?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Development of two biosensors to detect biogenic amines was carried out using immobilized diamine oxidase from Pisum sativum on 3-APTMS-functionalized SiO2 substrates with either glutaraldehyde or terephthalaldehyde as crosslinkers. The assembly process was validated by FT-IR, SEM-EDX, and AFM. Biosensor responses to biogenic amines were c.a. 3-fold lower compared to the free enzyme kinetics as assessed by UV/VIS spectrophotometry. The calculated initial velocities (ΔAbs·min-1) for glutaraldehyde and terephthalaldehyde biosensors in the given order were (3.2 ± 0.68) × 10–2, (0.48 ± 0.09) × 10–2 for putrescine; (4.6 ± 1.2) × 10–2, (0.35 ± 0.47) × 10–2 for cadaverine; (5.8 ± 2.4) × 10–3, (19.0 ± 155) × 10–3 for spermidine, and (3.0 ± 2.0) × 10–4, (0.0 ± 7) × 10–4for histamine. The 2-way t-tests indicated that the crosslinker type affected the catalytic activity of immobilized diamine oxidase as the responses of glutaraldehyde biosensors to putrescine and cadaverine were significantly higher than those of the terephthalaldehyde. Molecular Docking simulations showed that glutaraldehyde has a stronger affinity for DAO’s lysine and arginine residues while terephthalaldehyde to phenylalanine.
Introduction
Biogenic amines (BA) are biologically active polycations that can occur in food due to spoiling microbiota. As mono-, di- and poly-amines, these molecules can be either aliphatic, polycyclic, or aromatic. Cadaverine [Cad], putrescine [Put], spermine [Spm] and spermidine [Spdm] of the former kind; histamine [His] of the second type and tyramine [Tyr] and phenylethylamine [Phe] of the latter are some examples. Specifically, when His is ingested, it can elicit moderate-to-severe allergic-like symptoms, reason why food standards worldwide aim to control BA presence in food where they are most frequently in higher concentration, such as canned fish, semi-preserved fish, dry-fermented sausages, and raw milk cheese (Bover-Cid et al., Citation2014). Other biogenic amines can be found in varied food like vegetables, legumes, cured meat, cereals, wine, and beer, among others; these can produce hypertension, migraines and exacerbate the symptoms of histamine food poisoning.
It has been reported that those who eat fish with high His doses (≥50 mg·100 g−1) are at a higher risk of intoxication (Lehane & Olley, Citation2000). In general, ingestion of His doses higher than 400 mg·Kg−1 of body weight is considered to be dangerous, while doses of 1000 mg·Kg−1 trigger extremely dangerous intoxications (Rauscher-Gabernig et al., Citation2009). In animal models, it has been demonstrated that Tyr and Cad possess low toxicity in rats (<2000 mg·Kg−1) while Spdm and Spm’s is 600 mg·Kg−1 when given orally (Pegg, Citation2013; Zou et al., Citation2022).
Currently, the detection and quantification of BA in food are mostly based on chromatography. Both HPLC and GC have been extensively used for this purpose, more recently UHPLC has widely been implemented, saving run time (Tırıs et al., Citation2023). In both cases, a sample extraction and further derivatization is needed. In HPLC, a fluorescent compound, such as dansyl chloride, must be bonded to BA so they can be detected, on the other hand, GC derivatizing agents are used to ensure a proper elution because of their lack of volatility (Munir & Badri, Citation2020).
Biosensors are another approach to detect BA in food with remarkable advantages. They provide versatility as different types of biorecognition elements, transducers, and signal processing can be chosen according to the given needs. Antibodies, enzymes, and nanostructured materials can be used to detect the analytes (El-Nour et al., Citation2017; Verma et al., Citation2020); likewise, amperometric, voltametric and optical transducers are commonly utilized (Heerthana & Preetha, Citation2019). Some examples include amperometric immunosensors based on gold nanoparticles for histamine detection (Dong et al., Citation2017); spermine oxidase and polyamine oxidase co-immobilized by entrapment into gel matrices for polyamine detection using screen-printed electrodes (Tortolini et al., Citation2018); a genetically engineered putrescine oxidase that allows direct binding to gold surfaces with a controlled orientation where its activity is measured spectrophotometrically (Kamathewatta et al., Citation2021). In addition, biosensors are portable devices and generally do not require pre-treatment, modification, or destruction of samples; they can provide results within minutes (Curulli, Citation2021).
For enzymatic BA biosensors, amine oxidases are importantly used as bioreceptors, specifically, Diamine Oxidase (DAO), which is a copper containing amine oxidase that catalyze the oxidation of several BA to their corresponding aldehyde, producing hydrogen peroxide and ammonia in the process as follows
This enzyme is present in all types of organisms and exhibits different affinity towards distinct BA according to the source organism, often animal DAO has higher affinity to His than the ones from plants. In contrast DAO’s Km for aliphatic diamines and polyamines does not have a clear correlation between life domains (Chang et al., Citation2021). Bacteria use copper-containing amine oxidases as DAO in biochemical pathways that allows them to assimilate nitrogen and carbon from amines (Shoji et al., Citation2020). In humans, three types of DAO have been identified: AOC1 which is mainly involved in His degradation, retina-specific amine oxidase (AOC2) with higher affinity for monoamines and AOC3 which preferentially processes monoamines too, but it has been identified as a multifunctional cell-surface receptor (Vakal et al., Citation2020). On the other hand, plant DAO participate in complex mechanisms of biotic and abiotic stress response modulation by processing di- and polyamines (Fraudentali et al., Citation2021).
Because DAO recognizes and processes several types of BA, it has been extensively used as biorecognition element regardless the biosensor’s architecture (Kettner et al., Citation2022).
It is possible to use silicon dioxide substrates for enzyme immobilization; some forms of dopped crystalline SiO2 are transparent to infrared in the mid-IR region making them suitable for optical biosensing applications. In addition, their surfaces can be hydroxylated and further functionalized using self-assembled aminosilane monolayers exposing amino groups for further crosslinking. The advantages of silanized substrates with 3-aminopropyltrimetoxhysilane (3-APTMS) include biomolecule stability, improved analytical performance, leach-proof biomolecule binding, high immobilization density, also, they have a higher reactivity towards hydroxylated SiO2 surfaces than ethoxysilanes and, in the right conditions, can couple directly to its substrate without hydrolysis (Hermanson, Citation2013; Vashist et al., Citation2014).
The aim of this work is to compare the responses of DAO biosensors built using 3-APTMS functionalized SiO2 substrates with GA or TA as crosslinkers to Cad, Put, His and Spdm under controlled conditions.
Materials and methods
Chemicals and materials
Histamine dihydrochloride, putrescine dihydrochloride, cadaverine dihydrochloride, spermidine trihydrochloride, glutaraldehyde solution 25%, terephthalaldehyde, o-dianisidine, peroxidase from horseradish and (3-aminopropyl)trimethoxysilane (3-APTMS) were purchased from Merck (New Jersey, U.S.A.). DAO from Pisum sativum was purchased from IBEX (Montreal, Canada). 100 mm Ø, Type P, Boron-dopped silicon wafers, 355–395 μm thick were purchased from Pure wafer (California, U.S.A.). Ultrapure water was obtained from a Thermo Barnstead™ E-Pure™ Water Purification System (Massachusetts, U.S.A.).
SiO2 substrates cleaning and functionalization with 3-APTMS
Silicon wafers were cut into 0.9 mm2 pieces to make the biosensor substrates able to fit flat onto the bottom of 1 cm pathlength polystyrene spectrophotometer cuvettes. The supports were washed with ultrapure water three times and then sonicated in water for 10 min water was discarded, and acetone was used for one more round of sonication for 10 more minutes; next, the acetone was discarded and a solution of acetone and ethanol 50% (v/v) was used to sonicate the substrates for 10 min more, after which they were finally rinsed with ultrapure water 4 times and dried under a nitrogen stream.
The substrates went surface hydroxylation by immersion in piranha solution for 30 min under a fume hood. After that, they were rinsed with ultrapure water 4 times and dried under nitrogen. The hydroxylated substrates were functionalized with 3-APTMS using the vapor phase deposition protocol described by (Wang & Vaughn, Citation2008); an alternative silanization method by immersion in hot toluene was also implemented according to (Aissaoui et al., Citation2012).
Biosensors assembly
DAO was covalently bonded to functionalized SiO2 substrates using two crosslinkers, glutaraldehyde (GA) and terephthalaldehyde (TA). Solutions in ethanol of both crosslinkers were prepared at a concentration of 10% for the first and 1 mM for the second; the functionalized substrates were immersed in one of the two solutions at room temperature (ca. 26°C) for 2 h (Aissaoui et al., Citation2013). Once crosslinked, substrates were rinsed with ethanol three times and dried under a stream of nitrogen. Next, 2 μL of 91.1 U/mL DAO in 100 mM sodium phosphate buffer, pH 6.8 was applied on its surface followed by an incubation period of 24 h at 4°C. After that, the biosensors were rinsed with buffer three times to remove any unbonded DAO.
Biosensors assembly characterization
The biosensor assembly process was verified using Infrared Spectroscopy, Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM). A Bruker Tensor 27 FT-IR (Ettlingen, Germany) was used in the mid-IR region to assess the presence of the corresponding spectral bands for 3-APTMS functionalized substrate, crosslinkers binding and further DAO immobilization. For SEM, the biosensors were first covered with a gold thin film to improve the conductance using a Denton Vacuum Desk V sputter coater (New Jersey, U.S.A.) and then observed the surface changes with a Jeol JSM-6610 Scanning Electron Microscope (Tokyo, Japan). For AFM, a Quesant QSCOPE-250 (California, U.SA.) in tapping mode with an acoustic vibration isolation chamber was used; AFM images and the average surface roughness (RMS) of silanized substrates were generated and calculated with the software Gwyddion version 2.63 (Nečas & Klapetek, Citation2012).
DAO assay
Free DAO activity toward Put, Cad, His, and Spdm was determined in a coupled reaction with horseradish peroxidase. Hydrogen peroxide produced by DAO during BA oxidation (Equation. 1) is processed by peroxidase; released oxygen from H2O2 oxidizes o-dianisidine and produces a red chromophore as depicted in eq. 2 (Lebedeva et al., Citation1977).
For this purpose, 96.7 mM sodium phosphate monobasic, 4.98 mM of the corresponding BA as free base, 0.53 mM o-dianisidine, 200 U peroxidase and 0.6 U DAO were mixed in a 1 cm pathlength spectrophotometric cuvette. The cuvettes containing all reagents except DAO were preheated to 37°C in a dry bath; DAO was also equilibrated separately. The cuvettes were placed in a Thermo Scientific SPG 1A Peltier thermostatted cuvette holder at 37°C installed in a Thermo Scientific Genesys 10S UV/vis spectrophotometer. The reaction was started by adding DAO to the cuvette and mixing. The absorbance was recorded for 20 min at 440 nm.
Biosensors activity
To assess the biosensors’ activity the DAO assay protocol was used with minor variations: The biosensors were placed flat, horizontally into the bottom of the cuvettes with the immobilized enzyme side up along with all reagents except the corresponding BA. The cuvette was incubated for a few minutes at 37°C in a dry bath until; then, the cuvette was put into the thermostatted cell holder, and the BA was added and mixed to start the reaction. The final volume of the reagents mix in the cuvette was adjusted with buffer to be the same as the final volume of the DAO assay; the absorbance was recorded at 440 nm. Fresh prepared biosensors were used in every reaction.
Molecular docking simulation
Molecular docking is a computational methodology widely used to predict energies and bond modes by simulating the interaction between a small chemical structure (ligand) with a macromolecule (receptor). GA and TA molecules were used as ligands, and DAO as receptor. The purpose of these calculations is to describe the way in which ligands link to the protein and discover the main interactions between them. GA and TA crosslinking agents structures were built in GaussView software version 6.1.1 (Dennington et al., Citation2019). Subsequently, they were optimized at the B3LYP/6-311 level of theory in gas phase using Gaussian 16 software (Frisch et al., Citation2016). Ligands were prepared in AutodockTools 4.2 (ADT) (Morris et al., Citation2009) in two different ways: first, adding Gasteiger charges and setting all bonds nonrotatable and, second, adding Kollman charges and setting all bonds nonrotatable. The protein crystal structure of Pisum sativum DAO was retrieved from Protein Data Bank with ID 1KSI. It was prepared by adding polar hydrogens and assigning Kollman charges. To cover the surface of the protein, diverse grid boxes were built. The grid size was x = 73, y = 47, z = 47 with a point space of 1.000A, centered on superficial amino acids with amino group on its side chain. Autodock Vina software version 1.2.0 which uses a Lamarckian genetic algorithm was used to carry out all calculations (Eberhardt et al., Citation2021).
Data analysis
Data analysis was carried out using GraphPad Prism version 9.0.0 for Microsoft Windows.
Results and discussion
SiO2 functionalization and crosslinking strategy
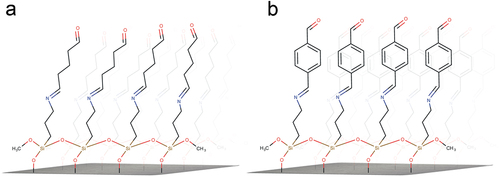
A scheme of the silanized substrates with GA or TA as crosslinkers is shown in ; in a first building step a self-assembled 3-APTMS layer is built onto the hydroxylated SiO2 substrate (Hermanson, Citation2013); then, they are exposed to GA or TA which reacts to the surface amine group and binds the two molecules through a Schiff base, at this point, the surface aldehyde group is ready to bind DAO with available surface amino groups.
FT-IR of biosensors assembly stages
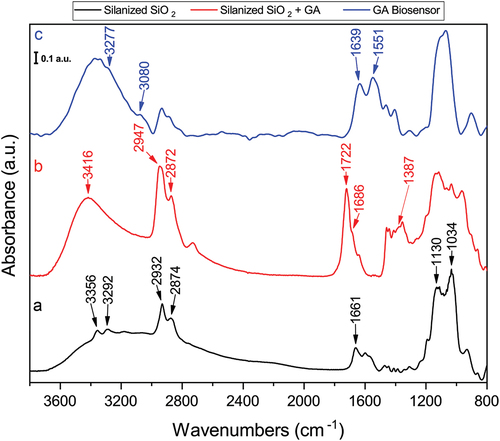
FT-IR spectra of every step of the biosensor assembly process was done in the mid-IR region using a clean 1 cm2 SiO2 piece as background; 40 scans were acquired when sampling. shows the spectral features associated with the layer-by-layer assembly of the GA crosslinked biosensor. 3-APTMS silanized SiO2 substrate shows 1130 cm−1 and 1034 cm−1 bands related to the LO and TO modes because of the silicon grafting lateral and transverse growth, respectively (Aissaoui et al., Citation2012). The 1661 cm−1 band corresponds to the NH2 bending vibration; the 2932 cm−1 and 2974 cm−1 bands correspond to CH2 and CH3 stretching vibrations of the aliphatic propyl chain, respectively; finally, the 3356 cm−1 and 3292 cm−1 bands are because of NH2 stretching () (Mostofi Sarkari et al., Citation2019). Once reacted with glutaraldehyde, the silanized SiO2 shows the O – H stretching at 3416 cm−1, also the absorbance increase of the methyl and methylene bands is noticeable, this is because the elongation of the aliphatic chain (). The 1722 cm−1 band correspond to the free carbonyl C=O stretching (Baranowska et al., Citation2015). The formation of the imine bond between 3-APTMS and glutaraldehyde can be observed at the 1686 cm−1 shoulder band (Socrates, Citation2004). The assembled glutaraldehyde biosensor spectrum is shown in , where DAO’s amide I and II bands are easily distinguishable at 1639 cm−1 and 1551 cm−1, respectively (Sadat & Joye, Citation2020). The weak bands at 3277 cm−1 and 3080 cm−1 correspond to amide A and B, respectively, arising from the enzyme’s NH2 stretching vibrations (Sahu et al., Citation2023).
Figure 2. FT-IR spectra of the glutaraldehyde biosensor assembly process. (a) silanized silicon wafer; (b) Silanized silicon wafer with glutaraldehyde as crosslinker and (c) complete biosensor with immobilized DAO. Spectra were normalized.
The infrared spectroscopy characterization of the TA biosensor building stages is shown in . As expected, silanized substrates after TA crosslinker reaction exhibit quite different spectral features than their GA homologues (), the methyl and methylene group bands are shifted to lower frequencies, also the C=O stretching vibration is recognizable as a sharp band at 1692 cm−1 that has been shifted to lower wavenumbers compared to the GA assembly one, this feature is typical of aryl aldehydes where carbonyl stretching vibration can be found between 1715 cm−1 and 1685 cm−1 (Socrates, Citation2004). The C–H stretching of the crosslinker aromatic ring can be detected because of the 3086 cm−1, 3047 cm−1, and 3020 cm−1 bands (Rogojerov et al., Citation2000). One intriguing aspect of the TA biosensor assembly process is that some amino groups from 3-APTMS did not appear to react with the crosslinker, as evidenced by the presence of weak bands at 3356 cm−1 and 3292 cm−1 within the NH2 stretching region (). The broad shoulder band at 1663 cm−1 corresponds to C–N bond stretching possibly with contributions from unbonded amine groups N–H deformation.
Figure 3. Normalized FT-IR spectra of the silanized substrate after TA reaction, (a); and DAO biosensor assembled with TA, (b).
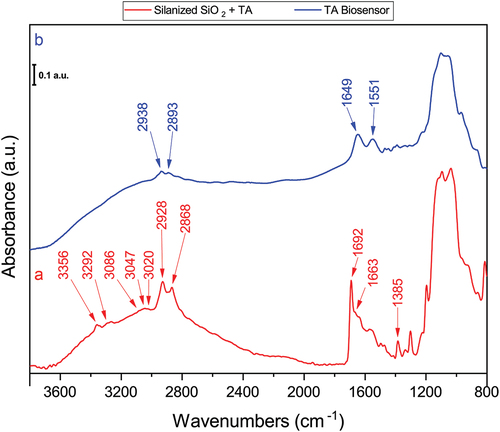
The FT-IR spectra of the TA biosensor complete assembly is shown in where methyl and methylene stretching are detected at 2938 cm−1 and 2893 cm−1; Amide II band preserves the same peak position between GA and TA biosensors, but Amide I of the latter one is shifted ~10 cm−1 towards higher wavenumbers, this could be an artifact introduced by a different moisture content rather than as a solely crosslinker interaction for we have observed this behaviour in biosensors dried under N2 flow in contrast with those mildly air dried, and it is widely known that drying produce changes at the secondary structure of proteins and polypeptides being deconvolution of Amide I band used to determine protein conformation (Cobb et al., Citation2020).
Electron microscopy – EDX and AFM
Changes in both composition and topology of the SiO2 substrates were observed as the biosensor assembly process progressed (). The hydroxylated substrate had an average elemental composition of 100% of Si as shown in (inset). Although the piranha solution can cause etching, the average roughness subsequently determined by AFM was 0.054 nm (), so the exposure times to the piranha solution were sufficient to hydroxylate the surface but not cause much etching (O’Mahony & Morris, Citation2021). The 3-APTMS layer is noticeable on the silanized substrate sample (), as well as changes in the average elemental composition compared to the previous stage which includes the decrease in Si content to 63.24% because of the presence of O at 14.14% and 20.69% of C, with minor traces of P (); N from 3-APTMS amine groups were not detected by EDX, although it was present as demonstrated by FT-IR (), it is known that this element together with B and Be is usually difficult to detect by Energy Dispersive Spectrometry (Wolfgong, Citation2016).
Figure 4. SEM of GA and TA biosensors assembly stages. (a) SiO2 substrates after hydroxylation with piranha solution; (b) 3-APTMS functionalized substrate; (c) 3-APTMS functionalized substrate after glutaraldehyde treatment; (d) 3-APTMS functionalized substrate after terephthalaldehyde substrate; (e) TA biosensor and (f) GA biosensor. Insets: averaged EDX-elemental composition of three zones.
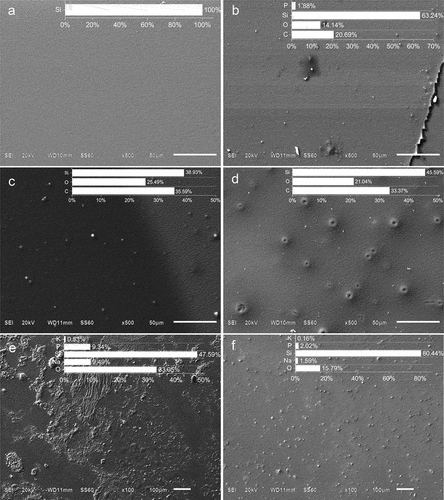
Figure 5. AFM images (top) and roughness profile plots (bottom) of silicon substrates after treatment with piranha solution, (a); after functionalization by gas phase, (b); after functionalization by toluene immersion, (c). White lines indicate the measurement range; roughness RMS values are plotted as dotted lines.
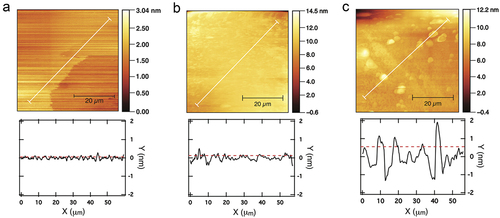
Interactions between silanol groups of 3-APTMS can lead to vertical polymerization and clump formation. These structures are susceptible to water attack and are more easily detached from substrates when immersed in aqueous solutions such as buffers, resulting in the loss of its bounded molecules. Therefore, it is critical to avoid clumping and achieve the lowest possible surface roughness and film thickness. AFM results showed that substrates silanized by vapor deposition had a mean roughness of 0.127 nm (), in contrast to 0.55 nm obtained by immersion in hot toluene (). This can be explained by the fact that the first technique produces thinner films of 3-APTES. The results presented here are consistent with those reported in similar studies, which also showed that vapor phase silanization generates more available NH2 groups than other silanization techniques (Gunda et al., Citation2014; Sypabekova et al., Citation2022).
The presence of O increased from the 3-APTMS to GA and TA crosslinked silanized substrates from 14.14% to 25.49% and 21.04%, respectively (); this can be explained because of the aldehyde groups present in both crosslinkers; it is noteworthy that the topology between both types of crosslinked silanized substrates is different being the TA one less homogeneous because of the presence of crater-like structures ().
SEM examination of the GA and TA biosensors showed different topologies between the two (). The surface of the TA biosensor has more irregular features than that of GA. This difference could be attributed to the distinct nature of interactions between the crosslinkers and DAO. It is known that various properties of the crosslinkers, such as their aliphatic or aromatic nature, the position of aromatic bonds (meta or para), or their length, can influence the binding and response of immobilized enzymes on SiO2 surfaces (Aissaoui et al., Citation2013; Fopase et al., Citation2020; Kujawa et al., Citation2021).
Molecular docking simulation
Molecular docking calculations between GA and TA crosslinking agents and DAO were carried out. Among all grid boxes generated to cover the protein surface, all of them including ASN, GLN, HIS and LYS amino acids, only one was useful. The selection was made considering the interaction between the aldehyde group of the crosslinker and the amino group of the side chain of the amino acid. This grid box was centered on x = 24.825, y = 49.368 z = 36.268 Cartesian coordinates. Ten poses were generated for each calculation and the best two were selected. Default parameters and Gasteiger chargers were used to run calculations, but useful information for the description sought was not obtained. Kollman chargers were assigned to both ligand and receptor for new dockings.
The complexes formed by crosslinker-DAO presented favorable and stable affinity energies, indicating strong binding interactions (). The utilization of Kollman charges revealed notably robust affinities between GA and LYS, as well as ARG. These residues are abundant on the enzyme surface, so Schiff bases can be readily formed during the crosslinked substrate incubation with DAO.
Table 1. Interaction type and affinity energies for crosslinker-DAO complexes.
Gasteiger charges were found to be useful only with TA, where, similarly, the aldehyde group showed a higher propensity to interact with LYS. The main interaction type was a conventional hydrogen bond, formed between the oxygen atom of the aldehyde group and the hydrogen atom of the amine group (). The presence of aromatic rings facilitated complex stabilization through π—π interaction formation with PHE. However, this residue is of a much lower abundance on DAO’s surface compared to LYS and ARG, making this interaction’s contribution to the enzyme immobilization considerably less significant.
Biosensors response to BA standards
Free DAO kinetics () showed that cadaverine and putrescine were the preferred substrates over spermidine, being histamine the one with the lowest DAO affinity for it as previously reported by Kivirand et al. (Citation2016). The GA and TA biosensor responses are shown in , respectively. In both cases, DAO activity was reduced approximately 3-fold compared to the free enzyme assay. This is an expected result, as it is known that immobilization of enzymes on solid surfaces can in some cases lead to a reduction in catalytic activity due to several factors, such as a reduction in the surface area available for interaction with substrates, conformational changes, or inefficient diffusion of substrate molecules to the active sites (Fopase et al., Citation2020). These results contrast with those of Verma, et al. in which Pisum sativum DAO was immobilized onto PVC (Verma et al., Citation2020) and nitrocellulose (Verma et al., Citation2020) strips using glutaraldehyde as crosslinker. In both studies, the relative activity at the highest immobilized concentration of DAO was only slightly lower than at the free one. In this context, it is possible that the loading capacity of these materials could be higher due to the increased availability of initial NH2 groups on their surfaces generated by ethylenediamine and chitosan used as functionalizing agents for PVC and nitrocellulose, respectively; In addition, the surface area of the substrates available for enzyme binding plays an important role in the biosensor response because the larger it is, the more enzymes can be accommodated. In this scenario, our surface area was 0.9 cm2 compared to 2 cm2 in the aforementioned reports.
Figure 7. DAO activity, biosensor response to different BA, and comparison of initial velocity between GA and TA cross-linked biosensors. A, free DAO activity; B, GA biosensor responses; C, TA biosensor response; and D, initial velocities of GA and TA biosensor responses. Each point represents the average ± SD; error bars are not shown if smaller than the symbol. ns, not significant; **(p < .05); ***(p ≤ .0001); n = 3.
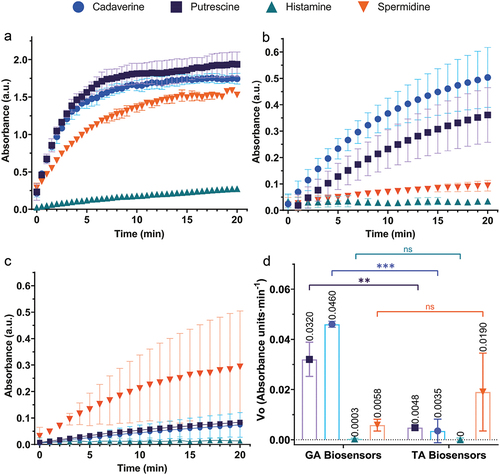
In general, the GA biosensors had higher activity for each BA than the TA (), except for spermidine. A two-tailed t-test was performed using the calculated initial velocities for each BA response for each type of biosensor. The averaged initial velocities (n = 3) of putrescine response were statistically different (t = 6.835, df = 4, p = .0024), including the ones for cadaverine, where the difference was highly significant (t = 15.13, df = 4, p = .0001). On the other hand, the averaged initial velocities of histamine were not significantly different between the GA and TA biosensors (t = 0.7738, df = 4, p = .4822), neither were those of spermidine (t = 1.455, df = 4, p = .2195).
From the free DAO assay, it was clear that detection of histamine would be challenging due to the lower catalytic activity of Pisum sativum DAO. In plants, aliphatic polyamines such as spermine and spermidine play an important role in their physiology as they are normally involved in organism development and stress response (Alcázar et al., Citation2020) so it is expected that DAO from many plant species would have a higher Km value to these BA. In animals, histamine is a key molecule for numerous physiological processes, including immunomodulation, wound healing, vascular permeability, protection against scombroid poisoning, and others (Hungerford, Citation2021) which is why animal DAO such as the commercially available porcine kidney DAO perform well in detecting histamine when used in biosensors, over other BA (Torre et al., Citation2020). In this scenario, although the response of both types of biosensors was not significantly different between each other, it was virtually undetectable by the assay, making this design unsuitable for this specific purpose, but useful for putrescine, cadaverine and spermidine detection. According to the outstanding compilation of BA food content reported by Bover-Cid et al. (Citation2014), putrescine, cadaverine and spermidine are generally found in higher amounts than histamine in fruits, nuts, legumes, vegetables, fresh meat and cheese so, in this case scenario the biosensors here built could work to provide information about the presence or absence of total BA with little-to-non histamine signal. An interesting case is the response of the biosensors to spermidine, where the average initial velocity of the TA biosensor was higher than that of its GA homolog, whereas the opposite was observed for the others BA ().
The influence of the crosslinker on the biosensor response is evident in the processing of Cad, Put, and Spdm. From the docking simulation results, it is clear that GA and TA yield different crosslinking results, potentially altering DAO’s ability to process its substrates due to the types of bonds that bind the enzyme. It is generally believed that both crosslinking mechanisms form Schiff bases when reacting with amino groups. In this context, Chung et al. (Citation2016) demonstrated that when glucose oxidase was crosslinked to carbon nanotubes using TA, only π-conjugated bonds were formed through aldol condensation. On the other hand, when GA was used, mixed C=N and C–N bonds were formed through a nucleophilic addition reaction after the initial formation of the C–N bonds. If this holds true for our model, considering that C – N bonds are less rigid than C=N bonds, it could provide a greater degree of movement for DAO, allowing for more efficient processing compared to its TA counterpart. We believe this bonding phenomena explains the observed differences in topologies between TA and GA biosensors, as revealed by SEM.
Conclusions
Two biosensors for in-vitro detection of different biogenic amines were built using SiO2 substrates functionalized with 3-APTMS with either glutaraldehyde or terephthalaldehyde crosslinkers. The assembly was confirmed by detecting the presence of specific functional groups throughout the process, e.g., amines, imines, carbonyls, aldehydes, and amide I and II bands using FT-IR, as well as by monitoring the topology changes during the process as its composition by AFM and SEM-EDX. Molecular Docking simulation was useful to predict the susceptible residues the crosslinkers interact with, and partially reproduced experimental data. These results show a first approach to clarify and propose the possible mechanism by which TA and GA interact with DAO. Immobilization of the enzyme reduced the responses of the biosensors almost 3-fold compared to free DAO. From the results of the initial velocities, GA biosensors performed best for Cad and Put, while TA biosensors had the best response to Spdm; histamine was nearly undetected by both types of biosensors. Finally, we recommend using the biosensors with food samples to evaluate its detection performance and continue with computer simulations to predict the possible mechanism by biosensors link to DAO.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aissaoui, N., Bergaoui, L., Landoulsi, J., Lambert, J. F., & Boujday, S. (2012). Silane layers on silicon surfaces: Mechanism of interaction, stability, and influence on protein adsorption. Langmuir, 28(1), 656–665. https://doi.org/10.1021/la2036778
- Aissaoui, N., Landoulsi, J., Bergaoui, L., Boujday, S., & Lambert, J.-F. (2013). Catalytic activity and thermostability of enzymes immobilized on silanized surface: Influence of the crosslinking agent. Enzyme and Microbial Technology, 52(6–7), 336–343. https://doi.org/10.1016/j.enzmictec.2013.02.018
- Alcázar, R., Bueno, M., & Tiburcio, A. F. (2020). Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells, 9(11), 2373. NLM (Medline). https://doi.org/10.3390/cells9112373
- Baranowska, M., Slota, A. J., Eravuchira, P. J., Alba, M., Formentin, P., Pallarès, J., Ferré-Borrull, J., & Marsal, L. F. (2015). Protein attachment to silane-functionalized porous silicon: A comparison of electrostatic and covalent attachment. Journal of Colloid and Interface Science, 452, 180–189. https://doi.org/10.1016/j.jcis.2015.04.022
- Bover-Cid, S., Latorre-Moratalla, M. L. L., Veciana-Nogués, M. T. T., & Vidal-Carou, M. C. C. (2014). Processing contaminants: Biogenic amines. In Encyclopedia of food safety (Vol. 2, pp. 381–391). Elsevier. https://doi.org/10.1016/B978-0-12-378612-8.00216-X
- Chang, A., Jeske, L., Ulbrich, S., Hofmann, J., Koblitz, J., Schomburg, I., Neumann-Schaal, M., Jahn, D., & Schomburg, D. (2021). BRENDA, the ELIXIR core data resource in 2021: New developments and updates. Nucleic Acids Research, 49(D1), D498–D508. https://doi.org/10.1093/nar/gkaa1025
- Chung, Y., Ahn, Y., Christwardana, M., Kim, H., & Kwon, Y. (2016). Development of a glucose oxidase-based biocatalyst adopting both physical entrapment and crosslinking, and its use in biofuel cells. Nanoscale, 8(17), 9201–9210. https://doi.org/10.1039/C6NR00902F
- Cobb, J. S., Zai-Rose, V., Correia, J. J., & Janorkar, A. V. (2020). FT-IR spectroscopic analysis of the secondary structures present during the desiccation induced aggregation of elastin-like polypeptide on silica. ACS Omega, 5(14), 8403–8413. https://doi.org/10.1021/acsomega.0c00271
- Curulli, A. (2021). Electrochemical biosensors in food safety: Challenges and perspectives. Molecules, 26(10), 2940. https://doi.org/10.3390/molecules26102940
- Dennington, R., Keith, T. A., & Millam, J. M. (2019). GaussView Version 6.
- Dong, X.-X., Yang, J.-Y., Luo, L., Zhang, Y.-F., Mao, C., Sun, Y.-M., Lei, H.-T., Shen, Y.-D., Beier, R. C., & Xu, Z.-L. (2017). Portable amperometric immunosensor for histamine detection using Prussian blue-chitosan-gold nanoparticle nanocomposite films. Biosensors and Bioelectronics, 98, 305–309. https://doi.org/10.1016/j.bios.2017.07.014
- Eberhardt, J., Santos-Martins, D., Tillack, A. F., & Forli, S. (2021). AutoDock Vina 1.2.0: New docking methods, expanded force field, and Python Bindings. Journal of Chemical Information and Modeling, 61(8), 3891–3898. https://doi.org/10.1021/acs.jcim.1c00203
- El-Nour, K. M. A., Salam, E. T. A., Soliman, H. M., & Orabi, A. S. (2017). Gold nanoparticles as a direct and rapid sensor for sensitive analytical detection of biogenic amines. Nanoscale Research Letters, 12(1), 231. https://doi.org/10.1186/s11671-017-2014-z
- Fopase, R., Paramasivam, S., Kale, P., & Paramasivan, B. (2020). Strategies, challenges and opportunities of enzyme immobilization on porous silicon for biosensing applications. Journal of Environmental Chemical Engineering, 8(5), 104266. https://doi.org/10.1016/j.jece.2020.104266
- Fraudentali, I., Rodrigues-Pousada, R. A., Angelini, R., Ghuge, S. A., & Cona, A. (2021). Plant copper amine oxidases: Key players in hormone signaling leading to stress-induced phenotypic plasticity. International Journal of Molecular Sciences, 22(10), 5136. MDPI AG. https://doi.org/10.3390/ijms22105136
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Petersson, G. A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A. V., Bloino, J., Janesko, B. G., Gomperts, R., Mennucci, B., Hratchian, H. P., Ortiz, J. V., and Fox, D. J. (2016). Gaussian 16 revision C.01.
- Gunda, N. S. K., Singh, M., Norman, L., Kaur, K., & Mitra, S. K. (2014). Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Applied Surface Science, 305, 522–530. https://doi.org/10.1016/j.apsusc.2014.03.130
- Heerthana, V. R., & Preetha, R. (2019). Biosensors: A potential tool for quality assurance and food safety pertaining to biogenic amines/volatile amines formation in aquaculture systems/products. Reviews in Aquaculture, 11(1), 220–233. Wiley-Blackwell. https://doi.org/10.1111/raq.12236
- Hermanson, G. T. (2013). Silane coupling agents. In J. Audet &, and M. Preap (Eds.), Bioconjugate Techniques (3rd ed., pp. 535–548). Elsevier. https://doi.org/10.1016/B978-0-12-382239-0.00013-3
- Hungerford, J. M. (2021). Histamine and Scombrotoxins. In Toxicon (Vol. 201, pp. 115–126). Elsevier Ltd. https://doi.org/10.1016/j.toxicon.2021.08.013
- Kamathewatta, N. J. B., Nguyen, T. M., Lietz, R., Hughes, T., Taktak Karaca, B., Deay, D. O., Richter, M. L., Tamerler, C., & Berrie, C. L. (2021). Probing selective self-assembly of putrescine oxidase with controlled orientation using a genetically engineered peptide Tag. Langmuir, 37(24), 7536–7547. https://doi.org/10.1021/acs.langmuir.1c01033
- Kettner, L., Seitl, I., & Fischer, L. (2022). Recent advances in the application of microbial diamine oxidases and other histamine-oxidizing enzymes. World Journal of Microbiology & Biotechnology, 38(12). https://doi.org/10.1007/s11274-022-03421-2
- Kivirand, K., Sõmerik, H., Oldekop, M. L., Rebane, R., & Rinken, T. (2016). Effect of spermidine and its metabolites on the activity of pea seedlings diamine oxidase and the problems of biosensing of biogenic amines with this enzyme. Enzyme and Microbial Technology, 82, 133–137. https://doi.org/10.1016/j.enzmictec.2015.09.007
- Kujawa, J., Głodek, M., Li, G., Al-Gharabli, S., Knozowska, K., & Kujawski, W. (2021). Highly effective enzymes immobilization on ceramics: Requirements for supports and enzymes. Science of the Total Environment, 801, 149647. https://doi.org/10.1016/j.scitotenv.2021.149647
- Lebedeva, O. V., Ugarova, N. N., & Berezin, I. V. (1977). Kinetic study of o-dianisidine oxidation by hydrogen peroxide in the presence of horseradish peroxidase. Biokhimiia, 42(8), 1372–1379.
- Lehane, L., & Olley, J. (2000). Histamine fish poisoning revisited. International Journal of Food Microbiology, 58(1–2), 1–37. https://doi.org/10.1016/S0168-1605(00)00296-8
- Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30(16), 2785–2791. https://doi.org/10.1002/jcc.21256
- Mostofi Sarkari, N., Doğan, Ö., Bat, E., Mohseni, M., & Ebrahimi, M. (2019). Assessing effects of (3-aminopropyl)trimethoxysilane self-assembled layers on surface characteristics of organosilane-grafted moisture-crosslinked polyethylene substrate: A comparative study between chemical vapor deposition and plasma-facilitated in situ grafting methods. Applied Surface Science, 497. https://doi.org/10.1016/j.apsusc.2019.143751
- Munir, M. A., & Badri, K. H. (2020). The importance of derivatizing reagent in chromatography applications for biogenic amine detection in food and beverages. Journal of Analytical Methods in Chemistry, 2020, 1–14. https://doi.org/10.1155/2020/5814389
- Nečas, D., & Klapetek, P. (2012). Gwyddion: An open-source software for SPM data analysis. Open Physics, 10(1). https://doi.org/10.2478/s11534-011-0096-2
- O’Mahony, T. F., & Morris, M. A. (2021). Hydroxylation methods for mesoporous silica and their impact on surface functionalisation. Microporous and Mesoporous Materials, 317, 110989. https://doi.org/10.1016/j.micromeso.2021.110989
- Pegg, A. E. (2013). Toxicity of polyamines and their metabolic products. Chemical Research in Toxicology, 26(12), 1782–1800. https://doi.org/10.1021/tx400316s
- Rauscher-Gabernig, E., Grossgut, R., Bauer, F., & Paulsen, P. (2009). Assessment of alimentary histamine exposure of consumers in Austria and development of tolerable levels in typical foods. Food Control, 20(4), 423–429. https://doi.org/10.1016/j.foodcont.2008.07.011
- Rogojerov, M., Jordanov, B., & Keresztury, G. (2000). Vibrational analysis of terephthalaldehyde from its IR and Raman spectra in isotropic and anisotropic solutions. Journal of Molecular Structure, 550–551, 455–465. https://doi.org/10.1016/S0022-2860(00)00401-4
- Sadat, A., & Joye, I. J. (2020). Peak fitting applied to Fourier transform infrared and raman spectroscopic analysis of proteins. Applied Sciences, 10(17), 5918. https://doi.org/10.3390/app10175918
- Sahu, R., Sooram, B., Sasidharan, S., Nag, N., Tripathi, T., & Saudagar, P. (2023). Applications of infrared spectroscopy to study proteins. In P. Saudagar & T. Tripathi (Eds.), Advanced spectroscopic methods to study biomolecular structure and dynamics (pp. 153–171 https://doi.org/10.1016/B978-0-323-99127-8.00005-2). Elsevier.
- Shoji, M., Murakawa, T., Boero, M., Shigeta, Y., Hayashi, H., & Okajima, T. (2020). Unique protonation states of aspartate and topaquinone in the active site of copper amine oxidase. RSC Advances, 10(63), 38631–38639. https://doi.org/10.1039/d0ra06365g
- Socrates, G. (2004). Infrared and Raman characteristic group frequencies (3rd ed.). John Wiley & Sons.
- Sypabekova, M., Hagemann, A., Rho, D., & Kim, S. (2022). Review: 3-aminopropyltriethoxysilane (APTES) deposition methods on oxide surfaces in solution and vapor phases for biosensing applications. Biosensors, 13(1), 36. https://doi.org/10.3390/bios13010036
- Tırıs, G., Sare Yanıkoğlu, R., Ceylan, B., Egeli, D., Kepekci Tekkeli, E., & Önal, A. (2023). A review of the currently developed analytical methods for the determination of biogenic amines in food products. Food Chemistry, 398, 133919. https://doi.org/10.1016/j.foodchem.2022.133919
- Torre, R., Costa-Rama, E., Nouws, H. P. A., & Delerue-Matos, C. (2020). Diamine oxidase-modified screen-printed electrode for the redox-mediated determination of histamine. Journal of Analytical Science and Technology, 11(1). https://doi.org/10.1186/s40543-020-0203-3
- Tortolini, C., Favero, G., & Mazzei, F. (2018). Development of amine-oxidase-based biosensors for spermine and spermidine analysis. Polyamines: Methods and Protocols, 1694, 75–80. https://doi.org/10.1007/978-1-4939-7398-9_7
- Vakal, S., Jalkanen, S., Dahlström, K. M., & Salminen, T. A. (2020). Human copper-containing amine oxidases in drug design and development. Molecules, 25(6), 1293. https://doi.org/10.3390/molecules25061293
- Vashist, S. K., Lam, E., Hrapovic, S., Male, K. B., & Luong, J. H. T. (2014). Immobilization of antibodies and enzymes on 3-aminopropyltriethoxysilane-functionalized bioanalytical platforms for biosensors and diagnostics. Chemical Reviews, 114(21), 11083–11130. https://doi.org/10.1021/cr5000943
- Verma, N., Hooda, V., Gahlaut, A., Gothwal, A., & Hooda, V. (2020). Enzymatic biosensors for the quantification of biogenic amines: A literature update. Critical Reviews in Biotechnology, 40(1), 1–14. https://doi.org/10.1080/07388551.2019.1680600
- Verma, N., Saini, R., Gahlaut, A., & Hooda, V. (2020). Stabilization and optimization of purified diamine oxidase by immobilization onto activated PVC membrane. Food Biotechnology, 34(4), 306–322. https://doi.org/10.1080/08905436.2020.1833912
- Verma, N., Sisodiya, L., Gahlaut, A., Hooda, V., & Hooda, V. (2020). Novel approach using activated cellulose film for efficient immobilization of purified diamine oxidase to enhance enzyme performance and stability. Preparative Biochemistry and Biotechnology, 50(5), 468–476. https://doi.org/10.1080/10826068.2019.1709976
- Wang, W., & Vaughn, M. W. (2008). Morphology and amine accessibility of (3-aminopropyl) triethoxysilane films on glass surfaces. Scanning, 30(2), 65–77. https://doi.org/10.1002/sca.20097
- Wolfgong, W. J. (2016). Chemical analysis techniques for failure analysis: Part 1, common instrumental methods. In Handbook of materials failure analysis with case studies from the aerospace and automotive industries (pp. 279–307). Elsevier Inc. https://doi.org/10.1016/B978-0-12-800950-5.00014-4
- Zou, D., Zhao, Z., Li, L., Min, Y., Zhang, D., Ji, A., Jiang, C., Wei, X., & Wu, X. (2022). A comprehensive review of spermidine: Safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Comprehensive Reviews in Food Science and Food Safety, 21(3), 2820–2842. https://doi.org/10.1111/1541-4337.12963