ABSTRACT
Methotrexate (MTX) is widely used chemotherapeutic and immune suppressant agent. Hepatotoxicity is a common side effect of chronic MTX therapy. This study investigated the hepatoprotective properties of camel milk (CM) and camel urine (CU) in hepatotoxicity induced by intraperitoneal injection of MTX 20 mg/kg. The MTX-intoxicated animals showed elevated serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, fasting blood glucose (FBG), liver DNA fragmentation marker (DFF-40), and serum and liver cytokeratin (CK-18), decreased serum proteins, albumin, fibrinogen, and total antioxidant capacity (TAC). Decreased antithrombin (AT) was associated with prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT). Treatment with CM and CU for four weeks decreased the liver enzymes, FBG, DFF-40, and CK-18 levels and increased total proteins, albumin, fibrinogen, and TAC. However, the changes in AT, PT, and APTT persisted. CM and CU showed promising abilities to counteract MTX hepatotoxicity and they exerted cytoprotective, antiapoptotic, antioxidative, antihyperglycemic, and antithrombotic effects.
Introduction
Methotrexate is a chemotherapeutic and immunosuppressant agent (Koźmiński et al., Citation2020). It is widely used as an effective treatment modality in malignant, inflammatory, and autoimmune disorders (Koźmiński et al., Citation2020; Naldi & Griffiths, Citation2005). The long-term use of methotrexate therapy is associated with hepatotoxicity which ranges in severity from increased hepatic enzymes and impairment in the synthetic liver function to manifest fibrosis and/or cirrhosis (Chakravarty et al., Citation2008). The coincidence of hemostatic derangements including venous thrombosis is not uncommon in the methotrexate-treated patients (Mahadeo et al., Citation2010). The toxic manifestations of methotrexate are in part related to the direct increase in the reactive oxygen species (ROS) production and the deficiency of the antioxidative enzymes such as S-Adenosyl methionine (SAM) and glutathione S-transferase (GST) (Saka & Aouacheri, Citation2017). Therefore, several studies demonstrated that the antioxidant compounds exerted protective effects against methotrexate hepatotoxicity (Abd Al-Azem et al., Citation2019; Mehrzadi et al., Citation2019). Furthermore, methotrexate was reported to induce and activate apoptosis through oxidative stress and by disrupting the mitochondrial membrane potential (Herman et al., Citation2005; Awad et al., Citation2018).
Nowadays, many research lines are aiming to study the naturally derived components that can improve and protect against the common diseases. As a point of fact, complementary and alternative medicine are widely used in some countries of Africa, Asia, and America for the treatment of multiple acute and chronic health problems (World Health Organization, Citation1993). Out of the naturally derived products of medicinal importance; camel milk (CM) and camel urine (CU) and their bioactive ingredients are gaining increased recognition in recent medical researches. CM is consumed for nutritive purposes by a considerable number of people in several arid and non-arid countries worldwide (World Health Organization, Citation1993). The camels favor grazing on natural vegetation particularly, desert bushes, salty plants, and herbs. This diet is responsible for the peculiar composition of CM which is rich in vitamins (e.g. A, B2, C, and E), minerals (e.g. sodium, potassium, copper, magnesium, and zinc), and the phytochemicals (Al-Humaid et al., Citation2010). A large body of recent evidences reported that CM has antioxidant and free radical scavenging activities that help in preventing tissue injury related to toxic agents (Arab et al., Citation2021; H. R. Ibrahim et al., Citation2018; Osman et al., Citation2021). Additionally, CM has immunomodulatory properties related to its rich contents of immunoglobulins, α-lactalbumin, lactoperoxidase, lysozyme, and lactoferrin, and many other proteins having multiple biological functions (Elagamy, Citation2000). The latter gives extra benefit to the chronically ill patients treated with CM such as allergy (Ehlayel et al., Citation2011), cancer, liver diseases, and renal disease (Arab et al., Citation2021; Ayyash et al., Citation2018; Wang et al., Citation2017). Recent studies demonstrated that most of the beneficial effects of CM are ascribed to the presence of extracellular nanovesicles known as exosomes which exhibit immunomodulatory and antioxidant properties in cyclophosphamide and diabetic nephropathy (H. M. Ibrahim et al., Citation2019; Shaban et al., Citation2022).
the CU is considered as one of the most famous urotherapeutic products (Gader & Alhaider, Citation2016) that is used as a traditional folk and complimentary medicine in the desert of the Arabian Peninsula, for centuries. Nowadays, the medical benefits of CU gained greater public interest and recent report revealed that CU consumption is safe and has no potential toxicities or genetic damaging effects on the normal tissues (Anwar et al., Citation2021). The detailed biochemical analysis by gas chromatography-mass spectrometer and inductively coupled plasma mass spectrometer revealed that CU contains multiple biologically active molecules such as benzene propanoic acid, fatty acid derivatives, amino acid derivatives, sugars, prostaglandins, and canavanines (Ahamad et al., Citation2017). Additionally some authors reported high levels of creatinine and uric acid components (Alkhamees & Alsanad, Citation2017) with antioxidant and free radical scavenging activities (Fabbrini et al., Citation2014; Giovannini et al., Citation2006). Furthermore, the antioxidant effect of creatinine was related to its capacity to buffer cellular ATP and lower intracellular Ca2+ levels (Tarnopolsky, Citation2011). This peculiar composition showed a powerful therapeutic anticancer action in Saudi patients (Abuelgasim et al., Citation2018), antibacterial activities against aerobic infections (Shoeib & Ba-Hatheq, Citation2007), antiplatelet (Al-Ghumlas, Citation2020), gastroprotective (Hu et al., Citation2017), and hepatoprotective effects against ethanol (Elhag & Mustafa, Citation2016), and carbon tetra chloride (CCl 4) toxicities (Mahmoud et al., Citation2019).
Owing to the widespread use of methotrexate in multiple chronic health problems, it is of great importance to find an approach to reduce its complications and to increase its safety. In view of the recent evidences of the organo-protective properties of CM and CU against several drug-induced toxicities, the current study aimed to investigate the hepatoprotective effects of CM and CU and to characterize their actions on the oxidative-antioxidative balance (redox status), hemostatic alterations, and the apoptotic pathway in methotrexate-induced toxicity in rats.
Materials and methods
Experimental design and animal grouping
The current study was performed on 40 adult male Wister rats (200–250 g) obtained from The Experimental Surgery and Laboratory Animals Care Unit of the College of Medicine King Saud University (KSU). All the experimental procedures followed the international guidelines of the use and care of laboratory animals. The study protocol was approved by the Institutional Review Board of KSU for experimental animal research (IRB number: KSU-SE-19-43). The animals were kept under standard housing conditions in an average room temperature 23°C and 12 h-light/dark cycles and relative humidity 50% ±10% with free access to rodent diet and drinking water throughout the study. After two weeks of acclimatization, animals were divided into four experimental groups (n = 10 in each). The control (C) group, contained healthy animals and received ordinary rodent diet and drinking water with no further treatment. In the methotrexate (MTX) group, the animals were subjected to methotrexate-induced toxicity with no further treatment. In the methotrexate-camel milk (MTX-M) group, rats were subjected to methotrexate-induced toxicity and received CM treatment. In methotrexate-camel urine (MTX-U) group, rats were subjected to methotrexate-induced toxicity and received CU treatment. Methotrexate (from EBEWE PHARMA) was administered intraperitoneally (i.p) as a single dose of 20 mg/kg body weight dissolved in 1 ml saline (Mukherjee et al., Citation2013) to the animals in the MTX, MTX-M, and MTX-U groups. CM and CU were brought from a camel breeding farm located in the desert 350 kilometer away from Riyadh city, Saudi Arabia. The milk and urine were collected from four lactating camels in their first month of the lactation period, fed on the desert plants and bushes. The camels were milked, in the early morning, manually using an aseptic technique in screw capped bottles, and the urine was collected from the same camel in sterile containers. The collected milk and urine were transported on ice to the laboratory where they were kept in the refrigerator at 4°C until administered to the animals. The next day after methotrexate administration the animals in the MTX-M and MTX-U groups started to receive oral CM 75 ml/kg/day and CU 25 ml/kg/day respectively from nozzle bottles hanged in the wall of the cages for four weeks (Elhag & Mustafa, Citation2016). The average food intake and the character of the stool passed by the animals were observed daily, and body weight was measured weekly for evaluation of any signs of gastrointestinal toxicity.
Blood and tissue samples collection
At the end of the experimental period, overnight fasting animals were anaesthetized by sodium phenobarbital (Nembutal) 50 mg/kg i.p. (AlNafea & Korish, Citation2021). Blood samples were collected from retro orbital venous plexus in sodium citrate and plain test tubes for the different biochemical assays. After blood collections, the animals were sacrificed by decapitation. The liver tissues were collected, washed with cold-iced saline, weighted and stored at −80°C for homogenization and determination of the apoptotic markers.
Assessment of methotrexate toxicity
Assessment of liver functions
The enzymatic markers of liver injury: The activity of alanine aminotransferase (ALT), Aspartate aminotransferase (AST), and Alkaline phosphatase (ALP) was determined in the serum calorimetrically using automated analyzer (Siemens Vista).
The liver function parameters: Serum levels of albumin, fibrinogen, and total proteins and bilirubin were determined calorimetrically using automated analyzer (Siemens Vista).
Assessment of the hemostatic functions
Coagulation screening tests: Prothrombin time (PT) and activated partial thromboplastin time (APTT) were measured with the STA compact automated coagulometer (Stago Diagnostica Reagents, France) using specific reagents of the system and according to the instructions of the manufacturer.
The natural anticoagulant antithrombin (AT) levels were assayed using colorimetric assay (Stachrom AT) (Stago Diagnostica Reagents, France) according to the manufacturer’s instructions.
Oxidative-anti oxidative status
The total antioxidant capacity (TAC) was determined in serum of the studied groups by enzyme linked immunosorbent assay. Using Oxiselect TM Total Antioxidant Capacity (TAC) Assay kit # STA-360 Cell Biolabs, Inc. San Diego, CA, U.S.A. Reading of the plate was performed at 490 nm the inter-assay and intra-assay % coefficient of variability (CV) were ≤ 9.8% and ≤ 5.1%, respectively.
The apoptotic markers
The apoptotic changes induced by MTX therapy were assessed by measuring the following parameters:
DNA fragmentation factor 40 kDa subunit beta (DFF-40) in the liver tissue
To remove excess blood, the liver tissue was rinsed with 1× PBS, homogenized in 20 ml of 1× PBS and stored over night at ≤ −20°C. The homogenate was subjected to two freeze-thaw cycles to break the cell membranes, centrifuged for 5 min at 5000 ×g. The supernatant was removed and assayed immediately using the Rat DNA fragmentation subunit beta ELISA kit #MBS288236 My BioSource, Inc. San Diego, CA, U.S.A. An optical density (OD) range of 0–3 or greater at 450 nm wavelength was used in absorbance measurement. The intra-assay and inter-assay % CV were ≤ 5.1% and ≤ 9.8%, respectively.
Cytokeratin-18 (CK-18) levels
The total CK-18 levels were detected in the serum and liver homogenate by an ELISA Rat (CK-18) assay kit #Cat No. MBS261799 My BioSource, Inc. San Diego, CA, U.S.A. To prepare the liver tissue homogenate; the tissue slices were washed in 0.01 PBS and the tissue protein extraction reagent was added according to the proportion of 1 G: 5–10 ml and mixed on ice water. After being blended, the mixture was centrifuged for 10 min at 5000–10000 rpm and the supernatant was collected for the biochemical assays. The OD was read at 450 nm. The intra-assay and inter-assay precisions of the kit were ≤ 8% and ≤ 12%, respectively.
Assessment of fasting blood glucose (FBG)
The FBG levels were determined by automated analyzer (Siemens Vista).
Statistical analysis
The obtained data was tabulated and statistically analyzed with Graph Prism software program (9.0). Comparison between multiple groups was carried out by one-way ANOVA test followed by the post hoc Tukey’s test. One Pearson’s correlation analysis was used to determine the relationship between some parameters. Data was presented as mean ± standard deviations (SD). Results were considered significant when p < .05.
Results
The changes in liver enzymes and bilirubin levels in methotrexate-induced toxicity and the effect of CM and CU treatment
High dose of methotrexate administration was associated with significant liver injury manifested by increased liver enzymes AST, ALT, and ALP in the MTX group in comparison with the control (C) group (p < .0001, p = .001, p < .0001, correspondingly) (). The CM treatment in the MTX-M group resulted in significant decline in the AST and ALP levels in comparison with the untreated MTX group (p = .04, p = .012, respectively). Additionally, the ALT levels showed a descending trend in the MTX-M group in comparison with the MTX group but it did not reach a significant level (p > .05). Meanwhile, the CU-treated MTX-U group showed significant reduction in AST, ALT, and ALP levels in comparison with the untreated MTX group (p = .0004, p = .002, p = .0005, respectively). In addition, shows that the MTX group exhibited significant increase in the total bilirubin levels in comparison with the C group (p = .03). However, the administration of CM and CU treatment decreased the total bilirubin levels in the MTX-M and MTX-U groups displaying no significance difference from the C group (p > .05 for both).
Table 1. The liver functions, the hemostatic parameters and the total antioxidant capacity (TAC) in the control, methotrexate (MTX), methotrexate-camel milk treated (MTX-M), and methotrexate-camel urine-treated (MTX-U) groups after 8 weeks of the study, (n = 10 in each group).
The changes in liver functions in methotrexate toxicity and the effect of CM and CU treatments
shows that the hepatic injury-induced by high dose methotrexate therapy altered the synthetic liver functions in the MTX group of animals. This leads to significant reduction of the total serum protein and albumin levels in the MTX group in comparison with the C group (p < .0001, p = .0177, respectively). The administration of CM or CU in association with methotrexate therapy in the MTX-M and MTX-U groups resulted in preservation of the albumin levels in both groups as compared to MTX group (p < .0001; for both). Interestingly, the serum albumin levels were significantly higher in the MTX-M and MTX-U groups in comparison with the C group (p = .0005, p = .0025, respectively). Additionally, the total serum protein level was preserved in the MTX-M group showing no significant difference from that of the C group (p > .05), but it remained significantly lower in the MTX-U group (p = .001) as compared to control C group. Therefore, the protective effect of CM treatment against hepatocyte cellular damage was more powerful than that of CU treatment.
The changes in the hemostatic functions in methotrexate toxicity and the effect of CM and CU treatment
shows that the methotrexate-induced hepatotoxicity was associated with impaired hemostatic functions manifested by prolonged PT and APTT in the untreated MTX-group as compared to the C group (p = .003, p = .0.2; respectively). There was no significant change in the PT in the MTX-M and MTX-U groups as compared to the MTX group (p > .05 for both). However, interestingly the PTT showed greater prolongation in the MTX-M group in comparison with both MTX and the control groups (p = .0001, p < .0001, respectively). Similarly, the MTX-U group showed prolonged APTT in comparison with the control groups (p < .0001). Additionally, the MTX group exhibited decreasing trend in AT levels but it did not reach a significant level as compared to the C group (p > .05). The use of CM and CU did not show any significant change in AT level in MTX-M and MTX-U as compared to the MTX group (p > .05 for both). shows that the serum fibrinogen levels were lower in the MTX group in comparison with the C group (p = .003). The MTX-M group showed an increased fibrinogen levels that were comparable to the C group but it is still not statistically different from the MTX group (p > .05). Similarly, there was no significant change in the fibrinogen levels in the MTX-U group in comparison with the MTX-group (p > .05).
Total antioxidant capacity (TAC) in methotrexate toxicity and the effect of CM and CU treatment
Methotrexate produced an oxidative stress condition in the MTX group of animals manifested by significant reduction of the TAC in comparison with the C group (p < .001). The treatment by CM and CU exhibited comparable increase in the TAC in the MTX-M and MTX-U groups as compared to the MTX group (p < .0001 for both) (). Remarkably, the TAC in the MTX-M and MTX-U groups were significantly higher than the C group (p < .0001 and p = .0028, respectively).
Markers of liver apoptosis in methotrexate toxicity and the effect of CM and CU treatment
Cytokeratin-18 (CK-18) levels
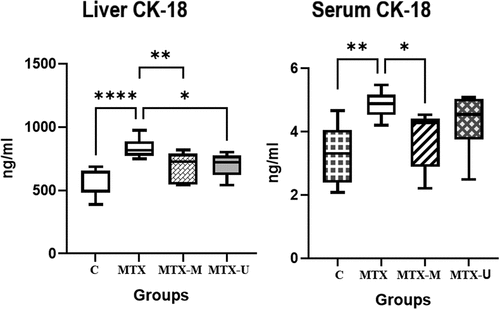
Methotrexate-induced necrosis and apoptosis of the liver cells as manifested by the elevated levels of the markers of early apoptosis (CK-18) in the serum and the liver tissue of the MTX group as compared to the serum and liver tissue of the C group (p < .001, p = .0006, respectively). The treatment by CM significantly lowered the CK-18 levels in the liver (p = .009) and serum (p = .029) of the MTX-M group as compared to the MTX group. The CU treatment exhibited significant reduction in the CK-18 in the liver tissues of the MTX-U group (p = .024) and showed no significant change in the serum CK-18 (p > .05) in comparison with the MTX group ().
Figure 1. Liver and serum cytokeratin-18 (CK-18) levels in methotrexate toxicity and the effect of camel milk and camel urine treatments. Control healthy (C), methotrexate (MTX), methotrexate-camel milk-treated (MTX-M), and methotrexate-camel urine-treated (MTX-U) groups. ****p < .0001, **p < .01, *p < .05.
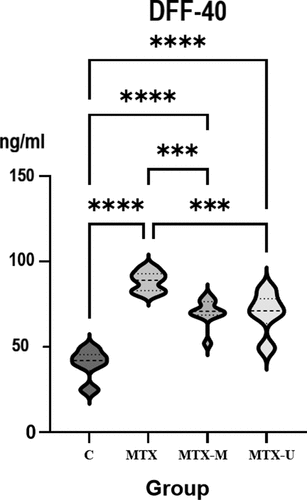
DNA fragmentation factor 40 kDa subunit beta (DFF-40)
As shown in , methotrexate toxicity increased the apoptotic changes in liver tissues. This was manifested by two-fold increase in the DFF-40 in the MTX group of animals in comparison with the control level in the C group (p < .0001). However, treatment by CM and CU comparably inhibited the DNA fragmentation of the liver tissue of the MTX-M and MTX-U groups in comparison with the MTX group (p = .0003, p = .0002; respectively).
Figure 2. The levels of DNA fragmentation factor 40 kDa subunit beta (DFF-40) in the liver tissue in the methotrexate toxicity and the effect of camel milk and camel urine treatments. Control healthy (C), methotrexate (MTX), methotrexate-camel milk treated (MTX-M), and methotrexate-camel urine-treated (MTX-U) groups. ****p < .0001, ***p < .001, **p < .01, *p < .05.
Changes in the fasting blood glucose levels (FBG) in methotrexate toxicity and the effect of CM and CU treatment
shows that methotrexate treatment was associated with significant fasting hyperglycemia in the untreated MTX group in comparison with the C group (p = .013). Interestingly, both CM and CU exerted hypoglycemic effect that was marked by the significant reduction of the FBG levels in the MTX-M and MTX-U groups compared to the MTX group (p < .0001, p = .001, respectively). The FBG levels in the MTX-M and MTX-U groups showed no significant difference from the normal FBG in the control C group (p > .05).
Changes in body weight in methotrexate toxicity and the effect of CM and CU treatment
The initial body weight (IBW) showed no significant difference between the studied groups (p > .05). However, after 4 weeks of the induction of the methotrexate toxicity, the untreated MTX group showed lower final body weight (FBW) in comparison with the C Group (p < .001). The MTX-M group showed significant increase in the FBW compared to the MTX group (p < .001). However, the MTX-U group showed no significant change in FBW compared to the MTX group (p > .05) ().
Table 2. The changes in body weight (B.Wt) and the liver/body weight (L/Wt.) ratio in the control, methotrexate (MTX), methotrexate-camel milk (MTX-M), and methotrexate-camel urine (MTX-U) groups of animals in the study.
Changes in the liver weight in relation to the body weight (liver/B.Wt) ratio due to methotrexate toxicity and the effect of CM and CU treatments
shows increased liver weight in relation to the final body weight (Liver/B.Wt.) ratio in the MTX group of animals in relation to the C group (p = .004). Treatment by CM or CU in the MTX-M and MTX-U groups, respectively, showed no significant change in (Liver/B.Wt) ratio in comparison with the MTX group (p > .05 for both).
Correlation analysis studies
Pearson’s correlation studies presented in showed significant negative correlation between the liver enzyme AST and the serum albumin and fibrinogen levels. However, AST correlated positively with ALT, CK-18, and DFF-40. The TAC showed inverse correlation with AST, ALT, CK-18, and DFF-40. However, TAC was positively correlated with serum albumin.
Table 3. Pearson’s correlation studies between the liver function parameters (AST, ALT, albumin, and fibrinogen), the total antioxidant capacity (TAC) and the apoptotic markers (CK-18 and DFF-40) in the studied groups.
Discussion
In view of the common long-term use of methotrexate in the management of the chronic inflammatory and malignant disorders; the methotrexate-induced hepatotoxicity is prevalent and needs to be counteracted (Conway & Carey, Citation2017). CM has recently proved to have protective role in several diseases due to its regulatory effects on the inflammatory mediators, immunomodulators, and the oxygen-free radicals. Therefore, the current study evaluated the effects of CM and CU on methotrexate-induced hepatotoxicity. The treatment by CM and CU produced hepatoprotection demonstrated by decreased serum ALT, AST, ALP, bilirubin, DNA fragmentation, and CK-18 levels. Additionally, the hepatocytes’ synthetic function was improved as evidenced by increase in serum total proteins, albumin, fibrinogen, and AT levels. Interestingly, we noticed that the CU showed a stronger protective effect against methotrexate-induced hepatocellular damage as compared to CM. This was evidenced by the lower levels of the hepatic enzymes activities in the MTX-U group of animals. This may be attributed to the powerful antioxidant and free radical scavenging activities of CU due to its high content of essential inorganic elements including K, Mg, Na, Ca, and Mn and active metabolites such as canavanine, and erythritol (Mahmoud et al., Citation2019). Alternatively, treatment by CM exerted greater preservation of the liver albumin synthesis in the MTX-M group of animals. This could be due to the rich amino acid contents and the strong antioxidant and free radical chelating effects of the small- and medium-sized bioactive peptides present in CM which support the hepatic synthetic process (Osman et al., Citation2021).
The current findings of the protective effects of CM against drug-induced hepatotoxicity supported our previous reports of the ability of CM to exert functional and histopathological liver protection in non-alcoholic steatohepatitis (Korish & Arafah, Citation2013) and thioacetamide-induced hepatotoxicity (Osman et al., Citation2021). Additionally, the present results were in accordance with similar reports of the hepato-protective effect of CU against ethanol and CCl4 toxicity (Elhag & Mustafa, Citation2016; Elhag et al., Citation2017; Mahmoud et al., Citation2019). Additionally, El-Fakharany et al. (Citation2017) reported that CM treatment in hepatitis C virus-infected subjects leads to improved liver functions and increased plasma proteins. The organo-protective functions of CM could be attributed to the modulation of the PI3K/Akt/eNOS signaling pathway and the inhibition of oxidative stress as recently reported by Arab et al. (Citation2021) in methotrexate and cyclosporin-induced renal toxicity.
The glucose homeostatic mechanisms are highly related to the normal liver functions. The methotrexate-induced liver damage in the present study resulted in significant hyperglycemia in the MTX group of animals that was corrected after CM and CU treatment. The ability of CM to counteract hyperglycemia is highly reported in diabetic patients (Agrawal et al., Citation2011) and experimental animals (Korish et al., Citation2020). This hypoglycemic effect of CM is attributed to its insulin-like activities (Agrawal et al., Citation2011), increased glucose-stimulated insulin secretion and incretin hormones (GIP, GLP) (Korish, Citation2014), inhibition of dipeptidyl peptidase (DPP-IV) enzyme that degrades incretin hormones (Nauck & Meier, Citation2018). Furthermore, CM has stimulatory role on the human insulin receptor (hIR) and the glucose uptake (Ashraf et al., Citation2021; Nongonierma et al., Citation2018).
The current findings of the hypoglycemic effect of CU are in accordance with the recent publication of Mustapha et al. Citation2021 who reported decreased blood glucose in in alloxan-induced diabetic rats receiving CU. The hypoglycemic effect of CU could be due to the presence of bioactive molecules and ingredients with antidiabetic action derived from the plants and bushes available in the food of the camel (Ahamad et al., Citation2017). Furthermore, the decrease of glucose absorption from the intestine by CU intake is also hypothesized.
The liver is the main producing organ of most of the coagulation factors and inhibitors. Therefore, liver diseases are not uncommonly associated with disturbed hemostatic functions. Up to the limit of our knowledge, few numbers of studies have evaluated the effect of methotrexate therapy on haemostasis. In the current study, methotrexate hepatotoxicity lowered the fibrinogen levels which contribute to the observed prolongation of PT and APTT in the MTX group of animals. Additionally, methotrexate reduced the natural anticoagulant antithrombin (AT) levels. The reduced fibrinogen and AT levels could be attributed to the deterioration in the synthetic liver functions. Correlation studies showed that AT had direct correlation with fibrinogen level and inverse correlation with AST, ALT, PT, and APTT levels.
Our results are in agreement with similar finding reported in methotrexate-treated patients by Totan et al. (Citation2001); and Fisgin et al. (Citation2004). Those authors reported prolonged PT and APTT associated with reduced coagulation factors; VII, IX, and X, and natural anticoagulants such as Protein C, Protein S, and AT.
In the current study, after CM and CU treatment in the MTX-M ans MTX-U groups of animals, there was unexpected further reduction in AT levels and increased prolongation in PT and APTT in the treated animals. This couldn’t be attributed to the decreased AT production because the improved synthetic liver function was demonstrated by increased serum total proteins, albumin, and fibrinogen levels. However, these changes in AT in MTX-M and MTX-U groups could reflect an increased consumption of AT or buffering of its action by the heparin like molecules present in the CM that could be also excreted in CU (Alhaider et al., Citation2013).
Oxidative stress has emerged as a key player in the pathogenesis of methotrexate-induced hepatotoxicity. Lipid peroxidation plays a vital role in the damage to the cell membrane through reactive oxygen radicals. methotrexate toxicity has been shown to increase malondialdehyde (MDA), an important index of lipid peroxidation in experimental studies, and this increase has been shown to be suppressed by antioxidant therapies (Dar et al., Citation2021; Malayeri et al., Citation2022).
In the current study, the methotrexate-treated animals showed marked reduction in their TAC. The latter showed significant inverse correlation with early markers of liver injury DFF-40 and CK-18 and with the traditional liver enzymes ALT, AST, and ALP. This indicates that oxidative stress and lack of the antioxidative activity play role in the methotrexate-induced liver injury. Additionally, TAC levels were directly correlated with serum albumin level, which means that the synthetic liver function is related to the TAC.
Administration of CM and CU to methotrexate-treated animals resulted in marked increase in the TAC which surprisingly exceeded the levels in the control animals. The current findings of increased TAC after CM treatment are in accordance with similar results obtained in different acute and chronic health problems including DM (Abdalla, Citation2018; Korish, Citation2014) and alcoholic and non-alcoholic hepatitis (Elhag et al., Citation2017; Korish & Arafah, Citation2013).
Similarly, protective effects of CU were reported against CCL4 hepatotoxicity (Elhag et al., Citation2017; Mahmoud et al., Citation2019) and cisplatin nephrotoxicity (Mahmoud et al., Citation2020) due to its strong ability to prevent the release of free radicals, scavenge the ROS, stimulate antioxidative enzymes catalase (CAT), and superoxide dismutase (SOD) activities, and increase levels of reduced glutathione (GSH).
Multiple disease problems can arise due to abnormal apoptosis. The DNA fragmentation (DFF-40) and CK-18 are used for detecting and characterizing cellular apoptotic processes (Majtnerová & Roušar, Citation2018). They have short half-life as compared to ALT, AST, ALP, and bilirubin which are used routinely to detect liver injury (Atkinson et al., Citation2020; Jaeschke et al., Citation2018). Therefore, DFF-40 and CK-18 accurately reflected early hepatocyte cellular injury in alcoholic hepatitis (Atkinson et al., Citation2020; Vatsalya et al., Citation2020), alcoholic fatty liver (Joka et al., Citation2012), acetaminophen toxicity (Antoine et al., Citation2020) and after therapeutic doses of anti-tuberculosis drugs (Thulin et al., Citation2014) injury.
The current study showed increased levels of DFF-40 and CK-18 in the MTX-treated animals which could be considered indirect biomarkers of mitochondrial dysfunction that reflect hepatocyte cellular injury (Jaeschke et al., Citation2018; Korver et al., Citation2021). The increased DFF-40 is due to upregulation of mRNA expression of CASP-3 gene (Hassan et al., Citation2015) and activation of caspases as indicated by the cleavage of the pro-enzymes and the increased respective enzyme activities (Jaeschke et al., Citation2019). The findings of our study were in accordance with recent reports of increased tissue caspase activity and DFF-40 in methotrexate (Awad et al., Citation2018) and acetaminophen (Jaeschke et al., Citation2018) hepatotoxicity.
The findings of the present study showed powerful antiapoptotic and anti-necrotic effect of CM and CU in protecting the liver against methotrexate toxicity as evidenced by the decreased levels of DFF-40 and CK18 levels in the MTX-M and MTX-U groups of animals. This could be related to their antioxidative properties that stabilizes the mitochondrial membrane and prevents its rupture and release of the mitochondrial enzyme, and thus stops the caspase activation and the DNA fragmentation (Xue et al., Citation2014). Furthermore, the antiapoptotic effect of CM was also recently reported against cyclosporin-induced nephrotoxicity through the inhibition of Nrf2/HO-1 and AKT/eNOS/NO pathways and increasing the renoprotective nitric oxide production (Arab et al., Citation2021).
Strengths and limitations
The current study investigated both CM and CU on the same model of liver toxicity. Up to the limit of our knowledge, we investigated for the first time, the antiapoptotic effect of CM and CU in methotrexate-hepatotoxicity. The current findings address multiple new actions of the camel-derived products including their abilities to counteract apoptosis, oxidative stress and hyperglycemia induced by methotrexate. These effects were associated with marked increase in TAC of the body. This helps us to give evidence of the powerful protective effects of the naturally derived CM and CU in overcoming the hepatotoxic effects of methotrexate. However, limited financial resources is an important limitation of our study, as other coagulation factors, natural anticoagulants and lipid peroxidation products, could not be measured.
Conclusion
The present findings clarified that the naturally derived CM and CU exerted a cytoprotective, antiapoptotic, antioxidative, antihyperglycemic and anticoagulant effects in methotrexate-induced hepatotoxicity. Therefore, administration of CM and CU either separately or in combination could be beneficial as an adjuvant therapy in combination with methotrexate to counteract its toxic effects. However, future large-scale clinical trials are needed to confirm the health benefits of CM and CU in the protection against methotrexate-induced liver injury in humans.
Acknowledgments
The authors would like to acknowledge Dallah Health Care Kingdom of Saudi Arabia for their financial support. The authors would like also to thank Mr. Lugman El-Sid, Physiology Department, College of Medicine, KSU for his technical assistance and Dr. Yasser Alshawakir and Dr. Abdullah Almubarak, The Experimental Surgery and Laboratory Animals Care Unit of the College of Medicine King Saud University (KSU) for their technical support in animal housing. The authors would also like to thank Mrs. Nashwa Othman in the Biochemistry unit of the Central Laboratory of the Female Scientific Colleges, KSU for her support in the biochemical assays.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd Al-Azem, D., Al Derawi, K. H., & Al-Saadi, S. A. M. (2019). The protective effects of syzygium aromaticum essential oil extract against methotrexate induced hepatic and renal toxicity in rats. Journal of Pure and Applied Microbiology, 13(1), 505–516. https://doi.org/10.22207/JPAM.13.1.57
- Abdalla, K. O. (2018). Camel milk is an alternative and a complementary treatment to the current parenteral insulin therapy of insulin-dependent diabetes mellitus. Gezira Journal of Health Sciences, 12(2).
- Abuelgasim, K. A., Alsharhan, Y., Alenzi, T., Alhazzani, A., Ali, Y. Z., & Jazieh, A. R. (2018). The use of complementary and alternative medicine by patients with cancer: A cross-sectional survey in Saudi Arabia. BMC Complementary and Alternative Medicine, 18(1), 1–8. https://doi.org/10.1186/s12906-018-2150-8
- Agrawal, R. P., Jain, S., Shah, S., Chopra, A., & Agarwal, V. (2011). Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. European Journal of Clinical Nutrition, 65(9), 1048–1052. https://doi.org/10.1038/ejcn.2011.98
- Ahamad, S. R., Alhaider, A. Q., Raish, M., & Shakeel, F. (2017). Metabolomic and elemental analysis of camel and bovine urine by GC–MS and ICP–MS. Saudi Journal of Biological Sciences, 24(1), 23–29. https://doi.org/10.1016/j.sjbs.2015.09.001
- Al-Ghumlas, A. K. (2020). Camel platelet aggregation responses and the antiplatelet effect of camel urine: Comparison between black and white camels. Heliyon, 6(10), e05353. https://doi.org/10.1016/j.heliyon.2020.e05353
- Alhaider, A., Abdelgader, A. G., Turjoman, A. A., Newell, K., Hunsucker, S. W., Shan, B., Ma, B., Gibson, D. S., & Duncan, M. W. (2013). Through the eye of an electrospray needle: Mass spectrometric identification of the major peptides and proteins in the milk of the one‐humped camel (Camelus dromedarius). Journal of Mass Spectrometry, 48(7), 779–794. https://doi.org/10.1002/jms.3213
- Al-Humaid, A. I., Mousa, H. M., El-Mergawi, R. A., & Abdel-Salam, A. M. (2010). Chemical composition and antioxidant activity of dates and dates-camel-milk mixtures as a protective meal against lipid peroxidation in rats. American Journal of Food Technology, 5(1), 22–30. https://doi.org/10.3923/ajft.2010.22.30
- Alkhamees, O. A., & Alsanad, S. M. (2017). A review of the therapeutic characteristics of camel urine. African Journal of Traditional, Complementary and Alternative Medicines, 14(6), 120–126. https://doi.org/10.21010/ajtcam.v14i6.12
- AlNafea, H. M., & Korish, A. A. (2021). Activation of the peroxisome proliferator-activated receptors (PPAR-α/γ) and the fatty acid metabolizing enzyme protein CPT1A by camel milk treatment counteracts the high-fat diet-induced nonalcoholic fatty liver disease. PPAR Research, 2021(9), 2021. https://doi.org/10.1155/2021/5558731
- Antoine, D. J., Jenkins, R. E., Dear, J. W., Williams, D. P., McGill, M. R., Sharpe, M. R., Craig, D. G., Simpson, K. J., Jaeschke, H., & Park, B. K. (2020). Retraction notice to “molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity”: J Hepatol 56 (2012) 1070–1079. Journal of Hepatology, 73(5), 1297. https://doi.org/10.1016/j.jhep.2020.08.022
- Anwar, S., Ansari, S. A., Alamri, A., Alamri, A., Alqarni, A., Alghamdi, S., Wagih, M. E., Ahmad, A., & Rengasamy, K. R. (2021). Clastogenic, anti-clastogenic profile and safety assessment of camel urine towards the development of new drug target. Food and Chemical Toxicology, 151, 112131. https://doi.org/10.1016/j.fct.2021.112131
- Arab, H. H., Eid, A. H., Gad, A. M., Yahia, R., Mahmoud, A. M., & Kabel, A. M. (2021). Inhibition of oxidative stress and apoptosis by camel milk mitigates cyclosporine‐induced nephrotoxicity: Targeting Nrf2/HO‐1 and AKT/eNOS/NO pathways. Food Science & Nutrition, 9(6), 3177–3190. https://doi.org/10.1002/fsn3.2277
- Ashraf, A., Mudgil, P., Palakkott, A., Iratni, R., Gan, C. Y., Maqsood, S., & Ayoub, M. A. (2021). Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. Journal of Dairy Science, 104(1), 61–77. https://doi.org/10.3168/jds.2020-18627
- Atkinson, S. R., Grove, J. I., Liebig, S., Astbury, S., Vergis, N., Goldin, R., Quaglia, A., Bantel, H., Guha, I. N., Thursz, M. R., & Newcombe, P. (2020). In severe alcoholic hepatitis, serum keratin-18 fragments are diagnostic, prognostic, and theragnostic biomarkers. Official Journal of the American College of Gastroenterology, 115(11), 1857–1868. https://doi.org/10.14309/ajg.0000000000000912
- Awad, M., Elsawy, S., Abdalfattah, A., & Nassar, A. (2018). The effect of taurine on methotrexate induced hepatorenal toxicity in rats. International Journal of Advanced Research, 6(2), 1778–1791. https://doi.org/10.21474/IJAR01/6618
- Ayyash, M., Al-Nuaimi, A. K., Al-Mahadin, S., & Liu, S. Q. (2018). In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chemistry, 239, 588–597. https://doi.org/10.1016/j.foodchem.2017.06.149
- Chakravarty, K., McDonald, H., Pullar, T., Taggart, A., Chalmers, R., Oliver, S., Mooney, J., Somerville, M., Bosworth, A., & Kennedy, T. (2008). BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology, 47(6), 924–925. https://doi.org/10.1093/rheumatology/kel216a
- Conway, R., & Carey, J. J. (2017). Risk of liver disease in methotrexate treated patients. World Journal of Hepatology, 9(26), 1092. https://doi.org/10.4254/wjh.v9.i26.1092
- Dar, A. A., Fehaid, A., Alkhatani, S., Alarifi, S., Alqahtani, W. S., Albasher, G., Almeer, R., Alfarraj, S., & Moneim, A. A. (2021). The protective role of luteolin against the methotrexate-induced hepato-renal toxicity via its antioxidative, anti-inflammatory, and anti-apoptotic effects in rats. Human & Experimental Toxicology, 40(7), 1194–1207.
- Ehlayel, M. S., Hazeima, K. A., Al-Mesaifri, F., & Bener, A. (2011). Camel milk: An alternative for cow’s milk allergy in children. Allergy and Asthma Proceedings, 32(3), 255. https://doi.org/10.2500/aap.2011.32.3429
- Elagamy, E. I. (2000). Effect of heat treatment on camel milk proteins with respect to antimicrobial factors: A comparison with cows’ and buffalo milk proteins. Food Chemistry, 68(2), 227–232. https://doi.org/10.1016/S0308-8146(99)00199-5
- El-Fakharany, E. M., El-Baky, N. A., Linjawi, M. H., Aljaddawi, A. A., Saleem, T. H., Nassar, A. Y., Osman, A., & Redwan, E. M. (2017). Influence of camel milk on the hepatitis C virus burden of infected patients. Experimental and Therapeutic Medicine, 13(4), 1313–1320. https://doi.org/10.3892/etm.2017.4159
- Elhag, A. E., Faye, B., & El Badwi, S. (2017). Protective activity of camel’s milk and urine mixture (Camelus dromedarius) against ethanol-induced hepatotoxicity in rats. Advances in Bioscience and Biotechnology, 8(10), 378–387. https://doi.org/10.4236/abb.2017.810027
- Elhag, A. E., & Mustafa, A. (2016). Hepatoprotective effect of urine of one-humped camel (Camelus dromedarius) against ethanol induced liver damage in rats. International Journal of Pharmaceutical Chemistry, 5, 1–8.
- Fabbrini, E., Serafini, M., Colic Baric, I., Hazen, S. L., & Klein, S. (2014). Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes, 63(3), 976–981. https://doi.org/10.2337/db13-1396
- Fisgin, T., Yarali, N., Kara, A., Bozkurt, C., Birgen, D., Erten, U., & Duru, F. (2004). Hemostatic side effects of high-dose methotrexate in childhood acute lymphoblastic leukemia. Pediatric Hematology and Oncology, 21(1), 77–83. https://doi.org/10.1080/pho.21.1.77.83
- Gader, A. G. M. A., & Alhaider, A. A. (2016). The unique medicinal properties of camel products: A review of the scientific evidence. Journal of Taibah University Medical Sciences, 11(2), 98–103. https://doi.org/10.1016/j.jtumed.2015.12.007
- Giovannini, I., Chiarla, C., Giuliante, F., Pallavicini, F., Vellone, M., Ardito, F., & Nuzzo, G. (2006). Serum uric acid, creatinine, and the assessment of antioxidant capacity in critical illness. Critical Care, 10(5), 1–2. https://doi.org/10.1186/cc5008
- Hassan, A. A. M., Ismail, M. F., & Mohamed, H. M. (2015). Effect of methotrexate combined with ginger, silymarin or propolis on the mRNA expression levels of cytochrome P450 oxidoreductase (POR), caspase 3 (CASP-3) and interlukin 6 (IL-6). African Journal of Biotechnology, 14(8), 695–701. https://doi.org/10.5897/AJB2014.14196
- Herman, S., Zurgil, N., & Deutsch, M. (2005). Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflammation Research, 54(7), 273–280. https://doi.org/10.1007/s00011-005-1355-8
- Hu, Z., Chang, X., Pan, Q., Gu, K., & Okechukwu, P. N. (2017). Gastroprotective and ulcer healing effects of camel milk and urine in HCl/EtOH, non-steroidal anti-inflammatory drugs (indomethacin), and water-restraint stress-induced ulcer in rats. Pharmacognosy Magazine, 13(52), 559. https://doi.org/10.4103/pm.pm_135_17
- Ibrahim, H. R., Isono, H., & Miyata, T. (2018). Potential antioxidant bioactive peptides from camel milk proteins. Animal Nutrition, 4(3), 273–280. https://doi.org/10.1016/j.aninu.2018.05.004
- Ibrahim, H. M., Mohammed-Geba, K., Tawfic, A. A., & El-Magd, M. A. (2019). Camel milk exosomes modulate cyclophosphamide-induced oxidative stress and immuno-toxicity in rats. Food & Function, 10(11), 7523–7532. https://doi.org/10.1039/C9FO01914F
- Jaeschke, H., Duan, L., Akakpo, J. Y., Farhood, A., & Ramachandran, A. (2018). The role of apoptosis in acetaminophen hepatotoxicity. Food and Chemical Toxicology, 118, 709–718. https://doi.org/10.1016/j.fct.2018.06.025
- Jaeschke, H., Ramachandran, A., Chao, X., & Ding, W. X. (2019). Emerging and established modes of cell death during acetaminophen-induced liver injury. Archives of Toxicology, 93(12), 3491–3502. https://doi.org/10.1007/s00204-019-02597-1
- Joka, D., Wahl, K., Moeller, S., Schlue, J., Vaske, B., Bahr, M. J., Manns, M. P., Schulze‐Osthoff, K., & Bantel, H. (2012). Prospective biopsy‐controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology, 55(2), 455–464. https://doi.org/10.1002/hep.24734
- Korish, A. A. (2014). The antidiabetic action of camel milk in experimental type 2 diabetes mellitus: An overview on the changes in incretin hormones, insulin resistance, and inflammatory cytokines. Hormone and Metabolic Research, 46(06), 404–411. https://doi.org/10.1055/s-0034-1368711
- Korish, A. A., & Arafah, M. M. (2013). Camel milk ameliorates steatohepatitis, insulin resistance and lipid peroxidation in experimental non-alcoholic fatty liver disease. BMC Complementary and Alternative Medicine, 13(1), 1–12. https://doi.org/10.1186/1472-6882-13-264
- A. A., Gader, A. G. M. A., & Alhaider, A. A. (2020). Comparison of the hypoglycemic and antithrombotic (anticoagulant) actions of whole bovine and camel milk in streptozotocin-induced diabetes mellitus in rats. Journal of Dairy Science, 103(1), 30–41.
- Korver, S., Bowen, J., Pearson, K., Gonzalez, R. J., French, N., Park, K., Jenkins, R., & Goldring, C. (2021). The application of cytokeratin-18 as a biomarker for drug-induced liver injury. Archives of Toxicology, 95(11), 3435–3448. https://doi.org/10.1007/s00204-021-03121-0
- Koźmiński, P., Halik, P. K., Chesori, R., & Gniazdowska, E. (2020). Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. International Journal of Molecular Sciences, 21(10), 3483. https://doi.org/10.3390/ijms21103483
- Mahadeo, K. M., Dhall, G., Panigrahy, A., Lastra, C., & Ettinger, L. J. (2010). Subacute methotrexate neurotoxicity and cerebral venous sinus thrombosis in a 12-year old with acute lymphoblastic leukemia and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Homocysteine-mediated methotrexate neurotoxicity via direct endothelial injury. Pediatric Hematology and Oncology, 27(1), 46–52. https://doi.org/10.3109/08880010903341904
- Mahmoud, H. S., Elsaed, W. M., & Gabr, S. A. (2019). Camel urotherapy and hepatoprotective effects against carbon tetrachloride-induced liver toxicity. International Journal of Pharmacology, 15(6), 696–705. https://doi.org/10.3923/ijp.2019.696.705
- Mahmoud, H. S., Elsaed, W. M., Khaled, H. E., Ezzat, T. M., & El Menyawi, M. A. I. (2020). Ameliorative effect of camel urotherapy to cisplatin induced urinary tract subacute and chronic toxicity in male albino rats. Biomedical and Pharmacology Journal, 13(3), 1311–1319. https://doi.org/10.13005/bpj/2000
- Majtnerová, P., & Roušar, T. (2018). An overview of apoptosis assays detecting DNA fragmentation. Molecular Biology Reports, 45(5), 1469–1478. https://doi.org/10.1007/s11033-018-4258-9
- Malayeri, A., Badparva, R., Mombeini, M. A., Khorsandi, L., & Goudarzi, M. (2022). Naringenin: A potential natural remedy against methotrexate-induced hepatotoxicity in rats. Drug and Chemical Toxicology, 45(2), 491–498.
- Mehrzadi, S., Mehrabani, M., Malayeri, A. R., Bakhshayesh, M., Kalantari, H., & Goudarzi, M. (2019). Ellagic acid as a potential antioxidant, alleviates methotrexate-induced hepatotoxicity in male rats. Acta chirurgica Belgica, 119(2), 69–77. https://doi.org/10.1080/00015458.2018.1455419
- Mukherjee, S., Ghosh, S., Choudhury, S., Adhikary, A., Manna, K., Dey, S., Sa, G., Das, T., & Chattopadhyay, S. (2013). Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways. The Journal of Nutritional Biochemistry, 24(12), 2040–2050. https://doi.org/10.1016/j.jnutbio.2013.07.005
- Mustapha, A., Marte, A. M., Makinta, A. A., & Benisheikh, A. A. (2021). Efficacy of camel urine in the management of diabetes mellitus in Alloxan induced Albino Rats. International Journal of Agricultural Science & Technology.
- Naldi, L., & Griffiths, C. E. M. (2005). Traditional therapies in the management of moderate to severe chronic plaque psoriasis: An assessment of the benefits and risks. The British Journal of Dermatology, 152(4), 597–615. https://doi.org/10.1111/j.1365-2133.2005.06563.x
- Nauck, M. A., & Meier, J. J. (2018). Incretin hormones: Their role in health and disease. Diabetes, Obesity & Metabolism, 20, 5–21. https://doi.org/10.1111/dom.13129
- Nongonierma, A. B., Paolella, S., Mudgil, P., Maqsood, S., & FitzGerald, R. J. (2018). Identification of novel dipeptidyl peptidase IV (DPP-IV) inhibitory peptides in camel milk protein hydrolysates. Food Chemistry, 244, 340–348. https://doi.org/10.1016/j.foodchem.2017.10.033
- Osman, A., El-Hadary, A., Korish, A. A., AlNafea, H. M., Alhakbany, M. A., Awad, A. A., & Abdel-Hamid, M. (2021). Angiotensin-I converting enzyme inhibition and antioxidant activity of papain-hydrolyzed camel whey protein and its hepato-renal protective effects in thioacetamide-induced toxicity. Foods, 10(2), 468. https://doi.org/10.3390/foods10020468
- Saka, S., & Aouacheri, O. J. J. B. A. (2017). The investigation of the oxidative stress-related parameters in high doses methotrexate-induced albino wistar rats. Journal of Bioequivalence & Bioavailability, 9(2), 372–376. https://doi.org/10.4172/jbb.1000327
- Shaban, A. M., Raslan, M., Qahl, S. H., Elsayed, K., Abdelhameed, M. S., Oyouni, A. A., Al-Amer, O. M., Hammouda, O., & El-Magd, M. A. (2022). Ameliorative effects of camel milk and its exosomes on diabetic nephropathy in rats. Membranes, 12(11), 1060. https://doi.org/10.3390/membranes12111060
- Shoeib, A., & Ba-Hatheq, A. (2007). Effect of camel’surfline on pathogenic Pseudomonas aeruginosa and E. coli isolates towards its maintains to their antibiotic (s) resistance and the presence of plasmid (s). Saudi Journal of Biological Sciences, 14(2), 177–184.
- Tarnopolsky, M. A. (2011). Creatine as a therapeutic strategy for myopathies. Amino Acids, 40(5), 1397–1407. https://doi.org/10.1007/s00726-011-0876-4
- Thulin, P., Nordahl, G., Gry, M., Yimer, G., Aklillu, E., Makonnen, E., Aderaye, G., Lindquist, L., Mattsson, C. M., Ekblom, B., & Antoine, D. J. (2014). Keratin‐18 and microRNA‐122 complement alanine aminotransferase as novel safety biomarkers for drug‐induced liver injury in two human cohorts. Liver International, 34(3), 367–378. https://doi.org/10.1111/liv.12322
- Totan, M., Dagdemir, A., Ak, A. R., Albayrak, D., & Kucukoduk, S. (2001). Effects of high‐dose methotrexate on the hemostatic system in childhood acute lymphoblastic leukemia. Medical and Pediatric Oncology: The Official Journal of SIOP—International Society of Pediatric Oncology Societé Internationale d’Oncologie Pédiatrique, 36(4), 429–443. https://doi.org/10.1002/mpo.1106
- Vatsalya, V., Cave, M. C., Kong, M., Gobejishvili, L., Falkner, K. C., Craycroft, J., Mitchell, M., Szabo, G., McCullough, A., Dasarathy, S., & Radaeva, S. (2020). Keratin 18 is a diagnostic and prognostic factor for acute alcoholic hepatitis. Clinical Gastroenterology and Hepatology, 18(9), 2046–2054. https://doi.org/10.1016/j.cgh.2019.11.050
- Wang, Z. X., Qiao, X. Y., Hao, S. N., & Ji, R. (2017). Demonstration of hepatoprotective action of camel milk through improving antioxidant activity and regulating gene expression in mice. Journal of Camel Practice and Research, 24(2), 169–174. https://doi.org/10.5958/2277-8934.2017.00026.1
- World Health Organization. (1993). Guidelines on the conservation of medicinal plants. International Union for Conservation of Nature and Natural Resources.
- Xue, X., Wang, W. S., Shi, J. Z., Zhang, S. L., Zhao, W. Q., Shi, W. H., Guo, B. Z., & Qin, Z. (2014). Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. Journal of Assisted Reproduction and Genetics, 31(9), 1161–1166. https://doi.org/10.1007/s10815-014-0287-z
