ABSTRACT
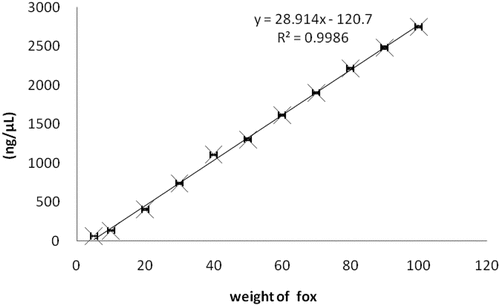
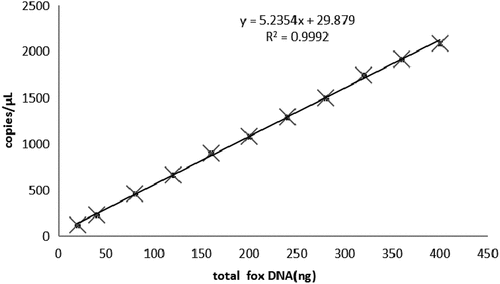
This paper reported a novel approach to quantification of adulterated fox-derived components in meat products by drop digital polymerase chain reaction (ddPCR). By using the F2 gene as the target gene of fox, a single primer was designed to identify the adulteration that had been added either inadvertently or deliberately during the process. In this paper, the fox meat was used as the experimental materials and a relationship was established between fox mass and copy number by extracting DNA and using DNA concentration as an intermediary. The results that across the dynamic range, the relationships between meat mass and DNA concentration were nearly linear (R2 = 0.9986), as was the relationship between DNA concentration and DNA copy number (R2 = 0.9992). Based on the DNA concentration, the following formulas were developed about the relationship between fox meat mass (Mfox) and DNA copy number (C): Mfox = 0.05C + 2.7.
1. Introduction
A global issue in recent years has been adulteration of food, particularly following the “horse meat storm” incident (O’Mahony, Citation2013), meat adulteration is a common occurrence. The most serious problem in meat adulteration is the incorporation of non-edible meat that has not been quarantined into meat products (Cavin et al., Citation2018; Tibola et al., Citation2018). The Wal Mart store in Jinan, China, previously disclosed an incident in which fox meat was fraudulently sold as donkey meat, leading to widespread public alarm (Fan, Citation2014). Meat adulteration not only seriously affects the health of consumers but also disrupts the market order. In order to determine whether meat products contain non-edible meat-derived ingredients, we need an effective, sensitive, and reproducible method. Therefore, the objective of this paper is to establish an adulteration method that can determine the quantification of inedible meat – fox meat in adulterated meat products.
Many analytical identification technologies have been employed for food adulteration, including polymerase chain reaction (PCR) (Amaral et al., Citation2014; Safdar et al., Citation2014), spectroscopy techniques (Ismail et al., Citation2014), enzyme-linked immunoassay techniques (Liu et al., Citation2006), electronic olfaction and electronic gustation techniques (Tian et al., Citation2013), chromatographic technology and mass spectrometry technology or their hyphenated techniques (Giaretta et al., Citation2013; Zhang et al., Citation2022). The PCR is the most accurate, sensitivity, and can detect specific genes in animal components that can identify heat-treated meat best among all technologies (Koppel et al., Citation2011; Zhao et al., Citation2015). The ordinary PCR procedure can only amplify, so it is used for qualitative analyses following electrophoresis detection (Abuzinadah et al., Citation2015). The real-time PCR, equipped with a fluorescence signal acquisition system and a computer analysis and processing system, can monitor the fluorescence intensity during the PCR process to compare the DNA levels of multiple samples and perform relative quantification of the samples (Cheng et al., Citation2014; Iwobi et al., Citation2015; Kppel et al., Citation2013). However, comparatively to real-time PCR, it is possible to directly determine DNA copy number with ddPCR without using standard curves or reference genes, and can achieve absolute quantification of samples (Falzone et al., Citation2020; Hindson et al., Citation2013; Pinheiro et al., Citation2011; Sanders et al., Citation2011). The ddPCR is a partial PCR using water-oil emulsion droplets and this reaction system contains millions of aqueous micro-droplets separated by oil phase. After amplification, the positive droplets were identified by detecting each droplet. According to the Poisson distribution principle, the concentration of template molecules in the original sample is inferred by the number of positive droplets (Massanella et al., Citation2013). At present, ddPCR technology is widely used for gene expression analysis (Brunetto et al., Citation2014), microbial detection (Blaya et al., Citation2016) and identification of animal-derived adulteration in food (Floren et al., Citation2015). A primer based on the single-copy gene β-actin of pigs and the single-copy gene prolactin receptor of sheep were designed by Song et al. (Citation2014) and realized the quantitative detection of pigs and sheep using ddPCR. In raw and processed foods, Ren et al. (Citation2017) developed a method to identify and quantify adulteration using ddPCR. Despite the high value of fox skin, its meat holds little economic worth and is subjected to deodorization processes before being blended with other edible meats to generate substantial profits (Ma, Citation2014). A large gap still exists in the identification and testing standards of meat sources, especially those that are non-edible, like fox (Zia et al., Citation2020). Therefore, in order to realize the quantitative research on whether there are fox-derived ingredients in meat products, this paper used ddPCR technology to quantitatively detect meat products.
In this study, ddPCR was utilized to establish a technique for quantitatively analyzing fox-derived adulterants in meat products. Fox lean meat was utilized as the experimental specimen, with primers and probes specifically crafted for the single-copy gene in the genome serving as the focus gene. The mass of fox meat, its DNA content, and its DNA copy number can be determined by two linear curves. Furthermore, as an intermediate conversion value, DNA content was used to establish the formula between of fox meat mass and its DNA copy number. For the purpose of verifying that it is the correctness and accuracy of the established formula, an artificial adulteration model was used to detect samples of meat with known adulteration ratios. Adulterated fox meat can be tested using this formula simply by knowing the DNA copy numbers obtained from ddPCR. The establishment of this method provides reliable technical support for the identification and control of adulteration in related meat, food and other products, and has important innovative practical significance for the rapid supervision of food safety.
2. Materials and methods
2.1. Materials
Fox meat was provided by Changli Fox Breeding Base (Qinhuangdao, China) and a variety of lean meat were collected from major supermarkets and farmers markets, including beef, sheep, chicken, pork, and duck. Shanghai Bioengineering Technology Co., Ltd. company provided primers and probes for this study.
2.2. Laboratory apparatus
We obtained the Bio-Rad Q×200ddPCR Droplet Generator by contacting Bio-Rad (U.S.A.) and the NanoDrop 2000 Micro Nucleic Acid Protein Analyzer by contacting American Termo (U.S.A.). Mettler Toledo Co., Ltd. (Shanghai, China) provided the ME204/02 Electronic Balance, and Tiangen Biochemical Technology Co., Ltd. (Beijing, China) provided the GMO food DNA extraction kit. Thermal Cycler Gene Amplification Instrument was purchased from Bio-Rad (U.S.A.).
2.3. Experimental method
2.3.1. Sample preparation for fresh lean meat
Separate samples of fresh lean meat was minced and dried at 80°C for 48 h using a vacuum drying oven. The samples were then ground in a pestle and mortar after the drying process. In order to prevent cross-contamination, the contents of the different samples were processed individually.
2.3.2. DNA extraction
Following manufacturer’s instructions, A GMO food DNA extraction kit was used to extract DNA from each sample after it was accurately weighed. 1000 μL of GMO1 buffer and 40 μL of proteinase K were added to the samples and the mixture was incubated at 60°C for 1.5 h. Then, 200 μL of GMO2 buffer was added, and the sample was mixed and allowed to rest for 10 min. After centrifugation at 12,000 rpm for 5 min, the supernatant was separated. In the supernatant, 700 μL of the isopropanol was introduced and thoroughly mixed. The solution was centrifuge for 4 min to remove the supernatant and retain the precipitate. A final step was to wash the precipitate twice with 70% ethanol, dry it, and dissolve it in water. Analyzing extracted DNA was done using a NanoDrop 2000 spectrophotometer as with its A260/280 in scope of compliance (1.8–2.0). All the centrifugation processes were performed at 4°C.
2.3.3. Fox primer design
The alignment of the conservative region of fox provided the basis for the design of gene-specific primers. Based on a previous reference (Ren, Citation2017), fox primers and probes were synthesized using the F2 gene as target gene. The 5′ end of the probe was tagged with FAM fluorophore, and the 3′ end was tagged with BHQ1. Approximately, 10 μM of primers and probes were used as the working concentration. The sequences of the specific primers are shown in .
Table 1. The list of primers and probes.
2.3.4. ddPCR reaction program
The ddPCR analysis required 30-fold dilution of the original DNA extraction sample, which was added to a reaction mixture consisting of ddPCR ™ Supermix for probes (No dUTP), primer, probe, and sterile double-distilled water to yield a total reaction volume of 20 μL. The reaction system is shown in . The reaction system was transferred to the droplet card and 70 μl of Droplet Generation Oil was added and processed in the Droplet Generator. After generating emulsified droplets, they were moved to a fresh 96-well PCR plate for PCR amplification. PCR reaction conditions: 95°C for 10 min; 40 cycles of 94°C for 30 s, 62°C for 1 min, and 98°C for 10 min.
Table 2. ddPCR reaction system.
2.3.5. Primer and probe specificity
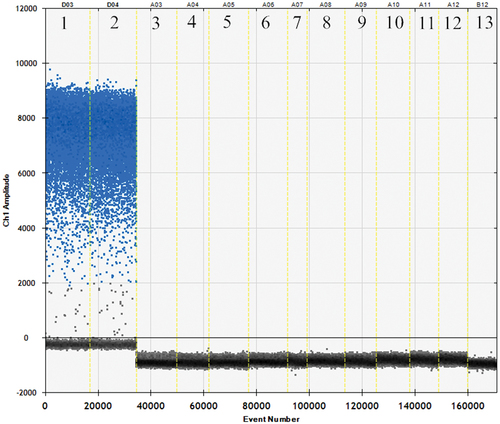
Beef, sheep, chicken, pork, and duck DNA templates served as negative controls, while fox DNA templates served as positive controls, and water served as the blank controls for species-specific probes and specially designed primers.
2.3.6. Formulation of fox mass and copy number
(1) A Relationship among the Mass of fox meat and its DNA Content
A precision electronic balance was used to weigh the meat samples of the fox, and the samples’ genomes were extracted. Using a NanoDrop 2000 spectrophotometer, the DNA concentration of the sample was determined.
(2) A Relationship among the DNA Content and DNA Copy Number of fox
Serial dilution of extracted DNA was performed, with fox dilution gradient of 5–100 ng/μL. Measurements were made three times for each gradient. A ddPCR was performed on the diluted genomic DNA, and samples with more than 12,000 droplets were accepted for analysis.
2.3.7. Models for detecting adulteration
For the purpose of verifying the accuracy of this experiment, the fox and pork adulteration ratio was set as follows: 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1. The total mass was 100 mg, and the DNA was extracted according to Section 2.3.2. After diluting the extracted DNA concentration by 30 times, the detection by ddPCR was carried out.
3. Results
3.1. Primer and probe specificity
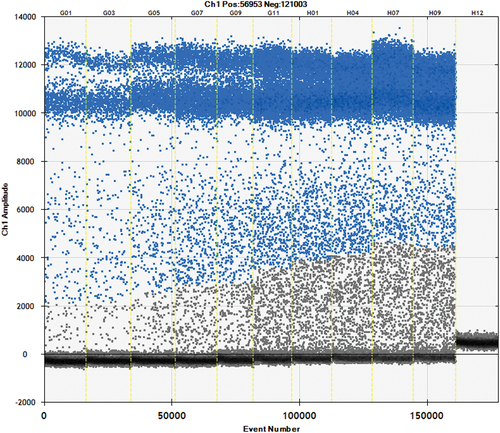
As shown in , the primers and probes had been evaluated for specificity by ddPCR. It can be seen that the fox primers and probes can amplify the fox components and other reference species did not amplify, indicating that the primers and probes exhibited strong specificity and were deemed suitable for the experiments.
3.2. A relationship among the mass of fox meat and its DNA content
The assay of relationship between the mass of fox meat and its DNA concentration was performed in three parallel experiments to avoid experimental error. The results are shown in and the coefficient of variation of detection about DNA content was below 7%. The results showed that the mass of the fox meat was found to have an excellent linear relationship with the content of DNA based on the gradient range of the mass data. (R2 = 0.9986) ().
Table 3. The DNA concentration extracted at different sample qualities.
3.3. DNA content of fox meat and its copy number relationship
A series of dilutions of DNA was performed (dilution gradients: 5,10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 ng/μL) and three parallel experiments were conducted per group. The samples with less than 12,000 droplets were not used for our analysis. The copy number measured at different DNA concentrations by ddPCR are shown in A good linear relationship was found among the DNA content and the copy number of fox samples (). The correlation coefficient of the standard curve was R2 = 0.9992.
Table 4. The copy number measured at different DNA concentrations by ddPCR.
3.4. The standard curve between the meat mass of the fox and its copy number
DNA content was used to calculate the formula among the fox meat mass and its DNA copy number (). The linear relationship can be described as: Mfox = 0.05C + 2.7, where C indicates number of copies (copies/μL), and M indicates meat mass of fox (mg).
Table 5. Establishment of the fox meat dose response curve.
3.5. Proportionally adulterated model detection
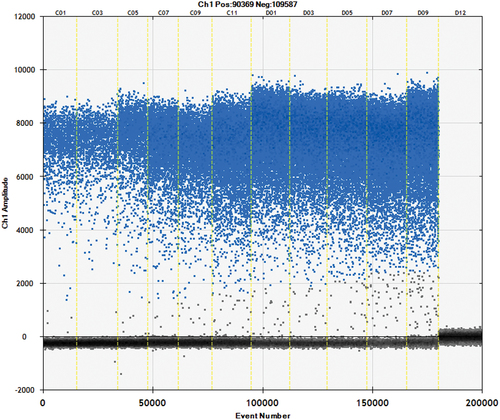
Repeating the experiment three times ensured accuracy and the map of adulterated fox can be seen from . The results indicate, comparing the predicting mass and real mass, the max relative error was 5.8%, and the minimum relative error was 0.3% (). There was little variation between proportions of fox adulterated meat samples in terms of relative standard deviation and the relative error rate of less than 6% was observed in the detection process. So the results obtained using the formula shown were in excellent agreement with the actual results and the developed method could provide a favourable figure of merit for monitoring the authenticity of meat products in the market.
Table 6. Adulteration ratios of mixed samples detected by ddPCR.
4. Discussion
Meat adulteration has become a growing focus of food research. Currently, scholarly investigations into the detection of adulteration primarily concentrate on edible meat sources, such as the inclusion of duck and chicken in beef and mutton, and the addition of horse meat to donkey meat. Nevertheless, there is a scarcity of research on the detection of adulteration in non-edible meat sources, and there is an absence of relevant standard specifications in this regard. Additionally, there exist numerous fox breeding facilities globally. If such inedible, non-quarantine meat infiltrates the market and becomes commingled with edible meat, it not only poses a significant threat to societal welfare but also jeopardizes consumer health. According to reports, 150 donkey meat products contained 65 samples of fox-derived ingredients (Bian et al., Citation2017). Therefore, it is urgent to establish a method for detecting fox components. In this study, ddPCR was used to detect accurately and quantitatively fox-derived components.
As compared with real-time PCR and conventional PCR, ddPCR showed higher precision. In a study conducted by Cai et al. (Citation2017), a dual droplet digital PCR detection and quantification system was developed to simultaneously identify and quantify meat sources in samples containing both beef and pork. ddPCR also was used by Chen et al. (Citation2020) for the quantitative detection of duck meat adulteration in samples of beef and beef meat. Moreover, ddPCR was used to establish duck genome reference materials for meat adulteration detection according to what was reported by X. Chen et al. (Citation2021). According to literature, there is a close linear relationship between between meat mass, DNA concentration, and DNA copy number (Cai et al., Citation2014; Chen et al., Citation2020). The quantitative method can accurately detect the amount of adulterants. It is generally believed that when the amount of adulteration is below 5%, it is accidentally mixed during processing, and when it is greater than 20%, it is deliberately adulterated (Cheng et al., Citation2020). Using this technique, we are able to distinguish whether the adulteration is caused by human factors or unintentionally introduced during the processing of meat products. Therefore, this paper established the standard linear relationship among meat mass and copy number curve by using the concentration of DNA as an intermediate conversion value. Quantitative analyses of fox-derived components were performed on meat products. A good set of standard curves was established for fox meat and R2 of both fits was above 0.99.
To validate the veracity of the calculation formula, the adulteration experiment was tested by artificially simulating a known fox meat adulteration model of a certain quality. The DNA was extracted from the meat samples with varying levels of adulteration quantified. Then, the theoretical meat mass of fox was obtained by using the standard curve of fox meat mass and its copy number. Results shows that the max relative error fox is 5.8%, so the theoretical values and the actual values were similar. It is possible to monitor the authenticity of fox meat in products by using this method.
5. Conclusion
A growing concern exists worldwide about adulteration of meat products, especially non-edible meat counterfeiting edible meat. The ddPCR was employed to set up a quantitative method to detect fox meat in meat products and the time required for a ddPCR run is 2 ~ 3 h. The method can precisely and rapidly detect the meat products that use fox meat instead of high-priced meat in the market, which non-edible meat sources can be detected, as well as artificially adulterated or naturally adulterated meat products can be distinguished. The established method provided a reliable technical means for the identification and detection of fox components in meat products.
This method of adulteration detection proves to be effective in discerning the presence of fox-derived ingredients, offering a reliable means of identifying non-edible meat that may enter the market without undergoing quarantine procedures. Additionally, it serves as a valuable technical tool for enforcing pertinent food regulations. Ultimately, this approach can help mitigate the adulteration of non-edible meat, thereby safeguarding the health and interests of consumers. This paper focused exclusively on quantitative detection of inedible meat components from foxes. It is anticipated that future research will focus on detecting edible meat components from other animals, including mice, raccoon, mink, etc.
Authors contributions
Hui Wang: Writing – Original Draft; Chen Chen: Formal analysis; Yan Zhang: Methodology; Boxu Chen: Conceptualization; Yongyan Li: Validation; Wenshen Jia: Investigation; Jia Chen: Supervision; Wei Zhou:Writing – Review and Editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Abuzinadah, O. H., Yacoub, H. A., El, A. H., & Ramadan, H. A. (2015). Molecular detection of adulteration in chicken products based on mitochondrial 12S rRNA gene. Mitochondrial DNA, 26(3), 337–7. https://doi.org/10.3109/19401736.2013.840593
- Amaral, J. S., Santos, C. G., Melo, V. S., Oliveira, M., & Mafra, I. (2014). Authentication of a traditional game meat sausage (alheira) by species-specific PCR assays to detect hare, rabbit, red deer, pork and cow meats. Food Research International, 60(June), 140–145. https://doi.org/10.1016/j.foodres.2013.11.003
- Bian, R., Fan, Y., Liu, Y., Huo, S., Sheng, Q., Tan, Q., Zhang, Q., Zhang, H., & Bu, X. (2017). Research on a rapid detection method for donkey, horse, and fox derived components. Feed Processing and Detection Techniques, 53(1), 100–104.
- Blaya, J., Lloret, E., Santísima-Trinidad, A. B., Ana, B., Ros, M., & Pascual, J. A. (2016). Molecular methods (digital PCR and real-time PCR) for the quantification of low copy DNA of Phytophthora nicotianae in environmental samples. Pest Management Science, 72(4), 747–753. https://doi.org/10.1002/ps.4048
- Brunetto, G. S., Massoud, R., Ohayon, J., Fenton, K., Cortese, I., & Jacobson, S. (2014). Digital droplet PCR for precise quantification of human T-lymphotropic virus 1 proviral loads. Retrovirology, 11(1suppl). https://doi.org/10.1186/1742-4690-11-S1-P13
- Cai, Y., He, Y., Lv, R., Chen, H., Wang, Q., Pan, L., & Te Pas, M. F. W. (2017). Detection and quantification of beef and pork materials in meat products by duplex droplet digital PCR. PLOS ONE, 12(8), e0181949. PMID: 28771608; PMCID: PMC5542382. https://doi.org/10.1371/journal.pone.0181949
- Cai, Y., Li, X., Lv, R., Yang, J., Li, J., He, Y., & Pan, L. (2014). Quantitative analysis of pork and chicken products by droplet digital PCR. Biomed Research International, 2014, 1–6. https://doi.org/10.1155/2014/810209
- Cavin, C., Cottenet, G., Cooper, K. M., & Zbinden, P. (2018). Meat vulnerabilities to economic food adulteration require new analytical solutions. Chimia (Aarau), 72(10), 697–703. https://doi.org/10.2533/chimia.2018.697
- Chen, C., Chen, J., Zhang, Y., Li, Y., Wang, Z., Li, X., Zhou, W., & Zhang, Z. (2020). Quantitative detection of beef and beef meat products adulteration by the addition of duck meat using micro drop digital polymerase chain reaction. Journal of Food Quality, 2020(6), 1–8. https://doi.org/10.1155/2020/2843056
- Cheng, X., He, W., Huang, F., Huang, M., & Zhou, G. (2014). Multiplex real-time PCR for the identification and quantification of DNA from duck, pig and chicken in Chinese blood curds. Food Research International, 60(June), 30–37. https://doi.org/10.1016/j.foodres.2014.01.047
- Cheng, Y., Wu, H., Liu, Y., Shen, W., Ren, M., & Liu, X. (2020). Quantification of animal adulteration ingredients in duck blood production by droplet digital PCR method. Food Technology, 45(2), 363–366. https://doi.org/10.13684/j.cnki.spkj.2020.02.059
- Chen, X., Ji, Y., Li, K., Wang, X., Peng, C., Xu, X., Pei, X., Xu, J., & Li, L. (2021). Development of a duck genomic reference material by digital PCR platforms for the detection of meat adulteration. Foods, 10(8), 1890. PMID: 34441667; PMCID: PMC8394454. https://doi.org/10.3390/foods10081890
- Chen, J., Zhang, Y., Chen, C., Zhang, Y., Zhou, W., & Sang, Y. (2020). Identification and quantification of cassava starch adulteration in different food starches by droplet digital PCR. PLOS ONE, 15(2), e0228624. https://doi.org/10.1371/journal.pone.0228624
- Falzone, L., Gattuso, G., Lombardo, C., Lupo, G., Grillo, C. M., Spandidos, D. A., Libra, M., & Salmeri, M. (2020). Droplet digital PCR for the detection and monitoring of legionella pneumophila. International Journal of Molecular Medicine, 46(5), 1777–1782. https://doi.org/10.3892/ijmm.2020.4724
- Fan, Y. (2014). Wal mart event exposes the problem of retail purchasing. China Logistics and Procurement, 03, 42–43. https://doi.org/10.16079/j.cnki.issn1671-6663.03.009
- Floren, C., Wiedemann, I. B., Brenig, E., & Beck, S. J. (2015). Species identification and quantification in meat and meat products using droplet digital PCR (ddPCR). Food Chemistry, 173, 1054–1058. https://doi.org/10.1016/j.foodchem.2014.10.138
- Giaretta, N., Giuseppe, A., Lippert, M., Parente, A., & Maro, A. D. (2013). Myoglobin as marker in meat adulteration: A UPLC method for determining the presence of pork meat in raw beef burger. Food Chemistry, 141(3), 1814–1820. https://doi.org/10.1016/j.foodchem.2013.04.124
- Hindson, C. M., Chevillet, J. R., Briggs, H. A., Gallichotte, E. N., Ruf, I. K., Hindson, B. J., Vessella, R. L., & Tewari, M. (2013). Absolute quantification by droplet digital PCR versus analog real-time PCR. Nature Methods, 10(10), 1003–1005. https://doi.org/10.1038/nmeth.2633
- Hui, W., Chen, C., Miaomiao, X., Yan, Z., Boxu, C., Yongyan, L., Wenshen, J., Jia, C., & Wei, Z. (2022). Quantification of adulterated fox-derived components in meat products by drop digital polymerase chain reaction. Preprint at. https://doi.org/10.1101/2022.11.29.518335
- Ismail, H. B., Reyhan, S. U., TüTüMay, T., & Halil, V. (2014). A rapid method for determination of the origin of meat and meat products based on the extracted fat spectra by using of Raman spectroscopy and chemometric method. European Food Research and Technology, 238(5), 845–852. https://doi.org/10.1007/s00217-014-2168-1
- Iwobi, A., Sebah, D., Kraeme, I., Losher, C., Fischer, G., Busch, U., & Huber, I. (2015). A multiplex real-time PCR method for the quantification of beef and pork fractions in minced meat. Food Chemistry, 169(169), 305–313. https://doi.org/10.1016/j.foodchem.2014.07.139
- Koppel, R., Jurg, R., & Jurg, R. (2011). Multiplex real-time PCR for the detection and quantification of DNA from beef, pork, horse and sheep. European Food Research and Technology, 232(6), 151–155. https://doi.org/10.1007/s00217-010-1371-y
- Kppel, R., Daniels, M., & Felderer, N. (2013). Multiplex real-time PCR for the detection and quantification of DNA from duck, goose, chicken, turkey and pork. European Food Research & Technology, 236(6), 1093–1098. https://doi.org/10.1007/s00217-013-1973-2
- Liu, L., Chen, F. C., Dorsey, J. L., & Hsieh, Y. P. (2006). Sensitive monoclonal antibody-based sandwich ELISA for the detection of porcine skeletal muscle in meat and feed products. Journal of Food Science, 71(1). https://doi.org/10.1111/j.1365-2621.2006.tb12393.x
- Ma, H. (2014). Unveiling the mysterious mask of fox meat - interview with Ma Guangyu. Food Safety Guide, 8(2), 38–39.
- Massanella, M., Singhania, A., Beliakova-Bethell, N., Pier, R., Lada, S. M., White, C. H., Pérez-Santiago, J., Blanco, J., Richman, D. D., Little, S. J., & Woelk, C. H. (2013). Differential gene expression in HIV-infected individuals following ART. Antiviral Research, 100(2), 420–428. https://doi.org/10.1016/j.antiviral.2013.07.017
- O’Mahony, P. J. (2013). Finding horse meat in beef products—a global problem. QJM: An International Journal of Medicine, 106(6), 595–597. https://doi.org/10.1093/qjmed/hct087
- Pinheiro, L. B., Coleman, V. A., Hindson, C. M., Herrmann, J., Hindson, B. J., Bhat, S., & Emslie, K. R. (2011). Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Analytical Chemistry, 84(2), 1003–1011. https://doi.org/10.1021/ac202578x
- Ren, J. (2017). Precise quantitative research on adulterated animal-derived components in mutton and its products by digital PCR technology. China Agricultural University.
- Ren, J., Deng, T., Huang, W., Chen, Y., Ge, Y., & Doi, H. (2017). A digital PCR method for identifying and quantifying adulteration of meat species in raw and processed food. PLOS ONE, 12(3), e0173567. https://doi.org/10.1371/journal.pone.0173567
- Safdar, M., Junejo, Y., Arman, K., & Abasıyanık, M. F. (2014). A highly sensitive and specific tetraplex pcr assay for soybean, poultry, horse and pork species identification in sausages: Development and validation - sciencedirect. Meat Science, 98(2), 296–300. https://doi.org/10.1016/j.meatsci.2014.06.006
- Sanders, R., Huggett, J. F., Bushell, C. A., Cowen, S., Scott, D. J., & Foy, C. A. (2011). Evaluation of digital PCR for absolute DNA quantification. Analytical Chemistry, 83(17), 6474–6484. https://doi.org/10.1021/ac103230c
- Song, L., Xue, C., Lu, Y., Zhao, L., Wang, D., Yang, H., Guo, M., & Yang, X. (2014). Detection and quantification pork in sheep products using real-time PCR. Food Science and Technology, 39(10), 319–322.
- Tian, X., Wang, J., & Cui, S. (2013). Analysis of pork adulteration in minced mutton using electronic nose of metal oxide sensors. Journal of Food Engineering, 119(4), 744–749. https://doi.org/10.1016/j.jfoodeng.2013.07.004
- Tibola, C. S., Da, S. S., Dossa, A. A., & Patricio, D. I. (2018). Economically motivated food fraud and adulteration in brazil: Incidents and alternatives to minimize occurrence. Food Science, 83(8), 2028–2038. https://doi.org/10.1111/1750-3841.14279
- Zhang, Y., Liu, M., Wang, S., Kang, C., Zhang, M., & Li, Y. (2022). Identification and quantification of fox meat in meat products by liquid chromatography–tandem mass spectrometry. Food Chemistry, 372, 131336. https://doi.org/10.1016/j.foodchem.2021.131336
- Zhao, X., Wang, Y., & Lan, Q. (2015). Identification of sheep derived components in meat products by fluorescent quantitative PCR. Science and Technology of Food Industry, 36(1), 299–302.
- Zia, Q., Alawami, M., Mokhtar, N., Nhari, R., & Hanish, I. (2020). Current analytical methods for porcine identification in meat and meat products. Food Chemistry, 324, 126664. https://doi.org/10.1016/j.foodchem.2020.126664