 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
“Granny Smith” apples are susceptible to quality deterioration and microbial decay during retail handling, and marketing display in plastic bags. Therefore, the effectiveness of coating as alternative packaging under retail condition was explored. Impact of edible coatings: zein, zein combined with nisin (zein-nisin) on maintaining various qualities microbial load on “Granny Smith” apples stored at 15°C for 21 days was investigated and compared with control samples. Results showed that weight loss (%) was highest in control samples (30%) by day 14, while zein edible coating significantly delayed weight loss till the end of storage day 21 (p ≤ .05). Overall, changes in hue angle (h°), titratable acidity (TA), total soluble solids (TSS), and firmness were not significantly impacted by coating. However, the application of Zein-nisin coatings effectively resulted in ~1.5 log and ~3 log reduction by the end of storage day 21 in yeast and mould, and TAMB count, respectively.
1. Introduction
Globally, apples are among the most popular and frequently consumed fruit due to their taste, nutritional composition, and health benefits. Apple fruit contains essential fatty acids, dietary fibre, antioxidant, and phytochemicals (Taher et al., Citation2018). Apple fruit is highly perishable and susceptible to various postharvest diseases, that leads to lose of quality during storage (Tarabay et al., Citation2018). From the various apple cultivars, “Granny Smith” is the most important export apple cultivar in South Africa (Brodie, Citation2023). “Granny Smith” apples, however, are most susceptible to pathogenic attack that leads to quality degradation and affects its marketability during postharvest (Florian et al., Citation2018). Maintaining fresh quality and eliminating the growth of microorganism after harvest constitute a serious challenge for the fruit industry (Rojas-Graü et al., Citation2007). The commonly available postharvest strategies for apple fruit include the use of chemical, controlled atmosphere, and cold storage. Optimal storage condition for apple mainly aims to slow down the quality degradation process and maintain freshness, texture, color, and bioactive compounds close to the level at harvest (Maletsika et al., Citation2014).
Edible coatings consist of a film forming biopolymer that creates barrier properties for O2, CO2 and moisture. The advantage of edible coating to reduce respiration rate, weight loss, loss of texture and the ability to reduce oxidation reactions for various fruits has been reported by several studies (Ebrahimi & Rastegar, Citation2020; Kumar et al., Citation2018; Lin et al., Citation2018; Y. L. Zhang et al., Citation2021). The most commonly used edible biopolymers are based on polysaccharides, proteins, and lipids (Alkan & Yemenicioğlu, Citation2016). Zein is a natural protein found in corn kernels, usually isolated from corn gluten meal, by-products of corn wet milling for wide application (Santos et al., Citation2018). According to grain South Africa, maize by-products are predominantly utilized for animal feed as a protein reach feed (Maluleke, Citation2020). However, due to several recent methodologies and recent developments zein can be used as a coating material for fresh produce. Edible coatings prepared from Zein delivered a promising potential to be used as a biopolymer, it is effective in preventing color change, weight loss and enhance shelf life (Jaski et al., Citation2022; Raghav et al., Citation2016). Zein has been reported as non-toxic, biocompatible, biodegradable, and as an excellent matrix to encapsulate bioactive compounds and to enhance their stability and bioavailability (Jaski et al., Citation2022; Khadka et al., Citation2017; Patel & Velikov, Citation2014; Raghav et al., Citation2016; Sharif et al., Citation2019).
For wider application, edible coatings can also carry antioxidants and antimicrobial compounds to improve their functional properties (Adiletta et al., Citation2021; Anjum et al., Citation2020; Bashir et al., Citation2022; Isopencu et al., Citation2021; Khadka et al., Citation2017; Sánchez-González et al., Citation2011). Antimicrobial compounds such as nisin have been getting popularity in food industry as an FDA and EU approved bio-preservative and low toxicity to human being. Nisin is a polycyclic antimicrobial peptide that inhibit the pathogenic food borne bacterial such as Listeria monocytogenes and many other gram-positive food spoilage microorganisms (Song et al., Citation2017). Nisin forms pore in cell membranes that disrupt the proton and pH, interferes with cell wall biosynthesis thus cause irons leakage and ATP hydrolysis and cell death (Liao et al., Citation2018). The effective antimicrobial potential of nisin as coating component has been explored for various fresh produce (Duran et al., Citation2016; Z. Song et al., Citation2017)
Various studies reported the application of edible coating for apple fruit (da Silva & Paillart, Citation2018; Jahanshahi et al., Citation2018; Rashid et al., Citation2020; Supapvanich et al., Citation2016; Thakur et al., Citation2019). The application of edible Aloe vera gel coating on fresh cut wax “Taaptimjaan” apple fruit showed a beneficial effect to reduce the weight loss, retard browning, and maintained antioxidant activities of the fruit (Supapvanich et al., Citation2016). In another study, coating of fresh cut-apple with Aloe vera gel containing 0.5% cysteine delayed browning and reduced microbial population during storage (H. Y. Song et al., Citation2013). Most recently, the development of coating with antioxidant chitosan film with banana peel extracts was reported for apple fruit by W. Zhang et al. (Citation2020). The above-mentioned studies indicate that coating of apple fruit maintained the fruit firmness, reduced weight loss, antioxidant, and physiological properties. The authors further reported the evidence of using the developed coating as a desirable alternative for active packaging.
Fresh apples even after coating are susceptible to contamination during handling, transportation, storage and during shelf life. Therefore, introducing sustainable solution by incorporating safe antimicrobial agent such as nisin to the coating matrix could be an alternative solution. However, studies investigating the effects of zein-nisin based edible coating for antimicrobial efficacy as well as the effects on the quality and physiological responses of apple fruit have been unexplored. Therefore, the objectives of this study were to determine the inhibitory effects of zein coating incorporating with nisin (an antimicrobial agent) on the surface microbial load on “Granny Smith” apples, and to evaluate the impact of these coatings on physicochemical and physiological attributes of “Granny Smith” apple during storage under retail conditions.
2. Materials and methods
2.1. Chemicals and fruit
Zein powder (G9012-L1) and nisin (N6764-5 G) were purchased from Sigma chemicals (St. Louist, MO, U.S.A), and ethanol (95%) and glycerol were purchased from Rochelle chemicals (South Africa). “Granny Smith” apples were obtained at commercial maturity from a commercial farm, Western Cape, South Africa, and transported under cooled conditions to the Agricultural Research Council (ARC), Infruitec-Nietvoorbij, Stellenbosch, South Africa. Only mature, healthy, and unblemished fruit were selected. Fruit samples were then treated with 1-Methylcyclopropen (1-MCP) (SmartFresh SmartTabs™, AgroFresh Inc., Durbanville, South Africa) and stored at −0.5°C for 6 months under optimum controlled atmosphere conditions (1.5% O2 and 1% CO2) prior to this investigation (this was done to mimic the local pack-house practices of medium to long-term storage of apples).
2.2. Preparation and application of zein coating solution
Zein solution was prepared by dissolving 2% of zein powder and 15 mL glycerol into 500 mL of warm (95°C) aqueous ethanol (95%) according to method described by Hager et al. (Citation2018). Zein coating with Nisin was prepared by blending 0.75 g of nisin with 50 g of zein powder in a warm (75°C) aqueous ethanol 95%. The mixture was obtained by using magnetic stirrer (LABOTEC, Model 105, South Africa) for 30 min. The solution was then cooled at room temperature with continuous stirring. To investigate the effect of zein based coating, 117 fruit were sorted in each three categories for the three treatments: (a) control (uncoated), (b) zein, and (c) zein-nisin coating, multiple triplicate samples were prepared to accommodate sampling in each sampling dates. In general, 361 fruit were used for the storage experiment.
Equal amount of the solution was applied on the surface of the apples using the dipping method, and for each treatment, fruit were dipped into the coating solutions for 1 min. Coated fruits were allowed to be air-dried at room temperature (23°C) on the laboratory bench. Apple fruit without any coating was designated as control. All samples were stored at 15°C (this temperature was selected to mimic the average retail outlet practice), and sampling was done at day 0, 7, 14 and 21.
2.3. Physicochemical quality analysis
2.3.1. Weight loss
The weight loss of apple fruit coated with zein, zein-nisin and control was measured according to the method by Nsumpi et al. (Citation2020). Fruit weight loss was determined by weighing each fruit of the different storage treatments on each sampling day using an electronic balance (UWE HGS-15 K, Taiwan). The difference in the mass between the initial and post-storage measurement was calculated and averaged as percentage mass loss using Equation (1):
where, WL is the weight loss (%); WO and Wf are the initial weight (g) and the final weight (g) of the apples, respectively.
2.3.2. Firmness
Firmness of the apple fruit treated and control were measured using texture analyser (FTA 20, Güss, South Africa) according to the method described by Nsumpi et al. (Citation2020). A compression prob of 7.9 - mm diameter was used for the penetration test with the speed of 10 mm s−1 and to a depth of 8.9 mm, the results were expressed in kg. All measurements were conducted in triplicate per treatment and tissue strength was expressed in kg.
2.3.3. Fruit colour
Colour change on each apple fruit was measured based on Commission International del’ Eclairage (CIE) colour system using a digital Chroma-meter (CR 400/410 Konica Minolta Sensing Inc., Japan). Colour calibration of the chroma-meter was performed against a white and black tile background prior to each measurement. Colour measurements were taken using individual fruit (n = 3) and data obtained were average of individual colour parameters L* denotes the lightness, a* describes red (+)/green (-) and b* for yellow (+)/blue (-). To describe the measured colour attributes hue angle (h°), which describes the qualitative attribute of colour shades (0° = red-purple and 180° = bluish green), and Chroma (C*), which denotes the quantitative attribute of colour intensity were calculated according to Caleb et al. (Citation2016) using Equations 2 and 3:
2.3.4. Total soluble solid (TSS) and titratable acidity (TA)
Three fruit per treatment were juiced using a juice extractor (4294 J700, Braun, China) to determine the effects of coating on TSS and TA of apple fruit during storage. Total soluble solid was measured using a pocket calibrated refractometer (PAL-1, ATAGO, and Japan), and the results were expressed as °Brix. Titratable acidity of each fruit was obtained from the titration of 53.7 mL of each fruit juice with 0.333 N of sodium hydroxide (NaOH) at a pH of 8.2, using Crison Titromatic 1S/2B (Crison Instruments, Barcelona, Spain) and the results were expressed as g 100 mL−1 of malic acid.
2.4. Physiological responses (Respiration rate (RR) and ethylene concentration)
Respiration rate (RR) and ethylene concentration were determined during the 21 days’ storage according to the method described by Nsumpi et al. (Citation2020). For these analyses, apple fruit of known weight were placed into a closed system developed in-house, which consisted of three hermetic jars glass. The headspace gas (about 100 μL) was sampled through the silicon septum provided in the jar lid. Gas samples (CO2) were taken after 1 h using a gas analyser (Isolcell Oxycarb 6, Italia). The RR was calculated as the amount of CO2 produced per unit mass of the fruit per unit time (mL kg−1 h−1) using Equation 4. The concentration of ethylene (ppm) was measured after an hour using ICA 56-ethylene analyser (Valencia, Spain). Ethylene production was calculated as the amount of ethylene produced per unit mass of the fruit per unit time (µL kg−1hr−1).
where and
are CO2 concentration (%) at time
(h) and time
(h), respectively.
is
due to CO2 production in mL/g day
is free volume of the containers and
is the total weight of the product (kg).
2.5. Microbiological analysis
Aerobic mesophilic bacteria and yeast and mould were determined using plate count agar (PCA) and potato dextrose agar (PDA) acidified with 10% tartaric acid, respectively. The surface area of the fruit was estimated based on the approach described by Nyamende et al. (Citation2022). Three whole fruit per treatment were placed inside a beaker containing 300 mL saline solution separately and vortex using an FMH200 Shaker for 1 h at 115 rpm to adequately remove the microorganisms on the fruit surface. This was followed by preparation of 3-fold dilutions up to 10−3 using 1 mL of diluent transferred into 9 mL saline solution. To enumerate the microbial load 1 mL of each dilution were pour-plated in triplicate on PCA and PDA plates. The PCA and PDA plates were then incubated at 30°C for 3 days and 5 days, respectively. After incubation, colonies between (30 and 300) on each plate were counted. The results were expressed as log colony forming unit (CFU) per surface area of samples (log CFU cm−2). All measurements were done in triplicate for each treatment and three levels of dilution (n = 9).
2.6. Statistical analysis
To elucidate on the impacts of coating material and storage duration on the physicochemical quality and microbial safety of apple during storage, factorial ANOVA was applied at 95% confident interval using Statistica Software (version 13, StatSoft Inc. TIBCO Software Inc., U.S.A). For the whole experiment multiple samples were prepared in triplicate (n = 10) for day 0, 7, 14 and 21. The Post-hoc test (Duncan multiple range test) was also used to determine the difference between mean combination values.
3. Results and discussion
3.1. Physicochemical quality analyses
3.1.1. Weight loss
Based on statistical results obtained, the application of coating and storage duration significantly (p ≤ .05) affected the weight loss of apples. Maximum weight loss was attained for the control samples by day 14, while coated samples significantly delayed weight loss until day 21 (). Comparing between the coated and control fruit, the coated samples relatively maintained the weight of apple fruit during storage. By the end of the storage day 21, apples in the control batch had lost about 30% of its initial weight, followed by the zein-nisin coated apple, which lost 25%, zein coated recorded the lowest weight (17%) of its initial weight.
Figure 1. Effects of zein and zein-nisin coating and storage duration on the weight loss of “Granny Smith” apple stored for 15°C for 21 days. Mean values (n = 3) with standard deviation bars. Different lower-case letters represent significantly different mean (p ≤ .05).
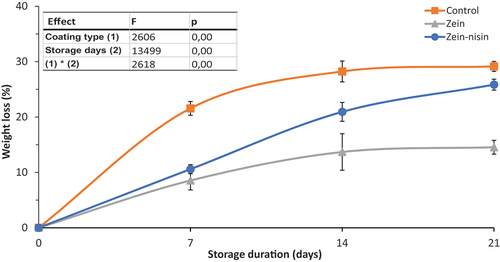
Fruits are highly susceptible to water loss through the process of transpiration, which causes them to shrink and become wrinkled thus lose weight and reduces their quality (Nawab et al., Citation2017; Shah & Hashmi, Citation2020). The observed delayed weight loss on coated fruit could be due to the ability of coating for imparting a modified water vapour barrier, through the blocking of the skin pores to transpiration and normal gas exchange of the fruit. The beneficial effects of coating to reduce the weight loss of various fruit have been reported by other studies. Supapvanich et al. (Citation2016) demonstrated coating apple fruit ‘Taaptimjaan‘ with glucomannan, and pineapple fruit extract effectively delayed the fruit weight loss compared to the control. The authors associated this to the low water vapour permeability of coating agent. W. Zhang et al. (Citation2020) demonstrated the significant reduction of apple fruit weight loss coated with chitosan-BPE during the storage period.
In another study, coating mango fruit with chitosan/zein-cinnamaldehyde reduced the weight loss during storage (Xiao et al., Citation2021). The authors specified that incorporation of zein might have a synergetic effect on mango preservation, which formed a barrier with protective function on the mango surface and blocked the movement of moisture, thus improve weight retention. The most common quality losses are associated with water vapor and oxygen (O2) transfer that led to loss of firmness. In this regard, the potential of zein-based edible coating to create barrier for O2 transfer has been reported by Pena Serna and Lopes Filho (Citation2015). These studies demonstrated that using zein significantly improved the barrier properties and moisture transfer. Zein is hydrophobic, contains nonpolar amino acids such as leucine or proline and resulting in film with excellent barrier properties to water vapor (Pena-Serna et al., Citation2016; Raghav et al., Citation2016). However, in the current study, the incorporation of nisin to zein reduced the effectiveness of zein to control the weight loss of apple fruit.
3.1.2. Firmness
Statistical analysis showed that, the firmness of “Granny Smith” apples stored at 15°C for 21 days was significantly (p ≤ .05) affected by treatments (control, coating with zein, and zein-nisin) and storage duration (). The firmness of apple fruit showed significant increase from the initial value of 7.1 ± 0.00 kg on day 0 to 7.6 ± 0.12 kg, 8.0 ± 0.20 kg and 7.8 ± 0.04 kg for control, zein and zein-nisin coated apples, respectively, on day 21. As it can be seen in this result, zein coated apples had the highest firmness. This was consistent on day 7 and 14, where the apple coated with zein had higher firmness values comparing to the control and zein-nisin coated apple fruit. Similar findings were reported by W. Zhang et al. (Citation2019), where the application of chitosan coating treatment significantly maintained the firmness of nectarine fruit (cv. Ruiguang 7) during storage at 25°C for 8 days. Similar, W. Zhang et al. (Citation2020) observed a continuous decline of apple fruit firmness during storage with the highest decline observed in week three that could be due to the respiratory climacteric of the fruit. According to Yao et al. (Citation2014), the main cause for reduction of firmness in harvested fruit is associated with the increase in cell wall hydrolase activity that can cause softness.
Table 1. Effects of zein and zein-nisin coating and storage duration on the total soluble solid (TSS), titratable acidity, colour attributes and texture of “Granny Smith” apples stored at 15°C for 21 days.
The retention of firmness could also be attributed to the restriction in metabolic activities associated with cell wall-degradation enzymes such as pectinesterase and polygalacturonase (Adhikary et al., Citation2022; Baraiya et al., Citation2015). According to Sapper and Chiralt (Citation2018) limiting the movement of gases in fruit such as maintaining low level of O2 and high level of CO2 reduces the activities of enzymes and allows retention of firmness during storage. The most common qualityof losses are associated with water vapor and oxygen transfer that led to loss of firmness.
3.1.3. Fruit colour
Colour of “Granny Smith” apple was evaluated in terms of the colour parameters such as, Chroma (C*) value and hue angle (h°). As shown in , the h° decreased in all samples but at different rate from 155.7 ± 0.00 at day 0 to 114.7 ± 0.83, 112.3 ± 0.66 and 111.7 ± 0.77 for control, zein and zein-nisin coating, respectively on day 21. There was no significant (p > .05) difference in the h° value between coated and control samples on a given sampling day. However, zein coated fruit had significantly lower h° angle (p ≤ .05) at day 7 that compared to the other samples. On the other hand, the effects of storage duration as well as the interaction effects of treatments and storage duration were significant (p ≤ .05). The decrease in h° could be a sign of degradation of chlorophyll in the skin of fruit (Nsumpi et al., Citation2020). Baysal et al. (Citation2010) reported that apricot coated with zein was able to maintain and delayed color change due to reduced respiration rate brought by the presence of the surface coating. The maintenance of h° coated fruit may be attributed to the modified atmosphere by an edible coating, which may indicate that the ripening process was delayed.
On the other hand, C* value increased (p > .05) for control fruit from its initial value of 44.4 ± 0.00 at day 0 to 44.7 ± 0.87 by day 21 (). On the contrary, the highest reduction in C* value of 37.8 ± 3.25 and 21.9 ± 3.48 on day 21 was observed for zein and zein-nisin coated samples, respectively. Results obtained showed that both treatments (control, zein and zein-nisin) and storage duration had a significant effect on the C* value of “Granny Smith” apple fruit stored at 15°C for 21 days. The colour parameters of all coated and uncoated fruit remained in acceptable conditions throughout the storage period, there was absent of visual decay, and visible color changes. However, Bai et al. (Citation2003) reported whiting of apples treated with zein during storage, that could influence the C* and h°. The authors further suggested that the degree of whitening could be controlled by varying the content of zein and glycerol and by defatting the zein before application. In the current study, the observed significant decrease in C* value could be attributed to the white color brought by the coating on the fruit at room temperature. It was evident that the L* values of coated fruit were significantly (p ≤ .05) higher compared to the control fruit throughout the storage duration (). Similarly, W. Zhang et al. (Citation2019) reported that chitosan coated nectarine fruit resulted in higher L* values than control fruit. In another study, Rawdkuen et al. (Citation2012) also demonstrated the highest L* value when incorporating catechin-lysozyme on gelatine-based matrix than the gelatine alone. The increase in L* value in the film matrix could be due to the light scattering effects of matrix compounds (Ghadermazi et al., Citation2019).
3.1.4. Total soluble solid (TSS) and titratable acidity (TA)
The TSS values of all treated and control fruit were not significantly different throughout the storage period (p > .05). All treatments maintained the initial TSS value till the end of storage (). Similarly, the coating (with zein and zein-nisin) did not have a significant (p > .05) effect on the TA values of “Granny Smith” apple fruit during storage (). However, the storage duration and the interaction effects of coating and storage duration significantly affected the TA values of the fruit (p ≤ .05). This was clearly observed from the results, wherein the initial TA value of 1.0 ± 0.00 g 100 mL−1 declined to 0.8 ± 0.06 g 100 mL−1 for control, 0.8 ± 0.05 g 100 mL−1 for zein and 0.8 ± 0.07 g 100 mL−1 for zein-nisin coated samples at the end of the storage, day 21. These results agreed with those found by Jahanshahi et al. (Citation2018), that demonstrated TA values of non-treated apple fruit (cvs. Golden Delicious and Red Delicious) significantly decreased, whereas TA values of fruit treated with tragacanth gum considerably maintained throughout the study (4 month at 1°C). Reduction of TA values in control fruit can be associated with presence of higher respiration rate that could result in degradation of organic acid. This was evident that organic acid can act as substrate for enzymatic reaction of respiration that result in reduction in acidity. On the other hand, coating fruit creates barrier to O2 and water vapour, reduce RR and consequently prevent acid oxidation.
On the study carried out by W. Zhang et al. (Citation2019), the TSS values of nectarine fruit (cv. Ruiguang 7) treated with low and high molecular weight chitosan coating were significantly suppressed, whereas the TSS value of the control fruit increased and reached 12.96% on day 8 at 25°C. In the same study, the authors reported that TA values of coated fruit were higher than the control at the end of storage. In another study, Ahmed et al. (Citation2009) reported that Aloe vera gel-coated nectarine (cv. Arctic Snow) fruit exhibited significantly lower TSS content than control fruit during ripening at 20°C stored for 8 days. Bai et al. (Citation2003) also found no significant difference in TA and TSS of “Granny Smith” and “Fuji” apples treated with 1-MCP and coated with candelilla wax, shellac, and carnauba wax. The differences in these reports could be attributed to various factors such as fruit type, selected cultivars and maturity of the fruit investigated, storage conditions and durations.
3.2. Physiological responses
3.2.1. Respiration rate
Respiration rate (RR) of apple fruit significantly (p ≤ .05) decreased at day 7 and 14 post-storage across all treatments. Statistical analysis indicated that the effect of both storage duration and treatments significantly (p ≤ .05) influenced the RR of “Granny Smith” apple stored at 15°C for 21 days. The significant climacteric increase, however, was observed after day 14, where the RR increased from 14.31 kg L−1 h−1 on day 0 to 39.82 kg L−1 h−1, 41.97 kg L−1 h−1, and 35.41 kg L−1 h−1 on day 21 for control, zein-nisin and zein, respectively (). Comparing the RR between the coated and control fruit throughout the storage duration, fruit coated with the zein had a lower RR compared to the zein-nisin coated fruit and control fruit during the 21 days of storage. Consistent with this study Ahmed et al. (Citation2009) found out that the coating treatment and time played significant role in the RR and ethylene production. On contrary, Aloe vera coated and control nectarine fruit did not exhibit significant changes in the RR for the first 3 days of storage, however, the RR of control fruit increased rapidly with the highest rate at day 8 which was 41% higher than the coated fruit (Ahmed et al., Citation2009).
Figure 2. Effects of zein and zein-nisin coating and storage duration on the respiration rate (a) and ethylene production rate (b) of “Granny Smith” apple stored for 15°C at 21 days. Mean values (n = 3) with standard deviation bars. Different lower-case letters represent significantly different mean (p ≤ .05).
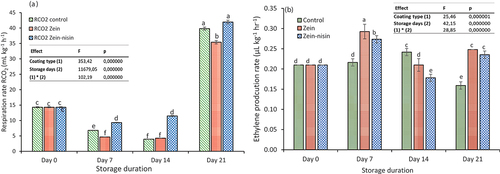
Similar result was observed by Shah and Hashmi (Citation2020) for mango fruit coated with chitosan and Aloe vera, where coating delayed the respiratory peak till the end of the storage. A study conducted by Bai et al. (Citation2003) reported the barrier properties of edible coatings resulting in a modified internal apple fruit atmosphere of relatively high CO2 and low O2. This combination of low internal O2 and high CO2 can lower RR and delay ethylene production, thus delaying ripening and senescence, this result was in contradiction with the results observed in the current study. W. Zhang et al. (Citation2019) revealed the single peak in RR of apple fruit coated with chitosan than uncoated fruit after two weeks of storage. The study further stated that the application of coating significantly reduced the RR throughout the storage duration. Respiration rate is directly related to the metabolic activity and is therefore considered an excellent indicator of fruit shelf-life. The results obtained in the current study thus suggested that coating treatment could change the respiratory activities of nectarine fruit during storage.
3.2.2. Ethylene production
An initial increase in ethylene production (p ≤ .05) was observed on day 7 across all the treatments from 0.21 µL kg−1 hr−1 on day 0 to 0.22 ± 0.06 µL kg−1 hr−1, 0.29 ± 0.02 µL kg−1 hr−1, and 0.27 ± 0.01 µL kg−1 hr−1 for control, zein- and zein-nisin coated apples, respectively (). On the contrary, the ethylene concentration significantly declined thereafter (p ≤ .05) until the end of storage, except control sample. The highest ethylene production was observed for apple fruit coated with zein alone (0.29 ± 0.02 µL kg−1 hr−1), whereas the lowest concentration was observed for control apple (0.16 ± 0.01 µL kg−1 hr−1), which did not receive any treatment. Similarly, Gardesh et al. (Citation2016) reported that the nano chitosan coating significantly declined ethylene production during storage of apple (cv. Golab Kohanz) at 1°C for nine weeks. In another study, Shah and Hashmi (Citation2020) observed the early ethylene production peaked for control (un-coated) samples, whereas chitosan and aloe vera coating delayed the ethylene production on mango.
Ethylene production is control by the enzyme ACC-synthase and ACC-oxidase (Bulens et al., Citation2012; Steffens et al., Citation2022), thus the decrease in ethylene production in control fruit by the end of the storage could be due to the decrease in activity of these enzymes. Furthermore, the highest ethylene production observed in the zein-nisin and control fruit could be the result of higher RR as ethylene production is influenced by the RR of the fruit. The burst of respiration on day 21 suggests that apples had accumulated ethylene endogenously. The results showed that all the treatments (zein, zein-nisin and control), storage duration as well as their interaction effects had a significant (p ≤ .05) impact on the ethylene production of “Granny Smith” apple post-storage. This study further illustrated that coating of apple fruit with zein or zein-nisin had no effect to suppress the ethylene production. In general, the ethylene production rate for all coated and control fruit were comparatively low throughout the storage. This could be due to the fact that the apple fruit used in this study received 1-MCP treatment prior to storage, that limit ethylene production (Bulens et al., Citation2012).
3.3. Microbial quality
As presented in , a significant (p ≤ .05) increase in aerobic mesophilic bacteria count was observed for control fruit throughout the storage duration. Total aerobic mesophilic bacteria count for control apple fruit increased from 2.9 ± 0.33 log CFU cm−2 on day 0 to 3.7 ± 0.06 log CFU cm−2 by the end of storage (day 21). In contrast, for samples coated with zein and zein-nisin the aerobic mesophilic bacteria count declined significantly in comparison to the control. The inhibitory effects of coatings on the apple fruit surface were evident, with ≈2 Log reduction on day 7 (). Furthermore, at the end of storage the total aerobic mesophilic bacteria count of 2.4 ± 0.37 log CFU cm−2 and 2.2 ± 0.03 log CFU cm−2 for zein and zein-nisin coated apples were significantly lower than the control (3.7 ± 0.06 log CFU cm−2). Overall, in the current study, the microbial load of both aerobic mesophilic bacteria was below the maximum acceptable limit of 7 log CFU mL−1 on fresh produce (Tango et al., Citation2018). Therefore, the results obtained demonstrated the potential of zein and zein-nisin as alternatives to polymeric films for the distribution of apples.
Figure 3. Effects of zein and zein-nisin coating and storage duration on the aerobic mesophilic bacteria (a) and the yeast and mould counts (b), on the surface of “Granny Smith” apple stored for 15°C for 21 days. Mean values (n = 9 plates) with standard deviation bars. Different lower-case letters represent significantly different mean (p ≤ .05).
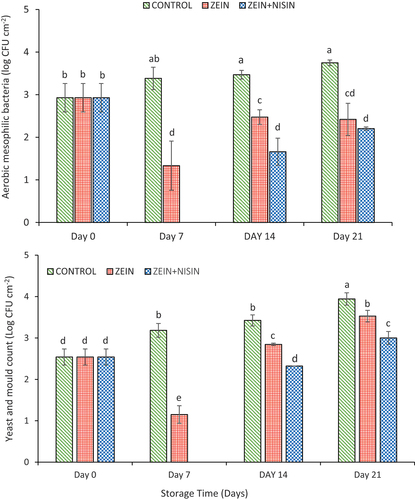
A similar trend was observed for yeast and mould count for coated and control apple fruit stored for 21 days (). The yeast and mould count showed a gradual increase throughout the study for on control samples, where the count increased from 2.5 ± 0.19 log CFU cm−2 on day 0 to ≈3.9 log CFU cm−2 at the end of day 21. Yeast and mould count were significantly lower on the coated samples with the highest inhibition of yeast and mould observed in zein-nisin samples from day 7 to day 21 (p ≤ .05). It is noteworthy to state that zein-nisin coated fruit completely inhibited the growth of yeast and mould on day 7, and a gradual increase was observed thereafter till day 21. Comparing the results obtained for zein alone and zein-nisin samples, the yeast and mould count increased to ≈3.5 log CFU cm−2 and 3.0 log CFU cm−2 (p ≤ .05), respectively, by the end of the storage. Therefore, this is evident that the microbial inhibitory efficacy of zein coating was enhanced by incorporating the nisin. Furthermore, the yeast and mould count in both treated and control were below the 5 log CFU mL−1.
It is important to note that, the growth of microbes was significantly inhibited during the first 7 days, with zein-nisin coated apples. This could be attributed to the zein solution being prepared with 95% ethanol, which could have increased the initial antimicrobial effect of zein on the first 7 days, the combination of zein with nisin resulted in a better control of microbial growth. These results agree with those of Baysal et al. (Citation2010), who worked with zein coating impregnated with potassium sorbate (0.1%) and ascorbic acid (1%) on moisture apricot and found a total reduction in microbial count. Hager at el. (2018) observed decrease in Listeria monocytogenes on fish fillets coated with zein and incorporated with nisin. Although zein can inhibit microbial growth alone an addition of nisin improved the antimicrobial characteristics of the edible coating. Nisin is a bacteriocin produced by Lactobacillus lactis, it inhibits microbes by incorporating itself into their cytoplasmic membranes, which results in a loss of intracellular ions and disruption of the pH gradient and proton motive force. Thus, the increased antimicrobial efficacy of zein-nisin film in the current study could be due to the dual effects of zein and nisin.
4. Conclusion
This study investigated the effects of zein and zein-nisin coatings on the quality of “Granny Smith” apple during 21 days of storage at 15°C. Storing apple fruit without treatment resulted in higher weight loss and texture loss, as well as higher aerobic mesophilic bacteria and yeast and mould. In contrast, zein-based coatings significantly maintained the quality attributes of “Granny Smith” apple and retarded microbial growth on the fruit surface during storage compared to the control fruit.
Furthermore, the incorporation of nisin enhanced the antimicrobial efficacy of zein coating, which was evident with the lower microbial count than all the treatments and control samples. In addition, zein-nisin had higher weight loss than zein alone and zein maintained texture better than zein-nisin but increased the respiration rate. However, the mechanism behind this physiological response and the change in the functionality of zein due to the addition of nisin has not been identified. Therefore, further study is recommended to study the mechanical property of zein-nisin films as well as to enhance the functional property of the film by applying plasma treatment. The results obtained demonstrated the potential of zein edible coating alone and/or in combination with nisin as effective alternative to polymeric films for the postharvest management of apples.
Acknowledgments
This work is based upon research supported wholly/in part by the National Research Foundation (NRF), South Africa (Grant No. 137990) awarded to Dr Caleb. National Research Foundation (NRF), South Africa (Grant No. 138128) awarded to Dr Belay. The authors thank Agricultural Research Council (ARC) Infruitec-Nietvoorbij for the support throughout the accomplishment of this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adhikary, T., Gill, P. P. S., Jawandha, S. K., & Sinha, A. (2022). Chitosan coating modulates cell wall degrading enzymes and preserved postharvest quality in cold-stored pear fruit. Journal of Food Measurement and Characterization, 16(2), 1395–1403. https://doi.org/10.1007/s11694-022-01291-8
- Adiletta, G., DiMatteo, M., & Petriccione, M. (2021). Multifunctional role of chitosan edible coatings on antioxidant systems in fruit crops: A review. International Journal of Molecular Sciences, 22(5), 2633. https://doi.org/10.3390/ijms22052633
- Ahmed, M. J., Singh, Z., & Khan, A. S. (2009). Postharvest aloe vera gel‐coating modulates fruit ripening and quality of ‘Arctic snow’ nectarine kept in ambient and cold storage. International Journal of Food Science & Technology, 44(5), 1024–1033. https://doi.org/10.1111/j.1365-2621.2008.01873.x
- Alkan, D., & Yemenicioğlu, A. (2016). Potential application of natural phenolic antimicrobials and edible film technology against bacterial plant pathogens. Food Hydrocolloids, 55, 1–10. https://doi.org/10.1016/j.foodhyd.2015.10.025
- Anjum, M. A., Akram, H., Zaidi, M., & Ali, S. (2020). Effect of gum arabic and aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’ guava fruits. Scientia Horticulturae, 271, 109506. https://doi.org/10.1016/j.scienta.2020.109506
- Bai, J., Alleyne, V., Hagenmaier, R., Mattheis, J., & Baldwin, E. (2003). Formulation of zein coatings for apples (Malus domestica Borkh). Postharvest Biology and Technology, 28(2), 259–268. https://doi.org/10.1016/S0925-5214(02)00182-5
- Baraiya, N. S., Rao, T. V. R., & Thakkar, V. R. (2015). Improvement of postharvest quality and storability of jamun fruit (Syzygium cumini L. Var. Paras) by zein coating enriched with antioxidants. Food and Bioprocess Technology, 8(11), 2225–2234. https://doi.org/10.1007/s11947-015-1577-x
- Bashir, S., Arshad, M. S., Khalid, W., Nayik, G. A., Al Obaid, S., Ansari, M. J., & Karabagias, I. K. (2022). Effect of antimicrobial and antioxidant rich pomegranate peel based edible coatings on quality and functional properties of chicken nuggets. Molecules, 27(14), 4500. https://doi.org/10.3390/molecules27144500
- Baysal, T., Bilek, S. E., & Apaydın, E. (2010). The effect of corn zein edible film coating on intermediate moisture apricot (Prunus armenica L.) quality. Gida, 35(4), 245–249.
- Brodie. (2023). Apple varieties fruit farming in South Africa. https://southafrica.co.za/apple-varieties.html
- Bulens, I., Van de Poel, B., Hertog, M. L. A. T. M., De Proft, M. P., Geeraerd, A. H., & Nicolai, B. M. (2012). Influence of harvest time and 1-MCP application on postharvest ripening and ethylene biosynthesis of ‘Jonagold’ apple. Postharvest Biology and Technology, 72, 11–19. https://doi.org/10.1016/j.postharvbio.2012.05.002
- Caleb, O. J., Wegner, G., Rolleczek, C., Herppich, W. B., Geyer, M., & Mahajan, P. V. (2016). Hot water dipping: Impact on postharvest quality, individual sugars, and bioactive compounds during storage of ‘Sonata’ strawberry. Scientia Horticulturae, 210, 150–157. https://doi.org/10.1016/j.scienta.2016.07.021
- da Silva, F. P., & Paillart, M. (2018). Effects of Starchy edible coating on shelf life of apples ( No. 1840). Wageningen Food & Biobased Research.
- Duran, M., Aday, M. S., Zorba, N. N. D., Temizkan, R., Büyükcan, M. B., & Caner, C. (2016). Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food and Bioproducts Processing, 98, 354–363. https://doi.org/10.1016/j.fbp.2016.01.007
- Ebrahimi, F., & Rastegar, S. (2020). Preservation of mango fruit with guar-based edible coatings enriched with Spirulina platensis and aloe vera extract during storage at ambient temperature. Scientia Horticulturae, 265, 109258. https://doi.org/10.1016/j.scienta.2020.109258
- Florian, V. C., Carmen, P. U. I. A., Groza, R., Suciu, L. A., & Florian, T. (2018). Study of the major pathogens that lead to apple fruit decay during storage. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 46(2), 538–545. https://doi.org/10.15835/nbha46211194
- Gardesh, A. S. K., Badii, F., Hashemi, M., Ardakani, A. Y., Maftoonazad, N., & Gorji, A. M. (2016). Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT, 70, 33–40. https://doi.org/10.1016/j.lwt.2016.02.002
- Ghadermazi, R., Hamdipour, S., Sadeghi, K., Ghadermazi, R., & Khosrowshahi Asl, A. (2019). Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose—A review. Food Science & Nutrition, 7(11), 3363–3377.
- Hager, J., Rawles, S., Xiong, Y., Newman, M., Thompson, K., & Webster, C. (2018). Listeria monocytogenesis inhibited on fillets of cold-smoked sunshine bass, Morone chrysops × Morone saxatilis, with an edible corn zein-based coating incorporated with lemongrass essential oil or nisin. Journal of the World Aquaculture Society, 50(3), 575–592. https://doi.org/10.1111/jwas.12573
- Isopencu, G. O., Stoica-Guzun, A., Busuioc, C., Stroescu, M., & Deleanu, I. M. (2021). Development of antioxidant and antimicrobial edible coatings incorporating bacterial cellulose, pectin, and blackberry pomace. Carbohydrate Polymer Technologies and Applications, 2, 100057. https://doi.org/10.1016/j.carpta.2021.100057
- Jahanshahi, B., Jafari, A., Vazifeshenas, M., & Gholamnejad, J. (2018). A novel edible coating for apple fruits. Horticulture and Postharvest Research, 1(1), 63–72.
- Jaski, A. C., Schmitz, F., Horta, R. P., Cadorin, L., da Silva, B. J. G., Andreaus, J., & Zimmermann, L. M. (2022). Zein-a plant-based material of growing importance: New perspectives for innovative uses. Industrial Crops and Products, 186, 115250. https://doi.org/10.1016/j.indcrop.2022.115250
- Khadka, R., Marasini, M., Rawal, R., Gautam, D., & Acedo, A. (2017). Effects of variety and postharvest handling practices on microbial population at different stages of the value chain of fresh tomato (Solanum lycopersicum) in Western Terai of Nepal. BioMed Research International, 2017, 1–6. https://doi.org/10.1155/2017/7148076
- Kumar, P., Sethi, S., Sharma, R. R., Srivastav, M., Singh, D., & Varghese, E. (2018). Edible coatings influence the cold-storage life and quality of ‘Santa Rosa’ plum (Prunus salicina L). Journal of Food Science and Technology, 55(6), 2344–2350. https://doi.org/10.1007/s13197-018-3130-1
- Liao, H., Jiang, L., Cheng, Y., Liao, X., & Zhang, R. (2018). Application of nisin-assisted thermosonication processing for preservation and quality retention of fresh apple juice. Ultrasonics Sonochemistry, 42, 244–249. https://doi.org/10.1016/j.ultsonch.2017.11.020
- Lin, M. G., Lasekan, O., Saari, N., & Khairunniza-Bejo, S. (2018). Effect of chitosan and carrageenan-based edible coatings on post-harvested longan (Dimocarpus longan) fruits. CyTA-Journal of Food, 16(1), 490–497. https://doi.org/10.1080/19476337.2017.1414078
- Maletsika, P., Nanos, G. D., Papoulia, I., & Vasilakakis, M. (2014). The effect of postharvest application of O3 and 1-MCP on ‘Red Chief’ apple quality during prolonged air cold storage. Acta Horticulturae Cyprus, 1079 (pp. 429–434).
- Maluleke, I. (2020). Mazie – What a versatile crop. Grain South Africa. https://www.grainsa.co.za/home
- Nawab, A., Alam, F., & Hasnain, A. (2017). Mango kernel starch as a novel edible coating for enhancing shelf-life of tomato (Solanum lycopersicum) fruit. International Journal of Biological Macromolecules, 103, 581–586. https://doi.org/10.1016/j.ijbiomac.2017.05.057
- Nsumpi, A., Belay, Z., & Caleb, O. J. (2020). Good intentions, bad outcomes: Impact of mixed-fruit loading on banana fruit protein expression, physiological responses and quality. Food Packaging and Shelf Life, 26, 100–594. https://doi.org/10.1016/j.fpsl.2020.100594
- Nyamende, N. E., Belay, Z. A., Keyser, Z., Oyenihi, A. B., & Caleb, O. J. (2022). Impacts of alkaline‐electrolyzed water treatment on physicochemical, phytochemical, antioxidant properties and natural microbial load on ‘Granny Smith’ apples during storage. International Journal of Food Science & Technology, 57(1), 447–456. https://doi.org/10.1111/ijfs.15426
- Patel, A. R., & Velikov, K. P. (2014). Zein as a source of functional colloidal nano-and microstructures. Current Opinion in Colloid & Interface Science, 19(5), 450–458. https://doi.org/10.1016/j.cocis.2014.08.001
- Pena Serna, C., & Lopes Filho, J. F. (2015). Biodegradable zein-based blend films: Structural, mechanical and barrier properties. Food Technology and Biotechnology, 53(3), 348–353. https://doi.org/10.17113/ftb.53.03.15.3725
- Pena-Serna, C., Penna, A. L. B., & Lopes Filho, J. F. (2016). Zein-based blend coatings: Impact on the quality of a model cheese of short ripening period. Journal of Food Engineering, 171, 208–213. https://doi.org/10.1016/j.jfoodeng.2015.10.039
- Raghav, P. K., Agarwal, N., & Saini, M. (2016). Edible coating of fruits and vegetables: A review. International Journal of Scientific Research and Modern Education, 1(1), 188–204.
- Rashid, Z., Khan, M. R., Mubeen, R., Hassan, A., Saeed, F., & Afzaal, M. (2020). Exploring the effect of cinnamon essential oil to enhance the stability and safety of fresh apples. Journal of Food Processing and Preservation, 44(12), e14926. https://doi.org/10.1111/jfpp.14926
- Rawdkuen, S., Suthiluk, P., Kamhangwong, D., & Benjakul, S. (2012). Mechanical, physico-chemical, and antimicrobial properties of gelatin-based film incorporated with catechin-lysozyme. Chemistry Central Journal, 6(1), 1–10.
- Rojas-Graü, M., Raybaudi-Massilia, R., Soliva-Fortuny, R., Avena-Bustillos, R., McHugh, T., & Martín-Belloso, O. (2007). Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biology and Technology, 45(2), 254–264. https://doi.org/10.1016/j.postharvbio.2007.01.017
- Sánchez-González, L., Vargas, M., González-Martínez, C., Chiralt, A., & Chafe, R. M. (2011). Use of essential oils in bioactive edible coatings: A review. Food Engineering Reviews, 3(1), 1–16. https://doi.org/10.1007/s12393-010-9031-3
- Santos, T. M., Men de Sá Filho, M. S., Silva, E. D. O., da Silveira, M. R., de Miranda, M. R. A., Lopes, M. M., & Azeredo, H. M. (2018). Enhancing storage stability of guava with tannic acid-crosslinked zein coatings. Food Chemistry, 257, 252–258. https://doi.org/10.1016/j.foodchem.2018.03.021
- Sapper, M., & Chiralt, A. (2018). Starch-based coatings for preservation of fruits and vegetables. Coatings, 8(5), 152. https://doi.org/10.3390/coatings8050152
- Shah, S., & Hashmi, M. S. (2020). Chitosan–aloe vera gel coating delays postharvest decay of mango fruit. Horticulture, Environment, and Biotechnology, 61(2), 279–289. https://doi.org/10.1007/s13580-019-00224-7
- Sharif, N., Fabra, M. J., & López-Rubio, A. (2019). Nanostructures of zein for encapsulation of food ingredients. Biopolymer Nanostructures for Food Encapsulation Purposes, 1, 217–245. https://doi.org/10.1016/B978-0-12-815663-6.00009-4
- Song, H. Y., Jo, W. S., Song, N. B., Min, S. C., & Song, K. B. (2013). Quality change of apple slices coated with Aloe vera gel during storage. Journal of Food Science, 78(6), C817–822. https://doi.org/10.1111/1750-3841.12141
- Song, Z., Li, F., Guan, H., Xu, Y., Fu, Q., & Li, D. (2017). Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control, 74, 34–44. https://doi.org/10.1016/j.foodcont.2016.11.026
- Steffens, C. A., Soardi, K., Heinzen, A. S., Amaral Vignali Alves, J., da Silva, J. C., Talamini Do Amarante, C. V., & Brackmann, A. (2022). Quality of “cripps pink” apples following the application of 1‐MCP, ethanol vapor and nitric oxide as pretreatments for controlled atmosphere storage. Journal of Food Processing and Preservation, 46(1), e16121. https://doi.org/10.1111/jfpp.16121
- Supapvanich, S., Mitrsan, P., Srinorkham, P., Boonyarithongchai, P., & Wongs-Aree, C. (2016). Effects of fresh Aloe vera gel coating on browning alleviation of fresh cut wax apple (Syzygium samarangenese) fruit cv. Taaptimjaan. Journal of Food Science and Technology, 53, 2844–2850. https://doi.org/10.1007/s13197-016-2262-4
- Taher, M. A., Tadros, L. K., & Dawood, D. H. (2018). Phytochemical constituents, antioxidant activity and safety evaluation of Kei-apple fruit (Dovyalis caffra). Food Chemistry, 265, 144–151. https://doi.org/10.1016/j.foodchem.2018.05.099
- Tango, C., Wei, S., Khan, I., Hussain, M., Kounkeu, P., Park, J., Kim, S., & Oh, D. (2018). Microbiological quality and safety of fresh fruits and vegetables at retail levels in Korea. Journal of Food Science, 83(2), 386–392. https://doi.org/10.1111/1750-3841.13992
- Tarabay, P. A., Chahine-Tsouvalakis, H., Tohmé Tawk, S., Nemer, N., & Habib, W. (2018). Reduction of food losses in Lebanese apple through good harvesting and postharvest practices. Annals of Agricultural Sciences, 63(2), 207–213. https://doi.org/10.1016/j.aoas.2018.11.006
- Thakur, R., Pristijono, P., Scarlett, C. J., Bowyer, M., Singh, S. P., & Vuong, Q. V. (2019). Starch-based edible coating formulation: Optimization and its application to improve the postharvest quality of “cripps pink” apple under different temperature regimes. Food Packaging and Shelf Life, 22, 100409. https://doi.org/10.1016/j.fpsl.2019.100409
- Xiao, J., Gu, C., Zhu, D., Huang, Y., Luo, Y., & Zhou, Q. (2021). Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. LWT, Food Science and Technology, 140, 110809. https://doi.org/10.1016/j.lwt.2020.110809
- Yao, B. N., Tano, K., Konan, H. K., Bédié, G. K., Oulé, M. K., Koffi-Nevry, R., & Arul, J. (2014). The role of hydrolases in the loss of firmness and of the changes in sugar content during the post-harvest maturation of Carica papaya L. var solo 8. Journal of Food Science and Technology, 51(11), 3309–3316. https://doi.org/10.1007/s13197-012-0858-x
- Zhang, Y. L., Cui, Q. L., Wang, Y., Shi, F., Liu, Y. P., Liu, J. L., & Nie, G. W. (2021). Effect of carboxymethyl chitosan-gelatin-based edible coatings on the quality and antioxidant properties of sweet cherry during postharvest storage. Scientia horticulturae, 289, 110462. https://doi.org/10.1016/j.scienta.2021.110462
- Zhang, W., Li, X., & Jiang, W. (2020). Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. International Journal of Biological Macromolecules, 154, 1205–1214. https://doi.org/10.1016/j.ijbiomac.2019.10.275
- Zhang, W., Zhao, H., Zhang, J., Sheng, Z., Cao, J., & Jiang, W. (2019). Different molecular weights chitosan coatings delay the senescence of postharvest nectarine fruit in relation to changes of redox state and respiratory pathway metabolism. Food Chemistry, 289, 160–168. https://doi.org/10.1016/j.foodchem.2019.03.047
