 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to optimize an extruded aquafeed for Oreochromis niloticus (Nile tilapia) using moringa as a partial protein source. Response surface methodology (RSM) with a Rotatable Central Composite Design (CCRD) was employed to evaluate the four factors influence: sardine meal (28.5–39.25%), moringa flour (34.85–45.60%), temperature (110–140°C) and moisture content (13–17%) on aquafeed’s physicochemical characteristics: expansion index (EI), bulk density, water solubility index (WSI), water absorption index (WAI), hardness and buoyancy. Numerical optimization identified 100 potential diets, of which four were selected and met the criteria for high WSI (11–14%), low hardness (22–32 N) and 100% buoyancy. The best diet consisted of 31.19% sardine meal and 42.91% moringa flour, processed at 132.5°C and 16% moisture, presenting 13.68% WSI, full buoyancy, 29.63 N hardness, 36.33% protein, 10.71% lipids, and 31.36% carbohydrates, applicable to fingerling O. niloticus.
1. Introduction
Feeding is a significant cost factor in aquaculture production, comprising 40% to 80% of the total production costs (Ansari et al., Citation2021; Hasan, Citation2017). Currently, developments in aquafeed focus on exploring new ingredients to reduce costs while maintaining nutritional balance and favourable physicochemical properties, such as, buoyancy, bulk density, expansion index (EI), water solubility index (WSI), water absorption index (WAI), and hardness. These attributes play a crucial role in the application of these aquafeeds (Hardy, Citation2010). WSI, hardness, and buoyancy are particularly important physicochemical characteristics in aquafeed formulation. The high WSI suggests greater starch disorganization in aquafeeds, potentially leading to improved assimilation and reduced processing costs by eliminating the need for pre-cooking to pregelatinize the starch (Chaabani et al., Citation2022; Samuelsen et al., Citation2013). Hardness in aquafeeds is essential since it must be firm enough to withstand transportation without cracking. However, excessive hardness may result in reduced intake, economic losses, and nutritional drawbacks (Khan et al., Citation2022; Ma et al., Citation2018). Increased buoyancy extends the time aquafeed can be consumed, and this characteristic can be obtained by applying a layer of oil to the product. However, extrusion technology can also provide this desirable characteristic of the product (Orire & Salihu, Citation2020; Soto-Rodríguez et al., Citation2020).
While the nutritional requirements of aquafeeds vary by species, sufficient protein content is essential for the growth and development of all organisms. For instance, in Oreochromis niloticus (Nile tilapia), a protein deficiency can impede growth. However, excessive protein intake is not recommended, as the surplus protein is utilized as an energy source and can increase nitrogen levels in wastewater (Singha et al., Citation2021). The protein content in tilapia aquafeed is in the range from 30% to 50%, depending on the fish’s growth stage and is mainly provided by fishmeal (Rokey et al., Citation2010).
However, new sources of vegetable meals and animals like insect meals for partial fishmeal substitution are being studied, without adversely impacting the final protein content of the aquafeed (Hodar et al., Citation2020). The protein content remains unaffected by the extrusion process. This procedure involves mixing, transportation, and thermoforming of ingredients under conditions of low moisture, high temperature, and high pressure in a short time, using shear forces exerted by a screw (Gil, Citation2010; Soto-Rodríguez et al., Citation2020). This process enables the production of extruded aquafeeds with desirable aquaculture properties, including buoyancy, achieved through expansion during processing (Das et al., Citation2018).
The inclusion of vegetable meals in aquafeeds also increases the carbohydrate content, serving as a vital energy source in diets, facilitating protein retention in the fish and reducing nitrogen discharge in wastewater (Kamalam & Panserat, Citation2016). Furthermore, the moisture content during extrusion has an influence on the quality of the aquafeed, affecting its EI, WSI, WAI and hardness, which, in turn, affects its various applications (Mugabi et al., Citation2022).
The scientific innovation of using a vegetable protein source, such as moringa, for optimizing aquafeed, addresses the environmental and sustainability changes associated with using animal protein in aquaculture diets. Traditional aquafeeds heavily rely on fishmeal, contributing to overfishing and the depletion of wild fish stocks (Grainger, Citation2016). Moringa offers an alternative to reduce dependence on fishmeal and other animal-origin proteins due to its high protein content (26%). It allows for partial or complete replacement of fishmeal while improving fish growth and weight gain. Additionally, the rich phytochemical compounds in moringa flour enhance fish health by bolstering the immune response and resistance to bacterial pathogens (Abdel-Latif et al., Citation2022). Although moringa flour contains some anti-nutritional factors, extrusion technology has proven effective in reducing these factors, such as tannins and phytates (Duguma et al., Citation2021; Rathod & Annapure, Citation2016). On the other hand, the inclusion of this meal promises to reduce the environmental footprint of aquaculture by increasing aquafeed digestibility and preserving water quality, including pH and dissolved oxygen levels, which are crucial for species development (Ajithkumar et al., Citation2021; Nsofor et al., Citation2012; Windarti & Kurniawan, Citation2023). Finally, the cultivation of moringa, a fast-growing and drought-resistant plant, offers economic benefits as it can be grown in various regions with minimal resource needs. The development of aquafeed formulation has the potential to transform aquaculture practices, making them more sustainable, cost-effective, and environmentally friendly. For that, the objective of this research was to develop and optimize an extruded aquafeed (in the form of pellets) to satisfy the physicochemical and nutritional requirements of O. niloticus, using moringa as a partial source of proteins and carbohydrates.
2. Methodology
2.1. Raw materials
Sardine flour (Maz Industrial S.A. de C.V., Mexico), moringa flour (Mi Granero S.A. de C.V., Mexico), gelatin (Coloidales Duche S.A. de C.V., Mexico), and vitamin and mineral mixes (Florida Aqua Farms Inc, U.S.A.) were donated by the Polytechnic University of Pachuca. Corn flour (SACSA, Mexico) was provided by the Autonomous University of the State of Hidalgo. The composition of the vitamin and mineral mixes is detailed in Appendix A. All flours underwent particle size reduction through grinding in a high-speed electric pulverization mill (JFIEEI, T-series Grinder, SV-MOL-100, CN) and were then sieved through a 70# ASTM mesh sieve, resulting in a particle size of 0.21 mm.
2.2. Proximal chemical analysis of raw materials
The proximal chemical analysis of the sardine meal, corn, and moringa flours, as well as, gelatin, was carried out using AOAC methods: 0.25.09 for moisture, 962.09 for lipids, 923.03 for ash, 962.09 for crude fiber, and 2001.11 for protein (AOAC, Citation1990). The carbohydrate content was calculated by difference according to EquationEquation (1)(1)
(1) :
2.3. Obtaining extruded aquafeed
To evaluate the influence of the addition of moringa flour in aquafeed formulation, a Rotatable Central Composite Design (RCCD) involving four factors was employed, as outlined in section 2.5.1.
2.3.1. Extrusion process
The extrusion process follows the methodology of Soto-Rodríguez et al. (Citation2020) with some modifications. The formulated diets based on the RCCD () were processed using a single-screw laboratory extruder 19/25 L/D (Brabender instrument, model 832005.007, GmbH & Co. KG. KG, Germany), with four heating zones, where the exit zone temperature was according to the RCCD (). The other heating zones were set to a ramp of 15°C below each other, except for the feeding zone, which was maintained at 60°C to prevent starch gelatinization. The screw used had a compression ratio of 2:1, with feed and screw speeds of 60 and 160 rpm, respectively. A 2 mm exit die was used.
Table 1. Rotatable central composite design.
2.4. Physicochemical characterization of the extruded aquafeed
2.4.1. Expansion index (EI)
It was determined according to the methodology reported by Gujska and Khan (Citation1990), dividing the diameter of the extruded aquafeed (pellet) by the diameter of the orifice of the extruder exit die.
2.4.2. Bulk density
It was determined according to the methodology reported by Wang et al. (Citation1993) dividing the weight of the extrudate (in g) by its volume (in cm3).
2.4.3. Water absorption index (WAI) and water solubility index (WSI)
WAI and WSI were determined using a modified version of the methodology described by Anderson et al. (Citation1970). One gram of the extruded aquafeed (in pellet form) was weighed (db) in a 50 mL falcon tube, followed by the addition of 10 mL of distilled water. The sample in the tube was stirred for 1 min in a vortex (IKA, Werke, Europe) and centrifuged for 30 min at 3000 × g, in a centrifuge (LAb-Tech, Hermle Z300, Germany). The supernatant was decanted into an aluminum tray, previously placed at constant weight. The tray was dried in an oven (Sel lab, U.S.A.) for 24 h at 110°C. The tube containing the solid sample was weighed again. WAI represented the water retained per gram of the sample, while WSI was expressed as the percentage of dissolved solids in the supernatant.
2.4.4. Hardness
Hardness was determined using a texturometer (Texturolab, TA. XT-Plus, the UK) following the method of Soto-Rodríguez et al. (Citation2020). A 25 mm cylindrical probe was used at a distance of 2 mm, a force of 0.049 N, a speed of 10 mm/s and a contact force of 2.65 N. The hardness was calculated as the force required to cut the product in a compression test. The results were reported as the average of 60 measurements in newtons (N).
2.4.5. Buoyancy
The buoyancy was carried out according to the methodology reported by Vargas (Citation2003) with some modifications. The result obtained was reported as the percentage of aquafeeds (in the form of pellets), which floated after 30 min of being suspended in a cylinder with 500 mL of water.
2.5. Design, statistical analysis, and optimization
2.5.1. Rotatable central composite design (RCCD)
A rotatable central composite design (RCCD) of four factors was used, the sardine meal and moringa flour contents, temperature, and moisture ().
2.5.2. Statistical analysis
A one-way ANOVA was used, and the comparison of means (p ≤ .05) was carried out with the Tukey test using Statistica version 8.5.1.
2.5.3. Aquafeed optimization and validation
Numerical optimization was carried out taking as responses the WSI, the hardness and the buoyancy of the aquafeed, since these are the main physicochemical characteristics of interest, obtaining a total of 100 optimal diets to generate aquafeeds of which the four optimal diets were taken considering the highest percentage of buoyancy (100%), low hardness (less than 30 N) and lower percentage of sardine flour in the formulation (31–32%). On the other hand, the desirability presented by these four optimal diets was 1 (). These optimal diets to generate the optimal aquafeed were validated according to the physicochemical characterization of the extruded aquafeed as described in section 2.4 with respect to those predicted by the Design Expert 9.0 program (). The validation of the optimal aquafeeds was complemented with the analysis of two commercial aquafeeds (controls 1 and 2) as a reference, with similar diameters to the aquafeed obtained. Finally, the proximal chemical analysis of the optimal and commercial aquafeeds was carried out as described in section 2.2, to identify the compliance of the requirements for O. niloticus.
Table 2. Optimal diets for the formulation of an aquafeed and predicted values of WSI, hardness and buoyancy for its validation.
3. Results and discussions
3.1. Proximal chemical analysis of raw materials
Statistically significant differences (p ≤ .05) were observed in the protein content of all raw materials due to their diverse sources (). The highest content was noted in gelatin, which is primarily used as a binder rather than a protein source due to incorporation challenges during the extrusion process if used in excess (Barreto-Curiel et al., Citation2018). Sardine meal emerged as the principal protein source, thanks to its complete essential amino acid profile and high nutrient digestibility (Daniel, Citation2018). Moringa flour also presented a significant protein content (20.24%), higher compared to other vegetable proteins such as wheat, barley, quinoa, amaranth, and rice (6.7%−16.49%). Its elevated protein content and favourable amino acid profile position it as a promising protein source for aquafeeds (Abdel-Latif et al., Citation2022; Awadalkareem et al., Citation2008; Awadelkarim et al., Citation2018; Manuel et al., Citation2019; Mota et al., Citation2016).
Table 3. Proximal chemical analysis of raw materials used in the formulation of an extruded food.
The highest lipid contents were found in moringa flour and sardine meal. The lipid content in moringa flour aligned with values reported by Nurcahyani et al. (Citation2019), indicating its suitability as a lipid source for aquafeed meeting O. niloticus requirements. In contrast, corn flour showed higher lipid values than the 1.7% reported by Sánchez et al. (Citation2014). Sardine meal exhibited notably high ash content but was lower than the approximately 21% reported for fishmeal by Tarhouni et al. (Citation2019) and Pérez-Viveros (Citation2017). Moringa flour had the highest crude fiber content, although it was lower than the 7.9% reported by Adewumi (Citation2014). This lower fiber content is advantageous for aquafeed formulation, as diets with less than 8% fiber are preferred to prevent potential issues with extruded aquafeed expansion (Martin et al., Citation2019; Mondal et al., Citation2016).
Moisture levels exhibited significant variation (p ≤ .05) in all raw materials. Corn flour had the highest moisture content, though it remained lower than the 12.43% reported by Pérez-Viveros (Citation2017). Except for corn flour, moisture content in all ingredients was below 10%. However, according to Magan and Aldred (Citation2007), the recommended moisture percentage for corn storage is 14%. Ash content also varied among all raw materials, with sardine meal having the highest value, similar to 16.13% reported by Kirimi et al. (Citation2017). This elevated ash content may be attributed to mineral content and presence of inorganic compounds in the raw materials, such as head bones, backbone, and viscera (Janbakhsh et al., Citation2018). Finally, corn flour showed the highest carbohydrate values, similar to 81.6% reported by Sánchez et al. (Citation2014). Moringa also had a high carbohydrate content, comparable to the values reported by Teixeira et al. (Citation2014) and Idris Maizuwo et al. (Citation2017), who reported values of 44.3% and 44.4%, respectively. Both sources have the potential to serve as an energy source for O. niloticus, as the species typically can use over 30% carbohydrates in its diet (Boonanuntanasarn et al., Citation2018).
Substituting sardine meal with moringa flour in aquafeed provides a sustainable and high-quality alternative. This substitution contributes to the reduction of issues of overfishing and limited resources derived from the obtention of fishmeal.
3.2. Physicochemical characterization of the extruded aquafeed
The estimated coefficients of the adjusted quadratic model for EI, bulk density, WSI, WAI, hardness, and buoyancy of the extrudates are presented in . Significance (p ≤ .05) was observed for the extrusion process parameters in all responses.
Table 4. Regression coefficient analysis.
The impacts of sardine meal and moringa flour concentrations, as well as moisture and temperature on the EI, bulk density, WSI, WAI, hardness, and buoyancy, are illustrated in and respectively.
Figure 1. Response surface plots showing the effect of moringa flour and sardine meal concentration on (a) expansion index (EI), (b) bulk density, (c) water solubility index (WSI) (d) water absorption index (WAI), (e) hardness and (f) buoyancy of extruded feeds.
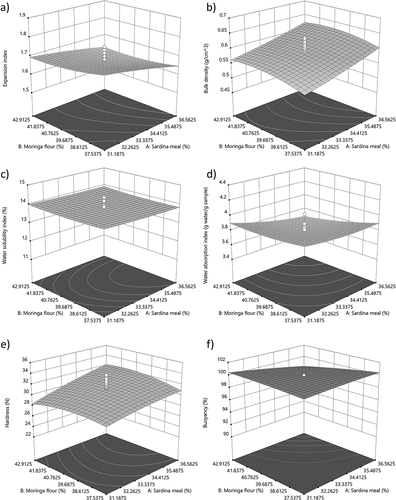
Figure 2. Response surface plots showing the effect of moisture and temperature process on (a) expansion index (EI), (b) bulk density, (c) water solubility index (WSI) (d) water absorption index (WAI), (e) hardness and (f) buoyancy of extruded.
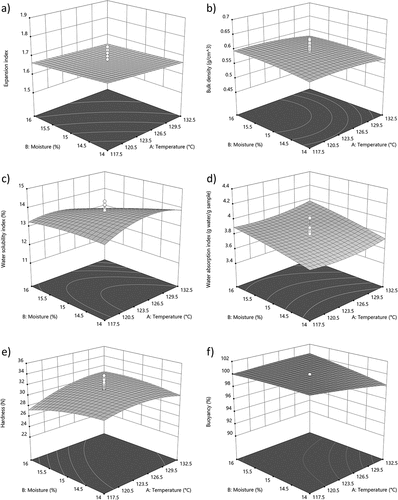
EI was negatively affected by higher concentrations of sardine meal and moringa flour (), possibly due to the lipid and fiber content in these raw materials (). Moringa flour contains a significant amount of lipids, which can interfere with shear forces, resulting in less mechanical damage to starch and fiber chains and consequently, reduced expansion (Delgado et al., Citation2021). This leads to a higher bulk density in extruded aquafeeds (). On the other hand, EI decreased at higher moisture (). Water in the mixture acts as a lubricant, increasing extruder outflow, reducing barrel pressure and screw shear, resulting in reduced expansion of the extruded aquafeed (Neder-Suárez et al., Citation2021).
WSI increased at lower concentrations of sardine meal and moringa flour (). This indicates enhanced structural disorganization of corn flour starch in aquafeed formulation, resulting in shorter amylose and amylopectin chains, which is beneficial for assimilation (Samuelsen et al., Citation2013). The higher temperature and moisture levels during the extrusion process () caused a decrease in WSI that can be attributed to the increased water content reducing the mechanical shear inside the barrel and resulting in less structural disorganization of starch and fibers (Delgado et al., Citation2021; Singh & Muthukumarappan, Citation2016; Ye et al., Citation2018; Yousf et al., Citation2017).
WAI increased with decreasing concentrations of sardine meal and moringa flour in the aquafeed, likely due to reduced lipid content in the mixture. This reduction allows for greater structural damage to the starch, resulting in a higher capacity for water absorption (Delgado et al., Citation2021). Furthermore, an increase in moisture content and processing temperature correlated with higher WAI values. These conditions enable partial gelatinization of starch, releasing amylose and amylopectin chains, which enhance the extruded feed’s water retention capacity (Neder‐Suárez et al., Citation2020; Singh et al., Citation2007).
Aquafeeds with higher moringa flour and sardine meal contents showed increased hardness values (), resulting in more compact extruded aquafeeds, as confirmed by the EI results (). This was associated with a decrease in WAI (), and an increase in bulk density () (Ahmad et al., Citation2019; Samuelsen et al., Citation2013). The buoyancy of the extruded aquafeeds was inversely proportional to the hardness results (). Moisture content during extrusion had a negative impact on extruded aquafeeds hardness. A smaller amount of water in the mixture resulted in fewer pores at the extruder outlet due to minimal water evaporation, leading to compact extrudates (Kannadhason et al., Citation2009; Wang et al., Citation2021). Consequently, this condition resulted in a decrease in buoyancy percentages ().
The observed impacts on the physicochemical properties of the aquafeed suggest that both the moringa flour and sardine meal concentration, as well as extrusion conditions, play a pivotal role in defining the product’s quality. The aquafeeds obtained showed improved WSI values and hardness, suggesting that replacing sardine meal with moringa flour does not compromise the feed’s physical properties, in fact, it can be beneficial for its application.
3.3. Optimization and validation of extruded aquafeed
The data from the analyzed optimal diets () show a correlation between lower EI and higher bulk density. The WSI values in these diets exceeded those in controls 1 and 2. The WSI values for diets 96 and 16 closely matched the numerical optimization predictions in , while diets 2 and 16 diverged from the predictions. These variations in solubility could be attributed to the fiber content contributed by sardine meal and moringa flour as protein sources, as well as the processing conditions (Yousf et al., Citation2017). Fiber content in extruder food impacts its solubility, as reported by Kallu et al. (Citation2017). Increased solubility in aquafeed is beneficial because it indicates better starch digestibility from corn flour, which correlates with its degree of gelatinization and structural disorganization during the extrusion (Ye et al., Citation2018).
Table 5. Physicochemical characterization of optimal extruded diets and commercial feeds.
Regarding WAI, optimal diets, except for diet 96, showed significant differences compared to control 1. Diets 2, 16, and 53 were statistically similar to control 2. These values are suitable for aquafeed application, indicating the presence of short starch chains post-extrusion, promoted by structural disorganization (Lu et al., Citation2019). Additionally, the inclusion of fiber in the diet affects the aquafeed’s absorption capacity, as reported by Kallu et al. (Citation2017), who found lower WAI values with fiber inclusion.
The hardness values of all optimal diets differed significantly from controls 1 and 2, being higher than control 1 and lower than control 2. These results can be attributed to the inclusion of moringa flour in the diet, which contributes to a higher fiber content, resulting in more fragile extrudates (Martin et al., Citation2019). However, the predicted results for diets 2 and 16 fell within the observed ranges in this study. Finally, the buoyancy results of all optimal diets were like control 2, achieving the predicted values of 100% for diets 2, 16, and 96, with diet 53 reaching 99.98%, fulfilling the buoyancy requirement for O. niloticus.
3.3.1. Proximal chemical analysis of optimal diets and control
The moisture content in the optimal aquafeed diets ranged from 7% to 9%, with statistically significant differences (p ≤ .05) compared to control 2 and control 1. All diets had moisture content below 10% (). This moisture range aligns with ideal conditions for aquafeed storage, preventing mold growth, and underscores the practical viability of these feeds for long-term storage and transport, which are essential considerations for large-scale aquaculture operations (Teruel, Citation2002).
Table 6. Proximal chemical analysis of optimal extruded diets and commercial feeds.
Regarding the lipid content (), all the optimal diets had lipid contents of less than 10%. Notably, diet 53 was statistically similar (p ≤ .05) to control 2, while all diets had lower contents than control. As per Ng and Chong’s (Citation2004) findings, which stipulate a minimum requirement of 5% and an optimal range of 10%−15% for lipid content, diet 16 fell below the minimum value, whereas diets 2 and 96 met the optimal range. These findings suggest that diets 2 and 96 are better suited to meet the lipid requirements of the fish being fed.
The crude fiber results of all the diets () were below 2%, which is lower than that of control 2. The high fiber content should not exceed 10% of the diet, as it negatively affects growth by decreasing gut passage time (Anderson et al., Citation1984; Liu et al., Citation2013). The protein content of the optimal diets for aquafeed production () ranges from 33.84% to 36.33%. Diet 16 was statistically similar (p ≤ .05) to control 2. In comparison to control 1, all diets had lower protein content. However, following the O. niloticus requirements reported by Liu et al. (Citation2013), diets 2, 96, and 53, with protein contents greater than 35%, are suitable for a fry stage culture, while diet 16, with a protein content of 33.84%, can be used in juvenile-stage culture.
The ash content in all the optimal diets () exceeds 10%, presenting statistically significant differences (p ≤ .05) compared to controls 1 and 2. This increase can be attributed to the incorporation of a 3% mineral mix into the diet and the presence of minerals and inorganic compounds in the sardine meal (Janbakhsh et al., Citation2018). Furthermore, the carbohydrate content in the diets () ranges between 30% and 41%. Diet 16 was statistically similar (p ≤ .05) to control 2. In comparison to control 1, all diets had higher carbohydrate values. Nevertheless, these carbohydrate contents can serve as an energy source for O. niloticus (Anderson et al., Citation1984; Boonanuntanasarn et al., Citation2018).
The formulated aquafeeds met the nutrient requirements for O. niloticus, demonstrating the feasibility of sustainable aquafeed production using alternative protein sources such as moringa meal. Despite the comprehensive study of aquafeed formulation and extrusion parameters to ensure physicochemical and nutritional quality, it remains important to assess the long-term effects of these aquafeeds on fish health and growth to validate their efficacy in aquaculture. Additionally, it is crucial to investigate the impact of moringa meal on fish immunity, disease resistance, and feed acceptability.
While this study provides a foundation for the use of alternative protein sources in aquafeeds, it is worth noting that the effects may vary among fish species due to their dietary preferences and digestive physiology, therefore, further research is necessary to extend this methodology to other species.
4. Conclusions
The concentrations of sardine meal and moringa flour, along with processing temperature and moisture, significantly influenced the physicochemical properties of the product. This allowed us to produce aquafeeds with 100% buoyancy and high WSI values (ranging from 12.4% to 13.67%), which will facilitate enhanced starch assimilation. Hardness values between 29.63 N and 32.01 N were lower than those of control 2, potentially contributing to improved feed digestibility.
Based on the above findings and considering the primary goal of substituting sardine meal, the most effective diet was formulated with 31.19% sardine meal and 42.91% moringa flour, processed at an extrusion temperature of 132.5°C with a moisture content of 16%. Under these conditions, a product with desirable physicochemical properties, such as a high WSI (13.68% ± 0.24), buoyancy (100% ± 0.00), and low hardness (29.63 N ± 2.78) was obtained. Furthermore, this product met the nutritional requirements (36.33% ± 0.26 protein, 10.71% ± 0.46 lipids, and 31.36% ± 1.18 carbohydrates) necessary for its application in the culture of O. niloticus during the fingerling stage.
Therefore, it can be concluded that moringa flour can replace up to 42.91% of sardine meal without compromising the physicochemical and nutritional quality of the aquafeed produced through extrusion.
Acknowledgments
The first author thanks the support to Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT) for the scholarship awarded (scholarship number 787722; CVU: 673637) to carry out the Doctorate of Sciences in Biotechnology in the postgraduate program of Universidad Politécnica de Pachuca, included in the Programa Nacional de Posgrados de Calidad. To the Universidad Politécnica de Pachuca and the Universidad Autónoma del Estado de Hidalgo for providing the raw materials, materials, equipment, and knowledge for the generation of this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdel-Latif, H. M., Abdel-Daim, M. M., Shukry, M., Nowosad, J., & Kucharczyk, D. (2022). Benefits and applications of Moringa oleifera as a plant protein source in aquafeed: A review. Aquaculture, 547, 737369. https://doi.org/10.1016/j.aquaculture.2021.737369
- Adewumi, A. A. (2014). Moringa oleifera (Lam) as a protein supplement in Clarias gariepinus diet. Advances in Research, 2(11), 580–589. https://doi.org/10.9734/AIR/2014/10399
- Ahmad, R., Oterhals, A., Xue, Y., Skodvin, T., & Samuelsen, T. A. (2019). Impact of fish protein concentrate on apparent viscosity and physical properties of soy protein concentrate subjected to thermomechanical treatment. Journal of Food Engineering, 259, 34–43. https://doi.org/10.1016/j.jfoodeng.2019.04.014
- Ajithkumar, M., Bhavatharanya, U., Manikandan, V., & Menaga, M. (2021). Effect of moringa (Moringa oleifera Lam.) leaf meal supplementation on growth and nutrient digestibility of rohu and PACU under different culture practices. The Pharma Innovation Journal, 10(6), 328–332. www.thepharmajournal.com
- Anderson, R. A., Conway, H. F., & Peplinski, A. J. (1970). Gelatinization of corn grits by roll cooking, extrusion cooking and steaming. Starch-stärke, 22(4), 130–135. https://doi.org/10.1002/star.19700220408
- Anderson, J., Jackson, A. J., Matty, A. J., & Capper, B. S. (1984). Effects of dietary carbohydrate and fibre on the tilapia Oreochromis niloticus (Linn.). Aquaculture, 37(4), 303–314. https://doi.org/10.1016/0044-8486(84)90296-5
- Ansari, F. A., Guldhe, A., Gupta, S. K., Rawat, I., & Bux, F. (2021). Improving the feasibility of aquaculture feed by using microalgae. Environmental Science and Pollution Research, 28(32), 43234–43257. https://doi.org/10.1007/s11356-021-14989-x
- AOAC. (1990). Association of official analytical chemists - official methods of analysis. The association of official analytical chemists.
- Awadalkareem, A. M., Mustafa, A. I., & El Tinay, A. H. (2008). Protein, mineral contents and amino acid profile of sorghum flour as influenced by soybean protein concentrate supplementation. Pakistan Journal of Nutrition, 7(3), 475–479. https://doi.org/10.3923/pjn.2008.475.479
- Awadelkarim, A. M., Ashraf, S. A., Adnan, M., Alshammari, E., & Sulieman, A. M. E. (2018). Nutritional characterization and Evaluation of wheat and barley cultivated in Ha’il and Al-Qassim province of Saudi Arabia. Advances in Bioresearch, 9(4), 35–40. https://doi.org/10.15515/abr.0976-4585.9.4.3540
- Barreto-Curiel, F., Ramirez-Puebla, S. T., Ringo, E., Escobar-Zepeda, A., Godoy-Lozano, E., Vazquez-Duhalt, R., Sanchez-Flores, A., & Viana, M. T. (2018). Effects of extruded aquafeed on growth performance and gut microbiome of juvenile Totoaba macdonaldi. Animal Feed Science and Technology, 245, 91–103. https://doi.org/10.1016/j.anifeedsci.2018.09.002
- Boonanuntanasarn, S., Kumkhong, S., Yoohat, K., Plagnes-Juan, E., Burel, C., Marandel, L., & Panserat, S. (2018). Molecular responses of Nile tilapia (Oreochromis niloticus) to different levels of dietary carbohydrates. Aquaculture, 482, 117–123. https://doi.org/10.1016/j.aquaculture.2017.09.032
- Chaabani, A., Labonne, L., Durrieu, V., Rouilly, A., Skiba, F., & Evon, P. (2022). Preconditioner influence on twin-screw extrusion cooking of starch-based feed pellets: The example of fish feed. Aquacultural Engineering, 98, 102268. https://doi.org/10.1016/j.aquaeng.2022.102268
- Daniel, N. (2018). A review on replacing fish meal in aqua feeds using plant protein sources. International Journal of Fisheries and Aquatic Studies, 6(2), 164–179.
- Das, K. C., Mohanta, K. N., Nayak, S. K., Mohanty, T., Toppo, S., & Swain, P. (2018). Effect of extrusion temperature on quality of carp floating feed prepared from local feed resources. Animal Nutrition and Feed Technology, 18(1), 117–123. https://doi.org/10.5958/0974-181X.2018.00011.2
- Delgado, E., Valles-Rosales, D. J., Flores, N. C., & Reyes-Jáquez, D. (2021). Evaluation of fish oil content and cottonseed meal with ultralow gossypol content on the functional properties of an extruded shrimp feed. Aquaculture Reports, 19, 100588. https://doi.org/10.1016/j.aqrep.2021.100588
- Duguma, H. T., Forsido, S. F., Belachew, T., & Hensel, O. (2021). Changes in anti nutritional factors and functional properties of extruded composite flour. Frontiers in Sustainable Food Systems, 5, 713701. https://doi.org/10.3389/fsufs.2021.713701
- Gil, A. (2010). Tratado de nutrición. Editorial Médica Panamericana.
- Grainger, R. (2016). Fisheries and aquaculture. In B. Pritchard, R. Ortiz, & M. Sheka (Eds.), Routledge handbook of food and nutrition security (1st ed., pp. 1–16). Routledge.
- Gujska, E., & Khan, K. (1990). Effect of temperature on properties of extrudates from high starch fractions of Navy, Pinto and garbanzo beans. Journal of Food Science, 55(2), 466–469. https://doi.org/10.1111/j.1365-2621.1990.tb06788.x
- Hardy, R. W. (2010). Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquaculture Research, 41(5), 770–776. https://doi.org/10.1111/j.1365-2109.2009.02349.x
- Hasan, M. R. (2017). Feeding global aquaculture growth. FAO Aquaculture Newsletter, 56, 1–3.
- Hodar, A. R., Vasava, R. J., Mahavadiya, D. R., & Joshi, N. H. (2020). Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. Journal of Experimental Zoology, India, 23(1), 13–21.
- Idris Maizuwo, A., Sharif Hassan, A., Momoh, H., & Andullahi Muhammad, J. (2017). Phytochemical constituents, biological activities, therapeutic potentials and nutritional values of Moringa oleifera (Zogale): A review. Journal of Drug Design and Medicinal Chemistry, 3(4), 60–66. https://doi.org/10.11648/j.jddmc.20170304.12
- Janbakhsh, S., Hosseini Shekarabi, S. P., & Shamsaie Mergan, M. (2018). Nutritional value and heavy metal content of fishmeal from the Southwest Caspian Sea. Caspian Journal of Environmental Sciences, 16(4), 307–317. https://doi.org/10.22124/CJES.2018.3200
- Kallu, S., Kowalski, R. J., & Ganjyal, G. M. (2017). Impacts of cellulose fiber particle size and starch type on expansion during extrusion processing. Journal of Food Science, 82(7), 1647–1656. https://doi.org/10.1111/1750-3841.13756
- Kamalam, B. S., & Panserat, S. (2016). Carbohydrates in fish nutrition. International Aquafeed, 20–23. https://doi.org/10.13140/RG.2.1.4570.6645
- Kannadhason, S., Muthukumarappan, K., & Rosentrater, K. A. (2009). Effects of ingredients and extrusion parameters on aquafeeds containing DDGS and tapioca starch. Journal of Aquaculture Feed Science and Nutrition, 1(1), 6–21. https://medwelljournals.com/abstract/?doi=joafsnu.2009.6.21
- Khan, G. Q., Prestløkken, E., Lund, P., Hellwing, A. L., & Larsen, M. (2022). Effects of the density of extruded pellets on starch digestion kinetics, rumen fermentation, fiber digestibility and enteric methane production in dairy cows. Journal of Animal Physiology and Animal Nutrition, 107(4), 981–994. https://doi.org/10.1111/jpn.13787
- Kirimi, J. G., Musalia, L. M., & Munguti, J. M. (2017). Effect of replacing fish meal with blood meal on chemical composition of supplement for Nile tilapia (Oreochromis niloticus). East African Agricultural and Forestry Journal, 82(1), 1–9. https://doi.org/10.1080/00128325.2016.1158898
- Liu, J., Li, Z., Li, X., & Wang, Y. (2013). On-farm feed management practices for Nile tilapia (Oreochromis niloticus) in southern China. FAO Fisheries and Aquaculture Technical Paper, 583, 71–99.
- Lu, X., Brennan, M. A., Narciso, J., Guan, W., Zhang, J., Yuan, L., Serventi, L., & Brennan, C. S. (2019). Correlations between the phenolic and fibre composition of mushrooms and the glycaemic and textural characteristics of mushroom enriched extruded products. Food Science and Tecnology, 118, 108730. https://doi.org/10.1016/j.lwt.2019.108730
- Magan, N., & Aldred, D. (2007). Post-harvest control strategies: Minimizing mycotoxins in the food chain. International Journal of Food Microbiology, 119(1–2), 131–139. https://doi.org/10.1016/j.ijfoodmicro.2007.07.034
- Ma, X., Gu, B. Y., Jin, T., Yoo, J. H., Kim, M. H., & Ryu, G. H. (2018). Physicochemical properties of extruded aquatic pellets at various conditions. Food Engineering Progress, 22(3), 214–219. https://doi.org/10.13050/foodengprog.2018.22.3.214
- Manuel, A. V., Shahanuddin, A. M., Saha, D., Chele, M. H., da Conceicao, K. O., Tsutsui, N., & Yoshimatsu, T. (2019). Growth and feed utilization of juvenile Nile tilapia fed with boiled Moringa oleifera meal diets: a preliminary report. Journal of International Cooperation for Agricultural Development, 17, 8–13. https://doi.org/10.50907/jicad.17.0_8
- Martin, A., Osen, R., Greiling, A., Karbstein, H. P., & Emín, A. (2019). Effect of rapeseed press cake and peel on the extruder response and physical pellet quality in extruded fish feed. Aquaculture, 512, 512. https://doi.org/10.1016/j.aquaculture.2019.734316
- Mondal, D. K., Siddiky, M. M., & Paul, M. (2016). Evaluation of nutritive value of three commercial fish feed and their effect on the growth and survival of tilapia (gift strain, Oreochromis niloticus) fry. International Journal of Natural and Social Sciences, 3(1), 67–72.
- Mota, C., Santos, M., Mauro, R., Samman, N., Matos, A. S., Torres, D., & Castanheira, I. (2016). Protein content and amino acids profile of pseudocereals. Food Chemistry, 193, 55–61. https://doi.org/10.1016/j.foodchem.2014.11.043
- Mugabi, R., Byakika, S., & Mukisa, I. M. (2022). Effects of feed moisture content, soybean ratio and barrel temperature on physical and functional properties of extruded maize-soybean flour blends. Tanzania Journal of Science, 48(2), 447–459. https://doi.org/10.4314/tjs.v48i2.19
- Neder‐Suárez, D., Amaya‐Guerra, C. A., Pérez‐Carrillo, E., Quintero‐Ramos, A., Mendez‐Zamora, G., Sánchez‐Madrigal, M. Á., & Lardizábal‐Gutiérrez, D. (2020). Optimization of an extrusion cooking process to increase formation of resistant starch from corn starch with addition of citric acid. Starch‐Stärke, 72(3–4), 1900150. https://doi.org/10.1002/star.201900150
- Neder-Suárez, D., Quintero-Ramos, A., Meléndez-Pizarro, C. O., de Jesús Zazueta-Morales, J., Paraguay-Delgado, F., & Ruiz-Gutiérrez, M. G. (2021). Evaluation of the physicochemical properties of third-generation snacks made from blue corn, black beans, and sweet chard produced by extrusion. LWT, 146, 111414. https://doi.org/10.1016/j.lwt.2021.111414
- Ng, W. K., & Chong, C. Y. (2004). An overview of lipid nutrition with emphasis on alternative lipid sources in tilapia feeds. In Proceedings of the Sixth International Symposium on Tilapia in Aquaculture (241–248). Bureau of Fisheries and Aquatic Resources.
- Nsofor, C. I., Igwilo, I. O., Avwemoya, F. E., & Adindu, C. S. (2012). The effects of feeds formulated with moringa oleifera leaves in the growth of the African Catfish, clarias gariepinus. Research & Reviews in BioSciences, 6, 121–126.
- Nurcahyani, R. O. D., Rahardja, B. S., & Al Arif, M. A. (2019). Moringa leaf flour (Moringa oleifera) as an alternative for the replacement of crude fat and energy in the commercial feed of siamese catfish (Pangasius hypothalamus). International Journal of Civil Engineering & Technology, 10(5), 404–412. https://doi.org/10.34218/IJCIET.10.5.2019.043
- Orire, A. M., & Salihu, Z. A. (2020). Effects of nutrient inclusion levels on aquafeed buoyancy. Tanzania Journal of Agricultural Sciences, 19(2), 161–166.
- Pérez-Viveros, K. J. (2017). Formulación y desarrollo de un alimento extrudido mediante la sustitución parcial de harina de pescado con harinas vegetales para Oreochromis niloticus (tilapia del Nilo). Tesis de maestría. Universidad Politécnica de Pachuca.
- Rathod, R. P., & Annapure, U. S. (2016). Effect of extrusion process on antinutritional factors and protein and starch digestibility of lentil splits. LWT-Food Science and Technology, 66, 114–123. https://doi.org/10.1016/j.lwt.2015.10.028
- Rokey, G. J., Plattner, B., & Souza, E. M. (2010). Feed extrusion process description. Revista Brasileira de Zootecnia, 39(suppl spe), 510–518. https://doi.org/10.1590/S1516-35982010001300055
- Samuelsen, T. A., Mjos, S. A., & Oterhals, A. (2013). Impact of variability in fishmeal physicochemical properties on the extrusion process, starch gelatinization and pellet durability and hardness. Animal Feed Science and Tecnology, 179(4), 77–84. https://doi.org/10.1016/j.anifeedsci.2012.10.009
- Sánchez, M., Martínez, E., Maldonado, M., & Aparicio, X. (2014). Comparative study on the nutritional and antioxidant properties of two Mexican corn (Zea mays) based meals versus processed cereal. Revista de la Sociedad Latinoamericana de Nutrición, 64(2), 116–123.
- Singha, K. P., Shamna, N., Sahu, N. P., Sardar, P., Harikrishna, V., Thirunavukkarasar, R., Chowdhury, D. K., Maiti, M. K., & Krishna, G. (2021). Optimum dietary crude protein for culture of genetically improved farmed tilapia (GIFT), Oreochromis niloticus (Linnaeus, 1758) juveniles in low inland saline water: Effects on growth, metabolism, and gene expression. Animal Feed Science and Technology, 271, 114713. https://doi.org/10.1016/j.anifeedsci.2020.114713
- Singh, S., Gamlath, S., & Wakeling, L. (2007). Nutritional aspects of food extrusion: A review. International Journal of Food Science and Technology, 42(8), 916–929. https://doi.org/10.1111/j.1365-2621.2006.01309.x
- Singh, S. K., & Muthukumarappan, K. (2016). Effect of feed moisture, extrusion temperature and screw speed on properties of soy white flakes based aquafeed: A response surface analysis. Journal of the Science of Food and Agriculture, 96(6), 2220–2229. https://doi.org/10.1002/jsfa.7339
- Soto-Rodríguez, D. L., Gómez-Aldapa, C. A., Cabrera-Canales, Z. E., & Cadena-Ramírez, A. (2020). Effect of the use of different types of fishmeal on the physicochemical properties of a fish feed for Oreochromis niloticus (Nile tilapia). ECORFAN Journal-Ecuador, 7(12), 8–14. https://doi.org/10.35429/EJE.2020.12.7.8.14
- Tarhouni, A., Zid, M. B., Talbi, O., Elbour, M., Sadok, S., & Boudhrioua, N. M. (2019). New integrated process for production of edible and fishmeal powders from sardines: Drying kinetics and quality attributes. Process Safety and Environmental Protection, 122, 352–365. https://doi.org/10.1016/j.psep.2018.12.019
- Teixeira, E. M. B., Carvalho, M. R. B., Neves, V. A., Silva, M. A., & Arantes-Pereira, L. (2014). Chemical characteristics and fractionation of proteins from moringa oleifera Lam. leaves. Food Chemistry, 147, 51–54. https://doi.org/10.1016/j.foodchem.2013.09.135
- Teruel, M. B. (2002). Evaluation of feedstuffs and aquafeeds. In O. M. Millamena, R. M. Coloso, & F. P. Pascual (Eds.), Nutrition in tropical aquaculture: Essentials of fish nutrition, feeds, and feeding of tropical aquatic species (pp. 149–168). Aquaculture Department, Southeast Asian Fisheries Development Center. http://hdl.handle.net/10862/3320
- Vargas, R. (2003). Evaluación preliminar del método utilizado en la determinación de la flotabilidad de alimentos piscícolas. Agronomia mesoamericana, 14(2), 193–199. https://doi.org/10.15517/am.v14i2.11948
- Wang, W. M., Klopfenstein, C. F., & Ponte, J. G. J. (1993). Effects of twin screw extrusion on the physical properties of dietary fiber and other components of whole wheat and wheat bran and on the baking quality of the wheat bran. Cereal Chemistry, 70(6), 707–711.
- Wang, H., Ma, S., Yang, J., Qin, Y., Cheng, H., Xue, M., Li, J., & Li, J. (2021). Optimization of the process parameters for extruded commercial sinking fish feed with mixed plant protein sources. Journal of Food Process Engineering, 44(1), e13599. https://doi.org/10.1111/jfpe.13599
- Windarti, I. E., & Kurniawan, R. (2023). Addition of moringa leaves to feed to improve growth performance and feed use of striped catfish (Pangasianodon Hypophthalmus). Nongye Jixie Xuebao/Transactions of the Chinese Society of Agricultural Machinery, 54(5), 196–208.
- Ye, J., Hu, X., Luo, S., Liu, W., Chen, J., Zeng, Z., & Liu, C. (2018). Properties of starch after extrusion: A review. Starch‐Stärke, 70(11–12), 1700110. https://doi.org/10.1002/star.201700110
- Yousf, N., Nazir, F., Salim, R., Ahsan, H., & Sirwal, A. (2017). Water solubility index and water absorption index of extruded product from rice and carrot blend. Journal of Pharmacognosy & Phytochemistry, 6(6), 2165–2168.
Appendix A
Table A1. Composition of vitamin and mineral mix.
