?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Some encapsulation process and formulation parameters influence on several physicochemical properties of the encapsulated products. In this study, the influence of the OEO:β-CD ratio and stirring speed on the total oil, surface oil, oil retention, entrapment efficiency, yield, size distribution (d10, d32, and d43), span value, bounding rectangle aspect ratio (BRAR), Feret’s aspect ratio (FAR), circularity, smoothness, and opacity of microparticles were examined. The superior entrapment efficiency (95.18%), highest oil retention (79.13%), and lowest surface oil content (10.02 mg oil/g powder microparticles) were achieved at an OEO:β-CD ratio of 0.15 (w/w) and stirring speed of 700 rpm. Likewise, all microparticles showed irregular and elongated shapes, in which the opacity distribution was more influenced. Finally, the obtained FTIR spectra contained OEO bands hidden inside β-CD peaks, indicating the occurrence of a complexation reaction after encapsulation.
Introduction
Encapsulated orange essential oil (OEO) exhibits various bioactive properties, such as antioxidant, antibacterial, antifungal, and larvicidal ones (Dehghan et al., Citation2020; Gomes et al., Citation2021; Savic et al., Citation2022). Therefore, OEO has been widely used as an active agent in juices, cakes, fruit coatings, and food packages (Bento et al., Citation2020; da Silva et al., Citation2018; Felix de Andrade et al., Citation2020; Kringel et al., Citation2020; Radi et al., Citation2018). However, changing the parameters of the encapsulation process can decrease the entrapment efficiency (EE), accumulate surface oil, and produce particles with inadequate sizes for a controlled release of bioactive compounds (Malekjani & Jafari, Citation2021; Ramos et al., Citation2021; Trojanowska et al., Citation2017).
Among various encapsulation techniques, a chemical method based on complexation via molecular inclusion is most promising owing to the low losses of volatile compounds and the highest particle stability (Razola-Díaz et al., Citation2021). In this method, β-cyclodextrin (β-CD) is a commonly used core material, and its truncated cone structure consists of seven glucopyranose units with a hydrophilic surface and a hydrophobic cavity suitable for oil entrapment (Kfoury et al., Citation2018; Wadhwa et al., Citation2017). A co-precipitation method was effectively used for the complexation of essential oils with β-CD by dissolving cyclodextrin in water and slowly aggregating oil in solution at a constant temperature (Bhandari et al., Citation1998). Under these conditions, Kringel et al. (Citation2021) achieved high OEO retention without reporting the microparticle yield.
The most actively investigated operational parameters of the OEO encapsulation by a coacervation method were time and temperature; however, the OEO:β-CD ratio (Torres-Alvarez et al., Citation2020) and stirring speed strongly influence the interactions between the oil and material core (Bai, Citation2021). According to Li et al. (Citation2018) and Torres-Alvarez et al. (Citation2020), the oil/core ratio correlates with other process parameters and determines the particle morphology, which is typically observed by scanning electron microscopy (SEM).
Therefore, the purpose of this study was to evaluate the influence of the OEO:β-CD ratio and stirring speed on physicochemical characteristics, such as the total oil (TO) content, surface oil (SO) content, oil retention (OR), entrapment efficiency (EE), yield (Y), and size distribution, span value, circularity, smoothness, and opacity of the produced microparticles by dynamic image analysis.
Materials and methods
Materials and chemicals
OEO was acquired from Lluch Essence (Spain). β-CD was obtained from Sigma–Aldrich (Germany). Absolute ethanol and food grade hexane were purchased from Merck (Peru).
Preparation of OEO/β-CD microparticles
OEO was encapsulated in β-CD using the coacervation method developed by Kringel et al. (Citation2017) with some modifications. First, β-CD was dissolved in 50 mL distilled water at 45°C on a hot plate (HSC-19T magnetic stirrer, Ningbo Joan Lab Equipment Co., China). The resulting mixture was cooled and maintained at 35°C with constant agitation followed by a slow OEO addition. According to previous studies, in lists various treatment conditions (T1–T4), including the OEO:β-CD ratios (w/w) of 0.15 and 0.84 and stirring speeds of 300 and 700 rpm, which were used for the evaluation of microparticle characteristics. After 120 min of agitation, the solution was cooled to room temperature (approximately 20–23°C), covered, and refrigerated overnight at 4°C. On the next day, the synthesized OEO:β-CD microparticles were precipitated, recovered by vacuum filtration, washed with absolute ethanol, and dried in an oven at 50°C for 24 h. Finally, the produced OEO microparticles were stored in dark bottles at 25°C until analysis.
Table 1. Effect of ratio and stirring speed on the total oil, surface oil, entrapment efficiency, and yield.
Total and surface OEO contents
The oil content in powder microparticles was determined using the methodology developed by Bhandari et al. (Citation1998) with some modifications. For TO extraction, 20 mg of microparticles was dissolved in 8 mL of distilled water in centrifuge tubes (50 mL) followed by the addition of 5 mL hexane. The tubes were sealed and placed in a water bath at 45°C for 45 min for the release of TO in hexane; after that, the tubes were centrifuged at 5000 × g (2904 rpm, rotor F-35-6-30, Eppendorf 5430 R Refrigerated Centrifuge, Germany) at 10°C for 20 min, and the upper phase was extracted three times to measure its absorbance by a spectrophotometer (Genesys 10S UV–Vis Spectrophotometer, Thermo Fisher Scientific Inc., U.S.A.) at 230 nm. To determine the SO content, the same amount of microparticles was mixed with 10 mL of hexane and manually shaken for 2 min, after which the samples were centrifuged under the conditions described above, and the supernatant was removed to measure the absorbance. In each case, the TO and SO contents were calculated using a calibration curve constructed for OEO solutions in hexane in the range of 0.04–0.42 mg/mL (Y = 2.503X + 0.0145, where Y is the absorbance at 230 nm, and X is the oil concentration; R2 = 0.999). Their values were expressed in mg/g of powder microparticles.
Oil retention (OR), entrapment efficiency (EE), and yield (Y)
The OR value (EquationEquation (1)(1)
(1) ) was calculated using the method developed by Bhandari et al. (Citation1998). The EE and Y values were computed via EquationEquations (2)
(2)
(2) and (Equation3
(3)
(3) ), respectively, used by Torres-Alvarez et al. (Citation2020):
Particle morphology
The morphology of the produced microparticles was determined by dynamic image analysis (Micromeritics Instrument Corp., Norcross, U.S.A.) according to the method developed by Ulusoy and Yekeler (Citation2014). The prepared microparticles were suspended in ethanol at a concentration of 0.1% (wt.%) under mechanical agitation and recirculation at 100 rpm. Particle Insight Software was utilized to determine the particle size and shape distributions as described below.
Particle size distribution
The mean particle size (d1,0), Sauter mean diameter (d3,2), and volume mean diameter (d4,3) were determined experimentally, and the span value was calculated via the following equation:
where D90, D10, and D50 are the particle sizes obtained for 90, 10, and 50% of microparticles, respectively.
Particle shape distribution
Particle shape distributions were expressed via the bounding rectangle aspect ratio (BRAR), Feret’s aspect ratio (FAR), circularity (C), smoothness (S), and opacity (O) computed by the equipment software. BRAR and FAR are the length-to-width ratios in the rectangular and irregular models, respectively (Ulusoy & Yekeler, Citation2014). The circularity is equal to 4A/πD2BC, smoothness is equal to 42, and opacity is the fraction of the particle area that is darker than the light threshold (1 is fully opaque, and 0 is fully transparent) (Micromeritics Instrument Corp., Citation2008). Here, A, DBC, and P are the experimentally determined area, bounding circle diameter, and perimeter of particles, respectively.
Fourier-transform infrared spectroscopy analysis
The microparticles obtained after different treatments were analyzed using a Fourier-transform infrared (FTIR) spectrometer (Nicolet iS10, Thermo Fisher Scientific Inc., U.S.A.) coupled with an attenuated total reflection accessory. Measurements included 130 scans performed in a frequency range from 4000 to 400 cm−1 at a resolution of 4 cm−1. The OMNIC FTIR software was used for peak identification.
Statistical analysis
All measurements were performed in triplicate, and the mean values with standard deviations were obtained for each treatment condition. Statgraphics Centurion 19 software (Statgraphics Technologies Inc., U.S.A.) was used for data analysis, and post hoc multiple comparisons of means by the Tukey test were considered significant at p-value < .05.
Results and discussion
Total and surface OEO contents
The TO and SO contents in powder microparticles are listed in . The OEO:β-CD ratio produced a significant effect (p-value < .05) on both contents at 700 rpm, in contrast to 300 rpm. According to investigations previously, the oil content encapsulated is influenced by stirring speed but also depends on the polymer to the core ratio (Bai et al., Citation2021; Bamba et al., Citation2018; Lemos et al., Citation2017). Treatments T1 and T3 with the lowest OEO:β-CD ratio of 0.15 resulted in the largest TO content in both stirring speeds, which might be related to the saturation capacity of the β-CD for OEO (Hadian et al., Citation2018); thus, TO was inversely proportional to the OEO:β-CD ratio. Similar results were obtained for lemon oil and other concentrated orange oils encapsulated in β-CD (Bhandari et al., Citation1998; Torres-Alvarez et al., Citation2020).
Moreover, T1 and T3 led to lower SO contents than those obtained after the T2 and T4 treatments. This indicated better oil entrapment inside the β-CD cavity at lower OEO:β-CD ratios, suggesting that the capacity of the β-CD hole was not saturated (Emadzadeh et al., Citation2021). In contrast, the treatments conducted at a higher OEO:β-CD ratio or larger oil amounts caused a capacity saturation and oil accumulation on the particle surface, which may result in wettability and dispersibility problems and promote oxidation reactions leading to the development of undesirable flavors (Bai et al., Citation2021; Balasubramani et al., Citation2015; Hadian et al., Citation2018).
Increasing the stirring speed from 300 to 700 rpm at the same ratio of 0.15 decreased the SO content; however, the opposite effect was observed when the ratio increased to 0.84 (p < .05). Bai et al. (Citation2021) reported that an excessive amount of oil resulted in an incomplete β-CD coating. T. A. Reineccius et al. (Citation2002) concluded that guest molecules and CD mainly experienced weak chemical interactions such as van der Waals forces, dipole–dipole interactions, and hydrogen bonds; therefore, the high stirring speed could induce the cleavage of bonds, which increased the SO content. This explains the results obtained after the T4 treatment conducted at a ratio of 0.84 and stirring speed of 700 rpm, which were significantly different from those obtained after the other treatments.
Oil retention (OR), entrapment efficiency (EE), and yield (Y)
The results of the Tukey test demonstrated the existence of statistically significant effects (p-value < .05) of the OEO:β-CD ratio and stirring speed on the OR, EE, and Y values. In particular, some treatments resulted in high EE values without considerably increasing OR and Y (). Oil retention was determined by dividing the oil content in the microparticles by the total oil initially added expressed in percentage (G. Reineccius et al., Citation2022). The highest value was 79.13 ± 0.13% at a ratio and stirring speed of 0.15 and 700 rpm (T3), respectively; similar values were obtained with other polymers (Savic et al., Citation2022; Tavassoli-Kafrani et al., Citation2018). The treatments with better OR were at a ratio of 0.15 (T1 and T3) than at a ratio of 0.84 (T2 and T4), the same trend was reported by Bhandari et al. (Citation1998) and Petrović et al. (Citation2010) for lemon and cinnamon oil. Furthermore, some researchers mentioned that the processing conditions, or after the complexation with β-CD could affect oil retention (El Kharraf et al., Citation2021; Ramos et al., Citation2021). The highest EE values were obtained in the treatment T1 and T3, which were much higher than those of other orange oils encapsulated in chitosan, gelatin, and alginate (Gajic et al., Citation2021; Tavassoli-Kafrani et al., Citation2018; Velmurugan et al., Citation2017). The obtained encapsulation entrapments are ranked in the order T3 > T1 > T2 > T4; an OEO:β-CD ratio of 0.15 (w/w) and 700 rpm achieved the best EE (95.18 ± 0.98%), this stirring speed likely caused the displacement of water from the CD cavity allowing complexation with hydrophobic molecules (Razola-Díaz et al., Citation2021). Torres-Alvarez et al. (Citation2020) reported a similar EE range (80.7–89.5%) and suggested that the limonene content, the polarity of the compounds, and the method selected influenced the EE value.
T4 obtained the highest value of the Y (51.26 ± 0.07%) indicating that only half of the total OEO and β-CD weight was recovered after the process; similar values were reported by Li et al. (Citation2018) for sweet orange oil, but Torres-Alvarez et al. (Citation2017) reported higher values (above 80%). The differences could due to oil volatility during the agitation process or the separation of surface oil after the complexation because T4 also presented more surface oil content. To respect, dos Passos Menezes et al. (Citation2017) revealed that limonene molecules interact with β-CD in 10 different ways during complexation and could be located inside or on the surface of the β-CD cavity.
Increasing the OEO:β-CD ratio and stirring speed also increased the yield of powder microparticles; the same effect was observed by Ma et al. (Citation2018) and Bai et al. (Citation2021) for clove essential oil and eugenol, respectively. Nevertheless, according to the authors, increasing the stirring time and stirring speed at a specific component ratio decreased the microparticle yield. Other studies also demonstrated that the characteristics of guest and host molecules as well as the encapsulation conditions strongly influenced the OR, EE, and Y values (Bhandari et al., Citation1998; Gharibzahedi & Jafari, Citation2017; Madene et al., Citation2006; Maraulo et al., Citation2021; Razola-Díaz et al., Citation2021; T. A. Reineccius et al., Citation2002).
Morphology characteristics
Particle size distribution
The particle size distributions obtained at different OEO:β-CD ratios and stirring speeds are presented in . The values of d10, d32, and d43 were higher than those of concentrated orange oil microparticles reported by Torres-Alvarez et al. (Citation2020) and lower than the values obtained for oregano oil: β-CD microparticles by Barbieri et al. (Citation2018). These results suggest that the size of microparticles depends on the type of essential oil and other compounds interacting with oil molecules (Marques, Citation2010). In addition, the agitation time was 240 min by Torres-Alvarez et al. (Citation2017) and 60 min by Barbieri et al. (Citation2018); in comparison to 120 min in this investigation.
Table 2. Particle size distribution of particle size at different OEO:β-CD ratios and stirring speeds.
In general, the microparticle size was strongly affected by the formulation (OEO:β-CD ratio) and process parameter (stirring speed) (p-value < .05). Increasing the ratio from 0.15 to 0.84 increased the particle size, which might be due to the addition of an excessive amount of oil to β-CD. Songkro et al. (Citation2012) and Kringel et al. (Citation2020) reported that β-CD complexes promoted particle agglomeration and increased particle size, which negatively affected their stability (Torres-Alvarez et al., Citation2020). In contrast, increasing the stirring speed from 300 to 700 rpm significantly reduced the particle size (p-value < .05); this trend has been reported previously by other researchers (Lemos et al., Citation2017; Trojanowska et al., Citation2017).
Meanwhile, the smallest particle size is correlated with the highest encapsulation efficiency (%EE). The same trend was observed by Mouffok et al. (Citation2016) for microspheres prepared at different stirring speeds, which suggested that the higher agitation speed produced broken particles, promoting the interactions with cavity walls and increasing the EE of guest molecules.
Finally, all treatments produced span values of approximately 1.00, which indicated a homogeneous granulometric dispersion (Chen et al., Citation2020). These values were smaller than 2.97 and 5.43 reported for OEO encapsulated in maltodextrin and modified starch dehydrated by spray drying and vacuum spray drying, respectively (Ramos et al., Citation2021). Moreover, different span values were obtained after the T3 and T4 treatments owing to the strong effect of the OEO:β-CD ratio at the highest agitation speed (in contrast to the T1 and T2 treatments), which might be caused by the relationship between the formulation and process parameters. Lemos et al. (Citation2017) observed a similar effect for the span values of microcapsules containing gelatin–sodium alginate due to stirring and different polymer concentrations.
Particle shape distribution
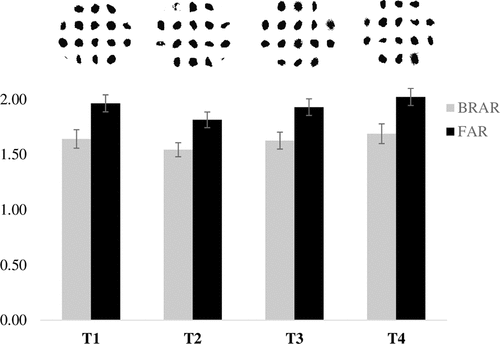
shows the BRAR and FAR values obtained for each treatment procedure. Here, BRAR is the ratio of the width to the length of the rectangular model, while FAR is the ratio of the width to the length of the Feret irregular model. Both parameters are greater than one in all cases; therefore, it corresponds to irregular shapes according to Ulusoy and Yekeler (Citation2014). to Ulusoy and Yekeler (Citation2014). To respect, Li et al. (Citation2018) found rectangular, rhombus, trapezoid, and parallelogram particle shapes that explain the obtained BRAR and FAR data. The treatment T2 exhibits shorter BRAR and FAR values as compared with those calculated for the other treatments but with a significant difference (p-value < .05) with T4, demonstrating the effect of the stirring speed on the particle shape at the highest ratio correlating to a significant reduction of the particle size evidenced in .
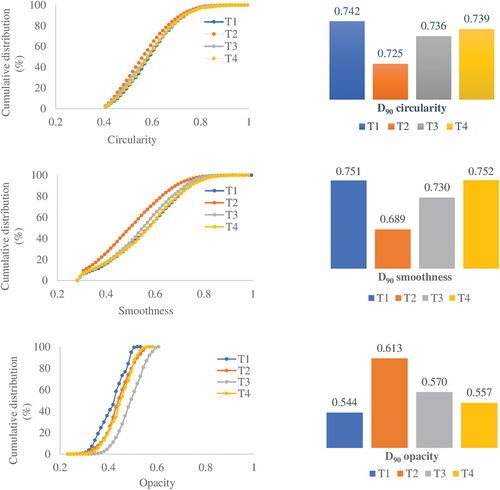
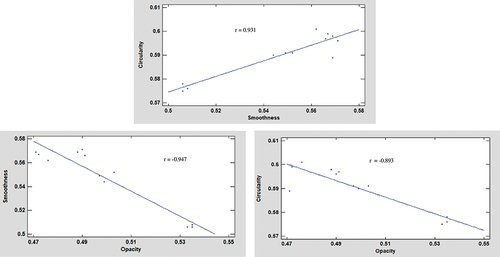
shows the cumulative distributions of the circularity, smoothness, and opacity of the produced microparticles as well as the D90 values computed for all shape parameters. The opacity distributions vary more significantly than the smoothness and circularity. The opacity distribution shows that the T3 curve is shifted to the right with respect to the T1 curve, probably by the increase in the stirring speed from 300 to 700 rpm, and this behavior only was observed at a ratio of 0.15; perhaps, these results are correlated at the oil retention. The obtained D90 ranges are equal to 0.725–0.742 for circularity, 0.689–0.752 for smoothness, and 0.544–0.613 for opacity. The correlations between various shape parameters are shown in . Here, a positive correlation is observed between circularity and smoothness, whereas negative correlations exist between opacity and smoothness and between circularity and opacity.
FTIR studies
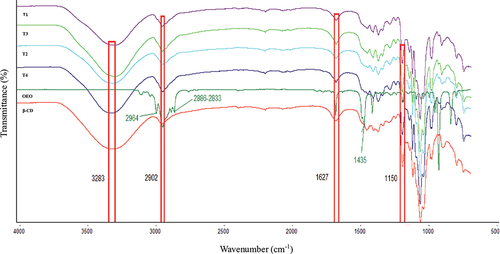
The FTIR spectra of pure β-CD, OEO, and the microparticles obtained after different treatments are shown in . β-CD exhibits a strong band at 3283 cm−1 due to the O–H vibrations of hydroxyl groups. Similarly, the weak band at 2902 cm−1 corresponds to the stretching vibrations of CH, CH2 groups and the bending vibrations of OH groups. The band at 1627 cm−1 is caused by the bending vibrations of H–O–H species and the peaks at 1406, 1361, and 1327 cm−1 are due to the bending vibrations of C–H groups. The bands at 1150, 1076, and 1022 cm−1 are attributed to the stretching vibrations of the C–O and C–O–C groups of α-1,4 links. On the other hand, the oscillating vibrations of the links C-H and skeletal vibrations C–C in the glucopyranose ring correspond to bands at 935–707 cm−1. Similar results were obtained by Bai et al. (Citation2021), and Torres-Alvarez et al. (Citation2020) for inclusion complexes with linalool, and citrus essential oil.
The OEO spectrum contains bands in the range from 2965 to 2832 cm−1 corresponding to different C–H links characteristic of OEO. The stretching vibrations in the bands of the range between 1644 and 1435 cm−1, 3071–2832 cm−1, and angular strain bands in the range from 1014 to 757 cm−1, corresponding to stretching vibrations of C-H species (Galvão et al., Citation2015; Hădărugă et al., Citation2020; Torres-Alvarez et al., Citation2020). The band 1435 cm−1, characteristic of OEO could be associated with elongation vibrations C=C of oleophilic groups of essential oils, which were not observed in inclusion complexes; therefore, these links could be involved in the complex formation (T1, T2, T3, T4) (Kringel et al., Citation2017; Sousa et al., Citation2022). Other investigations evaluated the complex formation with limonene, eucalyptol, orange, and thymol showing the disappearance of some bands or reduction of the intensity that suggested the essential oil interaction into the β-CD cavity (Bai et al., Citation2021; dos Passos Menezes et al., Citation2017; Galvão et al., Citation2015; Kringel et al., Citation2017; Torres-Alvarez et al., Citation2017).
The OEO:β-CD microparticle spectra exhibit broad and intense bands derived from β-CD (Mashaqbeh et al., Citation2021). The O–H wavenumbers corresponding to stretching vibrations decreased after treatment, while the wavenumbers obtained for CO stretching vibrations slightly increased. These changes are due to hydrophobic interactions between the OEO molecules and β-CD cavity, as well as hydrogen bonds, which were formatted among OEO hydroxyl groups with the cyclodextrins, disturbing the bands after complexation (Hădărugă et al., Citation2020; Mashaqbeh et al., Citation2021). Galvão et al. (Citation2015), and Torres-Alvarez et al. (Citation2017) reported similar results owing to the interactions that occurred at lower wavenumbers, while the OEO peaks at 2964, 2886, 2833, 1435 cm−1 disappeared in all treatments although there was surface oil on the particles; Hogenbom et al. (Citation2021) observed the same result, the authors indicated that there is no significant amount of oil outer surface of the complexes, demonstrating that the majority amount of oil is included within the complex as expected.
Conclusion
OEO was encapsulated in β-CD using the co-precipitation method to evaluate the effects of the OEO:β-CD ratio and stirring rate on the TO, SO, OR, EE, and Y values. The morphological characteristics and FTIR spectra of the produced microparticles were analyzed for each treatment. The lowest SO content and highest EE and OR values were achieved at an OEO:β-CD ratio of 0.15 and stirring speed of 700 rpm. The obtained BRAR and FAR magnitudes and particle sizes confirmed the formation of irregularly shaped (both elongated and rounded) microparticles, while their agglomeration increased the particle size, which was strongly affected by the formulation and operational parameter (stirring speed). Furthermore, the obtained distributions of opacity values were more strongly influenced by the treatment conditions than the circularity and smoothness characteristics. Meanwhile, correlations were observed between the circularity, smoothness, and opacity of microparticles. Finally, the FTIR spectra confirmed the existence of interactions between OEO and β-CD species, which resulted in the disappearance of some peaks representative of OEO and changes in the wavenumbers corresponding to the β-CD peaks, demonstrating high encapsulation efficiency. The interaction of the ratio and stirring speed demonstrates the affected physicochemical characteristics of the OEO:β-CD microparticles that can be optimized in future investigations.
CRediT authorship contribution statement
Diana Nolazco–Cama: conceptualization, methodology, formal analysis, investigation, writing original draft, visualization, resources. Angeles Sánchez–Contreras: methodology, review & editing, visualization. Lena Tellez–Monzón: formal analysis, writing original draft. Luis Vargas–Delgado: conceptualization, review & editing, supervision. Luis Condezo–Hoyos: conceptualization, methodology, formal analysis, writing – review & editing, visualization, supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bai, C., Xu, P., Qi, W., Kong, Q., Wang, J., Liao, T., & Xiong, G. (2021). Preparation and characterization of a sustained-release bio-preservative based on β-cyclodextrin encapsulated eugenol. Food Bioscience, 42. https://doi.org/10.1016/j.fbio.2021.101192
- Balasubramani, P., Palaniswamy, P. T., Visvanathan, R., Thirupathi, V., Subbarayan, A., & Prakash Maran, J. (2015). Microencapsulation of garlic oleoresin using maltodextrin as wall material by spray drying technology. International Journal of Biological Macromolecules, 72, 210–217. https://doi.org/10.1016/j.ijbiomac.2014.08.011
- Bamba, B., Shi, J., Tranchant, C., Xue, S., Forney, C., & Lim, L.-T. (2018). Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules, 23(7). https://doi.org/10.3390/molecules23071685
- Barbieri, N., Sanchez-Contreras, A., Canto, A., Cauich-Rodriguez, J. V., Vargas-Coronado, R., & Calvo-Irabien, L. M. (2018). Effect of cyclodextrins and Mexican oregano (Lippia graveolens Kunth) chemotypes on the microencapsulation of essential oil. Industrial Crops and Products, 121, 114–123. https://doi.org/10.1016/j.indcrop.2018.04.081
- Bento, R., Pagán, E., Berdejo, D., de Carvalho, R. J., García-Embid, S., Maggi, F., Magnani, M., de Souza, E. L., García-Gonzalo, D., & Pagán, R. (2020). Chitosan nanoemulsions of cold-pressed orange essential oil to preserve fruit juices. International Journal of Food Microbiology, 331. https://doi.org/10.1016/j.ijfoodmicro.2020.108786
- Bhandari, B. R., D’Arcy, B. R., Le, L., & Bich, T. (1998). Lemon oil to-cyclodextrin ratio effect on the inclusion efficiency of-cyclodextrin and the retention of oil volatiles in the complex.
- Chen, Z., Zong, L., Chen, C., & Xie, J. (2020). Development and characterization of PVA-Starch active films incorporated with β-cyclodextrin inclusion complex embedding lemongrass (Cymbopogon citratus) oil. Food Packaging and Shelf Life, 26. https://doi.org/10.1016/j.fpsl.2020.100565
- da Silva, C. F., de Oliveira, F. S. M., Caetano, V. F., Vinhas, G. M., & Cardoso, S. A. (2018). Orange essential oil as antimicrobial additives in poly(vinyl chloride) films. Polímeros, 40(4), 332–338. https://doi.org/10.1590/0104-1428.16216
- Dehghan, B., Esmaeilzadeh Kenari, R., & Raftani Amiri, Z. (2020). Nano-encapsulation of orange peel essential oil in native gums (Lepidium sativum and Lepidium perfoliatum): Improving oxidative stability of soybean oil. Journal of Food Processing and Preservation, 44(11). https://doi.org/10.1111/jfpp.14889
- dos Passos Menezes, P., dos Santos, P. B. P., Dória, G. A. A., de Sousa, B. M. H., Serafini, M. R., Nunes, P. S., Quintans-Júnior, L. J., de Matos, I. L., Alves, P. B., Bezerra, D. P., Mendonça Júnior, F. J. B., da Silva, G. F., de Aquino, T. M., de Souza Bento, E., Scotti, M. T., Scotti, L., & de Souza Araujo, A. A. (2017). Molecular modeling and physicochemical properties of supramolecular complexes of limonene with α- and β-cyclodextrins. AAPS Pharmaceutical Scientists Technology, 18(1), 49–57. https://doi.org/10.1208/s12249-016-0516-0
- El Kharraf, S., Farah, A., El Hadrami, E. M., El-Guendouz, S., Lourenço, J. P., Rosa Costa, A. M., & Miguel, M. G. (2021). Encapsulation of Rosmarinus officinalis essential oil in β-cyclodextrins. Journal of Food Processing and Preservation, 45(10). https://doi.org/10.1111/jfpp.15806
- Emadzadeh, B., Ghorani, B., Naji-Tabasi, S., Charpashlo, E., & Molaveisi, M. (2021). Fate of β-cyclodextrin-sugar beet pectin microcapsules containing garlic essential oil in an acidic food beverage. Food Bioscience, 42. https://doi.org/10.1016/j.fbio.2021.101029
- Felix de Andrade, M., Diego de Lima Silva, I., Alves da Silva, G., David Cavalcante, P. V., Thayse da Silva, F., Bastos de Almeida, Y. M., Vinhas, G. M., & Hecker de Carvalho, L. (2020). A study of poly (butylene adipate-co-terephthalate)/orange essential oil films for application in active antimicrobial packaging. LWT, 125. https://doi.org/10.1016/j.lwt.2020.109148
- Gajic, I. M. S., Savic, I. M., Gajic, D. G., & Dosic, A. (2021). Ultrasound-assisted extraction of carotenoids from orange peel using olive oil and its encapsulation in ca-alginate beads. Biomolecules, 11(2). https://doi.org/10.3390/biom11020225
- Galvão, J. G., Silva, V. F., Ferreira, S. G., França, F. R. M., Santos, D. A., Freitas, L. S., Alves, P. B., Araújo, A. A. S., Cavalcanti, S. C. H., & Nunes, R. S. (2015). β-cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: An alternative to control Aedes aegypti larvae. Thermochimica Acta, 608, 14–19. https://doi.org/10.1016/j.tca.2015.04.001
- Gharibzahedi, S., & Jafari, S. (2017). Nanoencapsule formation by cyclodextrins. In S. Jafari (Ed.), Nanocapsulation technologies for the food and nutraceutical industries (pp. 190–192). Academic Press.
- Gomes, B., Ogélio, H., Brant, F., Jesus Pereira-Pinto, C., Workman, M. J., Costa, M., Bento Pereira Lima, J., Jesus Martins, A., Ramalho-Ortigao, M., Durvasula, R., Hurwitz, I., Rocha David, M., & Ariel Genta, F. (2021). High larvicidal efficacy of yeast-encapsulated orange oil against Aedes aegypti strains from Brazil. Parasites Vectors, 14(1), 272. https://doi.org/10.1186/s13071-021-04733-2
- Hădărugă, N. G., Szakal, R. N., Chirilă, C. A., Lukinich-Gruia, A. T., Păunescu, V., Muntean, C., Rusu, G., Bujancă, G., & Hădărugă, D. I. (2020). Complexation of Danube common nase (Chondrostoma nasus L.) oil by β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. Food Chemistry, 303. https://doi.org/10.1016/j.foodchem.2019.125419
- Hadian, Z., Maleki, M., Abdi, K., Atyabi, F., Mohammadi, A., & Khaksar, R. (2018). Preparation and characterization of nanoparticle β-cyclodextrin: Geraniol inclusion complexes. Iranian journal of pharmaceutical research : IJPR, 17(1), 39–51. https://pubmed.ncbi.nlm.nih.gov/29755537/
- Hogenbom, J., Istanbouli, M., & Faraone, N. (2021). Novel β-cyclodextrin and catnip essential oil inclusion complex and its tick repellent properties. Molecules, 26(23), 7391. https://doi.org/10.3390/molecules26237391
- Kfoury, M., Landy, D., & Fourmentin, S. (2018). Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules, 23(5). MDPI AG https://doi.org/10.3390/molecules23051204.
- Kringel, D. H., Antunes, M. D., Klein, B., Crizel, R. L., Wagner, R., de Oliveira, R. P., Dias, A. R. G., & Zavareze, E. D. R. (2017). Production, characterization, and stability of orange or eucalyptus essential oil/β-cyclodextrin inclusion complex. Journal of Food Science, 82(11), 2598–2605. https://doi.org/10.1111/1750-3841.13923
- Kringel, D. H., da Silva, W. M. F., Biduski, B., Waller, S. B., Lim, L. T., Dias, A. R. G., & Zavareze, E. D. R. (2020). Free and encapsulated orange essential oil into a β-cyclodextrin inclusion complex and zein to delay fungal spoilage in cakes. Journal of Food Processing and Preservation, 44(5). https://doi.org/10.1111/jfpp.14411
- Kringel, D. H., Lang, G. H., Dias, Á. R. G., Gandra, E. A., Valente Gandra, T. K., & da Rosa Zavareze, E. (2021). Impact of encapsulated orange essential oil with β-cyclodextrin on technological, digestibility, sensory properties of wheat cakes as well as Aspergillus flavus spoilage. Journal of the Science of Food and Agriculture, 101(13), 5599–5607. https://doi.org/10.1002/jsfa.11211
- Lemos, Y. P., Mariano Marfil, P. H., & Nicoletti, V. R. (2017). Particle size characteristics of buriti oil microcapsules produced by gelatin-sodium alginate complex coacervation: Effect of stirring speed. International Journal of Food Properties, 20, 1438–1447. https://doi.org/10.1080/10942912.2017.1349139
- Li, D., Wu, H., Huang, W., Guo, L., & Dou, H. (2018). Microcapsule of sweet orange essential oil encapsulated in beta-cyclodextrin improves the release behaviors in vitro and in vivo. European Journal of Lipid Science and Technology, 120(9). https://doi.org/10.1002/ejlt.201700521
- Madene, A., Jacquot, M., Scher, J., & Desobry, S. (2006). Flavour encapsulation and controlled release - A review. International Journal of Food Science and Technology, 41(1), 1–21. https://doi.org/10.1111/j.1365-2621.2005.00980.x
- Malekjani, N., & Jafari, S. M. (2021). Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Comprehensive Reviews in Food Science and Food Safety, 20(1), 3–47. https://doi.org/10.1111/1541-4337.12660
- Maraulo, G. E., Lionello, M. E., Mazzobre, M. F., & dos Santos Ferreira, C. (2021). Obtention and characterization of cyclodextrins complexes for the development of food ingredients. https://doi.org/10.1007/978-1-0716-1649-9_13
- Marques, H. M. C. (2010). A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour and Fragrance Journal, 25(5), 313–326. https://doi.org/10.1002/ffj.2019
- Mashaqbeh, H., Obaidat, R., & Al-Shar’i, N. (2021). Evaluation and characterization of curcumin-β-cyclodextrin and cyclodextrin-based nanosponge inclusion complexation. Polymers, 13(23). https://doi.org/10.3390/polym13234073
- Ma, S., Zhao, Z., & Liu, P. (2018). Optimization of preparation process of β-cyclodextrin inclusion compound of clove essential oil and evaluation of heat stability and antioxidant activities in vitro. Journal of Food Measurement and Characterization, 12(3), 2057–2067. https://doi.org/10.1007/s11694-018-9820-6
- Micromeritics Instrument Corporation. (2008). Particle insight particle shape analyzer. www.particulatesystems.com
- Mouffok, M., Mesli, A., Abdelmalek, I., & Gontier, E. (2016). Effect of the formulation parameters on the encapsulation efficiency and release behavior of p-aminobenzoic acid-loaded ethylcellulose microspheres. Journal of the Serbian Chemical Society, 81(10), 1183–1198. https://doi.org/10.2298/JSC160308068M
- Petrović, G. M., Stojanović, G. S., & Radulović, N. S. (2010). Encapsulation of cinnamon oil in β-cyclodextrin. Journal of Medicinal Plants Research, 4(14), 1382–1390. https://doi.org/10.5897/JMPR10.146
- Radi, M., Akhavan Darabi, S., Akhavan, H. R., & Amiri, S. (2018). The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. Journal of Food Processing and Preservation, 42(2), e13441. https://doi.org/10.1111/jfpp.13441
- Ramos, F. D. M., Silveira Júnior, V., & Prata, A. S. (2021). Physical aspects of orange essential oil-containing particles after vacuum spray drying processing. Food Chemistry: X, 12, 12. https://doi.org/10.1016/j.fochx.2021.100142
- Razola-Díaz, M. D. C., Guerra-Hernández, E. J., García-Villanova, B., & Verardo, V. (2021). Recent developments in extraction and encapsulation techniques of orange essential oil. Food Chemistry, 354, 129575. https://doi.org/10.1016/j.foodchem.2021.129575
- Reineccius, G., Patil, S., & Anantharamkrishnan, V. (2022). Encapsulation of orange oil using fluidized bed granulation. Molecules, 27(6), 1854. https://doi.org/10.3390/molecules27061854
- Reineccius, T. A., Reineccius, G. A., & Peppard, T. L. (2002). Encapsulation of flavors using cyclodextrins: Comparison of flavor retention in alpha, beta, and gamma types. Journal of Food Science, 67(9), 3271–3279. https://doi.org/10.1111/j.1365-2621.2002.tb09577.x
- Savic, I. M., Savic Gajic, I. M., Milovanovic, M. G., Zerajic, S., & Gajic, D. G. (2022). Optimization of ultrasound-assisted extraction and encapsulation of antioxidants from orange peels in alginate-chitosan microparticles. Antioxidants, 11(2). https://doi.org/10.3390/antiox11020297
- Songkro, S., Hayook, N., Jaisawang, J., Maneenuan, D., Chuchome, T., & Kaewnopparat, N. (2012). Investigation of inclusion complexes of citronella oil, citronellal and citronellol with b-cyclodextrin for mosquito repellent. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 72(3–4), 339–355. https://doi.org/10.1007/s10847-011-9985-7
- Sousa, V. I., Parente, J. F., Marques, J. F., Forte, M. A., & Tavares, C. J. (2022). Microencapsulation of essential oils: A review. Polymers, 14(9), 1730. https://doi.org/10.3390/POLYM14091730
- Tavassoli-Kafrani, E., Goli, S. A. H., & Fathi, M. (2018). Encapsulation of orange essential oil using cross-linked electrospun gelatin nanofibers. Food and Bioprocess Technology, 11(2). https://doi.org/10.1007/s11947-017-2026-9
- Torres-Alvarez, C., Castillo, S., Sánchez-García, E., Aguilera González, C., Galindo-Rodríguez, S. A., Gabaldón-Hernández, J. A., & Báez-González, J. G. (2020). Inclusion complexes of concentrated orange oils and β-cyclodextrin: Physicochemical and biological characterizations. Molecules (Basel, Switzerland), 25(21). https://doi.org/10.3390/molecules25215109
- Torres-Alvarez, C., Núñez González, A., Rodríguez, J., Castillo, S., Leos-Rivas, C., & Báez-González, J. G. (2017). Perfil químico, actividad antimicrobiana y antioxidante del aceite esencial de naranja y sus aceites concentrados. CyTA - Journal of Food, 15(1), 129–135. https://doi.org/10.1080/19476337.2016.1220021
- Trojanowska, A., Nogalska, A., Valls, R. G., Giamberini, M., & Tylkowski, B. (2017). Technological solutions for encapsulation. Physical Sciences Reviews, 2(9). https://doi.org/10.1515/psr-2017-0020
- Ulusoy, U., & Yekeler, M. (2014). Dynamic image analysis of calcite particles created by different mills. International Journal of Mineral Processing, 133, 83–90. https://doi.org/10.1016/j.minpro.2014.10.006
- Velmurugan, P., Ganeshan, V., Nishter, N. F., & Jonnalagadda, R. R. (2017). Encapsulation of orange and lavender essential oils in chitosan nanospherical particles and its application in leather for aroma enrichment. Surfaces and Interfaces, 9. https://doi.org/10.1016/j.surfin.2017.08.009
- Wadhwa, G., Kumar, S., Chhabra, L., Mahant, S., & Rao, R. (2017). Essential oil–cyclodextrin complexes: An updated review. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 89(1–2), 39–58. Springer Netherlands https://doi.org/10.1007/s10847-017-0744-2