, Li, Sun, & Yao, Citation2005), in addition to covalent and hydrogen bonding and/or dipoles (Rivero et al., Citation2009). However, the antimicrobial activity of chitosan depends on different factors, such as pH, interaction with components and molecular weight, including the physical state (soluble and solid) (Kong et al., Citation2010). In case of intermediate and low moisture films, literature has shown possible interactions between the hydrocolloids (e.g. gelatin, carbohydrates) and chitosan, which could influence its antimicrobial effectiveness (Fernández-Saiz, Lagaron, & Ocio, Citation2009).
Molecular mobility of the films can be considered from a structural and macromolecular level and it could affect their physical properties and antimicrobial effectiveness, therefore, glass transition temperature, Tg, can be a descriptive parameter of the physical state of macromolecules, which differs from the molecular mobility of smaller molecules such as water (Vittadini & Chinachoti, Citation2003). Proton nuclear magnetic resonance (1H-NMR) measures transversal relaxation times (T2 ), which provide useful information on molecular mobility and water populations as being affected by the water–solid interactions (Kou, Dickinson, & Chinachoti, Citation2000).1H-NMR allows to assess differences in molecular mobility on polymers and foods by measuring the changes in spin–spin (T2 ) or spin–latice (T1 ) relaxation constants with temperature or water content (Farroni, Matiacevich, Guerrero, Alzamora, & Buera, Citation2008; Lin et al., Citation2006). The differences in relaxation times of protons from different environments have been exploited in NMR studies to measure the relative amounts of water with different degrees of interaction with solids and consequently, with different mobility (Acevedo, Schebor, & Buera, Citation2006; Farroni et al., 2008).
Therefore, the aim of the present work was to study the thermo-physical and antimicrobial properties of chitosan-gelatin films as a function of gelatin origin: commercial bovine-hide gelatin and a laboratory-obtained salmon-skin gelatin and as a function of the physical state with different molecular mobility (glassy or rubbery state) determined by their glass transition temperature. At the same time, this study proposed to verify the impact of chitosan incorporation in gelatin films on physical properties of films such as isotherms, film solubility and molecular mobility in order to establish their suitability as protective antimicrobial packaging materials.
Materials and methods
Materials
SG was extracted using Atlantic salmon skins (Salmo salar), from Chilean southern coast, kindly provided by Salmon Oil S.A. (Chile). The gelatin extraction was performed in the laboratory using an acidic–alkaline extraction. Briefly, the skins were cut out and immersed in 0.1 mol/L NaOH (Mallinckrodt, Mexico) at 10°C and stirred vigorously for 1 h and then immersed in 0.05 mol/L acetic acid (Winkler, Mexico) for 1 h in order to eliminate impurities. The gelatin extraction process was carried out at 64°C at pH ∼4.0 for 3.5 h. The supernatant liquid was vacuum filtered using a pump Arquimed (SU-660, Taiwan) and dried at 55°C in an oven (WiseVen WOF-105, Korea) for 24 h. The extraction yield of SG obtained was in approximately 180–190 g dry gelatin/kg of clean salmon skin, which is in accordance to non-mammalian extraction yield reported in literature (Jamilah & Harvinder, Citation2002).
Commercial type B Bovine Gelatin (BG) was provided by Rousselot, Brazil (Bloom 220). The isoelectric point was at approximately pH ∼5.0 calculated using the method described by Karim and Bhat (Citation2009).
Chitosan of low molecular weight (50 kDa) and a deacetylation degree of 92% was purchased from Sigma Aldrich Chemicals Ltd., USA.
Mueller Hinton agar (Merck, Germany) and broth (DIFCO, France) were used for microbial analysis. Gram-negative bacteria, Escherichia coli (ATCC 25922), Salmonella typhimurium (ISP Ty2) and Gram-positive bacteria, Listeria monocytogenes (ISP 65–08) were kindly provided by the Department of Chemistry of Materials from Santiago of Chile University.
All other reagents (acetic acid, NaOH, etc.) were analytical grade.
Film-forming suspensions (FFS)
BG and SGs were dissolved in distiller water to a final concentration in the suspension of 7% (w/w). The chitosan was previously dissolved in acetic acid solution (Winkler, Mexico) at a proportion of 1:1 (v/v) and then gradually added to the suspensions at final concentrations of 0, 0.25, 0.5 and 1% (w/w). Each gelatin-chitosan suspension was stirred moderately at 50°C until complete dissolution. The pH of the resultant mixture was adjusted to pH 5.5 using NaOH 2 mol/L (Mallinckrodt, Mexico).
pH and gel strength determinations on FFS
The pH of each FFS was carried out using a pH-meter (Jenway, UK) with a liquid electrode model 3505 (Jenway, 924–001), after calibration with pH 4.0 and 7.0 buffers (Chemix, Chile), following Official Methods of Analysis norms (AOAC, 1975).
The gel strength was measured on a 6.67% (w/w) gel concentration from gelatin (bovine and salmon) suspensions and mixtures of gelatin-chitosan, which was prepared with distilled water at initial pH of each FFS and then adjusted to pH 5.5, and followed by transferred into a standard Bloom jar. Gel strength indicated Bloom degree, is defined as the maximum force (in g) required for the probe to press the gel by 4 mm depression at a rate of 0.5 mm/s (Soekarto & Steinberg, Citation1981) (S.I. No 63/1953), which was carried out using a texture analyzer (Zwick/Roell model DO-FBO5TS, Zwick/Roell AG, Germany).
Physical properties of the films
Preparation of films
Films were obtained by cold casting method using each FFS over teflon rectangular molds and stored at 5 ± 1°C for 10 days, obtaining flat and clear films with an uniform thickness of 250 ± 5 μm, which was measured by a micrometer (Mitutoyo, Japan). Films were then cut to 70 mm in length and 10 mm in width. Subsequently, the films were dried at 20°C under 0% of relative humidity (RH) using P2O5 (Merck, Germany) for 7 days and then equilibrated under different RHs.
Sorption isotherm
All films were conditioned at 20°C in desiccators in presence of P2O5 during 7 days in order to obtain an adsorption isotherm. The dried films were then equilibrated under different RHs at 20°C using saturated salt solutions of LiCl (11% RH, Merck), KCH3COO (23% RH, Merck), MgCl2 (33% RH, Merck), K2CO3 (43% RH, Merck), Mg(NO3)2 (54% RH, Merck), CuCl2 (68% RH, Merck), NaCl (75% RH, Winkler, Mexico) and KCl (85% RH, Winkler, Mexico) (Greenspan, Citation1977). The water sorption was carried out for 3 weeks until equilibrium state (variations in mass were lower than 0.1%). Moisture content of films was determined gravimetrically at 105°C using an oven (WiseVen, model WOF-105, Korea) after 24 h and it was expressed as dry basis (g water/100 g dry sample) (% db).
The Guggenheim–Anderson–de Boer (GAB) model equation (EquationEquation (1)(1)) was applied to fit the sorption data. It has been claimed that this model can be used to predict moisture sorption by starch, protein, chitosan and quinoa protein-chitosan films with adequate accuracy (Abugoch et al., 2011). It is defined as:
The ability of the GAB model to fit experimental data was performed by minimization of the quadratic difference between the experimental and predicted values using Solver Excel (Office 2007, Microsoft Corp.). The fit of the experimental data was evaluated as the mean relative error (MRE) according to the following equation (Yanniotis & Blahovec, Citation2009):
Thermal properties
Thermal properties of each film were evaluated by differential scanning calorimetry (Diamond DSC, Perkin Elmer, USA), previously calibrated using indium (melting onset temperature 156.6 ± 1.6°C, ΔH = 28.6 ± 1 J/g). Approximately 20 mg of each film was loaded into aluminum 30 μl pans and then hermetically sealed. An empty pan was used as reference. Thermograms were obtained in the temperature range from –70 to 120°C at a heating rate of 10°C/min and at cooling rate of 40°C/min, under dry nitrogen purge (50 mL/min). The glass transition temperature values, defined as the midpoint of the change in heat capacity, were calculated from the second DSC heating scan using the instrument software (Pyris Software v 9.0.2. USA). All samples were analyzed in duplicate.
The glass transition temperature as a function of moisture content was fitted using the Gordon–Taylor EquationEquation (3)(3), which is widely applied to predict the theoretical ‘complete glass curve’ of food polymers in the presence of water. The Gordon–Taylor equation is defined as:
Moisture sorption kinetic
The kinetic of moisture absorption from the culture medium to the films was assessed Mueller Hinton agar (Merck, Germany) at 37°C and 4°C in order to obtain the differences on moisture absorption rate in microbiological growth conditions. Pieces of each (triplicates) film of an area of 1 cm2 was placed onto the agar plates, then were weighted using a balance Shimadzu (AUX 120, Japan) until 90 min (at 37°C) and 24 h (at 4°C). Moisture sorption rate (MAR) was obtained from the slope from the linear regression between % water absorbed in the film and time.
Film solubility
Film sections measuring 1 cm2 were placed in Petri plates (diameter = 55 mm) with 10 mL of distilled water and shaken gently at 37°C for 15 h. The solution was then filtered through Whatman Nº 1 filter paper to recover the remaining undissolved film, which was desiccated at 105°C for 24 h. Film solubility was calculated according to Gómez-Estaca et al. (Citation2009) by equation:
Color and opacity
Digital images from each film (white and black background) were captured through a computer vision system (CV) setup, which consisted of a black box with four natural daylight (D65) tubes of 18 W (Philips) and a camera (Canon 4 MP Powershot G3) placed in vertical position at 22.5 cm from samples. The camera lens angle and light was 45°, according to Pedreschi, León, Mery, & Moyano (Citation2006) and Matiacevich, Silva, Osorio, & Enrione (Citation2012). All images were acquired at the same conditions; the camera was remotely controlled by ZoomBrowser software (v6.0 Canon). Camera was calibrated using 30 color charts with a Minolta colorimeter. Color data were measured in the RGB space using Image J program and convert into CIEL*a*b* space standard. L*, a*, b* values obtained from image analysis were equal as those values from the colorimeter.
Variation of color between each gelatin-chitosan film and gelatin film were calculated using ΔE equation (CIE, Citation1978) and CIEΔE2000 (Luo, Cui, & Rigg, Citation2001).
The opacity was obtained using the values of lightness (L*) obtained from the films using white and black
background (EquationEquation (5)
(5)).
Molecular mobility
Transversal or spin–spin relaxation times (T2) were used to determine water and solids mobility and were measured by time resolved 1H-NMR in a Bruker Minispec mq20 (Bruker Biospin Gmbh, Rheinstetten, Germany) with a 0.47 T magnetic field operating at a resonance frequency of 20 MHz and at 30°C. Proton populations of different mobility were measured using two spin–echo sequences: (1) free induction decay (FID) for protons from solid matrix or from water strongly interacting with the solid matrix (Hansen, Kristiansen, & Pedersen, Citation1998) and (2) Carr–Purcell–Meiboom–Gill (CPMG) (Carr & Purcell, Citation1954; Meiboom & Gill, Citation1958), for more mobile protons. All samples were placed in 10 mm diameter glass tubes (to 5 cm height) and were previously equilibrated at 30.00 ± 0.01°C in a thermal bath (Haake, model Phoenix II C35P, Thermo Electron Corp., Germany).
| 1. | FID sequence. The spin–spin relaxation times obtained from FID following a single 90° pulse are affected by field inhomogenities. Nuclei in one part of the sample will experience a magnetic field slightly different from that experienced by identical nuclei in another region. This apparent relaxation time is designated T 2*. Only the relaxation times of fast relaxing protons (which are in the microsecond range) can be correctly measured without a 180° refocus pulse (Colquhouna, Ralet, Thibault, Faulds, & Williamson, Citation1994). In solid samples (like ours), we can consider that the intrinsic T 2 is very close to the T 2*, as reported previously by Fullerton and Cameron (Citation1988). The FID test itself if very fast, taking 10 s, and samples could be measured without appreciable temperature modification. The decay envelopes were fitted to mono-exponential behavior with the following equation: | ||||
| 2. | Longer relaxation times in rubber state films equilibrated at 85% RH (such as those corresponding to more mobile protons) can be measured after a refocusing pulse using CPMG sequence, which consists of 90°×—τ—[180°y—τ—echo—τ]n sequence, with the following setting: τ = 0.04, scans = 8, number points = 500, dummy shots = 0, gain = 68 dB; echoes:15. For sequence measurements, an exponential function (EquationEquation (6) | ||||
Antimicrobial activity of FFS and films
The antimicrobial activity of the FFS and the films was evaluated against E. coli (ATCC 25922), L. monocytogenes (ISP 65–08) and S. typhimurium (ISP Ty2). Bacteria were obtained from the ISP (Health Public Institute, Chile). The selection of the bacteria used is based on the common meat product contaminants (D'Aoust, Citation1991; Farber & Daley, Citation1994; Martin & Beutin, Citation2011).
Culture preparation
The bacteria were stored at –20°C in Mueller Hinton broth with 20% w/w skim milk until use. Each bacterium was previously grown in Mueller Hinton broth (DIFCO, France) at 37°C overnight. This culture served as the inoculums for the microbiological studies, these colony-forming units (CFU) counts were accurately and reproducibly obtained by absorbance value measured by optical density at 625 nm on a spectrophotometer (Shimadzu UVmini-1240, Japan), which corresponded to a 0.5 McFarland turbidity standard solution (approximately 106 CFU/mL) (CDCP & WHO, Citation2003) and diluted starting with a final concentration of each bacterium of 1 × 105 (CFU)/mL.
Inhibition zone method using FFS
Antibacterial activity tests of FFS were performed using inhibition zone method according to Pranoto, Rakshit and Salokhe (Citation2005). FFS (30 µL) were placed on Mueller Hinton (Merck, Germany) agar plates, which had been previously seeded homogenously in the agar medium with 1 × 105 CFU/(mL of agar) of each bacterial species. Later, agar plates were incubated at 37°C for 24 h and examined for inhibition halos. The appearance of a clean area under the FFS drops or under the film zones had been previously placed was an indication of positive antimicrobial activity.
Bacterial viability method from films
A novelty experimental design is described to measure the bacterial viability percentage from films instead from liquid suspensions containing the antimicrobial agent.
In order to obtain hydrated films with high molecular mobility and promote the diffusion chitosan into the broth, a piece of each film (1 cm2) was put in tubes containing 4.5 mL of Mueller Hinton broth (21 g/L) and incubated at 4°C for 24 h. Subsequently, bacteria were added at a concentration of 1 × 103 CFU/(mL of broth) and incubated at 37°C for 12, 24, 36 and 48 h. Later, an aliquot of the broth (100 µL) was then placed on a Petri plate containing Mueller Hinton agar and colonies were counted after incubation at 37°C for 24 h.
In an attempt to assess the diffusion of chitosan from the gelatin films to the liquid medium, 50, 100 and 200 µL aliquots of Mueller Hinton broth previously in contact with the hydrated films (without the addition of bacteria) were placed on agar plates inoculated with E. coli and incubated at 37°C for 24 h.
The results were expressed as percentage of growth inhibition (% inhibition).
Statistical analysis
All experiments were carried out in triplicate reporting the values of mean and standard deviation. The results were statistically analyzed by one-way analysis of variance (ANOVA) employing Graph Pad Prism software (v4). Differences between pairs of means were compared using Tukey test. The level of significance was set at p < 0.05.
Results and discussion
pH and Gel strength
The initial pH and gel strength determinations carried out on FFS () measured as Bloom degree revealed a decrease in the mechanical properties of the gelatin gels when chitosan was added in SG, which is attributed to the gelatin-chitosan interactions and the lowest initial pH of the samples. However, this behavior was not clear when it was added to BG based films.
Table 1 Gel strength or Bloom degree at different pH of bovine–salmon gelatine-chitosan based films forming suspensions.
Tabla 1. Fuerza del gel o Grados Bloom a diferentes pH de suspensiones formadoras de películas basadas en gelatina de bovino y salmón.
The suspensions of salmon-chitosan not gelled at pH lower than 4 and when the pH was adjusted to 5.5, near to isoelectric point of gelatin (∼5.1 ± 1) (CitationDíaz et al., 2011), a weak gel was obtained. Therefore, at the same pH (5.5) the salmon suspensions presented lower values of Bloom degree than BG suspensions. The differences of gel strength between both gelatins could be explained by (1) the different contents of imino-residues (proline and hidroxiproline), being less in SG; (2) the differences in the molecular weight distributions of gelatins (α and β chains), knowing that BG present a rate of 2 between chains α1/α2 (Gómez-Estaca et al., Citation2009) and (3) the temperature of animal habitats.
Songchotikunpan, Tattiyakul and Supaphol (Citation2008) suggested that gels are more compact and rigid when the pH is adjusted near to isoelectric point (pI), where protein chains are more neutral. Badii and Howell (Citation2006) indicated that cold gelatin not gelled in standard conditions for measuring Bloom grade, producing a viscous solution and that when changed the pH values from 4.5 to 6, the Bloom values increased, according to the results obtained in this work. It is important to emphasize that Bloom value measured by standard method can give a wrong impression of the gel strength in fish gelatin, due to that increases during storage compared to mammalian gelatin (Arnesen & Gildberg, Citation2007).
Sorption Isotherms
The moisture sorption isotherm allows the characterization of the water absorption property of the film, and that knowledge of the sorption isotherm is also important for predicting stability and quality changes during the packaging of food products. Experimental data for moisture sorption at 20°C for gelatin-chitosan films () showed typical sigmoid-shaped curves (sorption type II), which is usually associated to water soluble polymeric structures (Fennema, Citation2000). At high RH (> 60%), the moisture content was higher for gelatin-chitosan films compare to both pure BG and SG films (p < 0.05), showing that gelatin-chitosan blend films were more hydrophilic than gelatin pure films. It was observed that the presence of chitosan in the film increased their water content absorbed in the system, being more significant at RH > 60% (p < 0.05). This result indicates higher water content at the same RH as chitosan concentration increases, due to the high hygroscopic capacity of chitosan. This increase was shown to cause swelling as water activity increased (Abugoch et al., 2011; Sebti, Chollet, Degraeve, Noel, & Peyrol, Citation2007). Addition or removal of water may cause phase transitions in the macromolecular structure.
Figure 1. Sorption isotherm of bovine and salmon gelatin with chitosan (% w/w) films at 20°C. (a) Bovine gelatin and (b) salmon gelatin. Error bars indicate their corresponding standard deviation.Isoterma de sorción de películas basadas en gelatina de bovino y salmón con quitosano (% p/p) a 20°C. (a) Gelatina de bovino y (b) gelatina de salmón. Las barras de error indican su correspondiente desvío estándar.
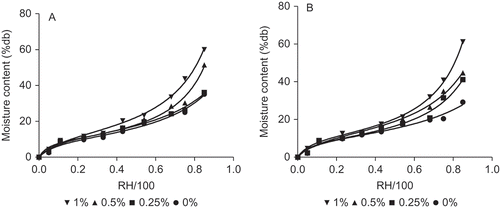
The GAB equation has been claimed to predict the moisture sorption of proteins and chitosan with adequate accuracy (Abugoch et al., Citation2011; Cho & Rhee, Citation2002; Despond, Espuche, & Domard, Citation2001). A good fitting of the experimental data using EquationEquation (1)(1) (GAB equation) was observed for both types of gelatin and its mixtures with a correlation coefficient (R
2) close to 1 and a MRE value 6.0% (EquationEquation (2)
(2)) in all cases (). The GAB parameters obtained using EquationEquation (1)
(1) is shown in . The monolayer value (mo), representing the critical hydration level was ∼10.6% (dry basis, db) for both gelatin types (p > 0.05). Therefore, the water adsorption on monolayer values of the films were not affected by the origin of the gelatin The reported mo and K values obtained for both gelatin films were similar to those reported in the literature (Carvalho et al., 2007; Chiou et al., Citation2009; Yakimets et al., Citation2005). However, a significant difference (p < 0.05) in mo with values of ∼13.5% (db) was observed for the BG films containing 1% (w/w) of chitosan compared to the control sample salmon-chitosan ∼11.3% (db). The addition of chitosan affected the adsorption properties of BG films, enhancing the moisture content of the film as chitosan concentration increased due to its high hygroscopic capacity.
Table 2 Parameters obtained by GAB model using EquationEquation (1) (1) for isotherms of bovine and salmon gelatin with different concentrations of chitosan films.
(1) for isotherms of bovine and salmon gelatin with different concentrations of chitosan films.
Tabla 2. Parámetros obtenidos ajustando el modelo de GAB (ecuación 1) a las isotermas de películas basadas en gelatina de bovino y salmón con diferentes concentraciones de quitosano.
Thermal properties of films
Thermal analysis, through glass transition temperature measurement, showed that both gelatin films equilibrated at 33% and 85% RH were obtained in a glassy and a rubbery state, respectively, at experimental temperature of 20°C, as shown in . Although the films equilibrated at 85% RH generated high moisture contents (>40% (db)) due to the hygroscopic nature of chitosan, the DSC thermograms did not show transitions associated to melting of ice at temperatures near 0°C (data not shown). This result reported that the rubbery samples contain non-freezable water, indicating a possible reduction of water availability for microorganism growth in the surface of the film in rubbery state.
Figure 2. Effect of chitosan concentration on the glass transition temperature of bovine and salmon gelatin films.Efecto de la concentración de quitosano en la temperatura de transición vítrea de películas de gelatina de bovino y salmón.
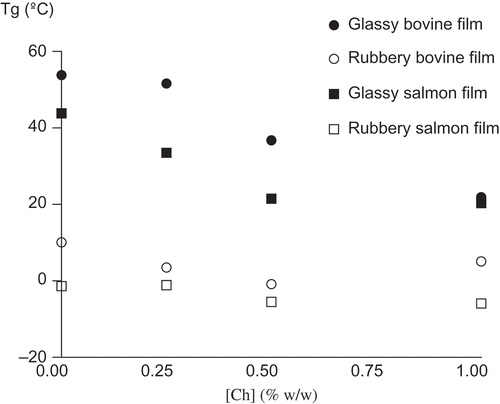
A single glass transition temperature is observed in DSC thermograms indicating a good miscibility between both gelatins gelatin and chitosan, according to results observed by Gómez-Estaca, Gómez-Guillén, Fernández-Martín and Montero (Citation2011), and Suyatma, Tighzert and Copinet (Citation2005). In this work, the Tg value was ∼ –1°C (rubbery state) on the bovine-0.5% w/w chitosan film equilibrated at 85% RH, increasing to ∼ 37°C for the same film equilibrated at 33% RH (glassy state). Also, Tg diminished as the concentration of chitosan increased, that is associated to the high hygroscopic nature of this antimicrobial biopolymer which increases the water content of the film at the same RH ( and 2).
Previous studies reported that the presence of plasticizer in the suspension can break the hydrogen bonds between the polymer and water, thus decreasing the Tg value (Barreto et al., Citation2003). The experimental results were fitted using the Gordon and Taylor equation (EquationEquation (3)(3)) for pure gelatin films and Cochman–Karasz equation for mixtures with chitosan. The application of these equations indicated a good fit of the models to experimental data and the adjustment error (% MRE) was less than 3.3% for all cases.
Significant differences (p < 0.05) were observed in thermal transitions between both gelatin sources, where the Tg of SG was 153°C and the Tg observed for BG was 194°C, both Tg values were obtained through Gordon and Taylor equation. These differences have been explained by the amino acid composition and the molecular weight distribution of the polymer (Díaz et al., Citation2011; Gómez-Guillén et al., Citation2002). These values were similar to those previously reported by Diaz et al. (Citation2011), for dry salmon (154°C) and bovine (194°C) gelatin films.
A and 3B showed the plasticizing effect of water on both gelatin and mixtures, since it decreases the Tg with increasing water content. However, when comparing the values of Tg of the films with and without the addition of chitosan, it was observed that at a particular value of water content, e.g. 10% (db) for BG films, the Tg value obtained in films with chitosan was lower (47°C) compared to the pure film (117°C). The reduction of Tg value, due to the presence of chitosan, was observed in both gelatins. This result could indicate that chitosan act as a plasticizer in matrix, increasing molecular mobility of the film. However, taking into account the molecular weight of chitosan (∼50 kDa), which is found in the order of the polymer chains of gelatin (∼100–200 kDa) and is greater than the plasticizers known as glycerol (0.092 kDa), sorbitol (0.182 kDa) and propylene (0.072 kDa), is not expected a plasticizing effect of chitosan that decrease the Tg observed value. It is known from the literature that the Tg value increases as increasing polymer molecular weight due to a decrease in free volume (Ferry, Citation1980). Therefore, the reduction of Tg value in presence of chitosan is attributed to a reduction of the total molecular weight of the matrix, produced by the addition of chitosan, due to the weight fraction of total solids (7% w/w) does not vary between samples.
Figure 3. Glass transition temperature (Tg) in function of moisture content of the gelatin-chitosan films (0–1% w/w chitosan) at different water contents was fitted using Gordon–Taylor equation. (a) Bovine gelatin and (b) salmon gelatin.Temperatura de transición vítrea (Tg) en función del contenido de humedad de películas de gelatina-quitosano (0–1% p/p quitosano) a diferentes contenidos de humedad fue ajustado usando la ecuación de Gordon-Taylor. (a) Gelatina de bovino y (b) gelatina de salmón.
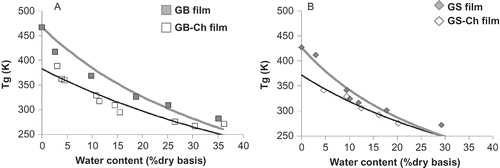
In agreement with the results obtained from Tg as a function of moisture content, both pure gelatin films and their mixtures were stored at 33% and 85% RH at 20°C in order to obtain films in glassy and rubbery state, respectively. Therefore, shows that all samples were obtained in the expected state matrix. Also this figure shows that Tg value was influenced by the origin of the gelatin, where Tg values in both glassy and rubbery state from SG films were lower than BG. This result was attributed to low concentration of amino acids proline and hydroxyproline in salmon-skin gelatin, which decreased by the molecular weight of the polymer chains of the gelatin, decreasing so the value of Tg.
Moisture sorption kinetic of films
The moisture sorption kinetic data showed the hygroscopic nature of chitosan, as suggested by Fernández-Cervera et al. (Citation2004) and Martínez-Camacho et al. (Citation2010) a protonated configuration in the films increasing the water content compared to chitosan in the powder form.
The rate of moisture sorption (MAR) of both gelatin-chitosan films in the glassy and rubbery states were obtained by incubation of each film in agar at 37°C and 4°C. shows that MAR at 37°C in glassy state films increased as chitosan concentration increased for both gelatin types, showing significant differences (p < 0.05) between the origin of the gelatin, obtaining the highest rate for SG where this value was not detected (ND) in the range time evaluated due to the fast dissolution of the film. These results also were observed at 4°C (data not shown). Although high moisture content (∼40% db) was obtained in rubbery state films due to their previous equilibration at 85% RH, MAR values were lower than glassy films, which also decreased as chitosan concentration increased at both temperature evaluated. This result was attributed to the highest initial water content as chitosan concentration increased in the films (see ).
Table 3 Moisture absorption rate at 37°C of bovine and salmon gelatin-chitosan films at different state of matrix (glassy or rubbery).
Tabla 3. Velocidad de absorción de humedad a 37°C de películas con diferentes estados iniciales de la matriz (vítreo o gomoso) basadas en gelatina de bovino y salmón adicionadas con quitosano.
It is important to note that the control films (pure gelatin) were dissolved after 20 min contact with the agar but the structure integrity was maintained in the presence of chitosan, showing a swelling effect up to 90 min at 37°C by water diffusing from the agar. However, the integrity of all films was observed at 4°C, although being visually lower in pure gelatin films. The results also showed that the sorption kinetic of the films in the glassy state was higher (at least the double) than the value obtained for the films in rubbery state for the same measuring time at both evaluated temperature. However, after 15 h the moisture uptake rate converged over this period (data not shown) due to the initial differences of structural state are lost, being both rubbery films.
Water film solubility
The solubility could determine the release of antimicrobial compounds when a film is placed over the food surface. No significant differences in film solubility were observed by the origin of gelatin films (p > 0.05) and independently of initial state of the film (glassy or rubbery) (p > 0.05). As expected, the moisture uptake is different between the initial states of the films; however, the final state after 15 h is the same (rubbery state), showing therefore similar final film solubility. The effect of chitosan concentration on film solubility obtained using EquationEquation (4)(4) is shown in . This figure shows that film solubility diminished as chitosan concentration increased (p 0.05). These results indicate an improvement of physical properties of gelatin films due to the presence of chitosan.
Figure 4. Film solubility at the different concentrations of chitosan-gelatin films. Different letters indicate significant differences (p < 0.05).Solubilidad de las películas a diferentes concentraciones de gelatina-quitosano. Las diferentes letras indican diferencias significativas (p < 0,05).
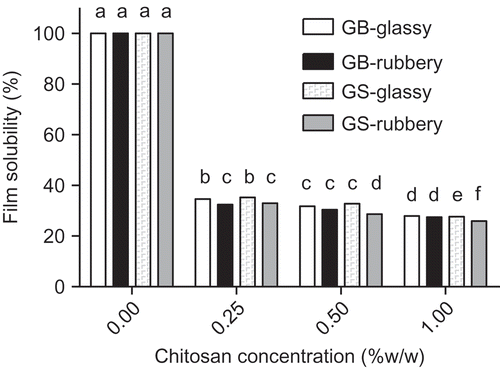
Although in the literature, it has been reported that bovine-hide gelatin based films showed similar water solubility of gelatin-chitosan films (Gómez-Estaca et al., 2009, Citation2010), the same authors (Gómez-Estaca et al., Citation2011) also reported that solubility of the gelatin-chitosan films was significantly lower (p < 0.05) than the gelatin films employed. Furthermore, in tuna-skin gelatin-chitosan mixtures the solubility was significantly (p < 0.05) lower than that of the tuna-skin gelatin (Gómez-Estaca et al., Citation2011). This fact could be due to specific interactions between gelatin and chitosan that stabilize the film structure. According to Taravel and Domard (Citation1995) and Gómez-Estaca et al. (Citation2011) results, gelatin and chitosan interact mainly by means of hydrogen bonding, which affects the physical properties of the mixtures but would maintain its integrity to a greater extent.
Color and opacity
The color parameters values obtained were L* = 76 ± 2; a* = 10.2 ± 0.7; b* = 10 ± 2 and chroma, C* = 14 ± 1. These values did not change significantly (p > 0.05) with (1) the state glassy or rubbery of the films (33% or 85% of equilibrium RH), (2) the origin of the gelatin (salmon or bovine) and (3) the concentration of chitosan. Only the color parameter Hue, H*, was significantly different (p 0.05) between the state of the film and chitosan concentration ().
Table 4 Color parameters of opacity and Hue (H*) of bovine gelatin-chitosan films equilibrated at 33% of relative humidity (film in glassy state) and 85% of relative humidity (film in rubbery state).
Tabla 4. Parámetros de color de opacidad y Hue (H*) obtenidos de películas de gelatina de bovino-quitosano equilibrado a 33% de humedad relativa (película en estado vítreo) y 85% de humedad relativa (película en estado gomoso).
The opacity values obtained using EquationEquation (5)(5) is shown in , where the results indicated that the opacity, as color, was not affected significantly (p > 0.05) by the concentration of chitosan and by the glassy and rubbery state of the matrix.
The variation of color calculated using ΔE* equation (CIE, Citation1978) was lower than 1, indicating imperceptible changes. However, this equation only use the parameters L*, a* and b*. Therefore, using the ΔE2000 equation (Luo et al., Citation2001) which also this parameters take into account the parameters C* and H*, the variation of color was lower than 3 between chitosan concentrations and RHs, indicating minima color changes, which is mainly due to differences observed in the Hue (H*) parameter.
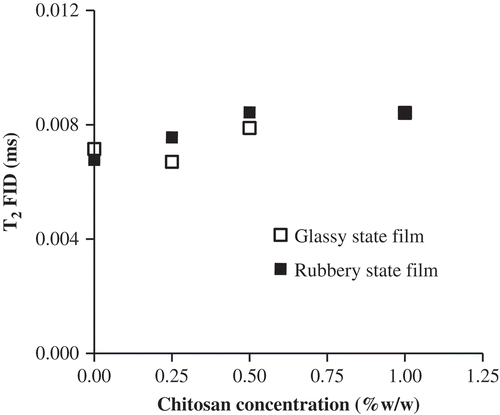
Molecular mobility
The study of NMR spin–spin transverse relaxation times (T
2) as a function of chitosan concentration and RH of the films provides information about the mobility of protons belonging to water and/or solids, according to the pulse sequence employed. shows the T
2* values obtained by FID analysis (T
2FID) for both gelatin-chitosan films in glassy and rubbery state using EquationEquation (6)(6). T2FID
values increased as chitosan concentration increased, due to the increasing mobility of protons in solids as water molecules strongly interacting with them. This result was attributed to the higher water content in gelatin-chitosan films equilibrated at same RH, which is due to hygroscopicity of chitosan. The spin–spin time relaxation, T
2, evaluated using a Hahn spin–echo sequence allows the measurement of proton magnetic relaxations characterized by higher T2
values than those determined by FID. In this way, this spin–echo pulse sequence (CPMG sequence) can be used to differentiate proton populations with different mobility as a function of water content and to study the relaxation of water protons occurring after the protons corresponding to solids have relaxed. However, although the films are in the rubbery state, the expected two sets of T
2 values were not obtained after the spin–echo sequence, which showed that not free water with high mobility are present in this samples. This result was also previously evidenced and confirms the results obtained in DSC analysis.
Figure 5. Transversal relaxation times (T2) obtained by FID sequence using EquationEquation (6)(6) measured by 1H-NMR at different chitosan concentrations added to bovine gelatin films on glassy and rubbery initial state of matrix. Error bars indicate their corresponding standard deviation.Tiempos de relajación transversal (T2) obtenidos a través de la secuencia FID ajustando la Ecuación (6) utilizando 1H-NMR a diferentes concentraciones de quitosano adicionados a películas de bovino en diferentes estados iniciales de la matriz (vítreo o gomoso). Las barras de error indican su respectivo desvío estándar.
Antimicrobial properties
Antimicrobial properties of FFS
As expected, the FFS for both gelatins with chitosan showed antibacterial properties against all the bacteria studied. As chitosan concentration increased in the FFS, the inhibition zone area became cleaner but the diameter of the inhibitory halo remained constant ( ). This result is due to the liquid media being dispensed directly on the surface of an inoculated agar, exerting their antimicrobial action only on the area described by the drop. However, the reduced inhibitory activity of FFS may be explained by the restricted diffusion phenomenon of chitosan from the gelatin matrix due to the interaction with the components of the films (gelatins), which is consistent with results previously reported by other authors (Coma, Citation2002; Pranoto et al., Citation2005).
Figure 6. Results of inhibition zone method using 30 µl of each film-forming suspension (FFS) in function to chitosan concentration (0%, 0.25%, 0.5% and 1% w/w) against (a) Escherichia coli, (b) Listeria monocytogenes and (c) Salmonella typhimurium.Resultados del método de zona de inhibición usando 30 µl de cada suspensión formadora de películas (FFS) en función de la concentración de quitosano (0%, 0,25%, 0,5% and 1% p/p) contra (a) Escherichia coli, (b) Listeria monocytogenes, y (c) Salmonella typhimurium.

However, it is necessary to take into account that the results obtained on antibacterial properties will be a combination of the effect of both chitosan and acetic acid, according to Liu et al. (Citation2006). As the solvent of chitosan, acetic acid with a concentration over 200 ppm (0.02% w/w) had antibacterial activity against E. coli at pH 5.4 (Liu et al., Citation2006). These authors also showed that low molecular weight of chitosan over 200 ppm (0.02% w/w) had antibacterial activity. Although some reported studies were performed using acetic acid concentration over this value and in a high proportion of chitosan compared to gelatin concentration, such as 1:50 (Devlieghere, Vermeulen, & Debevere, Citation2004) and 1:3 gelatin:chitosan (Gómez-Estaca et al., Citation2011), the antimicrobial activity observed was attributed only to chitosan by these authors. In this study, the relation of 1:1 w/w gelatin:chitosan was used with a concentration higher than 200 ppm was used, therefore, a control using both gelatins FFS and the acetic acid concentration without chitosan was performed showing antibacterial effect (data not shown). Therefore, the antimicrobial effect observed in the FFS is attributed at the combination of chitosan and acetic acid added.
Antimicrobial effect of films
The inhibition zone on agar using films was not possible to detect due to their high water adsorption capacity that dissolves the films after 24 h at 37°C. Therefore, several times of exposure on the surface of an inoculated agar (from 5 to 20 min) were not enough to produce some antibacterial activity.
Therefore in order to observe if the films are available to show antimicrobial activity, the antimicrobial properties of the gelatin films were tested only with E. coli in the presence of the highest concentration of chitosan (1% w/w) in order to evaluate the highest inhibitory effect of the films with the most sensitive bacteria to FFS. These results are shown in , where the CFU/(mL of broth) and total and related only to chitosan activity inhibition growth percentage are informed. It is important to note that the gelatin pure films had some inhibitory effect (∼13–20% at 24–48 h) on bacterial growth in the nutrient medium (), being slightly higher than the gelatin extracted from salmon (∼20%), however no significant differences (p 0.05) were observed by the initial structure state on both gelatin pure films. A possible explanation of this result (antimicrobial activity of gelatin pure) could be related to the presence of oligopeptides with antimicrobial properties such as amino groups present in the polymer chain as a result of the partial hydrolysis of gelatin, as reported previously by Pereda et al. (Citation2011).
Table 5 Total and related only to chitosan activity inhibition growth percentage of Escherichia coli by films based on gelatin pure film and 1% w/w chitosan-gelatin, in both initial structure glassy and rubbery state of the film matrix.
Tabla 5. Porcentaje de inhibición del crecimiento de Escherichia coli total y relacionada sólo a la actividad antimicrobiana del quitosano de películas basadas en gelatina de bovino y salmón puros y adicionadas con 1% p/p de quitosano a diferentes tiempos de incubación, tanto en el estado inicial vítreo como gomoso de la matriz.
shows the effect of chitosan in the films in the initial glassy and rubbery states on the growth of E. coli compared to the inhibition caused by pure gelatin; therefore, the reported data showed both the total inhibition growth caused by the mixture gelatin-chitosan and the inhibition percentage only attributed to the presence of the chitosan together with acetic acid. The results showed that the molecular mobility of the matrix affected the antimicrobial activity of gelatin-chitosan films. In the rubbery state, the amonio groups (R–NH3 +) of chitosan have greater mobility but they are not sufficiently exposed for growth inhibition (). The differences observed were not significant (p > 0.05) between salmon and BG-chitosan films as the incubation time increased (24–48 h) due to the moisture uptake rate converged over this period. However, a slight difference (p < 0.05) was observed depending on the initial structural glassy (∼73%) and rubbery (∼64%) state for both gelatin-chitosan films at 24–48 h. Nevertheless, when comparing only the effect caused by chitosan in films until 12 h of incubation, the highest percentage of bacterial viability was obtained in SG-chitosan film (46.6%) compared to the BG-chitosan films (17.4%), both in the glassy state. This differences observed by the initial mobility (glassy and rubbery) state could be attributed to the differences observed in the moisture uptake of the films, where the moisture uptake rate at 4°C was ∼12.3 ± 0.2% water adsorbed/h in the glassy state compared to the 2.25 ± 0.1% water adsorbed/h in the rubbery state for both gelatins, indicating a moisture uptake rate of 80% higher in glassy state than in rubbery state in the first hours.
However, the antimicrobial activity attributed to chitosan comparing glassy and rubbery structural films was significantly different between them but slightly less (17.4% and 13.6%, respectively) for BG-chitosan films than for SG-chitosan (46.6% in glassy state compared to 6.7% in rubbery state), showing a little influence of the structural state at 12 h for BG compared to SG.
Bacterial viability of the film diffusion control showed that there was no inhibition of bacterial growth by diffusion of chitosan, regardless of the incubation time of the film (12–24 h) and the volume (50–100 μL) used to measure total viable colony count. Literature showed that pure chitosan is capable to migrate to agar (Dutta et al., Citation2009; Pranoto et al., Citation2005), however, studies of mixtures of other hydrocolloids show that they are not capable of migrating due to interaction between them (Coma, Citation2002; Pereda et al., Citation2011). The results obtained confirmed that chitosan added to gelatin films was not able to migrate from the film to the nutrient broth, suggesting an interaction with the gelatin matrix.
Conclusions
The presence of chitosan increased the molecular mobility in the films by its hygroscopic nature, in other words by attracting more water and therefore increasing the water content of the films. However, chitosan does not act as a plasticizer in the films, it did not change significantly the color and opacity of the films and improved the physical performance (solubility) of both gelatin films, especially when fish gelatin is used. The antimicrobial activity was effective against E. coli, L. monocytogenes and Salmonella thyphimurium without the migration of the active agents and depends on the molecular mobility state (glassy or rubbery). The highest antimicrobial effect against E. coli was observed when the films were in the initial glassy state in first hours, due to the highest moisture uptake rate in this state.
Differences in physical characteristics were observed by the origin of the gelatin, principally on the gel strength, moisture uptake rate and sorption isotherms. Both pure gelatin films showed antimicrobial effect, being higher to salmon than BG, which was attributed to the presence of oligopeptides with antimicrobial activity in the sample obtained during gelatin extraction from skin collagen.
The scientific relevance of this work was focused on a complex issue as molecular mobility from glass and rubber state of a protein film matrix and their interaction and participation on physical state and antimicrobial effect of other polymer such as chitosan. Besides, a novelty experimental design was performed to measure the bacterial viability percentage from films instead from liquid suspensions containing the antimicrobial agent.
In conclusion, antimicrobial bio-based edible films by combining gelatin and chitosan were obtained by simple solvent-cast method. Before natural preservatives are applied to food, it is essential to evaluate their behavior in food matrices. In this study, it was observed that chitosan can be able to attack microorganism without diffusion from the film to the medium surrounded and the antimicrobial activity depended on the type of gelatin and the initial structure state (glass and rubber), which principally influenced in SG-chitosan films. Therefore, the implications of this work are associated to the evaluated gelatin-chitosan based films could be a potential application as bioactive edible films for fresh foods.
Acknowledgments
The authors acknowledge to Salmon Oil S.A. Company to provide the material, and the financial support from Fondecyt Projects Nº 11100209 and 1110607 and VRID-USACH.
References
- AOAC . 1975 . Official methods of analysis , 12th , Arlington , VA : Association of Official Analytical Chemists . doi: 10.1016/j.foodhyd.2010.08.008
- Abugoch , L. E. , Tapia , C. , Villamán , M. C. , Yazdani-Pedram , M. and Díaz-Dosque , M. 2011 . Characterization of quinoa protein-chitosan blend edible films . Food Hydrocolloids , 25 : 879 – 886 . doi: 10.1016/j.foodhyd.2010.08.008
- Acevedo , N. , Schebor , C. and Buera , M. P. 2006 . Water–solids interactions, matrix structural properties and the rate of non-enzymatic browning . Journal of Food Engineering , 77 : 1108 – 1115 . doi: 10.1016/j.foodhyd.2010.08.008
- Ahmad , M. , Benjakul , S. , Prodpran , T. and Agustini , T. W. 2012 . Physico-mechanicial and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils . Food Hydrocolloids , 28 : 189 – 199 . doi: 10.1016/j.foodhyd.2010.08.008
- Anderson , R. B. 1946 . Modification of the Brunauer, Emmett and Teller equation . Journal of the American Chemical Society , 68 : 686 – 691 . doi: 10.1016/j.foodhyd.2010.08.008
- Arnesen , J. and Gildberg , A. 2007 . Extraction and characterisation of gelatine from Atlantic salmon (Salmon salar) skin . Bioresource Technology , 98 : 53 – 57 . doi: 10.1016/j.foodhyd.2010.08.008
- Badii , F. and Howell , N. K. 2006 . Fish gelatin: Structure, gelling properties and interaction with egg albumen proteins . Food Hydrocolloids , 20 : 630 – 640 . doi: 10.1016/j.foodhyd.2010.08.008
- Barreto , P. , Roeder , J. , Crespo , J. , Maciel , G. , Terenzi , H. , Pires , A. and Soldi , V. 2003 . Effect of concentration, temperature and plasticizer content on rheological properties of sodium caseinate and sodium caseinate/sorbitol and glass transition of their films . Food Chemistry , 82 : 425 – 431 . doi: 10.1016/j.foodhyd.2010.08.008
- Carr , H. Y. and Purcell , E. M. 1954 . Effects of diffusion on free precession in nuclear magnetic resonance experiments . Physical Review , 94 : 630 – 638 . http://www.pascal-man.com/navigation/faq-java-browser/T2-nmr/carr-purcell.pdf doi: 10.1016/j.foodhyd.2010.08.008.
- Carvalho , R. A. , Sobral , P. J. , Thomazine , M. , Habitante , A. M. , Gimenez , B. , Guillen , M. C. G. and Montero , P. 2007 . Development of edible films based on differently processed Atlantic Halibut (Hippoglossus hippoglossus) skin gelatin . Food Hydrocolloids , 22 : 1117 – 1123 . doi: 10.1016/j.foodhyd.2010.08.008
- CDCP (Center for Disease Control and Prevention), & WHO (World Health Organization). (2003). Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world. 209–214.
- Celis , D. , Azocar , M. I. , Enrione , J. , Paez , M. and Matiacevich , S. 2012 . The effect of molecular mobility on the antimicrobial activity of chitosan-gelatin films . Journal of Food Research , 1 ( 4 ) : 184 – 193 . doi: 10.1016/j.foodhyd.2010.08.008
- Chiou , B.-S. , Avena-Bustillos , R. J. , Bechtel , P. J. , Imam , S. H. , Glenn , G. M. and Orts , W. J. 2009 . Effects of drying temperature on barrier and mechanical properties of cold-water fish gelatin films . Journal of Food Engineering , 95 ( 2 ) : 327 – 331 . doi: 10.1016/j.foodhyd.2010.08.008
- Cho , S. and Rhee , R. 2002 . Sorption characteristics of soy protein films and their relation to mechanical properties . Lebensmittel-Wissenschaft Und-Technologie , 35 : 151 – 157 . doi: 10.1016/j.foodhyd.2010.08.008
- CIE (Commission International de l'Eclairage). (1978). Recommendations on Uniform Color Spaces, Color Difference Equations, Psychometric Color Terms. CIE Publication 15, supplement 2, Colorimetry, Bureau Central de la CIE, Paris.
- Colquhouna , I. J. , Ralet , M.-C. , Thibault , J.-F. , Faulds , C. B. and Williamson , G. 1994 . Structure identification of feruloylated oligosaccharides from sugar-beet pulp by NMR spectroscopy . Carbohydrate Research , 263 : 243 – 256 . doi: 10.1016/j.foodhyd.2010.08.008
- Coma , V. 2002 . Bioactive packaging technologies for extended shelf life of meat-based products . Meat Science , 78 : 90 – 103 . doi: 10.1016/j.foodhyd.2010.08.008
- D'Aoust , J.-Y. 1991 . Psychrotrophy and foodborne Salmonella . International Journal of Food Microbiology , 13 : 207 – 215 . doi: 10.1016/j.foodhyd.2010.08.008
- DeBoer , J. H. 1968 . The dynamical character of adsorption , Oxford , , UK : Clarendon Press . doi: 10.1016/j.foodhyd.2010.08.008.
- Despond , S. , Espuche , E. and Domard , A. 2001 . Water sorption and permeation in chitosan films: Relation between gas permeability and relative humidity . Journal of Polymer Science Part B E Polymer Physics , 39 : 3114 – 3126 . doi: 10.1016/j.foodhyd.2010.08.008
- Devlieghere , F. , Vermeulen , A. and Debevere , J. 2004 . Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables . Food Microbiology , 21 : 703 – 714 . doi: 10.1016/j.foodhyd.2010.08.008
- Díaz , P. , López , D. , Matiacevich , S. , Osorio , F. and Enrione , J. 2011 . State Diagram of Salmon (Salmon salar) Gelatin Films . Journal of the Science of Food and Agriculture , 91 ( 14 ) : 2558 – 2565 . doi: 10.1016/j.foodhyd.2010.08.008
- Dutta , P. K. , Dutta , J. and Tripathi , V. 2004 . Chitin and chitosan: Chemistry, properties and applications . Journal of Scientific and Industrial Research , 63 : 20 – 31 . http://nopr.niscair.res.in/bitstream/123456789/5397/1/JSIR%2063%281%29%2020-31.pdf doi: 10.1016/j.foodhyd.2010.08.008.
- Dutta , P. , Tripathi , S. , Mehrotra , G. K. and Dutta , J. 2009 . Perspectives for chitosan based antimicrobial films in food applications . Food Chemistry , 114 ( 4 ) : 1173 – 1182 . doi: 10.1016/j.foodhyd.2010.08.008
- Fan , M. , Hu , Q. and Shen , K. 2009 . Preparation and structure of chitosan soluble in wide pH range . Carbohydrate Polymers , 78 : 66 – 71 . doi: 10.1016/j.foodhyd.2010.08.008
- Farber , J. M. and Daley , E. 1994 . Presence and growth of Listeria monocytogenes in naturally-contaminated meats . International Journal of Food Microbiology , 22 : 33 – 42 . doi: 10.1016/j.foodhyd.2010.08.008
- Farroni , A. E. , Matiacevich , S. B. , Guerrero , S. , Alzamora , S. and Buera , M. D. P. 2008 . Multi-level approach for the analysis of water effects in corn flakes . Journal of Agricultural and Food Chemistry , 56 : 6447 – 6453 . doi: 10.1016/j.foodhyd.2010.08.008
- Fennema , O. 2000 . Quimica de los Alimentos , New York : Acribia . doi: 10.1016/j.foodhyd.2010.08.008.
- Fernández-Cervera , M. , Karjalainen , M. , Airaksinen , S. , Rantanen , J. , Krogars , K. , Heinämäki , J. , Colarte , A. I. and Yliruusi , J. 2004 . Physical stability and moisture sorption of aqueous chitosan-amylose starch films plasticized with polyols . European Journal of Pharmaceutics and Biopharmaceutics , 58 : 69 – 76 . doi: 10.1016/j.foodhyd.2010.08.008
- Fernández-Saiz , P. , Lagaron , J. M. and Ocio , M. J. 2009 . Optimization of the biocide properties of chitosan for its application in the design of active films of interest in the food area . Food Hydrocolloids , 23 : 913 – 921 . doi: 10.1016/j.foodhyd.2010.08.008
- Ferry , J. D. 1980 . Viscoelastic properties of polymers , New York : Wiley & Sons, Inc . doi: 10.1016/j.foodhyd.2010.08.008.
- Fullerton , G. D. and Cameron , I. L. 1988 . “ Relaxation of biological tissues ” . In Biomedical magnetic resonance imaging: Principles, methodology and applications , Edited by: Wehrli , F. W. , Shaw , D. and Kneeland , J. B. pp. 1 – 115 . New York : VCH Publisher Inc . doi: 10.1016/j.foodhyd.2010.08.008.
- Gómez-Estaca , J. , Gómez-Guillén , M. C. , Fernández-Martín , F. and Montero , P. 2011 . Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin-chitosan films . Food Hydrocolloids , 25 : 1461 – 1469 . doi: 10.1016/j.foodhyd.2010.08.008
- Gómez-Estaca , J. , López de Lacey , M. E. , López-Caballero , M. C. , Gómez-Guillén , M. C. and Montero , P. 2010 . Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation . Food Microbiology , 27 : 889 – 896 . doi: 10.1016/j.foodhyd.2010.08.008
- Gómez-Estaca , J. , Montero , P. , Fernández-Martín , F. and Gómez-Guillén , M. C. 2009 . Physico-chemical and film-forming properties of bovine-hide and tuna-skin gelatin: A comparative study . Journal of Food Engineering , 90 : 480 – 486 . doi: 10.1016/j.foodhyd.2010.08.008
- Gómez-Guillén , M. , Turnay , J. , Fernández-Díaz , M. , Ulmo , N. , Lizarbe , M. and Montero , P. 2002 . Structural and physical properies of gelatin extracted from different marine species: A comparative study . Food Hydrocolloids , 16 : 25 – 34 . doi: 10.1016/j.foodhyd.2010.08.008
- Greener , I. and Fennema , O. 2002 . “ Edible films and costing: Characteristics, formation, definitions, and testing methods ” . In Coating and films to improve food quality , Edited by: Krochta , J. , Baldwin , E. and Nisperos-Carriedo , M. pp. 3 – 7 . Florida : Editorial CRC Press . doi: 10.1016/j.foodhyd.2010.08.008.
- Greenspan , L. 1977 . Humidity fixed points of binary saturated aqueous solutions . Journal of Research of the National Bureau of Standards , 81 A ( 1 ) : 89 – 96 . doi: 10.1016/j.foodhyd.2010.08.008
- Guggenheim , E. A. 1966 . Applications of stadistical mechanics , Oxford , , UK : Clarendon Press . doi: 10.1016/j.foodhyd.2010.08.008.
- Hansen , E. W. , Kristiansen , P. E. and Pedersen , B. 1998 . Crystallinity of polyethylene derived from solid-state proton NMR free induction decay . Journal of Physical Chemistry B , 102 : 5444 – 5450 . doi: 10.1016/j.foodhyd.2010.08.008
- Haug , I. , Draget , K. and Smidsrød , O. 2004 . Physical and rheological properties of fish gelatin compared to mammalian gelatin . Food Hydrocolloids , 18 : 203 – 213 . doi: 10.1016/j.foodhyd.2010.08.008
- Jamilah , B. and Harvinder , K. 2002 . Properties of gelatins from skins of fish-black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica) . Food Chemistry , 77 : 81 – 84 . doi: 10.1016/j.foodhyd.2010.08.008
- Karim , A. A. and Bhat , R. 2008 . Gelatin alternatives for the food industry: Recent developments, challenges and prospects . Trends in Food Science & Technology , 19 ( 12 ) : 644 – 656 . doi: 10.1016/j.foodhyd.2010.08.008
- Karim , A. A. and Bhat , R. 2009 . Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins . Food Hydrocolloids , 23 ( 3 ) : 563 – 576 . doi: 10.1016/j.foodhyd.2010.08.008
- Kong , M. , Chen , X.-G. , Xing , K. and Park , H. J. 2010 . Antimicrobial properties of chitosan and mode of action: A state of the art review . International Journal of Food Microbiology , 144 : 51 – 63 . doi: 10.1016/j.foodhyd.2010.08.008
- Kou , Y. , Dickinson , L. C. and Chinachoti , P. 2000 . Mobility characterization of waxy corn starch using wide-line 1H-nuclear magnetic resonance . Journal of Agricultural and Food Chemistry , 48 : 5489 – 5495 . doi: 10.1016/j.foodhyd.2010.08.008
- Li , B. , Wang , X. , Chen , R. , Huangfu , W. and Xie , G. 2008 . Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima . Carbohydrate Polymers , 72 ( 2 ) : 287 – 292 . doi: 10.1016/j.foodhyd.2010.08.008
- Lin , X. , Ruan , R. , Chen , P. , Chung , M. , Ye , X. , Yang , T. , Doona , C. and Wagner , T. 2006 . NMR state diagram concept . Journal of Food Science , 71 : R136 – R145 . doi: 10.1016/j.foodhyd.2010.08.008
- Liu , N. , Chen , X.-G. , Park , H.-J. , Liu , Ch.-G. , Liu , Ch.-S. , Meng , X.-H. and Yu , L.-Y. 2006 . Effect of MW and concentration of chitosan on antibacterial activity of E.coli . Carbohydrate Polymers , 64 : 60 – 65 . doi: 10.1016/j.foodhyd.2010.08.008
- Luo , M. R. , Cui , G. and Rigg , B. 2001 . The development of the CIE 2000 colour-difference formula: CIEDE2000 . Color Research & Application , 26 : 340 – 350 . doi: 10.1016/j.foodhyd.2010.08.008
- Martin , A. and Beutin , L. 2011 . Characteristics of Shiga toxin-producing Escherichia coli from meat and milk products of different origins and association with food producing animals as main contamination sources . International Journal of Food Microbiology , 146 : 99 – 104 . doi: 10.1016/j.foodhyd.2010.08.008
- Martínez-Camacho , A. P. , Cortez-Rocha , M. O. , Ezquerra-Brauer , J. M. , Graciano-Verdugo , A. Z. , Rodriguez-Félix , F. , Castillo-Ortega , M. M. , Yépiz-Gómez , M. S. and Plascencia-Jatomea , M. 2010 . Chitosan composite films: Thermal, structural, mechanical and antifungal properties . Carbohydrate Polymers , 82 ( 2 ) : 305 – 315 . doi: 10.1016/j.foodhyd.2010.08.008
- Matiacevich , S. , Silva , P. , Osorio , F. and Enrione , J. 2012 . “ Evaluation of blueberry colour during storage using image analysis ” . In Colour in food: Technological and psychophysical aspects , Edited by: Caivano , J. L. and Buera , M. P. pp. 211 – 218 . Buenos Aires : CRC Publisher . doi: 10.1016/j.foodhyd.2010.08.008.
- Meiboom , S. and Gill , D. 1958 . Modified spin-echo method for measuring nuclear relaxation times . Review of Scientific Instruments , 29 : 688 – 691 . doi: 10.1016/j.foodhyd.2010.08.008
- Pedreschi , F. , León , J. , Mery , D. and Moyano , P. 2006 . Development of a computer vision system to measure the colour of potato chips . Food Research International , 39 : 1092 – 1098 . doi: 10.1016/j.foodhyd.2010.08.008
- Pereda , M. , Ponce , A. , Marcovich , N. , Ruseckaite , R. and Martucci , J. 2011 . Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity . Food Hydrocolloids , 25 : 1372 – 1381 . doi: 10.1016/j.foodhyd.2010.08.008
- Pranoto , Y. , Rakshit , S. K. and Salokhe , V. M. 2005 . Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin . LWT-Food Science and Technology , 38 : 859 – 865 . doi: 10.1016/j.foodhyd.2010.08.008
- Rivero , S. , García , M. A. and Pinotti , A. 2009 . Composite and bi-layer films based on gelatin and chitosan . Journal of Food Engineering , 90 ( 4 ) : 531 – 539 . doi: 10.1016/j.foodhyd.2010.08.008
- Rivero , S. , García , M. A. and Pinotti , A. 2010 . Correlation between structural, barrier, thermal and mechanical properties of plasticized gelatin films . Innovative Food Science and Emerging Technology , 11 : 369 – 375 . doi: 10.1016/j.foodhyd.2010.08.008
- Sebti , I. , Chollet , E. , Degraeve , P. , Noel , C. and Peyrol , E. 2007 . Water sensitivity antimicrobial and physicochemical analyses of edible films based on HPMC and/or chitosan . Journal of Agricultural and Food Chemistry , 55 : 693 – 699 . doi: 10.1016/j.foodhyd.2010.08.008
- Soekarto , S. T. and Steinberg , M. P. 1981 . “ Determination of binding energy for three fractions of bound water ” . In Water activity: Influence on food quality , Edited by: Rockland , L. B. and Steward , G. F. pp. 265 New York , NY : Academic Press . doi: 10.1016/j.foodhyd.2010.08.008.
- Songchotikunpan , P. , Tattiyakul , J. and Supaphol , P. 2008 . Extraction and electrospinning of gelatin from fish skin . International Journal of Biological Macromolecules , 42 : 247 – 255 . doi: 10.1016/j.foodhyd.2010.08.008
- Suyatma , N. , Tighzert , N. and Copinet , A. 2005 . Effects of hydrophilic plasticizers on mechanical, thermal and surface properties of chitosan films . Journal of Agricultural and Food Chemistry , 53 : 3950 – 3957 . doi: 10.1016/j.foodhyd.2010.08.008
- Taravel , M. N. and Domard , A. 1995 . Collagen and its interaction with chitosan. Influence of the physicochemical characteristics of collagen . Biomaterials , 16 ( 11 ) : 865 – 871 . doi: 10.1016/j.foodhyd.2010.08.008
- Vittadini , E. and Chinachoti , P. 2003 . Effect of physico-chemical and molecular mobility parameters on Staphylococcus aureus growth . Journal of Food Science and Technology , 38 : 841 – 847 . doi: 10.1016/j.foodhyd.2010.08.008
- Yakimets , I. , Wellner , N. , Smith , A. C. , Wilson , R. H. , Farhat , I. and Mitchell , J. 2005 . Mechanical properties with respect to water content of gelatin films in glassy state . Polymer , 46 : 12577 – 12585 . doi: 10.1016/j.foodhyd.2010.08.008
- Yang , H. and Wang , Y. 2009 . Effects of concentration on nanostructural images and physical properties of gelatin from channel catfish skins . Food Hydrocolloids , 23 : 577 – 584 . doi: 10.1016/j.foodhyd.2010.08.008
- Yanniotis , S. and Blahovec , J. 2009 . Model analysis of sorption isotherms . LWT – Food Science and Technology , 42 ( 10 ) : 1688 – 1695 . doi: 10.1016/j.foodhyd.2010.08.008
- Yin , Y. , Li , Z. , Sun , Y. and Yao , K. 2005 . A preliminary study on chitosan/gelatine polyelectrolyte complex formation . Journal of Material Science (Letters) , 40 : 4649 – 4652 . doi: 10.1016/j.foodhyd.2010.08.008