Abstract
The phenolic compounds, total phenolic content, and antioxidant activity of Gemlik variety olive fruits obtained from four different locations in Turkey on five different harvesting dates (HDs) were investigated. The determination of phenolic compounds in olive flesh methanolic extracts was done by high performance liquid chromatography (HPLC) analysis. The total phenolic content and antioxidant activity values were estimated using Folin–Ciocalteu reagent and 2,2-diphenyl-1-picodrylhydrazyl reagent methods, respectively. Among 11 phenolic compounds identified, vanillic acid and hydroxytyrosol are the most abundant ones. In general, olive fruits from the Umurbey location had higher levels of phenolic compounds. The antioxidant activities of the olives were related to their phenolic content, and the olive fruits from the Umurbey location had a mean antioxidant activity 66.52% higher than that of other olives. There were differences among the quantities of phenolic compounds, the total phenolic contents, and the antioxidant activities of the olive fruits depending on HDs and location.
Para esta investigación, se analizó la presencia de compuestos fenólicos, el contenido total de fenólicos y la actividad antioxidante de aceitunas tipo Gemlik obtenidas de cuatro lugares diferentes de Turquía en cinco fechas de cosecha (FC) distintas. Para determinar los compuestos fenólicos presentes en los extractos metanólicos de la pulpa de aceituna se utilizó el análisis con cromatografía líquida de alta resolución (HPLC). El contenido fenólico total y los valores asociados a la actividad antioxidante fueron estimados usando los métodos reactivos Folin-Ciocalteu y 2,2-diphenyl-1-picodrylhydrazyl, respectivamente. Entre los once compuestos fenólicos identificados, los más abundantes fueron ácido vanílico y hidroxitirosol. En general, las aceitunas cosechadas en Umurbey mostraron niveles más elevados de compuestos fenólicos. Asimismo, se observó que las actividades antioxidantes de las aceitunas se relacionan con su contenido fenólico. En este sentido, la actividad antioxidante promedio de las aceitunas de Umurbey fue 66,52% más elevada que la de las demás aceitunas. A partir de esta investigación puede concluirse que existen diferencias en lo que respecta a las cantidades de compuestos fenólicos, los contenidos totales de fenólicos y las actividades antioxidantes de las aceitunas, vinculadas a su FC y a su lugar de origen.
Palabras clave:
Introduction
The olive tree (Olea europaea), one of the oldest known cultivated trees in the world, has been a part of Mediterranean civilization since before recorded history (Bartolini & Petruccelli, Citation2002; Uylaşer & Yildiz, Citation2014; Zamora, Alaiz, & Hidalgo, Citation2001). In favorable climatic conditions, such as those prevailing in Mediterranean countries, this tree is especially cultivated for the production of oil and table olives, which have significant economic value (Uylaşer & Yildiz, Citation2014). Turkey, as a Mediterranean country, is a major producer of olives. According to 2012 statistics, the world olive production reached approximately 16.5 million tons, and 11% of this quantity was produced in Turkey (FAO, Citation2012).
The Mediterranean diet has become universally associated with its positive effects on longevity and rates of heart disease, cancer, and other chronic diseases. The health-promoting properties of the Mediterranean diet have been largely attributed to the antioxidant and free radical-scavenging activity of phenolic compounds contained in the dietary components. Table olives and olive oil are important components of the Mediterranean diet that provide numerous health benefits and are largely consumed all over the world (Blekas, Vassilakis, Harizanis, Tsimidou, & Boskou, Citation2002; Cioffi et al., Citation2010; Owen et al., Citation2004; Stark & Madar, Citation2002). In fact, many studies have reported that olive oil may have a role in the prevention of coronary heart disease and cognitive impairment (e.g., Alzheimer’s disease) as well as it may have protective effects against cancers of the colon, breast, and ovary; diabetes accompanied by hypertriglyceridemia; and inflammatory and autoimmune diseases such as rheumatoid arthritis (Charoenprasert & Mitchell, Citation2012; Cicerale, Lucas, & Keast, Citation2010; Malheiro et al., Citation2012).
Phenolic compounds originate from general phenylpropanoid metabolism, which consists of three early steps in the conversion of l-phenylalanine to various hydroxycinnamic acids (Wang et al., Citation2012). The phenolic compounds in both olive fruits and their derived products (notably oil) are a complex mixture of components (Briante et al., Citation2002) that includes phenolic alcohols [3,4 dihydroxyphenylethanol (hydroxytyrosol), p-hydroxyphenylethanol (tyrosol)], phenolic acids (caffeic acid, vanillic acid, syringic acid), flavonoids (luteolin 7-O-glucoside, rutin and apigenin 7-O-glucoside, and the anthocyanins, cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside), lignans (pinoresinol, 1-acetoxypinoresinol), and secoiridoids (oleuropein, ligstroside, demethyloleuropein, oleuropein aglycone, elenolic acid, and verbascoside) (Esti, Cinquanta, & La Notte, Citation1998; Romani, Mulinacci, Pinelli, Vincieri, & Cimato, Citation1999; Romero, Tovar, Girona, & Motilva, Citation2002; Ryan & Robards, Citation1998; Soler-Rivas, Espin, & Wichers, Citation2000). In some cultivars, a delphinidin glycoside has also been described (Macheix, Fleuriet, & Billot, Citation1990). Oleuropein is the ester that consists of elenoic acid with 2–3,4-dihydroxyphenyl ethanol, and ligstroside is an ester of elenoic acid with 2–4-hydroxyphenyl ethanol. Demethyloleuropein, oleuropein aglycone, and elenoic acid are oleuropein derivatives; verbascoside is the main hydroxycinnamic derivative (Morelló, Romero, & Motilva, Citation2004; Romani et al., Citation1999; Servili et al., Citation2004).
Quantitative and qualitative differences in phenolic compounds affect the sensorial properties of olive fruits and the flavor, aroma, shelf life and nutritional quality of olive oil (Marsilio, Campestre, & Lanza, Citation2001). The phenolic contents of olive fruits have a direct effect on the chemical composition of olive products since it is dependent on the cultivar, the pedoclimatic production conditions, the agronomic techniques, and fruit ripening (Ben Othman, Roblain, Chammen, Thonart, & Hamdi, Citation2009; Romero et al., Citation2002; Vinha et al., Citation2005). Changes in phenol content during fruit development are important in determining the best harvesting period for olive fruits for olive oil and table olive production (Esti et al., Citation1998; Issaoui et al., Citation2011).
Several studies have focused on the phenolic content of olives. However, few studies have been conducted on the phenolic content of the Gemlik olive variety, which is approved as one of the best quality olive cultivars in the world and is cultivated and processed as black and green table olives in Bursa, located in the Marmara Region of Turkey. This variety has a high fruit and oil yield, early maturation, low periodicity, and sensory properties preferred by consumers. The origin of the Gemlik variety olive is Gemlik (Bursa) and this valuable olive variety has spread rapidly during the last years outside of its own origin. In addition, there is no information available regarding changes in the phenolic compounds of Gemlik variety olive fruits cultivated in Gemlik-Bursa. The purpose of this study was to investigate the effect of the ripening stage and location on the phenolic compounds of Gemlik variety olives harvested from August until the end of December. Moreover, the total phenolic contents and antioxidant activities of the olive fruits were determined during each ripening stage.
Materials and methods
Materials
The origin of the Gemlik olive variety is the Bursa province and districts in the Marmara region of Turkey. Gemlik variety of olives was collected from the olive garden located in Mudanya, Çağrışan, Kumla, and Umurbey provinces in Bursa. Olive fruits (approximately 2 kg) harvested by hand on five different harvest dates were placed in polyethylene bags and immediately transported to the laboratory in cool bags. The olive fruits were stored in a freezer (–18°C) until analysis. The olive harvesting dates (HDs) were designated as 22 August to 24 August (1st HD), 22 September to 24 September (2nd HD), 24 October to 25 October (3rd HD), 23 November to 25 November (4th HD), and 2 December to 27 December (5th HD).
Chemicals
HPLC grade methanol, acetic acid, hexane, and Folin–Ciocalteu’s phenol reagent were obtained from Merck (Darmstadt, Germany). The phenolic compound standards (protocatechuic acid, hydroxytyrosol, tyrosol, 4-hydroxybenzoic acid, 4-hydroxyphenylacetic acid, vanillic acid, syringic acid, p-coumaric acid, ferulic acid, cinnamic acid and oleanolic acid) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Fluka Chemie GmbH (Buchs, Switzerland).
Extraction method
Phenolic compound extraction from the olive fruits was carried out using a modified version of the method described by Arslan and Özcan (Citation2011). A quantity (15 g) of sample was extracted two times with 20 mL of methanol in water (80:20, v/v). The mixture was homogenized using an Ultraturrax homogenizer (T25 Digital, Ika Works Inc., USA) and centrifuged at 15,000g (Sigma 3K30, UK) for 10 min at 4°C. The pellet was re-extracted as described above, and the extracts were combined. The obtained supernatants were washed with hexane (2 × 10 mL) to remove the lipid fraction. The supernatants containing the phenolic compounds were filtered through 0.45 µm filters prior to HPLC analysis. The extracts obtained were also used for total phenol and antioxidant activity determination.
Total phenolic content
The total phenolic content in the extracts was determined with the Folin–Ciocalteau method, similar to Singh, Chidambara, and Jayaprakasha (Citation2002). To 0.3 mL of diluted extract, 1.5 mL of Folin-Ciocalteu reagent (diluted 10 times with distilled water) was added. After that, 1.2 mL of Na2CO3 (1 M) were added. The sample was incubated for 90 min. The absorbance was measured at 765 nm (Optizen 3220 UV, Mecasys, Korea). The results were expressed in milligrams of gallic acid (GAE) per kg of fresh weight (mg GAE/kg).
HPLC analysis of the phenolic compounds
The extracted phenolic fractions were analyzed by HPLC (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). The Flexar HPLC system included a diode array detector and a 4.6 mm × 250 mm i.d., 5 µm particle size, reversed-phase C18 column (PerkinElmer ODS-2, MA, USA). The mobile phase consisted of water ⁄acetic acid (98:2 v/v) as solvent A and methanol as solvent B. The solvent gradient changed according to the following conditions: from 5% to 15% B in 5 min, from 15% to 20% B in 10 min, from 20% to 25% B in 12 min, from 25% to 30% B in 10 min, from 30% to 35% B in 6 min, from 35% to 40% B in 10 min, from 40% to 50% B in 5 min, from 50% to 60% B in 2 min and isocratic at 100% B for 5 min. The column was maintained at 30°C. A flow rate of 1 mL/min was used, and the samples (40 μL) were detected at 280 and 320 nm. The phenolic compounds were expressed as mg/kg fresh weight.
Antioxidant Activity
The antioxidant activity was evaluated by measuring the radical scavenging effect of the methanolic extracts towards DPPH, as described by Boskou et al. (Citation2006), with minor modifications. A 3 mL volume of a 6 × 10–5 M methanolic solution of DPPH radical was added to 1 mL methanol extract from the olive fruit samples. Instead of methanolic extract of olive samples, pure methanol was used as control. The tubes were allowed to stand at room temperature for 60 min. The decrease in absorbance (A) at 515 nm was measured using a spectrophotometer (Optizen 3220 UV, Mecasys, Korea). The antioxidant activity was expressed as the inhibition percentage and was calculated using the following formula:
Statistical analysis
This research was conducted using a randomized plot factorial experimental design. The determination of the investigated components was performed in three replicates. The results were analyzed using the JMP (Version 7.0, SAS Institute Inc., Cary, NC, USA) software program. The mean differences were tested with a least significant difference test at a 5% level of significance.
Results
Total phenolic content of olive fruits
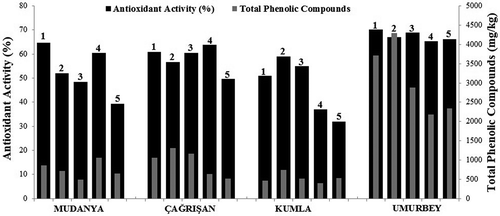
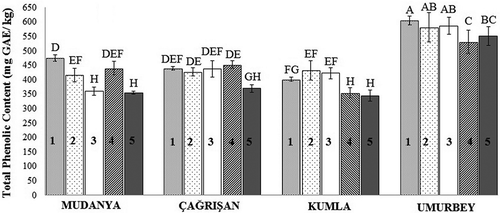
The total phenolic contents of the studied olive fruits are given in . All of the olive samples showed higher total phenolic contents on the 1st HD when compared to the 5th HD. The total phenolic content of the olive fruits showed a decreasing on the 5th HD compared to the 1st HD. The olive samples from the Mudanya (4742.33 mg GAE/kg) and Umurbey (6042.46 mg GAE/kg) locations had the highest total phenolic content on the 1st HD, while the olive fruits from the Çağrışan (4516.91 mg GAE/kg) and Kumla (4317.69 mg GAE/kg) locations had the highest total phenolic content on the 4th and 2nd HDs, respectively. However, there were no significant differences in the total phenolic contents of the fruits from the Çağrışan (4th HD) and Kumla (2nd HD) locations among 1st HD olive fruits (P > 0.05).
Figure 1. Total phenolic content of Gemlik variety olive fruits. Bars with different letters are significantly different (P < 0.05). (1–5: harvesting dates).
Figura 1. Contenido fenólico total de aceitunas tipo Gemlik. Las barras con letras distintas son significativamente diferentes (P < 0,05). (1–5: fechas de cosecha).
Phenolic compounds of olive fruits
The changes in the quantities of the phenolic compounds for five different HDs of Gemlik variety olive fruits obtained from Mudanya, Çağrışan, Kumla, and Umurbey are given . The chromatogram for the phenolic compounds from olive fruits from the Umurbey location is shown in . The identified and quantified phenolic compounds were protocatechuic acid, hydroxytyrosol, tyrosol, 4-hydroxybenzoic acid, 4-hydroxyphenylacetic acid, vanillic acid, syringic acid, p-coumaric acid, ferulic acid, cinnamic acid, and oleanolic acid. Among the identified compounds hydroxytyrosol, tyrosol, vanillic acid, syringic acid, cinnamic acid, and oleanolic acid were detected in olive fruits from all of the HDs for each location. For all of the locations and HDs studied, vanillic acid was the most abundant phenolic compound. Differences in the vanillic acid levels of the olive samples from the four different locations were observed (P < 0.05). The amount of vanillic acid ranged between 152.57 mg/kg and 2071.37 mg/kg. The olives from the Umurbey location showed the highest amount of vanillic acid, with a mean value of 1630.31 mg/kg. The vanillic acid reached its maximum level on the 2nd HD and then decreased until the 5th HD in the Çağrışan (971.30 mg/kg) and Kumla (485.27 mg/kg) locations. The Mudanya and Umurbey fruits both had the highest vanillic acid level on the 1st HD, while the lowest levels were identified on the 3rd (289.03 mg/kg) and 4th (1295.37 mg/kg) HDs.
Figure 2. HPLC chromatogram of phenolic extracts of olive fruit obtained from Umurbey locations. (1: Protocatechuic acid, 2: Hydroxytyrosol, 3: Tyrosol, 4: 4-Hydroxybenzoic acid, 5: 4-Hydroxyphenylacetic Acid, 6: Vanillic acid, 7: Syringic acid, 8: p-Coumaric acid, 9: Ferulic acid, 10: Cinnamic acid, 11: Oleanolic acid).
Figura 2. Cromatograma HPLC de extractos fenólicos de aceitunas cosechadas en lugares de Umurbey. (1: Ácido protocatecuico, 2: Hidroxitirosol, 3: Tirosol, 4: 4-Ácido hidroxibenzoico, 5: 4-Ácido hidroxifenilacético, 6: Ácido vinílico, 7: Ácido siríngico, 8: Ácido p-cumárico, 9: Ácido ferúlico, 10: Ácido cinámico, 11: Ácido oleanólico).
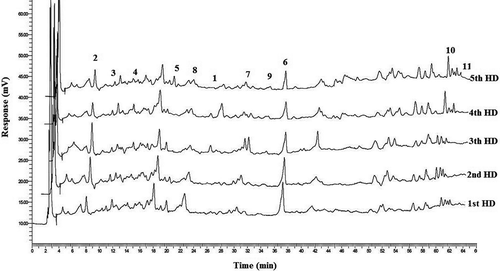
Table 1. Changes in phenolic compound level of the olive fruits of Gemlik variety during ripening (mg/kg).
Tabla 1. Cambios en los niveles de compuestos fenólicos de las aceitunas tipo Gemlik durante la maduración (mg/kg).
The second most abundant phenolic compound observed after vanillic acid was hydroxytyrosol, a phenolic alcohol, which is the principal product of oleuropein degradation during the ripening of olive fruit. The amount of hydroxytyrosol ranged from 1.20 mg/kg to 891.80 mg/kg, with a mean value of 205.77 mg/kg. The Umurbey fruits had the highest hydroxytyrosol value on the 3rd HD, while the lowest level was determined in the Kumla fruit on the 3rd HD (P < 0.05). The fruits cultivated in Umurbey were found to contain higher levels of hydroxytyrosol (varying from 189.57 to 891.80 mg/kg) than the fruits from the Mudanya, Çağrışan, and Kumla locations. Among all of the olive samples, hydroxytyrosol had the lowest values in the fruits from Mudanya (70.07 mg/kg) and Umurbey (189.57 mg/kg) on the 4th HD, whereas the Çağrışan (19.53 mg/kg) and Kumla (1.20 mg/kg) fruits showed the lowest amounts of hydroxytyrosol on the 3rd HD.
The highest tyrosol content was observed in fruits from the Umurbey location, which had a mean value of 52.01 mg/kg, followed by the Çağrışan (mean value of 32.67 mg/kg), Kumla (mean value of 29.51 mg/kg), and Mudanya fruits (mean value of 19.08 mg/kg). The lowest tyrosol levels were determined on the 1st HD for fruits from the Mudanya, Çağrışan, and Umurbey locations, while the fruits from the Kumla location showed the lowest tyrosol level on the 4th HD. The tyrosol content of the olive fruits displayed an irregular increase during fruit ripening.
The olive fruits from the Umurbey location contained a higher syringic acid, cinnamic acid, and oleanolic acid content compared to those of the fruits from the other locations. The Umurbey fruits had the highest level of syringic acid (66.50 mg/kg) and oleanolic acid (243.77 mg/kg) on the 3rd HD, while cinnamic acid (290.80 mg/kg) was present in the highest level in the Umurbey fruits on the 4th HD. An amount of 4-hydroxybenzoic acid was detected at all HDs only in the olive fruits from Mudanya. The 4-hydroxybenzoic acid level of the Mudanya fruits was 21.60 mg/kg on the 1st HD, followed by a decrease on the 2nd HD (9.30 mg/kg) and then a continuous decrease until the 4th HD (224.37 mg/kg). The fruit samples from Çağrışan on the 1st HD contained 148.40 mg/kg 4-hydroxybenzoic acid, and the level decreased progressively until the 4th HD (16.63 mg/kg). The amount of 4-hydroxyphenylacetic acid ranged between 1.23 mg/kg (Kumla) and 224.37 mg/kg (Mudanya). Olive fruits from the Mudanya, Çağrışan, and Umurbey locations displayed a decrease in the 4-hydroxyphenylacetic acid level as the fruit ripened. However, this phenolic compound was detected only on the 5th HD (11.90 mg/kg) in the Kumla fruits. Other phenolic acids detected in the olive fruits included protocatechuic acid (8.40–123.87 mg/kg), p-coumaric acid (2.63–1086.59 mg/kg), and ferulic acid (0.17–16.23 mg/kg).
Antioxidant activity of olive fruits
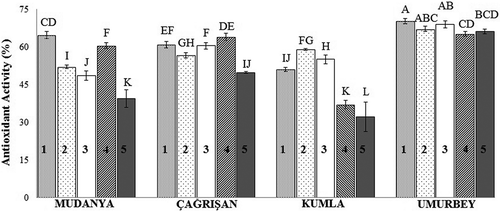
The antioxidant activities of the olive fruits are presented in . Among all of the olive fruits studied, the Umurbey olives on the 1st HD (70.18 %) had the highest antioxidant activity, and the Kumla olives on the 5th HD (32.04 %) had the lowest antioxidant activity. All the locations displayed similar changes in antioxidant activity, with a decrease in activity from the 1st HD to the 5th HD. The mean values of antioxidant activity of the olives decreased according to Umurbey > Çağrışan > Mudanya > Kumla. The antioxidant activities of the olive fruits were proportional to their total phenolic contents. The fruits obtained from the Umurbey location showed higher antioxidant activities compared to those from other locations, similar to the trend observed for total phenolic content. The phenolic compound levels (except 4-hydroxybenzoic acid) in the Umurbey fruits were higher than those from the other locations.
Figure 3. Antioxidant activities of Gemlik variety olive fruits. Bars with different letters are significantly different (P < 0.05). (1–5: harvesting dates).
Figura 3. Actividades antioxidantes de aceitunas tipo Gemlik. Las barras con letras distintas son significativamente diferentes (P < 0,05). (1–5: fechas de cosecha).
Discussion
The growth and ripening of olive fruit requires a lengthy period of approximately 5 months in Mediterranean climate conditions. The chemical structures and levels of phenolic compounds in olives are complex, and several factors are known to affect the phenolic profiles of olive fruits. These parameters include the cultivar and genetics, the degree of ripeness, the climate, the position on the tree, and the rootstock and agricultural practices (Ghanbari, Anwar, Alkharfy, Gilani, & Saari, Citation2012; Romero et al., Citation2004, Citation2002; Ryan & Robards, Citation1998; Vinha et al., Citation2005).
Compared to previous studies, differences were observed in both quantitative and qualitative fractions of phenolic compounds from the studied olive fruit samples. Dağdelen, Tümen, Özcan, and Dündar (Citation2013) investigated the phenolic profile of Gemlik variety olive fruits obtained from Edremit (Balıkesir) location at five different maturation stages. According to the results of their study, the highest levels of hydroxytyrosol (253.67 mg/kg), vanillic acid (30.98 mg/kg), tyrosol (28.70 mg/kg), syringic acid (3.28 mg/kg), p-coumaric acid (2.94 mg/kg), ferulic acid (0.85 mg/kg), cinnamic acid (0.21 mg/kg), and oleanolic acid were determined during the 5th, 2nd, 5th, 3rd, 2nd, 4th, and 5th harvest periods, respectively. Bouaziz, Chamkha, and Sayadi (Citation2004) reported that the hydroxytyrosol and tyrosol concentrations of the Chemlali olive cultivar increased during maturation. Morelló et al. (Citation2004), investigating the changes in phenolic compounds during ripening (September to November) of the Arbequina, Farga, and Morrut cultivars, noted that the levels of hydroxyrosol decreased from 8500 to 1580 mg/kg, from 7170 to 2380 mg/kg, and from 10,680 to 2480 mg/kg, respectively. The hydroxytyrosol, tyrosol, 4-hydroxyphenylacetic acid, vanillic acid, syringic acid, p-coumaric acid, ferulic acid and cinnamic acid levels found by Arslan and Özcan (Citation2011) during the ripening period of Sarıulak variety olive fruits ranged from 38.7 to 3596.4 mg/kg, from 20.1 to 872.4 mg/kg, from 3.8 to 471.8 mg/kg, from 7.4 to 172.3 mg/kg, from 0 to 60.8 mg/kg, from 0.1 to 28.6 mg/kg, from 8.2 to 56.9 mg/kg, and from 0.2 to 5.9 mg/kg, respectively.
In general, the values found for vanillic acid can also be considered as high, when compared with reported values by Dağdelen et al. (Citation2013) and Arslan and Özcan (Citation2011). Hydroxytyrosol levels determined for the Gemlik olive in this study from Umurbey location are higher than those reported by Dağdelen et al. (Citation2013). In addition, the tyrosol content of determined in this study are closer to reported values by Dağdelen et al. (Citation2013). Our findings were found to be lower than that reported by Vinha et al. (Citation2005) for 18 different cultivars from Portugal.
The higher antioxidant activity and total phenolic content in the Umurbey fruits could be strongly related to their phenolic content (). Previous studies reported that the antioxidant activity of olive fruit is likely due to their high levels of hydroxytyrosol and tyrosol (Issaoui et al., Citation2011; Vinha et al., Citation2005). Damak, Bouaziz, Ayadi, Sayadi, and Damak (Citation2008) showed that there was a decrease in the antioxidant activity of olive fruits from Chétoui variety with maturation. Cerretani et al. (Citation2004) reported that the total phenolics of olive fruits of the Nostrana di Brisighella and Ghiacciolo varieties decreased from 441.4 to 209.5 mg/kg and from 585.2 to 409.8 mg/kg, respectively, as the harvesting period progressed. In another study, the antioxidant activity and total polyphenol content of Cobrançosa variety olive fruits decreased from 236.6 to 43.2 mmol trolox/kg and from 61.3 to 15.7 mg GAE/g, respectively, between September and December, as found by Machado, Felizardo, Fernandes-Silva, Nunes, and Barros (Citation2013).
Conclusions
The present study characterized the phenolic profiles, total phenolic contents, and antioxidant activities of Gemlik variety olives from different locations during the ripening period. The results obtained showed that there are differences among the levels of phenolic compounds according to the HD and location. The main phenolic compounds were vanillic acid and hydroxytyrosol. In general, values found for hydroxytyrosol were higher in olives harvesting in December for Mudanya and Kumla locations, while in the case of Çağrışan and Umurbey locations, the olive fruits that were harvested between September and October had the higher hydroxytyrosol levels. The olive fruits from Umurbey had higher antioxidant activity and total phenolic content compared to the fruits from other three locations which could be attributed to total phenolic compounds, especially hydroxytyrosol. The obtained results will help identify the optimal harvest time for olive fruits used in olive products.
References
- Arslan, D., & Özcan, M. M. (2011). Phenolic profile and antioxidant activity of olive fruits of the Turkish variety “Sarıulak” from different locations. Grasas Y Aceites, 62(4), 453–461. doi:10.3989/gya.034311
- Bartolini, G., & Petruccelli, R. (2002). Classification, origin, diffusion and history of the olive. Rome: Food & Agriculture Organization of the United Nations (FAO).
- Ben Othman, N., Roblain, D., Chammen, N., Thonart, P., & Hamdi, M. (2009). Antioxidant phenolic compounds loss during the fermentation of chétoui olives. Food Chemistry, 116, 662–669. doi:10.1016/j.foodchem.2009.02.084
- Blekas, G., Vassilakis, C., Harizanis, C., Tsimidou, M., & Boskou, D. G. (2002). Biophenols in table olives. Journal of Agricultural and Food Chemistry, 50, 3688–3692. doi:10.1021/jf0115138
- Boskou, G., Salta, F. N., Chrysostomou, S., Mylona, A., Chiou, A., & Andrikopoulos, N. K. (2006). Antioxidant capacity and phenolic profile of table olives from the Greek market. Food Chemistry, 94, 558–564. doi:10.1016/j.foodchem.2004.12.005
- Bouaziz, M., Chamkha, M., & Sayadi, S. (2004). Comparative study on phenolic content and antioxidant activity during maturation of the olive cultivar Chemlali from Tunisia. Journal of Agricultural and Food Chemistry, 52, 5476–5481. doi:10.1021/jf0497004
- Briante, R., Patumi, M., Limongelli, S., Febbraio, F., Vaccaro, C., Di Salle, A. Nucci, R. (2002). Changes in phenolic and enzymatic activities content during fruit ripening in two Italian cultivars of Olea europaea L. Plant Science, 162, 791–798. doi:10.1016/S0168-9452(02)00022-5
- Cerretani, L., Bendini, A., Rotondi, A., Mari, M., Lercker, G., & Toschi, T. (2004). Evaluation of the oxidative stability and organoleptic properties of extra-virgin olive oils in relation to olive ripening degree. Progress in nutrition Casa Editrice Mattioli, 6, 50–56.
- Charoenprasert, S., & Mitchell, A. (2012). Factors influencing phenolic compounds in table olives (Olea europaea). Journal of Agricultural and Food Chemistry, 60, 7081–7095. doi:10.1021/jf3017699
- Cicerale, S., Lucas, L., & Keast, R. (2010). Biological activities of phenolic compounds present in virgin olive oil. International Journal of Molecular Sciences, 11, 458–479. doi:10.3390/ijms11020458
- Cioffi, G., Pesca, M. S., De Caprariis, P., Braca, A., Severino, L., & De Tommasi, N. (2010). Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chemistry, 121, 105–111. doi:10.1016/j.foodchem.2009.12.013
- Dağdelen, A., Tümen, G., Özcan, M. M., & Dündar, E. (2013). Phenolics profiles of olive fruits (Olea europaea L.) and oils from Ayvalik, Domat and Gemlik varieties at different ripening stages. Food Chemistry, 136, 41–45. doi:10.1016/j.foodchem.2012.07.046
- Damak, N., Bouaziz, M., Ayadi, M., Sayadi, S., & Damak, M. (2008). Effect of the maturation process on the phenolic fractions, fatty acids, and antioxidant activity of the Chétoui olive fruit cultivar. Journal of Agricultural Food Chemistry, 56, 1560–1566. doi:10.1021/jf072273k
- Esti, M., Cinquanta, L., & La Notte, E. (1998). Phenolic compounds in different olive varieties. Journal of Agricultural and Food Chemistry, 46, 32–35. doi:10.1021/jf970391+
- FAO. (2012). Food and Agriculture Organization of the United Nations, FAOSTAT.
- Ghanbari, R., Anwar, F., Alkharfy, K. M., Gilani, A. H., & Saari, N. (2012). Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.) - A review. International Journal of Molecular Sciences, 13, 3291–3340. doi:10.3390/ijms13033291
- Issaoui, M., Dabbou, S., Mechri, B., Nakbi, A., Chehab, H., & Hammami, M. (2011). Fatty acid profile, sugar composition, and antioxidant compounds of table olives as affected by different treatments. European Food Research and Technology, 232, 867–876. doi:10.1007/s00217-011-1455-3
- Machado, M., Felizardo, C., Fernandes-Silva, A. A., Nunes, F. M., & Barros, A. (2013). Polyphenolic compounds, antioxidant activity and L-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food Research International, 51, 412–421. doi:10.1016/j.foodres.2012.12.056
- Macheix, J. J., Fleuriet, A., & Billot, J. (1990). Fruit phenolics (pp. 1–126). Boca Raton, FL: CRC Press.
- Malheiro, R., Casal, S., Sousa, A., De Pinho, P. G., Peres, A. M., Dias, L. G. … Pereira, J. A. (2012). Effect of cultivar on sensory characteristics, chemical composition, and nutritional value of stoned green table olives. Food and Bioprocess Technology, 5, 1733–1742. doi:10.1007/s11947-011-0567-x
- Marsilio, V., Campestre, C., & Lanza, B. (2001). Phenolic compounds change during California-style ripe olive processing. Food Chemistry, 74, 55–60. doi:10.1016/S0308-8146(00)00338-1
- Morelló, J. R., Romero, M. P., & Motilva, M. J. (2004). Effect of the maturation process of the olive fruit on the phenolic fraction of drupes and oils from Arbequina, Farga, and Morrut cultivars. Journal of Agricultural and Food Chemistry, 52, 6002–6009. doi:10.1021/jf035300p
- Owen, R. W., Haubner, R., Würtele, G., Hull, E., Spiegelhalder, B., & Bartsch, H. (2004). Olives and olive oil in cancer prevention. European Journal of Cancer Prevention, 13, 319–326. doi:10.1097/01.cej.0000130221.19480.7e
- Romani, A., Mulinacci, N., Pinelli, P., Vincieri, F., & Cimato, A. (1999). Polyphenolic content in five Tuscany cultivars of Olea europaea L. Journal of Agricultural and Food Chemistry, 47, 964–967. doi:10.1021/jf980264t
- Romero, C., Brenes, M., Yousfi, K., García, P., García, A., & Garrido, A. (2004). Effect of cultivar and processing method on the contents of polyphenols in table olives. Journal of Agricultural and Food Chemistry, 52, 479–484. doi:10.1021/jf030525l
- Romero, M. P., Tovar, M. J., Girona, J., & Motilva, M. J. (2002). Changes in the HPLC phenolic profile of virgin olive oil from young trees (Olea europaea L. cv Arbequina) grown under different deficit irrigation strategies. Journal of Agricultural and Food Chemistry, 50, 5349–5354. doi:10.1021/jf020357h
- Ryan, D., & Robards, K. (1998). Critical review. phenolic compounds in olives. Analyst, 123, 31R–44. doi:10.1039/a708920a
- Servili, M., Selvaggini, R., Esposto, S., Taticchi, A., Montedoro, G., & Morozzi, G. (2004). Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. Journal of Chromatography A, 1054, 113–127. doi:10.1016/j.chroma.2004.08.070
- Singh, R. P., Chidambara, K. N., & Jayaprakasha, G. K. (2002). Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. Journal of Agricultural and Food Chemistry, 50, 81–86. doi:10.1021/jf010865b
- Soler-Rivas, C., Espin, J. C., & Wichers, H. J. (2000). Oleuropein and related compounds. Journal of the Science of Agriculture, 80, 1013–1023.
- Stark, A. H., & Madar, Z. (2002). Olive oil as a functional food: epidemiology and nutritional approaches. Nutrition Reviews, 60, 170–176. doi:10.1301/002966402320243250
- Uylaşer, V., & Yildiz, G. (2014). The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Critical Reviews in Food Science and Nutrition, 54, 1092–1101. doi:10.1080/10408398.2011.626874
- Vinha, A. F., Ferreres, F., Silva, B. M., Valentão, P., Gonçalves, A., Pereira, J. A. … Andrade, P. B. (2005). Phenolic profiles of Portuguese olive fruits (Olea europaea L.): ınfluences of cultivar and geographical origin. Food Chemistry, 89, 561–568. doi:10.1016/j.foodchem.2004.03.012
- Wang, Q., Tao, S., Dubé, C., Tury, E., Hao, Y.-J., Zhang, S., Zhoo, M., Wu, W., Khanizadeh, S. (2012). Postharvest changes in the total phenolic content, antioxidant capacity and L-phenylalanine ammonia-lyase activity of strawberries ınoculated with Botrytis cinerea. Journal of Plant Studies, 1(2), 11–18. doi:10.5539/jps.v1n2p11
- Zamora, R., Alaiz, M., & Hidalgo, F. J. (2001). Influence of cultivar and fruit ripening on olive (Olea europaea) fruit protein content, composition, and antioxidant activity. Journal of Agricultural and Food Chemistry, 49, 4267–4270. doi:10.1021/jf0104634