Abstract
Pectin and its acidic oligosaccharide derivatives are believed to have many potential applications in the food and pharmaceutical industries. In this article, we have investigated the effects of haw pectic oligosaccharide (HPOS) on hyperlipidemia (HL) and oxidative stress in mice induced by a high-fat diet. The results showed that HPOS significantly suppressed the fat deposition in the liver of HL mice. In addition, it significantly increased superoxide dismutase activity and suppressed the production and accumulation of malondialdehyde in liver. It may also be helpful in normalizing fatty acid metabolism in the liver of mice by altering the proportions of saturated fatty acid and monounsaturated fatty acid. These results reveal that HPOS has potentially beneficial effects against HL and oxidative stress in mice.
Se cree que la pectina y sus derivados oligosacáridos ácidos tienen muchas aplicaciones potenciales en la industria alimentaria y farmacéutica. En este trabajo, investigamos los efectos del oligosacárido péctico de espino chino (HPOS) en la hiperlipidemia (HL) y el estrés oxidativo de ratones inducida por una dieta alta en grasas. Los resultados mostraron que HPOS suprimió de forma significativa la deposición de grasa en el hígado de HL ratones. Además, los HPOS aumentaron significativamente la actividad de la superóxido dismutasa (SOD) y suprimió la producción y acumulación de malondialdehído (MDA) en el hígado. HPOS también pueden ser útiles para normalizar el metabolismo de ácidos grasos en el hígado de los ratones mediante la alteración de las proporciones de ácidos grasos saturados (SFA) y ácidos grasos monoinsaturados (MUFA). Estos resultados revelaron que HPOS tiene efectos potencialmente beneficiosos contra HL y el estrés oxidativo en ratones.
Introduction
Acidic oligosaccharides have attracted much interest from both the research community and the general public due to the potentially beneficial biological activities relevant to the food, drug, and agricultural industries. They are cheap, abundant, and easily isolated and prepared. These acidic oligosaccharides also possess the functions of antibacterial activity (El-Nakeeb & Yousef, Citation1970), removing toxins including heavy metal elements (Inoue, Mirvallev, & Makino, Citation2002) and promoting the adsorption of calcium ions in the body (Innami & Kiriyama, Citation1982). Previously, we successfully prepared a series of acidic oligosaccharides from haw pectin (HP) and confirmed that these oligosaccharides could significantly lower the levels of serum triacylglycerol (TG) and cholesterol in hyperlipidemic mice (Li, Wang, Du, & Guo, Citation2008; Li et al., Citation2010). In this study, the effects of haw pectic oligosaccharide (HPOS) on liver lipid metabolism and oxidative stress in hyperlipidemic mice were further investigated.
Materials and methods
Materials
Haw (Crataegus pinnatifida Bunge) fruit obtained from the Shenyang outskirt farm was sliced and dried to use. HP was prepared by hot water extraction from dried haw fruit (Wang, Li, Jin, & Li, Citation2008; Wang, Zhang, Qi, & Li, Citation2007) and hydrolyzed by pectinase (0.2 U/mL, DSM, China) in 0.02 M acetate buffer at pH 3.5 and 50°C. The hydrolyzate was subjected to ultra- and nano-filtration (Du, Li, Wang, Guo, & Zhang, Citation2009) to obtain low-molecular hydrolyzate (HPOS; MW < 6000) and high-molecular hydrolyzate (HPHM; MW > 6000) fractions. The total sugar contents of HP, HPHM, and HPOS were 92.2, 97.3, and 99.7%, and the uronic acid contents were 70.4, 59.8, and 93.6%, respectively. Quantification kits for superoxide dismutase (SOD) and malondialdehyde (MDA) were obtained from Bio-Sino Biotechnology & Science Inc. (Beijing, China).
Animals and diets
Pathogen-free male Kunming mice, aged 6 weeks and weighing 20–25 g, were fed a standard commercial diet (Beijing HFK Bioscience Co., Ltd., Beijing, China) ad libitum for 4 days before random assignment into different treatment groups (n = 10, ). The normal control group was fed a standard commercial diet, and the other groups were fed a high-fat (HF) diet (Beijing HFK Bioscience Co., Ltd.). Zhibituo, a commercially available Chinese herbal medicine (Qujing, China) for the treatment of hyperlipidemia, was used as a positive control. All groups were treated by oral infusion with the same volume of water (normal and HF control groups) or specified samples () daily. Animals were housed individually under natural light at a temperature of 20–23°C, with free access to standard or HF feed and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Shenyang Pharmaceutical University (SYXK-L-2010-0009). The compositions of the diets are presented in .
Table 1. Experimental group of animals.
Grupos experimentales de animales.
Table 2. Mass percent compositions (%) of the experimental diets.
Composición porcentual en masa (%) de las dietas experimentales.
Analytic methods
After 30 days of treatment, the animals were fasted for 24 h and then euthanized. The livers were removed and stored at −80°C. The levels of SOD and MDA in the liver were determined using commercially available kits (Nanjing Jiancheng, China) according to the manufacturer’s protocols. The total liver lipid fraction was extracted by chloroform/methanol (2:1, v/v), and the lipid mass was measured after removing the solvent completely. The composition of individual fatty acid was analyzed by gas chromatography-mass spectrometry (GC-MS) after chemical conversion to methyl esters (Metcalfe, Schmitz, & Pelka, Citation1966). All analysis experiments were performed in triplicates. An Agilent 6890-5973N gas chromatograph-mass spectrometer (USA) was used with an Agilent MP-1 MS capillary column (30 m × 250 μm). The column temperature program was 50°C for 1 min, rising from 50°C to 160°C at 10°C/min, then rising from 160°C to 240°C at 8°C/min and remaining at 240°C for 20 min.
Statistical analysis
Data are presented as mean ± SD. Analysis of variance was used to determine the significant differences between group means using SPSS software (version 13.0, SPSS Corp., Chicago, IL, USA). Statistical significance was accepted at p < 0.05.
Results
Effect of HPOS administration on liver weight and food intake of mice
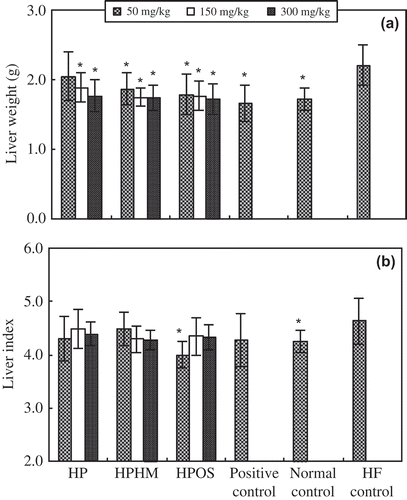
At the end of the experiment, the livers of the mice of the HF control group showed significant accumulations of fat, and the histomorphology of liver boundary was blurry with no clear edge. Except the lowest dose of HP (50 mg/kg), HPOS, HPHM, and HP all significantly (p < 0.05) reduced the final liver weight of mice after a 30-day HF diet compared to HF control mice (). In addition, these oligosaccharides lowered the liver index compared to HF control mice, and this suppressive effect was statistically significant at low dose of HPOS (). However, there was no significant difference in liver index among HP, HPHM, HPOS, and normal control. The average food intake between treatment groups (normal control group, 6.4 ± 0.8 g/day; HF groups, 6.7 ± 0.7 –7.0 ± 0.8 g/day) was not significantly different.
Figure 1. Effect of haw pectic oligosaccharide (HPOS) on liver weight (A) and index (B, Liver/body weight × 100) in mice fed a HF diet. Each value is mean ± SD (n = 10). * p < 0.05, compared with the HF control group.
Efecto de oligosacárido péctico de espino chino (HPOS) en el peso del hígado (A) y el índice (B, hígado/peso corporal × 100) en ratones alimentados con una dieta alta en grasas. Cada valor es la media ± SD (n = 10). * p < 0,05, en comparación con el grupo de control HF.
Effect of HPOS on the fat mass and fatty acid composition of liver
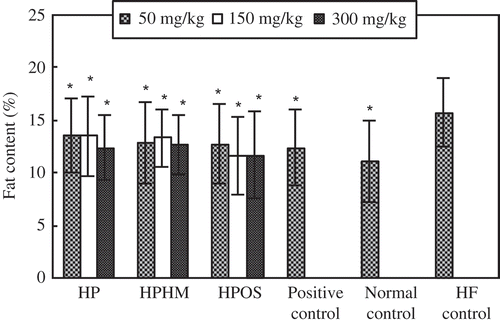
Treatment of mice with HPOS, HPHM, or HP significantly (p < 0.05) reduced fat deposition in liver compared to the HF group (). These suppressive effects were greater for higher doses (150–300 mg/kg) of HPOS compared to equivalent doses of HP and HPHM. In fact, at the doses of 150–300 mg/kg, the suppressive effect of HPOS was also somewhat superior to that of Zhibituo, a known anti-HL agent used as a positive control. However, there were no significant differences among HP, HPHM, HPOS, and normal control at higher doses (150–300 mg/kg).
Figure 2. Effect of haw pectic oligosaccharide (HPOS) on the liver lipid content in HF-fed mice. Each value is mean ± SD (n = 10). * p < 0.05, compared with the HF control group.
Efecto de oligosacárido péctico de espino chino (HPOS) en el contenido lipídico del hígado de ratones alimentados con una dieta alta en grasas. Cada valor es la media ± SD (n = 10). * p < 0,05, en comparación con el grupo de control HF.
The fatty acid composition of the total liver lipid fraction was moderately altered by the HF diet. At the dose of 150 mg/kg equaling that of the positive control, treatment with HPOS significantly altered (p < 0.05) the proportions of saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA) in the liver ( ) compared to the livers of HF mice. In contrast, there were no significant differences between SFA and MUFA in HPOS-treated mice compared to mice fed a control diet, suggesting that HPOS could normalize fatty acid metabolism in the liver even under a HF diet. Moreover, HP and HPHM showed a similar effect on the composition of the lipid fraction.
Table 3. Fatty acids composition in liver.
Composición de ácidos grasos en hígado.
Antioxidant effects of HPOS on the liver of HF-fed mice
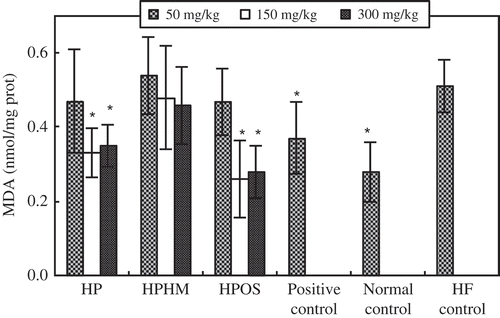
The antioxidant effects of HPOS on the liver are shown in and . Compared to the HF control mice, higher doses (150–300 mg/kg) of HPOS significantly (p < 0.05) increased SOD activity in the liver, while the synthesis and accumulation of the oxidation product MDA in liver were inhibited significantly (p < 0.05), consistent with the increase in SOD. However, there were no significant differences among HP, HPOS, and normal control at higher doses (150–300 mg/kg). The enhanced SOD activity and reduced MDA accumulation were also greater than that exerted by Zhibituo.
Figure 3. Effects of haw pectic oligosaccharide (HPOS) on superoxide dismutase (SOD) activity in the liver of HF-fed mice. Each value is mean ± SD (n = 10). * p < 0.05, compared with the HF control group.
Efecto de oligosacárido péctico de espino chino (HPOS) en la actividad de la superoxido dismutasa de ratones alimentados con una dieta alta en grasas. Cada valor es la media ± SD (n = 10). * p < 0,05, en comparación con el grupo de control HF.
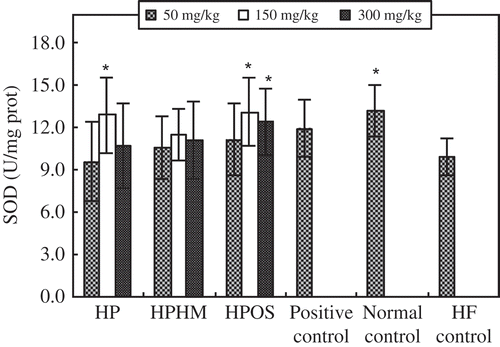
Figure 4. Effects of haw pectic oligosaccharide (HPOS) on the accumulation of malondialdehyde (MDA) in the liver of HF-fed mice. Each value is mean ± SD (n = 10). *p < 0.05, compared with the HF control group.
Efecto de oligosacárido péctico de espino chino (HPOS) en la acumulación de malondialdehído (MDA) en el hígado de ratones alimentados con una dieta alta en grasas. Cada valor es la media ± SD (n = 10). * p < 0,05, en comparación con el grupo de control HF.
Discussion
Liver is a very important organ for modulating and regulating lipid, sugar, and amino acid metabolism. Many animal studies (Tandy et al., Citation2010; Lee, Kim, Jo, & Hwang, Citation2010; Liu, Hung, & Huang, Citation1995) have demonstrated that prolonged administration of a HF and high-cholesterol diet accelerates the synthesis of TGs, inhibits the metabolism of fatty acids, and diminishes the secretion of TGs from the liver into blood by decreasing the β-oxidation of fatty acids. This leads to the accumulation of excess TGs within the liver. In the present study, the livers of HF mice showed significant accumulation of fat. The liver boundary was also blurry with no clear edge.
Fatty liver is commonly associated with metabolic syndromes such as lipid metabolic disturbance d6iseases and can progress to hepatic fibrosis and other cardiovascular diseases (Bayard, Holt, & Boroughs, Citation2006). As a soluble food fiber, pectin is known to decrease the levels of total cholesterol (TC) and TG in the blood (Lattimer & Haub, Citation2010; Terpstra, Lapre, Vries, & Beynen, Citation1998). We previously demonstrated that HPOS had a greater capacity to lower serum TG and TC (Li, Wang, Du, & Guo, Citation2008; Li et al., Citation2010). The present results further confirmed that HPOS inhibited fat deposition in the livers of hyperlipidemic mice and that this effect was relatively greater than that of both macromolecular HP and commercially available anti-HL drug Zhibituo (positive control). In addition, the liver index in low dose of HPOS was significantly lowered than that in HF control mice (), which could reflect a lower liver weight and a higher body weight. Moreover, HPOS could normalize the fatty acid metabolism in HF-fed mice by altering the proportions of SFA and MUFA in liver, similar to the results of Vial et al. (Citation2011) obtained in the liver of HF-fed rat. Although the underlying mechanism(s) about anti-hyperlipidemia of HPOS requires further study, the results obtained here and previously (Li, Wang, Du, & Guo, Citation2008; Li et al., Citation2010) suggest that HPOS holds great potential for the development of functional foods to protect against fatty liver diseases.
It has been reported (Strobel, Fassett, Marsh, & Coombes, Citation2011; Wilson et al., Citation2001) that the overproduction of reactive oxygen species or increased oxidative stress is a major pathogenic mechanism of vascular endothelial dysfunction, and in initiation and progression of liver and vascular diseases (Mika, Puntmann, & Kaski, Citation2007). It is now established that HF and high-cholesterol diet has an increasing effect on lipid peroxidation in plasma and tissues (Balkan et al., Citation2002). MDA is used frequently to evaluate products of lipid peroxidation (Sattler, Malle, & Kostner, Citation1998). Some food components could act as antioxidants to suppress the lipid oxidation. Al-Dosari (Citation2011) reported that avocado fruit pulp exhibited great antioxidant property in high-cholesterol-fed rats, representing as significantly reducing the MDA concentration. Chen, Liu, Zhu, Xu, and Li (Citation2011) found that soybean oligosaccharides were able to enhance SOD activity by reducing oxidative stress in the liver of HF-fed rats. In the present study, HPOS increased liver SOD activity and lowered the content of MDA in mice fed a HF diet, in agreement with the previous work showing reduced MDA in serum (Li et al., Citation2010). These results further highlight the benefits of HPOS for maintaining the normal morphology and function, reducing the oxidative stress of liver during hyperlipidemia, and for improving abnormal lipid metabolic diseases such as fatty liver.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (31071521), Liaoning Engineering Research Center for Food Bioprocessing and Shenyang Key Laboratory of Food Bioprocessing and Quality Control (F11-235-1-00).
References
- Al-Dosari, M. S. (2011). Hypolipidemic and antioxidant activities of avocado fruit pulp on high cholesterol fed diet in rats. African Journal of Pharmacy and Pharmacology, 5, 1475–1483.
- Balkan, J., Oztezcan, S., Aykaç-Toker, G., & Uysal, M. (2002). Effects of added dietary taurine on erythrocyte lipids and oxidative stress in rabbits fed a high cholesterol diet. Bioscience Biotechnology and Biochemistry, 66, 2701–2705.
- Bayard, M., Holt, J., & Boroughs, E. (2006). Nonalcoholic fatty liver disease. American Family Physician, 73, 1961–1968.
- Chen, H., Liu, L., Zhu, J., Xu, B., & Li, R. (2011). Effect of soybean oligosaccharides on blood lipid, glucose levels and antioxidant enzymes activity in high fat rats. Food Chemistry, 119, 1633–1636.
- Du, L., Li, T., Wang, N., Guo, M., & Zhang, H. (2009). Antioxidant activity of haw pectin hydrolyzates. Food Research and Development, 30, 8–21.
- El-Nakeeb, M., & Yousef, R. T. (1970). Study of antibacterial activity of pectin. Planta Medica, 18, 201–209.
- Inoue, K., Mirvallev, R. S., & Makino, K. (2002). Simple method for removing lead ion using orange juice residue. Journal of the Japan Society of Waste Management Experts, 13, 216–222.
- Innami, S., & Kiriyama, S. (1982). Dietary Fiber, Tokyo: Dai-Ichi syuppan.
- Lattimer, J. M., & Haub, M. D. (2010). Effects of dietary fiber and its components on metabolic health. Nutrition, 2, 1266–1289.
- Lee, M. S., Kim, D., Jo, K., & Hwang, J. K. (2010). Nordihydroguaiaretic acid protects against high-fat diet-induced fatty liver by activating AMP-activated protein kinase in obese mice. Biochemical and Biophysical Research Communications, 401, 92–97.
- Li, T., Li, S., Du, L., Wang, N., Guo, M., Zhang,Zhang, H. (2010). Effects of haw pectic oligosaccharide on lipid metabolism and oxidative stress in experimental hyperlipidemia induced by a high-fat diet in mice. Food Chemistry, 121, 1010–1013.
- Li, T., Wang, N., Du, L., & Guo, M. (2008). Effects of plant acidic oligosaccharides on the blood lipid metabolism in high fat fed mice. Annual meeting of glycobioscience, China.
- Liu, C. H., Hung, M. T., & Huang, P. C. (1995). Source of triacylglycerol accumulation in lives of rats fed a cholesterol-supplemented diet. Lipids, 30, 527–531.
- Metcalfe, L. D., Schmitz, A. A., & Pelka, J. R. (1966). Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Analytical Chemistry, 38, 514–515.
- Mika, J. T., Puntmann, V., & Kaski, J. C. (2007). Atherosclerosis and oxidant stress: The end of the road for antioxidant vitamin treatment. Cardiovascular Drug Therapy, 21, 195–210.
- Sattler, W., Malle, E., & Kostner, G. M. (1998). Methodological approaches for assessing lipid and protein oxidation and modification in plasma and isolated lipoproteins. Methods in Molecular Biology, 110, 167–191.
- Strobel, N. A., Fassett, R. G., Marsh, S. A., & Coombes, J. S. (2011). Oxidative stress biomarkers as predictors of cardiovascular disease. International Journal of Cardiology, 147, 191–201.
- Terpstra, A. H. M., Lapre, J. A., Vries, H. T., & Beynen, A. C. (1998). Dietary pectin with high viscosity lowers plasma and liver cholesterol concentration and plasma cholesteryl ester transfer protein activity in hamsters. Journal of Nutrition, 128, 1944–1949.
- Tandy, S., Chung, R. W. S., Kamili, A., Wat, E., Weir, J. M., Meikle, P. J., & Cohn, J. S. (2010). Hydrogenated phosphatidylcholine supplementation reduces hepatic lipid levels in mice fed a high-fat diet. Atherosclerosis, 213, 142–147.
- Vial, G., Dubouchaud, H., Couturier. K., Cottet-Rousselle, C., Taleux, N., Athias, A., … Leverve, X. M. (2011). Effects of a high-fat diet on energy metabolism and ROS production in rat liver. Journal of Hepatology, 54, 348–356.
- Wang, N., Li, J., Jin, S., & Li, T. (2008). The conditions for the production of pectic oligosaccharides from haw pectin. Food Research and Development, 29, 1–4.
- Wang, N., Zhang, C., Qi, Y., & Li, T. (2007). Extraction and food chemical characterizations of haw pectins. Science and Technology of Food Industry, 11, 87–92.
- Wilson, S. H., Caplice, N. M., Simari, R. D., Holmes, J. D. R.Carlson, P. J., & Lerman, A. (2001). Activated nuclear factor Kappa B is present in the coronary vasculature in experimental hypercholesterol mice. Atherosclersis, 148, 23–30.
