ABSTRACT
There are a variety of bioactive components in rice bran oil (RBO), among which the richer ones include γ-oryzanol, α-tocopherol and sitosterol. The activity of these ingredients is affected by the vehicle and formulation, and there is a significant synergy between these antioxidants. At present, the content of antioxidants in physically refined RBO has reached a high level. Therefore, comparing the activity of physically refined RBO with γ-oryzanol, α-tocopherol, and sitosterol as single components and the above three ingredients dissolved in the same base oil as RBO can provide information for better application of these antioxidants. The results showed that the antioxidant and anti-senescence capacities of physically refined RBO were significantly higher than those of dissolved γ-oryzanol, α-tocopherol, and sitosterol and better than those of the mixture. This study illustrates that the antioxidant and anti-senescence effects of highly active physically refined RBO are superior to those antioxidants derived from RBO.
KEYWORDS:
1. Introduction
Rice bran oil is a kind of vegetable oil that is further pressed or extracted from rice bran produced during rice processing. (Friedman, Citation2013). Oryzanols, tocotrienols, tricin, phytosterols, policosanols, squalene, tocopherols, and ferulic acid are the main bioactive components in RBO (Y. L. Liu et al., Citation2021; Maszewska et al., Citation2018). Many advantages have been found for RBO, such as cholesterol-lowering (Ardiansyah et al., Citation2006), anti-inflammatory (Chen & Cheng, Citation2006), and antioxidant activities (Xu et al., Citation2001). RBO contains 1 to 2% oryzanol, which acts as a powerful antioxidant that can reduce blood lipid levels, thereby lowering the risk of coronary heart disease (Bumrungpert et al., Citation2019). γ-oryzanol is a mixture of ferulic acid esters of phytosterols and triterpenoids (Kim et al., Citation2015), and the components of oryzanol were identified as D7-stigmastenyl ferulate, stigmasteryl ferulate, cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, D7-campestenyl ferulate, campesteryl ferulate, D7-sitotenyl ferulate, sitosteryl ferulate, campestanyl ferulate, and sitostanyl ferulate (Xu & Godber, Citation1999). In addition to γ-oryzanol, several studies have shown that α-Tocopherol functions as a chain-breaking antioxidant that prevents the production of free radical reactions (Muñoz & Munné-Bosch, Citation2019), and sitosterol is a relatively moderate antioxidant and exerts beneficial effects in vitro and in vivo by decreasing the level of reactive oxygen species (Yang et al., Citation2013).
At present, γ-oryzanol, α-tocopherol and sitosterol can be extracted and isolated from plant oils using different methods. Among them, γ-oryzanol has become a clinical drug, and α-tocopherol and sitosterol are also being utilized in the development of clinical drugs (Fernandez & Vega-López, Citation2005; Pang & Chin, Citation2019; Ramazani et al., Citation2021). However, during the preparation of rice bran oil and the extraction and isolation of these natural products, many factors affect its biological activity (Aryusuk et al., Citation2008; Junyusen et al., Citation2022; R. J. Liu et al., Citation2019). Owing to several technical and nontechnical problems in oil refining, the actual annual production of RBO does not meet demand (Pestana-Bauer et al., Citation2012). The major difficulties in processing crude RBO for edible purposes are its high levels of FFA, waxes, gums, and pigments. However, the chemical and physical deacidification process causes the loss of bioactive compounds such as γ-oryzanol and α-tocopherol.
Based on the components content in RBO, it is possible that the antioxidant capacity and biological activity of high-quality physically refined RBO is partially inferior to those of γ-oryzanol, α-tocopherol and sitosterol purified products at the same dose. Moreover, many studies have shown that the ratio between different antioxidants in RBO affects its bioactivity (R. R. Liu et al., Citation2020). When the ratio of α-tocopherol and β-carotene in liposomes was near 1:1, the synergistic effect was decreased, and when the ratio of α-tocopherol and myricetin was 1:1, the strongest synergistic effect was achieved (R. R. Liu et al., Citation2020). In addition, the total concentration of the mixture also affects the bioactivity. In this study, we compared the antioxidant and antisenescence activities of physically refined rice bran oil and purified γ-oryzanol, α-tocopherol, sitosterol, and a combination of these three active ingredients.
2. Methods
2.1. Materials and the preparation of samples
Physically refined RBO was obtained from HanKang Food Co., Ltd. (Jiangsu, China). γ-oryzanol (1479202, United States Pharmacopeia Reference Standard), α-tocopherol (258024, purity ≥ 95.5%) and sitosterol (1612947, United States Pharmacopeia Reference Standard) were purchased from Sigma‒Aldrich (Bellefonte, PA, U.S.A). Palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1n9c), and linoleic acid (C18:2n6c) were purchased from J&K Scientific (China). The volume ratios of fatty acids and the concentration of α-tocopherol, γ-oryzanol and sitosterol were determined through analyzing of physically refined rice bran oil according to Chinese standard methods (GB/T 25,223–2010 for the determination of individual and total sterols contents, GB/T 26,635–2011 for the determination of tocopherol and tocotrienol contents, LS/T 6121.1–2017 for the determination of γ-oryzanol content, and GB 5009 168–2016 for the determination of fatty acids contents). The base oil was prepared by mixing different fatty acids in the following volume ratios: C16:0 19%, C18:0 4%, C18:1n9c 42%, and C18:2n6c 35%. The single ingredient sample was prepared by adding a single component to the base oil, and the final concentrations of the three minor constituents were as follows: α-tocopherol 228 mg/kg, γ-oryzanol 20,300 mg/kg, and sitosterol 10,490 mg/kg. The mixed sample was obtained by mixing the above three single components together and then adding them to the base oil (γ-oryzanol plus α-tocopherol and sitosterol, OTS). The samples were mixed by mechanical vortexing and stored at 4°C.
2.2. Oxidative stability
The oxidative stability was determined using a 743 Rancimat apparatus (Metrohm Instruments, Herisau, Switzerland) (R. R. Liu et al., Citation2020), utilizing a sample of 2.50 g ± 0.01 g. A flow of air (20 L/h) was bubbled through the oil samples heated at 100°C, 110°C or 120°C. The test was applied in triplicate. The volatile organic acids from the oil sample were collected in cold water to increase the conductivity, and the conductivity was recorded continuously. The induction times [h] were printed automatically by the apparatus software with an accuracy of 0.005. The induction time is related to the antioxidant effect of the oil sample, and the longer the induction time, the stronger the antioxidant effect of the oil.
2.3. Scavenging capacity test
The scavenging capacity test were conducted as described previously (R. R. Liu et al., Citation2020). Briefly, a 0.1 mmol/L 2-Diphenyl-1-picrylhydrazyl (DPPH) solution was first prepared in ethyl acetate, and the samples were prepared at 25 mg/mL in ethyl acetate. A 2.0 mL sample solution was mixed with 2.0 mL DPPH solution, the mixtures were reacted in the dark at room temperature for 30 min, and the absorbance at 517 nm was recorded using a spectrophotometer. The experimental scavenging capacity (ESC) was calculated from the following equation: ESC = [1 - (Ai - Aj) A0] * 100%. where A0, Ai, and Aj are the absorbance values of 2.0 mL ethyl acetate + 2.0 mL DPPH solution, 2.0 mL sample solution + 2.0 mL DPPH solution and 2.0 mL sample solution + 2.0 mL ethyl acetate, respectively. The scavenging capacity is related to the antioxidant capacity of the sample, and the higher the scavenging capacity, the stronger the antioxidant capacity of the sample.
2.4. Analysis of the reducing capacity
The reducing capacity test were conducted as described previously (Arab et al., Citation2011). Different oil samples (0.375, 0.75, 1.5, 3.125, 6.25, 12.5, 25, 50 mg/ml) were mixed with phosphate buffer (2.5 ml, 2.0 M, pH 6.6), and then mixed with 2.5 ml of 1% potassium ferricyanide and incubated at 50°C for 20 min. 2.5 ml of 10% trichloroacetic acid was added to the mixture. After centrifugation for 10 min, the upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and 1% ferric chloride (0.5 ml), and the absorbance at 700 nm was recorded. The reducing capacity is related to the antioxidant capacity of the sample, and the higher the absorbance at 700 nm, the stronger the antioxidant capacity of the sample.
2.5. Determination of reactive oxygen species (ROS) in cells
The effect of the samples on excessive ROS generation was detected in 293T cells. 293T cells were exposed to 2 mM H2O2 for 30 min and incubated for 2 days in the presence of 0.1% (v/v) H2O2. The intracellular ROS were measured with a 2“,7”-dichlorodihydrofluorescein diacetate (DCFDA) cellular ROS detection assay kit according to the manufacturer’s instructions (Aranda et al., Citation2013). The intracellular fluorescence intensity is related to the amount of ROS in the cell, and the higher the DCFDA fluorescence intensity represents the more ROS in the sample.
2.6. Animals and treatments
Male C57BL/6J mice (18 g, 6–8 wk) and Sprague‒Dawley rats (180–200 g) were obtained from Vital River Laboratory Animal Technology (Beijing, China). All animal protocols conformed to the Guidelines for the Care and Use of Laboratory Animals approved by the Animal Care and Use Committee of the Chinese Academy of Medical Sciences and Peking Union Medical College. Mice had free access to food and water and were housed in an air-conditioned room with a 12-hr/12-hr light – dark cycle. The mice were divided randomly into seven groups (n = 10 each): the blank control group, model group, γ-oryzanol group, α-tocopherol group, sitosterol group, combination group (γ-oryzanol plus α-tocopherol and sitosterol, OTS), and the RBO group. Except for the blank control group, all groups were subcutaneously injected with D-galactose (D-gal) (120 mg kg−1 d−1) dissolved in normal saline solution (6%, w/v) for 8 weeks. All mice were orally gavaged with 2.5 ml/kg test samples. All animals were regularly monitored for weight on a weekly basis, and the dose was adjusted accordingly.
2.7. Morris water maze task
The Morris water maze equipment was used to test the learning and memory ability of mice in each group (Barnhart et al., Citation2015). The pool is 50 cm high, 120 cm in diameter, and 10 cm in diameter on the platform. The pool is divided into quadrants I, II, III, and IV. There is a video camera connected to the computer above the maze. The activity was tracked throughout the whole process, and the activity track of the experimental mice was displayed and analyzed through special computer software. The time required for each group of mice to successfully find the platform after the same learning training was recorded as the escape latency, and the number of times that each group of mice crossed the platform after the platform was removed was calculated. Learning and training: on the first day, the mice of each group were allowed to enter the water from different quadrants of the pool and swim freely for 2 min to familiarize themselves with the water maze environment. The swimming time was set to 2 min, and the mice were allowed to stand on the platform for 10 seconds to consolidate their memory each time they found the platform. Orientation navigation experiment: orientation navigation tests were carried out on the 2nd–6th day, and the result on the 6th day was used as the experimental data of this stage. At the same time, every day, the mice were put into the pool from 4 entry points facing the pool wall in turn, and the time required for them to successfully find the platform (escape latency) was calculated. Space exploration experiment: on the 7th day, the platform was removed, the mice were placed in the pool from the fourth quadrant, the movement of the mice was recorded within 2 min, and the number of times the mouse crossed the original platform was calculated. The escape latency time is related to the memory function and learning ability of the mouse, and longer escape latency time indicates worse memory function and learning ability.
2.8. Western blotting
Proteins were extracted from liver tissue using Radio-Immunoprecipitation Assay (RIPA) buffer containing 1% NP-40 and 1% sodium deoxycholate (Cell Signaling Technology, Danvers, MA). Protein concentrations were determined using a BCA Protein Assay Kit. SDS‒PAGE and Western blotting were conducted as described previously (Lv et al., Citation2022).
2.9. Cytokines and oxygen free radical damage analysis
The contents of cytokine and oxygen free radical markers in serum were performed according to the manufacturer’s instructions (R&D system, catalog M2000 for IL-2, M4000B for IL-4, DY442 for IL-8, and DY1679 for TGF-β. Beyotime, catalog S0131S for malondialdehyde (MDA), S0101S for superoxide dismutase (SOD), S0056 for phospholipid hydroperoxide glutathione peroxidase (GSH-Px)).
2.10. Tissue index
Tissue index was determined by thymus (mg) or spleen (mg) versus body weight (g).
2.11. Statistics
Data are expressed as the mean ± standard error of the mean (SEM). Groups were compared by one-way ANOVA followed by a Tukey‒Kramer’s multiple comparisons test. Comparisons between two groups were performed by unpaired Student’s t tests. A P value < .05 was considered to be significant. The Prism 9 was used for the statistical analysis in this study.
3. Results and discussion
3.1. The antioxidant capacity of RBO and its active ingredients
Because the free radical scavenging ability of antioxidants is usually affected by solvents, the same amount of γ-oryzanol, α-tocopherol and sitosterol as rice bran oil were dissolved in a base oil that has almost the same fatty acid composition as rice bran oil. In addition, a mixed sample of γ-oryzanol, α-tocopherol and sitosterol with the same content as RBO was prepared also. In this work, the Rancimat test and DPPH free radical scavenging method were performed to study the antioxidant capability of different samples. The antioxidant capacity in the Rancimat test of the samples could be generally ranked as follows: RBO > OTS > α-Tocopherol > γ-Oryzanol > Sitosterol at 100°C, 110°C and 120°C ( and ). The DPPH scavenging capacity and reducing power of RBO were significantly higher than those of γ-oryzanol and sitosterol (). The reducing power of RBO and other bioactive ingredients increased with increasing concentration ( and ). The highest values of absorption for reducing power were observed in the RBO group (). In addition, all the bioactive ingredients could decrease the level of ROS in 293T cells induced by H2O2 (), and among them, RBO showed the best ROS-clearing function ().
Figure 1. Antioxidant capacity of RBO and its active ingredients. (a) antioxidation stability of different samples by Rancimat test at 100°C, 110°C and 120°C. (b) DPPH radical scavenging activity of different samples. (c) Reducing capacity of different samples (10 mg/mL). (d) Reducing capacity of base oil, γ-oryzanol, α-tocopherol, sitosterol, OTS, and RBO at different concentration. All data are presented as Mean ± SEM, (n = 3, 3 independent experiments with 2 technical replicates). *P < .05, **P < .01, ***P < .001. RBO: rice bran oil, OTS: γ-oryzanol plus α-tocopherol and sitosterol.
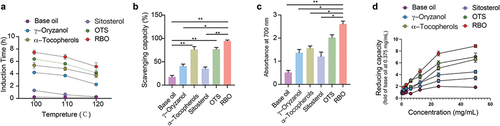
Figure 2. RBO can effectively scavenge oxygen free radicals. (a) Fluorescence images of DCF-DA stained HEK293T cells with indicated treatment after simulated with 2 mM H2O2., Scale bar = 50 µm. (b) DCF-DA fluorescence in HEK293T cells with indicated treatment after simulated with 2 mM H2O2. All data are presented as Mean ± SEM, (n = 3, 3 independent experiments with 3 technical replicates). *P < .05, **P < .01, ***P < .001. RBO: rice bran oil, OTS: γ-oryzanol plus α-tocopherol and sitosterol.
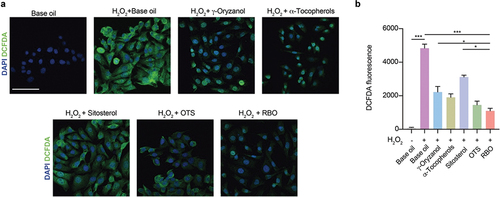
Table 1. The antioxidation stability of different samples by Rancimat test at 100°C, 110°C and 120°C.
Table 2. The reducing capacity of base oil, γ-oryzanol, α-tocopherol, sitosterol, OTS, and RBO at different concentration.
3.2. RBO inhibits oxygen free radical damage in senescent mice
Eight weeks after D-gal stimulation, the model group had a significant decrease in body weight compared with the control group, and the administration of RBO prohibited D-gal-induced weight loss (). The thymus index and spleen index of the model group were also significantly decreased compared with those of the control group. However, the administration of RBO increased the thymus index and spleen index compared to the D-gal-induced aging group ().
Figure 3. RBO improves antioxidant status in D-gal-induced senescence mice. (a) Changes in body mass in mice after indicated treatment of D-gal injured mice. Organ Index of thymus (b) and spleen (c) in D-gal injured mice after indicated treatment. Levels of SOD (d), MDA (e) and GSH-Px (f) in serum in D-gal mice. All data are presented as Mean ± SEM, (n = 8). *P < .05, **P < .01, ***P < .001. RBO: rice bran oil, OTS: γ-oryzanol plus α-tocopherol and sitosterol.
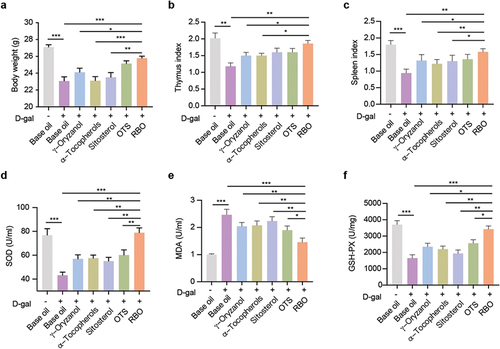
The oxidative damage marker Malondialdehyde (MDA) levels were significantly increased in the serum of aging mice (), whereas the activities of antioxidative marker superoxide dismutase (SOD) and phospholipid hydroperoxide glutathione peroxidase (GSH-Px) were significantly decreased in D-gal mice compared with the control group (). After RBO treatment, the activities of GSH-Px and SOD were increased, and the MDA level was decreased, and RBO treatment restored these indices to values closest to the control group compared with the other active ingredient treatments ().
3.3. RBO improves immune status in aging mice
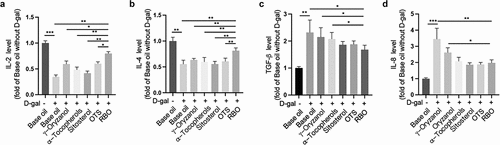
Immunosenescence-related inflammation in aging is mainly characterized by upregulation of proinflammatory cytokines or chemokines (such as TGF-β and IL-8) and downregulation of anti-inflammatory cytokines (such as IL-2 and IL-4). The levels of serum IL-2 and IL-4 were increased in RBO-treated mice compared with D-gal-exposed mice (). In contrast, the levels of serum TGF-β and IL-8 were reduced in RBO-treated mice compared with D-gal-treated mice (). Notably, there was no remarkable difference in the intervention effects on IL-2, IL-4 and TGF-β between the γ-oryzanol, α-tocopherol or sitosterol alone treatment groups and the model group (). In summary, these findings consistently demonstrated that RBO could effectively regulate the immune environment in mice exposed to D-gal.
Figure 4. RBO attenuates D-gal induced inflammation in mice. The level of IL-2 (a), IL-4 (b), TGF-β (c), and IL-8 (d) in serum of senescence mice after indicated treatment. All data are presented as Mean ± SEM, (n = 8). *P < .05, **P < .01, ***P < .001. RBO: rice bran oil, OTS: γ-oryzanol plus α-tocopherol and sitosterol.
3.4. Effect of RBO on spatial learning and memory
The Morris water maze (MWM) test was used to assess the spatial learning and memory functions of D-gal-treated senescent mice. The results suggested that during the 6-day training period, all mice showed a gradually shortened latency to locate the hidden platform. However, D-gal-exposed mice spent more time reaching the platform than control mice (), indicating that long-term exposure to D-gal significantly reduced the learning and memory abilities of mice. In addition, the number of target platform crossings in D-gal-exposed aging mice was dramatically reduced compared with that in the control group (), indicating a high possibility of impairment of learning and memory functions. Notably, impaired cognitive ability was effectively rehabilitated by RBO, as indicated by the escape latency and platform crossover number ( and ). However, γ-oryzanol or α-tocopherol and sitosterol alone did not effectively alleviate D-gal-induced learning and memory impairment ( and ).
Figure 5. RBO alleviates learning and memory impairment of D-gal exposed mice. (a) the escape latency of the mice during MWM training. (b) the number of platform crossing of the mice during MWM training. All data are presented as Mean ± SEM, (n = 8). *P < .05. RBO: rice bran oil, OTS: γ-oryzanol plus α-tocopherol and sitosterol.
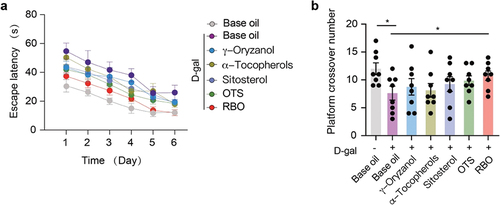
Table 3. The escape latency of the mice during MWM training.
3.5. RBO attenuates cellular senescence by inhibiting the p53/p21 pathway
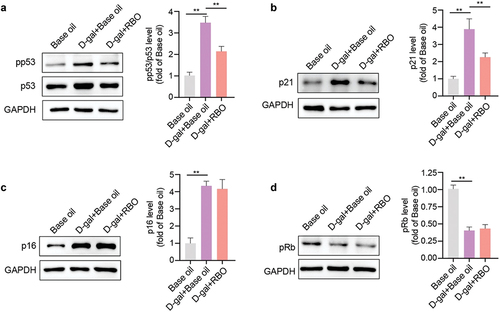
The p53/p21 and p16/Rb pathways are involved in cell senescence. We speculated whether RBO could inhibit cellular senescence by inhibiting the p53/p21 or p16/Rb pathway. After stimulation with D-gal, the pp53, p21, and p16 levels were increased, and pRb was decreased in the liver tissue of mice (), which indicates that D-gal induces senescence. The results showed that RBO significantly decreased the expression of pp53, p53 and p21 () but not p16 and pRb (). These data illustrate that RBO prevents cellular damage by blocking the p53/p21 pathway.
Figure 6. RBO inhibits p53/p21 pathway in D-gal exposed mice. The protein level of pp53/p53 (a), p21 (b), p16 (c), and pRb in liver of D-gal injured mice were detected using western blot after RBO treatment. All data are presented as Mean ± SEM, (n = 8). *P < .05, **P < .01, ***P < .001. RBO: rice bran oil.
4. Discussion
Plant oil contains many antioxidant and anti-inflammatory components, especially RBO, which contains a high abundance of γ-oryzanol, α-tocopherol or sitosterol (Maszewska et al., Citation2018). These bioactive components have shown good antioxidant, anti-inflammatory, and hypolipidemic effects in both in vitro and in vivo studies. However, the antioxidative and antisenescence effects produced by these single components extracted and isolated from RBO are not as good as those produced by natural physically refined RBO. Even if γ-oryzanol, α-tocopherol and sitosterol are mixed and redissolved in base oil according to the content of each component in RBO, their biological activity is still inferior to that of natural physically refined rice bran oil.
It has been confirmed that γ-oryzanol in oil form would be more bioactive than γ-oryzanol dissolved in corn oil, followed by crystalline γ-oryzanol (Dai, Citation2004). The results showed that the bioavailability of crystalline γ-oryzanol was lower than that of crystalline γ-oryzanol dissolved in corn oil, and the solvents largely affected the biological activity of γ-oryzanol. Crystalline γ-oryzanol might be excreted in much more intact forms than the dissolved oil form. Thus, γ-oryzanol is best consumed after being dissolved in vegetable oil. In addition, the bioavailability of γ-oryzanol in RBO is higher than that of the dissolved form of crystalline γ-oryzanol in corn oil since the compatibility of γ-oryzanol in RBO is higher than that in corn oil (Dai, Citation2004). Moreover, a large number of studies have shown that there is a clear synergistic effect between γ-oryzanol, α-tocopherol and phytosterol (R. R. Liu et al., Citation2020, Citation2022), so the combined use of γ-oryzanol, α-tocopherol and phytosterol in a certain proportion range can produce better antioxidant and anti-inflammatory effects. In our study, base oils with the same fatty acid composition as rice bran oil was used to dissolve γ-oryzanol, α-tocopherol or sitosterol. At the same time, the three bioactive components were dissolved in the base oil in the same proportion and concentration as rice bran oil to observe their antioxidant and anti-senescence effects. Unexpectedly, the results showed that simply dissolving γ-oryzanol in the base oil was far less effective than physically refining RBO. Even the OTS group had slightly worse antioxidant effects than RBO. This illustrates that the process of preparing pure γ-oryzanol, α-tocopherol or sitosterol may lead to a loss of activity. At the same time, we cannot rule out the synergistic effect of other active ingredients in RBO. In addition to γ-oryzanol, α-tocopherol or sitosterol and unsaturated fatty acids, RBO is rich in 24-methylenecholesterol, stigmasterol, γ-tocopherol, squalene, α-tocotrienol, and γ-tocotrienol, and these ingredients have certain antioxidant and anti-senescence effects also. Therefore, the antioxidant and anti-senescence effects of composition of ingredients are not as good as natural RBO. However, it is very difficult to study the synergy and mechanism between all the components in RBO.
ROS play important roles in tissue homeostasis, cellular signaling, differentiation, and survival (Schieber & Chandel, Citation2014). Overproduction of ROS is associated with the development of various human diseases. However, upregulation of antioxidant systems in cells can effectively scavenge ROS. MDA acts as a hallmark of oxidative damage in lipids. And the anti-oxidative system consists of reducing substances and anti-oxidative enzymes, such as, GSH-Px and SOD. RBO has a good antioxidant effect both in vitro and in vivo. In addition, the antioxidant effect of RBO in senescent mice was accompanied by weight gain and immune regulation. After RBO administration, the levels of IL-2 and IL-4 were significantly increased in senescent mice, while the levels of aging-inducing factors TGF-β and IL-8 were significantly decreased. This indicates that the anti-senescence effects of RBO not just dependent on antioxidation. More importantly, RBO has the effect of improving cognitive function, which fully shows that RBO is not only a nutrient, but also an active ingredient with clinical application prospects. Our results show that there is indeed a synergistic effect between these ingredients, but physically refined RBO which containing the same concentration of active ingredients has better antioxidant and anti-senescence effects than the manually combined active ingredients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arab, F., Alemzadeh, I., & Maghsoudi, V. (2011). Determination of antioxidant component and activity of rice bran extract. Scientia Iranica, 18(6), 1402–1406. https://doi.org/10.1016/j.scient.2011.09.014
- Aranda, A., Sequedo, L., Tolosa, L., Quintas, G., Burello, E., Castell, J. V., & Gombau, L. (2013). Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicology in Vitro, 27(2), 954–963. https://doi.org/10.1016/j.tiv.2013.01.016
- Ardiansyah, Shirakawa, H., Koseki, T., Ohinata, K., Hashizume, K., & Komai, M. (2006). Rice bran fractions improve blood pressure, lipid profile, and glucose metabolism in stroke-prone spontaneously hypertensive rats. Journal of Agricultural and Food Chemistry, 54(5), 1914–1920. https://doi.org/10.1021/jf052561l
- Aryusuk, K., Puengtham, J., Lilitchan, S., Jeyashoke, N., & Krisnangkura, K. (2008). Effects of crude rice bran oil components on alkali-refining loss. Journal of the American Oil Chemists’ Society, 85(5), 475–479. https://doi.org/10.1007/s11746-008-1215-0
- Barnhart, C. D., Yang, D. R., & Lein, P. J. (2015). Using the Morris water maze to assess spatial learning and memory in weanling mice. PloS One, 10(4), e0124521. https://doi.org/10.1371/journal.pone.0124521
- Bumrungpert, A., Chongsuwat, R., Phosat, C., & Butacnum, A. (2019). Rice bran oil containing gamma-oryzanol improves lipid profiles and antioxidant status in hyperlipidemic subjects: A randomized double-blind controlled trial. The Journal of Alternative and Complementary Medicine, 25(3), 353–358. https://doi.org/10.1089/acm.2018.0212
- Chen, C. W., & Cheng, H. H. (2006). A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. The Journal of Nutrition, 136(6), 1472–1476. https://doi.org/10.1093/jn/136.6.1472
- Dai, Z. L., (2004). Fecal steroid excretion of rats fed rice bran oil and oryzanol [LSU Master’s Theses]. 4303.
- Fernandez, M. L., & Vega-López, S. (2005). Efficacy and safety of sitosterol in the management of blood cholesterol levels. Cardiovascular Drug Reviews, 23(1), 57–70. https://doi.org/10.1111/j.1527-3466.2005.tb00157.x
- Friedman, M. (2013). Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. Journal of Agricultural and Food Chemistry, 61(45), 10626–10641. https://doi.org/10.1021/jf403635v
- Junyusen, T., Chatchavanthatri, N., Liplap, P., Junyusen, P., Phan, V. M., & Nawong, S. (2022). Effects of extraction processes on the oxidative stability, bioactive phytochemicals, and antioxidant activity of crude rice bran oil. Foods, 11(8), 1143. https://doi.org/10.3390/foods11081143
- Kim, H. W., Kim, J. B., Cho, S. M., Cho, I. K., Li, Q. X., Jang, H. H., Lee, S. H., Lee, Y. M., & Hwang, K. A. (2015). Characterization and quantification of γ-oryzanol in grains of 16 Korean rice varieties. International Journal of Food Sciences and Nutrition, 66(2), 166–174. https://doi.org/10.3109/09637486.2014.971226
- Liu, R. J., Liu, R. R., Shi, L. K., Zhang, Z. Y., Zhang, T., Lu, M. Y., Chang, M., Jin, Q. Z., & Wang, X. G. (2019). Effect of refining process on physicochemical parameters, chemical compositions and in vitro antioxidant activities of rice bran oil. LWT-Food Science and Technology, 109, 26–32. https://doi.org/10.1016/j.lwt.2019.03.096
- Liu, R. R., Xu, Y., Chang, M., Tang, L., Lu, M. Y., Liu, R. J., Jin, Q. Z., & Wang, X. G. (2020). Antioxidant interaction of α-tocopherol, γ-oryzanol and phytosterol in rice bran oil. Food Chemistry, 343, 128431. https://doi.org/10.1016/j.foodchem.2020.128431
- Liu, R. R., Xu, Y., Zhang, T., Gong, M. Y., Liu, R. J., Chang, M., & Wang, X. G. (2022). Interactions between liposoluble antioxidants: A critical review. Food Research International, 155, 111104. https://doi.org/10.1016/j.foodres.2022.111104
- Liu, Y. L., Zhang, H. B., Yi, C. P., Quan, K., & Lin, B. P. (2021). Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chemistry, 342, 128352. https://doi.org/10.1016/j.foodchem.2020.128352
- Lv, X. X., Liu, C., Liu, S. S., Li, Y. X., Wang, W. Y., Li, K., Hua, F., Cui, B., Zhang, X. W., Yu, J. J., Yu, J. M., & Hu, Z. W. (2022). The cell cycle inhibitor P21 promotes the development of pulmonary fibrosis by suppressing lung alveolar regeneration. Acta pharmaceutica Sinica B, 12(2), 735–746. https://doi.org/10.1016/j.apsb.2021.07.015
- Maszewska, M., Florowska, A., Dłużewska, E., Wronia, M., Marciniak, K., & Żbikowska, A. (2018). Oxidative stability of selected edible oils. Molecules, 23(7), 1746. https://doi.org/10.3390/molecules23071746
- Muñoz, P., & Munné-Bosch, S. (2019). Vitamin E in plants: Biosynthesis, transport, and function. Trends in Plant Science, 24(11), 1040–1051. https://doi.org/10.1016/j.tplants.2019.08.006
- Pang, K. L., & Chin, K. Y. (2019). The role of tocotrienol in protecting against metabolic diseases. Molecules, 24(5), 293. https://doi.org/10.3390/molecules24050923
- Pestana-Bauer, V. R., Zambiazi, R. C., Mendonça, C., Beneito-Cambra, M., & Ramis-Ramos, G. (2012). γ-Oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chemistry, 134(3), 1479–1483. https://doi.org/10.1016/j.foodchem.2012.03.059
- Ramazani, E., Akaberi, M., Emami, S. A., & Tayarani-Najaran, Z. (2021). Biological and pharmacological effects of gamma-oryzanol: An updated review of the molecular mechanisms. Current Pharmaceutical Design, 27(19), 2299–2316. https://doi.org/10.2174/1381612826666201102101428
- Schieber, M., & Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Current Biology, 24(10), R453–62. https://doi.org/10.1016/j.cub.2014.03.034
- Xu, Z., & Godber, J. S. (1999). Purification and identification of components of γ-Oryzanol in rice bran oil. Journal of Agricultural and Food Chemistry, 47(7), 2724–2728. https://doi.org/10.1021/jf981175j
- Xu, Z., Hua, N., & Godber, J. S. (2001). Antioxidant activity of tocopherols, tocotrienols, and γ-Oryzanol components from rice bran against cholesterol oxidation accelerated by 2,2‘-Azobis(2-methylpropionamidine) dihydrochloride. Journal of Agricultural and Food Chemistry, 49(4), 2077–2081. https://doi.org/10.1021/jf0012852
- Yang, C., Chen, Z. Y., Wong, S. L., Liu, J., Liang, Y. T., Lau, C. W., Lee, H. K., Huang, Y., & Tsang, S. Y. (2013). β-Sitosterol oxidation products attenuate vasorelaxation by increasing reactive oxygen species and cyclooxygenase-2. Cardiovascular Research, 97(3), 520–532. https://doi.org/10.1093/cvr/cvs370
