ABSTRACT
Gut microbes are considered as major factors contributing to human health. Nowadays, the vast majority of the data available in the literature are mostly exhibiting negative or positive correlations between specific bacteria and metabolic parameters. From these observations, putative detrimental or beneficial effects are then inferred. Akkermansia muciniphila is one of the unique examples for which the correlations with health benefits have been causally validated in vivo in rodents and humans.
In this study, based on available metagenomic data in overweight/obese population and clinical variables that we obtained from two cohorts of individuals (n = 108) we identified several metagenomic species (MGS) strongly associated with A. muciniphila with one standing out: Subdoligranulum. By analyzing both qPCR and shotgun metagenomic data, we discovered that the abundance of Subdoligranulum was correlated positively with microbial richness and HDL-cholesterol levels and negatively correlated with fat mass, adipocyte diameter, insulin resistance, levels of leptin, insulin, CRP, and IL6 in humans.
Therefore, to further explore whether these strong correlations could be translated into causation, we investigated the effects of the unique cultivated strain of Subdoligranulum (Subdoligranulum variabile DSM 15176 T) in obese and diabetic mice as a proof-of-concept. Strikingly, there were no significant difference in any of the hallmarks of obesity and diabetes measured (e.g., body weight gain, fat mass gain, glucose tolerance, liver weight, plasma lipids) at the end of the 8 weeks of treatment. Therefore, the absence of effect following the supplementation with S. variabile indicates that increasing the intestinal abundance of this bacterium is not translated into beneficial effects in mice.
In conclusion, we demonstrated that despite the fact that numerous strong correlations exist between a given bacteria and health, proof-of-concept experiments are required to be further validated or not in vivo. Hence, an urgent need for causality studies is warranted to move from human observations to preclinical validations.
Introduction
Human beings are superorganisms consisting of human cells coexisting with fungi, bacteria, and viruses, most of which reside in the gut. Recent human and animal studies have demonstrated that variations of the intestinal microbiota composition impact significantly on host physiology by influencing several processes including metabolism, immunity and inflammation, aging, and behavior.Citation1–4 Significant advances have been made in the study of gut bacteria-host interactions in the onset and progression of noninfectious diseases, particularly metabolic disorders such as obesity and insulin resistance, and diabetes. In this context, several bacterial taxa have been identified as being negatively (detrimentally) or positively (beneficially) correlated to metabolic health.Citation5 Unfortunately, most of the evidence so far is observational and associative in nature and the causal effect of specific species on the onset and development of obesity and associated metabolic disorders have been demonstrated only for a handful of species isolated from the human microbiota.Citation6–9 Therefore, given the potential role of specific bacteria in the physiologic regulation of host metabolic functions, identifying new bacterial taxa that mechanistically contributed to promote or maintain health can lead to the discovery of new targets for effective interventions in humans and bring new hope to slow down the metabolic disorders associated with obesity.
Among the novel probiotic candidates recently proposed, the most promising is Akkermansia muciniphila, for which the most advanced work has been done and both animal and human evidence have been published.Citation6,Citation10–14 A. muciniphila is commonly found in human and mouse gut microbiome and we showed previously that its administration could counteract diet-induced obesity and related disorders, such as glucose intolerance, insulin resistance, hepatic steatosis, and gut permeability in rodents.Citation6,Citation10,Citation11 These data have been largely confirmed by others.Citation12 Recently, we demonstrated the proof-of-concept that A. muciniphila administration is not only safe but can also improve cardiometabolic risk factors when given as a probiotic to humans with overweight or obesity.Citation13
In addition to showing that the administration of A. muciniphila can be causally linked with an improvement of several metabolic parameters, we noticed on several occasions that the higher abundance of A. muciniphila also co-occurs with the presence of another bacterium called Subdoligranulum sp. The genus Subdoligranulum belongs to the Ruminococcaceae family and is closely related to the Faecalibacterium genus. Indeed, we discovered that treatment of obese and diabetic mice with prebiotics (oligofructose) increased levels of A. muciniphila but that the levels of Subdoligranulum were also increased almost fourfold.Citation15 In obese humans, we noticed that a subgroup of the subjects with a mild improvement of their metabolic parameters during caloric restriction had significantly lower abundance of both Akkermansia and Subdoligranulum than the subgroup with a better metabolic response to the caloric restriction intervention.Citation16 Interestingly, the anti-diabetic drugs metformin and acarbose increase fecal Subdoligranulum relative abundance.Citation17,Citation18 Moreover, Subdoligranulum genus correlated negatively with glycated hemoglobin (HbA1c) and positively with HDL cholesterol.Citation18 Also, we found that alcoholic patients with poor gut integrity and high gut permeability had significantly reduced levels Subdoligranulum,Citation19 which confirmed previous observations who found less Subdoligranulum in cirrhotic compared to healthy individuals.Citation20,Citation21 Later, it was described that Subdoligranulum was underrepresented in subjects with NAFLD and that Subdoligranulum was negatively correlated with different parameters related to metabolic risks such as C-reactive protein (CRP), Fatty Liver Index, and Homeostasis Model Assessment-Insulin Resistance (HOMA-IR).Citation5,Citation22 Moreover, Subdoligranulum variabile, the only species of this genus isolated and described so far, was shown to produce butyrate,Citation23 a short-chain fatty acid, known for its health potential.Citation24 Several diseases, such as type-2 diabetes or inflammatory bowel diseases, were associated with lower abundance of butyrate producers like Subdoligranulum.Citation25,Citation26
Based on all these observations and associations found between Subdoligranulum and health, we postulated that Subdoligranulum could represent a promising probiotic candidate providing us with a handle to improve metabolic health. To make the leap from describing correlation to determining causation, we administered the only cultivated species of this genus to mice while inducing obesity by feeding them with a high-fat diet.
Results
Subdoligranulum is associated with Akkermansia muciniphila and correlates positively with a healthy metabolic status
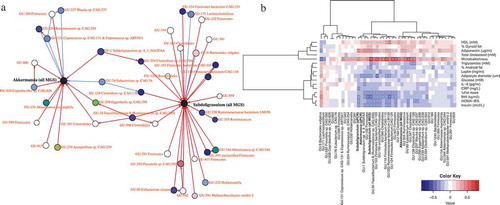
Based on available metagenomics and qPCR data, and clinical variables from two previously published cohorts of individuals with overweight and obesity,Citation16,Citation27,Citation28 we built a co-occurrence network that represents taxa as interconnected nodes if a co-occurrence relationship exists between them (). This allowed us to identify several metagenomic species (MGS) that were associated with A. muciniphila, including Subdoligranulum (). Moreover, Subdoligranulum and A. muciniphila relative abundances correlated positively (R = 0,35, p = 0,0003, Spearman correlation). We explored the link between Subdoligranulum genus in fecal samples and found that its abundance profile was correlated positively with fecal microbiota richness, HDL-cholesterol levels and adiponectin levels (). Microbiota richness is considered a hallmark of gut health and stability and individuals with low HDL cholesterol have a greater risk of atherosclerosis and cardiovascular disease.Citation29 In addition, we observed negative correlations between Subdoligranulum and fat mass, adipocyte diameter, a series of surrogate of insulin resistance, and levels of leptin, insulin, CRP, and IL-6. Adiponectin is an adipose tissue-derived hormone that has been involved in protecting against insulin resistance/diabetes and atherosclerosis. Decreased adiponectin levels are thought to play a central role in the development of type-2 diabetes, obesity and cardiovascular disease in humans. In contrast, elevated levels of leptin, another adipokine produced by adipocytes, have been associated with various health conditions including hypertension, low-grade systemic inflammation, and metabolic dysfunction in obese humans. IL-6 and CRP are commonly used systemic inflammatory markers. Taken together the results of this association study indicate that Subdoligranulum genus is associated with a healthier metabolic status.
Figure 1. Subdoligranulum is associated with Akkermansia muciniphila and correlates positively with a healthy metabolic status
Impact of S. variabile supplementation does not impact on the prevention of diet-induced obesity in mice
Although the genus Subdoligranulum is apparently composed of several representative putative species, only one species has been isolated and cultivated so far. This is the strain Subdoligranulum variabile DSM 15176 T.
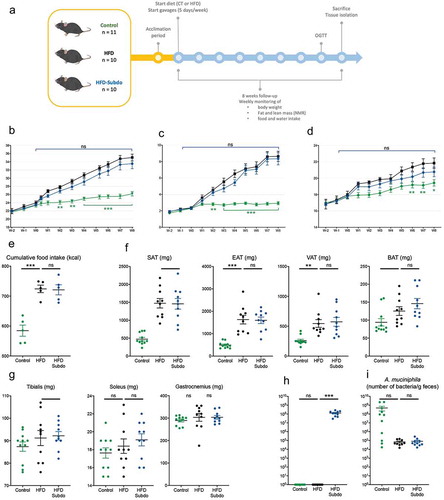
Therefore, to assess the in vivo effects of Subdoligranulum variabile we cultured this strain and administered 1.5 × 109 cultivable cells (cc) of bacteria frozen in glycerol to adult mice fed a high-fat diet (HFD), 5 days a week for 8 weeks (HFD-Subdo group, n = 10). This group was compared to two other groups of mice that were forced-fed with glycerol 25% and were fed either a control diet (Control group, n = 11) or a high-fat diet (HFD group, n = 10) ().
The gavages and treatment were well tolerated, and mice did not display any abnormal signs of stress or discomfort.
After 8 weeks of follow-up, there was a significant difference in body weight gain between the control fed and the high-fat fed mice (). Analysis of the weekly NMR measurements indicated that this was primarily due to an increased fat mass gain (), rather than lean mass (), and that this was the consequence of an increased caloric intake (), confirming the effectiveness of the diet-induced obesity model. However, we found differences neither in body weight gain between the HFD and HFD-Subdo groups, nor in lean or fat mass accumulation.
These observations were corroborated by the data of the tissue dissection, which showed no differences in weight of isolated adipose tissues (inguinal, epididymal, visceral and brown) () nor of selected muscles (tibialis, soleus, and gastrocnemius) () at the end of the 8-weeks period.
We found that Subdoligranulum abundance was very low in mice. However, the mice treated with S. variabile exhibited a clear elevation of Subdoligranulum compared to the control group (). As expected, A. muciniphila was reduced by the HFD. Supplementation with S. variabile did not induce an increase of A. muciniphila in treated mice ().
Figure 2. Impact of Subdoligranulum variabile supplementation on the prevention of diet-induced obesity in mice
S. variabile supplementation does not impact onlipid metabolism.
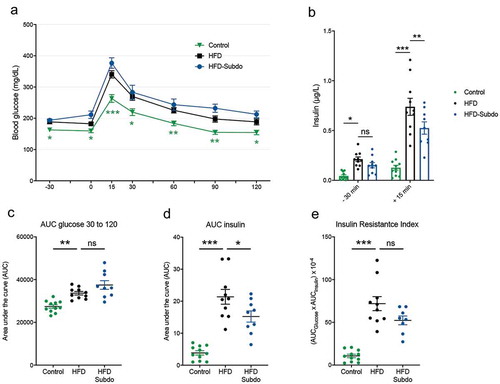
As we observed associations between Subdoligranulum and insulin levels as measures of insulin sensitivity in the overweight/obese group, we subjected the mice to an oral glucose test (OGTT) in the last week of S. variabile administration (). Compared to the control group, mice fed the HFD had higher fasting glucose levels after fasting (time points −30 min and 0 min) and a higher blood glucose profile after being given a glucose load (time points 15 min to 90 min). However, we did not observe an improvement of glucose tolerance in mice supplemented with S. variabile, as evidenced by similar glucose profiles () and corresponding areas under the curve (AUC, ), suggesting that S. variabile does not impact on overall glucose metabolism in diet-induced obese mice.
HFD-fed mice were hyperinsulinemic in the fasted state, as they exhibited more than two-fold higher levels of plasma insulin as compared to control mice (). They also displayed higher insulin levels after glucose challenge. There was a small, but significant reduction of insulin levels 15 min after the glucose load in the S. variabile treated mice, resulting in a lower area under the curve (). However, this was not sufficient to alter the insulin resistance index in a significant manner ().
Figure 3. Subdoligranulum variabile supplementation does not impact on glucose metabolism
S. variabile supplementation does not impact on lipid metabolism
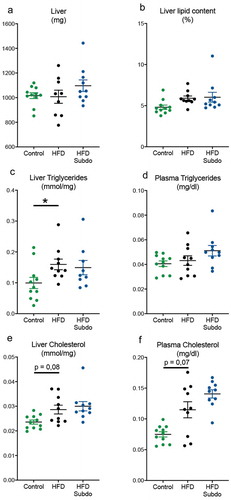
At the end of the 8 weeks of high-fat feeding and S. variabile administration, liver weight was comparable between all groups (). Total lipid contents of HFD-fed mice were somewhat increased, although this did not reach the significance threshold (p = 0,1) (). Hepatic triglyceride (p = 0,03), but not plasma triglycerides levels (p = 0,90) were increased by the higher dietary lipid supply in the HFD group (). Hepatic and plasma cholesterol levels were somewhat, but not significantly, elevated by HFD feeding (p = 0,08 and 0,07, respectively, ). There were no overt improvements or worsening of these parameters by the supplementation with S. variabile, indicating that increasing the intestinal abundance of this bacterium is not involved in lipid metabolism in mice.
Figure 4. Subdoligranulum variabile supplementation does not impact on lipid metabolism
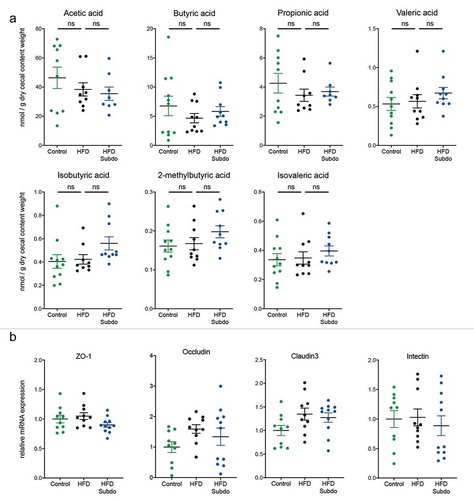
S. variabile supplementation does not impact on short-chain fatty acids or gut barrier
Since Subdoligranulum is a butyrate-producing organism and short-chain fatty acids (SCFA) have been linked to multiple beneficial health effects on host energy metabolism, we analyzed the SCFA concentrations in the cecal content of the mice. We found that the HFD slightly decreased the concentrations of the main SCFA: acetic acid, butyric acid, and propionic acid. Although most of the SCFA measured tended to be somewhat higher after S. variabile supplementation, none of the effects reached statistical significance (). Because butyrate is rapidly taken up by enterocytes where it serves as energy sourceCitation30 and where it enhances the intestinal barrier by regulating tight junction proteins,Citation31 we also measured some key markers of gut barrier integrity in the ileum. However, we found no increase in the mRNA expression of tight junction proteins (ZO-1, occludin and claudin 3), nor in the mRNA expression of intectin, a marker of intestinal epithelial cell renewal ().
Figure 5. Subdoligranulum variabile supplementation does not impact on short-chain fatty acids or gut barrier
Discussion
Different studies have started from observations and correlations between specific taxa and health, and then tentatively moved to causality by using different models. For example, mono-colonization of germ-free mice with specific bacterial strains, including Enterobacter cloacae B9,Citation32 Clostridium ramosum,Citation33 and Lachnospiraceae strain AJ110941Citation34 led to an increased propensity for obesity, identifying these strains as obesity-inducing pathogens. Another bacterium, Bilophila wadsworthia aggravated metabolic dysfunctions in conventional mice fed a high-fat diet.Citation8 In contrast, beneficial (probiotic) effects have been described for other bacterial strains, such as Faecalibacterium prausnitzii,Citation35 Anaerobutyricum soehngenii (formerly designated as Eubacterium hallii)Citation9 and Christensenella minuta.Citation7 Another key promising beneficial bacterium is Akkermansia muciniphila which has been extensively studied and finally confirmed in several proof-of-concept studies.Citation13
In our search for novel beneficial bacteria candidates, we identified Subdoligranulum, a strictly anaerobic, non-spore-forming, butyrate-producing, Gram-negative staining organism. Only one species belonging to this genus was isolated from human feces in 2004 and has since then been found in the gut of several other vertebrates.Citation23 Although the precise physiological role of Subdoligranulum is unknown, several interesting findings suggested a promising probiotic effect on host metabolism. Indeed, a series of human studies revealed that the increase of Subdoligranulum was associated with improved metabolic health including in intervention with prebiotics.Citation16,Citation19,Citation20,Citation22,Citation36
Thanks to a previous metagenomics approach in overweight/obese population, we discovered by using co-occurrence analysis that Subdoligranulum MGS (metagenomics species) was co-abundantly found with A. muciniphila and we confirmed the positive association between these two genera. Moreover, we found that Subdoligranulum was positively correlated with bacterial richness as well as with HDL cholesterol and surrogate measures of insulin sensitivity (HOMA). In addition, visceral fat mass was lower in individuals with high Subdoligranulum abundance. Taken together, our past and present data strongly supported the probiotic potential of Subdoligranulum and prompted us to investigate the role of this bacterium in vivo.
To firmly demonstrate causality, large, long-term clinical studies with well-defined criteria and protocols that yield robust outcomes are needed. However, initial exploratory research relies primarily on mechanistic studies performed in animals, in which confounding factors, such as age, diet, genetic background, environmental conditions, etc., can be omitted from the equation. Therefore, we treated mice with the only cultivated representative of this genus: Subdoligranulum variabile strain DSM 15176 T to determine whether it could counteract high-fat diet-induced obesity and associated metabolic disorders. After 8 weeks of S. variabile strain 15176T administration to HFD-fed mice, levels of Subdoligranulum in the feces of the mice were significantly increased. However, using Subdoligranulum variabile no improvement was observed on any of the measured parameters (body weight, fat mass, glucose metabolism and, lipid metabolism). Although S. variabile produces butyrate, a short-chain fatty acid with health-promoting abilities, we found only a modest, non-significative increase of this metabolite. It is possible that the lack of effect is the result of the dose of bacterium administered, however, the doses we used and the abundance recovered in the feces are well within range of the doses deemed relevant in other studies.Citation6,Citation37
Therefore, the lack of effect of S. variabile DSM 15176 T could have other potential explanations. It is, for example, possible that putative effects of Subdoligranulum in humans did not translate directly to rodents or that Subdoligranulum only has beneficial effects when co-occurring with specific bacteria or within a certain microbial community. Indeed, our previous and present data show that Subdoligranulum correlated with A. muciniphila. Moreover, both A. muciniphila and Subdoligranulum are highly increased upon metformin treatmentCitation17 or upon prebiotic treatment.Citation36 In this study, we found that administration of S. variabile did not result in a subsequent increase of A. muciniphila abundance. Another potential explanation would be that Subdoligranulum variabile is, in fact, not a determinant in obesity and that its correlation with improved metabolic parameters relies either on mere coincidence or on its inclination to thrive in a healthier microbial community. In other words, one may argue that Subdoligranulum is not a major bacterium acting on health but is rather an opportunistic bacterium growing either thanks to the presence of undigested compounds (e.g., fibers) or specific drugs (e.g., metformin, acarbose).
Importantly, it should be noted that in this study, we have tested only the impact of Subdoligranulum variabile, which is the only strain of this genus so far to be identified, isolated, and cultured. Meaning that several other, but unclassified, species within the Subdoligranulum genus may exist. Therefore, we may not rule out that the multiple correlations observed in humans are related to uncultured strains of Subdoligranulum. To support this hypothesis, we quantified the abundance of Subdoligranulum variabile versus Subdoligranulum spp. in human samples, and found that Subdoligranulum variabile only accounts for less than 1% of the total abundance of the Subdoligranulum genus in the human cohorts presented in this study (Supplementary Figure 1). Indeed, as depicted in , several other species exist and may be responsible for the numerous and strong associations found between the Subdoligranulum genus and a healthier metabolic profile.
In conclusion, the present study strongly supports the need to continue to move from correlations to causation when investigating the gut microbiota. Our study illustrates that the species and the strain specificity may be an issue. This requires appropriate sequencing methods with species identification. Finally, our study underlines the necessity to assess the validity of an associative observation between certain microbial components and health or disease conditions and urges the scientific community to be careful when stating conclusions based solely on co-occurrence data and correlations.
Material and methods
Patients
Using qPCR and shotgun metagenomic sequencing,Citation38 we examined Subdoligranulum in two already published population with a broad range of BMI range and metabolic complication recruited at Research Center on Human Nutrition, Pitié-Salpêtrière Hospital in Paris, France. Details regarding these overweight, obese and severely obese populations have been described elsewhere.Citation16,Citation27,Citation29 Briefly overweight and obese subjects were part of a dietary intervention (MICRO-Obes). Criteria to participate in the study included age between 25 and 65 years, body mass index (BMI) in the range of 27 to 38 kg/m2, absence of chronic disease, stable weight for 3 months prior to recruitment, and no antibiotic intake for 2 months before dietary intervention and stool collection. And, severely obese subjects were part of a bariatric surgery program (MICROBARIA). Since recruited in the same center, clinical and biological measures were harmonized. Anthropometric measurements included BMI, waist and hip circumference, and waist-to-hip ratio (WHR). Body composition was determined using dual energy x-ray absorptiometry (DXA), for the estimation of total body fat, lean body mass, and gynoid and android fat proportions. Blood samples were collected after an overnight fast at baseline, enabling measurements included markers of glucose homeostasis, blood lipids, and systemic inflammation. Fasting glycemia, insulinemia, Non-esterified fatty acids (NEFA), TG, and cholesterol were used to calculate various indexes of insulin sensitivity: the Homeostasis Model Assessment insulin resistance index (HOMA-IR) was calculated using the HOMA2Calculator system developed by Levy et al., which uses mathematical modeling and a healthy population as reference to determine insulin sensitivity.
Fecal microbiota richness was calculated as the number of metagenomic species present in the sample as described in Le Chatelier et al.Citation29
Culture of Subdoligranulum variabile
Subdoligranulum variabile DSM 15176 T was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ). The strain was routinely cultured in yeast extract-peptone medium supplemented with glucose, raffinose and anti-oxidants (detailed composition in supplementary Table 1). Attempts to enumerate S. variabile DSM 15176 T on solid medium failed despite separate autoclaving of phosphate and agar, as well as replacement of agar by gellan gum.Citation39,Citation40 Thus, live and cultivable bacteria were enumerated using most probable number calculation associated to dilution to extinction method: bacterial culture was serially diluted to the appropriate concentrations according to optical density (OD) measurements at 680 nm, then 10 vials per dilution were inoculated with 1 ml of dilution. The number of vials with visible growing within the three following days allowed the calculation of the live bacteria concentration in the culture.Citation41 For the administration to the mice, bacterial culture was centrifuged at 4000 g during 20 min at 4°C. Then supernatant was removed, and the pellet was washed twice in anoxic PBS supplemented with antioxidants and finally resuspended in glycerol (25% vol/vol) and stored at −80°C.
Mice
Ten-week-old male C57BL/6 J mice were housed in pairs in specific pathogen-free conditions and in controlled environment (room temperature of 22 +/- 2°C, 12 h daylight cycle) with free access to food and water. After an acclimatization period of one week, mice were randomly assigned to one of the three conditions: Control diet (10 kcal% fat, D12450Ji, Research Diet, New Brunswick, NJ, USA) gavaged with vehicle (Control group, n = 11), high-fat diet (60 kcal% fat, D12492i Research Diet) gavaged with vehicle (HFD Group, n = 10), or high-fat diet gavaged with 1.5 × 109 live cells of S. variabile DSM 15176 T (HFD-Subdo group, n = 10). Gavages were performed 5 days a week (Monday to Friday) for 8 weeks.
Body weight, food, and water intake were recorded weekly. Body composition (lean and fat mass) was assessed by using 7.5 MHz time domain-nuclear magnetic resonance (TD-NMR) (LF50 Minispec, Bruker, Rheinstetten, Germany).
All mouse experiments were approved by and performed in accordance with the guidelines of the local ethics committee. Housing conditions were specified by the Belgian Law of May 29, 2013, regarding the protection of laboratory animals (agreement number LA1230314).
Oral glucose tolerance test (OGTT)
After 7 weeks of treatment, an oral glucose tolerance test (OGTT) was performed as previously described.Citation42 Briefly, 6 h-fasted mice were given an oral glucose load (2 g glucose per kg body weight) and blood glucose levels were measured at different time points: 30 min before and 15, 30, 60, 90, and 120 min after oral glucose load. Blood glucose was measured with a standard glucose meter (Accu Check, Roche, Basel, Switzerland) on blood samples collected from the tip of the tail vein. Insulin resistance index was determined by multiplying the area under the curve of both blood glucose (−30 to 120 min) and plasma insulin (−30 and 15 min) obtained following the oral glucose tolerance test.
Insulin resistance index
Plasma insulin concentration was determined using an ELISA kit (Mercodia, Uppsala, Sweden) according to the manufacturer’s instructions. Insulin resistance index was determined by multiplying the area under the curve of both blood glucose (−30 to 120 min) and plasma insulin (−30 and 15 min) obtained following the oral glucose tolerance test.Citation43 Glucose-induced insulin secretion was calculated as the difference between plasma insulin levels 30 min before and 15 min after oral glucose load.
Tissue sampling
At the end of the treatment period (week 10), all animals were anesthetized with isoflurane (Forene, Abbott, Queenborough, Kent, England). After exsanguination, mice were killed by cervical dislocation. Subcutaneous adipose tissue depots, intestines, muscles, cecum, and liver were precisely dissected, weighed, and immediately immersed in liquid nitrogen followed by storage at −80°C for further analysis.
Feces collection
Feces were collected early in the morning. Mice were briefly separated and transferred to empty cages without bedding. Droppings were immediately collected using sterilized forceps, put in a tube and frozen on dry ice. Samples were stored at −80°C until further analysis.
Subdoligranulum spp, Subdoligranulum variabile and Akkermansia muciniphila quantification by qPCR
Genomic DNA was extracted from mice feces using the QIAamp DNA Stool Mini Kit (Qiagen, Germany), including a bead-beating step. DNA concentration was determined and purity (A260/A280) was checked using a NanoDrop2000 (Thermo Fisher Scientific, USA). Samples were diluted to an end concentration of 10 and 0.1 ng/μl in TE buffer pH 8. Total bacteria qPCR were performed on the 0.1 ng/μl dilution and the Subdoligranulum spp and Akkermansia muciniphila qPCRs were performed on the 10 ng/μl dilution. A standard curve was included on each plate by diluting genomic DNA from pure culture. Primers sequences targeting 16S rRNA gene are presented in .
Table 1. Primers used for the qPCR
Measurement of short-chain fatty acids
For SCFA analysis, the cecal content (50–60 mg wet material) was homogenized in water followed by sonication in an ice water bath. Acetonitrile was used for protein precipitation (in the presence of valproic acid as internal standard). Following centrifugation, the supernatant was recovered and a derivatization step (using 3-nitrophenylhydrazine in the presence of EDC and pyridine) performed. Samples were purified using liquid-liquid extraction to remove the remaining reagents. After evaporation, the final residue was analyzed using an LTQ Orbitrap XL mass spectrometer coupled to an Accela HPLC system (ThermoFisher Scientific). A Hypersil GOLD PFP (100 x 2.1 mm; 1.9 µm) column using a gradient of water-acetonitrile-acetic acid and acetonitrile-acetic acid allowed separating the different isomers. For ionization, an APCI probe was used in positive mode. Calibration curves were prepared using the same conditions to determine sample content. Xcalibur® software was used for data analysis. For each cecal content, an aliquot was freeze-dried to determine a dry residue that was used for data normalization.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 7.00 (GraphPad Software, San Diego, CA, USA) and R 3.5.1. Comparison between two groups was performed by Mann–Whitney–Wilcoxon test, comparison between three or more groups on one time-point was performed by one-way ANOVA followed by Tukey correction. Comparison between three or more groups at different time-points was performed by 2-way repeated measures ANOVA.
The co-abundance network was inferred using the Spectral Consensus Strategy (SCS) approach Citation44 with the scalenet R package applying default parameters, namely the “aracne” and “bayes_hc” inference modes. The dataset used to infer the network was composed of 776 variables (metagenomic species > 500 genes) plus two additional derived variables (i.e., targets) and 108 observations. Inferred edges were filtered by the estimated coefficient of mutual information and only those with |MI| > 0.2 were kept. From the global reconstructed, ecosystem only a subset was extracted with nodes that were directly linked to two targets. Both targets were computed as the sum of the relative abundance of all MGS annotated, respectively, as Akkermansia and Subdoligranulum at the genus level. The network was visualized with the igraph R package using Fruchterman-Reingold distribution of the vertices.
The heatmap was computed using Spearman correlations between metagenomic species and clinical information. The Manhattan distance along with the Ward algorithm were used for the hierarchical clustering of the variables.
Disclosure of potential conflicts of interest
P.D.C. is inventor of patent applications dealing with the use of A. muciniphila and its components in the context of obesity and related disorders. P.D.C. is co-founders of A-Mansia Biotech SA. K.C. is a consultant for Danone Research and LNC therapeutics for work unassociated with the present study.
Supplemental Material
Download Zip (363.5 KB)Acknowledgments
P.D.C. is a senior research associate at FRS-FNRS (Fonds de la Recherche Scientifique), Belgium.
We are grateful to Alexandre Barrois, Anthony Puel, and Henri Danthinne for excellent technical help.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80–101. DOI:10.1016/j.yfrne.2018.04.002
- Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 2018;16(3):171–181. DOI:10.1038/nrmicro.2017.149
- Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67:1716–1725.
- Cani PD, Van Hul M, Lefort C, Depommier C, Rastelli M, Everard A. Microbial regulation of organismal energy homeostasis. Nature Metabolism. 2019;1:34–46.
- Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279–297.
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. DOI:10.1073/pnas.1219451110
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. DOI:10.1016/j.cell.2014.09.053
- Natividad JM, Lamas B, Pham HP, Michel ML, Rainteau D, Bridonneau C, et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun. 2018;9(1):2802. DOI:10.1038/s41467-018-05249-7
- Udayappan S, Manneras-Holm L, Chaplin-Scott A, Belzer C, Herrema H, Dallinga-Thie GM, et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes. 2016;2(1):16009. DOI:10.1038/npjbiofilms.2016.9
- Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. DOI:10.1038/nm.4236
- Depommier C, Van Hul M, Everard A, Delzenne NM, De Vos WM, Cani PD. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020;1–15.
- Xu Y, Wang N, Tan HY, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219. DOI:10.3389/fmicb.2020.00219
- Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. DOI:10.1038/s41591-019-0495-2
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. DOI:10.1126/science.aan3706
- Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GM, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. DOI:10.2337/db11-0227
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Consortium M-O, Prifti E, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. DOI:10.1136/gutjnl-2014-308778
- Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. DOI:10.1038/nature15766
- Zhang X, Fang Z, Zhang C, Xia H, Jie Z, Han X, et al. Effects of acarbose on the gut microbiota of prediabetic patients: a randomized, double-blind, controlled crossover trial. Diabetes Ther. 2017;8(2):293–307. DOI:10.1007/s13300-017-0226-y
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485–E93. DOI:10.1073/pnas.1415174111
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–85.
- Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. DOI:10.1038/nature13568
- Louis S, Tappu RM, Damms-Machado A, Huson DH, Bischoff SC. Characterization of the gut microbial community of obese patients following a weight-loss intervention using whole metagenome shotgun sequencing. PLoS One. 2016;11(2):e0149564. DOI:10.1371/journal.pone.0149564
- Holmstrom K, Collins MD, Moller T, Falsen E, Lawson PA. Subdoligranulum variabile gen. nov., sp. nov. from human feces. Anaerobe. 2004;10:197–203.
- Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–876. DOI:10.3390/nu3100858
- Van Immerseel F, Ducatelle R, De Vos M, Boon N, Van De Wiele T, Verbeke K, et al. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J Med Microbiol. 2010;59(2):141–143. DOI:10.1099/jmm.0.017541-0
- Kaakoush NO, Day AS, Huinao KD, Leach ST, Lemberg DA, Dowd SE, et al. Microbial dysbiosis in pediatric patients with Crohn’s disease. J Clin Microbiol. 2012;50(10):3258–3266. DOI:10.1128/JCM.01396-12
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. DOI:10.1038/nature12480
- Dao MC, Belda E, Prifti E, Everard A, Kayser BD, Bouillot JL, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab. 2019;317:E446–E59.
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. DOI:10.1038/nature12506
- Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. DOI:10.1016/j.cmet.2011.02.018
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625.
- Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. Isme J. 2013;7(4):880–884. DOI:10.1038/ismej.2012.153
- Woting A, Pfeiffer N, Loh G, Klaus S, Blaut M. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. mBio. 2014;5(5):e01530–14. DOI:10.1128/mBio.01530-14
- Kameyama K, Itoh K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014;29(4):427–430. DOI:10.1264/jsme2.ME14054
- Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. Isme J. 2017;11(7):1667–1679. DOI:10.1038/ismej.2017.24
- Everard A, Lazarevic V, Derrien M, Girard M, Muccioli G, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. DOI:10.2337/db11-0227
- Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014–1023.
- Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68(1):70–82. DOI:10.1136/gutjnl-2018-316103
- Tamaki H, Hanada S, Sekiguchi Y, Tanaka Y, Kamagata Y. Effect of gelling agent on colony formation in solid cultivation of microbial community in lake sediment. Environ Microbiol. 2009;11(7):1827–1834. DOI:10.1111/j.1462-2920.2009.01907.x
- Tanaka T, Kawasaki K, Daimon S, Kitagawa W, Yamamoto K, Tamaki H, et al. A hidden pitfall in the preparation of agar media undermines microorganism cultivability. Appl Environ Microbiol. 2014;80(24):7659–7666. DOI:10.1128/AEM.02741-14
- Karunasagar A, Jalastagi R, Naik A, Rai P. Detection of bacteria by 16S rRNA PCR and sequencing in culture-negative chronic rhinosinusitis. Laryngoscope. 2018;128:2223–2225.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. DOI:10.2337/db06-1491
- Geurts L, Everard A, Van Hul M, Essaghir A, Duparc T, Matamoros S, et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat Commun. 2015;6(1):6495. DOI:10.1038/ncomms7495
- Affeldt S, Sokolovska N, Prifti E, Zucker JD. Spectral consensus strategy for accurate reconstruction of large biological networks. BMC Bioinform. 2016;17(S16):493. DOI:10.1186/s12859-016-1308-y
