Abstract
The bioavailability of major phenolics in Autumn Royal grape juice was measured in 16 subjects distributed into two groups: the experimental group (n = 8 receiving 300 mL of juice) and the placebo group (n = 8 receiving 300 mL of artificial beverage). Blood was obtained before and after 30, 90, 180, and 300 min after juice or placebo intake and 24-h urine was collected. At 30 min, catechin and gallic acid levels were 3.18 ± 0.06 and 0.33 ± 0.06 nmol/mL plasma (p < 0.01), respectively. The maximal plasma levels of catechin and gallic acid observed at 180 min were 7.11 ± 0.53 and 1.56 ± 0.07 nmol/mL, respectively. After 300 min, gallic acid was not detected and only two subjects exhibited measurable levels of catechin. After juice intake, urinary catechin and gallic acid contents were significantly higher than the basal values (p < 0.001). After a regular serving of grape juice, catechin and gallic acid are bioavailable and thus potentially capable of exerting their expected biological effects.
Se midió la biodisponibilidad de compuestos fenólicos en jugo de uvas Autumn Royal en dieciséis adultos en dos grupos (n = 8): experimental (recibió 300 mL de jugo), o placebo (recibió 300 mL bebida artificial). Se muestreó sangre antes y después de 30, 90, 180 y 300 min de la ingesta de jugo o placebo y se recolectó la orina por 24 h. A los 30 min, los niveles plasmáticos de catequina y ácido gálico fueron 3,18 ± 0,06 y 0,33 ± 0,06 nmol/mL (p < 0,01). Las concentraciones máximas de ambos compuestos fueron 7,11 ± 0,53 y 1,56 ± 0,07 nmol/mL (180 min). Luego de tomar el jugo, los niveles urinarios de catequina y ácido gálico fueron significativamente mayores que los basales (p < 0,001). Se concluye que luego de la ingesta de una porción regular de jugo de uvas, la catequina y el ácido gálico son biodisponibles y potencialmente capaces de ejercer sus efectos biológicos esperados.
Introduction
Grape juice (Vitis vinifera L) is considered a health-protecting beverage due to the presence of putatively bioactive phenolics, which provide a high antioxidant capacity (Anastasiadi, Pratsinis, Kletsas, Skaltsounis, & Haroutounian, Citation2010; Hogan, Zhang, Li, Zoecklein, & Zhou, Citation2009; Lutz, Cajas, & Henríquez, Citation2012; Lutz, Jorquera, Cancino, Ruby, & Henríquez, Citation2011). The intake of grape juice may contribute to reducing the risk of various diseases associated with oxidative stress (Vislocky & Fernández, Citation2010; Xia, Deng, Guo, & Li, Citation2010). Absorption of phenolics is an essential step to pass into the bloodstream and protect the body tissues against the oxidative damage. The rate and extent of intestinal absorption and the nature of the metabolites circulating in the plasma are determined by the chemical structure of phenolics, while their amounts found intact in urine vary among the ingested molecules. Although most phenolics are well characterized as powerful antioxidants in vitro, evidence indicates that they undergo significant metabolism and conjugation during absorption in the small intestine and in the colon, where the microflora degrade them to smaller phenolic acids, some of which may be absorbed (Selma, Espín, & Tomás Barberán, Citation2009).
In foods, most phenolics are usually glycosylated. After liberation of the glycosides, about 15% of the aglycones are absorbed with bile micelles into the epithelial cells and passed on to the lymph (Selma et al., Citation2009), and complex metabolic changes occur during absorption in the enterocytes (Petri et al., Citation2003). After hydrolysis, free aglycones are conjugated to facilitate their elimination from the body (Mullen, Edwards, & Crozier, Citation2006). It has been estimated that 90–95% of absorbed phenolics are converted to conjugates, primarily in the liver, while small amounts may be metabolized by the intestinal mucosa, and the liver further modifies the conjugates from the small intestine (Scalbert & Williamson, Citation2000). The absorbed products are secreted by organic acid transporters into the blood and subsequently excreted through the kidneys (Havsteen, Citation2002). However, a major part of the phenolics ingested (75–99%) is not found in urine due to the fact that they have not been absorbed through the gut barrier; they have been excreted in the bile or metabolized by the colonic microflora or the body tissues (Scalbert & Williamson, Citation2000).
Since substantial quantities of the ingested phenolics pass to the colon, affecting the microbiota, the level of urinary excretion also indicates that the colonic catabolites are absorbed into the portal vein and pass through the body into the circulatory system prior to excretion (Crozier, Del Rio, & Clifford, Citation2010). Consequently, the profile of urine metabolites may be markedly different to that of plasma (Mountzouris, McCartney, & Gibson, Citation2007; Mullen et al., Citation2006).
Studies on the bioavailability and urinary excretion of grape juice phenolics are scarce (Stalmach, Edwards, Wightman, & Crozier, Citation2011). A variety of table grapes was analyzed previously in order to select those that exhibit the highest antioxidant capacity (Lutz et al., Citation2011), and Autumn Royal red grape juice constitutes a suitable vehicle to deliver these putative healthy compounds.
The aim of this study is to describe the bioavailability and urinary excretion of major grape phenolics in Autumn Royal grape juice in healthy subjects in a postprandial test after the intake of a regular portion of this beverage.
Materials and methods
Plant material and preparation of grape juice
Ripe red grapes cv. Autumn Royal were harvested in San Felipe (32°45' S; 70°43' W), Chile, in summer 2010. They were placed in polyethylene bags and transported at 4°C to the CIDAF, Universidad de Valparaíso, immediately packed in 1 kg polyethylene bags and stored in a freezer (–20°C) until used. Damaged and poor-quality grapes were eliminated. The fruits were washed with tap water at room temperature and stems were manually removed. To obtain the juice the berries were crushed with a kitchen juice extractor, separating the skins. Juice was collected, measured, and homogenized by manual stirring. It was stored in glass bottles (300 mL), sealed, frozen, and kept at –20°C until used.
Subjects and study design
Sixteen volunteers (seven males and nine females), who were healthy, nonvegetarian, nonsmokers, not on any medication, were selected for the study. Obesity, pregnancy, lactation, or any condition that would impair compliance was excluded. Subjects who were asked to participate in the study were thoroughly informed about it and all gave their written consent. The subjects were aged between 20 and 37 years (26.1 ± 1.2), with body mass index (BMI) ranging from 20.1 to 28.6 kg/m2. The study protocol was approved by the Ethical Committee of the Faculty of Medicine, Universidad de Valparaíso. The study was an open, single-center study performed under controlled conditions at the CIDAF.
Subjects were randomly distributed into two groups: the experimental group (n = 8) received 300 mL of 100% grape juice and the placebo group (n = 8) received 300 mL of an artificial grape flavor beverage after an overnight fast. Both groups were submitted to the same experimental conditions. Subjects were instructed to follow a low phenolic diet avoiding some foods and beverages (various vegetables, fruits, legumes, whole cereals, fruit juice, wine, tea, cocoa drink, coffee, and beer) for 2 d prior to the assay. To aid adherence to the dietary guidelines, the restricted items were listed in a brochure. Subjects were given 300 mL of grape juice or placebo daily for 3 days before the study.
Blood and urine collection
Five milliliters of venous blood was collected in heparinized tubes (Vacutainer®, BD Vacutainer Systems, NJ, USA), immediately before baseline (t = 0) and after 30, 90, 180, and 300 min after the intake of 300 mL of grape juice or placebo between 9.00 and 9.30 am. Plasma was separated by centrifugation (4000g × 10 min at 4°C) (Medifuge Heraeus Sepatech, Berlin, Germany) and stored in amber vials at –20°C until analysis. The volunteers were asked to fast since dinner on the previous day. Basal urine samples were collected before the intake of the juice (t = 0) and individual urine was collected in plastic bottles over the next 24 h. The volume of each 24-h urine sample was measured, the samples were acidified to pH 4 with hydrochloric acid, and aliquots were stored frozen (−20°C) until analysis. During the sampling day, the volunteers remained at the facilities of the metabolic ward at the CIDAF, where they received a controlled diet throughout the day.
Phenolics assessment
All reagents and solvents were of analytical grade: methanol (high performance liquid chromatography (HPLC) grade), acetronitrile (HPLC grade), and orthophosphoric acid 85% were purchased from Merck, Darmstadt, Germany; β-glucuronidase (Helix pomatia type HP-2S), naringenin, gallic acid, catechin, and malvidin were purchased from Sigma, Chemical Co., St. Louis, MO, USA.
Grape juice: The phenolics composition was determined using the protocol reported by Frank, Netzel, Strass, Bitsch, and Bitsch (Citation2003). Grape juice was centrifuged at 2000g for 20 min. Then, 5 mL of supernatant was mixed with 5 mL methanol (HPLC grade) and filtered (Millipore teflon, 13 and 47 mm diameter, 0.2 μm pore size) before HPLC analysis.
Plasma: Phenolics in plasma were extracted using the protocol described by Bell et al. (Citation2000) with slight modifications. Duplicate plasma samples were analyzed. Briefly, to a 1 mL aliquot of plasma was added 100 μL 0.2 M phosphate buffer, pH 5 and 20 μL β-glucuronidase (Helix pomatia type HP-2S, Sigma). The mix was incubated for 45 min at 37°C and the enzyme was inactivated with 1 mL methanol (grade HPLC) and then centrifuged at 1500g at 4°C for 15 min. A solid-phase extraction column (Sep Pak C-18, Waters, Milford, MA, USA) pretreated with 10 mL methanol and 10 mL 1% H3PO4 in water was used to recover the phenolics, as reported by Bub, Watzl, Heeb, Rechkemmer, and Briviba (Citation2001) and Martínez-Ortega, García-Parrilla, and Troncoso (Citation2004). The C-18 nonbinding compounds were removed with 5 mL water containing 1% H3PO4, and bound phenolics were eluted with 6 mL 1% H3PO4 in methanol. The samples were dried under nitrogen, reconstituted in 1 mL methanol/water (80/20), and filtered through a 0.22 mm teflon membrane (polytetrafluoroethylene (PTFE), Millipore, Bedford, MA, USA) for HPLC analysis.
Urine: Phenolics in urine were extracted using the protocol described by Bitsch, Netzel, Frank, Strass, and Bitsch (Citation2004) and Ito et al. (Citation2005) with slight modifications. The acidified defrosted urine was centrifuged at 4500g at 4°C for 15 min. Duplicate urine samples were analyzed. Briefly, 1 mL of the supernatant was acidified with phosphate monobasic to pH 5 and incubated for 2 h at 37°C with 100 μL β-glucuronidase. The reaction was inactivated with 100 μL methanol. The sample was added with naringenin as internal standard (23 mg/L) and submitted to a solid-phase extraction, using a preconditioned column with 6 mL methanol and 6 mL acetate buffer, pH 4. The mix was washed with 9 mL acetate buffer and 1 mL methanol. Phenolics were eluted with 4 mL methanol. The combined organic layers were evaporated under nitrogen at 35°C, redissolved in 0.5 mL methanol, filtered through a 0.22 mm teflon membrane (PTFE, Millipore), and injected into the HPLC system.
Gallic acid, catechin, and malvidin were measured using a Merck-Hitachi LaChrom HPLC (Merck-Hitachi, Frankfurt–Tokyo, Germany–Japan) equipped with a quaternary pump programmable for gradients, thermostatically controlled column chamber, rheodyne injection valve (10 µL sample loop), and UV–visible detector. The detection of the phenolic compounds was realized at 280 nm for catechin, gallic acid, and naringenin and at 540 nm for malvidin. The column employed was LiChrospher C-18 (250 × 4.0 mm, 5 µm) fitted with a LiChrospher 100 RP-18 guard column. The mobile phase was a mixture of (A): 0.085% orthophosphoric acid dissolved in ultrapure water and (B): acetonitrile (injection volume: 20 μL at 30°C). The initial flow of the mobile phase was 0.5 mL/min during 35 min, then increased to 1 mL/min during 25 min to avoid the overlapping of peaks. Solvents gradient: 0–10 min, 100% A; 10–20 min, 90% A + 10% B; 20–30 min, 85% A + 15% B; 30–35 min, 65% A + 35% B; 35.0–35.1 min, 52% A + 48% B; 35.1–50 min, 52% A + 48% B; 50–55 min, 35% A + 65% B; 55–60 min, 85% A + 15% B; and 60–360 min, 100% A.
Urinary excretion of each phenolic species was obtained considering the amount excreted in the 24-h urine and the total volume collected in this period.
Statistical analysis
Data are expressed as individual values and mean ± SEM (n = 8). Analysis of variance was used to test for any significant difference among the baseline and the following time points (Meier & Zünd, Citation2000). Data were processed by Tukey–Kramer test. Statistical analyses were performed using SAS® software (SAS Institute Inc., Cary, NC, USA). Differences at p < 0.05 were considered significant.
Results and discussion
Characterization of grape juice
Crupi et al. (Citation2011, Citation2012) reported in a series of grape varieties that Autumn Royal is one of the red varieties that exhibits the highest total flavonoids content. In previous studies, Lutz et al. (Citation2011) and Lutz et al. (Citation2012) determined that the predominant phenolic species in Chilean red ground grapes, juice, and skin fractions was catechin.
The 300 mL juice portion contained 288.8 µg catechin (962.7 µg/L), 180 µg malvidin (600 µg/L), and 111.7 µg gallic acid (372.3 µg/L), while the placebo beverage did not contain any phenolics. These compounds represent 0.17%, 0.11%, and 0.07% of the total area determined by the HPLC profile, considering the three standard molecules used, since other derivatives of these compounds as well as a wide variety of other phenolics in the food matrix (Crupi et al., Citation2011) were not identified. Décordé, Teissèdre, Auger, Cristol, and Rouanet (Citation2008) also reported a high content of catechin in purple grape juice, and phenolic acids are reported as the major antioxidants found in grapes, with gallic acid prevailing over other compounds (Hogan et al., Citation2009; Yilmaz & Toledo, Citation2004).
Baseline characteristics of the subjects
Placebo and experimental groups were similar in age (22.7 ± 0.7 vs. 24.4 ± 0.7 years), sex (M/F 4/4 vs. 5/3), and BMI (24.5 ± 1.5 vs. 27.6 ± 1.8 kg/m2). According to the 3-day food records, the baseline diet was similar in both groups.
Phenolics in plasma
The bioavailability of phenolics differs, and there is no relationship between their amount in food and their bioavailability (Pandey & Rizvi, Citation2009). Plasma levels of dietary phenolics are dependent on their chemical structure, amount ingested, and food matrix, since all influence the rate and extent of absorption, determine the conjugation reactions, and affect the activity of digestive enzymes and colonic bacterial fermentation (Del Rio, Costa, Lean, & Crozier, Citation2010; Hollman, Citation2004). Moreover, they are also affected by the genetic and physiological conditions of the consumer (D’Archivio, Filesi, Vari, Scazzocchio, & Masella, Citation2010). Nevertheless, the presence of native phenolics in plasma and urine is a good indicator of their potential ability to exert biological actions.
We chose to analyze three major phenolics of grape juice which are likely to be absorbed as such after grape juice consumption, not considering a variety of phenolics which are in very small amounts in grapes and/or appear to have poor bioavailability due to their instability, large molecular weight, or fast excretion. The chosen phenolics were not detected in the subjects receiving placebo and confirmed their adhesion to diet prior to the study. The lack of detection of phenolics in fasting plasma of all subjects is in agreement with the fact that these molecules have short half-lives, and their concentrations may therefore not be detectable in this condition (Koli et al., Citation2010).
Since the major phenolics in grapes are in their free form and as glucuronides, we used β-glucuronidase prior to their determination by HPLC of these compounds in plasma. Although the total amount is underestimated, it gives a good approach to the actual amount of phenolics that are bioavailable. shows the plasma concentration of the phenolics detected in the subjects after drinking grape juice. At 30 min, catechin and gallic acid levels were 3.18 ± 0.06 and 0.33 ± 0.06 nmol/mL, respectively. In accordance with similar studies, our results show a high interindividual variation, which is partly due to biochemical factors such as differences in the expression or activity of enzymes involved in phenolics metabolism (Garcia-Alonso, Minihane, Rimbach, Rivas-Gonzalo, & de Pascual-Teresa, Citation2009) as well as their individual previous diet (Walsh et al., Citation2007). Changes in plasma concentrations reflect the effects of intestinal absorption, tissue metabolism, gut flora metabolism, and excretion of ingested catechin and gallic acid. Malvidin was not detected, which may be attributed to the fact that anthocyanins are absorbed faster than other phenolics and their bioavailability is very low (Hollman, Citation2004). Malvidin-3-glucoside has been measured in plasma after the intake of grape juice or red wine, while the aglycone has not been detected (Bitsch et al., Citation2004; Bub et al., Citation2001; Frank et al., Citation2003). In a recent study, Stalmach et al. (Citation2011) did not detect any phase II metabolites of procyanidins in plasma or urine using electrospray ionization-mass spectrometry (ESI-MS). The low bioavailability of anthocyanins has also been attributed to the low stability of these molecules in the mild alkaline condition of the small intestine (Pérez-Vicente, Gil-Izquierdo, & García-Viguera, Citation2002).
Figure 1. Plasma (A) gallic acid and (B) catechin after grape juice intake. The values are expressed as mean of three replications ± SE (n = 8).
Niveles plasmáticos de (A) Ácido gálico y (B) Catequina luego de la ingestión de jugo de uvas. Los valores se expresan como promedio de tres replicas ± error estándar (n = 8).
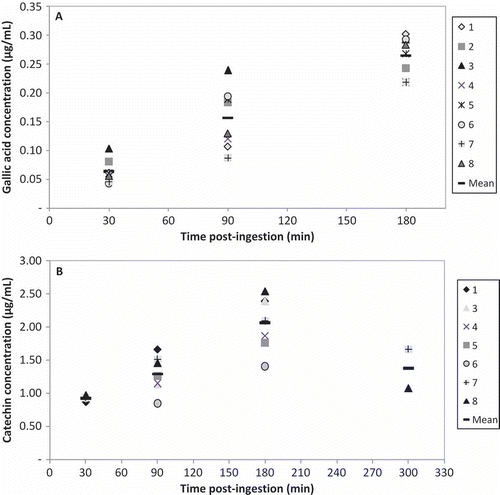
The time–response relationship between phenolics intake and their appearance in plasma is also dependent on the elimination rate of the compound, whether in the native form or metabolites (Koli et al., Citation2010). Plasma levels of catechin and gallic acid reached maximal values of 7.11 ± 0.53 and 1.56 ± 0.07 nmol/mL, respectively, 180 min after grape juice intake. These concentrations decreased later, and only two subjects exhibited measurable levels of catechin 300 min after juice intake. The percentage recovery for gallic acid ranged from 0.06% (30 min) to 0.24% (180 min), while the recovery for catechin ranged from 0.32% (30 min) to 0.71% (180 min). These low values reflect that these compounds are extensively metabolized, and metabolites appearing in plasma or excreted in urine can be very different from the native ingested (Stalmach et al., Citation2011). (+)-Catechin appears to be metabolized only if absorbed from the small intestinal lumen. The first step of conjugation is glucuronidation, and after these metabolites enter the blood circulation, methylation and sulfation are produced in the liver (Donovan et al., Citation2001). The use of sulfatase to remove sulfated moiety from catechin metabolites would probably increase the amount of catechin recovered. Donovan et al. (Citation1999) reported that catechin in red wine is highly metabolized, and after 1 h the level of free catechin was less than 2% of the unmethylated metabolites, while at 3–4 h no free catechin was detected. In addition to the metabolism that occurs in intestinal cells and liver, catechin can also be metabolized by gut microflora to produce phenolic acids (Kim et al., Citation1998). Gallic acid may be metabolized and transported or may experiment bacterial metabolism in the gut (Forester & Waterhouse, Citation2009). At 300 min, gallic acid level was below the detection limit. The plasma recoveries of catechin were 3.0 to 5.7-fold higher than the recoveries of gallic acid (p < 0.01). In addition, the maximal levels of catechin were 7.8 to 14.6-fold higher than those observed for gallic acid (p < 0.01).
Phenolics in urine
Extensive bacterial metabolism of phenolic acids occurs in the gut (Gonthier et al., Citation2006), and highly conjugated metabolites are more likely to be eliminated in bile, whereas small conjugates are preferentially excreted in urine. The urinary excretion of phenolics reveals that colonic metabolites pass through the circulatory system prior to excretion (Larrosa et al., Citation2009). As phenolics are mostly excreted during 24 h following their ingestion, the excretion in 24-h urine should be directly related to the amounts ingested during the sampling and the previous day (Koli et al., Citation2010).
Basal urine volumes (t = 0) were similar in experimental and control groups: 254 ± 20 and 309 ± 35 mL, respectively; and the 24-h total volumes were also similar: 1401 ± 99 and 1600 ± 175 mL, respectively (p > 0.05). The two phenolics species determined in 24-h urine samples were catechin and gallic acid. Similar results were reported by Bub et al. (Citation2001) after the ingestion of red wine, dealcoholized red wine, and red grape juice. These authors indicate that several factors could contribute to the low bioavailability of anthocyanins, such as the fecal excretion, decay at neutral pH in the intestine, metabolism by gut microflora, rapid accumulation in different tissues, or metabolism resulting in ring fission. In addition, the low plasma and urinary concentrations suggest that there is a combination of low absorption of anthocyanins with a rapid metabolism (Murkovic, Mülleder, Adam, & Pfannhauser, Citation2001). Additionally, the high sugar content of grape juice may affect the intestinal absorption of these compounds, thus reducing the bioavailability of anthocyanins (Frank et al., Citation2003).
shows the amounts of catechin (A) and gallic acid (B) excreted in 24-h urine by each subject after the intake of juice. The data exhibit a high interindividual variation: some exhibit very high excretion rates (subjects 3 and 8 for catechin and subjects 2 and 6 for gallic acid). Almost in all the subjects, the baseline values were lower than the 24-h urine values except for catechin in subjects 6 and 7 and gallic acid in subject 3. Urine samples contained total amounts of catechin and gallic acid of 5.6 ± 2.4 and 2.5 ± 0.7 µg, respectively. Considering the 24-h urine volume of each volunteer, total catechin excreted was 21.9 ± 8.1 nmol and total gallic acid excreted was 21.1 ± 6.9 nmol. Thus, the relative urinary excretions of catechin and gallic acid were below 2.2% and 3.2%, respectively (as percentage of the amounts of these phenolics in 300 mL juice) and may be considered as an estimation of the absorption efficiency.
Figure 2. Basal and 24-h urine content of (A) gallic acid and (B) catechin after grape juice intake. The values are expressed as mean of three replications ± SE (n = 8).
Contenido urinario basal y de 24 h de (A) ácido gálico y (B) catequina luego de la ingestión de jugo de uvas. Los valores se expresan como promedio de tres replicas ± error estándar (n = 8)
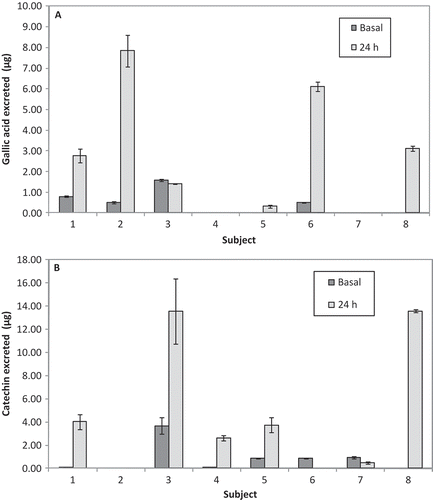
These results indicate that gallic acid was absorbed better and/or metabolized to a lesser extent than catechin. Donovan, Kasim-Karakas, German, and Waterhouse (Citation2002) determined that 3.0–10.3% of catechins in red wine are accounted for in urine over an 8-h period. According to these authors, the amount of free, native catechin is less than 0.3% of total catechin metabolites. In our study, the 24-h urinary catechin concentrations after the intake of grape juice were 3.7 to 35.5-fold higher than the basal values, while gallic acid concentrations were 3.5 to 15.5-fold higher than the basal values.
Although we did not measure biomarkers of antioxidant status in vivo, our plasma and urinary results are in agreement with the low maximal concentration values reported, which may be insufficient to exert significant direct systemic antioxidant effects (Halliwell, Citation2007). However, other forms of indirect beneficial effects of phenolics have been described; thus, the presence of these compounds in plasma and urine indicates that they may act as health-promoting bioactives in the organism by contributing to disease prevention through various mechanisms, including the modulation of gene expression (induction of the synthesis of antioxidant enzyme systems such as glutathione peroxidase, superoxide dismutase, and gluthation reductase; signaling pathways that participate in cell proliferation and differentiation; cell cycle detention and apoptosis; and/or the activity of enzymes that modulate xenobiotics), as well as the inhibition of lipid peroxidation by recycling other antioxidants, chelation of active metal ions preventing free radical formation, among other actions (Dragsted et al., Citation2004; Lampe Citation1999; Stevenson and Hurst Citation2007).
The results reveal that although catechin and gallic acid are poorly absorbed and extensively metabolized, they still are bioavailable as such and might contribute to the putative health benefits of grape juice intake. Consequently, a suitable natural alternative to increase the dietary intake of bioactives is red grape juice.
Conclusions
These results contribute to explain some putative health benefits of red grape juice consumption. After the intake of a regular portion of juice, major antioxidant phenolics (catechin and gallic acid) are bioavailable and thus capable of exerting their biological effects. The very low plasma and urinary levels observed indicate a rapid metabolism and clearance of the absorbed compounds, although they may be able to contribute to the whole antioxidant status of the body and exert other relevant biological effects.
Acknowledgment
This work was supported by CONICYT, Project FONDEF D07I1136, and CIDAF (CID 04/06), Universidad de Valparaíso.
References
- Anastasiadi, M., Pratsinis, H., Kletsas, D., Skaltsounis, A., & Haroutounian, S. (2010). Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Research International, 43, 805–813.
- Bell, J., Donovan, J., Wong, R., Waterhouse, A., German, B., Walzem, R., & Kasim-Karakas, S. (2000). (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. American Journal of Clinical Nutrition, 71, 103–108.
- Bitsch, R., Netzel, M., Frank, T., Strass, G., & Bitsch, I. (2004). Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. Journal of Biomedicine and Biotechnology, 5, 293–298.
- Bub, A., Watzl, B., Heeb, D., Rechkemmer, G., & Briviba, K. (2001). Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine, and red grape juice. European Journal of Nutrition, 40, 113–120.
- Crozier, A., Del Rio, D., & Clifford, M.N. (2010). Bioavailability of dietary flavonoids and phenolic compounds. Molecular Aspects of Medicine, 31, 446–467.
- Crupi, P., Coletta A., Milella R. A., Perniola R., Gasparro M., Genghi R., & Antonacci D. (2012). HPLC-DAD-ESI-MS analysis of flavonoid compounds in 5 seedless table grapes grown in Apulian region. Journal of Food Sciences, 77, C174–C181.
- Crupi, P., Milella, R. A., Perniola, R., Genghi, R., Coletta, M., Angela Giannandrea, A., ...Antonacci, D. (2011). The intake of unpeeled table grapes is important in dietary habits as a source of antioxidant and anticarcinogenic polyphenols. Bulletin de l'O.I.V, 84, 37–52.
- D’Archivio, M., Filesi, C., Vari, R., Scazzocchio, B., & Masella, R. (2010). Bioavailability of the polyphenols: Status and controversies. International Journal of Molecular Sciences, 11, 1321–1342.
- Décordé, K., Teissèdre, P. L., Auger, C., Cristol, J. P., & Rouanet, J. M. (2008). Phenolics from purple grape, apple, purple grape juice and apple juice prevent early atherosclerosis induced by an atherogenic diet in hamsters. Molecular Nutrition and Food Research, 52, 400–407.
- Del Rio, D., Costa, L. G., Lean, M. E. J., & Crozier, A. (2010). Polyphenols and health: What compounds are involved? Nutrition, Metabolism and Cardiovascular Diseases, 20, 1–6.
- Donovan, J. L., Bell, J. R., Kasim-Karakas, S., German, J. B., Walzem, R. L., Hansen, R. J., & Waterhouse, A. L. (1999). Catechin is present as metabolites in human plasma after consumption of red wine. Journal of Nutrition, 129, 1662–1668.
- Donovan, J. L., Crespy, V., Manach, C., Morand, C., Besson, C., Scalbert, A., & Remesy, C. (2001). Catechin is metabolized by both the small intestine and liver of rats. Journal of Nutrition, 131, 1753–1757.
- Donovan, J. L., Kasim-Karakas, S., German, J. B., & Waterhouse, A. L. (2002). Urinary excretion of catechin metabolites by human subjects after red wine consumption. British Journal of Nutrition, 87, 31–37.
- Dragsted, L., Pedersen, A., Hermetter, A., Basu, S., Hansen, M., Haren, G., … Sandström, B. (2004). The 6-a-day study: Effects of fruit and vegetables on markers of oxidative stress and antioxidative defense in healthy nonsmokers. American Journal of Clinical Nutrition, 79, 1060–1072.
- Forester, S., & Waterhouse, A. (2009). Metabolites are key to understanding health effects of wine polyphenolics. Journal of Nutrition, 139, 1824S–1831S.
- Frank, T., Netzel, M., Strass, G., Bitsch, R., & Bitsch, I. (2003). Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Canadian Journal of Physiology and Pharmacology, 81, 423–435.
- Garcia-Alonso, M., Minihane, A. M., Rimbach, G., Rivas-Gonzalo, J. C., & de Pascual-Teresa, S. (2009). Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. Journal of Nutritional Biochemistry, 20, 521–529.
- Gonthier, M. P., Remesy, C., Scalbert, A., Cheynier, V., Souquet, J. M., Poutanen, K., & Aura, A. M. (2006). Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human fecal microbiota in vitro. Biomedical Pharmacotherapy, 60, 536–540.
- Halliwell, B. (2007). Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovascular Research, 73, 341–347.
- Havsteen, B. H. (2002). The biochemistry and medical significance of the flavonoids. Pharmacology and Therapeutics, 96, 67–202.
- Hogan, S., Zhang, L., Li, J., Zoecklein, B., & Zhou, Z. (2009). Antioxidant properties and bioactive components of Norton (Vitis aestivalis) and Cabernet Franc (Vitis vinifera) wine grapes. LWT - Food Science and Technology, 42, 1269–1274.
- Hollman, P. C. (2004). Absorption, bioavailability, and metabolism of flavonoids. Pharmaceutical Biology, 42, 74–83.
- Ito, H., Gonthier, M. P., Manach, C., Morand, C., Mennen, L., Rémésy, C., & Scalbert, A. (2005). Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. British Journal of Nutrition, 94, 500–509.
- Kim, D. H., Jung, E. A., Sohng, I. S., Han, J. A., Kim, T. H., & Han, M. J. (1998). Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Archives of Pharmaceutical Research, 21, 17–23.
- Koli, R., Erlund, I., Jula, A., Marniemi, J., Mattila, P., & Alfthan, G. (2010). Bioavailability of various polyphenols from a diet containing moderate amounts of berries. Journal of Agriculture and Food Chemistry, 58, 3927–3932.
- Lampe, J. (1999). Health effects of vegetables and fruits: Assessing mechanisms of action in human experimental studies. American Journal of Clinical Nutrition, 70, 475S–490S.
- Larrosa, M., Luceri, C., Vivoli, E., Pagliuca, C., Lodovici, M., Moneti, G., & Dolara, P. (2009). Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Molecular Nutrition Food Research, 53, 1044–1054.
- Lutz, M., Cajas, Y., & Henríquez, C. (2012). Phenolics content and antioxidant capacity of Chilean grapes cv. País and Cabernet Sauvignon. CyTA Journal of Food, 10, 251–257.
- Lutz, M., Jorquera, K., Cancino, B., Ruby, R., & Henríquez, C. (2011). Phenolics and antioxidant capacity of table grape (Vitis vinifera L.) cultivars grown in Chile. Journal of Food Science, 76, C1088–C1093.
- Martínez-Ortega, M. V., García-Parrilla, M. C., & Troncoso, A. M. (2004). Comparison of different sample preparation treatments for the analysis of wine phenolic compounds in human plasma by reversed phase high-performance liquid chromatography. Analytica Chimica Acta, 502, 49–55.
- Meier, P. C., & Zünd, R. E. 2000. Statistical methods in analytical chemistry (2nd ed., pp. 115–118). New York: John Wiley & Sons.
- Mountzouris, K. C., McCartney, A. L., & Gibson, G. R. (2007). Intestinal microflora of human infants and current trends for its nutritional modulation. British Journal of Nutrition, 87, 405–420.
- Mullen, W., Edwards, C. A., & Crozier, A. (2006). Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. British Journal of Nutrition, 96, 107–116.
- Murkovic, M., Mülleder, U., Adam, U., & Pfannhauser, W. (2001). Detection of anthocyanins from elderberry juice in human urine. Journal of the Science of Food and Agriculture, 81, 934–937.
- Pandey, K. B., & Rizvi, S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity, 2, 270–278.
- Pérez-Vicente, A., Gil-Izquierdo, A., & García-Viguera, C. (2002). In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. Journal of Agriculture and Food Chemistry, 50, 2308–2312.
- Petri, N., Tannergren, C., Holst, B., Mellon, F., Bao, Y., Plumb, G.,… Williamson, G. (2003). Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metabolism and Disposition, 31, 805–813.
- Scalbert, A., & Williamson, G. (2000). Dietary intake and bioavailability of polyphenols. Journal of Nutrition, 130, 2073S–2085S.
- Selma, M. V., Espín, J. C., & Tomás Barberán, F. A. (2009). Interaction between phenolics and gut microbiota: Role in human health. Journal of Agriculture and Food Chemistry, 57, 6485–6491.
- Stalmach, A., Edwards, C. A., Wightman, J. D., & Crozier, A. (2011). Identification of (Poly)phenolic compounds in Concord grape juice and their metabolites in human plasma and urine after juice consumption. Journal of Agriculture and Food Chemistry, 59, 9512–9522.
- Stevenson, D., & Hurst, R. 2007. Polyphenolic phytochemicals - Just antioxidants or much more? Cellular and Molecular Life Sciences, 64, 2900–2916.
- Vislocky, L. M., & Fernández, M. L. (2010). Biomedical effects of grape products. Nutrition Reviews, 68, 656–670.
- Walsh, M. C., Brennan, L., Pujos-Guillot, E., Sébédio, J. L., Scalbert, A., Fagan, A.,… Gibney, M. J. (2007). Influence of acute phytochemical intake on human urinary metabolomic profiles. American Journal of Clinical Nutrition, 86, 1687–1693.
- Xia, E., Deng, G. F., Guo, Y. J., & Li, H. B. (2010). Biological activities of polyphenols from grapes. International Journal of Molecular Sciences, 11, 622–646.
- Yilmaz, Y., & Toledo, R. T. (2004). Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. Journal of Agriculture and Food Chemistry, 52, 255–260.
