?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Indonesia has an abundance of pigmented rice cultivars with high nutritional value. The purpose of this study was to identify and characterize the phytochemical profiles, anthocyanin properties, and biological activities of endogenous Indonesian black rice (Oryza sativa L.) cultivars from Java and East Nusa Tenggara (NTT) Islands. Thirteen black rice cultivars from eastern Nusa Tenggara and West, Central, and East Java were examined. The secondary metabolites of the black rice samples included flavonoids, tannins, phenolics, quinones, glycosides, anthraquinones, leucoanthocyanidins, alkaloids, and proteins. According to the FRAP (ferric reducing antioxidant power) assay, various levels of antioxidant activity were present. The results of an alpha amylase inhibition assay indicated that the black rice samples might have antidiabetic properties. We conclude that the Java and NTT endogenous black rice cultivars have rich phytochemical compounds with high nutritional value, and that their antioxidant and antidiabetic biological activities can promote a healthy life.
Introduction
Rice (Oryza sativa L.) is a major agricultural product for human consumption in many countries and constitutes the primary diet for more than 90% of Indonesian people. There are thousands of rice cultivars in Indonesia, which are generally divided into traditional cultivars that are native to a particular area, and superior cultivars. Oryza sativa var. Javanica and var. Indica are mostly grown in Indonesia (Irianto et al., Citation2009). In addition to various white rice cultivars, Indonesia also features many pigmented rice cultivars, such as black, red, and brown rice, which are grown as lowland rice, upland rice, or swamp rice. In other countries, black rice is widely used in the food industry (Ito & Lacerda, Citation2019). In Japan, black rice is utilized in the manufacturing of alcoholic beverages with high amounts of phenolic compounds (Ito & Lacerda, Citation2019; Katoh et al., Citation2011). The freeze-dried crude extract of black rice is used as a natural antioxidant source for mayonnaise production in Thailand (Ito & Lacerda, Citation2019; Tananuwong & Tewaruth, Citation2010). Flour made from black rice is used in the production of chiffon cakes and has more bioactive compounds and significant antioxidant activity (Ito & Lacerda, Citation2019; Mau et al., Citation2017). Rice contains many secondary metabolites including flavonoids, phenolic acids, terpenoids, alkaloids, and steroids (Wang et al., Citation2018). The main bioactive compounds in pigmented rice are anthocyanins, for instance cyanidin-3-O-glucosides and peonidin-3-O-glucosides, cyanidin 3-rutinoside, cyanidin 3,5-diglucoside, malvidin 3-galactoside, pelargonidin 3,5-diglucoside, proanthocyanidins, flavan-3-ols, isoflavones, γ-oryzano, and ferulic acid (Deng et al., Citation2013; Sari et al., Citation2021). These compounds have broad biological functions in promoting human health as nutraceuticals to regulate cellular metabolism (Fatchiyah, Safitri, et al., Citation2020; Samyor et al., Citation2017).
Pigmented rice is currently gaining more attention. The pigments contained in rice, especially anthocyanins, have been reported to have many health benefits. Anthocyanins are natural pigments that are responsible for the red, purple, and blue colors of many fruits and flowers and have water-soluble properties (Khoo et al., Citation2017; Mattioli et al., Citation2020). The therapeutic effects of anthocyanins are mainly due to their antioxidative activities. Anthocyanin chalcones and quinoidal bases with a double bond that conjugate to the keto group are effective antioxidants since they act as free radical scavengers (Khoo et al., Citation2017). In addition, the glycosylated B-ring structure of anthocyanin plays a role in the antioxidant activity, which is greatly enhanced by methoxylation and orthohydroxylation (Stintzing et al., Citation2002). Anthocyanins have many other beneficial functions in addition to their antioxidant activities, which include antiobesity, antiapoptosis, antidiabetes, and anticancer properties (Fatchiyah, Safitri, et al., Citation2020; Lin et al., Citation2017; Sari et al., Citation2019, Citation2020)
The genotypic diversity of phytochemicals associated with pigmented rice has been presented in numerous studies (Fatchiyah, Ratih, et al., Citation2020; Samyor et al., Citation2017; Sari et al., Citation2021). Nevertheless, most of the research on pigmented rice focused on the relationship between anthocyanins and antioxidants, with some health-stimulating effects. In addition, the antioxidant activity of pigmented rice is commonly higher than that of non-pigmented rice. This is because the phenolic and flavonoid contents and the antioxidant activity are positively correlated. Among pigmented rice types, black rice contains the highest concentrations of anthocyanins. Black rice is therefore considered to have higher biological activity and efficacy than other pigmented rice type s (Fatchiyah, Ratih, et al., Citation2020).
Indonesia has many cultivars of endogenous black rice. These cultivars are widely distributed throughout the country (Anggaraini et al., Citation2015; Apridamayanti et al., Citation2017). Previous studies showed that different black rice cultivars from Java have the black coloration in different parts of the rice grain (Purwanto et al., Citation2018; Sholikhah et al., Citation2019), which suggests that environmental factors contribute to the differences in the black color morphology (Bhuvaneswari et al., Citation2020). In addition, our previous study revealed that anthocyanin biosynthesis and composition were significantly influenced by the genomic and proteomic profiles (Sari et al., Citation2021). The various metabolites present in pigmented rice have been studied in detail (Fatchiyah, Ratih, et al., Citation2020; J. K. Kim et al., Citation2013; T. J. Kim et al., Citation2021). However, to the best of our knowledge, no comprehensive phytochemical profiling and analysis of the biological activities of various endogenous black rice cultivars from Indonesia has yet been performed.
In this study, we present a comprehensive identification of the chemical composition and biological activities of different cultivars of Indonesian black rice. We performed phytochemical analyses of the black rice cultivars Mentik, Melik, and Wajaloka from Java Island and cultivars endogenous to East Nusa Tenggara (NTT). We also determined the biological activities of these black rice cultivars based on antioxidant activity and alpha amylase inhibition assays.
Materials and methods
Rice samples and chemicals
The 13 Indonesian black rice cultivars used in this study were locally grown in East, Central, and West Java and in East Nusa Tenggara (NTT). The rice cultivars consisted of Mentik, Melik, Wajaloka, and endogenous cultivars of NTT. The eastern Java black rice cultivars were UB black rice from Batu Malang (BREJ Mentik 2), Lawang black rice (BREJ Mentik 3), Ngawi Mentik Wangi black rice (BREJ Mentik 4), Ngawi black rice (BREJ Mentik 5), Ngawi black rice 2 (BREJ Mentik 6), Jember Melik black rice (BREJ Melik), and Kepanjen Wajaloka black rice (BREJ Wajaloka). The Central Java black rice cultivars were Purworejo Mentik black rice (BRCJ Mentik 1), Klaten Mentik black rice (BRCJ Mentik 2), and Magelang Melik black rice (BRCJ Melik 2). The eastern Nusa Tenggara black rice cultivars were Blitar Endogenous black rice (BRNTT Endo 1), Sikka Bengawan Endogenous black rice (BRNTT Endo 2), and Ende Pulen Endogenous black rice (BRNTT Endo 3). Several previously studied black rice cultivars (Fatchiyah, Ratih, et al., Citation2020) were included in this work: Kepanjen Mentik black rice (BREJ Mentik 1), Semarang Melik black rice (BRCJ Melik 1), and Toraja-Sukabumi black rice (BRWJ Toraja).
All chemicals were purchased from Sigma – Aldrich (pro-analysis grade) and used without further purification. Alpha-amylase from Aspergillus oryzae (≥150 units/mg protein) was used for the enzyme inhibitory assay.
Extraction of black rice
The thirteen black rice cultivars were ground into fine powder, and extraction was performed using a maceration technique with 0.1% HCl (w/v) in methanol for 24 h, in a volume of 4× the dry weight. The extracts were collected through filtration using Whatman filter paper (0.45 μm) and concentrated using a rotary vacuum evaporator, with a slow speed of 700 rpm, at 45°C. The concentrated extracts were stored at 4°C for subsequent analysis. All extractions were performed in triplicate.
Phytochemical screening of black rice extracts
The phytochemical qualitative tests of the 13 black rice cultivars were conducted based on previous studies (Fatchiyah, Ratih, et al., Citation2020; Gul et al., Citation2017; Shaikh & Patil, Citation2020) to detect alkaloids, flavonoids, phenolic compounds, steroids, tannins, quinones, anthraquinone, leucoanthocyanidins, glycosides, and proteins. The absorbance of samples was determined using UV – Vis spectrophotometry at different wavelengths (210 nm for glycosides, 280 nm for phenolic compounds and proteins, 420 nm for quinones, 430 nm for flavonoids, 470 nm for alkaloids, 515 nm for anthraquinones, 535 nm for leucoanthocyanidins, and 700 nm for tannins) (Fatchiyah, Ratih, et al., Citation2020; Gul et al., Citation2017).
Total anthocyanin content (TAC) determination
The TAC of the rice extracts was determined with the pH differential method and expressed as cyanidin-3-glucoside (Kartini et al., Citation2020; Wu et al., Citation2015). The samples were dissolved in potassium chloride buffer (pH 1) and sodium acetate buffer (pH 4.5). The absorbance was measured at wavelengths of 520 nm and 700 nm with a UV – Vis spectrophotometer. The TAC was calculated as cyanidin-3-glucoside equivalent (% w/w), using the following formula:
where A = (A520 nm – A700 nm) at pH 1.0 – (A520 nm – A700 nm) at pH 4.5; MW = 449.2 g/mol (molecular weight of cyanidin-3-glucoside); DF = dilution factor of the solution; 103 = conversion from g to mg; ε = molar extinction coefficient (L.mol−1.cm−1); and L = pathlength (1 cm).
Characterization of anthocyanins in black rice
UV – Vis spectroscopy
Black rice extracts (0.15 mg) were diluted in 3 mL of potassium chloride buffer (pH 1) and sodium acetate buffer (pH 4.5). The absorbance was measured with a Shimadzu UV – Vis 1700 spectrophotometer in a wavelength range of 200–800 nm.
Thin-Layer Chromatography (TLC)
Black rice extract (0.2 mg/mL) was spotted on F254 silica gel paper and placed in a chamber containing the mobile phase n-butanol: glacial acetic acid: water in a 3:1:1 ratio (Sari et al., Citation2019). After separation occurred, the silica gel paper was air dried and visualized with UV light at a wavelength of 365 nm (Sari et al., Citation2019) for anthocyanin detection.
Fourier transform infrared spectroscopy
The Fourier transform infrared (FTIR) spectra of the black rice extracts were recorded using an FTIR spectrophotometer (Shimadzu IRSpirit). The FTIR spectra were used to identify the functional groups of anthocyanin in the samples by measuring the infrared absorption, emission, and photoconductivity spectra of the extracts (Agustika et al., Citation2022). The pigmented rice extracts were ground with KBr powder and compressed to form a pellet. The IR spectra were obtained in the wavenumber range of 500–4500 cm−1.
Antioxidant activity of pigmented rice using FRAP (ferric reducing antioxidant power) assay
Rice samples were diluted with 0.1% methanol containing HCl and prepared to yield concentrations of 0, 20, 40, 60, 80, and 100 μg/ml. The extracts were mixed with 2.5 mL of phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The solution was incubated at 50°C for 30 min. Then, 2.5 mL of 10% TCA was added to the solution and homogenized. The upper layer of the solution was mixed with 5 mL distilled water and a freshly prepared 0.1% ferric chloride solution (1 mL). The absorbance was measured using a Shimadzu UV – Vis spectrophotometer at 700 nm. Experiments were conducted in triplicate. Antioxidant activity was calculated as follows:
The percentage antioxidant inhibition (Y-axis) was plotted against the sample concentration (X-axis), and the IC50 values were obtained from the linear regression. Ascorbic acid was used as a positive reference.
Alpha amylase inhibitory activity of black rice
The alpha amylase inhibitory activity was determined based on the standard procedures (Agustin et al., Citation2021; Kazeem et al., Citation2013) with a minor modification. A volume of 250 μL of various concentrations of the pigmented rice extracts (10, 20, 40, 60, 80, and 100 µg/mL) was mixed with 250 µL of 50 µg/mL of alpha-amylase. The solution mixture was incubated for 20 min at 37°C. Then, 250 μL of 1% amylum starch solution was added to each sample and incubated for 10 min. Finally, 500 μL of dinitro salicylic acid reagent was added to the solution and boiled for 5 min. The absorbance was measured at 490 nm using a Shimadzu UV – Vis spectrophotometer. Experiments were performed in triplicate. The inhibition activity of the alpha amylase enzyme was calculated using the following equation:
The percentage of alpha amylase inhibition (Y-axis) was plotted against the sample concentration (X-axis), and the IC50 values were obtained from the linear regression. Acarbose was used as a positive reference.
Data analysis
Results were expressed as the mean ± standard deviation. Statistical analyses were conducted using the GraphPad Prism5 statistical package (Graphpad Software, San Diego, CA, U.S.A) (Favaro et al., Citation2018). One-way analysis of variance (ANOVA) followed by Tukey’s HSD test was used to determine differences among the cultivars. Differences at p < .05 were considered statistically significant.
Results
TLC results of phytochemical compounds and anthocyanin contents of black rice extracts
All black rice cultivars were subjected to phytochemical characterization and anthocyanin content analysis. The phytochemical characterization results are presented in . The phytochemical profiling showed that most of the black rice cultivars contained different types and amounts of secondary metabolites including flavonoids, tannins, phenolics, quinones, anthraquinones, glycosides, and leucoanthocyanidins (). In addition to the secondary metabolites, we also analyzed the level of total proteins. BREJ Mentik 2 had the lowest amounts of phytochemical compounds. BRCJ Mentik 2, BRCJ Melik 2, BRWJ Toraja, and BRNTT Endogenous 2 had higher amounts of alkaloid compounds than other cultivars (), and not alkaloids were detected in BREJ Mentik 2, BREJ Mentik 3, and BREJ Wajaloka. All samples contained flavonoids, tannins, phenolics, quinone, anthraquinone, leucoanthocyanidins, and glucoside compounds. Steroid compounds were detected only in BRWJ Toraja. Almost all black rice samples had high amounts of quinone compounds. BRWJ Toraja contained the highest amount of protein, and no protein was detected in BRWJ Wajaloka. These results suggest that most of the black rice cultivars are rich in phytochemical compounds, which can be beneficial for human health.
Figure 1. Qualitative phytochemical screening and anthocyanin and proanthocyanidin profiles based on thin-layer chromatography. a. Phytochemical identification, b. Phytochemical distribution, c. Qualitative anthocyanin and proanthocyanidin profiles. Three samples (BREJ Mentik 1, BRCJ Melik 1, BRWJ Toraja) were obtained from Fatchiyah et al. (Stintzing et al., Citation2002).
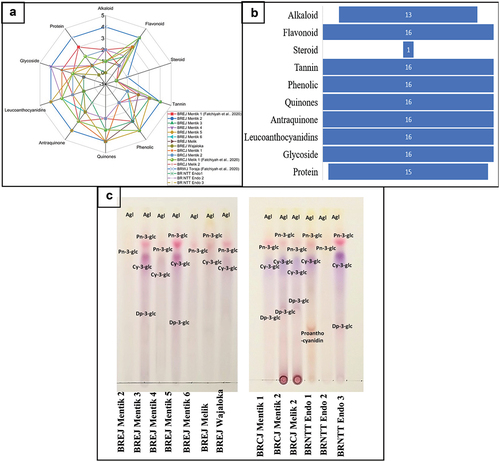
The separated fraction profile of the TLC test of the black rice samples showed several different spots (, ). Three major anthocyanin compounds, peonidin-3-O-glucoside, cyanidin-3-O-glucoside, and delphinidin-3-O-glucoside, were found in most of the black rice cultivars. All fractions from each sample had spots, which indicated the predicted peonidin-O-glucoside (Pn-3-glc) and aglycone (flavonoids). Cyanidin-O-glucoside compounds were detected in all samples except BREJ Mentik 2, BREJ Mentik 5, and BRNTT Endogenous 2. Delphinidin-O-glucoside was detected in BREJ Mentik 3, BRCJ Mentik 1, BREJ Mentik 2, BRCJ Melik 2, and BRNTT Endogenous 3. Observation of the TLC plate using UV light at 365 nm showed the presence of aglycone in all black rice cultivars. In addition, we measured the total anthocyanin content of the black rice extracts ().
Table 1. Anthocyanin prediction for black rice cultivars. Tukey’s HSD test was conducted in Graphpad Prism 9.3.1; significant differences are indicated by different letters (p < .05).
Characterization of the anthocyanins in black rice samples by UV – Vis spectroscopy
UV – Vis spectroscopy was performed to analyze the anthocyanin profile in the black rice samples. The total anthocyanin content (TAC) of each sample was calculated at different pH values. shows the UV – Vis spectra (200–800 nm) at pH 1 and pH 4.5, respectively. shows the total anthocyanin content based on the UV – Vis spectral results. In this study, anthocyanins had a specific profile with maximum absorbance in the range of 400–600 nm.
Figure 2. Anthocyanin characterization based on UV – Vis spectroscopy. A. pH 1, B. pH 4.5, C. Total anthocyanin content of black rice extracts. Tukey’s HSD test was conducted in Graphpad Prism 9.3.1; significant differences are indicated by different letters (p < .05).
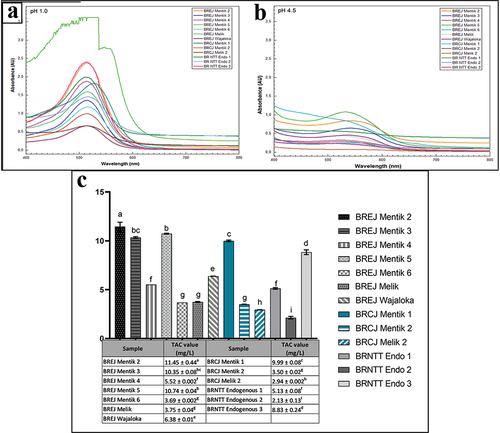
At pH 1, all black rice samples had maximum absorbance in the wavelength range of 512–528 nm (). BREJ Mentik 2, BREJ Mentik 5, and BRNTT Endogenous 3 showed the highest absorption of visible light (515 nm) at pH 1. At pH 4.5, when anthocyanins were degraded, the anthocyanin peak decreased (). BREJ Mentik 2, BREJ Mentik 3, BREJ Mentik 5, BREJ Wajaloka, and BRNTT Endogenous 3 exhibited a moderate anthocyanin peak when the absorbance was measured at pH 4.5. The decrease in absorbance indicated that the total anthocyanin content decreased due to the instability and degradation of anthocyanins at pH 4.5 ().
Table 2. Maximum wavelength and absorbance of spectral profiles at pH 1 and 4.5.
Different letters in symbolize a significant difference in TAC values between the samples based on Tukey’s HSD analysis; the absorbance values indicate the anthocyanin concentration in each sample. BREJ Mentik 2 had a higher TAC value than the other rice cultivars. BREJ Mentik 6 had a similar TAC value as BREJ Melik and BRCJ Mentik 2, and BRNTT Endogenous 2 had a significantly lower TAC value compared with other samples.
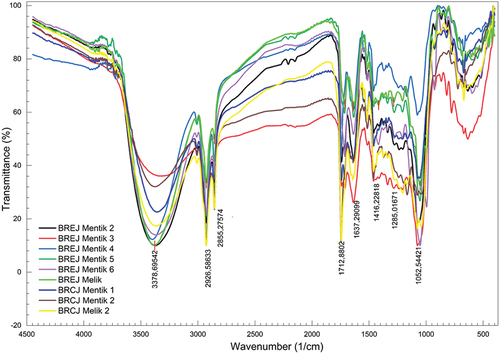
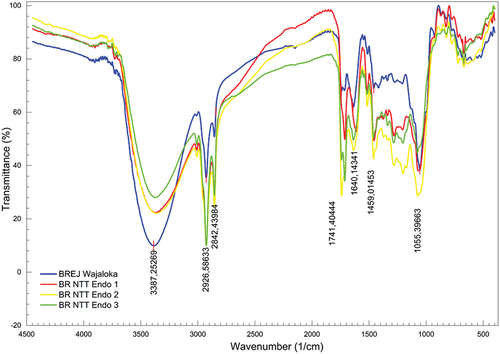
Functional group FTIR profiles of black rice
The functional group FTIR profiles of black rice are shown in . The FTIR spectra of the 13 tested black rice cultivars were similar. There were eight specific regions that appeared in the FTIR spectra of all samples: the O–H stretching region, C–H aliphatic region, C=C stretching from aromatics, C=C stretching from alkanes, N=O bending from amines, CH–OH stretching from alcohols, C–O stretching from ether or ester, and the fingerprint region. However, certain wavenumbers and functional groups shifted. The FTIR image of BR Mentik and Melik data from East Java and West Java was obtained using Origin Pro 21 and is presented in .
Based on the FTIR spectrum, shows the characteristics of the functional groups detected in the Mentik and Melik cultivars. These two cultivars had the same functional groups, i.e. O–H, C–H, C–O, and CH–OH (). The C–H aliphatic group was found in all samples except BREJ Mentik 3 and BREJ Melik. The aromatic groups, alkanes, ethers, and esters in the Mentik and Melik cultivars likely derived from secondary metabolites. Several anthocyanin functional groups such as O–H phenol, C–H aromatics, and carboxyl (C=O) were identified in the FTIR spectra. Protein was indicated by the amine group (N=O) in all samples of the Mentik and Melik cultivars.
Table 3. Wavenumbers and functional groups of the spectra of the Mentik and Melik black rice cultivars.
The IR spectra of the Wajaloka and NTT Endogenous black rice cultivars showed the characteristics of the functional groups (). Both cultivars had the same functional groups: O–H, C–H, C=O, alkane, aromatic, N=O, and CH–OH groups (). The aliphatic C–H group was not found in BREJ Wajaloka. The Wajaloka and NTT Endogenous black rice cultivars had identical fingerprint areas in the range of 670–998 cm−1.
Table 4. Wavenumber and functional groups of the spectra of the Wajaloka and NTT black rice cultivars.
Biological functions of black rice
Antioxidant activity
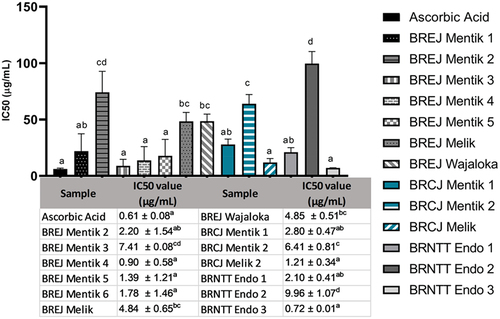
The biological activity of black rice was determined by the antioxidant activity (). The antioxidant activity of ascorbic acid served as a positive reference with an IC50 value of 0.61 ± 0.08 µg/mL. This was followed by BRNTT Endo 3 at 0.72 ± 0.01 µg/mL, BREJ Mentik 4 at 0.90 ± 0.58 µg/mL, BRCJ Melik 2 at 1.21 ± 0.08 µg/mL, BREJ Mentik 5 at 1.39 ± 1.21 µg/mL, and BREJ Mentik 6 at 1.78 ± 1.46 µg/m. The IC50 value of BRNTT Endo 2 was significantly different from that of ascorbic acid. The samples with IC50 values less than 100 g/mL were categorized as having high antioxidant activity.
Alpha amylase inhibition activity as a prediction of antidiabetic functions
To predict the antidiabetic biological function of the black rice extracts, we performed alpha amylase inhibition assay, with acarbose as a positive reference (). Acarbose had the highest activity with the lowest IC50 value of 1.38 µg/mL. The highest IC50 value was recorded in BREJ Wajaloka, at 98.95 ± 0.57 µg/mL, indicating that this cultivar had the lowest ability to inhibit alpha amylase activity. The IC50 values of BREJ Mentik 5 and BRNTT Endo 2 were not significantly different from that of acarbose. Other black rice samples had IC50 values between 14.76 and 87.73 µg/mL.
Figure 6. Alpha amylase inhibition activities of the black rice extracts. The designations of the samples correspond to those in the methods section. Tukey’s HSD test was conducted in Graphpad Prism 9.3.1; significant differences are indicated by different letters (p < .05).
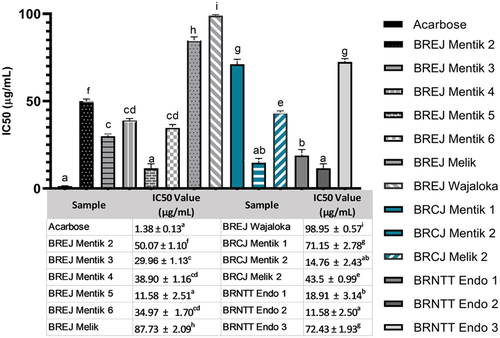
The IC50 values of most of the black rice samples were significantly different from that of the acarbose reference: BRCJ Mentik 2 and BRNTT Endo 1 at p < .05, BREJ Mentik 3, 4, and 6 at p < .001, and BREJ Mentik 2, BREJ Melik, BREJ Wajaloka, BRCJ Mentik 1, and BRNTT Endo 3 at p < .0001. These results indicate that most of the black rice samples have high potential as alpha amylase inhibitors and can be used as natural antidiabetic agents.
Discussion
The phytochemical investigation revealed that the 13 tested black rice cultivars from Java and East Nusa Tenggara contained phenolics, flavonoids, tannins, quinones, anthraquinones, leucoanthocyanidins, and glycosides. Alkaloids were absent in BREJ Mentik 2, Mentik 3, and Wajaloka. The phytochemical tests were carried out based on changes in color after the samples reacted with the standard reagents for identification of secondary metabolites.
The FTIR spectral results supported the phytochemical profiles. FTIR analyses were performed to identify functional groups, as each functional group shows a distinct frequency in the infrared spectrum. The FTIR spectra of all black rice cultivars were similar. The bands detected at 3400–3220 cm−1, 1240–1200 cm−1, and 1170–1040 cm−1 indicated the existence of –OH stretching regions that may have originated from carboxylic acid, ester, or carbonyl groups (Favaro et al., Citation2018). The C–H aliphatic group at 3010–3000 cm−1 is the C–H aliphatic group from the saturated hydrocarbon group or methylene group (C–H2). The C=C aromatic group (1740–1710 cm−1) and C=C (stretch) alkanes (1640–1500 cm−1) were from anthocyanins, flavonoids, or phenolic compounds (Moko & Rahardiyan, Citation2020). The N=O bending amines (1370–1330 cm−1) may have originated from proteins or alkaloids (Favaro et al., Citation2018). Finally, the IR region between 670 and 640 cm−1 is referred to as the fingerprinting region and is linked to the vibrations of the C–O, C–C, C–H, and C–N bonds. This region provides significant information related to organic compounds such as alcohols, organic acids, and carbohydrates that exist in black rice.
Analysis of the phytochemical composition is important to predict the biological and pharmacological activities of the plants of interest (Favaro et al., Citation2018; Lin et al., Citation2017; Sholikhah et al., Citation2019). All secondary metabolites detected in the phytochemical tests of the black rice samples have many biological activities. For example, phenolic and flavonoid compounds have been suggested to have high antioxidant activity (Gumul & Berski, Citation2021; Safitri et al., Citation2020; Swallah et al., Citation2020). Antioxidants protect the human body against oxidative stress (Rahal et al., Citation2014; Shahidi & Zhong, Citation2015).
Quinones, oxidized derivatives of aromatic compounds, are found in nature in diverse types with different properties based on the aromatic ring and chemical structure (Chien et al., Citation2015). In nature, quinones have aromatic ring structures, i.e. a 1-ring structure named benzoquinone, a 2-ring structure named naphtoquinone, and a 3-ring structure named anthraquinone (Chien et al., Citation2015). The biological functions of quinones in living organisms include the inhibition of bacterial growth (Pan et al., Citation2020), cancer treatment (Lu et al., Citation2014), and the prevention of protein misfolding and aggregation (Gong et al., Citation2014), as well as the inhibition of aldose reductase (Demir et al., Citation2019), which can be beneficial for diabetes treatment.
Interestingly, aglycone flavonoids were detected in all black rice samples from East, West, and Central Java, as well as East Nusa Tenggara. Aglycone has a potential role in health promotion and can reduce the risk of metabolic diseases (Koziara et al., Citation2019). Flavonoids are absorbed in the small intestine and transported to the colon and liver for further hydrolysis, and the liberated aglycones are easily digested by bacteria in the intestine (Kumar & Pandey, Citation2013). Glycosides are secondary metabolites that consist of a sugar portion that is linked to a non-sugar part (Xiao, Citation2017), and glycosidic residues improve pharmacokinetic parameters and are of great interest due to their bioactivity. Plant glycosides demonstrated antidiabetic, anti-inflammatory, antidegranulating, antistress, and antiallergic activities in many studies (Browning et al., Citation2010; Godinho et al., Citation2021; Ji et al., Citation2020; Xiao et al., Citation2016). Leucoantocyanidin, a flavan derivative, can be converted into anthocyanidin and subsequently into anthocyanin through the action of anthocyanin synthase (ANS) and glycosyl transferase (GT) (Sari et al., Citation2021). The leucoanthocyanidin test was positive in all black rice samples. The alkaloids and tannins detected in the black rice extracts are commonly used as antibacterial agents (Gul et al., Citation2017; Saha et al., Citation2021)
Anthocyanins are the most important flavonoid pigments in plants. The six main anthocyanin glycoside compounds are pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin. Three of these were discovered in samples in this study: peonidin-3-O-glucoside, cyanidin-3-O-glucoside, and delphinidin-3-O-glucoside. Similar to our study of black rice anthocyanins using UHPLC analysis (Sari et al., Citation2021), we detected peonidin-3-O-glucosides and cyanidin-3-O-glucoside in the black rice extracts from Java and Nusa Tenggara. In our previous study (Fatchiyah, Ratih, et al., Citation2020), those anthocyanins were also present in BREJ Mentik 1, BRCJ Melik, and BRWJ Toraja. The UV – Vis spectra of the black rice extracts provided anthocyanin profiles at two pH values and the anthocyanin concentrations in the samples. Anthocyanin peaks were identified in the visible light region (510–530 nm). In agreement with previous published studies (Ahliha et al., Citation2018; Saha et al., Citation2021; Wu et al., Citation2015), anthocyanin, as an unstable colorant, may undergo gradual degradation through changes in the environment, such as pH, light, and oxygen, and these changes can be followed to calculate the anthocyanin concentrations. The pH distinction method is a fast and simple spectrophotometric method based on the pH-dependent structural conversion of anthocyanin (oxonium form at pH 1.0 and hemiketal form at pH 4.5) (Favaro et al., Citation2018). At pH 1, anthocyanins are more stable than at higher pH values, which cause faster degradation rates (Saha et al., Citation2021). At pH 4.5, the anthocyanin peaks decreased, and there was a complete loss of anthocyanin peaks in some samples. The TAC value ranged from 2.13–11.45 mg/L; these values are lower than those in our previous study (Fatchiyah, Ratih, et al., Citation2020).
The biological functions of black rice presented here are based on antioxidant and alpha amylase inhibition assays. The FRAP assay was used to determine the antioxidant activity. The advantages of the FRAP assay are that this test is simple, rapid, affordable, and requires no special instruments. Antioxidant activity was calculated based on the reduction of the ferric ion (Fe3+)–ligand complex to the intensely blue-colored ferrous (Fe2+) complex by antioxidants in acidic media (Prastiwi et al., Citation2020). The results showed that even though black rice samples may have various levels of antioxidant activities, the ones used in this study had high antioxidant activities with IC50 values lower than 100 μg/mL. Several black rice cultivars used in this study had antioxidant activity comparable to that of ascorbic acid (BREJ Mentik 2, 4, 5, and 6, BRCJ Mentik 1, Melik 2, and BRNTT Endo 1 and 3). The highest TAC level was measured in BREJ Mentik 2, while the highest antioxidant activity was observed in BRNTT Endo 3. These findings suggest that the phytochemical compounds responsible for the antioxidant activity of black rice were not only anthocyanins but also other phytochemicals such as phenolics, flavonoids, or quinones. This is in accordance with a previous study, which showed that phenolic and flavonoid compounds were predominantly responsible for the antioxidant activity of pigmented rice (Ghasemzadeh et al., Citation2018).
The final in vitro biochemical assay was the alpha amylase inhibition assay. BREJ Mentik 2 had the highest TAC content, and BREJ Mentik 5 and BRNTT Endo 2 had the highest potential as inhibitors of alpha amylase. These results support the evidence that phytochemical compounds in black rice, other than anthocyanins, contribute to the biological functions of black rice. The inhibition of the enzyme involved in hydrolyzing carbohydrates (alpha amylase) is an important approach for reducing hyperglycemia (Meidinna & Fatchiyah, Citation2019; Oyedemi et al., Citation2017). Thus, alpha amylase inhibition activity can be used to determine potential antidiabetic activity. Acarbose, which was used as a positive control, is a known competitive inhibitor of alpha amylase and is used as a drug to treat diabetes mellitus (Dinicolantonio et al., Citation2015; Hedrington & Davis, Citation2019). Investigation of the biological activity of black rice extracts, especially their ability to inhibit alpha amylase, may reveal black rice as a potential functional food for patients with diabetes mellitus.
Several in silico studies of alpha amylase inhibitory activity have been performed and have shown that the polyphenolic compounds in black rice (flavonoids and anthocyanins) are tightly bound to alpha amylase by hydrogen bonds (Jhong & Chia, Citation2015; Meidinna & Fatchiyah, Citation2019; Safitri et al., Citation2020). Another proposed hypothesis is that polyphenolic compounds bind covalently to alpha amylase and change its activity due to the formation of quinones or lactones that react with nucleophilic groups on the enzyme molecule (Bhutkar & Bhise, Citation2012; Oyedemi et al., Citation2017). Nevertheless, further in vitro or in vivo studies are needed to elucidate the biological functions of the phytochemical compounds of Indonesian endogenous black rice.
Conclusion
This study presents a complete phytochemical profiling of Indonesian endogenous black rice cultivars as well as their in vitro biological activities, in particular as antioxidants and inhibitors of alpha amylase. The phytochemical compounds discovered in the black rice samples include flavonoids, tannins, phenolics, quinones, anthraquinones, and leucoanthocyanidins. Anthocyanins, which contribute to the pigment of black rice, were also detected in high concentrations. The black rice samples have high potential as antioxidants and also as alpha amylase inhibitors, as their IC50 values with regard to their biological activities were lower than 100 μg/mL. These findings provide an understanding of the relationship between black rice phytochemical compounds and their biological activities. This study also supports the application of Indonesian black rice as a functional food for healthy individuals and patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agustika, D. K., Mercuriani, I., Purnomo, C. W., Hartono, S., Triyana, K., Iliescu, D. D., & Leeson, M. S. (2022). Fourier transform infrared spectrum pre-processing technique selection for detecting PYLCV-infected chilli plants. Spectrochimica Acta Part A, Molecular and Biomolecular Spectroscopy, 278, 121339. https://doi.org/10.1016/j.saa.2022.121339
- Agustin, A. T., Safitri, A., & Fatchiyah, F. (2021). Java red rice (Oryza sativa L.) nutritional value and anthocyanin profiles and its potential role as Antioxidant and Anti-Diabetic. Indonesian Journal of Chemistry, 21(4), 968–978. https://doi.org/10.22146/ijc.64509
- Ahliha, A. H., Nurosyid, F., Supriyanto, A., & Kusumaningsih, T. (2018). Optical properties of anthocyanin dyes on TiO2 as photosensitizers for application of dye-sensitized solar cell (DSSC). IOP Conference Series: Materials Science and Engineering, 333, 012018. https://doi.org/10.1088/1757-899X/333/1/012018
- Anggaraini, T., Novelina, N., Limber, U., & Amelia, R. (2015). Antioxidant activities of some red, black, and white rice cultivar from West Sumatra, Indonesia. Pakistan Journal of Nutrition, 14(2), 112–117. https://doi.org/10.3923/pjn.2015.112.117
- Apridamayanti, P., Pratiwi, R., Purwestri, Y. A., Tunjung, W. A. S., & Rumiyati, R. (2017). Anthocyanin, nutrient contents, and antioxidant activity of black rice bran of Oryza sativa L. ‘Cempo Ireng’ from Sleman, Yogyakarta, Indonesia. Indonesian Journal of Biotechnology, 22(1), 49–54. https://doi.org/10.22146/ijbiotech.26401
- Bhutkar, M. A., & Bhise, S. B. (2012). In vitro assay of alpha amylase inhibitory activity of some indigenous plants. International Journal of Chemistry, 10(1), 457–462.
- Bhuvaneswari, S., Gopala Krishnan, S., Bollinedi, H., Saha, S., Ellur, R. K., Vinod, K. K., Singh, I. M., Prakash, N., Bhowmick, P. K., Nagarajan, M., Singh, N. K., & Singh, A. K. (2020). Genetic architecture and anthocyanin profiling of aromatic rice from Manipur reveals divergence of Chakhao Landraces. Frontiers in genetics, 11, 570731. https://doi.org/10.3389/fgene.2020.570731
- Browning, A. M., Walle, U. K., & Walle, T. (2010). Flavonoid glycosides inhibit oral cancer cell proliferation — role of cellular uptake and hydrolysis to the aglycones. The Journal of Pharmacy and Pharmacology, 57(8), 1037–1042. https://doi.org/10.1211/0022357056514
- Chien, S. C., Wu, Y. C., Chen, Z. W., & Yang, W. C. (2015). Naturally occurring anthraquinones: Chemistry and therapeutic potential in autoimmune diabetes. Evidence-Based Complementary and Alternative Medicine, 2015, 357357. https://doi.org/10.1155/2015/357357
- Demir, Y., Özaslan, M. S., Duran, H. E., Küfrevioğlu, Ö. İ., & Beydemir, Ş. (2019). Inhibition effects of quinones on aldose reductase: Antidiabetic properties. Environmental Toxicology and Pharmacology, 7, 103195. https://doi.org/10.1016/j.etap.2019.103195
- Deng, G. F., Xu, X. R., Zhang, Y., Li, D., Gan, R. Y., & Bin Li, H. (2013). Phenolic compounds and bioactivities of pigmented rice. Critical Reviews in Food Science and Nutrition, 53(3), 296–306. https://doi.org/10.1080/10408398.2010.529624
- Dinicolantonio, J. J., Bhutani, J., & O’keefe, J. H. (2015). Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart, 2(1), e000327. https://doi.org/10.1136/openhrt-2015-000327
- Fatchiyah, F., Ratih, D., Sari, T., Safitri, A., & Cairns, J. R. K. (2020). Phytochemical compound and nutritional value in black rice from Java Island, Indonesia. Systematic Reviews in Pharmacy, 11(7), 414–421.
- Fatchiyah, F., Safitri, A., Nikmatu Rohmah, R., Faraline Triprisila, L., Kurnianingsih, N., Nugraha, Y., Fajriani, S., Meidinna, H. N., & Cairns, J. R. K. (2020). The effect of anthocyanin of whole-grain pigmented rice attenuated visceral fat, cholesterol, LDL and PPARγ gene cascade in dyslipidemia rat. Systematic Reviews in Pharmacy, 11(10), 318–327.
- Favaro, L. I. L., Balcão, V. M., Rocha, L. K. H., Silva, E. C., Oliveira, J. M., Vila, M. M. D. C., & Tubino, M. (2018). Physicochemical characterization of a crude anthocyanin extract from the fruits of jussara (Euterpe edulis Martius): Potential for food and pharmaceutical applications. Journal of the Brazilian Chemical Society, 29(10), 2072–2088. https://doi.org/10.21577/0103-5053.20180082
- Ghasemzadeh, A., Karbalaii, M. T., Jaafar, H. Z. E., & Rahmat, A. (2018). Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chemistry Central Journal, 12(1), 17. https://doi.org/10.1186/s13065-018-0382-9
- Godinho, P. I. C., Soengas, R. G., & Silva, V. L. M. (2021). Therapeutic potential of glycosyl flavonoids as anti-coronaviral agents. Pharmaceuticals (Basel), 14(6), 546. https://doi.org/10.3390/ph14060546
- Gong, H., He, Z., Peng, A., Zhang, X., Cheng, B., Sun, Y., Zheng, L., & Huang, K. (2014). Effects of several quinones on insulin aggregation. Scientific reports, 4(1), 5648. https://doi.org/10.1038/srep05648
- Gul, R., Jan, S. U., Faridullah, S., Sherani, S., & Jahan, N. (2017). Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. The Scientific World Journal, 2017, 5873648. https://doi.org/10.1155/2017/5873648
- Gumul, D., & Berski, W. (2021). The polyphenol profile and antioxidant potential of irradiated rye grains. International Journal of Food Science, 2021, 8870754. https://doi.org/10.1155/2021/8870754
- Hedrington, M. S., & Davis, S. N. (2019). Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opinion on Pharmacotherapy, 20(18), 2229–2235. https://doi.org/10.1080/14656566.2019.1672660
- Irianto, S., Abdurrachman, H., Sembiring, S., Hendarsih, H., Samaullah, M., & Sasmita, P. (2009). Bagian-7, pedoman umum ip padi 40. Balai Besar Penelitian Tanaman Padi, Balitbang Pertanian.
- Ito, V. C., & Lacerda, L. G. (2019). Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chemistry, 301, 125304. https://doi.org/10.1016/j.foodchem.2019.125304
- Jhong, C., & Chia, Y. (2015). Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors, 41(4), 242–251. https://doi.org/10.1002/biof.1219
- Ji, Y., Li, B., Qiao, M., Li, J., Xu, H., Zhang, L., & Zhang, X. (2020). Advances on the in vivo and in vitro glycosylations of flavonoids. Applied Microbiology and Biotechnology, 104(15), 6587–6600. https://doi.org/10.1007/s00253-020-10667-z
- Kartini, K., Putri, L. A. D., & Hadiyat, M. A. (2020). FTIR-based fingerprinting and discriminant analysis of Apium graveolens from different locations. Journal of Applied Pharmaceutical Science, 10(12), 62–67. https://doi.org/10.7324/JAPS.2020.101208
- Katoh, T., Koguchi, M., Saigusa, N., & Teramoto, Y. (2011). Production and antioxidative activity of mead made from various types of honey and black rice (Oryza sativa var. Indica cv. Shiun). Food Science and Technology Research, 17(2), 149–154. https://doi.org/10.3136/fstr.17.149
- Kazeem, M. I., Ogunbiyi, J. V., & Ashafa, A. O. T. (2013). In vitro studies on the inhibition of α-Amylase and α- Glucosidase by leaf extracts ofpicralima nitida (stapf). Tropical Journal of Pharmaceutical Research, 12(5), 719–725. https://doi.org/10.4314/tjpr.v12i5.9
- Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research, 61(1), 1361779. https://doi.org/10.1080/16546628.2017.1361779
- Kim, T. J., Kim, S. Y., Park, Y. J., Lim, S. H., Ha, S. H., Park, S. U., Lee, B., & Kim, J. K. (2021). Metabolite profiling reveals distinct modulation of complex metabolic networks in non-pigmented, black, and red rice (Oryza sativa L.) Cultivars. Metabolites, 11(6), 367. https://doi.org/10.3390/metabo11060367
- Kim, J. K., Park, S. Y., Lim, S. H., Yeo, Y., Cho, H. S., & Ha, S. H. (2013). Comparative metabolic profiling of pigmented rice (Oryza sativa L.) cultivars reveals primary metabolites are correlated with secondary metabolites. Journal of Cereal Science, 57(1), 14–20. https://doi.org/10.1016/j.jcs.2012.09.012
- Koziara, Baranowska, Bartoszek, & Namieśnik. (2019). Comparison of Redox properties of flavonoid aglycones and corresponding Glycosides and their mixtures in the cellular model. Proceedings, 11(1), 25. https://doi.org/10.3390/proceedings2019011025
- Kumar, S., & Pandey, A. K. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 2013, 1–16. https://doi.org/10.1155/2013/162750
- Lin, B. W., Gong, C. C., Song, H. F., & Cui, Y. Y. (2017). Effects of anthocyanins on the prevention and treatment of cancer. British Journal of Pharmacology, 174(11), 1226–1243. https://doi.org/10.1111/bph.v174.11/issuetoc
- Lu, J.-J., Bao, J.-L., Wu, G.-S., Xu, W.-S., Huang, M.-Q., Chen, X.-P., & Wang, Y.-T. (2014). Quinones derived from plant secondary metabolites as anti-cancer agents. Anti-Cancer Agents in Medicinal Chemistry, 13(3), 456–463. https://doi.org/10.2174/1871520611313030008
- Mattioli, R., Francioso, A., Mosca, L., & Silva, P. (2020). Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules, 25(17), 3809. https://doi.org/10.3390/molecules25173809
- Mau, J.-L., Lee, C.-C., Chen, Y.-P., & Lin, S.-D. (2017). Physicochemical, antioxidant and sensory characteristics of chiffon cake prepared with black rice as replacement for wheat flour. LWT - Food Science Technol, 75, 434–439. https://doi.org/10.1016/j.lwt.2016.09.019
- Meidinna, H. N., & Fatchiyah, F. (2019). The potential role of rosmarinic acid and sinensetin as α-amylase inhibitor: In silico study. The Journal of Pure and Applied Chemistry Research, 8(1), 73–79. https://doi.org/10.21776/ub.jpacr.2019.008.001.460
- Moko, E. M., & Rahardiyan, D. (2020). Structure of stigmasterols in bran of red rice from Minahasa Regency, North Sulawesi, Indonesia. Fullerene Journal of Chemistry, 5(1), 16–22. https://doi.org/10.37033/fjc.v5i1.145
- Oyedemi, S. O., Oyedemi, B. O., Ijeh, I. I., Ohanyerem, P. E., Coopoosamy, R. M., & Aiyegoro, O. A. (2017). Alpha-amylase inhibition and antioxidative capacity of some antidiabetic plants used by the traditional healers in Southeastern Nigeria. The Scientific World Journal, 2017, 3592491. https://doi.org/10.1155/2017/3592491
- Pan, X., Ma, X., Jiang, Y., Wen, J., Yang, L., Chen, D., Cao, X., & Peng, A. (2020). Comprehensive review of natural products against liver fibrosis: Flavonoids, quinones, lignans, phenols, and acids. Evidence-Based Complementary and Alternative Medicine, 2020, 7171498. https://doi.org/10.1155/2020/7171498
- Prastiwi, R., Elya, B., Hanafi, M., Desmiaty, Y., & Sauriasari, R. (2020). The Antioxidant Activity of Sterculia stipulata Korth Woods and Leaves by FRAP Method. Pharmacognosy Journal, 12(2), 236–239. https://doi.org/10.5530/pj.2020.12.36
- Purwanto, E., Hidayati, W., & Nandariyah. (2018). The yield and quality of black rice cultivars in different altitude. IOP Conference Series: Earth and Environmental Science, 142, 012037. https://doi.org/10.1088/1755-1315/142/1/012037
- Rahal, A., Kumar, A., Singh, V., Yadav, B., Tiwari, R., Chakraborty, S., & Dhama, K. (2014). Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Research International, 2014, 761264. https://doi.org/10.1155/2014/761264
- Safitri, A., Fatchiyah, F., Sari, D. R. T., & Roosdiana, A. (2020). Phytochemical Phytochemical screening, in vitro anti-oxidant activity, and in silico anti-diabetic activity of aqueous extracts of Ruellia tuberosa L. Journal Applied Pharmaceutical Science, 10(03), 101–108. https://doi.org/10.7324/JAPS.2020.103013
- Saha, S., Singh, J., Paul, A., Sarkar, R., Khan, Z., & Banerjee, K. (2021). Anthocyanin profiling using UV-vis spectroscopy and liquid chromatography mass spectrometry. Journal of AOAC International, 103(1), 23–39. https://doi.org/10.5740/jaoacint.19-0201
- Samyor, D., Das, A. B., & Deka, S. C. (2017). Pigmented rice a potential source of bioactive compounds: A review. International Journal Food Science, 52(5), 1073–1081. https://doi.org/10.1111/ijfs.13378
- Sari, D. R. T., Cairns, J. R. K., Safitri, A., & Fatchiyah, F. (2019). Virtual prediction of the delphinidin-3-O-glucoside and peonidin-3-O-glucoside as anti-inflammatory of TNF-α signaling. Acta Informatica Medica, 27(3), 152–157. https://doi.org/10.5455/aim.2019.27.152-157
- Sari, D. R. T., Paemanee, A., Roytrakul, S., Cairns, J. R. K., Safitri, A., & Fatchiyah, F. (2021). Black rice cultivar from Java Island of Indonesia revealed genomic, proteomic, and anthocyanin nutritional value. Acta Biochimica Polonica, 68(1), 55–63. https://doi.org/10.18388/abp.2020_5386
- Sari, D. R. T., Safitri, A., Cairns, J. R. K., & Fatchiyah, F. (2020). Anti-apoptotic activity of anthocyanins has potential to inhibit caspase-3 signaling. Journal Tropical Life Science, 10(1), 15–25. https://doi.org/10.11594/jtls.10.01.03
- Shahidi, F., & Zhong, Y. (2015). Measurement of antioxidant activity. Journal of Functional Foods, 18, 757–781. https://doi.org/10.1016/j.jff.2015.01.047
- Shaikh, J. R., & Patil, M. (2020). Qualitative tests for preliminary phytochemical screening: An overview. International Journal of Chemical Studies, 8(2), 603–608. https://doi.org/10.22271/chemi.2020.v8.i2i.8834
- Sholikhah, U., Parjanto, Handoyo, T., & Yunus, A. (2019). Genetic diversity of black and aromatic rice cultivar (Oryza sativa L.) from various regions in Indonesia using random amplified polymorphic DNA markers (RAPD). International Journal on Advanced Science, Engineering and Information Technology, 9(3), 1046–1051. https://doi.org/10.18517/ijaseit.9.3.8382
- Stintzing, F. C., Stintzing, A. S., Carle, R., Frei, B., & Wrolstad, R. E. (2002). Color and antioxidant properties of cyanidin-based anthocyanin pigments. Journal of Agricultural and Food Chemistry, 50(21), 6172–6181. https://doi.org/10.1021/jf0204811
- Swallah, M. S., Sun, H., Affoh, R., Fu, H., & Yu, H. (2020). Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. International Journal of Food Science, 2020, 9081686. https://doi.org/10.1155/2020/9081686
- Tananuwong, K., & Tewaruth, W. (2010). Extraction and application of antioxidants from black glutinous rice. LWT - Food Science and Technology, 43(3), 476–481. https://doi.org/10.1016/j.lwt.2009.09.014
- Wang, W., Li, Y., Dang, P., Zhao, S., Lai, D., & Zhou, L. (2018). Rice secondary metabolites: Structures, roles, biosynthesis, and metabolic regulation. Molecules, 23(12), 3098. https://doi.org/10.3390/molecules23123098
- Wu, H., Johnson, M. C., Lu, C. H., Fritsche, K. L., Thomas, A. L., Cai, Z., & Greenlief, C. M. (2015). Determination of anthocyanins and total polyphenols in a cultivar of elderberry juices by UPLC-MS/MS and other methods. Acta horticulturae, 1061(1061), 43–51. https://doi.org/10.17660/actahortic.2015.1061.3
- Xiao, J. (2017). Dietary flavonoid aglycones and their glycosides: Which show better biological significance?. Critical Reviews in Food Science and Nutrition, 57(9), 1874–1905. https://doi.org/10.1080/10408398.2015.1032400
- Xiao, J., Capanoglu, E., Jassbi, A. R., & Miron, A. (2016). Advance on the flavonoid C-glycosides and health benefits. Critical Reviews in Food Science and Nutrition, 56(Suppl 1), S29–45. https://doi.org/10.1080/10408398.2015.1067595