Abstract
The present study was conducted with the objective of developing efficient method of extraction of nutraceutical compounds from protocorms of Dendrobium candidum for the food industry. Conventional heat reflux method was compared with ultrasonic-assisted method for extraction of polysaccharides, polyphenolics and flavonoids. Methanol, ethanol, acetone, acetonitrile and water were used as solvents for heat reflux extraction. Parameters tested for the optimization were methanol concentration (20, 40, 60, 80 and 100%) and duration of treatment (30 min, 1, 2, 4, 6 h). The treatment of samples with 80% methanol for 2-h period proved to be most suitable procedure for extraction of bioactive compounds. Heat reflux method was found superior to ultrasonic-assisted method since it yields higher amounts of bioactive compounds (463.0 mg g−1 DW polysaccharides, 10.75 mg g−1 DW polyphenolics and 5.30 mg g−1 DW flavonoids) and extracts obtained from heat reflux method also showed optimal antioxidant activity.
El presente estudio se realizó con el objetivo de desarrollar un método que permitiera la extracción eficiente del protocormo de Dendrobium candidum para la industria alimenticia. Para ello, se comparó el método convencional de reflujo de calor con la del método ultrasónico para la extracción de polisacáridos, de polifenólicos y de flavonoides. Para fines de la extracción por medio del reflujo de calor, se utilizaron como solventes metanol, etanol, acetona, acetonitrilo y agua. A su vez, con el objetivo de determinar la optimización, los parámetros examinados fueron la concentración de metanol (20, 40, 60, 80 y 100%) y la duración del tratamiento (30 min, 1, 2, 4 y 6 horas). Se constató que el procedimiento más adecuado para la extracción de compuestos bioactivos resultó ser el uso de metanol al 80% durante 2 horas. El método de reflujo de calor fue superior al método ultrasónico, ya que permite obtener mayores cantidades de compuestos bioactivos (463,0 mg g−1 dw de polisacáridos, 10,75 mg g−1 dw de polifenólicos, y 5,30 mg g−1 dw de flavonoides). Los extractos obtenidos empleando el método de reflujo de calor también mostraron una óptima actividad antioxidante.
Introduction
Plants produce diverse group of secondary metabolites which are used as food additives, nutraceuticals and antioxidant agents (Bourgaud, Gravot, Milesi, & Gontier, Citation2001) and hence, it is of interest to extract secondary metabolites from plants. However, plants contain a wide variety of chemical constituents and the abundance of the target secondary metabolites is usually low, which present a great challenge in the recovery and purification of secondary metabolites (Zhang, Yang, & Wang, Citation2011). Extraction technologies must be versatile, relatively simple, safe and inexpensive for the use. Soxhlet extraction, maceration and hydro-distillation methods have been followed for efficient extraction bioactive compounds from plant material. Nonconventional techniques such as ultrasound-assisted extraction, enzyme-assisted extraction, microwave-assisted extractions are also followed (Azmir et al., Citation2013).
Dendrobium candidum Wall ex Lindl. is a valuable medicinal plant in Chinese traditional medicine and it is one of the several Dendrobium species which were specified in the Chinese Pharmacopeia (Bao, Shun, & Chen, Citation2001). In China, more than 50 Dendrobium-based health food products have been approved by the State Food and Drug Administration. Besides phenols, alkaloids, coumarins, terpenes and flavonoids, polysaccharides are also considered one of the main active ingredients in Dendrobium plants. Polysaccharides obtained from D. candidum have shown obvious immunomodulatory, hepatoprotective and antioxidant activities (Ng et al., Citation2012). Natural populations of D. candidum have been depleted due to overexploitation and increasing demand for medicinal and health products. In the recent years plant organ cultures have become an alternative to whole plant for the production of valuable bioactive compounds. Protocorms were induced from D. candidum Wall ex Lindl. and have been cultivated in bioreactors for the production of biomass in large scale which can meet out the demand of the pharmaceutical, nutraceutical and herbal industry (Cui et al., Citation2014). However, extraction methodology of Dendrobium raw material is not available currently. Therefore, we have conducted experiments and evaluated heat reflux methodology for recovery of polysaccharide, polyphenolics and flavonoids from the protocorms of D. candidum. This paper describes the development of simple, convenient and rapid method of extraction by using varied solvents. The conventional solvent or heat reflux extraction method was compared with sonication-assisted extraction method and various experimental factors have been investigated with respect to the effect on extraction efficiency.
Materials and methods
Protocorm samples and extraction solvents
Protocorms of D. candidum were cultivated in suspension cultures (10-L capacity airlift bioreactors). Dried and powdered protocorms were used for extraction of bioactive compounds and analyzed the content of total polysaccharides, polyphenolics and flavonoids and free radical scavenging activity on 2, 2-diphenyl-1-picrylhdrzyl (DPPH) in the extract. The acetonitrile, ethanol, methanol and acetone were high pressure liquid chromatography (HPLC) grade (Fisher Scientific, Pittsburgh, USA). Water was purified by Millipore Q system (Millipore, Bedford, MA, USA). All solutions were filtered through a 0.45-µm hydrophilic polypropylene membrane before use.
Heat reflux extraction
Extraction was carried out in 100 mL round bottom flask fitted with a cooling condenser. To find out the suitability of material 0.5 g of dried, powered protocorm samples were put in round bottom flasks and extraction was carried at 80 ± 1°C for 1 h by using 50 mL of 80% methanol. In another set of experiments, samples (0.5 g powdered sample) were extracted according to the procedure mentioned above by using 50 mL of distilled water, absolute acetonitrile, ethanol, methanol and acetone to find out the suitability of solvent for extraction of bioactive compounds. In one more experiment, the samples (0.5-g powdered sample) were extracted as above by using 20, 40, 60, 80 and 100% methanol and ethanol to find out the suitability of concentration of solvents for extraction of bioactive compounds. In yet another experiment the samples (0.5-g powdered sample) were extracted as above by using 50 mL of 80% methanol and extraction was carried at 80°C for 0.5, 1, 2, 4, and 6 h to select out suitable duration of solvent treatment for extraction. In all the above experiments, when extraction was complete, the cooled extract was filtered on double layers of Whatmann no. 1 filter paper. The residue was taken back and re-extracted one more time using fresh solvent with the same conditions mentioned as above. The condenser was washed with 20 mL solvent. The washings were added to the extract, and then the flask was filled up to volume. The combined extract (100 mL) was used for the determination of total yield of polysaccharides, coumarins, polyphenolics and flavonoids.
Ultrasonic-assisted extraction
A 0.5 g powdered protocorm sample was mixed with 50 mL of the extraction solvent (80% methanol) in a 250 mL reagent bottle. The bottle was then closed and placed in the sonic bath with temperature maintained at 80°C for all extraction time for 30 min, 1, 2, 4 and 6 h by using ultrasonicator (40 kHz, heat power 150 W; Bransonic, MTH 1510, Sigma-Aldrich, Seoul, South Korea). Afterward, the protocorm extract was filtered using Whatmann no. 1 filter paper before analysis of bioactive compounds.
Determination of polysaccharide content
A sample of 0.2 mL extract was taken and mixed with 6 mL of concentrated sulphuric acid, held in a boiling water bath for 20 min and cooled. Then, 0.2 mL of carazole–absolute ethanol (0–15%, v/v) was added and the contents mixed vigorously. After a reaction time of 2 h in darkness at room temperature, a purplish red color developed and absorbance was measured at 530 nm. β-Galacturonic acid (0, 50, 100, 200, 400 and 600 mg mL−1) was used as standard (Cui et al., Citation2011).
Determination of total polyphenolic content
The content of total polyphenolics in protocorm extracts was analyzed spectrophotometrically by a modification of Folin–Ciocalteu colorimetric method (Kim, Murthy, Hahn, Lee, & Paek, Citation2007). One hundred micro-liter of extracts were mixed with 2.5 mL deionized water, followed by addition of 0.1 mL (2N) Folin–Ciocalteu reagent. They were mixed well and allowed to stand for 6 min before 0.5 mL of a 20% sodium carbonate solution was added. The color was developed after 30 min at room temperature and the absorbance was measured at 760 nm using a UV-visible spectrophotometer (UV-1650PC, Shimadzu, Japan). The measurement was compared to a standard curve of prepared gallic-acid solution and expressed as mean milligram of gallic acid equivalent per gram of plant material for the triplicate extracts.
Determination of total flavonoid content
The total flavonoid content in the methanolic root extract was determined by following the method of Cui et al. (Citation2011). To 0.25 mL of the methanolic protocorm extract or a (+)-catechin standard solution (Sigma Chemical Co., St. Louis, MO, USA), 1.25 mL distilled water and 0.075 mL 5% (w/v) aluminum chloride solution was added, and the mixture was allowed to stand for 5 min before 0.5 mL of 1 M sodium hydroxide solution was added. The absorbance was measured at 510 nm with a UV-visible spectrophotometer (UV-1650PC; Shimadzu, Kyoto, Japan). The absorbance measurements were integrated by comparison with an external standard calibration curve.
Scavenging effect on 1, 1-diphenyl-2-picrylhydrazyl (DPPH)
Free radical scavenging capacity was evaluated according to the previously reported procedure using 1, 1-diphynyl-2-picrylhydrazyl (DPPH) (Brand-Williams, Cuvelier, & Berset, Citation1995; Kim, Murthy, Hahn, Lee, & Paek, Citation2008). The initial concentration of DPPH radicals was 100 µM for all antioxidant–radical reactions. The antioxidant–radical reactions were conducted for 5 min in the dark at ambient temperature. The decrease in absorbance at 517 nm was measured against a blank of pure ethanol to estimate the radical scavenging capacity of each antioxidant sample.
Statistical analysis
All experiments were set up in a completely randomized design, and the data were collected from three replicates and mean values are subjected Duncan’s multiple range test using SPSS software (version 9.0; SPSS Inc., Chicago, USA).
Results and discussion
Comparison of different solvents for the extraction of bioactive compounds
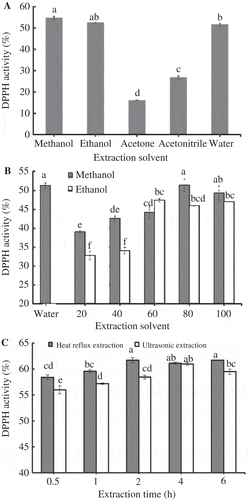
The quantitative and qualitative studies of bioactive compounds from plant material mostly relay on the selection of proper extraction method because it plays a crucial role on the final results and outcome (Azmir et al., Citation2013). The most common factors affecting extraction processes are matrix properties of the plant part, solvent, temperature, pressure and time (Hernandez, Lobo, & Gonzalez, Citation2009). Extraction of plant materials is done conventionally by using various chemical solvents and extraction efficiency mainly depends on the choice of solvents (Azmir et al., Citation2013; Cowan, Citation1999). Molecular affinity between solvent and solute, mass transfer, use of co-solvent, environmental safety, human toxicity and financial feasibility are also considered in selection of solvent for bioactive extraction. In the present study, methanol, ethanol, acetone, acetonitrile and water were used as solvents for the extraction of bioactive compounds from protocorm biomass of D. candidum and optimal polysaccharides (411.33 mg g−1 DW), polyphenolics (10.0 mg g−1 DW) and flavonoids (5.30 mg g−1 DW) were obtained when methanol was used as extraction solvent (). Free radical scavenging activity on DPPH was also high in this treatment (). Other solvents such as ethanol, acetone, acetonitrile and water were not efficient for the extraction of bioactive compounds from protocorms of D. candidum as the yield of bioactive compounds was lower when they were used as solvents for extraction. Various concentrations of solvents such as 20, 40, 60, 80 and 100% methanol, ethanol, acetone, acetonitrile were used to verify their effect on the yield bioactive compounds, results showed that 80% methanol was superior for extraction of polysaccharides (433.82 mg g−1 DW), polyphenolics (10.76 mg g−1 DW; ). However, the yield of flavonoids was optimal when 100% methanol was used as solvent. Methanol extracts showed highest radical scavenging activities when compared to other extracts (). Kim et al. (Citation2007) reported that the 70% ethanol found superior for ginsenoside extraction from Panax ginseng adventitious roots, whereas, 60% ethanol was found superior for the extraction of caffeic acid derivatives from adventitious roots of Echinacea purpurea (Wu, Murthy, Hahn, Lee, & Paek, Citation2008). Phenolics are very important plant constituents because of their scavenging ability and contribute directly to the anti-oxidative action (Duh, Tu, & Yen, Citation1999) and they are suggested to play a preventive role in the development of cancer and heart diseases. It is obvious that 80% methanol used in the present study could be able to yield optimal phenolics from protocorms of D. candidum and methanolic extract also showed very high DPPH scavenging activity (). The above results support the views of Cowan (Citation1999) that selection of suitable solvent and it concentration is very much important for optimal extraction of bioactive compounds from medicinal plant raw material.
Table 1. Effect of extraction solvents on the content of polysaccharides, polyphenolics and flavonoids during heat reflux processa.
Tabla 1. Efecto de los solventes de extracción en el contenido de polisacáridos, de polifenólicos y de flavonoides durante el proceso de reflujo de calor.
Figure 1. Radical scavenging activity on DPPH of D. candidum protocorm extract. (A) Effect of solvents, (B) effect of concentration of solvents (water, 20, 40, 60, 80 and 100% methanol and ethanol were used), (C) effect of extraction method. Mean values marked with different letters are significantly different at P ≤ 0.5.
Figura 1. Actividad de captación de radicales en dpph del extracto de protocormo de D. candidum. (A) Efecto de solventes; (B) efecto de la concentración de solventes (se utilizaron agua, 20, 40, 60, 80 y 100% de metanol y de etanol); (C) efecto del método de extracción. Los valores medios indicados con letras diferentes son significativamente diferentes en P ≤ 0,5.
The effect of duration of extraction
The duration of extraction is one of the important factors which influence the recovery of bioactive ingredients during heat reflux extraction (Kim et al., Citation2007). The samples were extracted with 80% methanol for different duration, viz., 30 min, 1, 2, 4 and 6 h. The results showed that the yield of bioactive compounds profound influence on the elution of active ingredients from the samples. The treatment of samples with 80% methanol for 2 h has yielded 463.0 mg g−1 DW polysaccharides, 10.75 mg g−1 DW polyphenolics and 5.30 mg g−1 DW flavonoids, respectively (). Free radical scavenging activity on DPPH was also high with this treatment (). This increment in polysaccharides, polyphenolics and flavonoids might be as a result of an increased diffusivity of solvents into cells and enhanced desorption of the components from the cells (Duh et al., Citation1999; Kahkonen et al., Citation1999).
Table 2. Effect of concentration of extraction solvents on the content of polysaccharides, polyphenols and flavonoids during the heat reflex extraction process.
Tabla 2. Efecto de la concentración de disolventes de extracción en el contenido de polisacáridos, polifenoles y flavonoides durante el proceso de extracción de calor reflejo.
Table 3. Effect of extraction method and duration of treatment on the content of polysaccharides, polyphenolics and flavonoids.
Tabla 3. Efecto del método de extracción y la duración del tratamiento sobre el contenido de polisacáridos, polifenoles y flavonoides.
Comparison of solvent extraction with ultrasonic-assisted extraction
Nonconventional method like ultrasonic-assisted extraction has been tested for the extraction of bioactive compounds from plants (Wang & Weller, Citation2006). In the present studies, heat reflux extraction was compared with ultrasonic method and results are presented in . Ultrasonic extraction was not suitable for extraction of bioactive compounds from the protocorms of D. candidum as it has yielded lower amounts of polysaccharides, polyphenolics and flavonoids (). The extract obtained from ultrasonic extraction showed lower DPPH scavenging activity (). Heat reflux extraction is a solid–liquid extraction, which is accomplished by allowing hot solvent to leach out the compounds from the solid tissue as interpreted by Kim et al. (Citation2007). However, ultrasonic-assisted extraction was superior to classical procedures for the extraction of polysaccharides from Zizypus jujube and Valeriana officinalis (Hromadkova, Ebringerova, & Valchovic, Citation2002; Li, Ding, & Ding, Citation2007).
Production of plant materials with high level of target compounds is the first important step for commercial manufacture of bioactive compound (Kumar et al., Citation2004). For example, industrial production of artemisinin, an antimalarial phytochemical mainly extracted from leaves of Artemisia annua, is frequently hindered by lack of vegetable materials (Ro et al., Citation2006; Zhang et al., Citation2008). D. candidum plant material is in high demand in China, natural populations of D. candidum have been depleted due to overexploitation and D. candidum is treated as threatened plant. We have adopted tissue-culture technology as an alternative strategy for the large-scale production of protocorms of D. candidum which enable us to obtain raw material throughout the year and in the present study we have developed reliable method of extraction of both carbohydrates along with phenolics and flavonoids. Antioxidant activity of phenolic substances extracted from the protocorms using solvent extraction was superior than that using ultrasonic-assisted extraction ().
For the extraction of food ingredients it is better to use the solvents that are less toxic and which are approved by the regulatory agencies. When methanol is used for extraction, the extract should be evaporated thoroughly and care should be taken to remove the methanol from the food ingredients to the permissible levels (Bart, Citation2011).
Conclusions
We have studied the conventional heat reflux and ultrasonic extraction methods for extraction of polysaccharides, polyphenolics and flavonoids from protocorm biomass of D. candidum. Heat reflux extraction was superior to ultrasonic-assisted extraction method. Comparison of different solvents (methanol, ethanol, acetonitrile, acetone and water), 80% methanol treatment for 2 h was found superior for extraction of polysaccharides, polyphenolics and flavonoids.
Acknowledgments
This study was supported by a grant of Korea Healthcare Technology R&D project, Ministry of Health and Welfare, Republic of Korea (grant no. A103017). Dr. H. N. Murthy is thankful to the Ministry of Education, Science and Technology, Republic of Korea for the award of Brainpool Fellowship (131S-4-3-0523), and this paper was studied with the support of the Ministry of Science, ICT and Planning (MSIP).
The authors declare that there are no conflicts of interest.
References
- Azmir, J., Zaidul, I. S. M., Rahman, M. M., Sharif, K. M., Mohamed, A., Sahena, F. … Omar, A. K. M. (2013). Techniques for extraction of bioactive compounds form plant materials: A review. Journal of Food Engineering, 117, 426–436. doi:10.1016/j.jfoodeng.2013.01.014
- Bao, X. S., Shun, Q. S., & Chen, L. Z. (2001). The medicinal plants of Dendrobium (shi-hu) in China, a coloured Atlas. Shanghai: Fudan University Press.
- Bart, H. J. (2011). Extraction of natural products from plant – An introduction. In H. J. Bart & S. Pilz (Eds.), Industrial scale natural product extraction (pp. 1–25). Weinheim: Wiley-VCH Verlag GmbH.
- Bourgaud, F., Gravot, A., Milesi, S., & Gontier, E. (2001). Production of plant secondary metabolites: A historical perspective. Plant Science, 161, 839–851. doi:10.1016/S0168-9452(01)00490-3
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT–Food Science and Technology, 28, 25–30. doi:10.1016/S0023-6438(95)80008-5
- Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews, 12, 564–582.
- Cui, H.Y., Murthy, H.N., Moh, S.H., Cui, Y.Y., Lee, E.J., & Paek, K.Y. (2014). Production of biomass and bioactive compounds in protocorm cultures of Dendrobium candidum Wall ex Lindl. using balloon type bubble bioreactors. Industrial Crops and Products, 53, 28–33. doi:10.1016/j.indcrop.2013.11.049
- Cui, X. H., Murthy, H. N., Jin, Y. X., Yim, Y. H., Kim, J. Y., & Paek, K. Y. (2011). Production of adventitious root biomass and secondary metabolites of Hypericum perforatum L. in a balloon type airlift bioreactor. Bioresource Technology, 102, 10072–10079. doi:10.1016/j.biortech.2011.08.044
- Duh, P. D., Tu, Y. Y., & Yen, G. C. (1999). Antioxidant activity of water extract of Harngjyur (Chrysanthemum morifolium Ramat.). LWT–Food Science and Technology, 32, 269–277. doi:10.1006/fstl.1999.0548
- Hernandez, Y., Lobo, M. G., & Gonzalez, M. (2009). Factors affecting simple extraction in the liquid chromatographic determination of organic acids in papaya and pineapple. Food Chemistry, 114, 734–741. doi:10.1016/j.foodchem.2008.10.021
- Hromadkova, Z., Ebringerova, A., & Valchovic, P. (2002). Ultrasound-assisted extraction of water-soluble polysaccharides from the roots of valerian (Valeriana officinalis L.). Ultrasonics Sonochemistry, 9, 37–44. doi:10.1016/S1350-4177(01)00093-1
- Kahkonen, M. P., Hopia, A. I., Vuorela, H. J., Rauha, J. P., Pihlaja, K., Kujala, T. S., & Heinonen, M. (1999). Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agricultural Food Chemistry, 47, 3954–3962. doi:10.1021/jf990146l
- Kim, S. J., Murthy, H. N., Hahn, E. J., Lee, H. L., & Paek, K. Y. (2007). Parameters affecting the extraction of ginsenosides from the adventitious roots of ginseng (Panax ginseng C.A. Meyer). Separation and Purification Technology, 56, 401–406. doi:10.1016/j.seppur.2007.06.014
- Kim, S. J., Murthy, H. N., Hahn, E. J., Lee, H. L., & Paek, K. Y. (2008). Effect of processing methods on the concentrations of bioactive components of ginseng (Panax ginseng C.A. Meyer) adventitious roots. LWT–Food Science and Technology, 41, 959–964. doi:10.1016/j.lwt.2007.06.012
- Kumar, S., Gupta, S. K., Singh, P., Bajpai, P., Gupta, M. M., Singh, D. … Sharma, S. (2004). High yields of artemisinin by multi-harvest of Artemisia annua crops. Industrial Crops and Products, 19, 77–90.
- Li, J. W., Ding, S. D., & Ding, X. L. (2007). Optimization of the ultrasonically assisted extraction of polysaccharides form Zizypus jujuba cv. Jinsiziaozao. Journal of Food Engineering, 80, 176–183. doi:10.1016/j.jfoodeng.2006.05.006
- Ng, T. B., Liu, J., Wong, J. H., Ye, X., Sze, S. C. W., Tong, Y., & Zhang, K. Y. (2012). Review of research on Dendrobium, a prized folk medicine. Applied Microbiology and Biotechnology, 93, 1795–1803. doi:10.1007/s00253-011-3829-7
- Ro, D. K., Paradise, E. M., Ouellet, M., Fisher, K. J., Newman, K. L., Ndungu, J. M. … Keasling, J. D. (2006). Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature, 440, 940–943. doi:10.1038/nature04640
- Wang, L., & Weller, C. L. (2006). Recent advances in extraction of nutraceuticals from plants. Trends in Food Science and Technology, 17, 300–312. doi:10.1016/j.tifs.2005.12.004
- Wu, C. H., Murthy, H. N., Hahn, E. J., Lee, H. L., & Paek, K. Y. (2008). Efficient extraction of caffeic acid derivatives from adventitious roots of Echinacea purpurea. Czech Journal of Food Science, 26, 254–258.
- Zhang, H. F., Yang, X. H., & Wang, Y. (2011). Microwave assisted extraction of secondary metabolites form plants: Current status and future directions. Trends in Food Science and Technology, 22, 672–688. doi:10.1016/j.tifs.2011.07.003
- Zhang, Y., Teoh, K. H., Reed, D. W., Maes, L., Goossens, A., Olson, D. J. … Covello, P. S. (2008). The molecular cloning of artemisinic aldehyde Delta11(13) reductase and its role in glandular trichome dependent biosynthesis of artemisinin in Artemisia annua. Journal of Biological Chemistry, 283, 21501–21508. doi:10.1074/jbc.M803090200
