ABSTRACT
Numerous studies point to the important role of probiotic bacteria in gastrointestinal health. Probiotics act through mechanisms affecting enteric pathogens, epithelial barrier function, immune signaling, and conditioning of indigenous microbiota. Once administered, probiotics reach the gastrointestinal tract and interact with the host through bacterial surface molecules, here called adhesion factors, which are either strain- or specie-specific. Probiotic adhesion, through structural adhesion factors, is a mechanism that facilitates persistence within the gastrointestinal tract and triggers the initial host responses. Thus, an understanding of specific probiotic adhesion mechanisms could predict how specific probiotic strains elicit benefits and the potential of adherence factors as a proxy to predict probiotic function. This review summarizes the present understanding of probiotic adherence in the gastrointestinal tract. It highlights the bacterial adhesion structure types, their molecular communication with the host and the consequent impact on intestinal diseases in both adult and pediatric populations. Finally, we discuss knockout/isolation studies as direct evidence for adhesion factors conferring anti-inflammatory and pathogen inhibition properties to a probiotic.
What is known:
Probiotics can be used to treat clinical conditions.
Probiotics improve dysbiosis and symptoms.
Clinical trials may not confirm in vitro and animal studies.
What is new:
Adhesion structures may be important for probiotic function.
Need to systematically determine physical characteristics of probiotics before selecting for clinical trials.
Probiotics may be genetically engineered to add to clinical efficacy.
1. Introduction
The human microbiota is a complex community that makes major contribution to human health.Citation1,Citation2 The gastrointestinal tract (GIT) microbiota has been referred to as an ancillary “organ” due to its impact on human well-being, including host metabolism, nutrition, physiology, and immune function.Citation3 The GIT microbiota harbors a complex microbial community, including prokaryotes, eukaryotes, and archaea. Several comprehensive microbial studies have focused on identification of individual organisms in this community.Citation4–7 However, identification alone does not explain which specific part of the “organ” is functionally responsible for human health benefits.
Probiotics have been shown to affect the human GIT and microbiota from birth when administered to neonates.Citation8 They are currently available as food supplements, either as prokaryotic probiotics (bacteria) or eukaryotic probiotics (yeast). Prokaryotic probiotics, typically belong to the genera Lactobacillus and Bifidobacteria, are most commonly used in treating disease.Citation9 Selecting probiotics from these genera with potential health benefits was initially studied using in vitro models such as intestinal epithelial cells (IECs), usually Caco-2 cell lines,Citation10 and animal models.Citation11 These models are used to screen for candidate strains isolated from food, humans, or animals as well as to investigate the mechanism of action of probiotics on the GIT. However, despite the final goal to translate knowledge gained from in vitro into human studies, the complexity of the human GIT hampers this transition. While in vitro experiments help to select a potentially effective strain, and animal studies may demonstrate efficacy, human clinical studies that can confirm health effects often fail.Citation12,Citation13
The possible reasons for human clinical studies failing to confirm probiotic effectiveness reported in vitro and animal studies are under discussion. One of the reasons is thought to be inefficient adherence of probiotics. Intrinsic molecular and structural characteristics of both bacteria and human hosts affect probiotic adherence. For example, oligo- or poly-saccharide structures, appendages and specialized surface proteins on bacteria interact with the host to induce innate immune signalingCitation14 or prevent pathogen attachment.Citation15 Some of these structures, such as pili on LGG (Lacticaseibacillus rhamnosus GG, formerCitation16 Lactobacillus rhamnosus), seem to be well characterized.Citation17 However, there is no clear-cut association between adhesion structures and health parameters reported in literature.
Adhesion of probiotics is defined as an initial bond or grip of probiotic bacteria and is based on unspecific physical interactions to a certain surface. Adhesion factors or adhesins are the molecular structures on the surface of the probiotic bacteria that facilitates this bond. This initial adhesion then initiates distinct interactions between adherence structures on bacteria and e.g. corresponding receptors on the host.Citation18 Some researchers have defined some of these adhesion factors as “cell-derived components”.Citation19 Adhesion of probiotic bacteria to GIT is considered a key aspect in relation with the host immune system modulation as well as for the exclusion of enteric pathogens.Citation20–22 Furthermore, an increasing number of studies are demonstrating that the viability of bacterial cells is not essential to exert immunomodulatory effects but rather their isolated adhesins can perform as well as when present as part of the bacterium.Citation23 Hence, in this review, we consider as adhesion factor both the adhesin in situ on the probiotic bacterium and the isolated/purified adhesin.
To establish whether a potential health benefit is shaped by probiotics, more attention is being focused on mechanistic knowledge, such as how do adherence mechanisms affect probiotic function.Citation24 For instance, adhesion of probiotics may be important for probiotic replication and colonization (Box 1), immunomodulation, inhibition of pathogen colonization. This direct interaction involves GIT immune and epithelial cells, as well as resident commensals. Hence, determining adhesion conservancy within the GIT can potentially be considered to predict probiotic function in humans.Citation25
The purpose of this review is to assess the surface structure of probiotics that are potentially responsible for adhesion. A review from Javanshir et al.Citation31 and a mini-review Monteagudo-Mera et al.Citation32 provide another perspective on the importance of the topic. Although the reviews focus more on the host perspective and some bacterial perspectives, more reviews are needed to supplement. We provide an additional overview of how probiotics can interact with the human host and suggest health implications deriving from such interactions. We focus on classes of bacterial surface structure molecules and consider the role they may play in GIT adhesion. Examples of pre- and clinical evidence are provided. Finally, we consider studies where these adhesion factors have been knocked out or purified to show their direct impact on probiotic function.
2. Adhesion Factors And Potential Downstream Mechanisms
2.1 Probiotic bacteria surface molecules involved in adhesion
Probiotics interact with the GIT mucosa through their bacterial surface structures. These structures are thought to facilitate adhesion and therefore contribute to probiotic persistence in the GIT mucosa. Consequently, surface structures may enhance probiotic function. In addition, adhesion is of major importance for probiotic colonization (Box 1) inside the host. Hence, adhesion to IECs in vitro is one of the leading selection criteria to determine whether a bacterial strain can potentially be a probiotic.Citation20 Traditionally adherence has been associated with the infectious mechanism of pathogenesis. While characterization of adhesion mechanisms and structures are ongoing for probiotic bacteria their surface structures resemble to a certain extent those of pathogens.Citation33 The surface structures on known probiotics are also referred to as adhesins, adhesion factors or ligands. Examples are exopolysaccharides (EPSs), pili and distinct surface proteins.Citation17 We discuss below and in some examples of adhesins and downstream interaction mechanisms.
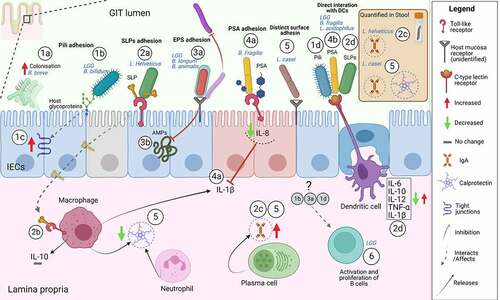
Figure 1. Examples of mode of action of probiotic adhesion factors. The figure shows binding and downstream effects on intestinal epithelial cells (IECs) and immune cells, reported for some probiotics with characterized adhesins. Probiotic pili-mediated adhesion favors a better colonizationCitation34 (1a), direct interaction to the intestinal mucus glycoproteinsCitation35 (1b), increased expression of tight junction-encoding genesCitation36 (1c) and direct interaction to antigen-presenting cells (dendritic cells)Citation37 (1d). SLPs-mediated adhesion through SLPs on bacteria or isolated SLPs occurs through interaction with TLR receptors on IECs (2a) and immune cells (macrophages)Citation23,Citation38,Citation39(2b) possibly this sustains production of fecal IgACitation40(2c). Studies using bacterial mutants of either SLPs or SLPs co-localized molecules show that SLPs trigger antigen-presenting cells (dendritic cells) inducing pro-inflammatory (IL-12, TNF-α, IL-1β) and anti-inflammatory cytokines (IL-6, IL-10)(2d).Citation41–43 Probiotic EPSs-mediated adhesion directly on other bacteria and/or competition for common adhesion sites on the host promotes “competitive exclusion” of pathogens or interferes with probiotic adhesion (3a).Citation44,Citation45 EPSs were shown to protect from innate immune mechanism via AMPs the probiotic LGG after adhesion to IECsCitation46 (3b). PSA on B. fragilis or isolated PSA has been shown to inhibit IL-1β induced inflammation through interaction with TLR2 and 4 on IECsCitation47,Citation48 (4a) and can also directly bind to antigen-presenting cells (dendritic cells) triggering downstream immune responsesCitation49,Citation50(4b). Distinct adhesins in the bacteria cells, not belonging to any of the aforementioned class, could support the sustained effects of L. casei on IgA and calprotectin Citation51–54(5). LGG probiotic triggers immediate adaptive immune response via B cells in humans.Citation13 Although the responsible upstream adhesion factors are not known the immediate effect is most possibly due to adhesion factors (6). The illustrative figure is shown without subdivision of GIT location (i.e. small or large intestine) but regional differences in the GIT immune system are of utmost importance.Citation55 In addition, the figure does not represent all mechanisms investigated by the studies in this review or literature but is meant to illustrate examples. Abbreviations: IECs, intestinal epithelial cells; PSA, polysaccharide A; SLPs, surface layer proteins; EPS, exopolysaccharides; IL, interleukin; IgA, immunoglobulin A; LGG, Lacticaseibacillus rhamnosus GG; AMPs, antimicrobial peptides. Created with BioRender.com.
2.2 Exopolysaccharides
Exopolysaccharides (EPSs) are polymers present on the surface of the bacterial cell wall, hence the prefix exo-, composed of polysaccharide structures.Citation56 EPSs are present on many bacteria, including Lactobacillus and Bifidobacterium genera.Citation44,Citation56–58 Functionally, EPSs enable communication between host cells and colonizing probiotics. EPSs support probiotic survival by adhering to GIT mucosal epithelium.Citation59–61 Hence, EPSs are involved in host interactions resulting in bacterial tolerance to harsh GIT conditions,Citation58 immunomodulatory activityCitation46 and have a role in biofilm formation.Citation56,Citation59 The first study to report the effect of probiotic EPSs as bacterial adhesins was a study assessing the EPSs function on LGG, Bifidobacterium longum NB667, and Bifidobacterium animalis IPLA-R1.Citation44,Citation62 The study showed that there is a dose-dependent effect of EPS that interfered with the adhesion of probiotics and pathogens to human intestinal mucus ().Citation44,Citation62 Since then further studies have investigated the EPSs of these three bacteria. The probiotic LGG seem to use EPSs to interfere with adherence of pathogens in a dose-dependent manner.Citation44–46 Similarly, for EPSs in B. animalis IPLA-R1Citation44 and B. animalis subsp. lactisCitation63,Citation64 it was shown a variable interference with other bacteria (probiotics and pathogen) via probiotic specific EPSs or biofilm formation. In conclusion, EPSs are thought to bind to external bacterial structures in both pathogens and commensals. Hence the bound EPS blocks the bacteria from adhering to the intestinal mucus.Citation44 This impact of probiotics on pathogens was named “competitive exclusion” and suggests the importance of EPS adhesins in this probiotic function in adults and neonates.Citation44,Citation65,Citation66
The EPSs on Bifidobacterium breve UCC2003 reduce the production of pro-inflammatory cytokines and immune cells.Citation67 EPSs help this probiotic to remain immunologically silent while exerting other functions such as pathogen exclusion. In addition, EPSs allowed this probiotic to be tolerated and to interfere with the persistence of the pathogen Citrobacter rodentium.Citation67 Citrobacter rodentium is a model used to investigate human GIT diseases (E. coli infections, inflammatory bowel disease, GIT tumors) hence interference with this pathogen could have implications for GIT chronic diseases.Citation68 Other B. breve strains, Bb99 and BBG-001, have been investigated in RCTs in infants. Bb99 was shown to modify beneficial microbiota in infants treated with antibiotics or delivered through cesarean section.Citation69 However, BBG-001 administration failed to protect from necrotizing enterocolitis (NEC) and sepsis in preterm babies.Citation70 NEC is a frequently encountered devastating condition in the premature neonates with symptoms similar to sepsis but differentiated by pneumatosis and portal venous gas.Citation71 Overall, although the adhesion factors of B. breve strains BBG-001 and Bb99 are not well investigated, the outcome of several studies suggests a direct effect of B. breve strains on other bacteria (pathogens, commensals) hence indirectly on the mucosa.
2.3 Glycolytic enzymes
Administration of Lactiplantibacillus plantarum HEAL9 (formerCitation16 Lactobacillus plantarum) and Lacticaseibacillus paracasei 8700:2 (formerCitation16 Lactobacillus paracasei) are thought to modulate the peripheral immune response in children with celiac disease autoimmunity.Citation72 This effect is enhanced by the ability of these two strains to attach to the human mucosa through a mannose-binding adherence mechanism.Citation73,Citation74 Interestingly, L. plantarum HEAL9 is genetically similar to the well-studied strain L. plantarum 299 v that could hypothetically behave in a similar manner.Citation72,Citation75 L. plantarum 299 v might adhere through multiple glycolytic enzymes.Citation74,Citation76 Although the contribution of each of these structures is difficult to study, because the mutants of this strain lose the metabolic activity conferred by these structures.Citation76 Administration of Lactiplantibacillus plantarum IS-10506 increased fecal immunoglobulin A (IgA).Citation77 IgA is an important mucosal humoral immunity antibody. The probiotic increased the immune response in two different clinical trials in children greater than two years of age and pre-school children.Citation77,Citation78 Once in contact with IECs, L. plantarum IS-10506 can adhere with its cell wall lipoteichoic acid and peptidoglycan.Citation77,Citation78 This adhesion potentially triggers signaling between IECs and nearby plasma cells which are the main producers of IgA.Citation78
2.4 Encapsulation
Bacterial encapsulations contribute directly and indirectly in the probiotic mechanisms of adhesion. Direct contribution is provided by engaging specific capsular components, such as polysaccharide A described in detail below, that interacts with IECs and immune cells. Indirectly, encapsulation confers probiotics the ability to persist and colonize longer in the GIT (Box 1). As such example, probiotic Bacillus coagulans Unique IS2, has been shown to be beneficial for children with irritable bowel syndrome (IBS).Citation79 This effect is due to strengthening of intestinal barrier function and reduction in bowel hypersensitivity. As opposed to other probiotic species, B. coagulans is a naturally encapsulated spore-forming bacterium. This encapsulation makes the bacteria potentially more viable and resistant to heat and acid degradation (gastric and bile acid). Hence, when administered it reaches the distal bowel and colon, mostly unaffected, where IBS symptoms appear.Citation79 Similar effects were observed in an RCT in adults suffering with IBS.Citation80 In addition, in this second study, with treatment, no changes were detected in pro- and anti-inflammatory cytokine levels suggesting that B. coagulans Unique IS2 could exert its effect by interacting with IECs and microbiota rather than a direct contact with innate immune cells.Citation80 It is important to note that adhesion of probiotics regards both that of bacteria cell (probiotic) to host cell and bacteria cell (probiotic) to bacteria cell (microbiota) with the second being defined either auto-aggregation or coaggregation.Citation81 Hence, benefits of encapsulation, with respect to adhesion and biofilm formation, are being widely explored to produce bioengineered probiotics mixtures. Probiotics such as Bacillus subtilis contain a natural extracellular matrix that surrounds the bacteria and facilitates their attachment to surfaces, hence can support their own and other bacterial survival to harsh industrial and GIT conditions.Citation82,Citation83
2.4.1 Polysaccharide A
Numerous gram-positive bacteria synthesize surface polysaccharides such as teichoic acids or lipoteichoic acids, which are important in the adherence of bacteria to biological surfaces. Polysaccharide A (PSA) is the immunodominant capsular polysaccharide of the human symbiont Bacteroides fragilis NCTC 9343. PSA is identified and isolated from the B. fragilis NCTC 9343 capsule and shown to be an important adherence factor communicating with the intestinal innate and adaptive immune cells of the neonatal and adult host.
First, bacteria containing PSA as well as purified PSA display the same anti-inflammatory properties through direct interaction to receptors on immature IECs as part of innate immune responses.Citation48 We have shown in our experimental studies, using an in vitro human fetal model, that PSA inhibits IL-1β-induced inflammation through toll-like receptors 2 (TLR2) and 4 on IECs.Citation48 In addition, our novel study described the anti-inflammatory role of Zona pellucida protein 4 (ZP4).Citation47 ZP4 is a distinctive protein on immature fetal IECs that mediates PSA anti-inflammation effects by involving TLR2 and IL-8Citation47 (). IL-1β is a pro-inflammatory cytokine released mainly from intestinal macrophages during cell activation and acute inflammation. Elevated cytokines such as IL-1β and IL-8, inhibited by PSA, are important because they have been associated with the diagnosis of necrotizing enterocolitis (NEC).Citation84 Hence, inhibition of IL-1β-induced intestinal inflammation by bacterial PSA is strategic for NEC prevention. Such bacterial components, that do not require a live bacterium and are able to maintain immunogenic effects, could potentially be introduced in formula for pre-term infants. Using bacterial components rather than using whole bacterial probiotics could potentially trigger a fine-tuned immune reaction, given the nature of the premature intestine.
Second, PSA binds directly to a C-type lectin receptor that is a glycan-binding receptor on dendritic cells () and this interaction is crucial for its processing and presentation to T cells as part of adaptive immune responses.Citation50,Citation85 PSA forms a complex with major histocompatibility complex class II of innate immune cells and is presented to the T cell receptor of CD4+ T cells. This antigen presentation mechanism was believed to apply exclusively to protein antigens.Citation86 However, PSA purified from B. fragilis NCTC 9343 was shown to protect animals from experimental colitis through induction of anti-inflammatory IL-10 producing CD4+ T cells.Citation49
Summarizing, PSA on B. fragilis has been studies in animals and in vitro in humans using inflammatory models of innate and adaptive immune system to try to prevent colitis/NEC/inflammation in adults and neonate models. PSA is an important and promising adhesion factor as part of the capsule of this bacterium with immunogenic properties. Although experimental data are promising, human studies are lacking and of great need for further confirming the application of such purified factors in nutritional recommendations.
2.5 Pili appendages
Pili, as probiotic appendages, consist of a protein called pilin, with the capacity to adhere to other bacteria mediating biofilm formation and bacterial aggregations, and to adhere to GIT surfaces mediating the probiotic interactions with the host. Various types of pili structures have been identified on both gram-positive and gram-negative bacteria and have received attention since the LGG probiotic comparative genomic study in 2009.Citation35 Since then, a immense number of studies, discussed in detail below, have investigated the pili of LGG.Citation17,Citation87–92 In addition to LGG, other bacteria also contain functional pili such as Bifidobacterium bifidum PRL2010,Citation93 Bifidobacterium breve UCC200,Citation34 Lactococcus lactis IL1403 and TIL448,Citation94,Citation95 Lactobacillus ruminis ATCC 25644Citation96 and Lacticaseibacillus casei LOCK 0919 (formerCitation16 Lactobacillus casei LOCK 0919).Citation97
2.5.1 Sortase-dependent pili on B. bifidum
Bifidobacterium bifidum PRL2010, a strain isolated from infant stools, is shown to have sortase-dependent pili proteins (). Pili conferred to this probiotic both adhesion properties to IECs and immunomodulatory properties in vivo. This was demonstrated by expressing the same coding sequence of the pili on a second bacteria without pili that manifested the same pili-conferring properties.Citation93 Bifidobacteria strains are predominant species colonizing the infants GIT and commensals themselves express common adhesion extracellular proteins with B. bifidum PRL2010. Investigating probiotic properties of bacteria isolated from healthy infant stool is important for designing pediatric GIT disease treatments where such strains could colonize and re-establish symbiosis. Effects of B. bifidum PRL2010 on intestinal barrier function, IECs and innate immune responses was confirmed by other studies.Citation36 These effects were achieved through the transcriptional regulation of tight junction genes for induced colitis in mice.Citation36 Hence, the piliation of B. bifidum and other pili containing probiotics is viewed with an expanded role as a niche‐adaptation factor.Citation98
2.5.2 Sortase-dependent pili on LGG
The probiotic LGG, one of the most frequently administered probiotics in adults and children, exert some of its probiotic properties via a well characterized pili adhesion factor. LGG contains two separate pilus gene clusters in its genome, SpaCBA and SpaFED.Citation17,Citation35,Citation99 However, SpaCBA pilus is functionally important for the well-organized adherence of this probiotic.Citation35,Citation87,Citation99 The functional pilin motifs SpaCBA on LGG contains 3 type of pilins.Citation35,Citation99,Citation100 These pilins are monomeric protein subunits joined together covalently by a pilus-specific sortase enzyme hence called sortase-dependent pilus type.Citation101 LGG employs this structure to assure strong adherence to glycoproteins on the intestinal mucus, colonization and functional probioticproperties.Citation17,Citation35,Citation87,Citation99 () (Box 1). It was shown that acid stress could also enhance the GIT adhesion capability of LGG by inducing pili-related genes on the bacterium.Citation102
Upon adhesion, LGG pili are important adhesins with immunomodulatory properties on the intestinal mucosal.Citation13,Citation103 In fact, glycans on LGG pili can be recognized by dendritic cells via a C-type lectin receptorCitation37 (). These interactions are of functional importance to induce dendritic cells hence pro-inflammatory cytokines IL-6 and IL-12 and anti-inflammatory cytokine IL-10Citation37 (). While this innate immune signaling can be induced also by several commensals, to what extent this relates to adaptive immunity is not known. Bornholdt et alCitation13 showed that only 2 h after administration of LGG, it was found in the jejunum of healthy participants. The study clustering analysis shows that adaptive immunity (B-cell activation) genes were upregulated only in one third of the participants.Citation13 This suggests that individual differences should be taken into account when designing human studies. Interestingly, the effects on adaptive immunity were immediate as B-cell gene changes were detected 2 h after LGG administration. In addition, another human study with a longer administration time (28 days) found transcriptional changes (in blood) of immune cell trafficking and inflammatory responses.Citation103 Expression was restored after the probiotic stopped, suggesting that the long term effect was due to the probiotic.Citation103 Although subjects in this study were healthy, induction of immune cells by LGG, if administered to inflamed intestine, could potentially maintain immunological tolerance while exerting anti-inflammatoryeffects.Citation13,Citation103 Finally, the effects of the pili on innate immune components, such as dendritic cells, could potentially render this probiotic useful in inflammatory conditions where this cells play key roles in diseases pathogenesisCitation104,Citation105 ().
Studies have also provided insights into the role that LGG can play in the host intestinal barrier function.Citation106–108 Intestinal barrier function is commonly studied using IEC models such as Caco-2 and by investigating tight junction expression (Zonula occludens-1, occludine, claudins) which are proteins holding together IECs hence keeping tight the intracellular passage of substances in the GIT. Hence, in vitro experimental models employ disruption of these tight junctions as a proxy for GIT disease models. LGG prevented interferon-gamma-induced epithelial barrier disruption used as a model for IBS. Barrier function via Zonula occludens-1 and occludine was protected in IBS-like enteroids but such protection was lost when using denatured LGG suggesting that pili might need viable bacteria to exert its function.Citation106 A similar protective effect of LGG was observed in a gliadin disrupted barrier function as a model for celiac disease. Only viable LGG in concomitant treatment with gliadin significantly increased Zonula occludens-1, claudin-1 and occludine gene expression in IECs Caco-2.Citation107 In conclusion, LGG protects intestinal barrier function and this protection is lost when employing not-viable bacterium. Differently to other adherence factors, there are no studies yet showing whether is possible to purify and use pili. However, LGG mutants are being engineered to adjust pili expression on bacteria mutants.Citation109 This anticipates a future of specialized mutants with varied capability to adhere.
2.6 Distinct surface adhesins
Some probiotics can have diverse surface adhesins, which are not associated with any of the abovementioned categories. These distinct adhesins have been considered in isolated strains with probiotic activity.Citation110 They are often of proteinaceous nature such as the Family 1 of solute binding proteins on B. infantis, a common member of infant intestinal microbiota.Citation111,Citation112 Adhesion factors of proteinaceous nature, defined here as distinct proteins, can perform more than one function but are also involved in adhesion. Groups of proteinaceous adhesins are for instance surface layer proteins (SLPs), collagen binding protein (Cbp), mucus-binding proteins (Mub), mucus adhesion promoting protein (MapA), sortase A, auto-aggregation promoting protein (AggLb). We will consider some of these in detail below.
2.6.1 Surface layer proteins
Surface layer proteins (SLPs), or S-Layer proteins, are a class of proteins that form the outermost interacting component of the bacterial cell wall of different Lactobacillus species.Citation113,Citation114 Presence of SLPs appendages is beneficial especially for Lactobacilli probiotic immunomodulatory action in the GIT. SLP and its homologue on Lactobacillus acidophilus NCFM affects immune response via dendritic cells () and interaction with IECs.Citation41,Citation42,Citation115 SLPs on L. acidophilus NCFM and ATCC 4365 are functionally involved ligands that interact with a C-type lectin receptor on dendritic cells and thereby prime these cells to regulate T cell function.Citation41,Citation43 L. acidophilus NCFM has been especially well-studied. Several type of SLPs have been identified for this probiotic (e.g. SlpA, SlpB).Citation116 It was shown, by using SLP mutants, that the type of SLP on L. acidophilus NCFM controls the cytokine type production.Citation41,Citation115,Citation117,Citation118 Compared to other probiotic Lactobacilli, L. acidophilus NCFM displays a slight proinflammatory profile with a very low IL-10/IL-12 cytokine ratio that has been directly linked to SLPs/SLP associated proteins.Citation41,Citation115,Citation117,Citation118 However, it is not clear to what extent each of these SLP protein types within L. acidophilus NCFM contributes to probiotic properties. The difficulty to study this is given by the fact that the knockdown of one protein has resulted in the changes in expression of another making it hard to attribute a single effect to a single SLP protein. Citation41,Citation115,Citation117,Citation118 SLP immunological properties are highly variable between different Lactobacilli as well despite their purified SLPs inducing the production of IL-12 p40 on macrophage cell line THP-1 from multiple strains.Citation118
Adhesion and colonization of Ligilactobacillus salivarius REN (formerCitation16 Lactobacillus salivarius REN) to human IECs is mediated by an S-layer protein called choline-binding protein A (CbpA).Citation119 The interaction was shown in vitro to be mediated via an enolase receptor on IEC HT-29 cells that can recognise CbpA.Citation119 It is not further documented how CbpA affects this probiotics properties but is interesting to know that detailed characterisation of each single Lactobacilli SLPs are starting to be elucidated.
2.6.2 Collagen-binding protein
Collagen-binding proteins (Cbp) are cell surface proteins on bacteria able to bind and adhere to GIT extracellular matrix components including collagen. This process was identified initially as pathogenic as it allowed colonization of pathogens once firmly bound to collagen, however probiotics can mimic the same mechanism without causing harm. Probiotic Lactobacillus strains, including Lactiplantibacillus plantarum LM3, 91 and W2 are able to bind to collagen.Citation120–122 Purified Cbp from L. plantarum 9, a strain selected for strong collagen binding among several L. plantarum, displays competitive exclusion (anti-adhesion) properties on pathogenic Escherichia coli 0157:H7.Citation123 Presence of adhesion factors like Cbp confer impactful colonization potential to probiotics under the harsh environment of the GIT and given that collagen is a component of mucus it is easily accessible.Citation123,Citation121 L. plantarum W2 was able to inhibit pathogen Penaeus vannamei. This probiotic cntains a Cbp in its genome although the study did not attribute the probiotic property solely to the Cbp.Citation122
Lacticaseibacillus casei supplementation during acute diarrhea in children of 6 months-6 years increased fecal IgA and reduced fecal lactoferrin and calprotectinCitation52 (). Induction of IgA deposition by this probiotic seems to be continuous and sustained.Citation51L. casei ATCC 393 has distinctive adhesion properties, although minimum adhesion to confer probiotic properties is observed in the large intestine it is comparable to other probiotics.Citation53 It is not clear form literature which adhesin is characteristic for which L. case strain. However, attempts have been made to genetically modify this probiotic to expresses collagen-binding protein gene cnb, which in turn enhances bacterial adhesion.Citation54
In conclusion, Cbp seems to naturally benefit L. plantarum strains to colonize better by adhering to GIT collagen. There seems to be some potential for pathogen inhibition, possibly due to common collagen-binding sites shared by probiotics and pathogens, but the studies are merely focusing on the bacteria and don’t have clinical implications yet. Interestingly, Cbp is purifiable and as such future research could consider administration of Cbp as adjuvant to infections without the complexity of assuring viable probiotics.
2.6.3 Mucus-binding or mucus adhesion proteins
Mucus-binding proteins (Mub) and mucus adhesion proteins (MapA) are the two key cell surface proteins expressed differentially among species of Lactobacilli, thus, promoting their attachment to GIT mucosa.Citation124 Mab and MapA mainly bind to mucins which represent the majority of proteins in the mucus of GIT. The nomenclature and classification of specific Mub and MapA, implicated in probiotic adhesion, is unclear from literature. In general, comparative genomic studies have assigned Mub and MapA names to genes or proteins identified in specific probiotic strains that can bind to GIT mucus components, usually of glycan nature. Mub is found in the surface of the bacterial cell and can contain mucin-binding repetitive domains (MucBP) that are functionally responsible for the interaction with the mucus.Citation125
Limosilactobacillus reuteri strains (formerCitation16 Lactobacillus reuteri) contains both Mub and MapA adhesion factors. Mub from L. reuteri 1063 was able to interact with mammalian Igs including IgA which is important for GIT mucosa homeostasis, and interacted also directly with GIT mucus.Citation126,Citation127 Similarly, a Mub on L. reuteri ATCC PTA 6475, named CmbA, was responsible for adhesion in IECs Caco-2. After evaluation of 5 potential adhesins this study observed strong loss of adhesion mainly in cmba bacteria mutants.Citation128 L. reuteri ATCC 53608, as an architype of a commensal bacteria, has 14 tandemly arranged Mub repeats and a motif called LPXTG that anchors to the bacteria cell side.Citation129 Mub of this bacteria was shown to be an adaption niche that organizes in a way to maximize the adhesion to GIT mucus glycans.Citation129 Both Mub on L. reuteri ATCC PTA 6475 and 53,608 have been shown to be able also to trigger an immune response and inhibition of E. coli. First, immunoregulatory properties are exerted via interaction of C-type lectin receptors on dendritic cells and hence influence production of both anti- and pro-inflammatory cytokines IL-10, TNF-α, IL-1β, IL-6, and IL-12.Citation130 Second, pathogen inhibition properties on enteropathogenic E. coli were shown both in mucus producing and non-mucus producing IEC lines as well as small intestine tissue.Citation131
Several studies have investigated the role of MapA as well on the probiotic strains L. reuteri. MapA on L. reuteri 104 R is considered a primary adhesion factor for adhesion of this probiotic in IECs and mucus.Citation132 This was shown by pre-treatment with purified MapA that bound to multiple receptor-like structures on IEC Caco-2 cells and subsequently inhibited L. reuteri in a dose-dependent manner showing a saturation of the receptors.Citation132 Parts of MapA, as a larger surface structure on L. reuteri LA92, were defined for their antimicrobial peptide properties and named AP48-MapA.Citation133 Such pleiotropic functions of MapA, both as GIT adhesion factor and for antimicrobial peptide properties, was proposed to be of major importance in establishing a healthy microbiota.Citation133 While L. reuteri 104 R and LA92 are not currently used in the clinical settings, L. reuteri DSM17938 is approved for human use to treat infant diseases. However, no consensus has been reached, as reported by meta-analysis, on the benefit of this strain, especially for formula-fed infants with colic.Citation134 In various RCTs using L. reuteri DSM17938, this probiotic failed to show efficacy alone, or compared to placebo (or control) treatment of acute diarrhea in infants.Citation135 For constipation, the results were contradictory in children aged 3–7, 2–16 and 2–4 years.Citation136–138 This lack of clarity could be attributed to differing administration length, doses of probiotic or to insufficient patient numbers. While Mub and MapA have not yet been investigated for DSM17938, their presence seem to be an advantage for several L. reuteri and worth investigating.Citation139 This was shown by the presence of MapA genes in eight tested L. reuteri that increased significantly upon co-culturing with Caco-2 cells as a model for intestinal barrier function.Citation139 Finally, the presence of Mub and MapA suggests opportunities for new L. reuteri strains to be introduced as potential probiotics.
L. plantarum 91 exhibits strong probiotic traits such as acid and bile tolerance and colonization, that in vivo were partially attributed to Mub genes which increased expression during the bacteria transit in the stomach of mice.Citation124 The Mub of L. plantarum 91 has 6 mucus-binding domains where the last 2 domains of the Mub are considered functionally responsible, named Mubs5s6, and exhibit high adhesion in human GIT tissue. It has been possible to purify Mub from L. plantarum 91 and further express it in other species.Citation140 Pre-treatment with purified Mubs5s6 of IEC lines Caco-2 and HT-29 inhibited the binding of enteropathogenic E. coli. This mechanism was attributed to the binding of Mubs5s6 to cytokeratins, Hsp90, and Laminin (all three ligands associated with infections) in the host mucosa. The effects of purified Mubs5s6 were potentially stronger than the effects of bacteria cells expressing Mubs5s6.Citation141Another L. plantarum, L. plantarum 423, showed putative probiotic genetic characteristics given by the presence of Mub, MapA and adhesion-like factor EF-Tu.Citation142 Gene expression changes of Mub, MapA and EF-Tu were evaluated in the presence of mucus, bile, pancreatin, different pH, and it was shown that the probiotic can adapt to such conditions of a healthy GIT simulation.Citation142
In conclusion, Mub and MapA seem to be very important adhesion factors for several different Lactobacilli that share common niche genes. Although adhesion on mucus-bearing or non-mucus bearing IECs and endurance of GIT conditions seem to be strongly guided by Mub and MapA presence, the clinical significance of such adhesion factors is still blurry. One interesting observation is that of the enteropathogenic E. coli inhibition both by Mub bearing strains as well as purified Mub. E. coli pathogenic strains are involved in sudden infant death.Citation143 The inhibition of such strains by purified Mub and Mub bearing Lactobacilli, possibly by the pathogen and probiotic sharing common adhesion sites, opens new frontiers in designing prevention treatments for at risk infants.
2.6.4 Multiple adhesion factors
Genomic analysis of commercially available Bacillus clausii ENTPro, revealed three proteins involved in adhesion: mucus-binding protein, a collagen-binding protein and a fibronectin-binding protein functionally responsible for adhesion.Citation144 The study also proposes that probiotic strains within B. clausii (i.e ENTPro, B106, and UBBC-07) are very similar to each other in this regard.Citation144 B. clausii UBBC-07 supplementation resulted clinically in improving diarrheic symptoms in children,Citation145 possibly by favoring colonization and resolving dysbiosis. This could be explained by distinct adhesion proteins, conferring anti-diarrheic properties to B. clausii UBBC-07 and its similar ENTPro.
Comparative genomic analysis of L. fermentum 3872 identified genes encoding putative mucus-binding proteins, collagen-binding proteins, and EPSs which all contribute to enhance probiotic function.Citation146 L. fermentum 3872 was isolated from breast milk of healthy human female and contains multiple vitamin synthesizing genes and adhesion genes. This would allow this probiotic candidate to persist in the GIT competing for similar sites with pathogens and favor nutritional processes which would make it an ideal candidate for addition in infant formula.Citation146
Finally, although we consider in this review one by one the adhesion factors that characterise a certain probiotic, a good number of probiotics or probiotic candidates have multiple distinct putative adhesion factors. Currently, in literature, most studies are genomic studies focused on identification rather than downstream function of these adhesins. Hence, to what extent each of adhesins contributes within a multi adherence factor system on a certain probiotic is yet to be attributed.
3. Evidence Of Involvement Of Adhesins In Probiotic Function By Isolation Or Knockout Of Adherence Factors
Probiotic mechanisms on the host GIT cells include cytoprotection, cell proliferation, cell nutrition, and synthesis of proteins with gene expression changes.Citation147 These can contribute to biological functions such as intestinal epithelial cell homeostasis and innate immune signaling regulation. Citation148,Citation149 A number of studies provide evidence that these effects are possibly attributable in part to probiotic adhesion capacity, using either knockout molecules or isolated specific adhesins. In and below we provide instances of studies in which changes in probiotic adhesion molecules have led to loss of probiotic function. Alternatively, specific adhesion molecules have been isolated to show a specific probiotic effect. For example, in several studies immune-related signaling by cytokines on host cells was affected when from the interacting probiotic was removed a specific structural molecule responsible for adhesion. From a broad literature screen, this was noted especially for Lactobacillus strains (). Overall, there was no apparent association between a specific adhesion factor and a known immunological response. Below are shown a few studies undertaking this approach.
Table 1. Modifications of adhesion structures affects the probiotic function. Specific modification of surface structures on probiotics, hypothesised to be potential adhesins, affected GIT rewiring with respect to direct interaction, immune stimulation through inflammatory cytokines production and pathogens inhibition.
3.1 Isolated SLP from Lactobacillus helveticus
SLPs on Lactobacillus helveticus were defined decades ago as a layer of non-glycosylated protein in the bacterial cell wall.Citation157 SLPs are thought to arbitrate a number of effects on host inflammatory mediators, innate immune signaling as well as in IECs homeostasis. A RCT with a parallel design, administering among others L. helveticus R0052 to healthy 3–12-month-old infants suggested an anti-inflammatory profile of this probiotic. The L. helveticus R0052 arm of this study showed an increase of the tumor necrosis factor alpha (TNF-α)/IL-10 ratio but no changes in fecal microbial composition.Citation158 Interestingly, another L. helveticus (the NS8), pre-selected for its adhesion and survival properties, was able to diminish the pro-inflammatory effects of LPS by inducing higher levels of IL-10 in a macrophage cell line.Citation39 This mechanism was possibly through SLP- mediated adhesion, however, when investigating the purified SLP on this strain it did not affect IL-10 (). Citation39
Isolated SLP from L. helveticus MIMLh5 and the bacterium itself, were investigated using in vitro and ex vivo models. The two triggered an innate immune response via expression of pro-inflammatory TNFα and COX-2 in a human macrophage cell lines via recognition by the TLR2. No effect on anti-inflammatory IL-10 by isolated SLP was shown in this study ().Citation23 In addition, anti-inflammatory effects were proposed by reducing the activation of NF-ƙB in IEC lines.Citation23
Both isolated SLP on L. helveticus SBT2171 as well as the probiotic bacteria itself induced antimicrobial peptide hBD2 expression in the host IECs via TLR2.Citation38 This study shows that isolated SLPs present in other Lactobacilli have a similar stimulatory effect and proposes it as a common feature of a number of Lactobacillus species. Given that the TLR2 signaling is an important pathway for host protection against infections,Citation159 isolated SLPs from L. helveticus could be considered for a supportive administration as probiotic molecules during human infant infections. Attempts have been made to use L. helveticus in infant infections. A multicenter clinical trial found that L. helveticus R0052, in combination with L. rhamnosus, did not prevent the development of gastroenteritis in 3–48-month-old children.Citation160 Others found that the same probiotic showed higher fecal IgA levels in 3.5-6-month-old healthy infants Citation40 (). However, L. helveticus R0052 in the second RCT was used in combination with two species of Bifidobacterium probiotics.
In summary, the role of both isolated SLPs on L. helveticus as well as L. helveticus bacteria alone have been directly compared and act through common pathways (i.e. TLR2). However, while the whole bacteria induced anti-inflammatory responses via dampening of e.g. IL-10 production this seems to be lost when using the purified SLPsCitation23,Citation39 (), possibly due to a weaker effect of the adhesin or inadequate dose. Thus far, no RCT or in vivo human study has investigated the effect of isolated SLPs. A few RCTs using combination probiotics have administered L. helveticus probiotic bacteria, but do not answer whether the effect of the study was due solely to L. helveticus strain or its SLPs.
3.2 EPS-knockout mutant of Lacticaseibacillus rhamnosus
Besides its surface pili, the prototypical probiotic LGG contains exopolysaccharides (EPSs) shown to be involved in adhesion hence probiotic function. EPSs protect LGG against anti-microbial peptides in the GIT ().Citation46 This was confirmed when the EPS LGG mutant exhibited a decreased persistence in the murine GIT and was more sensitive to the host´s innate defense mechanisms.Citation46 Interestingly, EPSs were also shown to change in the presence of different GIT conditions and despite different EPS molecules having similar mechanism of actions, their chemical structure seem to be sensitive to very small changes.Citation161 LGG bacteria EPS-mutant and isolated EPS molecules were shown to interfere with Candida infection compared to LGG wild-type bacteria,Citation45 suggesting that EPSs could confers anti-fungal properties. However, the study was performed in vaginal epithelial cells. Attempts have been made to study Lacticaseibacillus rhamnosus prevention of rectal colonization with Candida. A RCT of 150 12-year-old children on broad spectrum antibiotics were administered a mixture of probiotics containing among others Lacticaseibacillus rhamnosus and saw a decrease of prevalence of the fungal infection.Citation162
In conclusion, LGG is a complex multifaceted probiotic with pili adhesion factors that appear to be important for immune mechanisms. EPS effects were shown by creating bacterial mutants lacking EPSs. However, although EPSs are relevant for bacteria persistence in the human GIT, evidence is unclear whether isolation of EPSs as single molecules is possible and which disease can benefit from it. Candidiasis infection caused by broad spectrum antibiotics is a clinical problem in children. And since administration of antibiotics is more often accompanied with intervals of probiotics in children, finding an isolated adhesin with mechanisms to both protect from antibiotic-induced candidiasis as well as diarrhea would be ideal.
3.3 Sialidase-knockdown mutant of Bifidobacterium bifidum
Interaction of some B. bifidum strains with GIT mucosa is mediated via the bacterium extracellular sialidase domain. Sialidases are proteinic adhesins involved in probiotic-mucosal interactions. Through enzymatic activity sialidases process a variety of carbohydrates such as human milk oligosaccharides (HMOs) that are needed for the bacteria self-metabolism and to promote Bifidobacterial growth. HMOs are a glycan source for the infant GIT microbiota.Citation156,Citation163 Hence HMO are clinically relevant to infant homeostasis in addition to B. bifidum itself being part of the dominant colonizers of the breast-fed infants GIT.Citation164
Studies using B. bifidum ATCC 15696 mutant in sialidase domain (Siabb2) showed a decreased adhesion to human IECs and porcine mucin relative to the wild-type strain.Citation156 This suggests a key role of sialidases as adhesins. Another B. bifidum, B.bifidum PRL2010, seems to targets host-derived glycans in mucus for nutrient attainment.Citation165 This catabolic process, conserved in various strains, is an important colonization factor for B. bifidum.Citation165
In summary, sialidase adherence activity began to be shown by usage of a bacteria mutant lacking this protein and could be the mechanism through which the bacteria anchors on the GIT mucosa. More studies are needed to elucidate the role of sialidases in isolation with respect to colonization of commensals, immunity and infections. Since sialidases are need for self-colonization of bacteria, studies with a mutant overexpressing the protein ought to be performed to investigate dose response. Hence, re-colonization with this probiotic via sialidases could contribute to resolving dysbiosis and protecting infants who experience decreased abundance of B. bifidum during GIT diseases.
4. Adhesion Of Probiotics And Pathogen Inhibition
Probiotic action on enteric pathogens has been widely studied. Mechanisms such as inhibition of colonization by competitive exclusion or secretion of antimicrobial substances are direct (physical) mechanism on pathogens, while acting on the epithelium and immune component affects indirectly pathogens.Citation166 During competitive exclusion probiotics compete with pathogens for the same host mucosal receptors or for the same nutrition sources.Citation167 This probiotic mechanism is potentially due to specific adhesion factors, like those of pathogens, as sites of adhesions on IECs and immune cell receptors binding.
Lactiplantibacillus plantarum DM 69 through its purified antimicrobial factors may competitively exclude intestinal Salmonella enterica, as shown by adhesion studies in an HCT-116 cell line.Citation168 In addition, L. plantarum L15 strain was successfully established for prevention of pathogenic Escherichia coli adhesion.Citation167 However, although the two L. plantarum strains showed potential for pathogen-inhibition and anti-microbial factors production, the responsible adhesion factors are still not well characterized. The studies speculate that this is potentially due to the adhesion analogs between probiotic and pathogens.
In another study, a novel probiotic mechanism involving mucus-binding peptides of LGG was shown to outcompete Enterococcus faecium colonization.Citation90 Vancomycin-resistant enterococci peptides with known pathogenic properties were shown to share sequences with the peptides of SPCA-SRIP1 pili of the probiotic LGG.Citation90 Hence, immunological and functional similarities between LGG and the pathogen E. faecium strain E1165 opens new frontiers for prophylaxis and treatment of vancomycin-resistant enterococcus infections.Citation90 Supplementation of another L. rhamnosus, Lacticaseibacillus rhamnosus 19,070–2, to infants with intestinal colic was found to decrease crying and fuss time.Citation169 These benefits may be due to the fact that probiotic bacterial pili can better adhere, colonize (Box 1) and exclude gas-forming Clostridioides (previously Clostridium) difficile, Klebsiella pneumoniae, and Escherichia which are increased in colic.Citation169,Citation170
Further mechanisms have been proposed for probiotics during enteric infections. Supplementation of probiotic Streptococcus faecium (i.e. Enterococcus faecium) and Bacillus subtilis during Helicobacter pylori infections diminished antibiotic-induced dysbiosis.Citation171 This effect contributes to the H. pylori eradication success rate because it can restrict the growth of GIT antibiotic-resistant bacteria. On the other hand, Enterococcus faecium WEFA23 was shown to use the adhesion factor SLPs to inhibit five pathogens, and particularly Listeria monocytogenes CMCC54007.Citation152 Removal of the adhesion factor SLP on E. faecium WEFA23 significantly decreased its adhesion capacity, suggesting that the probiotic SLP adhesion factor is responsible for pathogenic exclusion.
During rotavirus infection, Lactobacillus acidophilus AD031 and Bifidobacterium longum BORI had an effect on the duration of the diarrhea while other effects were non-significant.Citation172 The supportive effect of L. acidophilus species during infections in infants were demonstrated by giving low doses of L. acidophilus (subsp. L. Gasseri) while E. faecium and B. infantum were found to decreased the frequency of late-onset sepsis in pre-term newborn.Citation173 Live or killed L. acidophilus bacteria retained similar benefits while reducing the incidence of necrotizing enterocolitis (NEC).Citation174 These data suggest that anti-microbial properties of L. acidophilus species are due to bacterial structures, possibly surface adhesion markers that persist after the probiotic is killed rather than to live probiotic metabolites.
In conclusion, from evaluation of pathogen inhibition studies several generalizable points come to light. First, the ability of probiotic bacteria to affect or eradicate pathogens has been studied mainly for pathogenic bacteria rather than e.g. viral infections. Second, most of these studies have widely speculated that the pathogen inhibition effect is due to similarities between adhesion factors of the probiotic bacteria and the pathogenic bacteria. However, very fewCitation152,Citation153 or no studies have shown which of these adhesins are responsible for the effect. Finally, the ability of a probiotic strain to eradicate a pathogen is clinically highly relevant in cases where the probiotic acts on an enteric bacteria that is antibiotic resistant,Citation90 hence alternative approaches are needed to eradicate the pathogen. In addition, it has been shown that in infants the choice of antibiotics affects both the healthy microbiota and the anti-bacterial resistance.Citation175 Hence, if we could find a way to shift the microbiota by using probiotics, e.g. by increasing the proportion of certain healthy strains, we could achieve a benefit on the outcome of antibiotic resitant infections.
5. Age Related To Probiotic Function And Adhesion
Unlike adults which have a mature fully developed GIT mucosa, infants have an immature GIT mucosa with an immune component that needs yet to establish tolerance to the external environment. Adults have a restricted macromolecular epithelial passage, while infants have a high endocytic capacity with enhanced passage of macromolecules and pathogens.Citation176 Earlier microbiota evaluations of Lactobacillus in feces showed that the infant intestine is initially colonized by only a few different strains whereas in adults there is a complex pattern with a higher diversity of strains.Citation177 Species such as L. rhamnosus and L. casei/paracasei were characteristic of adult feces, whereas L. gasseri and L. salivarius were common in infant feces.Citation177 Metagenomics and microbiome studies have shown also that children have a different functional microbiome with higher relative abundances of Bacteroides in children compared do adults.Citation178 On the other hand Bifidobacteria in human babies < 2 year old was shown to be higher than adults.Citation179 Human microbiota abundances are age-specific, but with increase of age a more diverse microbial colonization takes place shaping into adulthood GIT where intestinal microbiota is presumably relatively stable both in health and disease.Citation180,Citation181
Because of the age-specific nature of the GIT mucosa and microbiome, diseases affecting infants, children and adults require the use of tailored probiotics.Citation180 Why a certain probiotic is used at a certain age hasn’t been systematically studied in literature. Nevertheless, administration of probiotic mixtures was shown to accelerate the transition into a mature, term-like microbiome which was Bifidobacterium-driven in preterm infants.Citation182 Common GIT diseases affecting infants where probiotics are employed comprise necrotizing enterocolitis (NEC), infections, infant colic, use in preterm infants with the overall aim to improve short and long-term health.Citation183 Children and adults employ probiotics mainly for acute gastroenteritis,Citation180 acute diarrheaCitation184 (e.g., antibiotic induced) and chronic disease such as inflammatory bowel disease (IBD), irritable bowel syndromeCitation185,Citation186 celiac disease and infections eradication.Citation187 In and below are discussed a few probiotics strains in relationship to age.
Figure 2. Age and probiotics adhesion. The figure shows a schematic view of probiotic and GIT characteristics changing with age. (a) Conventionally probiotics are given in increasing doses with age.Citation184 (b) IECs, which are among the main cells interacting with probiotics in the GIT and form the intestinal barrier function, are less established early in life but with age they are progressively shaped into a fully functioning GIT barrier.Citation10,Citation188 Probiotic adhesion is strain- and age-dependent. In (c) are shown a few examples of probiotic strains, B. bifidum,Citation189 LGG,Citation10,Citation189 B. lactis Bb12Citation189, in which age-dependency has been investigated within a single study. Created with BioRender.com
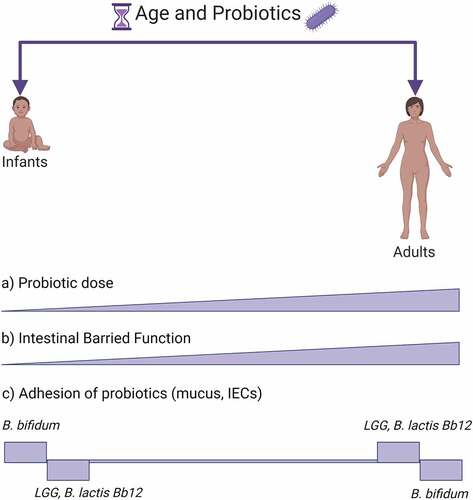
Eminent strains, such as LGG, are used in pediatric and adult populations regardless of age. Antibiotic-associated diarrhea (AAD) is an acute side effect that occurs during most antibiotic treatments and where probiotics are regularly used. Although in most cases probiotics are used during antibiotic treatment, more studies are supporting a preventive use before antibiotic treatment.Citation190 An elegant meta-analysis compared the current evidence of LGG use in AAD for children vs adults.Citation184 The study indicated that LGG was effective in preventing diarrhea in both. However, while for children there was a link between dose and effect size, for adults such link did not show up.Citation184 Why the later link did not show up is not clear, but possibly in the adult GIT LGG mechanism or adhesion and action might be different due to mucosal and microbiota differences with age. A methodological study showed that, immature IEC cell models HIEC-6 compared to the mature colonic Caco-2 were shown to react differently to LGG stimulation.Citation10 HIEC-6 cells form a scarce barrier with lack of some tight junction compared to Caco-2 cells. LGG treatment could not improve the experimental barrier function in immature HIEC-6 cells.Citation10 LGG employs a number of adhesion proteins (i.e. pili, EPS). Even thought LGG was able to prevent AAD in both pediatrics and adults, in neonates GIT mucus LGG adhesion was lower than adultsCitation189 hence the probiotic effect might be more a consequence of interaction with microbiota rather than strengthening the barrier function.Citation10 Possibly, LGG might employ a dose-dependent effect in children that is more related to its adherence factor EPS that interacts with the microbiota and pathogens,Citation44,Citation191 rather than a direct interaction on IECs through pili adhesion factor.
A few studies have investigated age-dependent immune effects of probiotics mixtures in animals. Jeong et al compared the effects of a probiotic mixture (L. casei, L. acidophilus, L. reuteri, B. bifidum, and S. thermophiles) in young and aged rats to study age-dependent colitis.Citation192 The mixture of probiotics seems to protect from LPS-induced inflammation, via NOS, COX2, TNF-α, IL-1β, CRP, and induce expression of intestinal barrier function markers (tight junctions, ZO-1, occluding) in an age-dependent manner. Kaushal et al showed that administration of probiotic Dahi consisting of Dahi bacteria along with L. acidophilus LaVK2 or L. acidophilus and B. bifidum BbVK3 improved age-related immune functions that are diminished with age. Peritoneal macrophage functions were enhanced by stimulating NOS and IL-6 and diminishing PGE2, and lymphocyte proliferation and IL-2 production was increased.Citation193 It is interesting to note that, although both studies used a combination of probiotics, making it hard to attribute the effect to a single strain, both studies included L. acidophilus and B. bifidum in this combination. While the use of a mixture makes it hard to distinguish to which strain the effect is due, however, these studies are highly relevant for humans as pro-inflammatory markers such as TNF-a IL-6 and CRP have been shown to be associated both with ageing and are prototypical pro-inflammatory markers in the pathogenesis of IBD.Citation194,Citation195
Vast limitations exist when searching for studies on probiotics in relation to age. First, the few available intestinal cell line models are either fetal/neonatal-derived or adult-derived, from either humans and animals.Citation10,Citation196 There are no “in between” cell lines to represent children, and isolating primary cell lines from different pediatric ages merely for studying probiotic mechanisms seems unfeasible. Second, there seem to be universal strains used both in adults and pediatrics (e.g. LGG, B. breve) and strains unique to pediatrics (B. infantis, L. rheuteri), however why that is the case does not seem to be evidence-driven but rather historical, general health- and safety-driven.Citation197 Third, a few studies directly comparing adhesion in infant and adult cell lines are general in referring to barrier function properties or mucus composition, and do not specifically discuss adhesins. Mechanistic studies comparing adhesion factors to age are not available and studies usually consider either infant or adult GIT.
6. Adhesion Factors As A Proxy To Predict The Effect On Probiotic Mechanism
It is suggested throughout the review that adhesion factors could be a proxy for probiotic mechanism and function. This idea is yet in its infancy given that adhesion factors are neither systematically studied nor formally classified in literature. However, a few important studies, discussed throughout the review and summarized in offer the strongest available evidence and may be considered as a starting point to suggest a specific adhesion factor as a proxy for a specific mechanism.
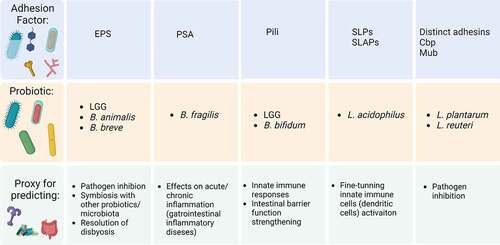
Figure 3. Key adhesion factors as potential proxy for a global probiotic function. Representation chart of some adhesion factors and potential application for global mechanisms with respective reference studies. First column: EPSs on LGG,Citation44–46 B. animalisCitation63 and B. breve.Citation67 Second column: PSA on B. fragilis.Citation47–50 Third column: Pili on B. bifidumCitation36,Citation93 and LGG.Citation37,Citation106,Citation108,Citation109 Fourth column: SLPs and SLAPs on L. acidophilus.Citation41–43,Citation115 Fifth column: distinct adhesins on L. plantarumCitation123,Citation141 and L. reuteri.Citation131 Abbreviations: EPS, exopolysaccharides; LGG, L. rhamnosus GG; PSA, polysaccharide A; SLPs, surface layer proteins; SLAPs, surface layer associated proteins; Cbp, collagen binding protein; Mub, mucus-binding protein. Created with BioRender.com
7. Concluding Remarks
This review aimed to gather knowledge on the pivotal role of adhesion factors in establishing a probiotic function in the GIT of adults and pediatrics. Numerous probiotic surface factors, defined here as adhesins, have shown promising results to be a proxy for predicting probiotic mechanisms in humans. However, only some of them (pili, SLPs, EPSs) are being widely investigated. Complex factors, related to both host and adhesion factors on bacteria, contribute to probiotic interactions with the host and these need to be understood individually before a systematic synthesis of this information. Possibly the age of the study population, probiotic dose, choice of the right in vitro models, the specific intestinal disease, and the individual variability of resident microbiota need to be considered in parallel to be able to interpret findings even for the same probiotic strain. Overall, observations made in vitro, in humans and animal models, on the role of probiotic adhesins, have great potential to guide probiotic function but are incomplete and can only be cautiously applied clinically in humans.
The few human clinical trials, using strains with well-characterized adherence factors, most often fail to consider the importance of adherence when a priori designing and discussing the study outcomes. We believe that a study design involving a bacterial mutant (e.g. genetic knockdown) and/or a purified/isolated adhesion factor is the best way to elucidate the role of an adhesin in probiotic function. For this purpose, more RCTs using clinically applied and probiotic candidates, are needed to assess the role of adhesins by using mutants or isolated adhesins in parallel with wild type strains. In we propose a step-by-step ideal planning of studies to systematically be conducted in order to reach a conclusion about a probiotic adhesin’s role and its clinical benefit.
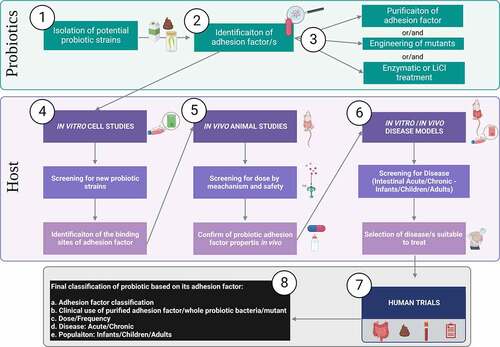
Figure 4. Ideal step-by-step workflow for studies of adhesion factors role in probiotic function. Given the mixed type of approaches found in literature the figure summarizes an ideal workflow for screening, experimental testing, and classification of probiotics considering adhesion factors. Bacteria from dairy, fermentation of fecal matter, etc, are isolated as potential probiotics (Step 1). Based on the bacteria type a hypothetical adhesion factor should be identified (Step 2). Then follows the purification of the adhesion factor (e.g. proteinic, polysaccharidic component), generation of mutants lacking, overexpressing the adhesion factor or enzymatic treatment of the bacteria (Step 3). In vitro studies, using most often IECs (e.g. Caco-2 cells) are the first experimental model used to investigate bacteria and adhesion factors for probiotic properties. Cell studies at this stage should ideally investigate the host receptors involved in the adhesion mechanism (Step 4). Once a probiotic and its adhesion mechanism have been identified, animal studies must aim to propose a dose range and mechanism of action in vivo (Step 5). Although adhesion factors are most often inert proteinic or complex carbohydrate by nature, hence considered safe, they could potentially be considered as drug-like when studying the metabolism in vivo to understand their half-life in the body. Once a preliminary hypothesis of the adhesin and healthy host is formulated, the next step should involve studies to decide which disease could benefit (Step 6). For instance, if it was hypothesized that an adhesion factor could benefit inflammatory conditions, established inflammatory models should be used to test this. Such models are for instance inflammation induced in IECs Caco-2 cell with inflammatory cytokines (e.g. IL-1β, TNF-α)Citation47 or/and animal models such as dextran sulfate sodium (DSS)-induced colitis in animals.Citation11 Next step comprises human trial conduction (Step 7). Although conduction of human trials in a stepwise manner takes longer, when possible, they should be performed in the following order: starting from pilot testing in healthy to study mainly safety, pilot trial in disease to mainly select a dose, and finally RCTs, Cross-Over or Parallel design trials with specific health outcomes. The 4 symbols represent samples used during human trials, intestinal biopsies, fecal samples, blood samples and symptoms questionnaires that can be used depending on the endpoint investigated and feasibility. Finally, considering both the probiotic and the host an adhesion factor-driven classification can be assigned to the probiotic (Step 8).
Finally, although more systematic studies are needed, there is preliminary evidence that probiotic adherence factors contribute to and guide the overall probiotic function in health and disease. The adhesion mechanisms, and consequent effects on the host, need to be considered in a strain-specific manner when selecting a probiotic for clinical trials with the overall aim of applying tailored probiotic therapies in GIT diseases.
Abbreviations
GIT Gastrointestinal tract
IECs Intestinal epithelial cells
SLP Surface layer protein
EPS Exopolysaccharide
LGG Lacticaseibacillus rhamnosus GG
IL Interleukin
NEC Necrotizing enterocolitis
RCT Randomized controlled trial
TLR Toll-like receptor
Author contributions
FG conceived the subject and wrote the review. WAW wrote the review.
Acknowledgments
We thank Dr. Ann Sattler and Dr. Stephen T.A Rush for proofreading the article. We want to acknowledge and apologize for all the studies we missed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al. 2016. The gut microbiota and host health: a new clinical frontier. Gut. 65(2):330–27. doi:10.1136/gutjnl-2015-309990.
- Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI. 2011. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 332(6032):970–974. doi:10.1126/science.1198719.
- O’Hara AM, Shanahan F. 2006. The gut flora as a forgotten organ. EMBO Rep. 7(7):688–693. doi:10.1038/sj.embor.7400731.
- Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, et al. 2012. Structure, function and diversity of the healthy human microbiome. Nature. 486:207–214.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, et al. 2011. Enterotypes of the human gut microbiome. Nature. 473(7346):174–180. doi:10.1038/nature09944.
- O’Malley MA, Skillings DJ. 2018. Methodological strategies in microbiome research and their explanatory implications. Perspect Sci. 26(2):239–265. doi:10.1162/POSC_a_00274.
- The Human Microbiome Project Consortium. 2012 Structure, function and diversity of the healthy human microbiome. Nature. 486(7402):207–214. doi:10.1038/nature11234.
- van Best N, Trepels-Kottek S, Savelkoul P, Orlikowsky T, Hornef MW, Penders J. 2020. Influence of probiotic supplementation on the developing microbiota in human preterm neonates. Gut Microbes. 12(1):1826747. doi:10.1080/19490976.2020.1826747.
- Otieno DO. 2011. Biology of Prokaryotic Probiotics. In: Liong M-T, editor. Probiotics. Berlin, Heidelberg: Springer Berlin Heidelberg; 1–28.
- Lopez-Escalera S, Wellejus A. 2022. Evaluation of Caco-2 and human intestinal epithelial cells as in vitro models of colonic and small intestinal integrity. Biochemi Biophys Rep. 31:101314. doi:10.1016/j.bbrep.2022.101314.
- Xia Y, Chen Y, Wang G, Yang Y, Song X, Xiong Z, Zhang H, Lai P, Wang S, Ai L. 2020. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J Funct Foods. 67:103854. doi:10.1016/j.jff.2020.103854.
- Reid G, Gaudier E, Guarner F, Huffnagle GB, Macklaim JM, Munoz AM, Martini M, Ringel-Kulka T, Sartor BR, Unal RR, et al. 2010. Responders and non-responders to probiotic interventions: how can we improve the odds? Gut Microbes. 1(3):200–204. doi:10.4161/gmic.1.3.12013.
- Bornholdt J, Broholm C, Chen Y, Rago A, Sloth S, Hendel J, Melsæther C, Müller CV, Juul Nielsen M, Strickertsson J, et al. 2020. Personalized B cell response to the Lactobacillus rhamnosus GG probiotic in healthy human subjects: a randomized trial. Gut Microbes. 12(1):1854639. doi:10.1080/19490976.2020.1854639.
- Jang YJ, Kim W-K, Han DH, Lee K, Ko G. 2019. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 10(6):696–711. doi:10.1080/19490976.2019.1589281.
- van Zyl WF, Deane SM, Dicks LMT. 2020. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes. 12(1):1831339. doi:10.1080/19490976.2020.1831339.
- Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, et al. 2020. A taxonomic note on the genus lactobacillus: description of 23 novel genera, emended description of the genus lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 70(4):2782–2858. doi:10.1099/ijsem.0.004107.
- Segers ME, Lebeer S. 2014. Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microb Cell Fact. 13(Suppl 1):S7. doi:10.1186/1475-2859-13-S1-S7.
- Guan C, Chen X, Jiang X, Zhao R, Yuan Y, Chen D, Zhang C, Lu M, Lu Z, Gu R. 2020. In vitro studies of adhesion properties of six lactic acid bacteria isolated from the longevous population of China. RSC Adv. 10(41):160–174. doi:10.1039/D0RA03517C.
- Jastrząb R, Graczyk D, Siedlecki P. 2021. Molecular and Cellular Mechanisms Influenced by Postbiotics. int J Mol Sci. 22(24):13475. doi:10.3390/ijms222413475.
- Garcia-Gonzalez N, Prete R, Battista N, Corsetti A. 2018. Adhesion properties of food-associated lactobacillus plantarum strains on human intestinal epithelial cells and modulation of il-8 release. Front Microbiol. 9: doi:10.3389/fmicb.2018.02392.
- Schiffrin EJ, Brassart D, Servin AL, Rochat F, Donnet-Hughes A. 1997. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am J Clin Nutr. 66(2):515S–520S. doi:10.1093/ajcn/66.2.515S.
- Bernet MF, Brassart D, Neeser JR, Servin AL. 1994. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 35(4):483–489. doi:10.1136/gut.35.4.483.
- Taverniti V, Stuknyte M, Minuzzo M, Arioli S, De Noni I, Scabiosi C, Cordova ZM, Junttila I, Hämäläinen S, Turpeinen H, et al. 2013. S-Layer Protein Mediates the Stimulatory Effect of Lactobacillus helveticus MIMLh5 on Innate Immunity. Appl Environ Microbiol. 79(4):1221–1231. doi:10.1128/AEM.03056-12.
- Martino C, Dilmore AH, Burcham ZM, Metcalf JL, Jeste D, Knight R. 2022. Microbiota succession throughout life from the cradle to the grave. Nat Rev Microbiol. 20(12):707–720. doi:10.1038/s41579-022-00768-z.
- Achi SC, Halami PM. 2019. In Vitro Comparative Analysis of Probiotic and Functional Attributes of Indigenous Isolates of Bifidobacteria. Curr Microbiol. 76(3):304–311. doi:10.1007/s00284-018-1615-9.
- Codex Alimentarius Commission. 2009 FAO, Weltgesundheitsorganisation, editors. Foods Derived Modern Biotechnol. 2. ed:Rome. Food and Agriculture Organization.
- Prilassnig M, Wenisch C, Daxboeck F, Feierl G. 2007. Are probiotics detectable in human feces after oral uptake by healthy volunteers? Wien Klin Wochenschr. 119(15–16):456–462. doi:10.1007/s00508-007-0808-1.
- Sanders ME. 2011. Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol. 45:45. doi:10.1097/MCG.0b013e3181dd1573.
- Han S, Lu Y, Xie J, Fei Y, Zheng G, Wang Z, Liu J, Lv L, Ling Z, Berglund B, et al. 2021. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: a Long Journey. Front Cell Infect Microbiol. 11:609722. doi:10.3389/fcimb.2021.609722.
- Sanders ME, Akkermans LMA, Haller D, Hammerman C, Heimbach JT, Hörmannsperger G, Huys G. 2010. Safety assessment of probiotics for human use. Gut Microbes. 1:164–185. doi:10.4161/gmic.1.3.12127.
- Javanshir N, Hosseini GNG, Sadeghi M, Esmaeili R, Satarikia F, Ahmadian G, Allahyari N. 2021. Evaluation of the Function of Probiotics, Emphasizing the Role of their Binding to the Intestinal Epithelium in the Stability and their Effects on the Immune System. Biol Proced Online. 23(1):23. doi:10.1186/s12575-021-00160-w.
- Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A. 2019. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol. 103(16):6463–6472. doi:10.1007/s00253-019-09978-7.
- Lebeer S, Vanderleyden J, De Keersmaecker SCJ. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 8(3):171–184. doi:10.1038/nrmicro2297.
- Motherway M O, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, O’Brien F, Flynn K, Casey PG, Moreno Munoz JA, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proceedings of the National Academy of Sciences 2011;108:11217–11222.
- Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx APA, Lebeer S, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proceedings of the National Academy of Sciences 2009; 106:17193–17198.
- Duranti S, Gaiani F, Mancabelli L, Milani C, Grandi A, Bolchi A, Santoni A, Lugli GA, Ferrario C, Mangifesta M, et al. 2016. Elucidating the gut microbiome of ulcerative colitis: bifidobacteria as novel microbial biomarkers. FEMS Microbiol Ecol. 92(12):fiw191. doi:10.1093/femsec/fiw191.
- Tytgat HLP, van Teijlingen NH, Sullan RMA, Douillard FP, Rasinkangas P, Messing M, Reunanen J, Satokari R, Vanderleyden J, Dufrêne YF, et al. 2016. Probiotic Gut Microbiota Isolate Interacts with Dendritic Cells via Glycosylated Heterotrimeric Pili. PLOS ONE. 11(3):e0151824. doi:10.1371/journal.pone.0151824.
- Kobatake E, Kabuki T. S-Layer Protein of Lactobacillus helveticus SBT2171 Promotes Human β-Defensin 2 Expression via TLR2–JNK Signaling. Front Microbiol. 2019;10. doi:10.3389/fmicb.2019.00010.
- Rong J, Zheng H, Liu M, Hu X, Wang T, Zhang X, Jin F, Wang L, Garin B, Breurec S. 2015. Probiotic and anti-inflammatory attributes of an isolate Lactobacillus helveticus NS8 from Mongolian fermented koumiss. BMC Microbiol. 15:15. doi:10.1186/s12866-015-0348-1.
- Xiao L, Gong C, Ding Y, Ding G, Xu X, Deng C, Ze X, Malard P, Ben X. 2019. Probiotics maintain intestinal secretory immunoglobulin A levels in healthy formula-fed infants: a randomised, double-blind, placebo-controlled study. Benef Microbes. 10(7):729–739. doi:10.3920/BM2019.0025.
- Konstantinov SR, Smidt H, de Vos WM, Bruijns SCM, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, et al. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA. 105(49):19474–19479. doi:10.1073/pnas.0810305105.
- Johnson BR, O’Flaherty S, Goh YJ, Carroll I, Barrangou R, Klaenhammer TR. 2017. The S-layer Associated Serine Protease Homolog PrtX Impacts Cell Surface-Mediated Microbe-Host Interactions of Lactobacillus acidophilus NCFM. Front Microbiol. 8:8. doi:10.3389/fmicb.2017.00008.
- Martínez MG, Prado Acosta M, Candurra NA, Ruzal SM. 2012. S-layer proteins of Lactobacillus acidophilus inhibits JUNV infection. Biochem Biophys Res Commun. 422(4):590–595. doi:10.1016/j.bbrc.2012.05.031.
- Ruas-Madiedo P, Gueimonde M, Margolles A, de Los REYES-GAVILÁN CG, Salminen S. 2006. Exopolysaccharides Produced by Probiotic Strains Modify the Adhesion of Probiotics and Enteropathogens to Human Intestinal Mucus. J Food Prot. 69(8):2011–2015. doi:10.4315/0362-028X-69.8.2011.
- Allonsius CN, Broek MFL, De Boeck I, Kiekens S, Oerlemans EFM, Kiekens F, Foubert K, Vandenheuvel D, Cos P, Delputte P, et al. 2017. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb Biotechnol. 10(6):1753–1763. doi:10.1111/1751-7915.12799.
- Lebeer S, Claes IJJ, Verhoeven TLA, Vanderleyden J, De Keersmaecker SCJ. 2011. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb Biotechnol. 4(3):368–374. doi:10.1111/j.1751-7915.2010.00199.x.
- Gorreja F, Rush ST, Kasper DL, Meng D, Walker WA. 2019. The developmentally regulated fetal enterocyte gene, ZP4 , mediates anti-inflammation by the symbiotic bacterial surface factor polysaccharide A on Bacteroides fragilis. American J Physiol-Gastrointestinal Liver Physiol. 317(4):G398–407. doi:10.1152/ajpgi.00046.2019.
- Jiang F, Meng D, Weng M, Zhu W, Wu W, Kasper D, Walker WA. 2017. The symbiotic bacterial surface factor polysaccharide A on Bacteroides fragilis inhibits IL-1β-induced inflammation in human fetal enterocytes via toll receptors 2 and 4. PLOS ONE. 12(3):e0172738. doi:10.1371/journal.pone.0172738.
- Mazmanian SK, Round JL, Kasper DL. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 453(7195):620–625. doi:10.1038/nature07008.
- Bloem K, García-Vallejo JJ, Vuist IM, Cobb BA, van Vliet SJ, van Kooyk Y, Cobb BA, van Vliet SJ, van Kooyk Y, van Vliet SJ, et al. Interaction of the Capsular Polysaccharide A from Bacteroides fragilis with DC-SIGN on Human Dendritic Cells is Necessary for Its Processing and Presentation to T Cells. Front Immunol. 2013;4. doi:10.3389/fimmu.2013.00004.
- Wan F, Wang H, Wang M, Lv J, Zhao M, Zhang H. 2022. Sustained release of Lactobacillus casei cell wall extract can induce a continuous and stable IgA deposition model. J Pathol. 257(3):262–273. doi:10.1002/path.5884.
- Lai -H-H, Chiu C-H, Kong M-S, Chang C-J, Chen -C-C. 2019. Probiotic Lactobacillus casei: effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients. 11(5):1150. doi:10.3390/nu11051150.
- Saxami G, Ypsilantis P, Sidira M, Simopoulos C, Kourkoutas Y, Galanis A. 2012. Distinct adhesion of probiotic strain Lactobacillus casei ATCC 393 to rat intestinal mucosa. Anaerobe. 18(4):417–420. doi:10.1016/j.anaerobe.2012.04.002.
- Hsueh H-Y, Yueh P-Y, Yu B, Zhao X, Liu J-R. 2010. Expression of Lactobacillus reuteri Pg4 Collagen-Binding Protein Gene in Lactobacillus casei ATCC 393 Increases Its Adhesion Ability to Caco-2 Cells. J Agric Food Chem. 58(23):12182–12191. doi:10.1021/jf1035756.
- Mowat AM, Agace WW. 2014. Regional specialization within the intestinal immune system. Nat Rev Immunol. 14(10):667–685. doi:10.1038/nri3738.
- Caggianiello G, Kleerebezem M, Spano G. 2016. Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl Microbiol Biotechnol. 100(9):3877–3886. doi:10.1007/s00253-016-7471-2.
- Sun Z, Zhang W, Bilige M, Zhang H. 2015. Complete genome sequence of the probiotic Lactobacillus fermentum F-6 isolated from raw milk. J Biotechnol. 194:110–111. doi:10.1016/j.jbiotec.2014.12.010.
- Nikolic M, López P, Strahinic I, Suárez A, Kojic M, Fernández-García M, Topisirovic L, Golic N, Ruas-Madiedo P. 2012. Characterisation of the exopolysaccharide (EPS)-producing Lactobacillus paraplantarum BGCG11 and its non-EPS producing derivative strains as potential probiotics. Int J Food Microbiol. 158(2):155–162. doi:10.1016/j.ijfoodmicro.2012.07.015.
- Flemming H-C, Neu TR, Wozniak DJ. 2007. The EPS Matrix: the “House of Biofilm Cells. J Bacteriol. 189(22):7945–7947. doi:10.1128/JB.00858-07.
- Tortora GJ, Funke BR, Case CL. 2016. Microbiology: an introduction. 12th ed., global ed. Harlow: Pearson;
- Liong M-T, editor. 2011. Probiotics: biology, genetics, and health aspects. Heidelberg Germany; New York: Springer.
- Ruas-Madiedo P, Hugenholtz J, Zoon P. 2002. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J. 12(2–3):163–171. doi:10.1016/S0958-6946(01)00160-1.
- Castro-Bravo N, Hidalgo-Cantabrana C, Rodriguez-Carvajal MA, Ruas-Madiedo P, Margolles A. 2017. Gene Replacement and Fluorescent Labeling to Study the Functional Role of Exopolysaccharides in Bifidobacterium animalis subsp. lactis. Front Microbiol. 8:1405. doi:10.3389/fmicb.2017.01405.
- Yuan L, Chu B, Chen S, Li Y, Liu N, Zhu Y, Zhou D. 2021. Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy. Microorganisms. 9(11):2363. doi:10.3390/microorganisms9112363.
- Romeo MG, Romeo DM, Trovato L, Oliveri S, Palermo F, Cota F, Betta P. 2011. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol. 31(1):63–69. doi:10.1038/jp.2010.57.
- Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C, Latino MA, Gomirato G. 2006. Oral Supplementation with Lactobacillus casei Subspecies rhamnosus Prevents Enteric Colonization by Candida Species in Preterm Neonates: a Randomized Study. Clin Infect Dis. 42(12):1735–1742. doi:10.1086/504324.
- Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, Motherway M O, Shanahan F, Nally K, Dougan G, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proceedings of the National Academy of Sciences 2012; 109:2108–2113.
- Collins JW, Keeney KM, Crepin VF, Rathinam VAK, Fitzgerald KA, Finlay BB, Frankel G. 2014. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 12(9):612–623. doi:10.1038/nrmicro3315.
- Korpela K, Salonen A, Vepsäläinen O, Suomalainen M, Kolmeder C, Varjosalo M, Miettinen S, Kukkonen K, Savilahti E, Kuitunen M, et al. 2018. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 6. cited 2020 Mar 11. Available from. https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-018-0567-4.
- Costeloe K, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, King A, Panton N, Stacey F, Whiley A, et al. 2016. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess (Rockv). 20(66):1–194. doi:10.3310/hta20660.
- Rich BS, Dolgin SE. 2017. Necrotizing Enterocolitis. Pediatr Rev. 38(12):552–559. doi:10.1542/pir.2017-0002.
- Håkansson Å, Andrén Aronsson C, Brundin C, Oscarsson E, Molin G, Agardh D. 2019. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the Peripheral Immune Response in Children with Celiac Disease Autoimmunity: a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 11(8):1925. doi:10.3390/nu11081925.
- Ahrné N, Jeppsson A, Wold M. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 85(1):88–94. doi:10.1046/j.1365-2672.1998.00480.x.
- Adlerberth I, Ahrne S, Johansson ML, Molin G, Hanson LA, Wold AE. 1996. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Environ Microbiol. 62(7):1753–1763. doi:10.1128/aem.62.7.2244-2251.1996.
- Lazou Ahrén I, Berggren A, Teixeira C, Martinsson Niskanen T, Larsson N. 2020. Evaluation of the efficacy of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 on aspects of common cold infections in children attending day care: a randomised, double-blind, placebo-controlled clinical study. Eur J Nutr. 59(1):409–417. doi:10.1007/s00394-019-02137-8.
- Glenting J, Beck HC, Vrang A, Riemann H, Ravn P, Hansen AM, Antonsson M, Ahrné S, Israelsen H, Madsen S. 2013. Anchorless surface associated glycolytic enzymes from Lactobacillus plantarum 299v bind to epithelial cells and extracellular matrix proteins. Microbiol Res. 168(5):245–253. doi:10.1016/j.micres.2013.01.003.
- Kusumo PD, Bela B, Wibowo H, Munasir Z, Surono IS. 2019. Lactobacillus plantarum IS-10506 supplementation increases faecal sIgA and immune response in children younger than two years. Benef Microbes. 10(3):245–252. doi:10.3920/BM2017.0178.
- Surono IS, Martono PD, Kameo S, Suradji EW, Koyama H. 2014. Effect of probiotic L. plantarum IS-10506 and zinc supplementation on humoral immune response and zinc status of Indonesian pre-school children. J Trace Elem Med Biol. 28(4):465–469. doi:10.1016/j.jtemb.2014.07.009.
- Sudha MR, Jayanthi N, Aasin M, Dhanashri RD, Anirudh T. 2018. Efficacy of Bacillus coagulans Unique IS2 in treatment of irritable bowel syndrome in children: a double blind, randomised placebo controlled study. Benef Microbes. 9(4):563–572. doi:10.3920/BM2017.0129.
- Madempudi RS, Ahire JJ, Neelamraju J, Tripathi A, Nanal S. Randomized clinical trial: the effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci Rep. 2019; 9.[cited 2021 Mar 1] Available from http://www.nature.com/articles/s41598-019-48554-x
- Konuray Altun G, Erginkaya Z. 2021. Identification and characterization of Bacillus coagulans strains for probiotic activity and safety. LWT. 151:112233. doi:10.1016/j.lwt.2021.112233.
- Yahav S, Berkovich Z, Ostrov I, Reifen R, Shemesh M. 2018. Encapsulation of beneficial probiotic bacteria in extracellular matrix from biofilm-forming Bacillus subtilis. Artif Cells Nanomed Biotechnol. 46(sup2):974–982. doi:10.1080/21691401.2018.1476373.
- Arnaouteli S, Bamford NC, Stanley-Wall NR, Át K. 2021. Bacillus subtilis biofilm formation and social interactions. Nat Rev Microbiol. 19(9):600–614. doi:10.1038/s41579-021-00540-9.
- Maheshwari A, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, McDonald SA, Thorsen P, Skogstrand K, Hougaard DM, et al. 2014. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 76(1):100–108. doi:10.1038/pr.2014.48.
- Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 414(6863):555–558. doi:10.1038/35107092.
- Mazmanian SK, Kasper DL. 2006. The love–hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 6(11):849–858. doi:10.1038/nri1956.
- Nishiyama K, Ueno S, Sugiyama M, Yamamoto Y, Mukai T. 2016. Lactobacillus rhamnosus GG SpaC pilin subunit binds to the carbohydrate moieties of intestinal glycoconjugates. Animal Sci J. 87(6):809–815. doi:10.1111/asj.12491.
- Mishra AK, Megta AK, Palva A, von Ossowski I, Krishnan V. 2017. Crystallization and X-ray diffraction analysis of SpaE, a basal pilus protein from the gut-adapted Lactobacillus rhamnosus GG. Acta Crystallogr Sect F Struct Biol Commun. 73(6):321–327. doi:10.1107/S2053230X17006963.
- Ardita CS, Mercante JW, Kwon YM, Luo L, Crawford ME, Powell DN, Jones RM, Neish AS. 2014. Epithelial Adhesion Mediated by Pilin SpaC Is Required for Lactobacillus rhamnosus GG-Induced Cellular Responses. Appl Environ Microbiol. 80(16):5068–5077. doi:10.1128/AEM.01039-14.
- Tytgat HLP, Douillard FP, Reunanen J, Rasinkangas P, Hendrickx APA, Laine PK, Paulin L, Satokari R, de Vos WM. 2016. Lactobacillus rhamnosus GG Outcompetes Enterococcus faecium via Mucus-Binding Pili: evidence for a Novel and Heterospecific Probiotic Mechanism. Appl Environ Microbiol. 82(19):5756–5762. doi:10.1128/AEM.01243-16.
- Sullan RMA, Beaussart A, Tripathi P, Derclaye S, El-Kirat-Chatel S, Li JK, Schneider Y-J, Vanderleyden J, Lebeer S, Dufrêne YF. 2014. Single-cell force spectroscopy of pili-mediated adhesion. Nanoscale. 6(2):1134–1143. doi:10.1039/C3NR05462D.
- Douillard FP, Rasinkangas P, Bhattacharjee A, Palva A, de Vos WM. 2016. The N-Terminal GYPSY Motif Is Required for Pilin-Specific Sortase SrtC1 Functionality in Lactobacillus rhamnosus Strain GG. PLOS ONE. 11(4):e0153373. doi:10.1371/journal.pone.0153373.
- Turroni F, Serafini F, Foroni E, Duranti S, Motherway M O, Taverniti V, Mangifesta M, Milani C, Viappiani A, Roversi T, et al. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium-host interactions. Proceedings of the National Academy of Sciences. 2013;110:11151–11156.
- Oxaran V, Ledue-Clier F, Dieye Y, Herry J-M, Péchoux C, Meylheuc T, Briandet R, Juillard V, Piard J-C. 2012. Pilus Biogenesis in Lactococcus lactis: molecular Characterization and Role in Aggregation and Biofilm Formation. PLoS ONE. 7(12):e50989. doi:10.1371/journal.pone.0050989.
- Meyrand M, Guillot A, Goin M, Furlan S, Armalyte J, Kulakauskas S, Cortes-Perez NG, Thomas G, Chat S, Péchoux C, et al. 2013. Surface Proteome Analysis of a Natural Isolate of Lactococcus lactis Reveals the Presence of Pili Able to Bind Human Intestinal Epithelial Cells. Mol Cell Proteomics. 12(12):3935–3947. doi:10.1074/mcp.M113.029066.
- Yu X, Jaatinen A, Rintahaka J, Hynönen U, Lyytinen O, Kant R, Åvall-Jääskeläinen S, von Ossowski I, Palva A. 2015. Human gut-commensalic lactobacillus ruminis ATCC 25644 displays sortase-assembled surface piliation Phenotypic Characterization of Its Fimbrial Operon through in Silico Predictive Analysis and Recombinant Expression in Lactococcus Lactis. PLOS ONE. 10:e0145718.
- Aleksandrzak-Piekarczyk T, Koryszewska-Bagińska A, Grynberg M, Nowak A, Cukrowska B, Kozakova H, Bardowski J. 2016. Genomic and functional characterization of the unusual pLOCK 0919 plasmid harboring the spaCBA pili cluster in lactobacillus casei LOCK 0919. Genome Biol Evol. 8(1):202–217. doi:10.1093/gbe/evv247.
- Krishnan V, Chaurasia P, Kant A. Pili in Probiotic Bacteria [Internet]. In: Rao V, Rao LG, editors. Probiotics and Prebiotics in Human Nutrition and Health. InTech; 2016 [cited 2017 Aug 24]. Available from: http://www.intechopen.com/books/probiotics-and-prebiotics-in-human-nutrition-and-health/pili-in-probiotic-bacteria
- von Ossowski I, Reunanen J, Satokari R, Vesterlund S, Kankainen M, Huhtinen H, Tynkkynen S, Salminen S, de Vos WM, Palva A. 2010. Mucosal adhesion properties of the probiotic lactobacillus rhamnosus GG SpaCBA and SpaFED Pilin Subunits. Appl Environ Microbiol. 76(7):2049–2057. doi:10.1128/AEM.01958-09.
- Hendrickx APA, Budzik JM, S-Y O, Schneewind O. 2011. Architects at the bacterial surface — sortases and the assembly of pili with isopeptide bonds. Nat Rev Microbiol. 9(3):166–176. doi:10.1038/nrmicro2520.
- von Ossowski I. 2017. Novel molecular insights about lactobacillar sortase-dependent piliation. Int J Mol Sci. 18(7):1551. doi:10.3390/ijms18071551.
- Bang M, Yong -C-C, Ko H-J, Choi I-G, Oh S. 2018. Transcriptional response and enhanced intestinal adhesion ability of lactobacillus rhamnosus GG after acid stress. J Microbiol Biotechnol. 28(10):1604–1613. doi:10.4014/jmb.1807.07033.
- Solano-Aguilar G, Molokin A, Botelho C, Fiorino A-M, Vinyard B, Li R, Chen C, Urban J, Dawson H, Andreyeva I, et al. 2016. Transcriptomic profile of whole blood cells from elderly subjects fed probiotic bacteria lactobacillus rhamnosus GG ATCC 53103 (LGG) in a phase i open label study. PLOS ONE. 11(2):e0147426. doi:10.1371/journal.pone.0147426.
- Coombes JL, Powrie F. 2008. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 8(6):435–446. doi:10.1038/nri2335.
- Baradaran Ghavami S, Asadzadeh Aghdaei H, Sorrentino D, Shahrokh S, Farmani M, Ashrafian F, Dore MP, Keshavarz Azizi Raftar S, Mobin Khoramjoo S, Zali MR. 2021. Probiotic-induced tolerogenic dendritic cells: a novel therapy for inflammatory bowel disease? IJMS. 22(15):8274. doi:10.3390/ijms22158274.
- Han X, Lee A, Huang S, Gao J, Spence JR, Owyang C. 2019. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes. 10(1):59–76. doi:10.1080/19490976.2018.1479625.
- Orlando A, Linsalata M, Notarnicola M, Tutino V, Russo F. 2014. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: the role of cellular polyamines. BMC Microbiol. 14(1):19. doi:10.1186/1471-2180-14-19.
- Lebeer S, Claes I, Tytgat HLP, Verhoeven TLA, Marien E, von Ossowski I, Reunanen J, Palva A, de Vos WM, De Keersmaecker SCJ, et al. 2012. Functional analysis of lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol. 78(1):185–193. doi:10.1128/AEM.06192-11.
- Costabile A, Bergillos-Meca T, Rasinkangas P, Korpela K, de Vos WM, Gibson GR. 2017. Effects of soluble corn fiber alone or in synbiotic combination with lactobacillus rhamnosus GG and the pilus-deficient derivative GG-PB12 on fecal microbiota, metabolism, and markers of immune function: a randomized, double-blind, placebo-controlled, crossover study in healthy elderly (saimes study). Front Immunol. 8(l). doi:10.3389/fimmu.2017.01443.
- Domínguez Rubio AP, Martínez JH, Martínez Casillas DC, Coluccio Leskow F, Piuri M, Pérez OE. Lactobacillus casei BL23 produces microvesicles carrying proteins that have been associated with its probiotic effect. Front Microbiol. 2017;8. doi:10.3389/fmicb.2017.01783.
- Garrido D, Kim JH, German JB, Raybould HE, Mills DA. 2011. Oligosaccharide binding proteins from bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS ONE. 6(3):e17315. doi:10.1371/journal.pone.0017315.
- Plummer EL, Bulach DM, Murray GL, Jacobs SE, Tabrizi SN, Garland SM for the ProPrems Study Group. 2018. Gut microbiota of preterm infants supplemented with probiotics: sub-study of the ProPrems trial. BMC Microbiol. 18:18. 10.1186/s12866-018-1161-4.
- Hynönen U, Palva A. 2013. Lactobacillus surface layer proteins: structure, function and applications. Appl Microbiol Biotechnol. 97(12):5225–5243. doi:10.1007/s00253-013-4962-2.
- Meng J, Zhu X, Gao S-M, Zhang Q-X, Sun Z, Lu -R-R. 2014. Characterization of surface layer proteins and its role in probiotic properties of three Lactobacillus strains. Int J Biol Macromol. 65:110–114. doi:10.1016/j.ijbiomac.2014.01.024.
- Klotz C, Goh YJ, O’Flaherty S, Barrangou R. 2020. S-layer associated proteins contribute to the adhesive and immunomodulatory properties of Lactobacillus acidophilus NCFM. BMC Microbiol. 20(1):248. doi:10.1186/s12866-020-01908-2.
- Guo Y, Li X, Yang Y, Wu Z, Zeng X, Nadari F, Pan D. 2018. Molecular cloning, expression and adhesion analysis of silent slpB of Lactobacillus acidophilus NCFM. AMB Expr. 8(1):103. doi:10.1186/s13568-018-0631-2.
- Foligne B, Guo J, Chan EWC, Chen S, Zeng Z. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 13(2):236. doi:10.3748/wjg.v13.i2.236.
- Suzuki S, Yokota K, Igimi S, Kajikawa A. 2019. Comparative analysis of immunological properties of S-layer proteins isolated from Lactobacillus strains. Microbiology. 165(2):188–196. doi:10.1099/mic.0.000766.
- Wang R, Jiang L, Zhang M, Zhao L, Hao Y, Guo H, Sang Y, Zhang H, Ren F. 2017. The adhesion of lactobacillus salivarius REN to a human intestinal epithelial cell line requires s-layer proteins. Sci Rep. 7(1):44029. doi:10.1038/srep44029.
- Salzillo M, Vastano V, Capri U, Muscariello L, Sacco M, Marasco R. 2015. Identification and characterization of enolase as a collagen-binding protein in Lactobacillus plantarum. J Basic Microbiol. 55(7):890–897. doi:10.1002/jobm.201400942.
- Kumar R, Grover S, Batish VK. 2011. Molecular identification and typing of putative probiotic indigenous lactobacillus plantarum strain Lp91 of human origin by specific primed-PCR assays. Probiotics Antimicro Prot. 3(3–4):186–193. doi:10.1007/s12602-011-9083-6.
- Wei C, Luo K, Wang M, Li Y, Pan M, Xie Y, Qin G, Liu Y, Li L, Liu Q, et al. 2022. Evaluation of potential probiotic properties of a strain of lactobacillus plantarum for shrimp farming: from beneficial functions to safety assessment. Front Microbiol. 13:854131. doi:10.3389/fmicb.2022.854131.
- Yadav AK, Tyagi A, Kaushik JK, Saklani AC, Grover S, Batish VK. 2013. Role of surface layer collagen binding protein from indigenous Lactobacillus plantarum 91 in adhesion and its anti-adhesion potential against gut pathogen. Microbiol Res. 168(10):639–645. doi:10.1016/j.micres.2013.05.003.
- Chandran A, Duary RK, Grover S, Batish VK. 2013. Relative expression of bacterial and host specific genes associated with probiotic survival and viability in the mice gut fed with Lactobacillus plantarum Lp91. Microbiol Res. 168(9):555–562. doi:10.1016/j.micres.2013.04.010.
- Devi SM, Halami PM. 2017. Diversity and evolutionary aspects of mucin binding (MucBP) domain repeats among Lactobacillus plantarum group strains through comparative genetic analysis. Syst Appl Microbiol. 40(4):237–244. doi:10.1016/j.syapm.2017.03.005.
- MacKenzie DA, Tailford LE, Hemmings AM, Juge N. 2009. Crystal structure of a mucus-binding protein repeat reveals an unexpected functional immunoglobulin binding activity. J Biol Chem. 284(47):32444–32453. doi:10.1074/jbc.M109.040907.
- Roos S, Jonsson H. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components The GenBank accession number for the sequence reported in this paper is AF120104. Microbiology. 148(2):433–442. doi:10.1099/00221287-148-2-433.
- Jensen H, Roos S, Jonsson H, Rud I, Grimmer S, van Pijkeren J-P, Britton RA, Axelsson L. 2014. Role of Lactobacillus reuteri cell and mucus-binding protein A (CmbA) in adhesion to intestinal epithelial cells and mucus in vitro. Microbiology. 160(4):671–681. doi:10.1099/mic.0.073551-0.
- Etzold S, Kober OI, MacKenzie DA, Tailford LE, Gunning AP, Walshaw J, Hemmings AM, Juge N. 2014. Structural basis for adaptation of Lactobacilli to gastrointestinal mucus. Environ Microbiol. 16(3):888–903. doi:10.1111/1462-2920.12377.
- Bene KP, Kavanaugh DW, Leclaire C, Gunning AP, MacKenzie DA, Wittmann A, Young ID, Kawasaki N, Rajnavolgyi E, Juge N. 2017. Lactobacillus reuteri surface mucus adhesins upregulate inflammatory responses through interactions with innate C-type lectin receptors. Front Microbiol. 8:8.
- Walsham ADS, MacKenzie DA, Cook V, Wemyss-Holden S, Hews CL, Juge N, Schüller S. 2016. Lactobacillus reuteri inhibition of enteropathogenic escherichia coli adherence to human intestinal epithelium. Front Microbiol. 7: doi:10.3389/fmicb.2016.00244.
- Miyoshi Y, Okada S, Uchimura T, Satoh E. 2006. A mucus adhesion promoting protein, mapa, mediates the adhesion of lactobacillus reuteri to Caco-2 human intestinal epithelial cells. Biosci Biotechnol Biochem. 70(7):1622–1628. doi:10.1271/bbb.50688.
- Bøhle LA, Brede DA, Diep DB, Holo H, Nes IF. 2010. Specific degradation of the mucus adhesion-promoting protein (mapA) of lactobacillus reuteri to an antimicrobial peptide. Appl Environ Microbiol. 76(21):7306–7309. doi:10.1128/AEM.01423-10.
- Sung V, D’Amico F, Cabana MD, Chau K, Koren G, Savino F, Szajewska H, Deshpande G, Dupont C, Indrio F, et al. 2018. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 141(1):e20171811. doi:10.1542/peds.2017-1811.
- Maragkoudaki M, Chouliaras G, Moutafi A, Thomas A, Orfanakou A, Papadopoulou A. 2018. Efficacy of an oral rehydration solution enriched with lactobacillus reuteri DSM 17938 and zinc in the management of acute diarrhoea in infants: a randomized, double-blind, placebo-controlled trial. Nutrients. 10(9):1189. doi:10.3390/nu10091189.
- Wegner A, Banaszkiewicz A, Kierkus J, Landowski P, Korlatowicz-Bilar A, Wiecek S, Kwiecien J, Gawronska A, Dembinski L, Czaja-Bulsa G, et al. 2018. The effectiveness of lactobacillus reuteri DSM 17938 as an adjunct to macrogol in the treatment of functional constipation in children. A randomized, double-blind, placebo-controlled, multicentre trial. Clin Res Hepatol Gastroenterol. 42(5):494–500. doi:10.1016/j.clinre.2018.03.008.
- Jadrešin O, Sila S, Trivić I, Mišak Z, Hojsak I, Kolaček S. 2018. Lack of benefit of lactobacillus reuteri DSM 17938 as an addition to the treatment of functional constipation. J Pediatr Gastroenterol Nutr. 67(6):763–766. doi:10.1097/MPG.0000000000002134.
- García Contreras A, Vásquez Garibay E, Sánchez Ramírez C, Fafutis Morris M, Delgado Rizo V. 2020. Lactobacillus reuteri DSM 17938 and agave inulin in children with cerebral palsy and chronic constipation: a double-blind randomized placebo controlled clinical trial. Nutrients. 12(10):2971. doi:10.3390/nu12102971.
- Singh TP, Tehri N, Kaur G, Malik RK. 2021. Cell surface and extracellular proteins of potentially probiotic Lactobacillus reuteri as an effective mediator to regulate intestinal epithelial barrier function. Arch Microbiol. 203(6):3219–3228. [[cited 2021 May 22]; Available from]. http://link.springer.com/10.1007/s00203-021-02318-2.
- Singh KS, Choudhary R, Bisht S, Grover S, Kumar S, Mohanty AK, Kaushik JK. 2017. Expression of recombinant truncated domains of mucus-binding (Mub) protein of Lactobacillus plantarum in soluble and biologically active form. Protein Expr Purif. 135:54–60. doi:10.1016/j.pep.2017.04.015.
- Singh KS, Kumar S, Mohanty AK, Grover S, Kaushik JK. 2018. Mechanistic insights into the host-microbe interaction and pathogen exclusion mediated by the Mucus-binding protein of Lactobacillus plantarum. Sci Rep. 8(1):14198. doi:10.1038/s41598-018-32417-y.
- Ramiah K, van Reenen CA, Dicks LMT. 2007. Expression of the mucus adhesion genes Mub and MapA, adhesion-like factor EF-Tu and bacteriocin gene plaA of lactobacillus plantarum 423, monitored with real-time PCR. Int J Food Microbiol. 116(3):405–409. doi:10.1016/j.ijfoodmicro.2007.02.011.
- Bettelheim KA, Goldwater PN, Bancu I, Lauzurica-Valdemoros R, Borràs FE. 2015. Escherichia coli and sudden infant death syndrome. Front Immunol. 6:6. doi:10.3389/fimmu.2015.00006.
- Khatri I, Sharma G, Subramanian S, Liu X, Dai F. 2019. Composite genome sequence of bacillus clausii, a probiotic commercially available as enterogermina®, and insights into its probiotic properties. BMC Microbiol. 19(1):19. doi:10.1186/s12866-018-1385-3.
- Sudha MR, Jayanthi N, Pandey DC, Verma AK. 2019. Bacillus clausii UBBC-07 reduces severity of diarrhoea in children under 5 years of age: a double blind placebo controlled study. Benef Microbes. 10(2):149–154. doi:10.3920/BM2018.0094.
- Lehri B, Seddon AM, Karlyshev AV. 2017. Potential probiotic-associated traits revealed from completed high quality genome sequence of Lactobacillus fermentum 3872. Stand in Genomic Sci. 12(1):19. doi:10.1186/s40793-017-0228-4.
- Gorreja F. 2019. Gene expression changes as predictors of the immune-modulatory effects of probiotics: towards a better understanding of strain-disease specific interactions. NFS J. 14–15:1–5. doi:10.1016/j.nfs.2019.02.001.
- Horvath A, Leber B, Schmerboeck B, Tawdrous M, Zettel G, Hartl A, Madl T, Stryeck S, Fuchs D, Lemesch S, et al. 2016. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther. 44(9):926–935. doi:10.1111/apt.13788.
- Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N. 2013. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. American J Physiol-Gastrointestinal Liver Physiol. 304(2):G132–41. doi:10.1152/ajpgi.00142.2012.
- Hecht AL, Casterline BW, Earley ZM, Goo YA, Goodlett DR, Bubeck Wardenburg J. 2016. Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep. 17(9):1281–1291. doi:10.15252/embr.201642282.
- Casterline BW, Hecht AL, Choi VM, Bubeck Wardenburg J. 2017. The Bacteroides fragilis pathogenicity island links virulence and strain competition. Gut Microbes. 8(4):374–383. doi:10.1080/19490976.2017.1290758.
- He Y, Xu X, Zhang F, Xu D, Liu Z, Tao X, Wei H. 2019. Anti-adhesion of probiotic Enterococcus faecium WEFA23 against five pathogens and the beneficial effect of its S-layer proteins against Listeria monocytogenes. Can J Microbiol. 65(3):175–184. doi:10.1139/cjm-2018-0031.
- Koo OK, Amalaradjou MAR, Bhunia AK, Ho PL. 2012. Recombinant Probiotic Expressing Listeria Adhesion Protein Attenuates Listeria monocytogenes Virulence In Vitro. PLoS ONE. 7(1):e29277. doi:10.1371/journal.pone.0029277.
- Archer AC, Kurrey NK, Halami PM. 2018. In vitro adhesion and anti-inflammatory properties of native Lactobacillus fermentum and Lactobacillus delbrueckii spp. J Appl Microbiol. 125(1):243–256. doi:10.1111/jam.13757.
- Konieczna C, Słodziński M, Schmidt MT. 2018. Exopolysaccharides produced by lactobacillus rhamnosus KL 53A and lactobacillus casei fyos affect their adhesion to enterocytes. Polish J Microbiol. 67(3):273–281. doi:10.21307/pjm-2018-032.
- Nishiyama K, Yamamoto Y, Sugiyama M, Takaki T, Urashima T, Fukiya S, Yokota A, Okada N, Mukai T. Bifidobacterium bifidum extracellular sialidase enhances adhesion to the mucosal surface and supports carbohydrate assimilation. mBio. 2017;8(5):e00928-17. doi:10.1128/mBio.00928-17.
- Lortal S, Van Heijenoort J, Gruber K, Sleytr UB. 1992. S-layer of Lactobacillus helveticus ATCC 12046: isolation, chemical characterization and re-formation after extraction with lithium chloride. J Gen Microbiol. 138(3):611–618. doi:10.1099/00221287-138-3-611.
- Andrés J D, Manzano S, García C, Rodríguez JM, Espinosa-Martos I, Jiménez E. 2018. Modulatory effect of three probiotic strains on infants’ gut microbial composition and immunological parameters on a placebo-controlled, double-blind, randomised study. Benef Microbes. 9(4):573–584. doi:10.3920/BM2017.0132.
- Dias ML, O’Connor KM, Dempsey EM, O’Halloran KD, McDonald FB. 2021. Targeting the Toll-like receptor pathway as a therapeutic strategy for neonatal infection. American J Physiol-Regul Integr Comp Physiol. 321(6):R879–902. doi:10.1152/ajpregu.00307.2020.
- Freedman SB, Williamson-Urquhart S, Farion KJ, Gouin S, Willan AR, Poonai N, Hurley K, Sherman PM, Finkelstein Y, Lee BE, et al. 2018. Multicenter Trial of a Combination Probiotic for Children with Gastroenteritis. New England J Med. 379(21):2015–2026. doi:10.1056/NEJMoa1802597.
- Lu Y, Han S, Zhang S, Wang K, Lv L, McClements DJ, Xiao H, Berglund B, Yao M, Li L. 2022. The role of probiotic exopolysaccharides in adhesion to mucin in different gastrointestinal conditions. Current Res Food Sci. 5:581–589. doi:10.1016/j.crfs.2022.02.015.
- Kumar S, Bansal A, Chakrabarti A, Singhi S. 2013. Evaluation of efficacy of probiotics in prevention of candida colonization in a PICU—A randomized controlled trial*. Crit Care Med. 41(2):565–572. doi:10.1097/CCM.0b013e31826a409c.
- Bunesova V, Lacroix C, Schwab C. 2016. Fucosyllactose and L-fucose utilization of infant bifidobacterium longum and bifidobacterium kashiwanohense. BMC Microbiol. 16(1). doi:10.1186/s12866-016-0867-4.
- Turroni F, Duranti S, Bottacini F, Guglielmetti S, Van Sinderen D, Ventura M. Bifidobacterium bifidum as an example of a specialized human gut commensal. Front Microbiol. 2014;5. doi:10.3389/fmicb.2014.00437.
- Turroni F, Bottacini F, Foroni E, Mulder I, Kim J-H, Zomer A, Sanchez B, Bidossi A, Ferrarini A, Giubellini V, et al. Genome analysis of bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proceedings of the National Academy of Sciences. 2010;107:19514–19519.
- Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. 2012. Probiotic mechanisms of action. Ann Nutr Metab. 61(2):160–174. doi:10.1159/000342079.
- Alizadeh Behbahani B, Noshad M, Falah F. 2019. Inhibition of Escherichia coli adhesion to human intestinal Caco-2 cells by probiotic candidate Lactobacillus plantarum strain L15. Microb Pathog. 136:103677. doi:10.1016/j.micpath.2019.103677.
- Mohanty D, Panda S, Kumar S, Ray P. 2019. In vitro evaluation of adherence and anti-infective property of probiotic Lactobacillus plantarum DM 69 against Salmonella enterica. Microb Pathog. 126:212–217. doi:10.1016/j.micpath.2018.11.014.
- Gerasimov S, Gantzel J, Dementieva N, Schevchenko O, Tsitsura O, Guta N, Bobyk V, Kaprus V. 2018. Role of lactobacillus rhamnosus (floraActiveTM) 19070-2 and lactobacillus reuteri (floraActiveTM) 12246 in infant colic: a randomized dietary study. Nutrients. 10(12):1975. doi:10.3390/nu10121975.
- Rhoads JM, Fatheree NY, Norori J, Liu Y, Lucke JF, Tyson JE, Ferris MJ. 2009. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr. 155(6):823–828.e1. doi:10.1016/j.jpeds.2009.05.012.
- Oh B, Kim B-S, Kim JW, Kim JS, Koh S-J, Kim BG, Lee KL, Chun J. 2016. The effect of probiotics on gut microbiota during the helicobacter pylori eradication: randomized controlled trial. Helicobacter. 21(3):165–174. doi:10.1111/hel.12270.
- Park M, Kwon B, Ku S, Ji G. 2017. The efficacy of bifidobacterium longum BORI and lactobacillus acidophilus AD031 probiotic treatment in infants with rotavirus infection. Nutrients. 9(8):887. doi:10.3390/nu9080887.
- Kanic Z, Micetic Turk D, Burja S, Kanic V, Dinevski D. 2015. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien Klin Wochenschr. 127(S5):210–215. doi:10.1007/s00508-015-0845-0.
- Awad H, Mokhtar G, Imam SS, Gad GI, Hafez H, Aboushady N. 2010. Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pakistan J Biol Sci. 13(6):253–262. doi:10.3923/pjbs.2010.253.262.
- Reyman M, van Houten MA, Watson RL, Chu MLJN, Arp K, de Waal WJ, Schiering I, Plötz FB, Willems RJL, van Schaik W, et al. 2022. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: a randomized trial. Nat Commun. 13(1):893. doi:10.1038/s41467-022-28525-z.
- Weström B, Arévalo Sureda E, Pierzynowska K, Pierzynowski SG, Pérez-Cano F-J. 2020. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front Immunol. 11:1153. doi:10.3389/fimmu.2020.01153.
- Wall R, Fitzgerald G, Hussey S, Ryan T, Murphy B, Ross P, Stanton C. 2007. Genomic diversity of cultivable Lactobacillus populations residing in the neonatal and adult gastrointestinal tract: cultivable lactobacillus populations in the GI tract. FEMS Microbiol Ecol. 59(1):127–137. doi:10.1111/j.1574-6941.2006.00202.x.
- Radjabzadeh D, Boer CG, Beth SA, van der Wal P, Kiefte-De Jong JC, Jansen MAE, Konstantinov SR, Peppelenbosch MP, Hays JP, Jaddoe VWV, et al. 2020. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci Rep. 10(1):1040. doi:10.1038/s41598-020-57734-z.
- Pachenari A, Suganthy M, Burczynska B, Dang V, Choudhury M, Pachenari A. 2016. A comparative study of bifidobacteria in human babies and adults. Biosci Microbiota Food Health. 35(2):97–103. doi:10.12938/bmfh.2015-006.
- Horne RG, Freedman SB, Johnson-Henry KC, Pang X-L, Lee BE, Farion KJ, Gouin S, Schuh S, Poonai N, Hurley KF, et al. 2022. Intestinal microbial composition of children in a randomized controlled trial of probiotics to treat acute gastroenteritis. Front Cell Infect Microbiol. 12:883163. doi:10.3389/fcimb.2022.883163.
- Öhman L, Lasson A, Strömbeck A, Isaksson S, Hesselmar M, Simrén M, Strid H, Magnusson MK. 2021. Fecal microbiota dynamics during disease activity and remission in newly diagnosed and established ulcerative colitis. Sci Rep. 11(1):8641. doi:10.1038/s41598-021-87973-7.
- Samara J, Moossavi S, Alshaikh B, Ortega VA, Pettersen VK, Ferdous T, Hoops SL, Soraisham A, Vayalumkal J, Dersch-Mills D, et al. 2022. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe. 30(5):696–711.e5. doi:10.1016/j.chom.2022.04.005.
- Wall R, Ross RP, Ryan CA, Hussey S, Murphy B, Fitzgerald GF, Stanton C. 2009. Role of gut microbiota in early infant development. Clinical Medicine. Pediatrics. 3:2008. doi:10.4137/CMPed.S2008.
- Szajewska H, Kołodziej M. 2015. Systematic review with meta-analysis: lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 42(10):1149–1157. doi:10.1111/apt.13404.
- Simrén M, Öhman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H. 2009. Clinical trial: the effect of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome (IBS) - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 31:2–218–227.
- Francavilla R, Miniello V, Magista AM, De Canio A, Bucci N, Gagliardi F, Lionetti E, Castellaneta S, Polimeno L, Peccarisi L, et al. 2010. A randomized controlled trial of lactobacillus gg in children with functional abdominal pain. PEDIATRICS. 126(6):e1445–52. doi:10.1542/peds.2010-0467.
- Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, Jiang Y, Zhang H, Yang Z, Wang Y, et al. 2011. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery - a double-blind study: randomised clinical trial: perioperative probiotics on colon cancer. Aliment Pharmacol Ther. 33:50–63.
- Man AL, Bertelli E, Rentini S, Regoli M, Briars G, Marini M, Watson AJM, Nicoletti C. 2015. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci. 129(7):515–527. doi:10.1042/CS20150046.
- Harata G, Yoda K, Wang R, Miyazawa K, Sato M, He F, Endo A. 2021. Species- and Age/Generation-Dependent Adherence of Bifidobacterium bifidum to Human Intestinal Mucus In Vitro. Microorganisms. 9(3):542. doi:10.3390/microorganisms9030542.
- Zhang L, Zeng X, Guo D, Zou Y, Gan H, Huang X. 2022. Early use of probiotics might prevent antibiotic-associated diarrhea in elderly (>65 years): a systematic review and meta-analysis. BMC Geriatr. 22(1):562. doi:10.1186/s12877-022-03257-3.
- Korpela K, Salonen A, Virta LJ, Kumpu M, Kekkonen RA, de Vos WM, Mistry N. 2016. Lactobacillus rhamnosus GG intake modifies preschool children’s intestinal microbiota, alleviates penicillin-associated changes, and reduces antibiotic use. PLoS ONE. 11(4):e0154012. doi:10.1371/journal.pone.0154012.
- Jeong -J-J, Woo J-Y, Ahn Y-T, Shim J-H, Huh C-S, S-H I, Han MJ, Kim D-H. 2015. The probiotic mixture IRT5 ameliorates age-dependent colitis in rats. Int Immunopharmacol. 26(2):416–422. doi:10.1016/j.intimp.2015.04.021.
- Kaushal D, Kansal VK. 2011. Age-related decline in macrophage and lymphocyte functions in mice and its alleviation by treatment with probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum. J Dairy Res. 78(4):404–411. doi:10.1017/S0022029911000537.
- Neurath MF. 2017. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 14(5):269–278. doi:10.1038/nrgastro.2016.208.
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. 2013. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 14:877–882.
- Langerholc T, Maragkoudakis PA, Wollgast J, Gradisnik L, Cencic A. 2011. Novel and established intestinal cell line models – an indispensable tool in food science and nutrition. Trends Food Sci Technol. 22:S11–20. doi:10.1016/j.tifs.2011.03.010.
- Stuivenberg GA, Burton JP, Bron PA, Reid G. 2022. Why are bifidobacteria important for infants? Microorganisms. 10(2):278. doi:10.3390/microorganisms10020278.
