ABSTRACT
The protective effect of ethanolic propolis extract (PE) in the toxic effects of doxorubicin (DOX), a chemotherapy drug, has been reported previously. Since ethanol usage has some restrictions, in the present study, olive oil as an alternative to ethanol has been tested in DOX-induced hepatorenal injury and the effectiveness of the two extracts were compared. Male rats were orally given PE or oily propolis extract (PO) for 15 days (50 mg/kg/day) and DOX was injected (15 mg/kg, intraperitoneally) on the 13th day. The animals were sacrificed and then hepatorenal oxidative stress and functional parameters were evaluated. DOX-induced reduction in hepatorenal functions and oxidative stress were significantly reduced in both propolis treatment groups. Treatment with PO was more effective in reducing DOX-induced oxidative stress, yet both extracts showed similar efficacy in improving hepatorenal functions. We suggest that the PO could be preferred to avoid the side effects of ethanol.
Introduction
Propolis as a resiny beehive product is gathered by honey bees from different botanical sources. The chemical composition of the propolis depends on its floral origin and collecting time, as well as climatic conditions and geographical properties. More than 300 compounds have been detected in propolis and the flavonoids and phenolic acid derivatives, as the principal components of propolis mainly determine its biological activity (Boutabet et al., Citation2011; Kosalec et al., Citation2004). Propolis has been used in traditional medicine for centuries and its antiviral, antibacterial, antifungal, antioxidant and anti-inflammatory, as well as immune supportive effects have been proven in many scientific studies (Kubiliene et al., Citation2015; Nakajima et al., Citation2009). An additional important finding is that propolis exhibits anti-cancer activity via affecting cell proliferation, angiogenesis and metastasis, which are the main processes for cancer development. It has been demonstrated that propolis and its components such as 3-O-methylquercetin, genistein, chrysin, galangin, caffeic acid and caffeic acid phenylethyl ester have shown significant proapoptotic activity in different cancer cell lines (Forma & Bryś, Citation2021). Moreover, in-vivo studies showed that treatment with propolis can prevent systemic side effects of some anti-cancer drugs including DOX (Ali et al., Citation2020; Mohamed et al., Citation2022; Wided et al., Citation2014).
DOX (also known as Adriamycin) is an antineoplastic drug that has been used for several decades in the treatment of many cancer types, including bladder, breast, lung, thyroid, ovarian and gastric cancer. Inhibition of DNA and RNA synthesis, production of free radicals, disruption of the cell membrane and mitochondrial dysfunction are the prominent cellular effects of DOX. However, those effects are not specific to cancer cells and non-targeted healthy cells are also affected by DOX treatment. Thus, in the clinic, DOX causes cardiac, renal and hepatic toxicities that limit its usage in cancer treatment and life-saving effects (Abbasnezhad et al., Citation2022; Carvalho et al., Citation2009). DOX-induced stimulation of reactive oxygen species (ROS), such as hydroxyl ion, superoxide radical and hydrogen peroxide (H2O2) production, is defined as the major contributor to its general side effects (Granados-Principal et al., Citation2010; Kalender et al., Citation2005). For example, in DOX-induced nephrotoxicity, glomerular and tubular damage, increased serum levels of creatinine and urea are accompanied by renal oxidative stress (Molehin et al., Citation2019). Similarly, the hepatic toxic effect of DOX can be monitored by serum alanine transaminase (ALT) and aspartate transaminase (AST) levels, resulting in vacuolation and degeneration of hepatocytes as well as focal necrosis and increased oxidative stress (Prasanna et al., Citation2020). Therefore, strategies to reduce oxidative damage including propolis treatment have been proposed to prevent the side effects caused by DOX (Ali et al., Citation2020; Mohamed et al., Citation2022; Wided et al., Citation2014).
The fact that propolis itself exhibits anti-cancer effects and reduces the toxic side effects of DOX is clinically important. Comprehensive preclinical evidence indicates that the use of propolis extract synergistically enhances the anti-cancer activity of DOX through the activation of apoptotic and antioxidant signaling pathways, while reducing the side effects on multiple organs (Hossain et al., Citation2022). Additional reports showed a decreased or completely prevented DOX-dependent oxidative damage of heart, liver and kidney tissues by propolis treatment (Ali et al., Citation2020; Mohamed et al., Citation2022; Wided et al., Citation2014). In these mentioned studies, the ethanolic extract of propolis has been preferred for treatment with a dose of 50 to 250 mg/kg for different durations (4–30 days). The ethanolic extraction is the simplest and most efficacious method for propolis extraction, since the active components of propolis are highly soluble in ethanol (Kubiliene et al., Citation2015). However, usage of ethanolic extracts has been limited in ophthalmology, otolaryngology and pediatrics or simply, it is not preferred by some believers for religious reasons. Thus, the preparation of propolis extract in an innocuous solvent might expand its utilization to a wider population, on the condition that its efficiency is not lower than that of the ethanolic extract. Vegetable oil, water, propylen glycol, chloroform and ethyl acetate are the solvents used for propolis extraction (Bankova et al., Citation2021). Among these solvents, olive oil may be superior compared to the others for its own antioxidant properties and being an edible vegetable oil (Mataix et al., Citation2006). However, the effects of olive oil extracts of propolis have not been extensively researched, and its efficiency in DOX-induced hepatorenal damage has not been studied before. In the present study, as an alternative to ethanol, extra virgin olive oil has been used in propolis extraction and the effects of PE and PO on DOX-induced hepatic and renal damage have been compared.
Material and methods
Preparation of extracts
Propolis samples were collected from certified beekeepers in the Middle Black Sea Region of Turkiye (41° 13′ 44″ N and 36° 35′ 30″ E) following the autumn season, characterized by a similar flora and stored at −20°C until the extraction process. The propolis extracts were prepared based on Altaee (Citation2014) and Kubiliene et al. (Citation2015) maceration methods with some modifications. For PE, the ground propolis was mixed with ethanol 96% at room temperature in the dark for 36 h on a magnetic stirrer and filtered using Whatman No.1 filter paper. Afterward, the ethanol content of the solvent was evaporated and mixed with drinking water until it reached 2% ethanol concentration and became homogeneous. For PO, the ground propolis was stirred with extra virgin olive oil (Tariş Olive Oil Trade Co. Ltd., Turkiye) at 35°C in the dark for 72 h. Then, it was cooled to room temperature and filtered.
In-vitro experiments
After preparation, the extracts were examined for the parameters described below and used in rats designated for propolis treatment.
Measurement of total phenolic compounds
The total phenolic contents of the two different extracts were measured by the spectrophotometric Folin-CioCalteu method. The propolis extract and Folin-Ciocalteu reagent were mixed, and the absorbance was recorded at 650 nm after incubation for 2 h by avoiding light. The results were expressed as μg gallic acid equivalent per g extract (Mathew & Abraham, Citation2006).
Measurement of total flavonoid content
To evaluate the total flavonoid content of PE and PO, a modified aluminum nitrate method was utilized. The extracted sample, sodium acetate solution and 10% (w/v) Al(NO3)3 were mixed and after the 40-min incubation at room temperature, the absorbance was read at 450 nm. A standard curve was used to calculate the total flavonoid contents of the samples, and the results were expressed as equivalent of μg quercetin per g extract (Chang et al., Citation2002).
Measurement of reducing power
A spectrophotometric method was used to determine the reducing power of the propolis extracts (Hwang et al., Citation2001). Briefly, the propolis extracts (1 μg/ml) were diluted with distilled water (1:20) and incubated with a mixture of phosphate buffer and potassium ferricyanide solution. After 20-min incubation at 50°C, trichloroacetic acid (TCA) was added to each sample and 15-min centrifugation was performed at 3000 rpm. The supernatants were mixed with FeCl3.6 H2O solution and incubated at room temperature (in the dark, for 24 h) and the absorbance values were recorded at 700 nm. Butylated hydroxytoluene (BHT) was used as a standard and the reducing power of the extracts was calculated using the following formula: the reducing power (%) = [(AC-AS)/(AC)] × 100 (AC: Absorbance of control, AS: Absorbance of the sample or standard).
Measurement of 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity
A spectrophotometric method was performed to measure the DPPH radical scavenging activity of the samples (Blois, Citation1958). Briefly, propolis extracts (20 mg/ml) or standards (Trolox) were mixed with the DPPH solution (20 mg/l), and absorbance changes were recorded at 517 nm following different incubation periods (5, 10, 15 and 30 min). Calculation of the result was done using the following formula: the DPPH radical scavenging activity (%) = [(AC-AS)/(AC)] × 100 (AC: Absorbance of control, AS: Absorbance of the sample or standard).
Measurement of 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity
To measure the ABTS radical scavenging activity, a methanolic ABTS solution and the propolis extracts or standard solution (Trolox) were mixed and placed in a dark place for 2 h incubation. At 734 nm, the absorbance values were recorded, and using the following formula, results were calculated; the ABTS radical scavenging activity (%) = [(AC-AS)/(AC)] × 100 (AC: Absorbance of control, AS: Absorbance of the sample or standard) (Re et al., Citation1999).
In-vivo experimental design
In this study, 2.5–3 months old male Sprague-Dawley rats weighing 250–300 g were used, and the animals were divided into six groups as follows (n = 6).
In the control and DOX groups, rats were given water orally for 15 days. In the DOX group, a single dose of DOX was injected intraperitoneally (ip, 15 mg/kg) on the 13th day of the experiment to induce toxicity, as indicated in previous studies (El-Moselhy & El-Sheikh, Citation2014; Mansour et al., Citation1999).
In the PE group – the ethanolic propolis extract (50 mg/kg) and in the PO group – the oily propolis extract (50 mg/kg) was daily given to animals by intragastric gavage for 15 days (Mohamed et al., Citation2022).
In the ethanolic propolis + DOX (PED) group, on the 13th day of the ethanolic propolis treatment (50 mg/kg/day) and in the oily propolis + DOX (POD) group on the 13th day of the oily propolis treatment (50 mg/kg/day), DOX was injected at a dose of 15 mg/kg (ip).
The rats were fed a standard laboratory rat chow containing 19.2% protein, 67.3% carbohydrates and 4.3% fat (Casein, DL-Methionine, L-Cystine, Corn Starch, Maltodextrin, Cellulose, Palm Oil, Corn Oil, Mineral Mix, DCP, Calcium Carbonate, Potassium Citrate, Vitamin Mix, Choline; Arden Arastirma Deney, Ankara, Turkiye). The access to food and drinking water was unlimited. All animals were placed in the metabolic cage, and 24-h urine samples were collected prior to sacrificing. On the 15th day of the study, which relates to 2 days after the DOX injection for the DOX, PED and POD groups, the rats were sacrificed under anesthesia induced by ketamine (80 mg/kg, ip) and xylazine (10 mg/kg, ip), and then blood and tissue samples were harvested. Serum, urine, liver and kidney samples were kept in a deep freezer (−80°C) until the biochemical parameters were studied as described below. All experimental procedures in the present study have been approved by the local ethics committee (2023/09).
Determination of malondialdehyde (MDA) level
Spectrophotometric MDA measurements were performed in homogenized kidney and liver tissue samples (in TCA) according to the thiobarbituric acid reagent formation method (Buege & Aust, Citation1978). Briefly, after homogenization, the supernatants were obtained by centrifugation at 1000 g for 10 min, and then the supernatants were mixed with thiobarbituric acid (0.8%), acetic acid (20%) and sodium dodecyl sulfate (8.1%) and kept in boiling water for 30 min. The absorbances of the reaction mixtures were measured at 535 nm.
Determination of reduced glutathione (GSH) level
Kidney and liver tissues were homogenized in TCA, and supernatants were used for the determination of GSH spectrophotometrically, according to the modified Ellman method (Aykaç et al., Citation1985). Briefly, the homogenates were mixed with TCA (10%) in equal volume and centrifuged at 3000 g for 15 min. The supernatants were mixed with Tris buffer (0.4 M) and 5,5’-Dithiobis (2-Nitro Benzoic Acid) (10 mM), and after 5 min, the absorbance was read at 412 nm.
Measurement of superoxide dismutase (SOD) activity
SOD activity in renal and hepatic tissues was measured by spectrophotometric method, based on the formazone chromogen formation from nitroblue tetrazolium in the presence of the superoxide radicals, which are produced by xanthine and xanthine oxidase (Sun et al., Citation1988). The SOD activity was measured spectrophotometrically reading the red color of the reaction mixture at a wavelength of 505 nm.
Measurement of catalase (CAT) activity
CAT activity of renal and hepatic tissues was detected according to the method based on the spectrophotometric monitoring of the H2O2 decrements per unit time at 240 nm (Aebi, Citation1984).
Activities of ALT and AST
Serum ALT and AST activities were measured colorimetrically by an auto-analyzer (Mindray-BS400) using commercial kits (OttoBC128 for ALT and OttoBC127 for AST, Otto Scientific, Ankara, Turkiye).
Albumin levels
Albumin levels were measured in serum samples by an auto-analyzer (Mindray-BS400) using commercial kits (OttoBC123, Otto Scientific, Ankara, Turkey).
Creatinine level
The spectrophotometric Jaffe method was used to analyze serum and urinary levels of creatinine. Then, these results and urine flow rate values were used to calculate glomerular filtration rate (GFR) with the following formula; GFR (ml/min) = (Urinary creatinine concentration (g/ml) × Urine flow rate (ml/min))/Serum creatinine concentration (g/ml) (Newman & Price, Citation1999).
Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (Kim-1) concentration
NGAL and Kim-1 levels in 24-h urine samples were determined spectrophotometrically using commercial ELISA kits (E-EL-R0662 for NGAL and E-EL-R3019 for Kim-1, Elabscience Biotechnology, Wuhan, China).
Statistical evaluation
All results are given as mean ± standard deviation (SD). Statistical evaluation was performed by one-way analysis of variance, and the post-hoc analysis was carried out by Tukey test using GraphPad 4.0 software. Values of p < .05 were accepted as statistically significant.
Results
In-vitro experiments
Characteristics of propolis extracts
The total amount of phenolic compound was determined as 88.20 ± 0.75 μg gallic acid/g sample for the PE and 129.05 ± 1.04 μg gallic acid/g sample for the PO. The total flavonoid content of the samples was 2.99 ± 0.11 μg quercetin/g sample for the PE and 5.12 ± 0.42 μg quercetin/g sample for the PO.
The reducing power was determined as 0.142 ± 0.01% for the PE (50 μg/mL), 0.168 ± 0.01% for the PO (50 μg/mL) and 0.195% for the BHT. The DPPH radical scavenging activity of the samples was 61.52 ± 1.02% for the PE, 70.80 ± 0.97% for the PO and 88.50% for the Trolox. The ABTS radical scavenging activity of the samples was calculated as 67.48 ± 2.25% for the PE, 74.03 ± 1.11% for the PO and 94.30% for the Trolox.
In-vivo experiments
Evaluation of renal oxidative stress
The renal MDA level was 104.67 ± 5.13 nmol/g wet tissue (wt) in the control group, and it was at the similar level in the PE and PO groups. The value significantly increased to 197.50 ± 6.20 nmol/g wt in the DOX group (p < .001). Both extracts of propolis significantly reduced DOX-induced MDA increments, but the PO was more effective in reducing MDA (p < .05) ().
Figure 1. Effects of the ethanolic propolis extract (PE) and oily propolis extract (PO) on oxidative stress status at the basal and DOX-induced conditions (PED, POD) in rat kidneys. Oxidative stress was evaluated by renal MDA level (a), GSH level (b), SOD activity (c) and CAT activity (d) in all groups. Results are expressed as mean ± SD (n = 6). Statistical difference from control *p < .01, **p < .001; from DOX # p < .001; from PE ϕp < .05, ϕϕp < .001; from PO θ p < .001; from ⊥ p < .05, ⊥⊥ p < .001 PED.
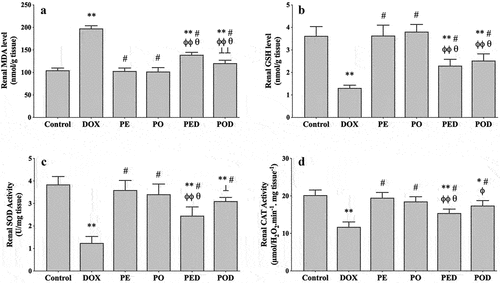
The renal GSH level was 3.63 ± 0.41 µmol/g wt in the control group. It did not change in the PE and PO groups but significantly reduced in the DOX group (1.31 ± 0.12 µmol/g wt, p < .001). Similar prevention in DOX-induced renal GSH depletion was observed in the PED and POD groups ().
The renal SOD activity was 3.84 ± 0.35 U/mg wt in the control group, and similar activity levels were observed in the PE and PO groups. In the DOX group, SOD activity significantly reduced to 1.25 ± 0.29 U/mg wt (p < .001). Treatment with propolis extracts significantly restored DOX-induced decrements in SOD activity. Compared to the PED group, SOD activity was higher in the POD group (p < .001) ().
The renal CAT activity was 20.22 ± 1.37 µmol.H2O2/min/mg wt in the control group. In the PE and PO groups, the CAT activity was not different from the control. In the DOX group, it significantly reduced to 11.74 ± 1.33 µmol.H2O2/min/mg wt (p < .001) and this effect was significantly restored in the PED and POD groups ().
Evaluation of liver oxidative stress
The hepatic MDA level was 110.58 ± 15.17 nmol/g wt in the control rats and did not change significantly after treatment with the PE or PO. In the DOX group, the MDA level significantly increased to 617.92 ± 21.92 nmol/g wt (p < .001). Treatment with the propolis extracts caused a reduction in DOX-induced hepatic MDA increments, yet the PO was more effective (p < .001) ().
Figure 2. Effects of the ethanolic propolis extract (PE) and oily propolis extract (PO) on oxidative stress status at the basal and DOX-induced conditions (PED, POD) in rat livers. Oxidative stress was evaluated by hepatic MDA level (a), GSH level (b), SOD activity (c) and CAT activity (d) in all groups. Results are expressed as mean ± SD (n = 6). Statistical difference from control *p < .001; from DOX # p < .001; from PE ϕp < .001; from PO θ p < .001; from PED ⊥ p < .01, ⊥⊥ p < .001.
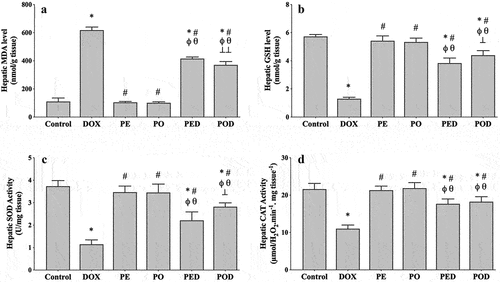
In the control group, the hepatic GSH concentration was 5.73 ± 0.14 µmol/g wt and did not change in the PE and PO groups. A notable GSH depletion was observed in the DOX group (1.30 ± 0.11 µmol/g wt, p < .001), but it was significantly alleviated by treatment with PE (p < .001) or PO (p < .001). Compared to the PED group, the hepatic GSH level was higher in the POD group (p < .01) ().
The hepatic SOD activity was 3.73 ± 0.25 U/mg wt and equivalent activity levels were observed in the PE and PO groups. After DOX injection, SOD activity significantly reduced to 1.15 ± 0.19 U/mg wt (p < .001). The reduction in enzyme activity caused by DOX was significantly attenuated by treatment with two of the propolis extract. However, PO appeared to restore SOD activity more effectively (p < .01) ().
The hepatic CAT activity was measured as 21.62 ± 1.53 µmol.H2O2/min/mg wt in the control group and no significant changes were observed in the PE and PO groups. In the DOX group, enzyme activity significantly reduced to 11.03 ± 0.97 µmol.H2O2/min/mg wt (p < .001). Compared to the DOX group, CAT activity prominently increased in the PED (p < .001) and POD groups (p < .001) at a similar level ().
Evaluation of serum and urinary markers
To evaluate the liver and kidney functions, some well-known parameters such as GFR and serum levels of creatinine, ALT, AST and albumin, as well as urinary levels of Kim-1 and NGAL, were studied, and the results are presented in . In the control, PE and PO groups, all those parameters were alike and not significantly different from each other. However, in the DOX group, DOX-induced renal injury and functional loss were confirmed by decreased GFR and increased serum creatinine, as well as increased levels of urinary Kim-1 and NGAL. Additionally, increased AST and ALT levels and decreased serum albumin levels suggesting hepatic injury and functional loss were also detected in the DOX group. Treatment with the PE or PO caused a significant alleviation of DOX-induced GFR decrements, serum creatinine, ALT and AST increments and urinary NGAL and Kim-1 increments. For those parameters, there was no significant difference between the PED and POD groups, which suggested that both extracts were capable of reducing the hepatorenal injury with similar efficiency ().
Table 1. Effects of the ethanolic propolis extract (PE) and oily propolis extract (PO) on serum and urinary parameters at the basal and DOX-induced conditions (PED, POD). Results are expressed as mean ± SD (n = 6).
Discussion
In the present study, the effects of propolis extract prepared in extra virgin olive oil against DOX-induced hepatorenal injury were examined for the first time, and the results were compared to the effects of the PE at the same conditions. Present data showed that the PO is more effective in reducing DOX-induced oxidative stress, both in kidney and liver tissues. However, according to the serum and urinary markers, which were used to evaluate the functional condition and damage status of the kidney and liver, both types of propolis extract exhibited similar protective effects.
It is quite well known that oxidative stress is an important contributor to DOX-related damage of the heart, liver and kidneys. DOX-induced ROS production mainly depends on its mitochondrial effects, and a substantial quantity of ROS is generated due to the disruption of the redox cycle at complex I during DOX treatment (Songbo et al., Citation2019). In hepatorenal tissues, the presence of oxidative stress after DOX administration has been confirmed by increased lipid peroxidation (Ali et al., Citation2020; Mohamed et al., Citation2022; Wided et al., Citation2014). Intracellular antioxidant enzymes such as SOD, CAT and glutathione peroxidase typically work to neutralize ROS; however, during DOX toxicity decreased activities of these defensive enzymes have been reported. Additionally, the concentration of GSH, which provides a non-enzymatic defense during oxidative stress, has been reported to be reduced during DOX treatment (Ali et al., Citation2020; Mohamed et al., Citation2022; Wided et al., Citation2014). In the present study, consistent with previous studies, 2 days after a single dose of DOX injection, an increase in oxidative stress, evidenced by an increase in MDA and a decrease in GSH levels in both kidney and liver tissues, and a decrease in SOD and CAT activities were observed.
According to the literature findings, increased hepatic and renal oxidative stress was accompanied by significant functional decrements of the liver and kidneys, respectively (Ali et al., Citation2020; Boutabet et al., Citation2011; Lahouel et al., Citation2010; Mohamed et al., Citation2022; Omar et al., Citation2016; Singla et al., Citation2014; Wided et al., Citation2014). DOX-induced functional loss of the liver was demonstrated by increased serum ALT and AST and decreased serum albumin levels in this study. Likewise, renal functional loss was approved by increased serum creatinine levels and decreased GFR. DOX-induced renal damage was also verified by increased urinary levels of NGAL and Kim-1, which are the main tubular injury markers in the urine (Zou et al., Citation2022). These results showed that single-dose injection of DOX successfully induced hepatic and renal toxicity in our experimental design, in accordance with the literature (El-Moselhy & El-Sheikh, Citation2014; Mansour et al., Citation1999). Present results showed that treatment with the PE for 2 weeks successfully reduced hepatorenal oxidative stress induced by DOX. Hepatic and renal MDA increments, GSH depletion, SOD and CAT activity loss evoked by DOX were partially reversed by treatment with the PE. In a similar way, DOX-induced changes in serum ALT, AST and creatinine levels, GFR value as well as urinary NGAL and Kim-1 concentrations were diminished by treatment with the PE. Although a histopathological evaluation has not been performed, which is a major deficiency of our study, present results suggested that treatment with a relatively low dose (50 mg/kg) of PE attenuated but did not completely repair DOX-induced hepatorenal damage. Since previous studies using propolis extract at higher doses than ours have shown a complete reversal of DOX-induced changes, we suggest that higher doses or longer treatment durations may be needed to restore parameters to control values (Boutabet et al., Citation2011; Lahouel et al., Citation2010; Wided et al., Citation2014). However, there are different studies reporting that hepatic or renal damage did not completely recover, even at higher doses or longer treatment durations of propolis (Ali et al., Citation2020; Mohamed et al., Citation2022; Omar et al., Citation2016; Singla et al., Citation2014).
Although ethanol is the most popular one, other solvents such as water, chloroform, polyethylene glycol and seed oil can be also used in propolis extraction (Forma & Bryś, Citation2021). Among these solvents, the olive oil is suggested to be safer than chemical solvents such as ethanol, polyethylene glycol or chloroform (Forma & Bryś, Citation2021; Silici et al., Citation2023) in addition to possessing antioxidant properties (Mataix et al., Citation2006). However, olive oil extraction of propolis has been less extensively used and its efficiency in DOX-induced hepatorenal toxicity has not been studied before. Thus, besides ethanol, the olive oil was also used to prepare propolis extract, and then total phenolic and flavonoid content, reducing power and radical scavenging activities of both extracts were evaluated in this work. In contrast to previous findings showing that ethanol is a better solvent than olive oil to get phenolic compounds (Kubiliene et al., Citation2015), our results presented comparable properties for the two extracts. A longer extraction time in olive oil (72 h) compared to ethanol (36 h) may have brought the extraction properties to a similar level in this study.
Apart from their characteristics, the effectiveness of these two different extracts was compared in DOX toxicity, and our results showed that PO was as successful as PE, even more effective for some parameters in reducing DOX-induced oxidative stress. For example, treatment with the PO caused a more efficient inhibition in DOX-induced MDA increments in both liver and kidney tissues, than the PE did. DOX-induced reduction in SOD activity was more markedly inhibited by PO in both liver and kidney. However, liver tissue seemed to get more benefits from oily extract, since pretreatment with PO caused a greater prevention of hepatic GSH depletion, compared to the kidney tissue.
As seen from our results, both the PE and PO treatments significantly reduced DOX-induced hepatorenal oxidative stress. These effects might be related to the scavenging of free radicals by antioxidant flavonoids found in propolis extracts or upregulation of the antioxidant enzyme system via its bioactive components, such as caffeic acid phenethyl ester (Ichikawa et al., Citation2002; Yang et al., Citation2017). Indeed, it has been reported that caffeic acid phenethyl ester can activate the nuclear factor E2-related factor 2 pathway, which upregulates a variety of antioxidant enzymes including SOD, CAT, glutathione peroxidase, glutathione reductase and heme oxygenase-1 (Yang et al., Citation2017). Better results obtained from the oily extract suggest that propolis and extra virgin olive oil may have a combined effect. Literature findings showing the beneficial effects of extra virgin olive oil itself in DOX-induced cardiotoxicity support this consideration (AlMalki & Shahid, Citation2020). On the other hand, the improvement in decreased serum and urine markers was comparable in the two treatment groups, the PED and POD. Considering the efficacy of oily extract in reducing oxidative stress, it may seem contradictory that quite similar changes in functional parameters were observed in the PED and POD groups. This can be explained by the inability of propolis and/or olive oil to affect all mechanisms governing DOX-induced tissue damage, except oxidative stress.
While oxidative stress remains a prominent factor, it is important to note that other cellular mechanisms such as inflammation and apoptosis also play a role in DOX-induced damage. As a limitation of our study, the effectiveness of propolis extracts has only been compared in terms of oxidative stress. Nevertheless, reducing oxidative stress may not always mean a reduction in functional loss. For example, Ali et al. (Citation2020) showed that the renal and cardiac oxidative stress induced by DOX was completely inhibited by the ethanol-based propolis extract (200 mg/kg, 4 weeks). However, at the same conditions, unimproved circulating levels of urea, B-type natriuretic peptide and troponin T suggested that kidneys and heart were not completely recovered, although oxidative stress was prevented after propolis treatment.
In conclusion, while the effects of the ethanol-based propolis extract have been investigated in more detail, the therapeutic potential of the olive oil-based propolis extract in DOX-induced hepatorenal damage has not been studied to date. Present results for the first time showed that the olive oil extract of propolis is quite effective in reducing DOX-induced damage to the liver and kidneys, and it is more capable than the ethanolic extract in terms of reducing oxidative stress. Although further studies are needed to get a more detailed comparison of the two extracts regarding their biologically active components or cellular action mechanisms, we conclude that olive oil extracts of propolis can be preferred in cases where ethanol cannot be used or is undesirable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbasnezhad, A., Salami, F., & Mohebbati, R. (2022). A review: Systematic research approach on toxicity model of liver and kidney in laboratory animals. Animal Models and Experimental Medicine, 5(5), 436–444. https://doi.org/10.1002/ame2.12230
- Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
- Ali, S. Y., Abdel-Moneim, A., & Abdel-Reheim, E. S. (2020). Protective effect of propolis on doxorubicin induced cardio-and nephrotoxicity. Annals of Cardiology and Vascular Medicine, 3(1), 1028.
- AlMalki, W. H., & Shahid, I. (2020). Characterization of antihypertensive and cardioprotective effects of extra virgin olive oil against doxorubicin induced cardiomyopathy in rats. Journal of Pharmacy & Pharmacognosy Research, 8(4), 316–326.
- Altaee, M. F. (2014). Cytogenetic analysis for the effect of alcoholic and water extracts of Iraqi propolis in mice. Current Research in Microbiology and Biotechnology, 2(1), 310–315.
- Aykaç, G., Uysal, M., Yalçin, A. S., Koçak-Toker, N., Sivas, A., & Oz, H. (1985). The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology, 36(1), 71–76. https://doi.org/10.1016/0300-483x(85)90008-3
- Bankova, V., Trusheva, B., & Popova, M. (2021). Propolis extraction methods: A review. Journal of Apicultural Research, 60(5), 734–743. https://doi.org/10.1080/00218839.2021.1901426
- Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181(4617), 1199–1200. https://doi.org/10.1038/1811199a0
- Boutabet, K., Kebsa, W., Alyane, M., & Lahouel, M. (2011). Polyphenolic fraction of Algerian propolis protects rat kidney against acute oxidative stress induced by doxorubicin. Indian Journal of Nephrology, 21(2), 101–106. https://doi.org/10.4103/0971-4065.82131
- Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–310. https://doi.org/10.1016/s0076-6879(78)52032-6
- Carvalho, C., Santos, R. X., Cardoso, S., Correia, S., Oliveira, P. J., Santos, M. S., & Moreira, P. I. (2009). Doxorubicin: The good, the bad and the ugly effect. Current Medicinal Chemistry, 16(25), 3267–3285. https://doi.org/10.2174/092986709788803312
- Chang, C. C., Yang, M. H., Wen, H. M., & Chern, J. C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10(3), 178–182. https://doi.org/10.38212/2224-6614.2748
- El-Moselhy, M. A., & El-Sheikh, A. A. (2014). Protective mechanisms of atorvastatin against doxorubicin-induced hepato-renal toxicity. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 68(1), 101–110. https://doi.org/10.1016/j.biopha.2013.09.001
- Forma, E., & Bryś, M. (2021). Anticancer activity of propolis and its compounds. Nutrients, 13(8), 2594. https://doi.org/10.3390/nu13082594
- Granados-Principal, S., Quiles, J. L., Ramirez-Tortosa, C. L., Sanchez-Rovira, P., & Ramirez-Tortosa, M. C. (2010). New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 48(6), 1425–1438. https://doi.org/10.1016/j.fct.2010.04.007
- Hossain, S., Yousaf, M., Liu, Y., Chang, D., & Zhou, X. (2022). An overview of the evidence and mechanism of drug-herb interactions between propolis and pharmaceutical drugs. Frontiers in Pharmacology, 13, 876183. https://doi.org/10.3389/fphar.2022.876183
- Hwang, J. Y., Shue, Y. S., & Chang, H. M. (2001). Antioxidative activity of roasted and defatted peanut kernels. Food Research International, 34(7), 639–647. https://doi.org/10.1016/S0963-9969(01)00083-7
- Ichikawa, H., Satoh, K., Tobe, T., Yasuda, I., Ushio, F., Matsumoto, K., Endo, K., & Ookubo, C. (2002). Free radical scavenging activity of propolis. Redox Report: Communications in Free Radical Research, 7(5), 347–350. https://doi.org/10.1179/135100002125000965
- Kalender, Y., Yel, M., & Kalender, S. (2005). Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology, 209(1), 39–45. https://doi.org/10.1016/j.tox.2004.12.003
- Kosalec, I., Bakmaz, M., Pepeljnjak, S., & Vladimir-Knezević, S. (2004). Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharmaceutica (Zagreb, Croatia), 54(1), 65–72.
- Kubiliene, L., Laugaliene, V., Pavilonis, A., Maruska, A., Majiene, D., Barcauskaite, K., Kubilius, R., Kasparaviciene, G., & Savickas, A. (2015). Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complementary and Alternative Medicine, 15(1), 156. https://doi.org/10.1186/s12906-015-0677-5
- Lahouel, M., Boutabet, K., Kebsa, W., & Alyane, M. (2010). Polyphenolic fractions of Algerian propolis reverses doxorubicin induced acute renal oxidative stress. African Journal of Pharmacy and Pharmacology, 4(10), 712–720.
- Mansour, M. A., El-Kashef, H. A., & Al-Shabanah, O. A. (1999). Effect of captopril on doxorubicin-induced nephrotoxicity in normal rats. Pharmacological Research, 39(3), 233–237. https://doi.org/10.1006/phrs.1998.0432
- Mataix, J., Ochoa, J. J., & Quiles, J. L. (2006). Olive oil and mitochondrial oxidative stress. International Journal for Vitamin and Nutrition Research, 76(4), 178–183. https://doi.org/10.1024/0300-9831.76.4.178
- Mathew, S., & Abraham, T. E. (2006). In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 44(2), 198–206. https://doi.org/10.1016/j.fct.2005.06.013
- Mohamed, H. K., Mobasher, M. A., Ebiya, R. A., Hassen, M. T., Hagag, H. M., El-Sayed, R., Abdel-Ghany, S., Said, M. M., & Awad, N. S. (2022). Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing nephrotoxicity induced by doxorubicin in male albino rats. Antioxidants (Basel, Switzerland), 11(5), 1029. https://doi.org/10.3390/antiox11051029
- Molehin, O. R., Adeyanju, A. A., Adefegha, S. A., Oyeyemi, A. O., & Idowu, K. A. (2019). Protective mechanisms of protocatechuic acid against doxorubicin-induced nephrotoxicity in rat model. Journal of Basic and Clinical Physiology and Pharmacology, 30(4), 10.1515/jbcpp-2018–0191. https://doi.org/10.1515/jbcpp-2018-0191
- Nakajima, Y., Tsuruma, K., Shimazawa, M., Mishima, S., & Hara, H. (2009). Comparison of bee products based on assays of antioxidant capacities. BMC Complementary and Alternative Medicine, 9(1), 4. https://doi.org/10.1186/1472-6882-9-4
- Newman, D. J., & Price, C. P. (1999). Renal function and nitrogen metabolites. In B. CA and E. Ashwood (Eds.), Tietz textbook of clinical chemistry (pp. 1204–1270). WB Saunders Company.
- Omar, N. A. A., Allithy, A. N. E. A., Baghdadi, H., Zolaly, M., Abdel-Haleem, M., Helmy, M. M., Ayat, M. M., & El Sayed, S. M. (2016). Hepatoprotective effects exerted by propolis against doxorubicin-induced rat liver toxicity: A biochemical and histopathological study. American Journal of Cancer Prevention, 4, 36–40. https://doi.org/10.12691/ajcp-4-3-1
- Prasanna, P. L., Renu, K., & Valsala Gopalakrishnan, A. (2020). New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sciences, 250, 117599. https://doi.org/10.1016/j.lfs.2020.117599
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26(9–10), 1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
- Silici, S., Okan, A., Köklü, B., Demiray, S., & Doğanyiğit, Z. (2023). Toxicity of propylene glycol extract of propolis on central nervous system and liver in pregnant and neonatal rats. Zeitschrift für geburtshilfe und neonatologie. Advance online publication. https://doi.org/10.1055/a-2010-4009
- Singla, S., Kumar, N. R., & Kaur, J. (2014). In vivo studies on the protective effect of propolis on doxorubicin-induced toxicity in liver of male rats. Toxicology International, 21(2), 191–195. https://doi.org/10.4103/0971-6580.139808
- Songbo, M., Lang, H., Xinyong, C., Bin, X., Ping, Z., & Liang, S. (2019). Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicology Letters, 307, 41–48. https://doi.org/10.1016/j.toxlet.2019.02.013
- Sun, Y., Oberley, L. W., & Li, Y. (1988). A simple method for clinical assay of superoxide dismutase. Clinical Chemistry, 34(3), 497–500. https://doi.org/10.1093/clinchem/34.3.497
- Wided, K., Hassiba, R., & Mesbah, L. (2014). Polyphenolic fraction of Algerian propolis reverses doxorubicin induced oxidative stress in liver cells and mitochondria. Pakistan Journal of Pharmaceutical Sciences, 27(6), 1891–1897.
- Yang, N., Shi, J. J., Wu, F. P., Li, M., Zhang, X., Li, Y. P., Zhai, S., Jia, X. L., & Dang, S. S. (2017). Caffeic acid phenethyl ester up-regulates antioxidant levels in hepatic stellate cell line T6 via an Nrf2-mediated mitogen activated protein kinases pathway. World Journal of Gastroenterology, 23(7), 1203–1214. https://doi.org/10.3748/wjg.v23.i7.1203
- Zou, C., Wang, C., & Lu, L. (2022). Advances in the study of subclinical AKI biomarkers. Frontiers in Physiology, 13, 960059. https://doi.org/10.3389/fphys.2022.960059
