 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The evaluation of different methods for kafirin extraction was made to obtain techno-functional concentrates from two sorghum cultivars. The concentrates were rheologically evaluated in a “dough-like” resin system to assess their potential as a gluten substitute. Under acidic extractions (ethanol 70%, pH 2.5), yields of 56.98% and 46.31% for white (WS) and red sorghum (RS) were obtained, with the highest purity for WS (74.54%). A characteristic electrophoretic pattern was obtained for kafirins, including fractions α1, α2, β and γ. The WS protein was used to prepare resins with lactic acid (LA) as a plasticizing agent, showing similar extensibility to zein (11.37 vs. 11.89 mm respectively) but below wheat gluten (42.61 mm). The microstructure of kafirin resins showed air encapsulation and alignment as fibers. This study allowed to explore new food-compatible extractions for kafirin and evaluate how they will perform rheologically at the kneading stage using a simple resin method.
Resumen
En este estudio se evaluaron diferentes métodos de extracción de kafirina con el objetivo de obtener concentrados tecnofuncionales de dos cultivares de sorgo. Los concentrados fueron evaluados de manera reológica en un sistema de resina “tipo masa” para valorar su potencial como sustitutos del gluten. Bajo extracciones ácidas (etanol 70%, pH 2.5), se obtuvieron rendimientos de 56.98% y 46.31% para el sorgo blanco (WS) y el rojo (RS), respectivamente, constatándose la mayor pureza en el caso del WS (74.54%). Asimismo, se obtuvo un patrón electroforético característico de las kafirinas, que incluía las fracciones α1, α2, β y γ. La proteína del WS se utilizó para preparar resinas empleando ácido láctico (LA) como agente plastificante, tras lo cual mostró una extensibilidad similar a la de la zeína (11.37 mm frente a 11.89 mm, respectivamente), pero inferior a la del gluten de trigo (42.61 mm). Además, la microestructura de las resinas de kafirina presentó encapsulación de aire y alineación como fibras. Mediante el uso de un método sencillo de resinado, este estudio permitió examinar nuevas extracciones de la kafirina compatibles con los alimentos, así como evaluar su comportamiento reológico en la fase de amasado.
1. Introduction
Celiac disease (CD) has a global prevalence of 1.4% (Lebwohl & Rubio-Tapia, Citation2021), and it is defined as an autoimmune condition caused by gluten consumption (Aljada et al., Citation2021). Gluten proteins are classified into two categories according to their solubility: gliadins and glutenins. During the baking process, these proteins crosslink due to the formation of disulfide bonds by the oxidation of the cysteine sulfhydryl groups (Wieser, Citation2007). The cross-linked proteins form a viscoelastic three-dimensional network that allows the dough to retain gases (a property necessary in the baking process) (Rosell, Citation2011). Due to the growing number of persons with CD and the growing popularity of gluten-free diets as a trend, the market needs high-quality gluten-free (GF) bakery products. However, according to Toth et al. (Citation2020), in a recent study, 70.8% of the consumers are disappointed with GF bread because of its texture and flavor. Therefore, finding a protein to substitute the role of gluten in bakery applications represents a unique challenge for the food industry.
It has been found that corn zein, caroubin from carobo, and soybean β-conglycinin could be a good alternative to replace gluten in foods for celiacs, as they try to mimic the characteristics that gluten gives to the structure of the bread crumb (Espinosa Ramírez, Citation2017; Schober et al., Citation2008). Kafirin is a sorghum protein whose potential as a gluten substitute has not yet been fully explored, although its hydrophobicity and tendency to form intramolecular disulfide bonds can facilitate its incorporation in bakery products (Belton et al., Citation2006; de Mesa-Stonestreet et al., Citation2010; Widowati & Luna, Citation2022).
Sorghum (Sorghum bicolor L. Moench) kafirins account for up to 43% of total proteins in this cereal and are safe to eat for celiac patients (Pontieri et al., Citation2013; Serna-Saldivar & Espinosa Ramírez, Citation2018), but because of the challenges to increase extractability, many of the reagents and protocols applied to this quest are incompatibles with food processes either because of their cost or toxicity (2-mercaptoethanol, for example) (Ioerger et al., Citation2007; Pontieri et al., Citation2019).
A way to evaluate the potential of proteins as a gluten substitute (when a reduced protein amount is available) is to study their rheological behavior in small-scale bread-making models. This can be observed in “resins” made up of a protein and a plasticizing agent that simulate bread dough. For example, Oom et al. (Citation2008) reported the rheological study of zein and kafirin resins with oleic acid as a plasticizer. However, kafirin resin became far more stiff, difficult to stretch, and started to expel the plasticizing agent. Such stiffening is associated with heat-induced disulfide cross-linking due to the extraction process. Many kafirin studies focus on extraction, digestibility, and crosslinking (Belton et al., Citation2006; Emmambux & Taylor, Citation2003; Musigakun & Thongngam, Citation2007), but the information related to their potential as a gluten substitute is limited. Until today, the available information concludes that current kafirin extraction methods modify its functionality (Oom et al., Citation2008; Taylor et al., Citation2018), affecting its rheological properties, making it difficult to observe gluten-like viscoelasticity. Therefore, this work aimed to obtain high purity kafirin extracts using food-compatible reagents and methods to produce resins with lactic or acetic acid to be compared with gluten counterparts in small-scale bread-making protocols to study their potential as a gluten substitute in baking.
2. Materials and methods
2.1. Raw materials
The Nutriomics Research group provided vital gluten and corn zein (Sigma Aldrich, Lot: Z3625). The Center for Research and Protein Development (CIDPRO) provided white (type 1) and red sorghum (type 2 without tannins) seeds. Seeds were milled in a coffee grinder (GX4100–11, KRUPS, Mexico). Sorghum flour was defatted with hexane (1:5, flour: hexane) for 24 h at 32°C. Then hexane was removed, and the flour was left overnight at room temperature under a fume hood.
2.2. Reagents
Hexane, sulfuric acid, ethanol, sodium hydroxide (NaOH), sodium metabisulfite (Na2S2O5), hydrochloric acid (HCl), lactic acid, glacial acetic acid, sodium and potassium tartrate tetrahydrate, potassium iodide, potassium sulfate, boric acid, glycerol, and methanol were obtained from Desarrollo de Especialidades Quimicas (Nuevo Leon, Mexico). Mixed indicator 5 was obtained from Merck (New Jersey, USA). BSA protein standard (Bovine Serum Albumin) was obtained from VWR (Pennsylvania, USA). Corn zein, copper sulfate pentahydrate, acrylamide, N’N’’-methylenebisacrylamide, and glycine were obtained from Sigma-Aldrich (Missouri, USA). Electrophoresis reagents, including Coomassie Brilliant Blue, Tris, bromophenol blue, SDS, 2-mercaptoethanol, molecular marker (SDS-PAGE Standard Broad Range), ammonium persulfate, and TEMED, were obtained from Bio-Rad (California, USA).
2.3. Proximate analysis of white and red sorghum
Hectoliter weight (Serna-Saldívar, Citation2013), 1000 grain weight (Vignoni et al., Citation2006), moisture 925.10, and protein content 978.02 (N x 6.25) (AOAC, Citation1992) were performed.
2.4. Extraction of kafirins from white (WSF) and red sorghum (RSF) flours
The experimental runs shown in were executed. A dispersion of 1:5 of defatted sorghum flour: ethanol 70% was adjusted to pH 2.5 (HCl 15% v/v) and placed in a water bath (VWR, OR, USA) set at 70°C. After 2 h, contents were centrifuged for 10 min at 3500 g (5804 R, Eppendorf, Germany), and the resulting supernatant was maintained for 2 h in a container before adding distilled water (<10 °C). The solution was kept overnight at −19°C, and the suspension was centrifuged for 10 min at 3500 g. The curd was washed three times with distilled water to obtain kafirins and dried at 49°C in a food dehydrator (T5160D, Cabela´s, Nebraska, USA). The percentage of protein extraction was calculated using equation 1.
Table 1. Experimental runs carried out in the kafirin extraction from white (WS) and red sorghum (RS).
Tabla 1. pruebas experimentales realizadas durante la extracción de kafirina de sorgo blanco (WS) y rojo (RS).
2.5. Structural characterization of white and red sorghum kafirins (WSK and RSK)
2.5.1. Protein determination by Biuret
The Biuret method (Lubran, Citation1978) was used to determine the soluble protein concentration; 500 μL of the sample were placed in a 15 mL tube, 2.5 mL of Biuret reagent was added, and allowed to stand for 30 min. Absorbance at 540 nm was read. A calibration curve with bovine serum albumin (BSA) was used to calculate the protein concentration in the sample.
2.5.2. SDS-PAGE electrophoresis
Kafirin characterization was performed by SDS-PAGE electrophoresis under reducing conditions. Protein extracts were dissolved in electrophoretic sample buffer [10% (w/v) SDS, 25% (v/v) glycerol, 1% (w/v) bromophenol blue, and Tris-HCl pH 6.8]. Samples were heated in boiling water for 5 min and then loaded (20 μg protein) in each well. Electrophoresis (Mini-Protean I cell, Bio-Rad) was conducted at 200 V until the tracking dye reached the bottom of the resolving gel consisting of acrylamide (15%, w/v). Protein bands were stained with Coomassie Brilliant Blue R-250 (Bio-Rad). The distaining process was performed with solution 1 (10% acetic acid, 45% ethanol, water) per one hour and solution 2 (7% methanol) with several washes until the excess dye was removed and the bands were readable.
2.5.3. FTIR analysis
FTIR analysis was made in an ATR-FTIR (Perkin Elmer, Spectrum ONE, Norwalk, VA, USA). Data was collected in absorbance mode with a 4 cm−1 resolution. The spectra were analyzed with Spectrum software (V.5.3.0) in the region from 4500 to 650 cm−1 to evaluate the secondary structure (Taylor et al., Citation2018).
2.6. Resin preparation
A modified Sly, King et al. (Citation2016) methodology was followed for resins preparation. The tests were carried out with corn zein, vital wheat gluten, and white sorghum kafirin. In addition, lactic acid (85%) and glacial acetic acid were evaluated as plasticizing agents. Glacial acetic acid (140 μL) and lactic acid (62.5 μL) were used for every 100 mg of protein. Each component (protein and plasticizing agent) was preheated separately in a water bath for 15 min at 60°C. Both materials were mixed to obtain a homogeneous resin and then stood for 30 min at room temperature before being hand-kneaded for 7 min and stored in an airtight container.
2.7. Characterization of kafirin, zein, and gluten resins
2.7.1. Measurement of extensibility
The previously prepared resins were characterized using the method of King et al. (Citation2016) briefly described: 1) formation of resin cylinders in mold (60 mm x 3 mm internal diameter) to obtain pieces of uniform size and shape; 2) test on TAXT2 Plus texturometer (Stable Micro Systems) with the Kieffer Rig probe. The analysis was performed thrice at 3.3 mm/s over 200 mm. Extension, maximum force, and area under the curve were recorded.
2.7.2. Fluorescence microscopy
An inverted microscope with fluorescence Axio Observer (Zeiss) was used under natural fluorescence. The resins were characterized as 1) unstretched and 2) stretched to observe the alignment of their fibers. In both cases, samples were placed on a slide and examined with the 2.5x objective in the channels green at 488 nm, red at 568 nm, and bright field.
2.8. Statistical analysis
All treatments were performed at least in triplicate. Data evaluation was made with analysis of variance (ANOVA; Minitab Statistical Software version 19, Minitab, Ltd., Coventry, UK) procedures. The means were compared with Tukey’s test (P ≤ 0.05).
3. Results and discussion
3.1. Proximate analysis of white (WS) and red (RS) sorghum
The WS, showed higher values in all evaluated parameters than RS. Hectoliter weight in WS was higher (78.2 ± 0.45 kg/hL) than RS (75.90 ± 0.38 kg/hL), both similar to reported by Serna-Saldívar (Citation2013) (68.5–77.3 kg/hL). The weight obtained for 1000 grains was 33.97 ± 0.02 and 25.49 ± 0.07 g for WS and RS, respectively, being RS near the range previously reported by Serna-Saldívar (Citation2013) (23–25 g). Moisture content in white sorghum flour (WSF) (13.85 ± 0.06%) was higher than red sorghum flour (RSF) (9.96 ± 0.03%) and also higher than that reported by Liu et al. (Citation2012) for WSF (9.2%). Protein content was lower in RSF (8.06 ± 0.24%) compared to WSF (10.26 ± 0.47%), similar to what was reported by Serna-Saldívar (Citation2013) and Tasie and Gebreyes (Citation2020), ranging from 7.3 to 16.48%. These differences are given by the grain cultivars, the environmental conditions on the field, and the grain storage conditions. However, the protein content made them suitable for extracting and manufacturing protein concentrates or isolates, as described in the following sections.
3.2. Extraction of kafirins from white (WSF) and red (RSF) sorghum flour
Three experiments were carried out to isolate the kafirin fraction from the WSF and RSF, each with two experimental levels. Kafirin extractions were performed in two main stages: 1) kafirin solubilization and isolation from the flour, and 2) precipitation and recovery of the total protein fraction from the solubilization. In the first stage, ethanol was chosen as a solvent since it can be used in food applications, and different pH conditions were evaluated.
Experiments 1.1 and 1.2 () show no precipitation of kafirin fraction despite reducing agent. It is well known that kafirins are the more abundant protein fraction in sorghum endosperm representing almost 43% of the total proteins (Amoura et al., Citation2020; Selle et al., Citation2020; Serna-Saldivar & Espinosa Ramírez, Citation2018); however, their high disulfide bond content makes extraction and digestibility difficult (Tasie & Gebreyes, Citation2020). Therefore, a reducing agent is necessary to promote an efficient sorghum protein extraction. Sodium metabisulfite (Na2S2O4) has been tested before as a reducing agent in sorghum products and other applications such as wine and bread manufacturing (Castro-Jácome et al., Citation2020; Pontieri et al., Citation2019)
Table 2. Percentage of protein extraction in the different experiments carried out with white and red sorghum
Tabla 2. Porcentaje de extracción de proteínas en los diferentes experimentos realizados con sorgo blanco y rojo.
In experiments 2.1 and 2.2 (), kafirin extraction was enhanced by 16.63% and 10.92% for WSF and RSF, respectively, using Na2S2O4. These results were similar to what Ioerger et al. (Citation2007) reported, 17.4% higher for kafirin protein extraction using Na2SO4 for WSF. Protein extraction increases because the reducing agent weakens the protein matrix due to the reduction of disulfide bonds, facilitating the solubilization of kafirin in ethanol. However, in an alkaline medium (NaOH, excess of Na+ ions) has been seen that when adding a reducing agent, a salt precipitated in the solution due to the decrease in solubility of Na2S2O4 under the common ion effect (Skoog & West, Citation2018), which could affect the performance of the reducing agent and the final purity of the extract. Regarding the precipitation step, the pH 5 adjustment has been reported by Pontieri et al. (Citation2019), and it is commonly used as a strategy for protein precipitation by isoelectric point. However, in this case, the wide range of isoelectric points reported for the different kafirins subclasses (Blackwell & Bean, Citation2012) results in a challenge when using pH change for protein separation. So, it is possible that only a fraction of the total kafirin could be obtained under this protocol.
In experimental set 3, new extraction conditions were applied. At the solubilization step, ethanol 70% at two different pH levels was evaluated: 2.5 (acid) and 9.5 (basic); the precipitation was promoted by the addition of water <10°C at the end of the extraction process. depicts experiments 3.1 (acid conditions) and 3.2 (basic conditions). Higher extraction percentages for acid and basic conditions were obtained for WSF than RSF.
The higher protein extraction (%) for acid conditions shows that the amount of kafirin extracted from sorghum flour depends on pH. The increase or decrease of pH during extraction will change the net negative or positive charge on proteins, affecting the solubility, final yield, and purity (Xu et al., Citation2007). Based on these results, an acidic pH is the most suitable for obtaining a higher kafirin protein extraction than an alkaline pH during solubilization. This effect is attributed to the ester’s hydrolysis and ether linkages between proteins and polysaccharides, facilitating the partial break of the protein matrix and its solubility in ethanol. The medium polarity was changed by diluting the ethanolic extract with cold water to achieve the kafirin precipitation. The hydrophobic amino acids present in the kafirin were polarized as the percentage of water in the solution increased with the effect of temperature; since it is well known that with a lower medium temperature, the solubility of a substance decreases. The combination of both factors favored kafirin precipitation. Comparing all experimental sets (), the best conditions for further experiments were the experimental set 3.
3.3. Structural characterization of white (WSK) and red (RSK) sorghum kafirins
3.3.1. Purity
The purity of protein extracts obtained by acid solubilization of WSF and RSF was 74.54 ± 0.90% and 52.00 ± 0.57%, respectively, while in alkaline medium was 7.20 ± 0.56% and 15.08 ± 0.15% also for WSF and RSF, respectively. The purity of WSK and RSK under alkaline conditions was lower than those of the acid medium, similar to the results reported by Gao et al. (Citation2005), where the purity of the extracts decreased from 73% to 67% when using NaOH during the solubilization step. The lower purity is due to non-protein contaminants, such as carbohydrates in the extract. It has been previously reported in other prolamin extractions, such as corn zein, where the coextraction of lipoproteins, non-protein compounds, and carbohydrates was confirmed (Darie-Ion et al., Citation2021). Strong alkaline conditions can also cause protein hydrolysis, reducing the final protein yield (Tan et al., Citation2022). The higher purity in an acid medium for sorghum kafirins showed that the medium conditions, together with the solvent, are more specific for the protein and not for other compounds, agreeing with previous results obtained by Xu et al. (Citation2007) for zein extraction from distillers dried grains with solubles.
3.3.2. Electrophoretic profile
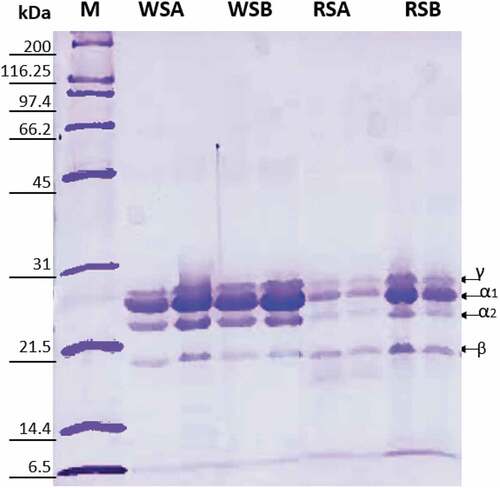
Because extraction methods modify protein characteristics, we obtained the protein profile for WSK and RSK under reducing conditions (). Although in the analysis, four bands with different molecular weights are depicted, these bands correspond to all the kafirin fractions (α1 and α2, β, and γ) and agree with electrophoretic profiles previously published by other authors (Muhiwa et al., Citation2017; Taylor et al., Citation2005; Wang et al., Citation2009; Zhu et al., Citation2022). This way, it was verified that the chemical composition of extracted proteins did not change when NaOH or HCl was added during the solubilization, and that ethanol was a specific solvent for prolamin extraction, despite the pH adjustment.
Figure 1. SDS-PAGE of protein extracts under reducing conditions. M, molecular marker; WSA, White Sorghum Acid medium; WSB, White Sorghum Basic medium; RSA, Red Sorghum acid Medium and RSB, Red Sorghum Basic Medium.
Figura 1. SDS-PAGE de extractos de proteínas en condiciones reductoras. M, marcador molecular; WSA, medio ácido de sorgo blanco; WSB, medio básico de sorgo blanco; RSA, medio ácido de sorgo rojo y RSB, medio básico de sorgo rojo.
3.3.3. Analysis of secondary structures of kafirins by FTIR
FTIR is used to characterize functional groups of molecules; in the case of proteins, it gives information about its secondary structure. shows the FTIR spectra of three prolamins (corn zein, vital wheat gluten, and WSK). As can be seen, even though the prolamins come from different cereals, they have similar spectra due to their similar amino acid composition, where glutamine, proline, and leucine are in higher proportion, yielding similar secondary structures (Wrigley et al., Citation2004). Based on the results obtained in the extract’s identity under different conditions, we can confirm that these correspond to sorghum kafirins and that the solubilization conditions were specific for their extraction. In addition to this, the different kafirin extracts obtained were analyzed. These spectra are depicted in and agree with the results previously reported by Xiao et al. (Citation2015) for kafirin from WS with 60% tert-butanol and for the kafirin extraction with a NaOH-ethanol method (Shah et al., Citation2021; Wang et al., Citation2009; Zhu et al., Citation2022).
Figure 2. IR spectra of white sorghum kafirin (WSK), corn zein and vital wheat gluten.
Figura 2. Espectros IR de la kafirina de sorgo blanco (WSK), la zeína de maíz y el gluten vital de trigo.
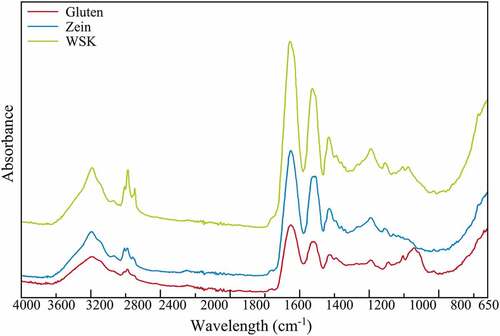
Figure 3. IR spectra of protein extracts of sorghum; where WSA, White Sorghum Acid Medium and WSB, White Sorghum Basic Medium, RSA, Red Sorghum Acid Medium and RSB, Red Sorghum Basic Medium.
Figura 3. Espectros IR de extractos proteicos de sorgo; WSA, medio ácido de sorgo blanco y WSB, medio básico de sorgo blanco, RSA, medio ácido de sorgo rojo y RSB, medio básico de sorgo rojo.
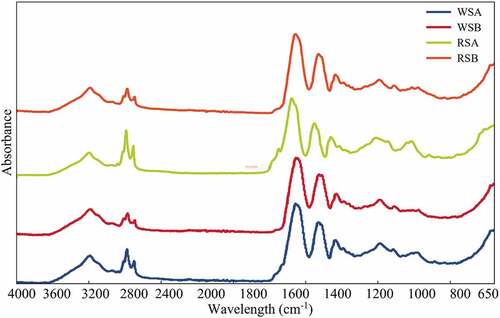
In both FTIR spectra, the signals with higher intensity where amide I, amide II, and amide A. Amide I and II signals in proteins correspond to the amide linkage of amino acids. Amide I signal (1600–1700 cm−1) represents vibrations and stretching of the C=O group and C-N stretching. Amide II corresponds to N-H and C-N linkage (1510–1580 cm−1). Amide A signal (3280 cm−1) corresponds to Fermi resonance between the first overtone of amide 2 and the bending of the N-H bond. For example, in , the spectra for the red sorghum acid (RSA) treatment depict a signal that appears at 1710 cm−1 and could be associated with the acid’s interaction (from the solubilization step) with the amino acids chain present. As reported in the literature, this signal has been assigned to glutamic acid when found as a side chain in the amino acid sequence (Barth, Citation2007).
3.4. Evaluation of resin extensibility of kafirin, zein, gluten and determination of their microstructures by fluorescence microscopy
Rheological tests can be used for the functional evaluation of wheat flours since the physical properties of hydrated gluten formed by kneading can be determined. A texturometer with Kieffer Rig attachment is one of the most useful tests for material characterization in baking processes because it is analogous to Brabender’s extensometer. In this type of equipment, the instrument measures the rheological properties of an optimally formed dough. In addition, this technique seems to help correlate rheological behavior with baking as a gluten substitute (Yue et al., Citation2020).
The rheological parameters obtained for the three prolamins with two different plasticizing agents are shown in . In the glacial acetic acid (GA) systems, corn zein was the resin with the higher area down the curve, followed by wheat vital gluten. In the case of kafirin, the resin generated was too rigid to show any extensibility values. The three resins with 85% lactic acid (LA) could be formed and evaluated (). As can be seen, the acids worked as plasticizing agents, a definition that can be assigned to small molecules that are added to soften a polymer since, when added, they separate the chains of the proteins and occupy a space between them, allowing greater molecular movement and therefore higher flexibility (Pienaar, Citation2015).
Table 3. Rheological parameters of resins prepared with white sorghum kafirin, vital gluten and zein and two plasticizing agents (lactic or acetic acid).
Tabla 3. Parámetros reológicos de las resinas preparadas con kafirina de sorgo blanco, gluten vital y zeína y dos agentes plastificantes (ácido láctico o acético).
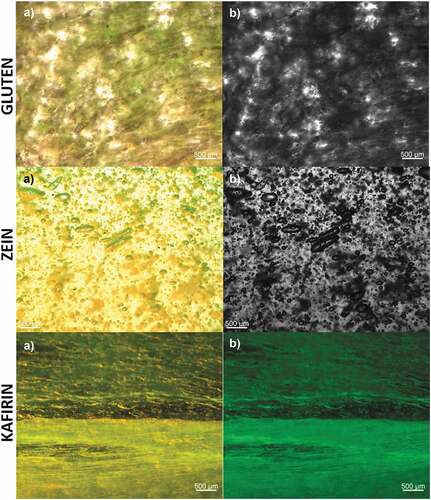
Previously, the formation of viscoelastic resins with zein and kafirins has been evaluated with oleic and acetic acid, but without success, probably due to the extraction methods used since the treatments modify their functional properties (Taylor et al., Citation2018). It has been demonstrated that the only extraction method that allows the formation of resins is when 70% ethanol is used. At the same time, alternatives with NaOH or reducing agents decrease the capacity of the proteins to form resins, losing their functionality (Oom et al., Citation2008). In this case, zein and kafirin resins presented no statistically different values regarding extensibility results with LA, and this value represents the third part of gluten extensibility results. Gluten shows higher extensibility due to a better plasticization with LA, resulting from the disulfide linkages interaction with this acid. Although kafirin and zein are more hydrophobic, this cross-linking degree probably prevented a correct protein plasticization (Pienaar, Citation2015), affecting its elasticity. The microscopic structure of the resins (LA as plasticizing agents) was evaluated to detect differences between three different prolamins. The results obtained can be observed in . In all micrographs, bubbles within the resin systems are depicted because of air encapsulation. In zein and kafirin resins, the material is aligned in fibers when stretched, similar to previously reported by Ncube et al. (Citation2022), who reported this kind of fiber arrangement with zein-kafirin coacervated resins. This arrangement has been previously reported as an important step in forming viscoelastic materials (Taylor et al., Citation2018), and the best fiber alignment, the better integration between the protein and the plasticizing agent. Based on the results obtained in the characterization of the resins in terms of extensibility and their microscopic structure analysis, it is concluded that these proteins, even though showing lower values of extensibility compared to wheat gluten, display viscoelasticity and the ability to encapsulate air, which is an important feature in the bakery manufacture.
Figure 4. Micrographs of films made with 85% lactic acid, where gluten is shown in a) yellow field and b) bright field; zein in a) yellow field and b) bright field and white sorghum kafirin in a) yellow field and b) green field.
Figura 4. Micrografías de películas elaboradas con ácido láctico al 85%, en las que el gluten se muestra en a) campo amarillo y b) campo brillante; la zeína en a) campo amarillo y b) campo brillante y la kafirina de sorgo blanco en a) campo amarillo y b) campo verde.
4. Conclusions
Kafirin extracts from two sorghum cultivars were obtained using food-compatible reagents and processes. The best extraction yield and purity were obtained with ethanol 70% at pH 2.5 as a solvent. For those conditions, 56.98% and 30.88% of the total protein for WSF and RSF was extracted with purities of 74.5% and 52.0%, respectively. Extracts were characterized using SDS-PAGE (including fractions α1, α2, β and γ) and FTIR, indicating that the process was specific for kafirins. Resins were manufactured using LA, discovering that kafirin extracts from WSF depicted similar rheological properties compared with zein. These resins were prepared to assess how a protein in a “dough-like system” will perform related to its rheological properties during kneading and baking. The rheological test used in this work allows a good evaluation of the potential behavior of doughs during baking. Further research is still needed to optimize extraction methods and obtain higher kafirin yields and purity, which is believed to affect their rheological performance directly and its potential applications in baking as a gluten substitute.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge the Research Center for Protein Development (CIDPRO) at Tecnologico de Monterrey for providing facilities and equipment and Dr. Grissel Trujillo de Santiago for her support in the microscopic analysis.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Aljada, B., Zohni, A., & El-Matary, W. (2021). The gluten-free diet for celiac disease and beyond. Nutrients, 13(11). https://doi.org/10.3390/nu13113993
- Amoura, H., Mokrane, H., & Nadjemi, B. (2020). Effect of wet and dry milling on the functional properties of whole sorghum grain flour and kafirin. Journal of Food Science and Technology, 57(3), 1100–1109. https://doi.org/10.1007/s13197-019-04145-2
- AOAC. (1992). Official methods 925.10, 978.02. moisture content, total nitrogen content. Association of Official Analytical Chemists, 75(1).
- Barth, A. (2007). Infrared spectroscopy of proteins. Biochimica et Biophysica Acta - Bioenergetics, 1767(9), 1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
- Belton, P. S., Delgadillo, I., Halford, N. G., & Shewry, P. R. (2006). Kafirin structure and functionality. Journal of Cereal Science, 44, 272–286. https://doi.org/10.1016/j.jcs.2006.05.004
- Blackwell, D. L., & Bean, S. R. (2012). Separation of alcohol soluble sorghum proteins using non-porous cation-exchange columns. Journal of Chromatography A, 1230, 48–53. https://doi.org/10.1016/j.chroma.2012.01.063
- Castro-Jácome, T. P., Alcántara-Quintana, L. E., & Tovar-Pérez, E. G. (2020). Optimization of Kafirin extraction conditions and identification of potential bioactive peptides. BioResearch Open Access, 9(1), 198–208. https://doi.org/10.1089/biores.2020.0013
- Darie-Ion, L., Jayathirtha, M., Hitruc, G. E., Zaharia, M. M., Gradinaru, R. V., Darie, C. C., Pui, A., & Petre, B. A. (2021). A proteomic approach to identify zein proteins upon eco-friendly ultrasound-based extraction. Biomolecules, 11(12), 1–13. https://doi.org/10.3390/biom11121838
- de Mesa-Stonestreet, N. J., Alavi, S., Bean, S. R., Mesa-Stonestreet, N. J., De Alavi, S., & Bean, S. R. (2010). Sorghum proteins: The concentration, isolation, modification, and food applications of kafirins. Journal of Food Science, 75(5), https://doi.org/10.1111/j.1750-3841.2010.01623.x
- Emmambux, N. M., & Taylor, J. R. N. (2003). Sorghum kafirin interaction with various phenolic compounds. Journal of the Science of Food and Agriculture, 83(5), 402–407. https://doi.org/10.1002/jsfa.1379
- Espinosa-Ramírez, H. (2017). Extraction and characterization of gluten-free proteins from sorghum, chickpeas and soybean with potential to be used as structuring agents in the production of gluten-free yeast-leavened bread. Instituto Tecnológico Y de Estudios Superiores de Monterrey. School of Engineering and Sciences. Master Thesis.
- Gao, C., Taylor, J., Wellner, N., Byaruhanga, Y. B., Parker, M. L., Mills, E. N. C., & Belton, P. S. (2005). Effect of preparation conditions on protein secondary structure and biofilm formation of kafirin. Journal of Agricultural and Food Chemistry, 53(2), 306–312. https://doi.org/10.1021/jf0492666
- Ioerger, B., Bean, S. R., Tuinstra, M. R., Pedersen, J. F., Erpelding, J., Lee, K. M., & Herrman, T. J. (2007). Characterization of polymeric proteins from vitreous and floury sorghum endosperm. Journal of Agricultural and Food Chemistry, 55(25), 10232–10239. https://doi.org/10.1021/jf0716883
- King, B. L., Taylor, J., & Taylor, J. R. N. (2016). Formation of a viscoelastic dough from isolated total zein (α-, β- and γ-zein) using a glacial acetic acid treatment. Journal of Cereal Science, 71, 250–257. https://doi.org/10.1016/j.jcs.2016.09.005
- Lebwohl, B., & Rubio-Tapia, A. (2021). Epidemiology, presentation, and diagnosis of Celiac disease. Gastroenterology, 160(1), 63–75. https://doi.org/10.1053/j.gastro.2020.06.098
- Liu, L., Herald, T. J., Wang, D., Wilson, J. D., Bean, S. R., & Aramouni, F. M. (2012). Characterization of sorghum grain and evaluation of sorghum flour in a Chinese egg noodle system. Journal of Cereal Science, 55(1), 31–36. https://doi.org/10.1016/j.jcs.2011.09.007
- Lubran, M. M. (1978). The measurement of total serum proteins by the biuret method. Annals of Clinical and Laboratory Science, 8(2), 106–110. http://www.annclinlabsci.org/content/8/2/106.full.pdf
- Muhiwa, P. J., Taylor, J., & Taylor, J. R. N. (2017). Extraction and film properties of kafirin from coarse sorghum and sorghum ddgs by percolation. Cereal Chemistry, 94(4), 693–698. https://doi.org/10.1094/CCHEM-01-17-0003-R
- Musigakun, P., & Thongngam, M. (2007). Characteristics and functional properties of Sorghum Protein (Kafirin). Kasetsart Journal of Natural Science, 41(5), 313–318. https://www.thaiscience.info/journals/Article/TKJN/10471506.pdf
- Ncube, M. B., Taylor, J., Bean, S. R., Ioerger, B. P., & Taylor, J. R. N. (2022). Modification of zein dough functionality using kafirin as a coprotein. Food Chemistry, 373(PB), 131547. https://doi.org/10.1016/j.foodchem.2021.131547
- Oom, A., Pettersson, A., Taylor, J. R. N., & Stading, M. (2008). Rheological properties of kafirin and zein prolamins. Journal of Cereal Science, 47(1), 109–116. https://doi.org/10.1016/j.jcs.2007.02.005
- Pienaar, S. W. (2015). Optimisation of kafirin extraction parameter for commercial applications [University of Pretoria]. https://repository.up.ac.za/handle/2263/56103
- Pontieri, P., Mamone, G., De Caro, S., Tuinstra, M. R., Roemer, E., Okot, J., De Vita, P., Ficco, D. B. M., Alifano, P., Pignone, D., Massardo, D. R., & Del Giudice, L. (2013). Sorghum, a healthy and gluten-free food for celiac patients as demonstrated by genome, biochemical, and immunochemical analyses. Journal of Agricultural and Food Chemistry, 61(10), 2565–2571. https://doi.org/10.1021/jf304882k
- Pontieri, P., Troisi, J., Bean, S. R., Tilley, M., DiSalvo, M., Boffa, A., Pignone, D., Del Giudice, F., Aletta, M., Alifano, P., & Del Giudice, L. (2019). Comparison of extraction methods for isolating kafirin protein from food grade sorghum flour. Australian Journal of Crop Science, 13(8), 1297–1304. https://doi.org/10.21475/ajcs.19.13.08.p1695
- Rosell, C. M. (2011). The science of doughs and bread quality. In Flour and breads and their fortification in health and disease prevention (3rd ed., pp. 3–14). Elsevier Inc. https://doi.org/10.1016/B978-0-12-380886-8.10001-7.
- Schober, T. J., Bean, S. R., Boyle, D. L., & Park, S. H. (2008). Improved viscoelastic zein-starch doughs for leavened gluten-free breads: Their rheology and microstructure. Journal of Cereal Science, 48(3), 755–767. https://doi.org/10.1016/j.jcs.2008.04.004
- Selle, P. H., McInerney, B. V., McQuade, L. R., Khoddami, A., Chrystal, P. V., Hughes, R. J., & Liu, S. Y. (2020). Composition and characterisation of kafirin, the dominant protein fraction in grain sorghum. Animal Production Science, 60(9), 1163–1172. https://doi.org/10.1071/AN19393
- Serna-Saldívar, S. O. (2013). Química, almacenamiento e industrialización de los cereales (2nd ed.). AGT editors.
- Serna-Saldivar, S. O., & Espinosa Ramírez, J. D. P. (2018). Grain structure and grain chemical composition of Sorghum. In J. Taylor and K. G. Duodu (Eds.), Sorghum and millets: Chemistry, technology, and nutritional attributes (2nd ed., pp. 85–129). Elsevier.
- Shah, U., Dwivedi, D., Hackett, M., Al-Salami, H., Utikar, R. P., Blanchard, C., Gani, A., Rowles, M. R., & Johnson, S. K. (2021). Physicochemical characterisation of kafirins extracted from sorghum grain and dried distillers grain with solubles related to their biomaterial functionality. Scientific Reports, 11(1), 1–11. https://doi.org/10.1038/s41598-021-94718-z
- Skoog, D., & West, D. (2018). Chapter 9. Chemical equilibrium. In Sandra Kiselica (Ed.), Fundamentals of analytical chemistry (9th ed., pp. 209–211). Cengage Learning.
- Tan, H., Zhou, H., Guo, T., Li, J., Zhang, C., Wang, S., Zhang, Y., & Ma, L. (2022). Zein structure and its hidden zearalenone: Effect of zein extraction methods. Food Chemistry, 374(2), 131563. https://doi.org/10.1016/j.foodchem.2021.131563
- Tasie, M. M., & Gebreyes, B. G. (2020). Characterization of nutritional, antinutritional, and mineral contents of thirty-five sorghum varieties grown in Ethiopia. International Journal of Food Science, 2020. https://doi.org/10.1155/2020/8243617
- Taylor, J., Anyango, J. O., Muhiwa, P. J., Oguntoyinbo, S. I., & Taylor, J. R. N. (2018). Comparison of formation of visco-elastic masses and their properties between zeins and kafirins. Food Chemistry, 245(August 2017), 178–188. https://doi.org/10.1016/j.foodchem.2017.10.082
- Taylor, J., Taylor, J. R. N., Dutton, M. F., & De Kock, S. (2005). Glacial acetic acid - a novel food-compatible solvent for kafirin extraction. Cereal Chemistry, 82(5), 485–487. https://doi.org/10.1094/CC-82-0485
- Toth, M., Vatai, G., & Koris, A. (2020). Consumers’ acceptance, satisfaction in consuming gluten-free bread: A market survey approach. International Journal of Celiac Disease, 8(2), 44–49. https://doi.org/10.12691/ijcd-8-2-1
- Vignoni, L. A., Césari, R. M., Forte, M., & Mirábile, M. L. (2006). Determinación de Indice de Color en Ajo Picado. Información Tecnológica, 17(6), 63–67. https://doi.org/10.4067/S0718-07642006000600011
- Wang, Y., Tilley, M., Bean, S., Susan Sun, X., & Wang, D. (2009). Comparison of methods for extracting kafirin proteins from sorghum distillers dried grains with solubles. Journal of Agricultural and Food Chemistry, 57(18), 8366–8372. https://doi.org/10.1021/jf901713w
- Widowati, S., & Luna, P. (2022). Nutritional and functional properties of sorghum (Sorghum bicolor (L.) Moench)-based products and potential valorisation of Sorghum Bran. IOP Conference Series: Earth and Environmental Science, 1024(1), 012031. https://doi.org/10.1088/1755-1315/1024/1/012031
- Wieser, H. (2007). Chemistry of gluten proteins. Food Microbiology, 24(2), 115–119. https://doi.org/10.1016/j.fm.2006.07.004
- Wrigley, C., Corke, H., & Walker, C. (2004). Celiac disease. In C. Wrigley (Ed.), Encyclopedia of grain science (1st ed., pp. 184–185). Elsevier Academic Press.
- Xiao, J., Li, Y., Li, J., Gonzalez, A. P., Xia, Q., & Huang Q. (2015). Structure, morphology, and assembly behavior of kafirin. Journal of Agricultural and Food Chemistry, 63(1), 216–224. PMID: 25510968; PMCID: PMC4298357. https://doi.org/10.1021/jf504674z
- Xu, W., Reddy, N., & Yang, Y. (2007). An acidic method of zein extraction from DDGS. Journal of Agricultural and Food Chemistry, 55(15), 6279–6284. https://doi.org/10.1021/jf0633239
- Yue, Q., Li, M., Liu, C., Li, L., Zheng, X., & Bian, K. (2020). Comparison of uniaxial/biaxial extensional rheological properties of mixed dough with traditional rheological test results: Relationship with the quality of steamed bread. International Journal of Food Science and Technology, 55(7), 2751–2761. https://doi.org/10.1111/ijfs.14528
- Zhu, L., Li, X., Song, X., Li, Q., Zheng, F., Li, H., Sun, J., Huang, M., & Sun, B. (2022). Characterization of prolamin recycled from the byproduct of the Baijiu brewing industry (Jiuzao) by SDS-PAGE, multispectral analysis, and morphological analysis. Food Bioscience, 49(11), 101854. https://doi.org/10.1016/j.fbio.2022.101854
