ABSTRACT
The study examined the effect of refrigerated storage on antioxidant activities, lipid and protein oxidation, fatty acids (FAs), drip loss and color of semimembranosus (SM) muscle from goats. Samples of SM were obtained from carcasses of 15 Boer bucks (7 months old; body weight, 32.18 ± 0.81 kg) subjected to an 8 d storage at 4°C. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) activities were stable while carotenoid, tocopherol, water holding capacity and redness declined (P < 0.05) as storage progressed. Carbonyl content increased from 1.85 to 4.73 nmol/mg protein while thiol content reduced from 54.22 to 42.82 nmol/mg protein. The TBARS value increased from 0.2 to 0.8 mg MDA/kg. SDS-PAGE expression of myosin heavy chain (MHC) decreased (P < 0.05) from 72.45 to 49.82 density/mm2 while actin reduced (P > 0.05) from 14.00 to 13.08 density/mm2. The concentrations of n-3 and n-6 FA decreased while the saturated FA increased over storage. Correlations (P < 0.05) were found between antioxidant vitamins and quality indicators of chevon.
RÉSUMÉ
Este estudio examinó el efecto del almacenamiento por refrigeración en las actividades antioxidantes, la oxidación lipídica y proteínica, los ácidos grasos (FA), la pérdida por goteo y el color del músculo semimembranoso (SM) en las cabras. Se obtuvieron muestras de SM de los cuerpos de quince cabras bóer (7 meses; peso 32.18±0.81 kg) sujetos a un almacenamiento de 8 días a 4°C. Las actividades de la superóxido dismutasa, la catalasa y la glutationa peroxidasa fueron estables mientras que disminuyeron los carotenoides, los tocoferoles, la pérdida por goteo y el color rojizo (P< 0,05) a medida que el almacenamiento se alargaba. El contenido de carbonilo aumentó de 1,85 a 4,73 nmol/mg de proteína mientras que el contenido de tiol se redujo de 54.22 a 42.82 nmol/mg de proteína. El valor TBARS aumentó de 0,2 a 0,8 mg MDA/kg. La expresión SDS-PAGE de la cadena pesada de miosina disminuyó (P< 0,05) de 72,45 a 49,82 densidad/mm2 mientras que la actina se redujo (P> 0,05) de 14,00 a 13,08 densidad/mm2. La concentración de los FA de n-3 y n-6 disminuyó mientras que los FA saturados aumentaron con el almacenamiento. Se encontraron correlaciones (P< 0,05) entre las vitaminas antioxidantes y los indicadores de calidad de la carne de ejemplares adultos.
Introduction
Postmortem aging of meat at refrigeration temperature encourages proteolytic degradation of myofibrillar proteins by endogenous proteases, thereby improving tenderness (Lawrie & Ledward, Citation2006). Nonetheless, loss of antioxidants during aging is inevitable (Renerre, Dumont, & Gatellier, Citation1996), creating a condition in which the balance between antioxidant and pro-oxidant capacity favors oxidative damage. Prominent among the changes influenced by postmortem aging are oxidative deterioration of lipids (Liu, Xu, Dai, & Ni, Citation2015; Popova, Marinova, Vasileva, Gorinov, & Lidji, Citation2009; Sabow et al., Citation2015a) and proteins (Astruc, Marinova, Labas, Gatellier, & Santé-Lhoutellier, Citation2007; Martinaud et al., Citation1997). Both lipid and protein oxidation could jeopardize the safety, nutritional and sensory properties and shelf life of meat (Astruc et al., Citation2007; Martinaud et al., Citation1997; Olorunsanya, Adeyemi, & Babatunde, Citation2011; Sola-Ojo et al., Citation2013). The major factor controlling postmortem oxidative stability is the antioxidant/pro-oxidant status of muscle (Popova et al., Citation2009; Renerre, Poncet, Mercier, Gatellier, & Métro, Citation1999). Muscle tocopherol plays a dominant role in the maintenance of oxidative stability of meat (Ponnampalam et al., Citation2014; Renerre et al., Citation1999). There are indications that the onset of oxidative spoilage in meat could be delayed by endogenous antioxidant enzymes (Pradhan, Rhee, & Hernández, Citation2000; Renerre et al., Citation1999; Rhee, Anderson, & Sams, Citation1996).
There is a growing demand for goat meat (Devendra, Citation2015; Gandhi, Citation2015) because its quality attributes are concordant with the present-day consumers’ demand (Webb, Citation2014). Despite the importance of goat meat in human diet, little attention has been paid to the impact of processing conditions on its physicochemical properties (Webb, Casey, & Simela, Citation2005). For instance, compared with beef (Kang, Kang, Seong, Park, & Cho, Citation2014; Renerre et al., Citation1996) and mutton (Díaz et al., Citation2011; Muíño et al., Citation2014; Petron et al., Citation2007), the effects of chill storage on antioxidant vitamin and enzyme activities and changes in fatty acid (FA) composition are poorly documented in goats. The impact of lipid oxidation on meat quality has been studied extensively, but protein oxidation and its associated effects on meat quality have not been completely elucidated (Astruc et al., Citation2007; Lund, Heinonen, Baron, & Estevez, Citation2011). In particular, the impact of chill storage on protein oxidation and changes in myofibrillar proteins in relation to meat quality in goat meat remain elusive. Thus, the objective of this study was to assess the effect of refrigerated storage on antioxidant status, FA composition, lipid and protein oxidation, drip loss and color of semimembranosus (SM) muscle in goats.
Materials and methods
Animal welfare
The study was conducted following the guidelines of research policy of the Universiti Putra Malaysia on animal welfare and animal ethics.
Animal slaughtering and muscle sampling
Fifteen Boer bucks (7 months old; average body weight, 32.18 ± 0.81 kg) were slaughtered according to the halal procedure as outlined in MS1500:2009 (Department of Standards Malaysia, Citation2009). The carcasses were subjected to chill storage (4°C) for 8 d. On 0, 1, 4 and 8 d, SM muscle was sampled from the right hind limb and divided into three parts. The first part was snap frozen in liquid nitrogen, stored at −80°C and assigned for the determination of antioxidants and lipid and protein oxidation. The second part was vacuum packaged, weighed, stored in the chiller (4°C) and assigned for the determination of drip loss. The third part was assigned for the determination of color.
Determination of color
Meat color was assessed by measuring L* (lightness), a* (redness) and b* (yellowness) values using a colorimeter Color Flex spectrophotometer (Hunter Lab Reston, Reston, VA, USA). The colorimeter was calibrated against black and white reference tiles prior to use and the color coordinates of samples were determined after a 30 min blooming period using illuminant D65 as the light source.
Determination of drip loss
Drip loss was measured according to the method of Sabow et al. (Citation2015b). Fresh meat samples (25 g) obtained on d 0 were weighed and recorded as initial weight (W1). The weighed samples were placed into polyethylene plastic bags, labeled, vacuum packaged and stored at 4 °C. After 1, 4 and 8 d postmortem, the samples were removed from the bags, gently blotted dry, weighed and recorded as W2. Drip loss was calculated and expressed as the percentage of difference between initial and final weights of sample after storage divided by the initial weight of sample.
Antioxidant enzyme activity
The glutathione peroxidase (GPX) was measured with the aid of EnzyChromTM Glutathione Peroxidase Assay Kit EGPX-100 (BioAssay Systems, USA), the superoxide dismutase (SOD) activity was measured with the aid of Cayman SOD Assay kit 706002 (Cayman chemical), while the catalase (CAT) activity was measured using Cayman Catalase Assay Kit 707002 (Cayman chemical) following the manufacturer’s procedure.
Total carotenoid content
Meat carotenoid contents were extracted and quantified as described by Okonkwo (Citation2009). Briefly, 4 g of each sample was homogenized with 20 ml acetone. The contents were stirred for 30 min and two 10 ml aliquots of acetone were used to rinse the flask and re-extract the residue. The extracts were pooled and 2 ml of deionized water was added. The mixture was transferred into 10 ml n-hexane and centrifuged at 3500 g for 15 min. The absorbance of the hexane layer was read at 450 nm using a spectrophotometer (Secomam, Domont, France). The total carotenoid content was estimated using the following formula:
where A = absorbance
V = volume of n-hexane (ml)
W = sample weight
Determination of tocopherol contents
Extraction of tocopherol from meat samples followed the method of Kamal-Eldin et al. (Citation2000). Quantification of tocopherol contents was done using Agilent 1200 series HPLC as described by Pegg and Amarowicz (Citation2009). The column used was C30 YMCTM carotenoid (250 mm x 4.6 mm. i.d, 5 μm) (YMC, USA). An isocratic mobile phase made up of 99% n-hexane and 1% Isopropanol was used. The flow rate was 0.5 ml/min and the injection volume was 20 μL. UV detection was monitored at 295 nm. The isomers of tocopherol were quantified by comparing the peak area of samples with those of tocopherol standards.
Determination of lipid oxidation
Lipid oxidation was measured as 2-thiobarbituric acid reactive substances (TBARS) using QuantiChromTM TBARS Assay Kit (DTBA-100, BioAssay Systems, USA) following the description of the manufacturer.
Determination of free thiol content
The free thiol contents were quantified according to Elman’s method using 2,2-dithiobis (5-nitropyridine) DTNP (Winterbourn, Citation1990). The thiol concentration was measured using a spectrophotometer (Spectronic instruments, USA) at 386 nm and was calculated using an absorption coefficient of 14 g−1 cm−1. The results were expressed as nanomoles of free thiols per milligram of protein.
Determination of carbonyl content
The carbonyl content in muscles was determined using a Cayman protein carbonyl colorimetric assay kit (10005020) following the manufacturer’s procedure. Results were expressed as nanomoles of 2, 4 dinitrophenylhydrazine (DNPH) per milligram of myofibrillar protein.
Extraction of myofibrillar proteins
Myofibrillar proteins were isolated using an extraction buffer containing 150 mM NaCl, 25 mM KCl, 3 mM MgCl2 and 4 mM EDTA at pH 6.5 as described by Morzel, Gatellier, Sayd, Renerre, and Laville (Citation2006). The total protein concentration of the sample was determined by the method of Bradford (Citation1976) using Protein Assay Kit II 500–0002 (Bio-Rad, USA). Bovine serum albumen (BSA) was used to prepare the protein standards.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Myofibrillar proteins were incubated for 10 min at 90 ºC in a sample buffer containing 30% (v/v) glycerol, 5% (v/v) ß-mercaptoethanol, 2.3% (w/v) SDS, 62.5 mM Tris–HCl (pH 6.8) and 0.05% (w/v) bromophenol blue at a ratio of 1:1(v/v). One-dimensional SDS-PAGE was performed according to the method of Laemmli (Citation1970) using polyacrylamide gels of 8 cm × 5.5 cm x 0.8 mm (length × width x thickness). Twelve percent resolving gels were prepared for actin, whereas 5% resolving gels were prepared for myosin heavy chain (MHC). The resolving gels were overlaid with 4% stacking gel solution and kept overnight at 4°C to allow complete polymerization. The protein load was adjusted to 30 μg per lane. Proteins were separated in running buffer containing 0.025 M Tris base, 0.192 M glycine, 0.1% SDS at pH 8.3 under constant voltage of 120 V and 400 mA for 90 min, following which the tracking dye reached the bottom of the gel (Adeyemi, Mislan, Aghwan, Sarah, & Sazili, Citation2014). Protein bands were stained with 0.05% Coomassie blue staining solution for 60 min and destained with destaining solution for 30 min. The bands of myofibrillar proteins were visualized using GS-800 Calibrated Imaging Densitometer (Bio-Rad, USA).
Western blotting
The fractionated proteins that were initially separated from the samples based on their molecular weight through gel electrophoresis were transferred from the gel onto polyvinylidene difluoride (PVDF) membranes using Trans-Blot® SD semi-dry transfer system cell (Bio-Rad, USA). MHC was transferred at a constant amperage of 250 mA per gel, voltage limit of 25 V for 135 min, whereas actin was transferred at the same amperage and voltage for 45 min. After transfer, membranes were blocked for 3 h at room temperature in a blocking solution [5% bovine serum albumin (BSA) in TBS-T buffer (100 mM Tris-HCl; 150 mM NaCl; 0.05% Tween 20)]. Blots were washed thrice (10 min per wash) in PBS-T incubated overnight at room temperature with the primary antibody, which was diluted 1: 500 in TBS-T containing 3% BSA. Monoclonal Anti-Myosin (Skeletal, Fast), produced in mouse; Cat #. M4276, Monoclonal Anti-Myosin (Skeletal, Slow), antibody produced in mouse; Cat # M842 and monoclonal Anti actin antibody produced in rabbit; Cat # A2066 227 from Sigma- Aldrich, USA, were the primary antibodies used for MHC (fast), MHC (slow) and actin, respectively. Subsequently, the membranes were incubated with secondary antibody [anti-mouse IgG (whole molecule) – peroxidase, antibody developed in rabbit; Cat # A9044 from Sigma- Aldrich, USA] diluted 1:10000 in 3% BSA in TBS-T buffer for 90 min at room temperature. This was followed by three times washing with TBS-T buffer. The blocked membranes were detected using a DAB substrate kit Code: E885 (DAB SUBSTRATE SYSTEM (aMReSCO®, Solon, DH, USA)). MHC and actin band intensities were measured using a GS-800 Calibrated Imaging Densitometer (Bio-Rad, USA) followed by quantification of the bands’ intensity using Quantify One® software.
Determination of FA composition
The FAs of the muscle samples were extracted in chloroform: methanol (2:1, v/v) mixture following the method of Folch, Lees, and Sloane-Stanley (Citation1957) modified by Rajion, McLean, and Cahill (Citation1985) and described by Adeyemi et al. (Citation2015a). The FAs were transmethylated into their fatty acid methyl esters (FAMEs) using 0.66 N KOH in methanol and 14% methanolic boron trifluoride (BF3) in accordance with the method of Association of Official Analytical Chemists (AOAC Citation1990). Heneicosanoic acid was used as internal standard. FAME was separated in a gas chromatograph (Agilent 7890A) equipped with a flame ionization detector (FID) and a splitless injector. The column used was fused silica capillary (Supelco SP-2560, 100 m, 0.25 mm ID, 0.20 mm film thickness). High-purity nitrogen was used as the carrier gas at 40 ml/min. Compressed air and high-purity hydrogen were used for the FID in the chromatograph. To facilitate optimal separation, the oven temperature was set at 100 °C for 2 min and warmed to 170 °C at 10 °C/min, held for 2 min, warmed to 230 °C at 5°C/min, and then held for 20 min. Identification of sample FAs was done by comparing the relative retention times of FAME peaks from samples with those of standards.
Statistical analysis
The data obtained were analyzed as repeated measures using the General Linear Model (GLM) procedure of SAS (SAS Institute Inc., Cary, NC, USA). Means were separated by Tukey HSD test and the P value was set at 0.05. Correlations between the measured variables were estimated using the CORR procedure of SAS.
Results and discussion
Color
Meat color is an important parameter used for the appraisal and selection of meat (Lawrie & Ledward, Citation2006). Chill storage had a significant impact on the a* and L* values of SM muscle from goats (). Chill storage had no effect (P > 0.05) on b* throughout storage. The L* and a* values observed on d 0 were similar to those observed on d 1 and 4. However, on d 8, the a* value was lower (P < 0.05) while the L* value was higher (P < 0.05) compared with those observed on other storage days. This observation could be due to the oxidation of myoglobin to form metmyoglobin due to decreased metmyoglobin reducing activity (Xue, Huang, Huang, & Zhou, Citation2012). The decrease in a* and the increase in L* over storage is consistent with the findings in chilled beef (Liu et al., Citation2015) and chevon (Sabow et al., Citation2015b). The current results also indicate that the color stability of SM muscle from goats could be maintained for up to 4 d during chill storage.
Table 1. Effect of chill storage on color and drip loss in semimembranosus muscle in goats.
Tabla 1. Efecto del almacenamiento en frío en el color y la pérdida por goteo del músculo semimembranoso en las cabras.
Drip loss
The ability of meat to keep its inherent water is called the water-holding capacity (WHC). WHC can affect the quality and yield of the end product (Lonergan, Huff-Lonergan, Rowe, Kuhlers, & Jungst, Citation2001). In fresh uncooked meat, WHC is called drip loss or purge (Lawrie & Ledward, Citation2006). The impact of chill storage on drip loss in SM muscle in goats is shown in . Drip loss increased (P < 0.05) as storage continued. This observation could be due to the weakening of myofibrillar proteins by proteolytic enzymes during aging, thereby affecting the ability of myofibrillar proteins to hold water (Adeyemi & Sazili, Citation2014; Lawrie & Ledward, Citation2006). Increase in protein oxidation has been linked to decrease in WHC of meat (Lonergan et al., Citation2001). The current finding is consistent with those observed during chill storage of pork (Lonergan et al., Citation2001) and chevon (Sabow et al., Citation2015b).
Antioxidant enzymes and vitamins
The antioxidant profile of SM muscle in goats during chill storage is shown in . The concentration of total carotenoids and α, γ and δ-tocopherol decreased (P < 0.05) as chill storage advanced. This finding is akin to those of Irie, Fujita, and Sudou (Citation1998) who observed a decrease in the concentration of the vitamin E content of beef aged for 10 d.
Table 2. Effect of chill storage on antioxidant status, lipid oxidation and protein oxidation in semimembranosus muscle in goats.
Tabla 2. Efecto del almacenamiento en frío en el estado antioxidante, la oxidación lipídica y la oxidación proteínica del músculo semimembranoso en las cabras.
The SOD and catalase (CAT) activities observed in the current study were lower than the values reported for different beef muscles (Renerre et al., Citation1996) but higher than the values reported for rabbit (Hernández, López, Marco, & Blasco, Citation2010), turkey (Renerre et al., Citation1999) and pork (Hernández, Park, & Rhee, Citation2002). In contrast, chevon had higher GPX activity compared with beef (Renerre et al., Citation1996), but comparable to that of turkey (Renerre et al., Citation1999).
Chill storage had no effect (P > 0.05) on antioxidant enzyme activities. The stability of CAT during chill storage is in tandem with the findings in beef (Pradhan et al., Citation2000; Renerre et al., Citation1996) and fish (Watanabe, Goto, Abe, & Nakano, Citation1996). The stability of GPX contrasts the findings in rabbit (Hernández et al., Citation2010) and turkey (Renerre et al., Citation1999), where GPX decreased over storage. Contrary to the stability of SOD activity in the current study, SOD activity in beef declined over storage (Renerre et al., Citation1996) while SOD activity in turkey increased over storage (Renerre et al., Citation1999).
Lipid oxidation
Oxidative deterioration of lipid is a leading cause of quality deterioration of muscle foods during processing and storage (Liu et al., Citation2015; Popova et al., Citation2009). The presence of unsaturated lipids, metal catalyst, heme pigments and myriad oxidizing agents in the muscle tissue makes it susceptible to oxidative spoilage (Liu et al., Citation2015; Popova et al., Citation2009; Renerre et al., Citation1999). Chill storage had a significant impact on lipid oxidation. The TBARS values increased (P < 0.05) from 0.2 to 0.8 mg MDA/kg sample. This observation could be due to the generation of reactive oxygen species resulting from the collapse of antioxidant defense systems. This finding concurs with the earlier reports on refrigerated broiler meat (Adeyemi, Olorunsanya, & Akanji, Citation2011; Xiao, Zhang, Lee, Ma, & Ahn, Citation2011), beef (Liu et al., Citation2015; Renerre et al., Citation1996), mutton (Díaz et al., Citation2011; Muíño et al., Citation2014; Petron et al., Citation2007) and chevon (Sabow et al., Citation2015a). The TBARS value observed in the current study was lower than 5 mg MDA/kg, indicating discernable abnormal flavor development in meat (Insausti et al., Citation2001).
Carbonyl content
Carbonyl is one of the prominent products of oxidized proteins whose quantification has been widely used as a reliable indicator for protein oxidation in food and biological systems (Popova & Marinova, Citation2013; Zhang, Xiao, & Ahn, Citation2013). In this study, the carbonyl content increased from 1.85 nmol/mg protein on d 0 to 4. 73 nmol/mg protein on d 8, representing a 61% increase (a 2.5-fold increase). A 2- to 5-fold increase in carbonyl content was observed in mackerel fillet between 0 and 8 d of chill storage (Srinivasan & Hultin, Citation1994). Similarly, Martinaud et al. (Citation1997) reported that the carbonyl content increased from 3.1 to 5.1 nmol/mg protein (70% increase) in beef longissimus lumborum and from 4.8 to 6.9 nmol/mg protein (44% increase) in diaphragma pedialis from 1 to 10 d of chill storage. Astruc et al. (Citation2007) observed that chill storage (4°C) induced protein oxidation at the inner and outer surfaces of bovine rectus abdominis. The authors observed that the carbonyl content of myofibrillar proteins increased from 1.5 nmol/mg protein on day 0 to 2.8 nmol/mg protein on d 10 of chill storage. The authors also observed higher carbonyl content in the outer surface compared with the inner surface of bovine rectus abdominis.
Free thiol content
Free thiol, a sulfhydryl group (SH) of cysteine residue, is an important parameter for accessing the extent of protein oxidation in tissues (Nieto, Jongberg, Andersen, & Skibsted, Citation2013). Chill storage influenced (P < 0.05) free thiol concentration. The free thiol content reduced from 54.20 nmol/mg protein on d 0 to 42.82 nmol/mg protein on d 8, representing about 20.99% decrease. The free thiol content observed on d 0 in the present study was comparable to the 50–62 nmol/mg protein reported for pork (Nieto et al., Citation2013), but lower than the 66–70 nmol/mg protein in beef (Martinaud et al., Citation1997). The decrease in thiol group over storage is consistent with the findings of Martinaud et al. (Citation1997), who observed a 10% decrease in thiol (from 66.2 to 59.6 nmol/mg protein) in longissimus lumborum and a 17% decrease in thiol (from 71.9 to 60.2 nmol/mg protein) in diaphragma pedialis during a 10 d aging of beef at 0–5°C. In addition, Nieto et al. (Citation2013) observed a reduction in thiol content during a 9 d chill storage of pork. Loss of protein thiol resulted from oxidation reactions involving the side chains of amino acids (Lund et al., Citation2011). This reaction is often accompanied by loss of solubility, catalytic activities and increased susceptibility to protein degradation and aggregation (Rowe, Maddock, Lonergan, & Huff-Lonergan, Citation2004).
Degradation of myofibrillar proteins
The SDS-PAGE pattern of myofibrillar proteins of SM muscle in goats during an 8 d chill storage is shown in while the reflective density is presented in . The band intensity and reflective density of MHC decreased (P < 0.05) over storage. The reflective density of MHC decreased (P < 0.05) from 72.45 density/mm2 on d 0 to 49.82 density/mm2 on d 8, representing a 31.24% decrease. The MHC constitutes about 45% of the total myofibrillar protein in the skeletal muscles of animals (Lefaucheur, Citation2010) and it is highly susceptible to oxidation (Lund et al., Citation2011; Morzel et al., Citation2006). Oxidation of chicken myofibrils brought about changes in MHC, particularly intermolecular cross-linking of MHC and modifications of thiol groups at the myosin ATPase active site (Ooizumi & Xiong, Citation2004). In addition, Addeen, Benjakul, Wattanachant, and Maqsood (Citation2014) and Sun, Cui, Zhao, Zhao, and Yang (Citation2011) observed that the protein band corresponding to MHC was protein most sensitive to oxidation, while proteins of lower molecular weights appeared to oxidize later. In contrast, MHC of beef SM was stable throughout a 28 d chill storage at 4 °C (Bandman & Zdanis, Citation1988). In addition, Koohmaraie (Citation1994) posited that myosin was stable at refrigeration temperature (0–5°C), but can undergo substantial degradation at temperatures higher than 25°C.
Figure 1. SDS-PAGE of myofibrillar proteins of semimembranosus muscle in goats at 0, 4 and 8 d postmortem. Equal amounts of protein (30 μg) of each sample were loaded and electrophoresed on a separate 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at a constant voltage (120 V) for 90 min. The gels were stained with Coomassie blue staining for 60 min and destained with a destaining solution for 45 min.
Figura 1. SDS-PAGE de proteínas miofibrilares del músculo semimembranoso en las cabras a 0, 4 y 8 días postmortem. Se cargaron las mismas cantidades de proteína (30 μg) de cada muestra y estuvieron sujetas a electroforesis en un 12% separado de electroforesis en gel de poliacrilamida de sulfato dodecílico de sodio (SDS-PAGE) a un voltaje constante (120 V) durante 90 min. Los geles fueron manchados con azul de Coomassie durante 60 min y decolorados con solución decolorante durante 45 min.
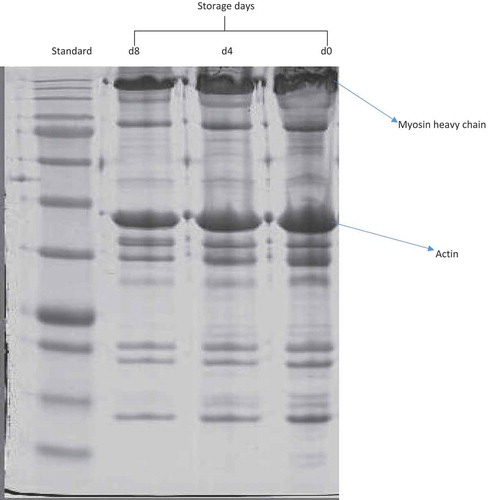
Actin and actin-bundling proteins play significant roles in muscle contraction (Xue et al., Citation2012). Unlike MHC, the band intensity corresponding to actin underwent (P > 0.05) slight degradation as storage progressed. The reflective density of actin reduced from 14.00 density/mm2 on d 0 to 13.08 density/mm2 on d 8 of chill storage (), representing a 6.57% loss. In line with the current observation, Gil et al. (Citation2006) did not observe significant degradation of actin during aging of rabbit meat at 0–5°C for 7 d. In addition, Koohmaraie (Citation1994) reported that actin was stable at refrigeration temperature (0–5°C) but can undergo substantial degradation at temperatures higher than 25°C. The oxidative stability of actin could be due to the unavailability of oxidation sites, which might have been hidden by the reaction of actin with myosin chain in myofibrillar suspensions (Xue et al., Citation2012).
Table 3. Effect of chill storage on the reflective density of myofibrillar proteins in semimembranosus muscle in goats.
Tabla 3. Efecto del almacenamiento en frío en la densidad de reflexión de las proteínas miofibrilares del músculo semimembranoso en las cabras.
FA composition
The FA composition of SM muscle from goats during chill storage is shown in . The major FAs were C18:1n-9, C18:0 and C16:0. Similarly, the major FAs in longissimus dorsi muscle from sheep (Díaz et al., Citation2011; Muíño et al., Citation2014; Popova, Citation2014) and triceps brachii muscle from goats (Adeyemi et al., Citation2015b) were C16:0, C18:0 and C18:1n-9. The concentrations of C14:0, C16:0 and C18:0 increased (P < 0.05) over storage. The concentrations of C18:2n-6, C20:5n-3 and total polyunsaturated fatty acids (PUFA) decreased (P < 0.05) as storage advanced. The concentrations of C18:3n-3, C20:4n-6, C22:6n-3, C22:5n-3 and total n-3 FA on d 0 were similar (P < 0.05) to those observed on d 4 but higher than those observed on d 8 of chill storage. The decrease in the concentration of PUFA could be due to lipid oxidation resulting from the decline in the concentration of antioxidant vitamins over storage. The decline in PUFA concentration could be responsible for the increase in SFA over storage. Similar findings were observed during chill storage of mutton for 7 d (Díaz et al., Citation2011) and 12 d (Muíño et al., Citation2014). The concentrations of C16:1n-7, C18:1n-9, C18:1t11, total monounsaturated fatty acids (MUFA), CLA c9t11 and CLA c12t10 were stable throughout storage. This could be due to the higher oxidative stability of MUFA and CLA compared with the n-3 and n-6 PUFA.
Table 4. Effects of chill storage on fatty acid composition (mg/kg muscle) of semimembranosus muscle in goats.
Tabla 4. Efectos del almacenamiento en frío en la composición de ácidos grasos (mg/kg de músculo) del músculo semimembranoso en las cabras.
Correlations between antioxidants and quality indicators of SM muscle from goats
shows the correlations between antioxidant activities and quality indicators in SM muscle in goats. The concentration of antioxidant vitamins (total carotenoids and α, γ and δ-tocopherol) was positively correlated (P < 0.05) with redness (a*) and negatively correlated (P < 0.05) with lightness (L*). This observation could be attributed to the role of antioxidant vitamins in the prevention of myoglobin oxidation and the accumulation of metmyoglobin in meat. Thus, a decline in the concentration of antioxidant vitamins would be expected to lead to a reduction in the redness and an increase in the lightness of meat during chill storage. This observation is consistent with that of Liu et al. (Citation2015), who observed that the addition of antioxidants to ground beef patties enhanced its redness during chill storage. There was no significant correlation (P > 0.05) between antioxidant enzyme activities and the color coordinates of chevon. Redness was negatively correlated (P < 0.05) with TBARS, carbonyl content and L*. This observation is in tandem with that of Zakrys, Hogan, O’sullivan, Allen, and Kerry (Citation2008), who observed that the changes in a* values are related to lipid oxidation and are strongly correlated with the TBARS values.
Table 5. Correlation coefficient (r) between antioxidants and quality attributes of semimembranous muscle in goats.
Tabla 5. Coeficiente de correlación (r) entre los antioxidantes y los atributos cualitativos del músculo semimembranoso en las cabras.
Drip loss was positively correlated (P < 0.05) with carbonyl content but negatively correlated (P < 0.05) with antioxidant vitamins, free thiol content and the reflective density of MHC and actin. This indicates that oxidative degradation of myofibrillar proteins could be responsible for the increase in drip loss as chill storage progressed. In line with the current observation, Lonergan et al. (Citation2001) observed a positive correlation between drip loss and storage time, indicating that oxidative processes taking place in both protein and lipid fractions during postmortem storage can influence the ability of myofibrillar proteins to hold water, resulting in poor WHC. In addition, Estévez, Ventanas, Heinonen, and Puolanne (Citation2011) posited that the loss of amino groups from acid side chains of proteins can modify the electronic arrangement and the isoelectric point of myofibrillar proteins, which can lower the WHC. There was no significant (P > 0.05) correlation between drip loss and antioxidant enzyme activities.
The concentration of total carotenoids, α, γ and δ-tocopherol was negatively correlated (P < 0.05) with TBARS values and carbonyl content and negatively correlated with free thiol content and the reflective density of MHC and actin. This observation suggests that the increase in lipid and protein oxidation over storage resulted from the decline in the concentration of antioxidant vitamins. The protective role of alpha tocopherol against lipid and protein oxidation has been documented (Astruc et al., Citation2007; Rowe et al., Citation2004; Xiao et al., Citation2011). There was no significant correlation (P > 0.05) between antioxidant enzyme activities and the quality indicators of goat meat. This indicates that antioxidant enzymes play little or no role in postmortem antioxidant defense system in goat meat. Contrarily, earlier studies have espoused the role of antioxidant enzymes in maintaining the postmortem oxidative stability of meat (Pradhan et al., Citation2000; Renerre et al., Citation1999). No significant correlation (P > 0.05) was established between antioxidant enzyme activities and the concentration of antioxidant vitamins (total carotenoids and α, γ and δ-tocopherol).
The TBARS value was positively correlated (P < 0.05) with carbonyl content, and negatively correlated with free thiol and the reflective density of MHC and actin. This observation indicates that lipid oxidation was positively correlated with protein oxidation. This observation concurs with that of Xiao et al. (Citation2011), who found that carbonyl content was positively (P < 0.05) correlated with TBARS value during a 7 d chill storage of chicken meat. In addition, a timely coincidence of protein and lipid oxidation has been reported (Estévez, Kylli, Puolanne, Kivikari, & Heinonen, Citation2008). The onset of lipid oxidation in meat systems appears to occur faster than protein oxidation; thus it is more likely that lipid-derived radicals and hydroperoxides promote protein oxidation than the vice versa (Estévez et al., Citation2008). For instance, it has been documented that peroxyl radicals formed during lipid oxidation abstract hydrogen atoms from protein molecules, leading to a radical-mediated chain reaction similar to that of lipid oxidation (Stadtman & Levine, Citation2003).
Conclusion
The results of this study demonstrate that chill storage reduced the concentration of total carotenoids, and α, γ and δ-tocopherol, but had no effect on antioxidant enzyme activities in goat meat. Chill storage decreased the WHC, color, lipid and protein stability of goat meat. MHC decreased while actin was stable over storage. There were significant correlations between antioxidant vitamins and quality deterioration in goat meat during chill storage. Antioxidant enzymes did not affect the oxidative stability of goat meat.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Addeen, A., Benjakul, S., Wattanachant, S., & Maqsood, S. (2014). Effect of Islamic slaughtering on chemical compositions and post-mortem quality changes of broiler chicken meat. International Food Research Journal, 21, 897–907.
- Adeyemi, K., Mislan, N., Aghwan, Z., Sarah, S., & Sazili, A. (2014). Myofibrillar protein profile of pectoralis major muscle in broiler chickens subjected to different freezing and thawing methods. International Food Research Journal, 21, 1089–1093.
- Adeyemi, K., Olorunsanya, A., & Akanji, M. (2011). Effect of watermelon (Citrulus lantus) extracts on oxidative stability of broiler meat. African Journal of General Agriculture, 7, 103–108.
- Adeyemi, K. D., Ebrahimi, M., Samsudin, A. A., Alimon, A. R., Karim, R., Karsani, S. A., & Sazili, A. Q. (2015a). Influence of Carotino oil on in vitro rumen fermentation, metabolism and apparent biohydrogenation of fatty acids. Animal Science Journal, 86, 270–278. doi:10.1111/asj.2015.86.issue-3
- Adeyemi, K. D., & Sazili, A. Q. (2014). Efficacy of carcass electrical stimulation in meat quality enhancement: A review. Asian-Australasian Journal of Animal Sciences, 27, 447–456. doi:10.5713/ajas.2013.13463
- Adeyemi, K. D., Sazili, A. Q., Ebrahimi, M., Samsudin, A. A., Alimon, A. R., Karim, R. … Sabow, A. B. (2015b). Influence of dietary blend of canola oil and palm oil on nutrient intake and digestibility, growth performance, rumen fermentation and fatty acids in goats. Animal Science Journal. doi:10.1111/asj.12549
- Association of Official Analytical Chemists. (1990). Official methods of analysis (15th ed.). Washington, DC: Author.
- Astruc, T., Marinova, P., Labas, R., Gatellier, P., & Santé-Lhoutellier, V. (2007). Detection and localization of oxidized proteins in muscle cells by fluorescence microscopy. Journal of Agricultural and Food Chemistry, 55, 9554–9558. doi:10.1021/jf0717586
- Bandman, E., & Zdanis, D. (1988). An immunological method to assess protein degradation in post-mortem muscle. Meat Science, 22, 1–19. doi:10.1016/0309-1740(88)90023-X
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3
- Department of Standards Malaysia. (2009). MS1500:2009 (1st revision) 520 Halal food-production, preparation, handling and storage-general guideline. Cyberjaya: Department of Standards Malaysia.
- Devendra, C. (2015). Dynamics of goat meat production in extensive systems in Asia: Improvement of productivity and transformation of livelihoods. Agrotechnology, 4, 2–21.
- Díaz, M., Cañeque, V., Sánchez, C., Lauzurica, S., Pérez, C., Fernández, C. … De La Fuente, J. (2011). Nutritional and sensory aspects of light lamb meat enriched in n− 3 fatty acids during refrigerated storage. Food Chemistry, 124, 147–155. doi:10.1016/j.foodchem.2010.05.117
- Estévez, M., Kylli, P., Puolanne, E., Kivikari, R., & Heinonen, M. (2008). Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Science, 80, 1290–1296. doi:10.1016/j.meatsci.2008.06.004
- Estévez, M., Ventanas, S., Heinonen, M., & Puolanne, E. (2011). Protein carbonylation and water-holding capacity of pork subjected to frozen storage: Effect of muscle type, premincing, and packaging. Journal of Agricultural and Food Chemistry, 59, 5435–5443. doi:10.1021/jf104995j
- Folch, J., Lees, M., & Sloane-Stanley, G. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
- Gandhi, S. (2015). A study of goat meat (chevon) market value chains in Kumaon region, Uttarakhand state. New Delhi: Report of International Livestock Research Institute.
- Gil, M., Ramirez, J. A., Pla, M., Arino, B., Hernández, P., Pascual, M. … Szerdahelyi, E. N. (2006). Effect of selection for growth rate on the ageing of myofibrils, meat texture properties and the muscle proteolytic potential of m. longissimus in rabbits. Meat Science, 72, 121–129. doi:10.1016/j.meatsci.2005.06.014
- Hernández, P., López, A., Marco, M., & Blasco, A. (2010). Influence of muscle type, refrigeration storage and genetic line on antioxidant enzyme activity in rabbit meat. World Rabbit Science, 10, 141–146. doi:10.4995/wrs.2002.486
- Hernández, P., Park, D., & Rhee, K. S. (2002). Chloride salt type/ionic strength, muscle site and refrigeration effects on antioxidant enzymes and lipid oxidation in pork. Meat Science, 61, 405–410. doi:10.1016/S0309-1740(01)00212-1
- Insausti, K., Beriain, M., Purroy, A., Alberti, P., Gorraiz, C., & Alzueta, M. (2001). Shelf life of beef from local Spanish cattle breeds stored under modified atmosphere. Meat Science, 57, 273–281. doi:10.1016/S0309-1740(00)00102-9
- Irie, M., Fujita, K., & Sudou, K. (1998). Changes in α-tocopherol concentrations in plasma and tissues from Japanese Beef cattle fed by two methods of Vitamin E supplementation. Asian-Australasian Journal of Animal Science, 12, 810–814. doi:10.5713/ajas.1999.810
- Kamal-Eldin, A., Frank, J., Razdan, A., Tengblad, S., Basu, S., & Vessby, B. (2000). Effects of dietary phenolic compounds on tocopherol, cholesterol, and fatty acids in rats. Lipids, 35, 427–435. doi:10.1007/s11745-000-541-y
- Kang, S. M., Kang, G., Seong, P., Park, B., & Cho, S. (2014). Evaluation of various packaging systems on the activity of antioxidant enzyme, and oxidation and color stabilities in sliced Hanwoo (Korean cattle) beef loin during chill storage. Asian-Australasian Journal of Animal Sciences, 27, 1336–1344. doi:10.5713/ajas.2014.14136
- Koohmaraie, M. (1994). Muscle proteinases and meat aging. Meat Science, 36, 93–104. doi:10.1016/0309-1740(94)90036-1
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. doi:10.1038/227680a0
- Lawrie, R., & Ledward, D. (2006). Lawrie’s meat science. Cambridge, UK: Woodhead Publishing Ltd.
- Lefaucheur, L. (2010). A second look into fibre typing – Relation to meat quality. Meat Science, 84, 257–270. doi:10.1016/j.meatsci.2009.05.004
- Liu, F., Xu, Q., Dai, R., & Ni, Y. (2015). Effects of natural antioxidants on colour stability, lipid oxidation and metmyoglobin reducing activity in raw beef patties. Acta Scientiarum Polonorum. Technologia Alimentaria, 14, 37–44. doi:10.17306/J.AFS
- Lonergan, S. M., Huff-Lonergan, E., Rowe, L., Kuhlers, D., & Jungst, S. (2001). Selection for lean growth efficiency in duroc pigs influences pork quality. Journal of Animal Science, 79, 2075–2085.
- Lund, M. N., Heinonen, M., Baron, C. P., & Estevez, M. (2011). Protein oxidation in muscle foods: A review. Molecular Nutrition & Food Research, 55, 83–95. doi:10.1002/mnfr.201000453
- Martinaud, A., Mercier, Y., Marinova, P., Tassy, C., Gatellier, P., & Renerre, M. (1997). Comparison of oxidative processes on myofibrillar proteins from beef during maturation and by different model oxidation systems. Journal of Agricultural and Food Chemistry, 45, 2481–2487. doi:10.1021/jf960977g
- Morzel, M., Gatellier, P., Sayd, T., Renerre, M., & Laville, E. (2006). Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Science, 73, 536–543. doi:10.1016/j.meatsci.2006.02.005
- Muíño, I., Apeleo, E., De La Fuente, J., Pérez-Santaescolástica, C., Rivas-Cañedo, A., Pérez, C. … Lauzurica, S. (2014). Effect of dietary supplementation with red wine extract or vitamin E, in combination with linseed and fish oil, on lamb meat quality. Meat Science, 98, 116–123. doi:10.1016/j.meatsci.2014.05.009
- Nieto, G., Jongberg, S., Andersen, M. L., & Skibsted, L. H. (2013). Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Science, 95, 177–184. doi:10.1016/j.meatsci.2013.05.016
- Okonkwo, J. (2009). Effects of breed and storage duration on the beta-carotene content of egg yolk. Pakistan Journal of Nutrition, 8, 1629–1630. doi:10.3923/pjn.2009.1629.1630
- Olorunsanya, A., Adeyemi, K., & Babatunde, I. (2011). Effect of bamboo (Bambusa valgaris) and elephant grass (Pennisetum purpureum) leaf extracts on oxidative stability of cooked and raw broiler meat. Journal of Agricultural Research and Development, 10, 1–10.
- Ooizumi, T., & Xiong, Y. L. (2004). Biochemical susceptibility of myosin in chicken myofibrils subjected to hydroxyl radical oxidizing systems. Journal of Agricultural and Food Chemistry, 52(13), 4303–4307. doi:10.1021/jf035521v
- Pegg, R. B., & Amarowicz, R. (2009). Content of tocopherol isomers in oilseed radish cultivars-a short report. Polish Journal of Food and Nutrition Sciences, 59, 129–133.
- Petron, M., Raes, K., Claeys, E., Lourenço, M., Fremaut, D., & De Smet, S. (2007). Effect of grazing pastures of different botanical composition on antioxidant enzyme activities and oxidative stability of lamb meat. Meat Science, 75, 737–745. doi:10.1016/j.meatsci.2006.10.010
- Ponnampalam, E. N., Norng, S., Burnett, V. F., Dunshea, F. R., Jacobs, J. L., & Hopkins, D. L. (2014). The synergism of biochemical components controlling lipid oxidation in lamb muscle. Lipids, 49, 757–766. doi:10.1007/s11745-014-3916-5
- Popova, T. (2014). Fatty acid composition of longissimus dorsi and semimembranosus muscles during storage in lambs reared indoors and on pasture. Emirates Journal of Food and Agriculture, 26, 302–308.
- Popova, T., & Marinova, P. (2013). Protein oxidation in M. longissimus dorsi and M. semimembranosus lambs reared indoors and on pasture. Iranian Journal of Applied Animal Science, 3(4), 673–677.
- Popova, T., Marinova, P., Vasileva, V., Gorinov, Y., & Lidji, K. (2009). Oxidative changes in lipids and proteins in beef during storage. Archiva Zootechnica, 3, 30–38.
- Pradhan, A., Rhee, K., & Hernández, P. (2000). Stability of catalase and its potential role in lipid oxidation in meat. Meat Science, 54(4), 385–390. doi:10.1016/S0309-1740(99)00114-X
- Rajion, M., McLean, J., & Cahill, R. N. (1985). Essential fatty acids in the fetal and newborn lamb. Australian Journal of Biological Sciences, 38, 33–40.
- Renerre, M., Dumont, F., & Gatellier, P. (1996). Antioxidant enzyme activities in beef in relation to oxidation of lipid and myoglobin. Meat Science, 43, 111–121. doi:10.1016/0309-1740(96)84583-9
- Renerre, M., Poncet, K., Mercier, Y., Gatellier, P., & Métro, B. (1999). Influence of dietary fat and vitamin E on antioxidant status of muscles of turkey. Journal of Agricultural and Food Chemistry, 47, 237–244. doi:10.1021/jf9805000
- Rhee, K., Anderson, L., & Sams, A. (1996). Lipid oxidation potential of beef, chicken, and pork. Journal of Food Science, 61(1), 8–12. doi:10.1111/jfds.1996.61.issue-1
- Rowe, L., Maddock, K., Lonergan, S. M., & Huff-Lonergan, E. (2004). Influence of early post mortem protein oxidation on beef quality. Journal of Animal Science, 82, 785–793.
- Sabow, A. B., Sazili, A. Q., Zulkifli, I., Goh, Y. M., Ab Kadir, M. Z. A., Abdulla, N. R. … Adeyemi, K. D. (2015a). A comparison of bleeding efficiency, microbiological quality and lipid oxidation in goats subjected to conscious halal slaughter and slaughter following minimal anesthesia. Meat Science, 104, 78–84. doi:10.1016/j.meatsci.2015.02.004
- Sabow, A. B., Sazili, A. Q., Zulkifli, I., Goh, Y. M., Ab Kadir, M. Z. A., & Adeyemi, K. D. (2015b). Physico-chemical characteristics of longissimus lumborum muscle in goats subjected to halal slaughter and anesthesia (halothane) pre-slaughter. Animal Science Journal. doi:10.1111/asj.12385
- Sola-Ojo, F. E., Adeyemi, K. D., Toye, A. A., Bolu, S. A., Fayeye, T. R., Annongu, A. A. … Garba, S. O. (2013). Performance, carcass profile and oxidative stability of broiler chickens fed processed baobab seed meal. Bulletin of Environment Pharmacology and Life Sciences, 2, 94–99.
- Srinivasan, S., & Hultin, H. O. (1994). Hydroxyl radical modification of fish muscle proteins. Journal of Food Biochemistry, 18, 405–425. doi:10.1111/jfbc.1994.18.issue-6
- Stadtman, E., & Levine, R. (2003). Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids, 25, 207–218. doi:10.1007/s00726-003-0011-2
- Sun, W., Cui, C., Zhao, M., Zhao, Q., & Yang, B. (2011). Effects of composition and oxidation of proteins on their solubility, aggregation and proteolytic susceptibility during processing of Cantonese sausage. Food Chemistry, 124, 336–341. doi:10.1016/j.foodchem.2010.06.042
- Watanabe, F., Goto, M., Abe, K., & Nakano, Y. (1996). Glutathione peroxidase activity during storage of fish muscle. Journal of Food Science, 61, 734–735. doi:10.1111/jfds.1996.61.issue-4
- Webb, E. (2014). Goat meat production, composition, and quality. Animal Frontiers, 4, 33–37. doi:10.2527/af.2014-0031
- Webb, E., Casey, N., & Simela, L. (2005). Goat meat quality. Small Ruminant Research, 60, 153–166. doi:10.1016/j.smallrumres.2005.06.009
- Winterbourn, C. C. (1990). Oxidative reactions of hemoglobin. Methods in Enzymology, 186, 265.
- Xiao, S., Zhang, W. G., Lee, E. J., Ma, C. W., & Ahn, D. U. (2011). Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poultry Science, 90, 1348–1357. doi:10.3382/ps.2010-01244
- Xue, M., Huang, F., Huang, M., & Zhou, G. (2012). Influence of oxidation on myofibrillar proteins degradation from bovine via μ-calpain. Food Chemistry, 134, 106–112. doi:10.1016/j.foodchem.2012.02.072
- Zakrys, P., Hogan, S., O’sullivan, M., Allen, P., & Kerry, J. (2008). Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Science, 79, 648–655. doi:10.1016/j.meatsci.2007.10.030
- Zhang, W., Xiao, S., & Ahn, D. U. (2013). Protein oxidation: Basic principles and implications for meat quality. Critical Reviews in Food Science and Nutrition, 53, 1191–1201. doi:10.1080/10408398.2011.577540
