ABSTRACT
It is well known that beef produces volatile molecules. In this work, the detection of volatiles released by post-mortem bovine fast-twitch muscles (Musculus longissimus dorsi and Musculus cutaneus trunci) was done using GC/MS–SPME (gas chromatography/mass spectrum–solid-phase microextraction). The releases of volatile molecules were modeled against three factors (rigor-mortis, animal age and oxidative capacity) using a chemometrics approach (experimental design and partial least squares regression). The GC/MS–SPME technique produced more than 30 reproducible chromatographic peaks, but only 13 were associated significantly with two factors (rigor-mortis and animal age). The volatile profile was composed mainly of alcohols, aldehydes and alkanes. The factor “animal age” was the main variable related to the release of volatile molecules. The results strongly suggest that the release of volatile molecules change according to post-mortem metabolism and the animal age.
RESUMEN
Es conocido que la carne produce moléculas volátiles. En este trabajo, la detección de moléculas volátiles emanadas por músculos bovinos post-mortem de contracción rápida (Musculus longissimus dorsi y Musculus cutaneus trunci) fue realizada usando GC/MS–SPME. Las emanaciones de las moléculas volátiles fueron modeladas contra tres factores (rigor-mortis, edad del animal y capacidad oxidativa) usando un enfoque quimiométrico (diseño experimental y regresión de mínimos cuadrados parciales). La técnica GC/MS–SPME produjo más de 30 picos cromatográficos reproducibles, pero solo 13 fueron asociados significativamente con dos factores (rigor-mortis y edad del animal). El perfil volátil estuvo compuesto principalmente por alcoholes, aldehídos y alcanos. El factor “edad del animal” fue la principal variable relacionada con la emanación de las moléculas volátiles. Los resultados sugieren fuertemente que la emanación de moléculas volátiles cambia de acuerdo al metabolismo post-mortem y la edad del animal.
PALABRAS CLAVES:
1. Introduction
It is well known that volatile composition influences the odorant characteristics of foods. Different kinds of volatile molecules are present in meat and beef (Elmore et al., Citation2004; Insausti, Goñi, Petri, Gorraiz, & Beriain, Citation2005; Osorio, Zumalacárregui, Cabeza, Figueira, & Mateo, Citation2008; Vasta, Ratel, & Engel, Citation2007; Vasta et al., Citation2010), which can be classified into several groups according to their functional groups such as hydrocarbons, aldehydes, ketones, alcohols, esters, aromatics and others (Rivas-Cañedo, Fernández-García, & Nuñez, Citation2009). Several of these molecules are produced by meat maturation. However, fresh meat has a volatile profile before maturation, hence the question “How does muscle produce these volatile molecules?” The information about the biochemistry and metabolic source of these odorants is not totally available in scientific literature.
Many factors and biological processes affect the production of volatile compounds and their releases. It has been reported that environmental factors like feeding (Elmore et al., Citation2004), weather (Zhang, Cai, Ruan, & Li, Citation2005) and animal age (Arredondo et al., Citation2014; Santander et al., Citation2013) affect the behavior of volatile profiles. Different kinds of beef also present differences in the volatile profiles (Watanabe, Ueda, Higuchi, & Shiba, Citation2008). Moreover, biological processes like rigor-mortis or post-mortem glycolytic fluxes might modify the volatile release of fresh meat (Acevedo et al., Citation2012). Considering the importance of odors in food science, it is very important to understand the origin of volatile compounds, because this information could be used to develop technologies to certify beef and to enhance the flavor of fresh meat.
When an animal is slaughtered, the glycolytic flux of its muscles immediately increases. The anaerobic glycolytic activity of the post-mortem muscle is the main mechanism of energy production and it has been reported that glycolysis might be a key pathway in the release of volatiles by mammal cells (Acevedo, Sánchez, Reyes, & Young, Citation2010) and raw meat (Acevedo et al., Citation2012). Since muscles can be classified according their metabolic characteristics into slow-twitch (essentially oxidative metabolism maintaining energy levels aerobically) and fast-twitch (essentially glycolytic metabolism maintaining ATP supply anaerobically) (Ylä-Ajos, Ruusunen, & Puolanne, Citation2006), we hypothesized that post-mortem fast-twitch muscles might be a good scientific model to use as an exploratory pattern analysis of volatile profiles by chemometrics analysis.
In this work, we analyzed volatiles released during rigor-mortis (fresh meat) in two bovine fast-twitch muscles by using GC/MS–SPME (gas chromatography/mass spectrum–solid-phase microextraction). Then, we analyzed the complex data system by using chemometrics tools based on partial least square regression to obtain a relationship between the volatile release and post-mortem metabolism of beef carcasses.
2. Materials and methods
2.1. Samples of beef carcasses
Fresh meat samples were obtained from male bovines (Holstein, Bos taurus). After slaughtering, the animals were skinned, eviscerated and split into two half carcasses. The half carcasses were stored at 0°C. Six half carcasses of newborn calves (<3 days old) and six half carcasses of 2 year old bovines were used.
2.2. Gas chromatography
Release of volatile molecules from beef was analyzed by means of a GC/MS–SPME. A headspace vials were loaded with 2.5 g of sample and equilibrated at extraction temperature (50°C) for 30 minutes. Then, a DVB/CAR/PDMS fiber (50/30 µm Divinylbenzene/Carboxen/Polydimethylsiloxane) (Supelco, USA) (Martin, Antequera, Muriel, Perez-Palacios, & Ruiz, Citation2009; Santander et al., Citation2013) was introduced inside the headspace vial to extract the volatiles, through a silicon septum for 30 minutes. Analyses were made in triplicate.
Targeted analyses loaded in the fiber were analyzed in a Hewlett-Packard (USA) HP 6890 gas chromatography (with a HP MD5973 quadrupole mass spectrometer) in splitless mode (5 minutes). Desorption and cleaning of fiber was carried out at 250°C for 20 minutes. Helium at a constant flow (1.3 mL/min) served as a carrier gas.
Separation was conducted in a 60 m length × 0.32 mm i.d. × 1.0 μm film BPX5 column (SGE, Australia). The oven was programmed as follows: The temperature program was 40°C for 10 minutes, then raised to 200°C at a rate of 5°C/min (maintained 200°C for 1 minute) and then raised to 250°C at a rate of 20°C/min (maintained 250°C for 5 min).
Operation of the GC/MS was done by using the Chemstation software (Agilent Technologies). Scan mode was used.
Potential emanations were analyzed by matching sample mass spectrums with those of the National Institute of Standards and Technology (NIST) MS spectral library (USA) for peaks presented in the chromatograms. Chromatographic peaks were considered “unknown,” when their mass spectral match quality (MQ) was less than 85%, and discarded in this identification process (Acevedo et al., Citation2010, Citation2012). MQ value is referred to the degree the target spectrum matches the standard spectrum in the NIST library (100% indicates a perfect fit). Chromatographic peaks with mass spectral fit value >85% were confirmed with their respective commercial chemical standards.
2.3. Experimental design
In order to maximize the experimental information, the experimental factors studied were arranged as informed in . The sex of the animals was not taken into consideration (all bovines were male).
Table 1. Description of samples and factors.
Tabla 1. Descripción de muestras y factores.
The influence of rigor-mortis (X1) was analyzed taken samples before rigor-mortis and advanced rigor-mortis. For that, the carcasses was sampled twice: the first sampling (45 minutes post-mortem) was considered as pre rigor-mortis and the second sampling (36 hours post-mortem) was considered as advanced rigor-mortis.
To analyze influence of animal age (X2), we sampled beef obtained from newborn calves (<3 days old) and animals of 2 years old.
To analyze the influence of the oxidative capacity of the fast-twitch muscles (X3), two tissues were used: M. longissimus dorsi (essentially glycolitic fast-twitch) (Hwang, Kim, Jeong, Hur, & Joo, Citation2010; Ylä-Ajos et al., Citation2006) and M. cutaneus trunci (fast-twitch with oxidative capacity) (Devine, Ellery, & Averill, Citation1984).
To obtain an adequate number of experimental run repetitions, six half carcasses of newborn calves (<3 days old) and six half carcasses of 2-year-old bovines were used. Each sample of tissue taken was sectioned into three parts to obtain triplicates of each experimental run.
2.4. Chemometrics analysis
The three predictors studied (X1, X2 and X3; see ) were associated with relative chromatographic areas of the volatile molecules present in chromatograms (response variables). The purpose of the statistical analysis was to relate the chromatograms to the proposed predictors. Our analysis was based on the multivariate linear regression model, given by
where Y is the response matrix of order n × q; X is an n × p design matrix; B is an unknown p × q matrix of parameters called regression coefficients; and U is n × q matrix of random disturbances, with n being the sample size, q the number of Y variables and p represent the number of X variables.
The estimation of the regression coefficients matrix B was carried out using partial least square (PLS) (Frank & Friedman, Citation1993). Before using the PLS regression, a simple pre-processing method of the variables X and Y was done based on autoscale of the values (Procopio, Krause, Hofmann, & Becker, Citation2013). The selection of the number of components was based on cross-validation procedure (Wold, Sjöström, & Eriksson, Citation2001). As suggested, for instance in Wold et al. (Citation2001), the standard errors were estimated from the data by using Jackknife formula (Efron & Gong, Citation1983). Thus, we tested the hypothesis that individual coefficients brs are zero, for r = 1,…, p; s = 1,…, q by applying an approximate t statistic. Importance of the factors was studied by mean of variable importance on projection (VIP) and shown as a VIP plot. This approach has been used to model volatile compounds in food systems (Acevedo, Díaz-Calderón, López, & Enrione, Citation2015; Procopio et al., Citation2013).
All computations were done using software SIMCA-P (Umetrics, Sweden) (Eriksson et al., Citation2006).
3. Results and discussion
3.1. Chromatographic profile
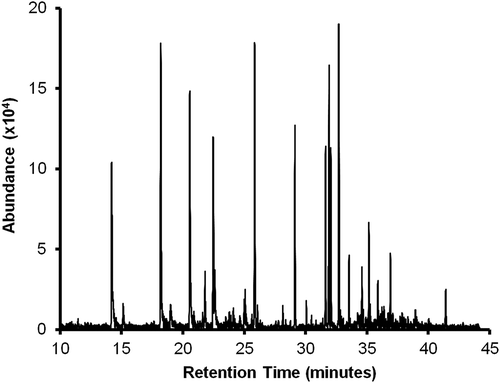
Application of the GC/MS–SPME technique to measure volatiles in samples produced 30 chromatographic peaks. shows a selected chromatogram where several of these peaks can be distinguished. This later strongly suggests that the fast-twitch muscles of beef carcasses release volatile molecules and their profile can be identified by using GC/MS–SPME.
3.2. Analysis of the chromatographic data
The putative volatile compounds or chromatographic peaks of the chromatographic profile were modeled against all factors (see ), by using the multivariate regression model previously described. It was used to represent each response (Y variable) against each factor (X variable).
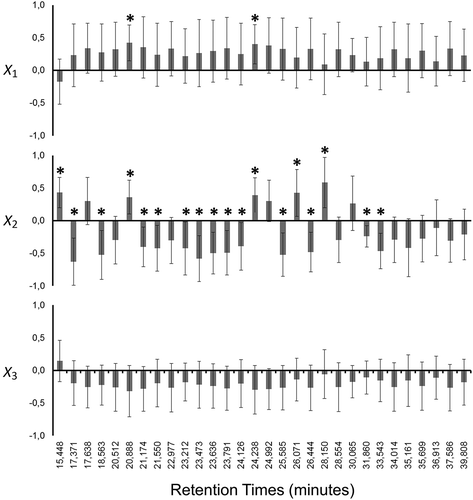
shows a bar plot for the estimated coefficients of matrix B. When applied the t test, there is a big group of volatiles (12 types of molecules) that have no significant (p > 0.05) relationship with any X variable, and the presence can be attributed to random behavior or other factors. The volatiles with significant regression coefficient in the matrix B (p < 0.05) and their respective confidence interval (95%) are indicated in the . Only 18 volatiles have relationship with two factors (X1 and X2). In particular, the oxidative capacity (X3) was not directly associated with the release of volatile molecules, suggesting likely a similar behavior of the post-mortem volatile metabolism among fast-twitch muscles.
Figure 2. Coefficients of B matrix.
Figura 2.. Coeficientes de la matriz B.
Note: Error bars correspond to confidence interval (95%). Asterisks (*) indicate significant coefficients (p < 0.05).
Nota: Barras de error corresponden a intervalo de confianza (95%). Asteriscos (*) indican coeficientes significativos (p < 0.05).
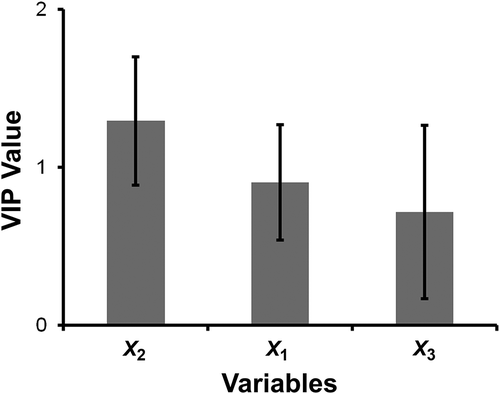
shows the VIP plot (which indicates the importance of X variables). Rigor-mortis (X1) and animal age (X2) have large and significant VIP values (larger than 1 and p < 0.05, respectively). Previous works (Arredondo et al., Citation2014; Santander et al., Citation2013) have reported that volatiles molecules release by fresh meat can be used as biomarker related to animal age. In our case, the factor animal age was the most important of the variables studied, indicating that the volatiles could be used to identify the animal age.
3.3. Volatile molecules identified
Eighteen chromatographic peaks display dependence with some of the factors considered in this study. Thirteen of these 18 volatiles were chemically identify by using mass spectrometry and chemical standards (). One of these 13 identified compounds was related with both main factors (X1 and X2). Only xylene – which presents a characteristic sweet and pungent odor – shows a strong dependence with animal age and rigor-mortis, simultaneously.
Table 2. Volatile molecules selected by chemometrics analysis and identified using mass spectrometry and chemical standards.
Tabla 2.. Moléculas volátiles seleccionadas por análisis quimiométrico e identificadas usando espectrometría de masas y estándares químicos.
Xylene released by mammal cells (in vitro) has been correlated to glycolytic activity (Acevedo et al., Citation2010). It has been reported in raw lamb meat (Vasta et al., Citation2007, Citation2010), cooked beef (Insausti et al., Citation2005), grilled bovine and lamb meat (Elmore et al., Citation2004, Citation2005) and human blood (Deng, Zhang, & Li, Citation2004).
In addition, four alcohols (1-pentanol, heptanol, 1-octen-3-ol, 1-octanol), four aldehydes (hexanal, heptanal, octanal, nonanal) and four alkanes (undecane, dodecane, tetradecane, hexadecane) were identified and associated strongly with the variable animal age, being this very important to perform further chemometrics studies such as classification algorithms.
3.4. Alcohols identified
Identification of 1-pentanol is associated with the presence of lactic acid bacteria (Ercolini, Russo, Nasi, Ferranti, & Villani, Citation2009; Hernández-Macedo et al., Citation2012; Holm, Adamsen et al., Citation2013; Jääskeläinem et al., Citation2013; Muriel, Antequera, Petrón, Andrés, & Ruiz, Citation2004) and grass intake in the diet (Vasta, D’Alessandro, Priolo, Petrotos, & Martemucci, Citation2012). Regarding 1-heptanol, this compound appears in lipid thermal oxidation of beef and pig meat (Elmore, Mottram, Enser, & Wood, Citation1999; Elmore et al., Citation2004, Citation2005; Shi et al., Citation2012), 1-octen-3-ol is associated with lactic bacteria as well as monounsaturated lipid oxidative reactions in different species such as sheep, cattle and pigs (Elmore et al., Citation1999, Citation2004, Citation2005; Ercolini et al., Citation2009; Holm, Schäfer, Koch, & Petersen, Citation2013; Holm, Adamsen et al., Citation2013; Osorio et al., Citation2008; Resconi, Escudero, & Campo, Citation2013; Watanabe et al., Citation2008). Concerning 1-octanol, it is reported as a lipid oxidation product generated during fat thermal oxidation (Elmore et al., Citation1999, Citation2004, Citation2005; Osorio et al., Citation2008; Shahidi, Citation1998; Shi et al., Citation2012; Watanabe et al., Citation2008).
The alcohol 1-octanol has been used a marker to identify beef obtained from OTM (over 30 months) cattle (Arredondo et al., Citation2014). Interestingly, the main result of this study is that the volatiles (including 1-octanol) are strongly associated with factor X2 (animal age).
3.5. Aldehydes identified
Aldehyde compounds such as hexanal, heptanal, octanal and nonanal are related to lipid oxidation (Elmore et al., Citation1999, Citation2004, Citation2005; Jääskeläinem et al., Citation2013; Muriel et al., Citation2004; Resconi et al., Citation2013; Shahidi, Citation1998; Shi et al., Citation2012; Watanabe et al., Citation2008) and regarding hexanal and nonanal, they were found as products associated with the growth of lactic acid bacteria. Insausti et al. (Citation2005) and Ercolini et al. (Citation2009) have referred to hexanal, octanal and nonanal as the predominant volatiles compounds in meat, being hexanal one of the most abundant volatiles in beef because it is originated during linoleic acid (C18:2 n-9) oxidation (Elmore et al., Citation2005). In addition, other reports have used the nonanal as a putative marker related with the animal age with high accuracy (Arredondo et al., Citation2014).
3.6. Alkanes identified
The analysis of possible routes for obtaining alkanes proposed by Shahidi (Citation1998) is only associated with lipid degradation, especially for saturated fatty acid degradation. Some alkanes, such as tetradecane and hexadecane, have been implicated as potent markers of grass intake in the diet (Sivadier, Ratel, Bouvier, & Engel, Citation2008) and linked by other authors as products provided by feeding and deposited in fat (Meynier, Genot, & Gandemer, Citation1999; Tejeda, García, Petrón, Andrés, & Antequera, Citation2001).
It has been informed that the alkanes as undecane and tetradecane released by beef have relationship with the animal age (Arredondo et al., Citation2014), confirming the results obtained in the chemometrics analysis.
4. Conclusion
Our results show that bovine fresh meat from fast-twitch muscles release volatile molecules. The chromatograms obtained by GC/MS–SPME showed 30 peaks, but only 18 were associated with two of the three factors studied (rigor-mortis and animal age).
The factors rigor-mortis and animal age were the main variables associated with the whole chromatographic profile. The factor oxidative capacity was not associated with the release of volatile molecules, suggesting a similar behavior of the post-mortem volatile metabolism among fast-twitch muscles.
The factor animal age was the most important of the variables studied. The volatile profile associated with animal age was composed mainly of alcohols, aldehydes and alkanes, indicating that these volatiles could be used to discriminate the animal age.
The evidence strongly suggests that the release of these volatile molecules change according to post-mortem metabolism and animal age.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Acevedo, C. A., Creixell, W., Pavez-Barra, C., Sánchez, E., Albornoz, F., & Young, M. E. (2012). Modeling volatile organic compounds released by bovine fresh meat using an integration of solid phase microextraction and databases. Food and Bioprocess Technology, 5, 2557–2567. doi:10.1007/s11947-011-0571-1
- Acevedo, C. A., Díaz-Calderón, P., López, D., & Enrione, J. (2015). Assessment of gelatin–chitosan interactions in films by a chemometrics approach. CyTA – Journal of Food, 13, 227–234. doi:10.1080/19476337.2014.944570
- Acevedo, C. A., Sánchez, E. Y., Reyes, J. G., & Young, M. E. (2010). Volatile profiles of human skin cell cultures in different degrees of senescence. Journal of Chromatography B, 878, 449–455. doi:10.1016/j.jchromb.2009.12.033
- Arredondo, T., Oñate, E., Santander, R., Tomic, G., Silva, J. R., Sánchez, E., & Acevedo, C. A. (2014). Application of neural networks and meta-learners to recognize beef from OTM-cattle by using volatile organic compounds. Food and Bioprocess Technology, 7, 3217–3225. doi:10.1007/s11947-014-1289-7
- Deng, C., Zhang, X., & Li, N. (2004). Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography-mass spectrometry. Journal of Chromatography B, 808, 269–277. doi:10.1016/j.jchromb.2004.05.015
- Devine, C. E., Ellery, S., & Averill, S. (1984). Responses of different types of ox muscle to electrical stimulation. Meat Science, 10, 35–51. doi:10.1016/0309-1740(84)90030-5
- Efron, B., & Gong, G. (1983). A leisurely look at the bootstrap, the Jack-knife, and cross-validation. American Statistician, 37, 36–48.
- Elmore, J. S., Cooper, S. L., Enser, M., Mottram, D. S., Sinclair, L. A., Wilkinson, R. G., & Wood, J. D. (2005). Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Science, 69, 233–242. doi:10.1016/j.meatsci.2004.07.002
- Elmore, J. S., Mottram, D. S., Enser, M., & Wood, J. D. (1999). Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. Journal of Agricultural and Food Chemistry, 47, 1619–1625. doi:10.1021/jf980718m
- Elmore, J. S., Warren, H. E., Mottram, D. S., Scollan, N. D., Enser, M., Richardson, R. I., & Wood, J. D. (2004). A comparison of the aroma volatiles and fatty acid compositions of grilled beef muscle from Aberdeen Angus and Holstein–Friesian steers fed diets based on silage or concentrates. Meat Science, 68, 27–33. doi:10.1016/j.meatsci.2004.01.010
- Ercolini, D., Russo, F., Nasi, A., Ferranti, P., & Villani, F. (2009). Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Applied and Environmental Microbiology, 75, 1990–2001. doi:10.1128/AEM.02762-08
- Eriksson, L., Johansson, E., Kettaneh-Wold, N., Trygg, J., Wikstrom, C., & Wold, S. (2006). Multi and megavariate data analysis. Part I: Basic principles and applications (3rd ed.). Umea: Umetrics Academy.
- Frank, L. E., & Friedman, J. H. (1993). A statistical view of some chemometrics regression tools. Technometrics, 35, 109–135. doi:10.1080/00401706.1993.10485033
- Hernández-Macedo, M. L., Contreras-Castillo, C. J., Tsai, S. M., Da Cruz, S. H., Sarantopoulos, C. I. G. L., Padula, M., & Dias, C. T. S. (2012). Gases and volatile compounds associated with micro-organisms in blown pack spoilage of Brazilian vacuum-packed beef. Letters in Applied Microbiology, 55, 467–475. doi:10.1111/lam.12004
- Holm, E. S., Adamsen, A. P. S., Feilberg, A., Schäfer, A., Løkke, M. M., & Petersenet, M. A. (2013). Quality changes during storage of cooked and sliced meat products measured with PTR-MS and HS-GC–MS. Meat Science, 95, 302–310. doi:10.1016/j.meatsci.2013.04.046
- Holm, E. S., Schäfer, A., Koch, A. G., & Petersen, M. A. (2013). Investigation of spoilage in saveloy samples inoculated with four potential spoilage bacteria. Meat Science, 93, 687–695. doi:10.1016/j.meatsci.2012.11.016
- Hwang, Y.-H., Kim, G.-D., Jeong, J.-Y., Hur, S.-J., & Joo, S.-T. (2010). The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Science, 86, 456–461. doi:10.1016/j.meatsci.2010.05.034
- Insausti, K., Goñi, V., Petri, E., Gorraiz, C., & Beriain, M. J. (2005). Effect of weight at slaughter on the volatile compounds of cooked beef from Spanish cattle breeds. Meat Science, 70, 83–90. doi:10.1016/j.meatsci.2004.12.003
- Jääskeläinem, E., Johansson, P., Kostiainen, O., Nieminen, T., Schmidt, G., Somervuo, P., … Björkroth, J. (2013). Significance of heme-based respiration in meat spoilage caused by leuconostoc gasicomitatum. Applied and Environmental Microbiology, 79, 1078–1085. doi:10.1128/AEM.02943-12
- Martin, D., Antequera, T., Muriel, E., Perez-Palacios, T., & Ruiz, J. (2009). Volatile compounds of fresh and dry-cured loin as affected by dietary conjugated linoleic acid and monounsaturated fatty acids. Meat Science, 81, 549–556. doi:10.1016/j.meatsci.2008.10.010
- Meynier, A., Genot, C., & Gandemer, G. (1999). Oxidation of muscle phospholipids in relation to their fatty acid composition with emphasis on volatile compounds. Journal of the Science of Food and Agriculture, 79, 797–804. doi:10.1002/(ISSN)1097-0010
- Muriel, E., Antequera, T., Petrón, M. J., Andrés, A. I., & Ruiz, J. (2004). Volatile compounds in Iberian dry-cured loin. Meat Science, 68, 391–400. doi:10.1016/j.meatsci.2004.04.006
- Osorio, M. T., Zumalacárregui, J. M., Cabeza, E. A., Figueira, A., & Mateo, J. (2008). Effect of rearing system on some meat quality traits and volatile compounds of suckling lamb meat. Small Ruminant Research, 78, 1–12. doi:10.1016/j.smallrumres.2008.03.015
- Procopio, S., Krause, D., Hofmann, T., & Becker, T. (2013). Significant amino acids in aroma compound profiling during yeast fermentation analyzed by PLS regression. LWT - Food Science and Technology, 51, 423–432. doi:10.1016/j.lwt.2012.11.022
- Resconi, V. C., Escudero, A., & Campo, M. M. (2013). Review: The development of aromas in ruminant meat. Molecules, 18, 6748–6781. doi:10.3390/molecules18066748
- Rivas-Cañedo, A., Fernández-García, E., & Nuñez, M. (2009). Volatile compounds in fresh meats subjected to high pressure processing: Effect of the packaging material. Meat Science, 81, 321–328. doi:10.1016/j.meatsci.2008.08.008
- Santander, R., Creixell, W., Sánchez, E., Tomic, G., Silva, J. R., & Acevedo, C. A. (2013). Recognizing age at slaughter of cattle from beef samples using GC/MS-SPME chromatographic method. Food and Bioprocess Technology, 6, 3345–3352. doi:10.1007/s11947-012-0998-z
- Shahidi, F. (1998). Flavor of meat, meat products and seafoods (2nd ed.). London: Blackie Academic and Professional, and Imprint of Charpman & Hall.
- Shi, X., Zhang, X., Song, S., Tan, C., Jia, C., & Xia, S. (2012). Identification of characteristic flavour precursors from enzymatic hydrolysis-mild thermal oxidation tallow by descriptive sensory analysis and gas chromatography-olfactometry and partial least squares regression. Journal of Chromatography B, 913–914, 69–76. doi:10.1016/j.jchromb.2012.11.032
- Sivadier, G., Ratel, J., Bouvier, F., & Engel, E. (2008). Authentication of meat products: Determination of animal feeding by parallel GC-MS analysis of three adipose tissues. Journal of Agricultural and Food Chemistry, 56, 9803–9812. doi:10.1021/jf801276b
- Tejeda, J. F., García, C., Petrón, M. J., Andrés, A. I., & Antequera, T. (2001). N-alkane content of intramuscular lipids of Iberian fresh ham from different feeding systems and crossbreeding. Meat Science, 57, 371–377. doi:10.1016/S0309-1740(00)00114-5
- Vasta, V., D’Alessandro, A. G., Priolo, A., Petrotos, K., & Martemucci, G. (2012). Volatile compound profile of ewe’s milk and meat of their suckling lambs in relation to pasture vs. indoor feeding system. Small Ruminant Research, 105, 16–21. doi:10.1016/j.smallrumres.2012.02.010
- Vasta, V., Jerónimo, E., Brogna, M. R., Dentinho, T. P., Biondi, L., Silva, J. S., … Bessa, J. B. (2010). The effect of grape seed extract or Cistus ladanifer L. on muscle volatile compounds of lambs fed dehydrated lucerne supplemented with oil. Food Chemistry, 119, 1339–1345. doi:10.1016/j.foodchem.2009.09.010
- Vasta, V., Ratel, J., & Engel, E. (2007). Mass spectrometry analysis of volatile compounds in raw meat for the authentication of the feeding background of farm animals. Journal of Agricultural and Food Chemistry, 55, 4630–4639. doi:10.1021/jf063432n
- Watanabe, A., Ueda, Y., Higuchi, M., & Shiba, N. (2008). Analysis of volatile compounds in beef fat by dynamic-headspace solidphase microextraction combined with gas chromatography–mass spectrometry. Journal of Food Science, 73, C420–C425. doi:10.1111/j.1750-3841.2008.00764.x
- Wold, S., Sjöström, M., & Eriksson, L. (2001). PLS-regression: A basic tool of chemometrics. Chemometrics and Intelligent Laboratory Systems, 58, 109–130. doi:10.1016/S0169-7439(01)00155-1
- Ylä-Ajos, M., Ruusunen, M., & Puolanne, E. (2006). The significance of the activity of glycogen debranching enzyme in glycolysis in porcine and bovine muscles. Meat Science, 72, 532–538. doi:10.1016/j.meatsci.2005.09.004
- Zhang, Z., Cai, J., Ruan, G., & Li, G. (2005). The study of fingerprint characteristics of the emanations from human arm skin using the original sampling system by SPME-GC/MS. Journal of Chromatography B, 822, 244–252. doi:10.1016/j.jchromb.2005.06.026