?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A specific and sensitive monoclonal antibody (mAb) for ribavirin (RBV) was generated by immunizing BALB/c mice with RBV – bovine serum albumin. Using the mAb, a highly sensitive indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and a colloidal gold immunochromatographic (CGI) test strip were developed for detecting RBV in pig urine. Under optimum assay conditions, IC50 was 0.08 ng/mL in 0.05 M phosphate-buffered saline and the recovery rate in real-world samples ranged between 77.3% and 99%. The cut-off limit of the CGI test strip for RBV was 5 ng/mL in pig urine. The cross-reactivity rate of the test strip to the tested antiviral drugs including acyclovir and moroxydine hydrochloride and the metabolites 1,2,4-triazole-3-carboxamide and 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxylic acid was below 0.1%. The results indicate that ic-ELISA and the CGI test strip have high sensitivity and specificity and that these methods are suitable for the rapid detection of RBV in pig urine.
1. Introduction
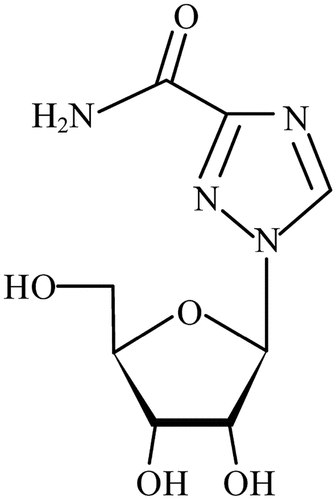
Ribavirin (1-β-D-ribofuranosyl-1 H–1,2,4-triazole-3-carboxamide) (RBV) is the first synthetic broad-spectrum antiviral drug (Casaos et al., Citation2019), and has high efficacy and good clinical application in the treatment of viral diseases. The chemical structure of ribavirin is shown in . China’s Ministry of Agriculture released No. 560 Document in 2005 which specified that RBV and its relative single and compound preparations are prohibited for use in veterinary medicine. However, because of its low cost and high efficacy, it may be unreasonably used for the prevention and treatment of viral diseases in animals. This can result in residues of the drug being found in subsequent animal products that can then get into humans via the biological chain, harming human health as well as causing ecological and environmental damage (Wang et al., Citation2018). The drug can accumulate in red blood cells for weeks. Ribavirin may cause biological toxicity such as causing erythropenia (Liu et al., Citation2022) and fetal malformations (Magdy et al., Citation2023). Both hemolysis and bone marrow inhibition underlie the fall in red cells, but the quantitative contribution by each of these factors in not yet clear. Ribavirin is metabolized in the liver, Tl/2 elimination is about 0.5–2 hours after intravenous administration, and is mainly excreted through the kidney, with urinary excretion rate of 30%-55% at 72–80 hours and fecal excretion rate of about 15% at 72 hours.
Various methods have been developed for the detection of RBV residues in different kinds of materials. At present, instrumental methods mainly include liquid chromatography (Qie et al., Citation2019), gas chromatography – mass spectrometry (Haggag et al., Citation2014), high performance liquid chromatography – tandem mass spectrometry (Aouri et al., Citation2013), and liquid chromatography – mass spectrometry (Jimmerson et al., Citation2015). Zheng xin developed a method to detect RBV residue in eggs and was able to eventually achieve a limit of detection (LOD) of 1 ng/mL with the recovery rate ranging between 86.4% and 97.1% in 10–1000 ng/mL samples. Although this method is relatively specific and sensitive, it requires expensive instruments and highly trained operators and is not suitable for large-scale food sample screening
To ensure food safety and human health (Way et al., Citation2023), and to strengthen veterinary drug residue monitoring, the establishment of an efficient and reliable test is required. At present ELISA methods and colloidal gold immunochromatographic (CGI) test strip tests are used for detecting various drug residues in real systems.
The aim of this study was to develop a more sensitive monoclonal antibody (mAb) against RBV, and using this RBV mAb, develop an indirect competitive-ELISA (ic-ELISA) and CGI test strip that can be used to detect RBV residues in pig urine. We proposed a simpler antigen synthesis method, shortened the antigen synthesis time, and added sucrose during CGI production to increase the affinity of antigen and antibody, and obtained a more sensitive detection
2. Materials and methods
2.1. Chemicals and reagents
RBV, Tris-HCl buffer (pH 7.4), bovine serum albumin (BSA), ovalbumin (OVA), complete Freund’s adjuvant, incomplete Freund’s adjuvant, carbon dimethylamine, and n-hydroxysuccinyl amines (NHS) were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). All reagents for cell fusion and cell cultures, and tetramethylbenzidine and horseradish peroxidase, were obtained from Bioyou Biological Technology (Beijing, China). Enzyme immunoassay-grade horseradish peroxidase-labeled goat anti-mouse immunoglobulin (Ig) was purchased from Kangwei Century Technology (Beijing, China). Other reagents and chemicals were from Beijing Chemical Factory (Beijing, China). Nitrocellulose membranes (UniSart CN 140) were from Sartorius Stedim Biotech (Aubagne, France). The glass fiber membrane used for the sample pad, the polyvinyl alcohol backing material, and the absorbance pad (470 paper) were supplied by Jiening Biological Technology (Shanghai, China).
2.2. Immunogen and coating the antigen
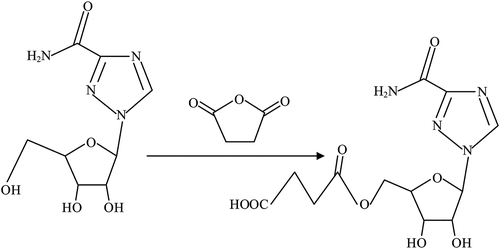
We used the succinic anhydride method. First, RBV (100 mg) was added to 20 mL of methanol in a 100-mL beaker equipped with a mechanical stirrer and the drug was stirred for 6 h until it had dissolved. All liquid was then transferred into a round-bottomed flask for rotary evaporation for 12 h in a water bath at 80°C. Absolute ethyl alcohol (30 mL) was added and the mixture stirred for 4 h at room temperature. Succinic anhydride (90 mg) was added and continuously stirred for 72 h before we were able to obtain RBV hapten (Chen et al., Citation2014). Based on previous studies, we added 1.5 mL Tris-HCl buffer (pH 7.4) into the RBV hapten mixture and then added carbodiimide (80 mg) and NHS (32 mg) and stirred for 12 h. Separately, 40 mg BSA was dissolved in Tris-HCl buffer (pH 7.4) and this solution was added in a dropwise manner into the RBV hapten solution. The reaction continued for 24 h at room temperature before being dialyzed with phosphate-buffered saline (PBS) (0.01 M, pH 7.4) for 2 days at 4°C. The preparation method for coating the antigen was the same as that for the immunogen antigen. Hapten synthesis of ribavirin is shown in .
2.3. Development of monoclonal anti-RBV antibody
2.3.1 Immunization and generation of monoclonal antibody (mAb)
Initially, RBV – bovine serum albumin (RBV-BSA) solution and Freund’s complete adjuvant at a dosage of 100 μL per mouse (containing 200 μg antigen) were administered to immunize six 8-week-old female BALB/c mice, followed by 100 μL per mouse (containing 100 mg antigen) and Freund’s incomplete adjuvant once every 2 weeks. Immunization was via a subcutaneous injection into the backs of the mice. Seven days after each immunization, antisera from mice were tested using ic-ELISA. When antisera titers were higher than 128 000, antisera were collected for ic-ELISA. The antisera of the mouse that had the highest titer and best inhibition with free RBV by ic-ELISA was selected for cell fusion.
Splenocytes from the selected mouse were fused with Sp2/0 murine myeloma cells. Hybridomas were screened using ic-ELISA. The selected hybridoma cells were expanded and injected into BALB/c mice to produce the mAb (Deng et al., Citation2012). Ascites was collected and then purified using the caprylic acid – ammonium sulfate precipitation method (Kuang et al., Citation2013). The purified antibody solution was divided into small aliquots and stored at − 20°C until further use.
2.3.2. Evaluation of the mAb by ic-ELISA
The concentrations of the antibody and the coating antigen (RBV-OVA) were optimized using bi-dimensional titration experiments. We used a blank group, a negative serum group, and a positive control group, every group for two holes. We first identified the antibody’s maximum dilute concentration by ELISA. We selected dilute concentration close to an optical density (OD) value of 1 as the antibody concentration in ic-ELISA. An optimal ic-ELISA was then developed as follows: A 96-well microplate was coated with 100 μL/well of RBV-OVA diluted with PBS (0.01 M) and incubated for 12 h at 4°C. Plates were washed three times with 5% (w/v) PBS-Tween 20 each time for 2 min. The remaining binding sites were blocked with blocking buffer at 37°C for 2 h followed by washing. Standard solutions were prepared by serial dilutions (0, 0.01, 0.1, 1, and 10 ng/mL) and 50 μL of different concentrations of RBV and the same volume of RBV antibody were added to each well and incubated for 30 min at 37°C. Diluted (1:8000) goat anti-mouse IgG – horseradish peroxidase (100 μL/well) was added to the washed plates and incubated for another 30 min at 37°C. Finally, after five washes, 100 μL of TMB substrate solution was added to each well at room temperature. After 15 min, color development was stopped with 2 M H2SO4 per well (50 μL) and read the OD450 value using an ELISA reader at 450 nm (Barshevskaya et al., Citation2019; Li et al., Citation2011).
The OD450 value of the concentration at 0 ng/mL was set as B0. Different OD values at different concentrations of RBV were set as B, with B/B0 as the ordinate and the numerical of the antibody concentration as the abscissa. From this a standard curve was determined (Lin et al., Citation2011; Liu, Kuang, et al., Citation2014; L. Liu et al., Citation2015). The half inhibition concentration (IC50) value of the mAb was evaluated, which characterized the sensitivity of the mAb (Jiang et al., Citation2011).
2.3.3. Cross-reactivity
We used antiviral drugs including acyclovir and moroxydine hydrochloride and metabolites including 1,2,4-triazole-3-carboxamide and 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxylic acid to determine cross-reactivity in the ic-ELISA. The cross-reactivity was calculated using the following equation:
2.3.4. Recovery rate
We used the method described in 2.3.2 (above) for development of ic-ELISA to test the recovery rate of RBV in pig urine but also including 0.1, 1, and 10 ng/mL. Assays were repeated three times and each time used 10 samples. To establish the regression equation, we calculated the drug content and added the recovery rate. Recovery rate was determined using the following equation:
2.4. Development of CGI test strip
2.4.1. Preparation of colloidal gold particles
We used sodium citrate reduction method to get the colloidal gold. We first added 100 mL of pure water and 1 mL of 1% (w/v) HAuCl4 and the appropriate sized magnetic rotor to a clean conical flask. The mixture was stirred at 300°C at 300 ×g until it boiled. The temperature was then reduced to 150°C and the rotation speed reduced to 150 ×g. Sodium citrate 0.8 mL of 1% (w/v) was quickly added. Three min after the color of the solution stabilized, the magnetic stirrer was stopped. The solution was cooled at room temperature and stored at 4°C in the dark for future use. After cooling, we used spectral scanning to test the maximum absorption peak to determine the size of the colloidal gold particles. We judged particle uniformity according to the width of the absorption of colloidal gold peak. According to the formula, we obtain its particle size. The formula is as follows:
2.4.2. Preparation of colloidal gold-labeled mAb
The anti-RBV mAb with an IC50 value of 0.08 ng/mL was prepared previously. We added 10 μL of mAb into 10 mL of colloidal gold and mixed before leaving to stand for 10 min. Then 20 μL of 1% trisodium citrate solution was added followed by blending and then the mixture was again left to stand for 10 min. Blocking buffer (200 μL of 10% (w/v) BSA) was added and the mixture was stirred before being left to stand for 20 min. The mixture was centrifuged for 10 min at 10 000 ×g to remove the supernatant and the precipitate was collected. We dissolved the precipitate in a solution containing 0.01 M PBS, 2% (w/v) sucrose, 0.5% (w/v) BSA. All steps were performed gently (Peng et al., Citation2016).
2.4.3. Preparation of immunochromatographic test strips
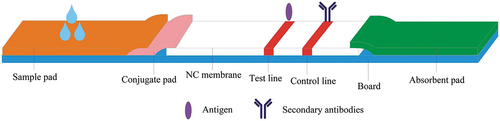
The preparation of test strips was as follows: A glass fiber was prepared with dilution buffer (0.01 M PBS buffer (pH 7.4) containing 0.5% (w/v) BSA, and 0.5% (w/v) Tween 20) and dried at 37°C for 2 h. The prepared glass fiber was cut into sections (30 × 1 cm) and sprayed with 1, 2, and 3 μL/cm of colloidal gold-labeled mAb. The fiber was then dried at 37°C for 30 min before being made into a conjugate pad and stored under dry conditions at room temperature for future use. The sample pad (glass fiber) was soaked with 0.02 M PBS and dried for 2 h at 37°C. The sample layer was cut into sections (30 × 1.5 cm) and stored under dry conditions at room temperature.
The nitrocellulose membrane was treated as follows: The control line was goat anti-mouse IgG. According to specifications, I diluted the goat anti-mouse IgG for two levels of concentrations (12.5, 20 μg/mL) for use. The test line was the coating antigen (RBV-BSA), and I diluted it for two levels of concentrations (55.8, 139.5 μg/) for use. The antigen and goat anti-mouse IgG coatings were sprayed onto the nitrocellulose membrane at 1 μL/cm using a dispenser to produce the test line and a control line on the strip. The membrane was then dried at 37°C for 2 h and stored under dry conditions at room temperature until use. The diluent selected was 0.01 M PBS solution. Finally, the test strips were assembled as shown in below.
2.4.4. Test procedure and principle
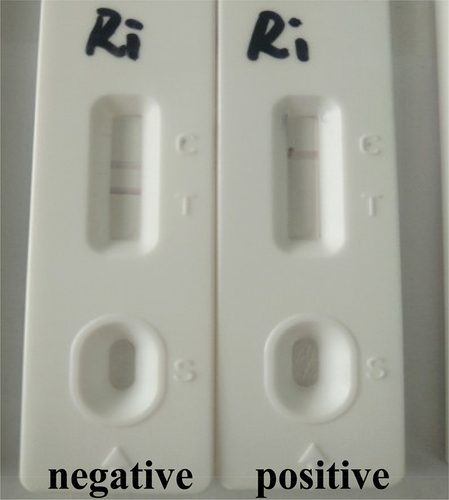
We added 80 μL of the sample to the sample layer (Liu et al., Citation2014). Because of capillary action, the solution flows in the direction of the absorbent paper. The control line should be always be present. If RBV is present in the sample, it will bind to the colloidal gold-labelled anti-RBV mAb. The more RBV present in the sample, the weaker the color of the test line will become. If there is no RBV in the sample, the colloidal gold-labelled mAb can be trapped by the immobilized RBV-BSA conjugations, which in turn will create a clearly visible red test line. The strips were observed after 5 min ().
2.4.5. Detection of RBV in pig urine
The sensitivity of the test strip was determined by testing some RBV reference samples in pig urine. Pig urine was centrifuged at 6 000 ×g for 15 min. The supernatant was used for the tests. RBV was diluted at concentrations of 0, 1, 2, 3, and 5 ng/mL in pig urine. Every sample was subject to five detection tests, and the detection limit was determined (Wang et al., Citation2016).
The repeatability of the test strip was determined by replicate trials. After optimizing all conditions, we produced three batches of test strips and tested the strips at random. Each batch was tested three times, each time set three groups. Every group set a negative sample and a positive sample.
The stability of the test strip was determined. Optimized strips were stored in self-styled bags with desiccant away from light and at 4°C. Contrast experiments were carried out on days 1, 10, 15, 30, and 60 from storage. The line color and detection limits for each group were tested 10 times (Fang, Citation2010).
3. Results and discussion
3.1. Preparation of antibody
3.1.1. Synthesis of RBV-BSA
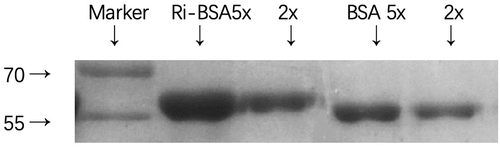
It was showed in that the transfer speed of RBV-BSA was slower than that of BSA, which indicated that the immunogenic molecule weight was larger than BSA. Therefore, the complete antigen had conjugated successfully. The density of the immunogenicity antigen was 2.79 mg/mL, and the density of the coating antigen was 2.87 mg/mL.
3.1.2. Immunization and generation of monoclonal antibody (mAb)
Selected mice that meet the criteria for cell fusion. The ratio of mouse spleen cells to myeloma cells is 6:1. Each fusion experiment involved six plates, totaling 576 wells. Among them, 326 wells formed hybridoma cells, resulting in a fusion rate of 56.6%. There were 72 positive Wells detected by ic-ELISA, with a positive rate of 12.5%. After 4 subclonings, one stable hybridoma cell line named 6D3 was obtained. This cell line demonstrated continued secretion of specific antibodies during stable passage and cultivation. After the detection titer met the qualification, the cloned and stable hybridoma cell line was injected into mice intraperitoneally at a dose of approximately 5 × 105-8 × 105 cells per mouse. After one week, the mice showed abdominal swelling and sluggish movement. Subsequently, ascitic fluid was collected and purified, resulting in an antibody concentration of 6.4 mg/mL.
3.2. Establishment of Elisa assay for RBV
3.2.1. Optimization and specificity of ic-ELISA
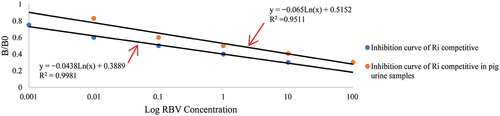
We selected B/B0 as the ordinate and the numerical of the antibody concentration as the abscissa to get a standard curve. The working concentration of RBV ranged between 0.001 and 10 ng/mL. The standard curve showed good linearity. The linear equation was y = −0.0438Ln(x) + 0.3889, where R2 was 0.9981, and IC50 was 0.08 ng/mL. The standard curve is shown below ().
The cross-reactivity rate of the RBV test strip to the tested antiviral drugs including acyclovir and moroxydine hydrochloride and metabolites 1,2,4-triazole-3-carboxamide and 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxylic acid were zero. The results are shown in .
Table 1. Cross-reactivity of other drugs/metabolites with the monoclonal antibody.
3.2.2. Analysis of samples by ic-ELISA
We used ic-ELISA to test the recovery rate of RBV in pig urine. The standard curve was y = −0.065Ln(x) + 0.5152, where R2 was 0.9511, IC50 was 1.71 ng/mL, and LOD was lower than 0.1 ng/mL. The standard curve is shown below (). The properties of the antibody were influenced by the pig urine. IC50 was higher than that in PBS. It is possible that there are enzymes or other chemical substances in pig urine that can affect the assay.
Three different concentrations of RBV (0.1, 1, and 10 ng/mL) were added to pig urine. We were able to achieve a recovery rate ranging between 77.3% and 99%. Average intra-batch and inter-batch percentages were 3.19% and 3.15%.The results are shown in .
Table 2. The rate of recovery of the sample using ELISA.
3.3. Establishment of a colloidal gold strip assay for RBV
3.3.1. Optimization of the test strip
The concentrations of the antibody and pH are very important during conjugation of colloidal gold with mAb (Shuang et al., Citation2016). The optimal conditions can be determined by multiple attempts at different concentrations. Based on laboratory studies, we selected a colloidal gold particle size of 33.8 nm. The results have been represented in the table below (). In the current study, to evaluate the effect of pH on the strip, we changed the amount of trisodium citrate (1, 2, and 3 μL) for the conjugation reaction in the optimization process. The results showed that the colloidal gold solution was stable at 2 μL trisodium citrate. The amount of labelled antibody was optimized in these tests using different levels (6.4, 12.8, and 25.6 μg/mL) in conjugation with 1 mL colloidal gold. The optimum amount of mAb was 12.8 μg in 1 mL colloidal gold. In addition, the optimum amount of goat anti-mouse IgG was 12.5 μg/mL. The optimum amount of coating antigen (RBV-BSA) was 55.8 μg/mL.
Table 3. The relation of particle size and amount of sodium citrate added.
3.3.2 Specificity of the test strip
In this evaluation, sensitivity was investigated with a series of diluted RBV standards. We added 0, 1, 2, 3, and 5 ng/mL RBV in pig urine to the strip. The results are shown in . The signal color on the test lines changed from strong to weak and disappeared completely at 5 ng/mL, giving a detection limit of 5 ng/mL.
3.3.3 Detection of RBV in samples
We testing the stability of the strips. The optimized strips were stored in self-styled bags with desiccant away from light and at 4°C and showed no significant differences on the 1st, 10th, 15th, and 30th day from storage. When the strips were tested on day 60, we found that there was a bit of a hollow in the test line, but no difference in the results. The results are shown in .
Table 4. The repeatability of the colloidal gold strip.
4. Conclusions
In this assay, we produced a mAb with high sensitivity and specificity based on the immunogen. We then developed ic-ELISA and used the mAb to create a GCI test strip that can be used to detect RBV residues in pig urine. The IC50 for ic-ELISA was 1.71 ng/mL and the LOD was lower than 0.1 ng/mL. Our results indicate that the cut-off limit of the semi-quantitative test strip for RBV can be as low as 5 ng/mL in pig urine. Under optimum assay conditions, IC50 was 0.08 ng/mL in 0.05 M phosphate-buffered saline and the recovery rate in real-world samples ranged between 77.3% and 99%. The cut-off limit of the CGI test strip for RBV was 5 ng/mL in pig urine. The cross-reactivity rate of the test strip to the tested antiviral drugs including acyclovir and moroxydine hydrochloride and the metabolites 1,2,4-triazole-3-carboxamide and 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxylic acid was below 0.1%.
Ethic review
This study has been approved by the Animal Welfare and Ethics Committee of the College of Animal Science and Technology, Beijing University of Agriculture.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aouri, M., Moradpour, D., Cavassini, M., Mercier, T., Buclin, T., Csajka, C. … Decosterd, L. A. (2013). Multiplex liquid chromatography-tandem mass spectrometry assay for simultaneous therapeutic drug monitoring of ribavirin, boceprevir, and telaprevir. Antimicrobial Agents and Chemotherapy, 57(7), 3147–7. https://doi.org/10.1128/aac.00281-13
- Barshevskaya, L. V., Sotnikov, D. V., Zherdev, A. V., Khassenov, B. B., Baltin, K. K., Eskendirova, S. Z., Mukanov, K. K., Mukantayev, K. K., & Dzantiev, B. B. (2019). Triple immunochromatographic system for simultaneous Serodiagnosis of Bovine Brucellosis, Tuberculosis, and Leukemia. Biosensors, 9(4), 115. https://doi.org/10.3390/bios9040115
- Casaos, J., Gorelick, N. L., Huq, S., Choi, J., Xia, Y., Serra, R., Felder, R., Lott, T., Kast, R. E., Suk, I., Brem, H., Tyler, B., & Skuli, N. (2019). The use of ribavirin as an anticancer therapeutic: Will it go viral? Molecular Cancer Therapeutics, 18(7), 1185–1194. https://doi.org/10.1158/1535-7163.MCT-18-0666
- Chen, G., Huang, X., Li, S., Kong, X., & Huai, B. (2014). Synthesis of a newly designed artificial antigen and preparation of a polyclonal antibody against salbutamol. Food and Agricultural Immunology, 25(3), 322–331. https://doi.org/10.1080/09540105.2013.791970
- Deng, X., Liu, L., Ma, W., Xu, C., Wang, L., & Kuang, H. (2012). Development and validation of a sandwich ELISA for quantification of peanut agglutinin (PNA) in foods. Food and Agricultural Immunology, 23(3), 265–272. https://doi.org/10.1080/09540105.2011.617358
- Fang, Z. (2010). Development of Colloidal gold immunochromatographic assay for rapid detection of Sulfamethoxydiazine Residue. [ Master’s degree]. Yangzhou University.
- Haggag, R. S., Belal, S. F., Hewala, I. I., & El Rouby, O. A. (2014). Stability-indicating HPLC–DAD determination of ribavirin in capsules and plasma. Journal of Chromatographic Science, 52(6), 493–500. https://doi.org/10.1093/chromsci/bmt067
- Jiang, Y., Huang, X., Hu, K., Yu, W., Yang, X., & Lv, L. (2011). Production and characterization of monoclonal antibodies against small hapten-ciprofloxacin. African Journal of Biotechnology, 10(65), 14342–14347. https://doi.org/10.5897/AJB11.1546
- Jimmerson, L. C., Ray, M. L., Bushman, L. R., Anderson, P. L., Klein, B., Rower, J. E., Zheng, J. H., & Kiser, J. J. (2015). Measurement of intracellular ribavirin mono-, di-and triphosphate using solid phase extraction and LC–MS/MS quantification. Journal of Chromatography B, 978, 163–172. https://doi.org/10.1016/j.jchromb.2014.11.032
- Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L., & Xu, C. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors (Basel, Switzerland), 13(4), 4214–4224. https://doi.org/10.3390/s130404214
- Li, X., Luo, P., Tang, S., Beier, R. C., Wu, X., Yang, L., Li, Y., & Xiao, X. (2011). Development of an immunochromatographic strip test for rapid detection of melamine in raw milk, milk products and animal feed. Journal of Agricultural and Food Chemistry, 59(11), 6064–6070. https://doi.org/10.1021/jf2008327
- Lin, F., Song, S., Liu, L., Kuang, H., Wang, L., & Xu, C. (2011). Development of the detection of benzophenone in recycled paper packaging materials by ELISA. Food and Agricultural Immunology, 22(1), 39–46. https://doi.org/10.1080/09540105.2010.523781
- Liu, L., Kuang, H., Peng, C., Wang, L., & Xu, C. (2014). Structure-specific hapten design for the screening of highly sensitive and specific monoclonal antibody to salbutamol. Analytical Methods, 6(12), 4228–4233. https://doi.org/10.1039/C4AY00062E
- Liu, L., Luo, L., Suryoprabowo, S., Peng, J., Kuang, H., & Xu, C. (2014). Development of an immunochromatographic strip test for rapid detection of ciprofloxacin in milk samples. Sensors, 14(9), 16785–16798. https://doi.org/10.3390/s140916785
- Liu, N., Song, S., Lu, L., Nie, D., Han, Z., Yang, X., Zhao, Z., Wu, A., & Zheng, X. (2014). A rabbit monoclonal antibody-based sensitive competitive indirect enzyme-linked immunoassay for rapid detection of chloramphenicol residue. Food and Agricultural Immunology, 25(4), 523–534. https://doi.org/10.1080/09540105.2013.847065
- Liu, Y., Thaker, H., Wang, C., Xu, Z., & Dong, M. (2022). Diagnosis and treatment for Shiga toxin-producing Escherichia coli associated hemolytic uremic syndrome. Toxins, 15(1), 10. https://doi.org/10.1080/09540105.2017.1359498
- Liu, L., Yan, H., Zhang, X., Kuang, H., & Xu, C. (2015). Development of an anti-chlorothalonil monoclonal antibody based on a novel designed hapten. Food and Agricultural Immunology, 26(3), 410–419. https://doi.org/10.1080/09540105.2014.938319
- Magdy, M., Ghareeb A E W, E., Eldebss, T M A., & Abd El Rahman, H. A. (2023). Investigation of the embryo-toxicity of the antiviral drug “Ribavirin” in Wistar rats during different gestation periods[J]. Egyptian Journal of Basic and Applied Sciences, 10(1), 396–409. https://doi.org/10.1080/2314808X.2023.2217650
- Peng, S., Song, S., Liu, L., Kuang, H., & Xu, C. (2016). Rapid enzyme-linked immunosorbent assay and immunochromatographic strip for detecting ribavirin in chicken muscles. Food and Agricultural Immunology, 27(4), 449–459. https://doi.org/10.1080/09540105.2015.1104657
- Qie, M., Zheng, S., Xiaoyun, B., Bo, Z., Guozhen, F., & Shuo, W. (2019). Specific recognition of ribavirin in animal-derived foods by high performance liquid chromatography combined with magnetic solid-phase extraction based on highly selective Zr-Fe3 O4. Journal of Separation Science, 42(16), 2602–2611. https://doi.org/10.1002/jssc.201900245
- Shuang, P., Shan, S. S., Li, Q. L., Kuang, H., & Xu, C. (2016). Rapid enzyme-linked immunosorbent assay and immunochromatographic strip for detecting ribavirin in chicken muscles. Food and Agricultural Immunology, 27(4), 449–459. https://doi.org/10.1080/09540105.2015.1104657
- Wang, X. C., Fan, H. X., Fan, M. X., Li, F. H., Feng, S. B., Li, J. C., Wu, J. J., Li, Y., & Wang, J. S. (2016). A sensitive immunochromatographic assay using colloidal gold–antibody probe for rapid detection of fumonisin B1 in corn. Food Additives & Contaminants: Part A, 33(9), 1435–1443. https://doi.org/10.1080/19440049.2016.1213429
- Wang, J., Wang, Q., Zheng, Y., Peng, T., Yao, K., Xie, S., Zhang, X., Xia, X., Li, J., & Jiang, H. (2018). Development of a quantitative fluorescence-based lateral flow immunoassay for determination of chloramphenicol, thiamphenicol and florfenicol in milk. Food and Agricultural Immunology, 29(1), 56–66. https://doi.org/10.1080/09540105.2017.1359498
- Way, H., Roh, J., Venteicher, B., Chandra, S., & Thomas, A. A. (2023). Synthesis of ribavirin 1,2,3- and 1,2,4-triazolyl analogs with changes at the amide and cytotoxicity in breast cancer cell lines. Nucleosides, Nucleotides & Nucleic Acids, 42(1), 38–64. https://doi.org/10.1080/15257770.2022.2107218