?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Natural pigments derived from microorganisms have many advantages and are widely studied in the food industry. A filamentous fungus, DBFL05, was identified as Aspergillus ustus, which produces a bright and abundant brown pigment. This extracellular, water-soluble pigment is highly polar and slightly soluble in alcohol but insoluble in other organic solvents. The pigment is stable under sunlight, at pH 2–10, and below 100°C. However, it is sensitive to Zn2+, Fe3+, Fe2+, and Cu2+. The pigment is mainly composed of two chemical components, pyrropyrazine diketone and dianthrone, and is classified as a polyketone pigment. Both components of the pigment exhibit inhibitory effects on bacteria and scavenging abilities for •OH and DPPH. The pigment showed no significant toxicity to plants and low toxicity to brine shrimp larvae. These findings indicate that the easily obtainable A. ustus pigment is a polyketone with attractive colour, stability, safety, and bioactivity.
1. Introduction
Pigment is widely used in the food industry, which can improve the color, flavour, taste, and nutritional quality of food and is an indispensable food additive (Darwesh et al., Citation2019). Pigments used in food mainly include synthetic and natural. However, synthetic pigments have teratogenicity, toxicity, and carcinogenicity. Compared with synthetic pigments, natural pigments have the advantages of natural, bright colors, high safety, significant nutritional value, and pharmacological features (Cho et al., Citation2002). Hence, the trend of replacing synthetics with natural pigments is growing (Y. E. Kim et al., Citation2020). Natural pigments come from the tissues or metabolites of animals, plants, and microorganisms. Among natural pigments, pigments of microbial origin have many advantages, such as low cost, resistance to various environmental factor influences, easy to be industrialized, can overcome many defects of animal and plant origin of pigments, and have higher application potential (Indra Arulselvi et al., Citation2014). Although most microorganisms are able to produce pigments, there are only carotenoid pigments and Monascus pigments currently used in the food industry, and the development of microbial-derived pigments is seriously under development. In addition, many microbial strains produce little or intracellular pigments, while some other strains produce toxic pigments that are difficult to separate and purify. Therefore, it is particularly important to screen out efficient, safe, and easily extractable pigment-producing strains (Narsing Rao et al., Citation2017). Filamentous fungi in microorganisms are easily obtained, with developed mycelia that grow rapidly, and the pigments produced by them are widely used in the food industry, such as polyketone pigments produced by Monascus (Dufosse et al., Citation2014), anthraquinone pigment produced by Penicillium oxalate (Alberti et al., Citation2017), and β-carotene produced by Streptomyces coronae (Hernández-Almanza et al., Citation2014). The pigments produced by filamentous fungi have the advantages of rich and diverse color, significant biological activity, antibacterial and anti-inflammatory, convenient production, nutritional and health-care functions, etc., which makes filamentous fungi a reliable source of natural food pigments (F. W. Li et al., Citation2017). In addition, the pigments of filamentous fungi can be used not only as byproducts of biorefineries but also as substitutes for synthetic pigments or other limited natural pigments to meet the increasing demand for natural pigments (Kum et al., Citation2015), further increasing the interest in studying filamentous fungi as potential pigment producers.
Fermentation is an important biotechnological process to enhance the nutritional and sensory value of food, increase phenolic compounds to reduce or eliminate oxidative damage (Darwesh et al., Citation2023; Eweys et al., Citation2022). Broad bean paste, mainly fermented by a variety of microorganisms to produce small molecular flavour substances such as sugars, amino acids, aldehydes, phenols, alcohols, and ketones is usually used in preparing Asian cuisine, since it is not only flavor-enhancing but also highly nutritional and functional, including antioxidant, anti-inflammatory, and anti-cancer activities (M. J. Kim et al., Citation2018; Wolfe & Dutton, Citation2015). Broad bean paste is one of the most popular condiments in China, and its annual production value has exceeded 100 billion RMB in recent years (C. Niu et al., Citation2018). In the postfermentation stage of broad bean paste, fungi, especially filamentous fungi, play a key role in the flavour and color of broad bean paste (Z. Li et al., Citation2016). Therefore, this study is intended to isolate and screen filamentous fungi with high safety and strong pigment-producing abilities from broad bean paste to develop a new natural and edible microbial source pigment.
2. Materials and methods
2.1. Source of the strain and culture medium
The target strains were isolated and purified from fermented bean paste. Tested strains including E. coli, Bacillus subtilis, Staphylococcus aureus, Saccharomyces cerevisiae, Penicillium chrysogenum, Rhizopus oryzae, and Streptomyces longispororuber were isolated, purified, and identified by the Microbiology Laboratory of Hefei Normal University.
The media including Martini medium (Glucose 12.0 g, Peptone 4.5 g, KH2PO4 1.2 g, MgSO4·7H2O 0.6 g, Agar 20 g, Distilled water 1000 mL, pH natural), GAO’s No. 1 medium (Soluble starch 20 g, KNO3 1 g, K2HPO4 0.5 g, MgSO4·7H2O 0.5 g, NaCl 0.5 g, FeSO4·7H2O 0.01 g, Agar 20 g, Distilled water 1000 mL, pH 7.4–7.6), and beef extract peptone medium (Beef Paste 3 g, Peptone 10 g, NaCl 5 g, Agar 20 g, Distilled water 1000 mL, pH 7.4–7.6) were sterilized for 30 min at 121°C and 0.1 MPa.
2.2. Separation, screening, and liquid fermentation culture of pigment-producing filamentous fungi
Approximately 1 g ripe bean paste was diluted 103 times with sterile water, and diluents of 0.1 mL coating were cultivated on the surface of Martini’s solid medium at 28°C for 72 h. The filamentous fungal colonies were transformed and purified many times, and the test tube slant was made and stored for later use. The slant of the screening strains was washed with sterile water, and a spore suspension of 1 × 106 spores/mL was prepared. A spore suspension of 1 mL was added to 150 mL of Martini liquid medium, which was oscillated at 28°C and 150 rpm for 120 h. The pigment production of liquid fermentation was observed, and the dominant pigment-producing strain was selected as the target strain.
2.3. Microscopic observation and molecular sequencing of the dominant pigment-producing strains
The dominant pigment-producing strains were cultured on Martini’s solid medium, when the colonies were mature, the mycelia were selected and placed on slides, and aqueous tablets were prepared with carbolic cotton blue to observe the morphology of mycelia and spores.
Molecular identification of the dominant pigment-producing strains: The DNA genome of the dominant pigment-producing strain was extracted by DNA extraction kit, the fungal universal primer ITS5 (5S-GGAAGTAAAAGTCGTAACAAGG-3”)/ITS4 (5I-TCCTCCGCTTATTGATATGC-3”) was used to amplify the rDNA-ITS sequence of the strain by PCR. PCR reaction system (25 µL): PCR Buffer mixture 2.5 µL, 10 mmol/L dNTP 1 µL, 1 µl of each of the ITS4/ITS5 (10 µmol/L), template DNA 2 µL, 5 U/µL TaqDNA polymerase 0.4 µL, ddH2O,17.1 µL. The mixture was subjected to a first step of denaturation for 2 min at 95°C, followed by 35 cycles of 30 s at 94°C, annealing temperature of 55°C for 30 s and 40 s at 72°C. The last step at 72°C for 10 min was then carried out, and the PCR products were sent to the Shanghai Bioengineering Co., Ltd. for sequencing.
Phylogenetic analysis: The sequencing columns were analysed with the NCBI GenBank database for homology. The corresponding reference strain sequence was selected, Aspergillus niger (accession number OQ726219) as out-group, and MEGA7.0 ClustalX method was used for multiple sequence alignment, and adjacency method Molecular phylogeny was constructed by neighbor-joining method Tree, the system tree was tested 1000 times using bootstrap method.
2.4. Solubility, maximum absorption peak, and stability of pigment
A 1 mL spore suspension of 1 × 106 spores/mL, which was the dominant pigment-producing strain, was inoculated in 150 mL of Martini liquid medium and cultured at 28°C and 150 rpm for 120 h. The fermentation solution was filtered, and the filtrate was centrifuged at 12,000 rpm for 10 min. The pigment filtrate was extracted and placed in a metal disc of the freeze-dryer, sealed with a perforated transparent film and prefrozen at −20°C for 12 h. Then, the filtrate was dried in a vacuum freeze-dryer for 12 h, soaked in anhydrous ethanol for 24 h, centrifuged for 10 min at 12,000 rpm, and dried by vacuum rotary evaporation at 60°C to obtain pigment powder. Approximately 0.05 g pigment powder was dissolved in 20 mL of different organic solvents to study its solubility. The maximum absorption peak of the pigment was obtained by UV–vis scanning between 200 nm and 800 nm, and distilled water was used as a blank control. The pigment aqueous solution was placed at different temperatures, pH values, light times, and metal ions (0.05%), and the OD value was detected below the maximum absorption peak to study the pigment stability.
2.5. HPLC of the pigments and UV–vis scanning of the isolated components
The pigment powder (0.05 g) was dissolved in chromatographic-grade methanol, filtered through 0.22 μm microporous membranes, and centrifuged at 12,000 rpm for 10 min to obtain the pigment supernatant, which was used for HPLC component analysis (chromatographic column: Agilent TC-C18 semi-prepared column [250 mm × 10 mm, 5 µm]; mobile phase: methanol–water [50:50, v/v]; equal degree elution, flow rate: 5.0 mL/min; column temperature: 30°C; detector: diode array detector (DAD); detection wavelength: 298 nm; injection volume: 20 µL; elution time of the samples: 10 min). The distillate was collected at the half-peak of each HPLC separation component, and the pigment samples and the group of components were scanned UV–vis in the range of 200–800 nm to obtain the UV–vis absorption spectra of the pigment samples and the distillate to study the separation of pigments by HPLC.
2.6. Analysis of the pigment samples and HPLC components by GC–MS
The pigment samples and HPLC components were dried by rotary evaporation, dissolved in chromatographic-grade methanol, filtered through 0.22 μm microporous membranes, and centrifuged at 12,000 rpm for 10 min. The pigment samples and HPLC components were examined with GC–MS (Trace GC Ultra and ISQIIMS). The GC–MS column was a TG-35 ms quartz capillary column, and the column temperature was set from 100°C to 300°C (10°C/min). The flow rate of the carrier gas (helium) was 1 mL/min. The syringe and detector temperatures were set at 300°C, and the injection volume was 10 µL. Mass spectrometry was carried out in EI mode at 70 eV to study the separation of pigments in the gas phase, and the separation components were analysed by mass spectrometry to determine the basic chemical composition of the pigments.
2.7. Antibacterial and antioxidant effects of the pigment components
Filter paper pieces with a diameter of 0.5 cm soaked with pigment components were pasted on the surface of solid beef extract peptone and Martini medium coated with suspensions of Escherichia coli, Staphylococcus aureus, Bacillus subtilis, and Saccharomyces cerevisiae. The culture was carried out under the corresponding conditions, and the antibacterial zone size was observed to examine the antibacterial effect of the pigment components. Approximately 1 mL of pigment component was added to 100 mL of Martini’s and GAO’s No. 1 solid medium and poured into the petri dish for mixing solidification. The cultured colonies of Penicillium chrysogenum, Rhizopus oryzae, and Streptomyces longispororuber were drilled with hole punches with 1 and 0.2 cm diameters. The resulting mushroom cake was placed in the corresponding groove of the mixed and solidified medium, and the culture was carried out under corresponding conditions to observe the growth of mushroom cake in the solid medium with different pigment components.
The scavenging ability of the pigment components to •OH was determined by the (Moukette et al., Citation2015) method. The pigment components were diluted 1, 2, 3, 4, 5, and 6 times; 60 μL FeCl3 (1 mM), 90 μL 1,10-phenanthroline (1 mM), 2.4 mL phosphate buffer (0.2 M; pH 7.8), and 150 μL 0.17 M H2O2 were added to 1.5 mL of pigment component diluents and evenly mixed. The reaction was carried out at room temperature for 5 min. With the addition of component diluents as the sample group, three parallel experiments were performed. The mixed solution without diluents was used as the blank control group, the absorbance of the blank and sample groups was measured at 560 nm, and the scavenging rate of the pigment components to •OH was calculated according to Formula (1).
A0 – blank absorption value; AS – sample absorption value
Free radical scavenging ability was determined by the DPPH method (Moukette et al., Citation2015). A 1 mL DPPH (0.1 mM) methanol solution was mixed with a 3 mL dilution of pigment components. The mixture was kept at room temperature for 30 min away from light, with the addition of component diluents as the sample group, three times in parallel. The mixed solution without diluents was used as the blank control group. The absorbance of the blank and sample groups was measured at 517 nm, and the DPPH scavenging rate of the pigment components was calculated according to Formula (2).
A0 – blank absorption value; AS – sample absorption value
2.8. Brine shrimp toxicity bioassay
Brine shrimp eggs were incubated for 24 h under a 40 w fluorescent lamp with a salinity of 3.5%, pH 8°C and 28°C. Pigment solutions of 10, 100, and 1000 mg/L were prepared with 3.5% brine and cultured in a 24-well plate with 3 mL pigment solutions. The 3.5% brine was used as a blank control, and the samples and experimental group were parallel. Ten brine shrimp larvae were placed in each well and incubated at 25°C for 24 h. The fatality rate of brine shrimp was recorded. The weighted probability unit method (Bliss method) was used to calculate the median lethal concentration (LC50) and the 95% confidence interval by SPSS 16.
2.9. Phytotoxicity test
Wheat and broad bean seeds with full grain and similar volume were selected for phytotoxicity of pigment. Then, the wheat and broad bean seeds were soaked in distilled water for 24 h. Twenty wheat seeds were selected and transferred to 90 mm petri dishes covered with two layers of gauze, five broad bean seeds were inserted into 90 mm petri dishes filled with sand, and six groups of parallel petri dishes were made separately. Three groups of seeds containing wheat and broad beans were irrigated with distilled water and 0.05% of the pigment solution separately, 10 mL once a day. The germination rate, root length, stem length, rhizosphere colony number, and root total nitrogen content of wheat and broad bean seeds were measured at 7 and 14 days to determine the effects of pigment on plant growth.
3. Results
3.1. Screening and liquid fermentation culture of pigment-producing filamentous fungi
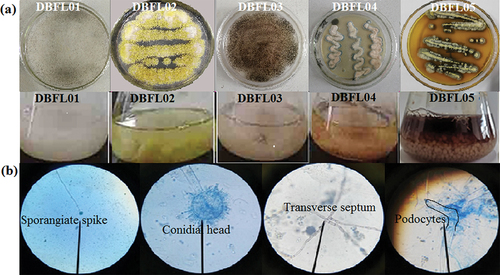
Five strains of filamentous fungi, namely, DBFL01, DBFL02, DBFL03, DBFL04, and DBFL05, were isolated and screened from broad bean paste. These strains were then purified and cultured using liquid fermentation techniques. The characteristics of the colonies and pigment production of each strain are shown in . All five strains exhibited developed aerial and substrate mycelium, typical of filamentous fungi. Strains DBFL02, DBFL04, and DBFL05 showed pigment production. Strain DBFL05 produced a brown-red pigment that permeated the entire medium, while strains DBFL02 and DBFL04 produced intracellular pigments that were dull in colour compared to the liquid fermentation culture’s background. In contrast, strain DBFL05 produced a water-soluble, extracellular pigment with a bright brown-red colour. The pigment was entirely secreted into the liquid culture medium, resulting in the fermentation liquid becoming brown-red and the mycelium pellets turning greyish white. Based on these characteristics, strain DBFL05 was selected as the dominant pigment-producing strain for further study.
3.2. Microscopic observation and molecular sequencing of strain DBFL05
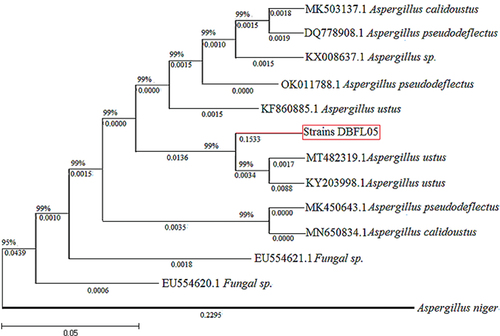
Microscopy was used to observe the morphology of mycelia and spores of strain DBFL05. The branch mycelia of strain DBFL05 contained transverse septa and podocytes, and conidiophores were observed on the podocytes. The tips of the conidiophores enlarged to form a rounded apical sac with many single or double pedicels, and chained conidia were found on the apical sac, indicating that strain DBFL05 belongs to the Aspergillus genus (). A molecular sequencing analysis was performed on strain DBFL05, and a phylogenetic tree was constructed to further determine its species. The gene sequence of strain DBFL05 was found to be 99% similar to the alignment sequence (). Based on the colony and mycelia morphology observed in , strain DBFL05 (accession number OQ726220) was identified as Aspergillus ustus.
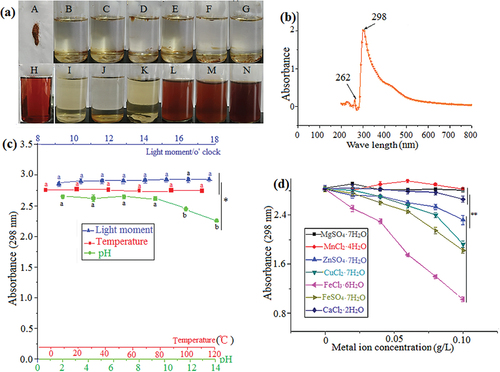
3.3. Solubility, maximum absorption peak, and stability of the A. ustus pigment
The solubility, maximum absorption peak, and stability of the A. ustus pigments were investigated. The pigment powder was found to be insoluble in organic solvents, slightly soluble in methanol and ethanol, easily soluble in water and aqueous ethanol, and easily deliquescent, indicating that the pigment was water-soluble and had strong polarity (). The maximum absorption peak of the pigment was found to be 298 nm, indicating that it contained an aldehyde-ketone structure. The fine structure in the near ultraviolet region indicated that it had an aromatic ring, and a secondary absorption peak was observed at 262 nm. The pigment may be an aldehyde-ketone compound with an aromatic ring or composed of multiple compounds containing aldehyde-ketone structures (). The stability of the pigment was minimally affected by light and temperature, and it exhibited higher stability under acidic and medium-strong alkaline conditions. However, it was significantly influenced by strong alkaline conditions, causing the colour to change from brown to purple red (). Mg2+, Mn2+, and Ca2+ had little influence on the pigment stability, while Zn2+, Fe3+, Fe2+, and Cu2+ ions had an influence on the pigment stability, with all of them forming floccules. Among these ions, Fe2+ had the greatest influence, causing the pigment solution to become clarified, forming a large amount of floccule precipitation ().
Figure 3. Solubility of pigment (a – Pigment powder, b – Ethyl acetate, c – Acetone, d – Petroleum ether, e – Chloroform, f – Normal butanol, g – Methylbenzene, h – Fermentation broth, I – Methyl alcohol, J – Ethanol, K – 80% ethanol, L – 60% ethanol, M – 40% ethanol, N – 20% ethanol) (a). Maximum absorption peak of pigment (b). Effects of light, temperature, and pH on pigment stability. The wavelength for absorbance was 298 nm (c). Effect of metal ions on pigment stability. The wavelength for absorbance was 298 nm (d). The data represent the average of three independent experiments. Error bars represent the standard deviation. *P < .05, calculated by t test.
3.4. HPLC separation and UV–vis scanning of the pigment components
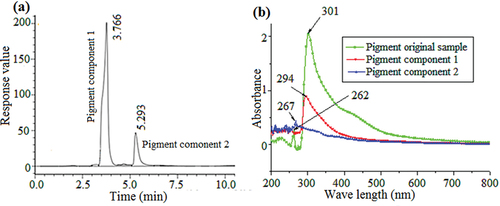
The separation conditions of the pigment HPLC were explored based on solubility and TLC experiments performed at the early stage of pigment analysis. The mobile-phase methanol-water (50:50, v/v) was used for isocratic elution, and the pigment was separated into two components by HPLC (F. W. Li et al., Citation2017; Suwannarach et al., Citation2019), with retention times of 3.766 min and 5.293 min, respectively (). The distillate of the two components was collected at the half-peak, and UV–vis scanning was performed to determine the changes in the absorption spectra of the original pigment and the two separated components. The UV–vis absorption spectra of the original pigment and the two components were significantly different. Both components had only one absorption peak at 294 and 267 nm, and the two absorption peaks were similar to those of the original pigment, indicating that the pigment was composed of two components ().
3.5. GC–MS of the original pigment sample and components
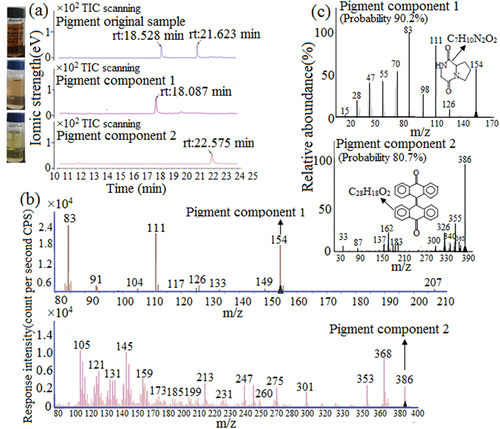
The original pigment sample was analysed using GC–MS, which revealed two peaks on the chromatogram, suggesting the presence of two substances with the retention times of 18.528 and 21.623 min, respectively. Separation of the pigment components 1 and 2 by HPLC and subsequent GC–MS detection revealed their retention times were 18.087 and 22.575 min, respectively. These retention times were similar to those of the two substances separated from the original pigment (), indicating that A. ustus pigment was composed of two components and could vaporize at the set program temperature, the gasification temperatures for pigment components 1 and 2 were 235°C and 265°C, respectively (Zhou et al., Citation2022). MS was used to detect the chemical structure of the pigment components 1 and 2, under the bombardment of ion source energy, the pigment components were cracked into many molecular fragments with a positive charge and entry mass analyzer, showing many different molecular weights and abundances in the mass spectrum. Based on the rules of molecular ion composition and instrumental analysis (Lederberg, Citation1964; Y. X. Zhao & Sun, Citation2010), the m/z of characteristic molecular ion fragments of pigment component 1 in were mainly as follows: 154, 126, 111, 83, response intensity from weak to strong was 126, 154, 111, 83, compared with NIST image library (Peng et al., Citation2013), the matching degree relating to characteristic molecular ion fragments and relative abundance was high, indicating that the structure of pigment component 1 was highly likely to be pyrrolipyrazine diketone (Molecular weight 154 Da), the probability was 90.2% (, pigment component 1). The m/z of characteristic molecular ion fragments of pigment component 2 was mainly as follows (): 386, 368, 353, 275, 247, 213, 145, 105, response intensity from weak to strong was 275, 386, 247, 353, 213, 368, 145, 105, compared with NIST image library (Peng et al., Citation2013), the molecular weight of large molecular ion fragments was similar (386, 355), but there was a certain gap relating to the relative abundance and small molecular weight, hence, the matching degree is not high enough to be dianthrone (molecular weight 386 Da), the probability was 80.7% (, pigment component 2).
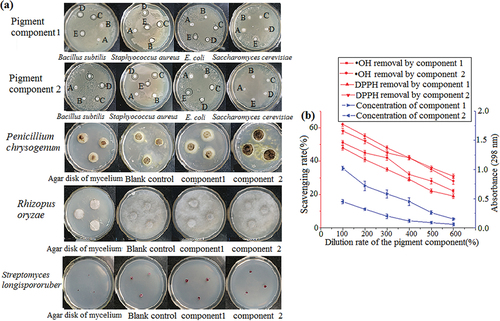
3.6. Inhibition of the microorganisms and antioxidant effects of the pigment components
The inhibition of pigment components 1 and 2 on microorganisms and their antioxidant properties were studied using filter paper and drilling methods. As shown in , transparent rings were observed around the filter paper containing pigment components 1 and 2, indicating that components 1 and 2 had inhibitory effects on Staphylococcus aureus, Bacillus subtilis, and Escherichia coli. The inhibitory effect on gram-positive bacteria was greater than that on gram-negative bacteria, and the two components had no inhibitory effect on Saccharomyces cerevisiae. The mold cake of Penicillium chrysogenum and Rhizopus oryzae was cultured for 72 h in the petri dish containing components 1 and 2, and there were no significant differences in the colony diameter between the control and the experiment, indicating that the pigment components 1 and 2 had no obvious inhibitory effect on filamentous fungi. The Streptomyces longispororuber colony diameter of component 1 was similar to that of the blank control, indicating that pigment component 1 had no evident inhibitory effect on Streptomyces longispororuber. Meanwhile, the Streptomyces longispororuber colony diameter of component 2 was smaller than that of the blank control, indicating that component 2 had a certain inhibitory effect on Streptomyces longispororuber. Pigment components 1 and 2 exhibited good scavenging ability for •OH and DPPH, with the scavenging rate of component 2 reaching more than 60%, and the scavenging ability decreased with the decrease in component concentration. Furthermore, the scavenging rate of pigment component 2 was higher than that of pigment component 1, indicating a stronger antioxidant capacity, as shown in .
Figure 6. Inhibition effect of pigment components 1 and 2 on microorganisms (a). Antioxidant capacity of pigment components 1 and 2. The wavelength for absorbance was 298 nm (b). The data represent the average of three independent experiments. Error bars represent the standard deviation. *P < .05, calculated by t test.
3.7. Brine shrimp acute toxicity bioassay
shows that A. ustus pigment had a certain lethality on brine shrimp larvae. Statistical analysis by SPSS showed that the goodness of fit of the model was P = .963, indicating a good fit. As a general guideline, a crude pigment LC50 >1000 mg/L can be considered nontoxic for brine shrimp (Meyer et al., Citation1982). The LC50 of pigment on brine shrimp was 842 mg/L, which was close to 1000 mg/L, and the 95% confidence limit was 1338–2259 mg/L. Thus, the pigment would be expected to have low toxicity and nontoxicity.
Table 1. Toxicity test of Aspergillus ustus pigment to brine shrimp.
3.8. Phytotoxicity test
To investigate the effect of pigment on plant growth, wheat and broad bean seeds were watered with 0.05% pigment solution and tap water. Figure S1 depicts that the wheat and broad bean seedlings in both the control and experimental groups exhibited green leaves without spots, yellowing, curling, wilting, and discolouration or quenching of stems throughout the growth process. The root system of both plants remained milky white without black rot, indicating that the wheat and broad bean did not exhibit any symptoms of poisoning on the plant organs, even when watered with 0.05% pigment solution. In , the germination rates of wheat and broad bean exceeded 90%, with no significant difference observed between the control and experimental groups. After 7 days of culture, a noticeable difference was observed in the rhizome lengths of wheat and broad bean seedlings between the control and experimental groups, with the experimental group exhibiting slower growth. However, 14 days later, no significant difference was found in the rhizome lengths of wheat between the control and experimental groups, while the rhizome length of the broad bean control group was still better than that of the experimental group. The rhizosphere colony number of the wheat and broad bean groups varied significantly, with the blank group showing significantly higher levels than the experimental group, indicating that the pigment had an evident bacteriostatic effect. Regarding nitrogen content, no significant difference was found between the wheat control group and the experimental group, while broad bean seedlings exhibited significant variation. Legume plants have a closer relationship with nitrogen-fixing bacteria, so they had a greater impact on broad bean seedlings.
Table 2. Effect of Aspergillus ustus pigment on germination rate, stem length, root length, number of rhizosphere colonies, and nitrogen content of rhizosphere.
4. Discussion
Natural pigments have more advantages than synthetic pigments in terms of safety, environmental protection, health, and cost, making them increasingly popular (Darwesh et al., Citation2020; Tirumale & Wani, Citation2018). Microbial pigments, which are an important source of natural pigments, have many advantages, such as quickly and cheaply producing pigments without being affected by the climate environment (Mumtaz et al., Citation2019; Sen et al., Citation2019). Furthermore, microbial pigments have biological activities, such as antibacterial, anticancer, antiradiation, and antioxidative activities, making them the first choice in the food and pharmaceutical industries (Habimana et al., Citation2022; Ramesh et al., Citation2019). However, the practical applications of microbial pigments are limited due to the susceptibility of their stability to external physical and chemical factors, the lack of excellent pigment-producing strains, the presence of accompanying toxins during pigment production, and the difficulty in pigment purification (F. W. Li et al., Citation2017). Filamentous fungi can ferment to produce many commercial products, such as enzymes, antibiotics, feed products, and pigments. However, the presence, solubility, and stability of mycotoxins limit the application of filamentous fungi pigments in food (Ferreira et al., Citation2016; Mourad et al., Citation2020). Broad bean paste is condiment which is fermented mainly by Aspergillus oryzae. It is popular in China, with attractive color and unique flavor (C. Niu et al., Citation2018).
In this study, a filamentous fungus was isolated from broad bean paste and identified as A. ustus, which can rapidly produce pigments with a bright brown colour, strong sensory attraction, and no special odor. The extracellular water-soluble pigment from A. ustus can be easily separated and purified. The water-soluble pigment from microorganisms is generally not accompanied by toxin production (Ramesh et al., Citation2019). The pH value affects natural pigment by altering its structure or composition, resulting in a change of color (Andrés-Bello et al., Citation2013). Additionally, studies have also found that a balance between protonation and deprotonation in natural pigments and synthetic pigments, such as methyl red, exhibiting different colors under different pH value (Andrés-Bello et al., Citation2013; Gong et al., Citation2022). Analyses of A. ustus pigment structure has identified active groups capable of ionization and maintaining equilibrium, which shifts as pH changes and leads to color variations.
Some metal ions can significantly affect the stability of natural pigments and directly react with pigment compounds, potentially leading to color changes or acceleration of oxidation that causes fading. For example, anthocyanin pigments are particularly susceptible to forming complexes with Fe2+, Cu2+, Fe3+, Zn2+, etc., which can alter their color (T. X. Zhao et al., Citation2023). Therefore, A. ustus pigment may form complexes with Zn2+, Fe3+, Fe2+, and Cu2+, resulting in flocculent precipitation. Among these, Fe3+ has strong oxidation properties and both Fe3+ and Fe2+ are easily hydrolyzed, thereby having a greater influence on color change. A. ustus pigment is stable below pH 10, light, and high temperature, and the water-solubility of pigment is the first choice in many foods, cosmetics, and drugs, so the practicality and safety of the pigment are greatly improved.
It has been reported that carotenoids, melanin, riboflavin, Monascus pigments and other microbial pigments can inhibit or remove various pathogens (Dufossé, Citation2018). The A. ustus pigment isolated in this study also has inhibitory effects on Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Streptomyces longispororuber, but there is no obvious inhibition on fungi; therefore, it has certain application potential in preventing bacterial contamination of food. Microbial pigment is usually produced in the cytoplasm, which can resist adverse environmental effects, such as nutrient restriction and ultraviolet irradiation, and has strong antioxidant activity, which helps improve the survival rate of fungi (Pagano & Dhar, Citation2015; Wang et al., Citation2012). The main methods for the determination of free radical scavenging ability based on electron transfer include DPPH method, •OH method, and ABTS method (Y. R. Li et al., Citation2020; D. F. Niu et al., Citation2019). In this study, the antioxidant function of pigment components was qualitatively studied using the DPPH and •OH method, with the pigment component stock solution being diluted to establish a linear relationship between components concentration and scavenging ability. In the antioxidant test, both components of A. ustus pigment have the ability to remove hydroxyl and DPPH radicals, indicating that the pigment has biological activity. The plant rhizosphere is a diverse habitat with a high abundance of microbial communities, and bacterial species are the most dominant microorganisms in the rhizosphere, which colonize the microenvironment for mutual benefit (Zhang et al., Citation2016). Through the plant toxicity test, it was found that the pigment showed no toxic symptoms to the plants, but the growth rate was significantly lower than that of the blank group. Through the detection of rhizosphere bacterial colony number, the number of bacteria in the experimental group was significantly lower than that in the control group, which broke the symbiotic relationship between plants and rhizosphere microorganisms and reduced nitrogen absorption, resulting in slow growth of plants in the experimental group, while nitrogen-fixing bacteria are more important for leguminous plants (Olanrewaju et al., Citation2019), so the influence of pigment on broad bean was greater than that on wheat. The larvae of brine shrimp are sensitive to toxic substances, and the lethal test of brine shrimp is an effective means to preliminarily assess the toxicity of biological toxins, heavy metals, and insecticides. The method has the advantages of simple operation, is rapid and economical, and has been widely used in toxicity tests (Finger et al., Citation2019; Solis et al., Citation1993). The A. ustus pigment in this experiment had certain antibacterial and antioxidant capacities, so it had biological activity, and it has been seen that the toxicity for Artemia is related to bioactivity, the LC50 of this pigment was 820 mg/L, the value was sufficiently large to be considered, in the worst case, as having a low toxicity (caffeine has LC50 = 0.31 g/L on Artemia) (Meyer et al., Citation1982). In summary, the A. ustus pigment fulfils key requirements such as abundant yield, good colouration, water solubility, high stability, convenient extraction, safety, and environmental protection, indicating its significant potential for various applications (Fernández-López et al., Citation2018; Wrolstad & Culver, Citation2012).
Among the natural pigments produced by fungi, the well-known ones are Monascus pigment, carotenoids, and melanin (Alberti et al., Citation2017; Gmoser et al., Citation2017). Carotenoids consist of carotene-containing hydrocarbon elements (such as α-carotene, β-carotene, and lycopene) and lutein-containing hydroxyl, ketone, carboxyl, methoxyl, and other oxygen-containing functional groups, in addition to hydrocarbon elements (Avalos & Limón, Citation2015). Melanin, on the other hand, is a type of negatively charged, high molecular weight, hydrophobic pigment that is oxidized and polymerized by phenolic or indole compounds. Its structure is highly compact, and it usually combines with proteins (Ambrico, Citation2016). Monascus pigment has a similar molecular structure and chemical properties, and it belongs to the polyketone pigments containing anthraquinone, naphthoquinone, and azoquinone, which consist of three organic heterocyclic rings with lactone, unsaturated ketone, and pyrane (R2=O) or pyridine (R2=NH) (Yu et al., Citation2022). Recent research has reported that filamentous fungi, such as Penicillium and Aspergillus, can synthesize anthraquinone and naphthoquinone via the polyketone synthesis pathway (Lin & Xu, Citation2020).
LC/MS, HPLC–MS, and GC-MS are rapid methods for qualitative and quantitative analysis of pigments in different species (Obanolu & Yazc, Citation2021). Analysis software running on MS stations was assisted by the NIST05 MS library (National Institute of Standards and Technology, Gaithersburg, MD, U.S.A.), and manual interpretation identifies MS peaks (Peng et al., Citation2013). In this study, pigment component 1 had a high probability with NIST database (90.2%), so confirmatory experiments were conducted and the standard product of pyrrolipyrazine diketone was detected by GC-MS under the same conditions as pigment component 1. The results showed that the relative retention time and chemical structure were consistent with the characteristics of component 1, and the probability was 95.2%, indicating that pigment component 1 was pyrrolipyrazine diketone with high confidence (Figure S2). The structure of pyrropyrazine diketone is similar to the Azaphilone family and contains a highly oxidized pyrroquinone ring structure (), show orange, have biological activity also, and it could be an Azaphilone derivative. Azaphilone compound exhibits yellow, red colours and possesses several biological activities, such as nerve protection, antibacterial, anticancer, and antioxidant activities (Pavesi et al., Citation2021; Sandeep et al., Citation2013). The biosynthesis mechanism of Azaphilone compounds has not been fully elucidated, but through genome sequencing, it has been found that there is a pathway of Azaphilone synthesis in Aspergillus, which can protect the fungus from the hydrogen peroxide stress generated by itself and enables it to survive in its ecological niche (Pang et al., Citation2020).
Dianthrone, the parent structure, is typically yellow, and its derivatives are mainly found in narrow or pointed leaves of legume shrubs and tomatoes, which are plant polyketone pigments that possess purgative, hemostatic, antibacterial, and antiviral functions (Liu et al., Citation2016; Tong et al., Citation2021). There are few reports about the presence of dianthrone derivative pigments in fungi. The dianthrone structure of pigment component 2 is relatively complex with a larger molecular weight, and only an 80.7% probability with the NIST library () may be a new substance; therefore, it is difficult to accurately characterize using GC-MS. Although mass spectrometry is widely used, it had some limitations in analyzing unknown natural pigment compounds, such as no pole ability, insufficient qualitative ability, low resolution (unit resolution), there are isotopes and other m/z approximate ion interference, and lack of internal standard in the identification of unknown objects (Chen et al., Citation2018; Qiu et al., Citation2019). Hence, more precise chemical structures of the pigment component 2 need further investigation.
5. Conclusions
In this study, a filamentous fungus, DBFL05, with abundant brown pigment was isolated from broad bean paste and identified as A. ustus. The brown pigment was found to be composed of two main fractions, pyrropyrazine diketone and dianthrone, both of which are polyketone pigments. Preliminary tests showed that the pigment is highly water-soluble and has low toxicity. The A. ustus pigment has significant potential for application due to its characteristics of abundant yield, good colouration, extracellular water solubility, high stability, ease of extraction, safety, and bioactivity.
Abbreviations
ITS: Internal transcribed spacer; LC50: Lethal concentration; TLC: Thin-layer chromatography; HPLC: High-performance liquid chromatography; GC–MS: Gas chromatography-mass spectrometer; UV–vis: Ultraviolet–visible; DPPH: 1,1-diphenyl-2-trinitrophenylhydrazine.
Ethical approval
This article does not include any studies conducted by any of the authors with human or animal participants.
Figure S2.
Download TIFF Image (173.9 KB)Figure S1. Effect of Aspergillus ustus pigment on plant growth
Download TIFF Image (825.9 KB)Acknowledgements
We would like to thank Yajun Chen and Xue Fang for their kind support during the sampling process and analysis, and financial support from Dr Yan Zhang’s Funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2023.2207613
Additional information
Funding
References
- Alberti, F., Foster, G. D., & Bailey, A. M. (2017). Natural products from filamentous fungi and production by heterologous expression. Applied Microbiology and Biotechnology, 101(2), 493–500. https://doi.org/10.1007/s00253-016-8034-2
- Ambrico, M. (2016). Melanin, a long lasting history bridging natural pigments and organic bioelectronics. Polymer International, 65(11), 1249–1250. https://doi.org/10.1002/pi.5239
- Andrés-Bello, A., Barreto-Palacios, V., García-Segovia, P., Mir-Be, L. J., & Martínez-Monzó, J. (2013). Effect of pH on color and texture of food products. Food Engineering Reviews, 5(3), 158–170. https://doi.org/10.1007/s12393-013-9067-2
- Avalos, J., & Limón, M. C. (2015). Biological roles of fungal carotenoids. Current Genetics, 61(3), 309–324. https://doi.org/10.1007/s00294-014-0454-x
- Chen, Y., Xie, B., Yang, J., & Sun, Z. (2018). Identification of microbial carotenoids and isoprenoid quinones from Rhodococcus sp. B7740 and its stability in the presence of iron in model gastric conditions. Food Chemistry, 240, 204–211. https://doi.org/10.1016/j.foodchem.2017.06.067
- Cho, Y. J., Park, J. P., Hwang, H. J., Kim, S. W., Choi, J. W., & Yun, J. W. (2002). Production of red pigment by submerged culture of Paecilomyces sinclairii. Letters in Applied Microbiology, 35(3), 195–202. https://doi.org/10.1046/j.1472-765X.2002.01168.x
- Darwesh, O. M., Barakat, K. M., Mattar, M. Z., Sabae, S. Z., & Hassan, S. H. (2019). Production of antimicrobial blue green pigment pyocyanin by marine Pseudomonas aeruginosa. Biointerface Research in Applied Chemistry, 9(5), 4334–4339. https://doi.org/10.33263/BRIAC95.334339
- Darwesh, O. M., Eweys, A. S., & Zhao, Y. S. (2023). Application of environmental-safe fermentation with Saccharomyces cerevisiae for increasing the cinnamon biological activities. Bioresources and Bioprocessing, 10(1), 12. https://doi.org/10.1186/s40643-023-00632-9
- Darwesh, O. M., Ibrahim, A. M., Hesham, S. A., Sulaiman, A. A., & You-Kwan, O. (2020). Isolation and optimization of Monascus ruber OMNRC45 for red pigment production and evaluation of the pigment as a food colorant. Applied Sciences, 10(24), 8867. https://doi.org/10.3390/app10248867
- Dufossé, L. (2018). Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries. Natural and Artificial Flavoring Agents and Food Dyes, 7, 113–132. https://doi.org/10.3390/microorganisms7070186
- Dufosse, L., Fouillaud, M., Caro, Y., Mapari, S. A., & Sutthiwong, N. (2014). Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Current Opinion in Biotechnology, 26(26), 56–61. https://doi.org/10.1016/j.copbio.2013.09.007
- Eweys, A. S., Zhao, Y. S., & Darwesh, O. M. (2022). Improving the antioxidant and anticancer potential of Cinnamomum cassia via fermentation with Lactobacillus plantarum. Biotechnology Reports, 36, e00768. https://doi.org/10.1016/j.btre.2022.e00768
- Fernández-López, J. A., Roca, M. J., Angosto, J. M., & Obón, J. M. (2018). Betaxanthin-rich extract from cactus pear fruits as yellow water-soluble colorant with potential application in foods. Plant Foods for Human Nutrition, 73(2), 146–153. https://doi.org/10.1007/s11130-018-0664-3
- Ferreira, J. A., Mahboubi, A., Lennartsson, P. R., & Taherzadeh, M. J. (2016). Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresource Technology, 215, 334–345. https://doi.org/10.1016/j.biortech.2016.03.018
- Finger, S., Godoy, F. A., Wittwer, G., Aranda, C. P., Calderón, R., & Miranda, C. D. (2019). Purification and characterization of indochrome type blue pigment produced by Pseudarthrobacter sp. 34LCH1 isolated from Atacama desert. Journal of Industrial Microbiology & Biotechnology, 46(1), 101––111. https://doi.org/10.1007/s10295-018-2088-3
- Gmoser, R., Ferreira, J. A., Lennartsson, P. R., & Taherzadeh, M. J. (2017). Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biology and Biotechnology, 4(4), 1–25. https://doi.org/10.1186/s40694-017-0033-2
- Gong, X. B., Luo, H., Wu, X., Liu, H., Sun, C. W., & Chen, S. C. (2022). Production of red pigments by a newly isolated Talaromyces aurantiacus strain with led stimulation for screen printing. Indian Journal of Microbiology, 62(2), 280–292. https://doi.org/10.1007/s12088-022-01008-x
- Habimana, P., Jiang, Y., Gao, J., Ndayambaje, J. B., Darwesh, O. M., & Mwizerwal. (2022). Enhancing laccase stability and activity for dyes decolorization using ZIF-8@MWCNT nanocomposite. Chinese Journal of Chemical Engineering, 48, 66–75. https://doi.org/10.1016/j.cjche.2021.05.044
- Hernández-Almanza, A., Montañez-Sáenz, J., Martínez-Ávila, C., Rodríguez-Herrera, R., & Aguilar, C. N. (2014). Carotenoid production by Rhodotorula glutinis YB-252 in solid-state fermentation. Food Bioscience, 7, 31–36. https://doi.org/10.1016/j.fbio.2014.04.001
- Indra Arulselvi, P., Umamaheswari, S., Ranandkumar, S. G., Karthik, C., & Jayakrishna, C. (2014). Screening of yellow pigment producing bacterial isolates from various eco-climatic areas and analysis of the carotenoid produced by the isolate. Journal of Food Processing & Technology, 5(1), 1–4. https://doi.org/10.4172/2157-7110.1000292
- Kim, M. J., Kwak, H. S., & Kim, S. S. (2018). Effects of salinity on bacterial communities, Maillard reactions, isoflavone composition, antioxidation and antiproliferation in Korean fermented soybean paste (Doenjang). Food Chemistry, 245, 402–409. https://doi.org/10.1016/j.foodchem.2017.10.116
- Kim, Y. E., Matter, I. A., Lee, N., Jung, M., Lee, Y. C., Choi, S. A., Lee, S. Y., Kim, J. R., & Oh, Y. K. (2020). Enhancement of astaxanthin production by Haematococcus pluvialis using magnesium aminoclay nanoparticles. Bioresource Technology, 307, 123270. https://doi.org/10.1016/j.biortech.2020.123270
- Kum, S. J., Yang, S. O., Lee, S. M., Chang, P. S., Choi, Y. H., Lee, J. J., Hurh, B. S., & Kim, Y. S. (2015). Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (Doenjang). Journal of Agricultural and Food Chemistry, 63(5), 1401–1418. https://doi.org/10.1021/jf5056002
- Lederberg, J. (1964). Computation of molecular formulas for mass spectrometry. Holden-Day.
- Li, Z., Dong, L., Huang, Q., & Wang, X. (2016). Bacterial communities and volatile compounds in doubanjiang, a Chinese traditional red pepper paste. Journal of Applied Microbiology, 120(6), 1585–1594. https://doi.org/10.1111/jam.13130
- Lin, L., & Xu, J. (2020). Fungal pigments and their roles associated with human health. Journal of Fungi, 6(4), 280. https://doi.org/10.3390/jof6040280
- Liu, J., Kanetake, S., Wu, Y. H., Tam, C., Cheng, L. W., Land, K. M., & Friedman, M. (2016). Antiprotozoal effects of the tomato tetrasaccharide glycoalkaloid tomatine and the aglycone tomatidine on mucosal trichomonads. Journal of Agricultural and Food Chemistry, 64(46), 8806–8810. https://doi.org/10.1021/acs.jafc.6b04030
- Li, F. W., Xue, F., & Yu, X. (2017). GC–MS, FTIR and Raman analysis of antioxidant components of red pigments from Stemphylium lycopersici. Current Microbiology, 74(4), 532–539. https://doi.org/10.1007/s00284-017-1220-3
- Li, Y. R., Zhou, W. W., & Wang, Z. Y. (2020). Relationship between antioxidant activity and spectrum-effect of hawthorn leaf extracts. Chinese Pharmaceutical Journal, 55(20), 1673–1679. https://doi.org/10.11669/cpj.2020.20.004
- Meyer, B. N., Ferrigni, N. R., Putnam, J. E., Jacobsen, L. B., Nichols, D. E. J., & McLaughlin, J. L. (1982). Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica, 45(5), 31–34. https://doi.org/10.1055/s-2007-971236
- Moukette, B. M., Pieme, C. A., Njimou, J. R., Biapa, C. P. N., Marco, B., & Ngogang, J. Y. (2015). In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biological Research, 48(1), 1–17. https://doi.org/10.1186/s40659-015-0003-1
- Mourad, R., Darwesh, O., & Abdel-Hakim, A. (2020). Enhancing physico-mechanical and antibacterial properties of natural rubber using synthesized ag-sio2 nanoparticles. International Journal of Biological Macromolecules, 164, 3243–3249. https://doi.org/10.1016/j.ijbiomac.2020.08.063
- Mumtaz, R., Bashir, S., Numan, M., Shinwari, Z. K., & Ali, M. (2019). Pigments from soil bacteria and their therapeutic properties: A mini review. Current Microbiology, 76(6), 783–790. https://doi.org/10.1007/s00284-018-1557-2
- Narsing Rao, M. P., Xiao, M., & Li, W. J. (2017). Fungal and bacterial pigments: Secondary metabolites with wide applications. Frontiers in Microbiology, 8, 1113. https://doi.org/10.3389/fmicb.2017.01113
- Niu, C., Fan, Z., Zheng, F., Li, Y., Liu, C., Wang, J., & Li, Q. (2018). Isolation and identification of gas-producing spoilage microbes in fermented broad bean paste. Food Control, 84, 8–16. https://doi.org/10.1016/j.foodcont.2017.07.004
- Niu, D. F., Wang, B., & Zhang, J. (2019). Study on antioxidant activity of different solvent extracts of rape bee pollen and rape bee pollen and rape bee bread. Food Research and Development, 40(6), 42–46. https://doi.org/10.3969/j.issn.10056521.2019.06.008
- Obanolu, E., & Yazc, A. (2021). Isolation, characterization, and antibiofilm activity of pigments synthesized by Rhodococcus sp. sc1. Current Microbiology, 79(1), 15. https://doi.org/10.1007/s00284-021-02694-4
- Olanrewaju, O. S., Ayangbenro, A. S., Glick, B. R., & Babalola, O. O. (2019). Plant health: Feedback effect of root exudates-rhizobiome interactions. Applied Microbiology and Biotechnology, 103(3), 1155–1166. https://doi.org/10.1007/s00253-018-9556-6
- Pagano, M. C., & Dhar, P. P. (2015). Fungal pigments: An overview. In V.K. Mach, R. L. Gupta, & S. Sreenivasaprasad. Fungal bio-molecules: Sources, applications and recent developments (pp.173–181). Wiley.
- Pang, G., Sun, T. T., Yu, Z. Z., Yuan, T., Liu, W., Zhu, H., Gao, Q., Yang, D. Q., Kubicek, C. P., Zhang, J., & Shen, Q. R. (2020). Azaphilones biosynthesis complements the defence mechanism of Trichoderma guizhouense against oxidative stress. Environmental Microbiology, 22(11), 4808–4824. https://doi.org/10.1111/1462-2920.15246
- Pavesi, C., Flon, V., Mann, S., Leleu, S., Prado, S., & Franck, X. (2021). Biosynthesis of azaphilones: A review. Natural Product Reports, 38(6), 1058–1071. https://doi.org/10.1039/D0NP00080A
- Peng, C. X., Wang, Q. P., Liu, H. R., Gao, B., Sheng, J., & Gong, J. S. (2013). Effects of Zijuan pu-erh tea theabrownin on metabolites in hyperlipidemic rat feces by Py-GC/MS. Journal of Analytical and Applied Pyrolysis, 104, 226–233. https://doi.org/10.1016/j.jaap.2013.07.011
- Qiu, X. D., Zhang, X., & Bai, Y. (2019). Application of direct analysis in real time mass spectrometry in environmental pollutant screening. Journal of Chinese Mass Spectrometry Society, 44(2), 146–157. https://doi.org/10.7538/zpxb.2022.0194
- Ramesh, C., Vinithkumar, N. V., Kirubagaran, R., Venil, C. K., & Dufossé, L. (2019). Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms, 7(7), 186. https://doi.org/10.3390/microorganisms_7070186
- Sandeep, K., Nikhil, K., Partha, R., & Sondhi, S. M. (2013). Efficient synthesis of piperazine-2,6-diketone and 4-(1H-indole-2-carbonyl) piperazine-2,6-diketone derivatives and their evaluation for anticancer activity. Medicinal Chemistry Research, 22(10), 4600–4609. https://doi.org/10.1007/s00044-012-0438-7
- Sen, T., Barrow, C. J., & Deshmukh, S. K. (2019). Microbial pigments in the food industry challenges and the way forward. Frontiers in Nutrition, 6(7), 1–14. https://doi.org/10.3389/fnut.2019.00007
- Solis, P. N., Wright, C. W., Anderson, M. M., Gupta, M. P., & Phillipson, J. D. (1993). A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Medica, 59(03), 250–252. https://doi.org/10.1055/s-2006-959661
- Suwannarach, N., Kumla, J., Nishizaki, Y., Sugimoto, N., Meerak, J., Matsui, K., & Lumyong, S. (2019). Optimization and characterization of red pigment production from an endophytic fungus, Nigrospora aurantiaca CMU-ZY2045, and its potential source of natural dye for use in textile dyeing. Applied Microbiology and Biotechnology, 103(17), 6973–6987. https://doi.org/10.1007/s00253-019-09926-5
- Tirumale, S., & Wani, N. A. (2018). Biopigments: Fungal Pigments. In P. Gehlot and J. Singh (Eds.), Fungi and their role in sustainable development: Current Perspectives (pp. 413–426). Springer.
- Tong, S., Wang, H., Sha, A. L., Bai, T. N., Gong, J. H., Jin, W. J., Dai, L. L., Ba, G. N., Cho, S. B., & Fu, M. H. (2021). Protective effect and mechanisms of action of Mongolian medicine Sulongga-4 on pyloric ligation-induced gastroduodenal ulcer in rats. World Journal of Gastroenterology, 27(16), 1770–1784. https://doi.org/10.3748/wjg.v27.i16.1770
- Wang, H. L., Li, P., Liu, Y. F., Ren, Z. F., & Wang, G. (2012). Overproduction of a potential red pigment by a specific self-immobilization biomembrane-surface liquid culture of Penicillium novae-zeelandiae. Bioprocess and Biosystem Engineering, 35(8), 1407–1416. https://doi.org/10.1007/s00449-012-0729-x
- Wolfe, B. E., & Dutton, R. J. (2015). Fermented foods as experimentally tractable microbial ecosystems. Cell, 161(1), 49–55. https://doi.org/10.1016/j.cell.2015.02.034
- Wrolstad, R. E., & Culver, C. A. (2012). Alternatives to those artificial FD&C food colorants. Annual Review of Food Science and Technology, 3(1), 59–77. https://doi.org/10.1146/annurev-food-022811-101118
- Yu, X., ZHANG, W., & WU, Y. J. (2022). Production mechanism and biological activity of microbial pigments. Acta Microbiologica Sinica, 62(4), 1231–1246. https://doi.org/10.13343/j.cnki.wsxb.20210465
- Zhang, B., Li, S., Chen, S., Ren, T., & Han, X. (2016). Arbuscular mycorrhizal fungi regulate soil respiration and its response to precipitation change in a semiarid steppe. Scientific Reports, 6(1), 19990. https://doi.org/10.1038/srep19990
- Zhao, T. X., Min, Y., Luo, J. X., Chen, S. Z., Qiu, B. H., Wang, Y. Q., Qiao, H. Z., & Wang, J. (2023). Antioxidant activity and stability of Rehmannia glutinosa residues pigment. Feed Research. https://kns.cnki.net/kcms/detail//11.2114.S.20230217.1702.011
- Zhao, Y. X., & Sun, X. Y. (2010). Spectral identification of organic molecular structure. Science press.
- Zhou, M. H., Zhang, Y., Chen, Y. J., Zhang, F. Y., & Yang, D. H. (2022). Optimization of the decolorization conditions of Rose Bengal by using Aspergillus niger TF05 and a decolorization mechanism. Microbiology (Reading, England), 168(1), 001128. https://doi.org/10.1099/mic.0.001128