?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The study was aimed to examine the HMG-CoA reductase (HMGCR) inhibition potential. The in-silico investigations followed the assessments of molecular docking, druggability and molecular dynamics. The molecular dynamics was performed at 100 ns by calculating interaction free energies, RSMD, RSMF, radius of gyration and SASA. Consequently, in-vivo examinations were performed by using a hypercholesterolemic rabbit animal model. The molecular docking showed significant interaction capabilities of myricetin as revealed by binding energy data up to −8.4 Kcal/mol. Accordingly, the data of molecular dynamics i.e. free energy, solvation free energy, radius of gyration, RSMD, RSMF and SASA were shown significant interaction capabilities of myricetin with HMGCR. Supportively, significant ameliorations were made in lipid profile, dyslipidemia indices and oxidative stress by the treatments of myricetin compared to quercetin and atorvastatin. Thus, it can be concluded that myricetin is a potent flavanol with significant potential for HMGCR inhibition and free radical scavenging capability.
1. Introduction
Since the 1990s, there has been a flourishing interest in dietary flavonoids, a unique kind of polyphenolic phytocompounds naturally possessed in fruits, vegetables, and different herbal products (Sarian et al., Citation2017; Suganya, Natarajan, et al., Citation2017). Charan et al., (Citation2022) These phytocompounds are reported for various biological activities (such as anticancer, antidiabetic, hypocholesterolemic and others) as well as leading components of different nutraceutical approaches (Mutha et al., Citation2021). Accordingly, myricetin and quercetin are two leading polyphenolic phytocompounds that primarily occur in the Myricaceae, Anacardiaceae, Polygonaceae, Pinaceae, and Primulaceae families and from consumables such as vegetables, fruits and tea (Sultana & Anwar, Citation2008). The quercetin, a prominent dietary heterocyclic polyphenol, differs from myricetin by number and positioning of hydroxyl group. The myricetin and quercetin are significant mediators of the biological actions attributed to healthy diets as purported by several studies (Septembre-Malaterre et al., Citation2020; Sultana & Anwar, Citation2008; Taheri et al., Citation2020). The Mediterranean diet-rich ingredients of quercetin are associated with decreased cardiovascular diseases (Agraharam et al., Citation2022). Accordingly, numerous researchers reported the effects consistent with pharmacological applications of quercetin and myricetin in cardiovascular diseases (Agraharam et al., Citation2022; Papakyriakopoulou et al., Citation2022), neurological ameliorations (Ma et al., Citation2022; Zhang et al., Citation2021) and anticancer activities (Asgharian et al., Citation2022; Lu et al., Citation2006). Along with these effects, the both selected compounds and its derivatives serves as potent therapeutic agents for COVID-19 and made some patents (Derosa et al., Citation2021; Di Pierro et al., Citation2021; Fintelman-Rodrigues et al., Citation2022). Therefore, the current study was aimed to explore the systematic inhibition potential of HMG-CoA reductase of myricetin and quercetin by in-silico and in-vivo approaches compared to a leading statin, i.e. atorvastatin.
2. Materials and methods
2.1. Selection of phytocompounds
The PubMed search was carried out by filtering the “polyphenols+phytocompound+ hypercholesterolemia+ dyslipidemia +2022” for searching the potent phytocompounds for management of hypercholesterolemia shown results of 20 pages, i.e. Items: 1 to 20 of 84521 where Myricetin and quercetin are highlighted in maximum studies as bioactive compounds. Therefore, the selection was opted to explore the hypocholesterolemic potential of myricetin and quercetin.
2.2. Chemicals and reagents
Myricetin (CAS no. 529-44-2) and quercetin (CAS no. 6151-25-3) were obtained from Sigma Aldrich Mumbai, India. The standard hypolipidemic drug i.e. atorvastatin (Lipitor@ 20 mg/day from Pfizer) was obtained from a registered local pharmacy store as per the veterinarian’s advice. Others required chemicals were purchased from the chemical supplier of Loba chemie ltd, India, and biochemical diagnostic assays kits were obtained from Erba diagnostics Mannheim GmbH, Germany.
2.3. In-silico investigations
The in-silico investigations followed the sequential steps of molecular docking, molecular dynamics and druggability parameters.
2.3.1. Molecular docking
The molecular docking was performed using the Autodock 4.1 for ligand – Protein molecular docking through sequential analysis steps (Rizvi et al., Citation2013). The myricetin and quercetin were retrieved from PubChem databank in SDF format, and further changes were made as per requirements. Consequently, the HMG-CoA reductase (HMGCR) molecular structure was retrieved from the protein data bank of NCBI.
2.3.2. Molecular dynamics
The top-docked complexes Atorvastatin, Quercetin, and Myricetin with HMG CoA reductase (HMGCR), showing the highest binding affinity, were subjected to molecular dynamics simulations using GROMACS 2020.2 package to study the structural deviations in a dynamic environment for a time scale of 100 ns (James et al., Citation2015; Usha et al., Citation2018). The molecular dynamic simulations were examined based on Interaction energy, Free energy of solvation (DGsolv), Root-Mean-Square Fluctuation (RMSF), Solvent Accessible Surface Area (SASA), Radius of gyration (Rg), and Root-Mean-Square Deviation (RMSD) values as a function of time.
2.3.3. Druggability prediction
The druggability prediction was performed by following the ADMET pharmacokinetics assessment through Drulito software to determine the ideal pharmacokinetic profile of the test compounds measured for drug development (Ramya et al., Citation2022). The toxicity of test compounds was examined using the ProTox II software (Bhat & Chatterjee, Citation2021).
2.4. Experimental animals, induction of hypercholesterolemia and experimental design
Healthy, male New Zealand white rabbits were used as an animal model of hypercholesterolemia at the age of 7–8 months and weighed up to 1.7 to 1.9 kg. Animals were housed under standard environmental status as per CPCSEA norms by routine monitoring. The experimental protocol and schedule were approved by Institutional Animal Ethical Committee (IAEC), Department of Zoology, Jai Narain Vyas University, Jodhpur, Rajasthan, India (IAEC NO.1646/GO/ERe/S/19/CPCSEA).
The hypercholesterolemia was induced by high-fat diet and oral supplementation of cholesterol powder at the dose of 500 mg/kg body weight with 5 ml of coconut oil for 15 to 20 days. The induction of hypercholesterolemia was confirmed by examinations of lipid profile parameters from the ear vein samplings.
After the confirmation of hypercholesterolemia, the five experimental groups were formulated by dividing the five animals into each group for 45 days as follows:
Group I- Vehicle control: received only vehicle olive oil for 45 days.
Group II- Hypercholesterolemic control: received only a high fat diet for 45 days.
Group III- Animals were treated with 20 mg/kg per day by myricetin for 45 days.
Group IV- Animals were treated with 20 mg/kg per day by quercetin for 45 days.
Group V – Animals were treated with 20 mg/kg per day by atorvastatin for 45 days.
After completion of the experiment, overnight fasted animals were autopsied under prolonged anaesthesia. The blood was collected from the hepatic portal vein, and serum was separated by centrifugation at 3000 rpm for 15 min for biochemical examinations.
2.4.1. Lipid profile investigation
The total cholesterol (Abel et al., Citation1952), HDL (High Density Lipoprotein) (Hirano et al., Citation2008) and triglyceride (Bucolo & David, Citation1973) were examined by following the standard methods, whereas the VLDL (Very Low Density Lipoprotein) and LDL (Low Density Lipoprotein) calculated by following formulas (Friedewald et al., Citation1972).
The LDL calculation was made by following the Friedewald’s equation as following.
2.4.2. Calculations of dyslipidemia indices
The dyslipidemia was calculated by incorporating the Castelli indices I & II, atherogenic indices of plasma and atherogenic coefficient (Kamoru et al., Citation2017).
The Castelli – I and II indices were calculated by following formulas.
Accordingly, the atherogenic indices of plasma (AIP) and atherogenic coefficient were calculated as follows:
2.4.3. Antioxidant stress investigation
The antioxidant stress was investigated by standard assessment methods of catalase (Hadwan, Citation2018), GSH (Rahman et al., Citation2007), LPO (Lipid peroxidation) (Buege & Aust, Citation1978) and FRAP (Ferric Reducing Antioxidant Power Assay) (Benzie & Strain, Citation1996) from serum samples by following against the total protein at a respective absorptive wavelength of end products.
2.5. Statistical analysis
The values of different lipid profile parameters and antioxidants were calculated by mean ± standard error of the 5 values where n = 5 (Assaad et al., Citation2014). Data were performed one-way ANOVA, and the statistical variances were carried out, followed by a post hoc mean separation test.
3. Results
The in-silico and in-vivo assessments were made sequentially by following the suitable software and standard methods. The results were obtained as follows:
3.1. Molecular docking
The myricetin and quercetin are different in numbers and positioning of hydroxyl groups which were used as ligands (). The binding energy was obtained up to −8.4 Kcal/mol by myricetin and quercetin against the target of HMGCR, which is depicted by 2D and 3D structures (). Accordingly, the maximum number of H bonds were obtained from myricetin interactions with HMGCR compared to quercetin and atorvastatin. Consequently, myricetin’s bond length was significant compared to quercetin and atorvastatin ().
Figure 2. 3D Figures posing the molecular interactions of ligand-protein myricetin(1a) and quercetin(1b) with HMG CoA reductase studied through docking analysis. The dotted line indicates the hydrogen bond interactions.
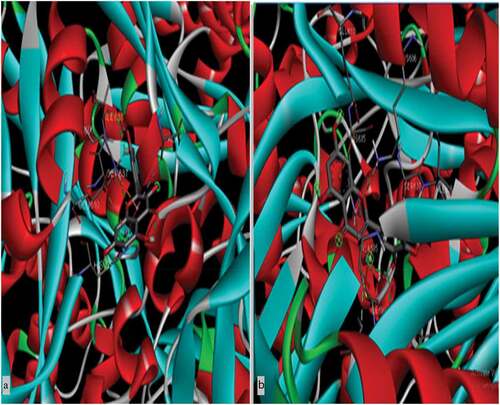
Figure 3. 2D figures posing the ligand-protein interactions of Myricetin(2a) and Quercetin(2b) with HMG CoA reductase.
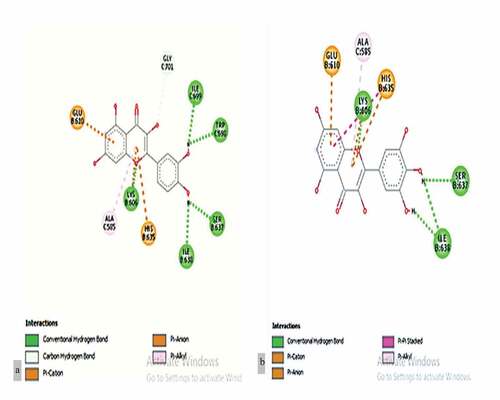
Table 1. Molecular docking of myricetin and quercetin compound against target enzyme of HMG CoA reductase.
3.2. Draggabilities predictions of pharmacokinetics of ADME and toxicity
The ilog P and Clog P were significantly lower in myricetin profile compared to quercetin and atorvastatin. Consequently, myricetin’s TPSA (Total Polar Surface Area) was more significant than quercetin and atorvastatin (). Subsequently, the toxicity profile was assessed using ProTox software that predicted Toxicity class 3 for myricetin and quercetin, whereas class 5 for atorvastatin ().
Table 2. Pharmacokinetics ADMET predictions of myricetin and quercetin by SwissADME.
Table 3. In silico toxicity study of myricetin and quercetin compound by ProTox-II.
3.3. Molecular dynamics
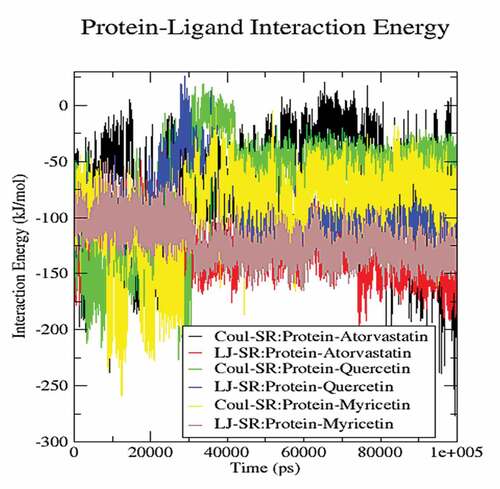
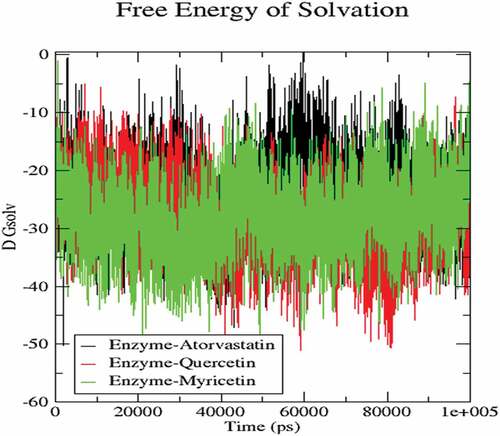
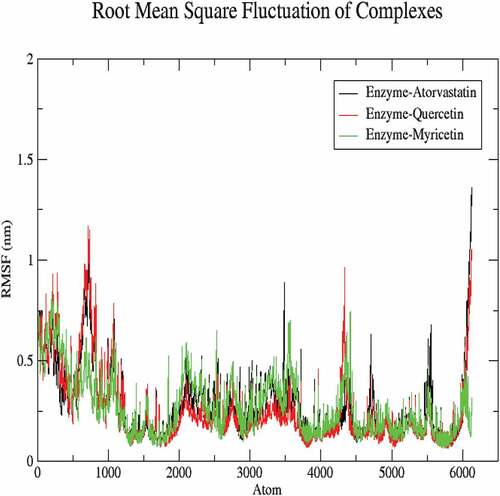
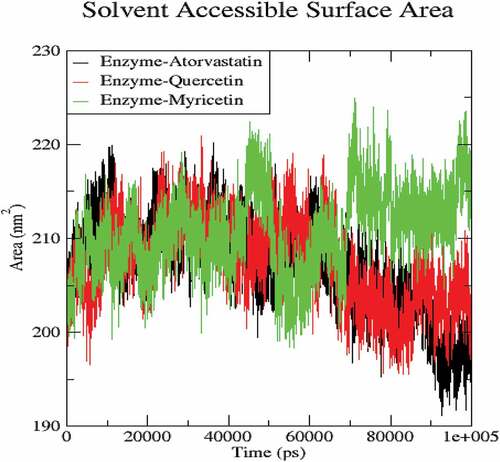
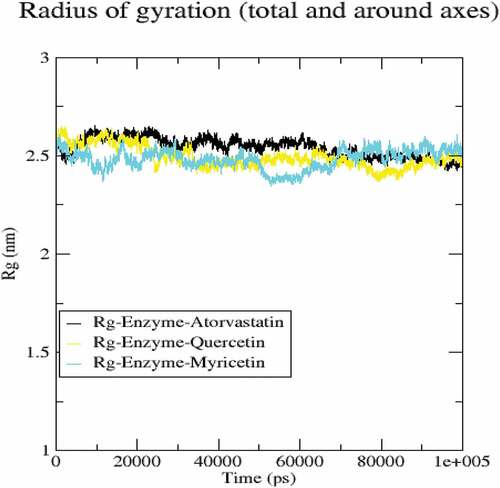
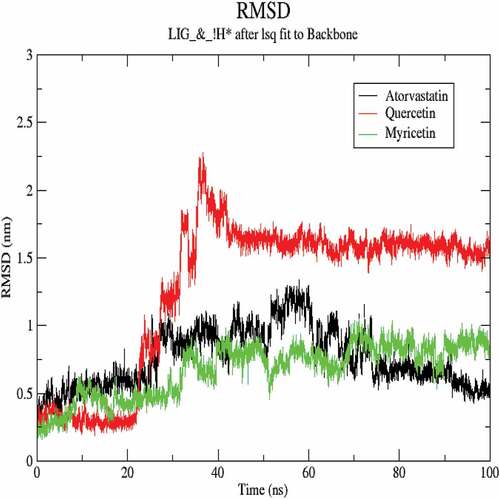
The molecular dynamics performed at 100 ns and showed significant validations of ligand-protein interactions. The Coul-SR values for Atorvastatin, Quercetin and Myricetin were recorded with values of −68.29 KJ/mol, −73.45 KJ/mol and −92.44 KJ/mol, respectively. The Lennard-Jones short-range (LJ-SR) values for Atorvastatin, Quercetin and Myricetin were recorded with values-129.11 KJ/mol, 93.12 KJ/mol and 122.42 KJ/mol, respectively (). Sequentially, the solvation-free energy of all three complexes remains static, with an average value of −25 DGsolv (). The RMSF values of all three complexes were calculated, which remained under 0.5 nm. Consequently, Myricetin complex expresses the least RMSF value (). Throughout the simulations, SASA fluctuates around 210 nm2. SASA values for Atorvastatin, Quercetin and Myricetin complexes remain comparable up to 70ns, but after 70 ns, the SASA of Atorvastatin and Quercetin decreases and SASA for Myricetin increases (). A plot of the radius of gyration (Rg) spanning over 100 ns is analyzed to display the compactness of the protein during MD simulations. Throughout simulations, the radius of gyration for all three complexes remains stable, having a value of 2.5 nm, indicating that the complexes remain stable during simulation studies (). The RMSD value for Myricetin was recorded lowest with a value below 0.75 nm., indicating the stability of Myricetin over Atorvastatin and Quercetin during 100 ns MD simulations ().
3.4. In-vivo studies
The in-vivo examinations were made to examine the lipid metabolism and circulation by packaging through different cholesterol fractions and indices compared to standard statin.
3.4.1. Effects of treatment of myricetin and quercetin on lipid profile
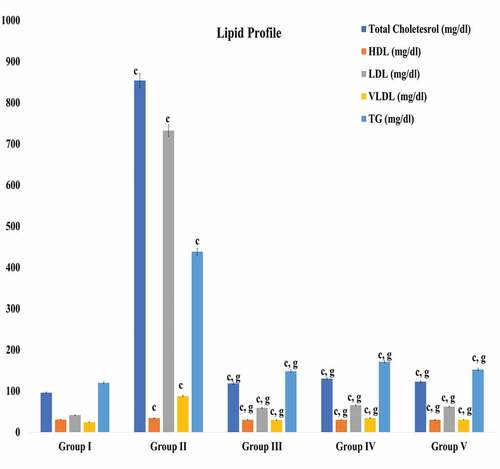
The high fat diet and cholesterol powder supplementation caused ten folds elevations in total cholesterol and LDL-cholesterol in hypercholesterolemic rabbits. Accordingly, the triglyceride and VLDL (Very Low-Density lipoprotein) increased significantly except HDL-cholesterol. Whereas the treatments of test compounds (myricetin and quercetin) caused significant reductions in total cholesterol, LDL-cholesterol, VLDL -cholesterol and triglyceride ().
Figure 10. Effect of treatments of myricetin and quercetin on lipid profile. Data are means ± S.E.M. (n = 5); c P ≤ .001 as significant; and d was non-significant as compared to the respective control values. g P ≤ .001 as significant; and h was non-significant as compared to the respective values of the hypercholesterolemic control group.
3.4.2. Effect of test compounds on dyslipidemia indices
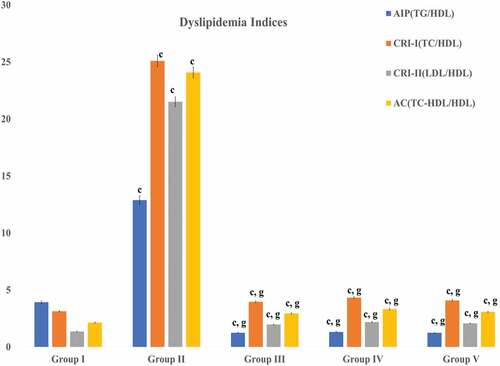
The treatments of test compounds (myricetin and quercetin) caused significant reductions in dyslipidemia indices, i.e. Castelli Indices I and II, atherogenic indices of plasma (AIP), and atherogenic coefficient. The reductions in castelli indices I and II made as per levels of circulated total cholesterol, LDL-cholesterol, and HDL-cholesterol ratios. Accordingly, atherogenic indices of plasma (AIP) altered as per triglyceride levels. Consequently, the atherogenic coefficient (AC) altered as per levels of total cholesterol and HDL – cholesterol ().
Figure 11. Effect of treatments of myricetin and quercetin on lipid profile. Data are means ± S.E.M. (n = 5); c P ≤ .001 as significant; and d was non-significant as compared to the respective control values. g P ≤ .001 as significant; and h was non-significant as compared to the respective values of the hypercholesterolemic control group.
3.4.3. Alterations in antioxidant stress
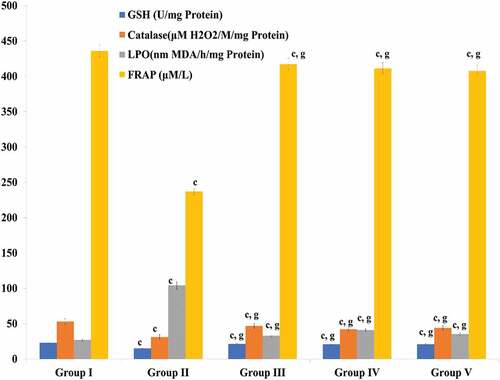
The hypercholesterolemia resulted in abnormal alterations in GSH (reduced glutathione), catalase, FRAP (Ferric Reducing Ability of Plasma) and LPO (lipid Peroxidation). Whereas the treatments of myricetin and quercetin resulted in the normalization of antioxidant levels (GSH, catalase and FRAP). Accordingly, the level of lipid peroxidation decreased up to normalcy by the treatments of test compounds compared to the atorvastatin ().
Figure 12. Effect of treatments of myricetin and quercetin on oxidative stress. Data are means ± S.E.M. (n = 5); c P ≤ .001 as significant; and d was non-significant as compared to the respective control values. g P ≤ .001 as significant; and h was non-significant as compared to the respective values of the hypercholesterolemic control group.
4. Discussion
The HMG-CoA reductase (HMGCR) inhibitors (statins) are widely used to treat hypercholesterolemia as well as reported for some significant associated benefits (Lu et al., Citation2019; Markowska et al., Citation2020; Vettor & Serra, Citation2018). The potent bioactive phytocompounds follow the five rules of the Lipinski which can be serving as an ideal drug molecule (Abbas et al., Citation2017; Keller et al., Citation2006). Accordingly, the myricetin and quercetin have shown the ideal profile of ADMET which may be an potent druggability potential as seen by the ADEMT data. Primarily, the enzyme inhibition potential of selected compounds can be checked by interactions capabilities with the targets through binding energies and related parameters which further validated by molecular simulations or dynamics (Al-Karmalawy et al., Citation2021; Sampangi-Ramaiah et al., Citation2020). The molecular interactions fluctuate and vary as per cytosolic and different environmental conditions needed further validation by molecular dynamics. Consequently, myricetin was documented with values-129.11 KJ/mol free energy of interaction which validated the higher interaction capabilities (Fatriansyah et al., Citation2022). Consequently, the LJ-SR value and Coul-SR value received significantly by myricetin and HMGCR interaction energy which represents total internal energy due to the energy of Coulombic interaction (Coul) and van der Waals interaction energy. In this context, the molecular dynamics indicate varying RSMF, which reveals the average deviation of interactive residue (Chouhan et al., Citation2021). In contrast, the RSMD presents the difference between two static structures (interactive atom and target with reference source). Similarly, the significant values of RSMF and RSMD are shown by myricetin – HMCGR interaction compared to quercetin and atorvastatin. Supportively, the radius of gyration denotes the compactness of secondary structure of target protein and SASA is a crucial characteristic of proteins for resolving their folding and stability, indicating the interaction abilities (Ali et al., Citation2014; Lobanov et al., Citation2008). In this point of view, the molecular dynamics of the study depicted significant values of radius of gyration and SASA. In continuation to molecular dynamics simulation of the study revealed substantial interaction potential of myricetin compared to quercetin and atorvastatin.
Consequently, the in-vivo assessments showed significant interpretations where the availability of high fat content through diet and supplementation of cholesterol powder resulted in hypercholesterolemia through increased biosynthesis of cholesterol (Francis & Pierce, Citation2011). Whereas the treatments of myricetin and quercetin (in comparison to atorvastatin) caused significant reductions in total cholesterol, LDL-cholesterol, VLDL cholesterol and other parameters. This kind of result follows the effects of standard statin drug, i.e., atorvastatin (HMGCR inhibitor), which denotes inhibition of HMGCR as revealed by in-silico analyses (Jiang et al., Citation2018; Mannino et al., Citation2021; Suganya, Nandagopal, et al., Citation2017). Supportively, the dyslipidemia indices, i.e. castelli – I & II, AIP and AC represent the packaging of free cholesterol in circulation (Bhardwaj et al., Citation2013; Koleva et al., Citation2015). The castelli – I, present a ratio of initial uptake form and high packaging cholesterol fraction, i.e. HDL, whereas the castelli-II shows a ratio of the loosely bound cholesterol and compact bound cholesterol. Accordingly, AIP and AC were validated by further calculations by logarithm and compact boundness of cholesterol. These results again validate the following results of atorvastatin by HMGCR inhibition as depriving the free cholesterol in circulation. Supportively, the hypercholesterolemia resulted in abnormal and deprived status of antioxidants which ameliorated by treatments of aforesaid test compounds. These kinds of results reveal the antioxidant potential of myricetin and quercetin (Kaliora et al., Citation2006). Based on the results, myricetin interfered significantly, as shown potent hypocholesterolemic agent.
5. Conclusion
It can be concluded that myricetin has significant interaction capabilities with HMG-CoA reductase as proven by in-silico assessments (molecular docking and Molecular dynamics) and in-vivo studies in comparison to quercetin. Supportively, its antioxidant potential may promote efficient inhibition of HMGCR. Therefore, myricetin or myricetin like derivatives may serve as potent HMGCR inhibitors after following human subjects and further validation from molecular biology.
Authors’ contributions
PR (Priyanka Riyad), AP (Ashok Purohit) – In-vivo experimentation, KS (Karishma Sen)-Molecular docking, AP (Anil Panwar) – Molecular dynamics and HR (Heera Ram)-Designed and supervised the study.
Research involving human participants and/or animals
The part of animal experimentations for in-vivo study was approved and provided ethical clearance by the Institutional Animal Ethics Committee (IAEC), department of Zoology, Jai Narain Vyas University, Jodhpur (Rajasthan)-342001, India is registered under CPCSEA (Reg. No.1646/GO/a/12/CPCSEA valid up to 27.03.23).
Consent for publication
The authors agreed with the final version to proceed with publication.
Acknowledgment
The author would like to acknowledge the Council of Scientific and Industrial Research, New Delhi Senior Research Fellow (File No. 09/098(0137)/2019-EMR-I).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data have already been incorporated into the manuscript.
References
- Abbas, M., Saeed, F., Anjum, F. M., Afzaal, M., Tufail, T., Bashir, M. S., Ishtiaq, A., Hussain, S., & Suleria, H. A. R. (2017). Natural polyphenols: An overview. International Journal of Food Properties [Internet], 20(8), 1689–1699. Taylor & Francis. https://doi.org/10.1080/10942912.2016.1220393
- Abel, L. L., Levy, B. B., Brodie, B. B., & Kendall, F. E. (1952). A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. The Journal of Biological Chemistry, 195(1), 357–366. https://doi.org/10.1016/S0021-9258(19)50907-3
- Agraharam, G., Girigoswami, A., & Girigoswami, K. (2022). Myricetin: A multifunctional flavonol in biomedicine. Current Pharmacology Reports [Internet], 8(1), 48–61. Springer International Publishing. https://doi.org/10.1007/s40495-021-00269-2
- Ali, S., Hassan, M., Islam, A., & Ahmad, F. (2014). A review of methods available to estimate solvent-accessible surface areas of soluble proteins in the folded and unfolded states. Current Protein and Peptide Science, 15(5), 456–476. https://doi.org/10.2174/1389203715666140327114232
- Al-Karmalawy, A. A., Dahab, M. A., Metwaly, A. M., Elhady, S. S., Elkaeed, E. B., Eissa, I. H., & Darwish, K. M. (2021). Molecular docking and dynamics simulation revealed the potential inhibitory activity of ACEIs against SARS-CoV-2 targeting the hACE2 receptor. Frontiers in Chemistry, 9, 1–21. https://doi.org/10.3389/fchem.2021.661230
- Asgharian, P., Tazekand, A. P., Hosseini, K., Forouhandeh, H., Ghasemnejad, T., Ranjbar, M., Hasan, M., Kumar, M., Beirami, S. M., Tarhriz, V., Soofiyani, S. R., Kozhamzharova, L., Sharifi-Rad, J., Calina, D., & Cho, W. C. (2022). Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer cell international [Internet], 22(1), 1–20. BioMed Central. https://doi.org/10.1186/s12935-022-02677-w
- Assaad, H. I., Zhou, L., Carroll, R. J., & Wu, G. (2014). Rapid publication-ready MS-Word tables for one-way ANOVA. Springerplus, 3(1), 1–8. https://doi.org/10.1186/2193-1801-3-474
- Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
- Bhardwaj, S., Bhattacharjee, J., Bhatnagar, M. K., & Tyagi, S. (2013). Atherogenic index of plasma, castelli risk index and atherogenic coefficient - New parameters in assessing cardiovascular risk. International Journal of Pharmacy and Biological Sciences [Internet], 3, 359–364. https://www.ijpbs.com/ijpbsadmin/upload/ijpbs_526938e855804.pdf
- Bhat, V., & Chatterjee, J. (2021). The use of in silico tools for the toxicity prediction of potential inhibitors of SARS-CoV-2. Alternatives to Laboratory Animals, 49(1–2), 22–32. https://doi.org/10.1177/02611929211008196
- Bucolo, G., & David, H. (1973). Quantitative determination of serum triglycerides by the use of enzymes. Clinical Chemistry, 19(5), 476–482. https://doi.org/10.1093/clinchem/19.5.476
- Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–310. https://doi.org/10.1016/s0076-6879(78)52032-6
- Charan, J., Riyad, P., Ram, H., Purohit, A., Ambwani, S., Kashyap, P., Singh, G., Hashem, A., Abd_Allah, E. F., Gupta, V. K., & Kumar, A. (2022). Ameliorations in dyslipidemia and atherosclerotic plaque by the inhibition of HMG-CoA reductase and antioxidant potential of phytoconstituents of an aqueous seed extract of Acacia senegal (L.) Willd in rabbits. Plos One, 264646. https://doi.org/10.1371/journal.pone.0264646
- Chouhan, H., Purohit, A., Ram, H., Chowdhury, S., Kashyap, P., Panwar, A., & Kumar, A. (2021). The interaction capabilities of phytoconstituents of ethanolic seed extract of cumin (Cuminum cyminum L.) with HMG-CoA reductase to subside the hypercholesterolemia: A mechanistic approach. Food Frontiers, 3(2), 1–16. https://doi.org/10.1002/fft2.122
- Derosa, G., Maffioli, P., D’Angelo, A., & DiPierro, F. (2021). A role for quercetin in coronavirus disease 2019 (COVID-19). Phytotherapy Research, 35(3), 1230–1236. https://doi.org/10.1002/ptr.6887
- DiPierro, F., Iqtadar, S., Khan, A., Ullah Mumtaz, S., Masud Chaudhry, M., Bertuccioli, A., Derosa, G., Maffioli, P., Togni, S., Riva, A., Allegrini, P., & Khan, S. (2021). Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second, pilot, randomized, controlled and open-label clinical trial. International Journal of General Medicine, 14, 2807–2816. https://doi.org/10.2147/IJGM.S318949
- Fatriansyah, J. F., Rizqillah, R. K., Yandi, M. Y., Fadilah, S. M., & Sahlan, M. (2022). Molecular docking and dynamics studies on propolis sulabiroin-A as a potential inhibitor of SARS-CoV-2. Journal of King Saud University - Science [Internet], 34(1), 1–9. The Author(s). https://doi.org/10.1016/j.jksus.2021.101707
- Fintelman-Rodrigues, N., Wang, X., Sacramento, C. Q., Temerozo, J. R., Ferreira, A. C., Mattos, M., Mattos, M., Pereira Dutra, F., Bozza, P. T., Castro-Faria-Neto, H. C., Russo, J. J., Ju, J., & Souza, T. M. L. (2022). Commercially available flavonols are better SARS-CoV-2 inhibitors than isoflavone and flavones. Viruses, 14(7), 1–17. https://doi.org/10.3390/v14071458
- Francis, A. A., & Pierce, G. N. (2011). An integrated approach for the mechanisms responsible for atherosclerotic plaque regression. Experimental and Clinical Cardiology, 16(3), 77–86.
- Friedewald, W. T., Levy, R. I., & Fredrickson, D. S. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry, 18(6), 499–502. https://doi.org/10.1093/clinchem/18.6.499
- Hadwan, M. H. (2018). Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochemistry, 19(1), 1–8. https://doi.org/10.1186/s12858-018-0097-5
- Hirano, T., Nohtomi, K., Koba, S., Muroi, A., & Ito, Y. (2008). A simple and precise method for measuring HDL-cholesterol subfractions by a single precipitation followed by homogenous HDL-cholesterol assay. Journal of Lipid Research, 49(5), 1130–1136. https://doi.org/10.1194/jlr.D700027-JLR200
- James, M., Murtola, T., Schulz, R., Smith, J. C., Hess, B., Lindahl, E., & Lindahl, E. (2015). GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. Softw X, 2, 19–25. https://doi.org/10.1016/j.softx.2015.06.001
- Jiang, S. Y., Li, H., Tang, J. J., Wang, J., Luo, J., Liu, B., Wang, J. K., Shi, X. J., Cui, H. W., Tang, J., Yang, F., Qi, W., Qiu, W. W., & Song, B. L. (2018). Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nature Communications [Internet], 9(1), 1–13. https://doi.org/10.1038/s41467-018-07590-3
- Kaliora, A. C., Dedoussis, G. S. V., & Schmidt, H. (2006). Dietary antioxidants in preventing atherogenesis [Internet]. Atherosclerosis, 187(1), 1–17. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L43817017%0A10.1016/j.atherosclerosis.2005.11.001%0Ahttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=00219150&id=doi:10.1016%2Fj.atherosclerosis.2005.11.001&atitle=
- Kamoru, A. A., Japhet, O. M., Adetunji, A. D., Musa, M. A., Hammed, O. O., & Akinlawon, A. A., Abdufatah, O. A., Taofik, A. A., Kabiru, A. A., & Roji, S. M. (2017). Castelli risk index, atherogenic index of plasma, and atherogenic coefficient: Emerging risk predictors of cardiovascular disease in HIV-treated patients. International Journal Clinical Trials & Case Studies [Internet], 2, 8–15. http://scholarsmepub.com/sjmps/Website:http://scholarsmepub.com/
- Keller, T. H., Pichota, A., & Yin, Z. (2006). A practical view of ‘druggability’. Current Opinion in Chemical Biology, 10(4), 357–361. https://doi.org/10.1016/j.cbpa.2006.06.014
- Koleva, D. I., Andreeva-Gateva, P. A., Orbetzova, M. M., & Atanassova, I. B. (2015). Atherogenic index of plasma, castelli risk indexes and leptin/adiponectin ratio in women with metabolic syndrome. International Journal of Pharmaceutical and Medicinal Research [Internet], 6, 12–18. https://www.woarjournals.org/admin/volissue2/upload Image/IJPMR031506.pdf
- Lobanov, M. Y., Bogatyreva, N. S., & Galzitskaya, O. V. (2008). Radius of gyration as an indicator of protein structure compactness. Molecular biology, 42(4), 623–628. https://doi.org/10.1134/S0026893308040195
- Lu, J., Papp, L. V., Fang, J., Rodriguez-Nieto, S., Zhivotovsky, B., & Holmgren, A. (2006). Inhibition of mammalian thioredoxin reductase by some flavonoids: Implications for myricetin and quercetin anticancer activity. Cancer Research, 66(8), 4410–4418. https://doi.org/10.1158/0008-5472.CAN-05-3310
- Lu, D., Shen, L., Mai, H., Zang, J., Liu, Y., Tsang, C. K., Li, K., & Xu, A. (2019). HMG-CoA reductase inhibitors attenuate neuronal damage by suppressing oxygen glucose deprivation-induced activated microglial cells. Neural Plasticity, 2019, 1–15. https://doi.org/10.1155/2019/7675496
- Ma, J., Liu, J., Chen, Y., Yu, H., & Xiang, L. (2022). Myricetin improves impaired nerve functions in experimental diabetic rats. Frontiers in Endocrinology, 13, 1–9. https://doi.org/10.3389/fendo.2022.915603
- Mannino, G., Iovino, P., Lauria, A., Genova, T., Asteggiano, A., Notarbartolo, M., Porcu, A., Serio, G., Chinigò, G., Occhipinti, A., Capuzzo, A., Medana, C., Munaron, L., & Gentile, C. (2021). Bioactive triterpenes of protium heptaphyllum gum resin extract display cholesterol-lowering potential. International Journal of Molecular Sciences, 22(5), 1–22. https://doi.org/10.3390/ijms22052664
- Markowska, A., Antoszczak, M., Markowska, J., & Statins, H. A. (2020). HMG-CoA reductase inhibitors as potential anticancer agents against malignant neoplasms in women. Pharmaceuticals, 13(12), 1–13. https://doi.org/10.3390/ph13120422
- Mutha, R. E., Tatiya, A. U., & Surana, S. J. (2021). Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future Journal of Pharmaceutical Sciences, 7(1), 1–13. https://doi.org/10.1186/s43094-020-00161-8
- Papakyriakopoulou, P., Velidakis, N., Khattab, E., Valsami, G., Korakianitis, I., & Kadoglou, N. P. E. (2022). Potential pharmaceutical applications of quercetin in cardiovascular diseases. Pharmaceuticals, 15(8), 1–23. https://doi.org/10.3390/ph15081019
- Rahman, I., Kode, A., & Biswas, S. K. (2007). Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nature protocols, 1(6), 3159–3165. https://doi.org/10.1038/nprot.2006.378
- Ramya, S., Murugan, M., Krishnaveni, K., Sabitha, M., Kandeepan, C., & Jayakumararaj, R. (2022). In-silico ADMET profile of ellagic acid from syzygium cumini: a natural biaryl polyphenol with therapeutic potential to overcome diabetic associated vascular complications. Journal of Drug Delivery and Therapeutics, 12(1), 91–101. https://doi.org/10.22270/jddt.v12i1.5179
- Rizvi, S. M. D., Shazi, S., & Haneef, M. (2013). A simple click by click protocol to perform docking: Autodock 4.2 made easy for non-bioinformaticians. EXCLI journal, 12, 831–857.
- Sampangi-Ramaiah, M. H., Vishwakarma, R., & Shaanker, R. U. (2020). Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Current Science, 118(7), 1087–1092. https://doi.org/10.18520/cs/v118/i7/1087-1092
- Sarian, M. N., Ahmed, Q. U., Mat So’Ad, S. Z., Alhassan, A. M., Murugesu, S., Perumal, V., Syed Mohamad, S. N. A., Khatib, A., & Latip, J. (2017). Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. BioMed Research International, 2017, 1–14. https://doi.org/10.1155/2017/8386065
- Septembre-Malaterre, A., Boina, C., Gasque, P., Guiraud, P., Jimmy, S., Guiraud, P., & Sélambarom, J. (2020). Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomedicine Plus, 2(1), 1–18. https://doi.org/10.1016/j.phyplu.2022.100220
- Suganya, S., Nandagopal, B., & Anbarasu, A. (2017). Natural inhibitors of HMG-CoA reductase—An insilico approach through molecular docking and simulation studies. Journal of Cellular Biochemistry, 118(1), 52–57. https://doi.org/10.1002/jcb.25608
- Suganya, S., Natarajan, S., Chamundeeswari, D., Anbarasu, A., Balasubramanian, K. A., Schneider, L. C., & Nandagopal, B. (2017). Clinical evaluation of a polyherbal nutritional supplement in dyslipidemic volunteers. Journal of Dietary Supplements [Internet], 14(6), 679–690. Taylor & Francis. https://doi.org/10.1080/19390211.2017.1305478
- Sultana, B., & Anwar, F. (2008). Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chemistry, 108(3), 879–884. https://doi.org/10.1016/j.foodchem.2007.11.053
- Taheri, Y., Suleria, H. A. R., Martins, N., Sytar, O., Beyatli, A., Yeskaliyeva, B., Seitimova, G., Salehi, B., Semwal, P., Painuli, S., Kumar, A., Azzini, E., Martorell, M., Setzer, W. N., Maroyi, A., & Sharifi-Rad, J. (2020). Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complementary Medicine and Therapies, 20(1), 1–14. https://doi.org/10.1186/s12906-020-03033-z
- Usha, T., Shanmugarajan, D., Goyal, A. K., Kumar, C. S., & Middha, S. K. (2018). Recent updates on computer-aided drug discovery: Time for a paradigm shift. Current Topics in Medicinal Chemistry, 17(30), 3296–3307. https://doi.org/10.2174/1568026618666180101163651
- Vettor, R., & Serra, R. (2018). Management of hypercholesterolemia, appropriateness of therapeutic approaches and new drugs in patients with high cardiovascular risk. Italian Journal of Medicine, 12(3), 203–212. https://doi.org/10.4081/itjm.2018.1062
- Zhang, Q., Song, W., Zhao, B., Xie, J., Sun, Q., Shi, X., Yan, B., Tian, G., & Liang, X. (2021). Quercetin attenuates diabetic peripheral neuropathy by correcting mitochondrial abnormality via activation of AMPK/PGC-1α pathway in vivo and in vitro. Frontiers in Neuroscience, 15, 1–16. https://doi.org/10.3389/fnins.2021.636172