ABSTRACT
The gut microbiota is believed to be a critical factor in the pathogenesis of IBS, and its metabolic byproducts, such as short-chain fatty acids (SCFAs), are known to influence gut function and host health. Despite this, the precise role of SCFAs in IBS remains a topic of debate. In this study, we examined the bacterial community structure by 16S rRNA gene profiling and SCFA levels by UPLC-MS/MS in fecal samples from healthy controls (HC; n = 100) and non-constipated patients (IBS-D and IBS-M; NC-IBS; n = 240) enrolled in 19 hospitals in Italy. Our findings suggest a significant difference between the fecal microbiomes of NC-IBS patients and HC subjects, with HC exhibiting higher intra-sample biodiversity. Furthermore, we were able to classify non-constipated patients into two distinct subgroups based on their fecal SCFA levels (fecal catabotype “high” and “low”), each characterized by unique taxonomic bacterial signatures. Our results suggest that the fecal catabotype with higher SCFA levels may represent a distinct clinical phenotype of IBS that could have implications for its diagnosis and treatment. This study provides a new perspective on the intricate relationship between the gut microbiome and bowel symptoms in IBS, underscoring the importance of personalized strategies for its management.
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder that affects a large proportion of the population worldwide, with an estimated prevalence of 7–21%, depending on the geographical location and diagnostic criteria usedCitation1. IBS is characterized by recurrent abdominal pain associated with altered bowel habits, including diarrhea (IBS-D), constipation (IBS-C), or a mixture of both (IBS-M), which can significantly impair the quality of life of affected individuals. Despite its high prevalence and impact, the pathophysiology of IBS still needs to be better understood, and the available treatments result effective only for a limited portion of patientsCitation2,Citation3.
A growing body of evidence suggests that alterations in the gut microbiota and its metabolic products may play a crucial role in the pathogenesis of IBSCitation4. The gut microbiota is a complex community of microorganisms (prevalently bacteria) that colonize the human gastrointestinal tract, interacting with the host immune system, modulating gut motility and secretion, and participating in the digestion and absorption of nutrientsCitation5. The main metabolic products of the gut microbiota are short-chain fatty acids (SCFAs), a group of organic acids that are mainly produced by the bacterial fermentation of dietary fiber and other carbohydrates in the colon. In particular, the most abundant SCFAs in the human bowel are the bacterial catabolites acetate (C2), propionate (C3), and butyrate (C4), which are reported to reach up to hundreds of millimoles per liter in the proximal colon and cecumCitation6. In addition, other organic acids deriving from the bacterial catabolism detected in the human gut are valerate (C5), lactate, and succinateCitation7.
SCFAs are essential for maintaining gut health and function since they provide energy to the colonic epithelium, stimulate the production of mucus and antimicrobial peptides, and regulate gut motility and inflammationCitation8. Moreover, SCFAs have been shown to have systemic effects beyond the gut, including regulating glucose and lipid metabolism and the modulation of immune function and gut-brain communicationCitation9,Citation10. Despite the demonstrated beneficial effects of intestinal SCFAs on human physiology and metabolism, the role of these microbial metabolites in IBS remains controversial. Some studies have reported lower levels of SCFAs in IBS patients compared to healthy controls, while others have reported higher levels or no significant differencesCitation11. This heterogeneity in results may be due to the different subtypes of IBS, as well as the differences in dietary habits, medication use, and other confounding factors that can influence gut microbiota and SCFA production. Recently, we reported chronically elevated SCFA levels in the feces of a small cohort of IBS patients with diarrhea (n = 11; IBS-D) compared to IBS patients with constipation (n = 12; IBS-C) and healthy controls (n = 25) over a period of 16 weeksCitation12. Similarly, a meta-analysis by Sun et al. evidenced a significant increase of fecal butyrate in IBS-D patients compared to healthy controlsCitation13. Nonetheless, contradicting results are also reported, such as reduced SCFAsCitation14, and improved gastrointestinal symptoms upon oral intake of butyrate in IBS-D patientsCitation15,Citation16.
To investigate the role of the intestinal microbiome in patients with IBS, we studied fecal short-chain fatty acid (SCFA) levels and bacterial community structure in a cohort of 240 non-constipated patients (IBS-D and IBS-M; NC-IBS) recruited from 19 hospitals in Italy in comparison to healthy control (HC) subjects. Our findings suggest that NC-IBS patients can be stratified into distinct subgroups based on fecal SCFA levels (catabotypes), which are characterized by specific taxonomical bacterial signatures and clinical profiles.
Results
The fecal microbiome of NC-IBS patients and HC subjects differs significantly
Metataxonomic analysis evidenced several significant differences between HC (n = 100) and NC-IBS patients (n = 235; ). Intra-sample biodiversity (α-diversity) was found to be significantly higher in HC as measured with four different indexes: observed-features (measure of richness), Shannon’s entropy (considering both evenness and richness), Pielou’s (measure of evenness), and Faith’s phylogenetic diversity (PD) (). Conversely, we did not find significant differences in β-diversity between IBS and HC as determined through both unweighted and weighted UniFrac (Not shown). Nonetheless, it was possible to separate NC-IBS from HC samples by performing a PLS discriminant analysis (PLSDA) based on the CLR-transformed abundance of bacterial taxa in fecal samples (outcome HC vs. NC-IBS: 0.96; accuracy: 0.67; ; Supplementary Figure S1).
Figure 1. Diagram reporting the number of patients for which specific indicated data are available. (a), quantification of organic acids in feces; (b), metataxonomics of fecal samples through 16S rRNA gene profiling; (c), abdominal pain and bowel habits data.
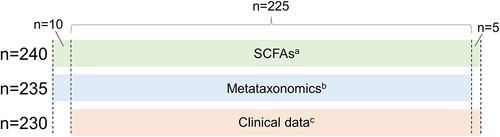
Figure 2. Comparison of the intra-sample biodiversity of the fecal bacterial communities between non-constipated patients (NC-IBS; n = 235) and healthy control subjects (HC; n = 100) according to four different α-diversity indexes. Statistics is according to the Mann-Whitney test. **, P < .01; ****, P < .0001).
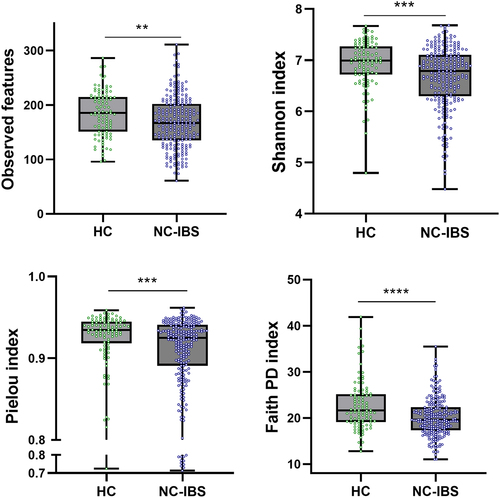
Figure 3. PLS discriminant analysis (PLSDA) with prediction background for non-constipated IBS patients (NC-IBS) and healthy control subjects (HC). The Receiver Operating Characteristic (ROC) curve of the PLSDA model is shown in supplementary Figure S1.
The analysis of metataxonomic data was also conducted while keeping the IBS-D and IBS-M patients separate. The results revealed no significant differences between these two subgroups, both in alpha-diversity and taxonomic community structure (see Supplementary Figure S2), thus supporting the decision to analyze these two subgroups together.
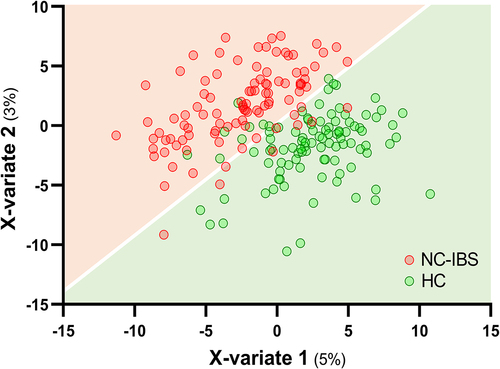
Several taxa were found to be differently represented between NC-IBS and HC samples. Specifically, the phylum Bacteroidetes, the Firmicutes families Ruminococcaceae and Christensenellaceae, and the order Erysipelotrichales were underrepresented in NC-IBS samples, as revealed by LEfSe analysis (; Supplementary Figure S3). Conversely, we observed an overrepresentation of the Actinobacteria genera Rothia and Collinsella (in particular, the species C. aerofaciens), and several Firmicutes taxa, including the genera Clostridium (family Clostridaceae) and [Ruminococcus] (now reclassified as Mediterraneibacter; family Lachnospiraceae), and the species [Ruminococcus] gnavus in NC-IBS samples (; Supplementary Figure S3).
Figure 4. Bacterial taxa in fecal samples exhibiting a significantly different abundance between individuals with non-constipated irritable bowel syndrome (NC-IBS) and healthy subjects (HC). (a) cladogram based on LEfSe analysis (LDA scores for the single taxa are reported in Supplementary Figure S3). (b) significantly different taxa determined through the Mann-Whitney test carried out with CLR-transformed bacterial abundances. Higher and lower abundances for each taxon are reported with a red and cyan background, respectively. The black and yellow heatmap represents the mean CLR-transformed abundances of the reported taxonomic units. The taxonomic lineage of each taxon is shown: p, phylum; c, class; o, order; f, family; g, genus; s, species.
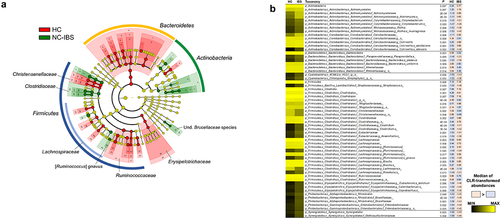
Due to the compositional nature of 16S rRNA gene profiling data [17], we inferred the bacterial taxa that were differently represented between NC-IBS and HC samples after CLR-transformation of taxonomic abundances. The obtained results confirmed several findings obtained with the LEfSe analysis, including the overrepresentation of the species C. aerofaciens and [R.] gnavus in NC-IBS samples (). Overall, at the higher taxonomic levels, the analysis of CLR-transformed data showed that NC-IBS patients had increased Actinobacteria and Firmicutes (order Clostridiales), and decreased Bacteroidales compared to HC. At the family level, the main bacteria overrepresented in the IBS patients were Actinomycetaceae, Clostridiaceae, Lachnospiraceae, Brucellaceae, and Enterobacteriaceae. Additionally, within Bacteroidetes, we found that the species Bacteroides uniformis was more abundant in HC, whereas Bacteroides plebeius was more abundant in NC-IBS ().
Subsequently, we quantified the concentration of the primary organic acids derived from bacterial catabolism in the human gut, including acetate, butyrate, propionate, valerate, lactate, and succinate, in fecal samples collected from 100 HC and 240 NC-IBS individuals (). Acetate and butyrate were the most abundant organic acids detected in most samples, with propionate being the most abundant in ten NC-IBS patients and one HC, and succinate being the most abundant in four NC-IBS patients and one HC. Valerate and lactate were the most abundant organic acids in four and one NC-IBS patients, respectively. NC-IBS patients had a significantly lower abundance of butyrate (median concentrations of 3.9 vs. 3.1 mmol/100 g of feces; P = 0.007 according to the Mann-Whitney test), succinate (0.04 vs. 0.00 mmol/100 g of feces; P = 3.8 × 10–7), and valerate (1.5 vs. 1.2 mmol/100 g of feces; P = 4.3 × 10–4) compared to HC subjects (; ). The propionate/butyrate ratio, which was proposed as a potential biomarker for IBSCitation17,Citation18, was not significantly different between IBS and HC samples. Furthermore, we found no significant differences in fecal organic acids between IBS-D and IBS-M subtypes (Supplementary Figure S2).
Figure 5. Organic acids in fecal samples exhibiting a significantly different abundance between individuals with non-constipated irritable bowel syndrome (NC-IBS) and healthy subjects (HC). The statistical analysis was performed using the Mann-Whitney test and indicated a statistically significant difference compared to HC samples. **, P < .01; ***, P < .001; ****, P < .0001. Red numbers refer to the σCitation2 variance of the corresponding data.
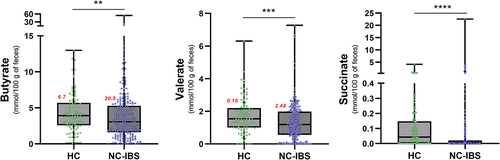
Table 1. Concentration of organic acids in fecal samples collected from healthy subjects (HC) and non-constipated patients (NC-IBS). Data for NC-IBS samples are also shown after stratification in catabotypes FC-H and FC-L. All data are reported in mmol/g of feces. Prp/but, ratio between the concentrations of propionate and butyrate. Statistics is according to Mann-Whitney test and indicate statistically significant difference compared to HC samples. *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001.
Overall, our results indicate that NC-IBS patients exhibit significant alterations in their fecal microbiome compared to healthy individuals. Specifically, our data suggest a general expansion of Actinobacteria, particularly the species C. aerofaciens, as well as a reduction in Bacteroidetes, butyrate, valerate, and succinate in the fecal microbiome of NC-IBS patients.
Stratification based on the fecal levels of SCFAs revealed two distinct microbiome clusters in NC-IBS
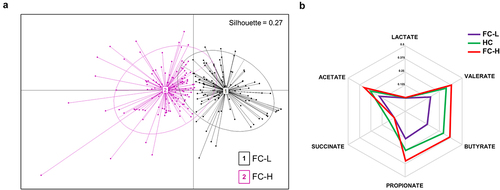
We performed an unsupervised clustering analysis of the 240 NC-IBS patients under investigation using principal coordinate analysis (PCoA) based on the concentrations of six microbial catabolites in their fecal samples: acetate, butyrate, propionate, lactate, succinate, and valerate (catabotyping analysis). Based on the highest Silhouette coefficient (SI = 0.27) and variance ratio criterion (CH index = 77), we were able to classify the IBS samples into two distinct clusters, which we named fecal catabotype “high” (FC-H; n = 128) and “low” (FC-L; n = 112) (). The FC-H group was characterized by significantly higher concentrations of all six metabolites, except for succinate, compared to FC-L. Additionally, the fecal levels of acetate, butyrate, and propionate were lower in HC than in FC-H, while succinate was higher. Notably, HC subjects had significantly higher fecal concentrations of all metabolites compared to FC-L NC-IBS patients (). Moreover, we observed that the propionate/butyrate ratio was higher in FC-L (median value of 0.48) compared to HC (0.34; P = 0.011) and FC-H (0.38; P = 0.066). These findings suggest that NC-IBS patients can be categorized into two clusters characterized by higher and lower fecal SCFA levels than HC, as evidenced by the spider chart based on the relative abundances of bacterial catabolites (). Furthermore, the identified metabotypes do not overlap with IBS subtypes, as it emerged that IBS-D samples distributed in 60% and 40% between FC-H and FC-L, respectively, while IBS-M samples distributed in 45% and 55% between FC-H and FC-L, respectively.
Figure 6. Stratification analysis of non-constipated IBS patients (NC-IBS) based on the fecal levels of the organic acids. (a) principal-coordinate analysis (PCoA) generated through catabotyping based on the fecal concentrations of acetate, butyrate, propionate, lactate, succinate, and valerate. (b) spider chart based on the abundances of bacterial catabolites expressed as relative abundance.
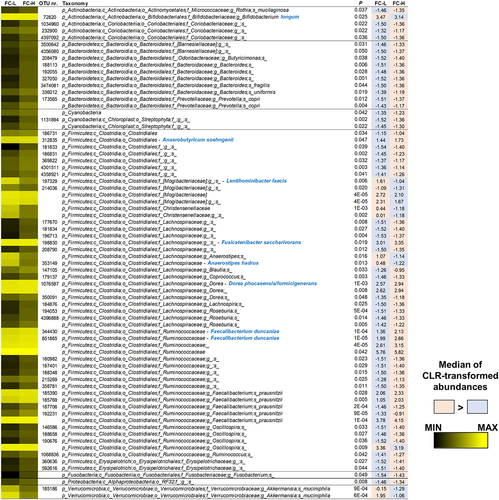
Since intestinal SCFA levels directly result from microbial metabolism in the gut, we compared the fecal bacterial community structure of the IBS subgroups FC-H and FC-L. Statistical analysis of CLR-transformed abundances revealed 73 bacterial taxa with different representations, including 54 OTUs (). Only 12 taxa were more abundant in FC-L than FC-H, including OTUs ascribed to the species Bifidobacterium longum (phylum Actinobacteria), Lentihominibacter faecis, Anaerostipes hadrus, and an undefined Oscillospira species (order Clostridiales), and Akkermansia muciniphila (). Conversely, among the most abundant taxa overrepresented in FC-H, we found several bacteria known to ferment fiber to produce SCFAs, such as Anaerobutyricum soehngenii, Fusicatenibacter saccharivorans, Dorea phocaeensis/formicigenerans, Faecalibacterium duncaniae, and Faecalibacterium prausnitzii ().
Figure 7. Bacterial taxa in fecal samples of NC-IBS patients exhibiting a significantly different abundance between catabotypes FC-H and FC-L. Significantly different taxa have been determined through the Mann-Whitney test carried out with CLR-transformed bacterial abundances. The black-yellow heatmap represents the mean CLR-transformed abundances of the reported taxonomic units. Higher and lower abundances for each taxon are reported with a red and cyan background, respectively. The taxonomic lineage of each taxon is shown: p, phylum; c, class; o, order; f, family; g, genus; s, species. Taxonomic names written in blue were determined through a manual BLASTN search in GenBank using the sequence of the corresponding reads.
Comparison with HC showed that NC-IBS catabotypes had lower α-diversity (Supplementary Figure S4). In addition, we found that 102 bacterial taxa (including 62 OTUs) significantly distinguished HC from FC-L samples. In contrast, the number of differently represented bacterial taxa between HC and FC-H was 276 (including 206 OTUs) (Supplementary Figure S5). In general, most of the significantly different bacterial taxa were overrepresented in the IBS catabotypes compared to HC samples (86 and 263 significantly different taxa respectively increased in FC-L and FC-H compared to HC). Specifically, both FC-L and FC-H had significantly more Firmicutes than HC, mostly due to the overrepresentation of the order Clostridiales and the family Lachnospiraceae within it. Also, Actinobacteria (particularly the species Collinsella aerofaciens) showed higher abundance in both IBS bacterial catabotypes than HC. On the contrary, dominant members of the phylum Bacteroidetes were depleted in the IBS groups compared to HC, such as the order Bacteroidales and the species Bacteroides uniformis reduced in FC-L, and the family Rikenellaceae depleted in FC-H. Notably, the butyrate producer F. prausnitzii was higher in FC-H and lower in FC-L compared to HC (Supplementary Figure S5).
Overall, these data show that IBS patients can be stratified according to the fecal level of SCFAs into two groups (catabotypes), which the diverse representation of several bacterial taxa can distinguish. In particular, the catabotype FC-H, which had significantly higher concentrations of SCFAs, was characterized by the overrepresentation of many taxonomic units (including numerous fiber degraders and SCFA producers) compared to both HC and the NC-IBS group FC-L.
Fecal catabotypes may be clinically relevant
Subsequently, we compared the IBS subgroups FC-H and FC-L in relation to the clinical parameters registered two weeks before fecal collection. We considered abdominal pain (Numeric Pain Rating Scale, NRS scale), fecal type, and stool frequency. Notably, patients in the catabotype FC-H (n = 120) had higher levels of abdominal pain (normal distribution: mean value of 3.8 vs. 3.2; P < .01) and fecal type (non-normal distribution: median value of 4.9 vs. 4.4; P < .0001) than those in the FH-L catabotype (n = 99) ().
Table 2. Clinical symptoms in the non-constipated IBS patients in the PROBE-IBS/2 cohort of the fecal catabotypes FC-L and FC-H. (a) comparison of the FC-H and FC-L catabotypes at baseline. Statistics is according to the Mann-Whitney U test (fecal type and evacuation number) or the unpaired t-test (abdominal pain), depending on the normal distribution of data. (b) comparison between the initial (baseline) and final (after 16 weeks) visit. Statistics is according to the Wilcoxon signed-rank test (fecal type and evacuation number) or the unpaired t-test (abdominal pain) depending on the normal distribution of data. *, P < .05; **, P < .01.
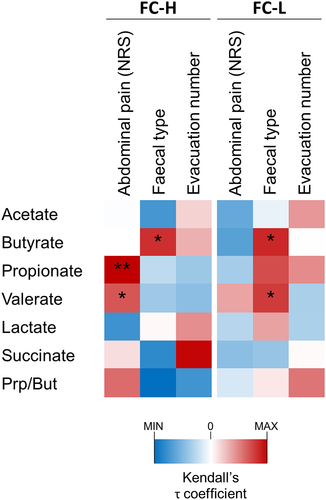
Subsequently, we performed a Kendall’s correlation analysis, which showed a significant positive association of abdominal pain toward valerate and propionate, and succinate toward evacuation number, but only in the FC-H catabotype (). In addition, fecal type and butyrate correlated positively in both catabotypes, whereas fecal type and valerate correlated only in the catabotype FC-L ().
Figure 8. Correlation analysis between fecal organic acids and IBS symptoms in catabotypes FC-L (n = 105) and FC-H (n = 125). The heatmap represents the τ coefficient of Kendall rank correlation. Asterisks indicate significance in the Kendall rank correlation: *, P < .05; **, P < .01. NRS, numeric rating scale.
Finally, we analyzed the clinical data of the PROBE-IBS/2 cohort of IBS patients for 16 weeks during which they followed a conventional therapeutic protocol for IBS based on dietary and lifestyle recommendations (n = 202). Interestingly, the patients in the FC-H group responded better than those in FC-L. In fact, over a period of 16 weeks, FC-H patients reported a significant reduction of abdominal pain and fecal type, whereas only the stool frequency decreased in the FC-L group (). In particular, the initial difference between FC-L and FC-H in abdominal pain was not anymore significant after 16 weeks due to a better responsiveness of the FC-H patients during the first 4 weeks ().
Figure 9. Progression of abdominal pain over 16 weeks in the PROBE-IBS/2 cohort of non-constipated IBS patients stratified in the fecal catabotype FC-L and FC-H. Each point refers to the median value of the numeric rating scale (NRS) for pain assessment. Statistics is according to the Mann-Whitney test between FC-L and FC-H at each specific time point. The numbers in gray close to each point refer to the number of patients. **, P < .01; *, P < .05; n.s., not significant.
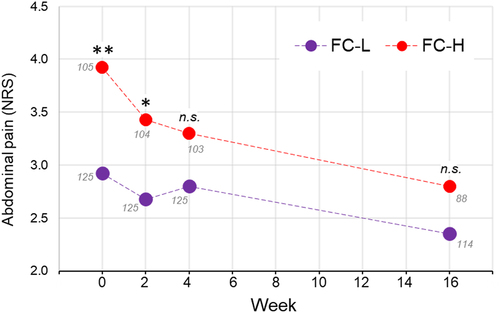
Overall, these data showed that the fecal catabotypes FC-H and FC-L are characterized by different IBS symptom severity and suggest that the direct association between fecal SCFA and IBS symptoms can be stronger in the FC-H catabotype.
Discussion
Numerous studies investigated the role of the intestinal microbial ecosystem in IBS, providing several different hypotheses on the potential microbial signatures for this conditionCitation19. The fact that IBS is a remarkably heterogeneous condition both at the etiological level and in its symptomatic manifestations plausibly justifies the contradicting findings and suggests the need to distinguish between potentially different subtypes within IBS. In this context, bowel habits represent the most commonly used criterion to categorize among IBS patients. In support of this discrimination strategy, several studies highlighted significant differences between IBS patients with diarrhea compared to those with constipation, which seem to be particularly clear at the level of the intestinal microbiome in terms of both specific taxaCitation20 and microbial metabolites such as SCFAsCitation21. Therefore, in order to reduce the existing variability in the context of IBS, in this study, we restricted our analysis to IBS patients with diarrhea (IBS-D and IBS-M), excluding constipation-predominant IBS. Two factors substantiate the inclusion of mixed-type IBS within a group with IBS-D: (i) existing scientific literature demonstrating similarities in characteristics and responses to clinical treatments among patients with IBS lacking constipationCitation22,Citation23; (ii) the absence of significant differences in the taxonomic community structure of the fecal microbiome and fecal levels of SCFAs between IBS-D and IBS-M, as revealed in this study.
The comparison of the fecal bacterial communities of a cohort of 235 NC-IBS patients with 100 age- and sex-matched healthy controls highlighted some significant differences that already emerged in the literature, such as a significantly lower alpha-diversityCitation20 and an expansion of the Actinobacteria phylumCitation24,Citation25. Furthermore, here we observed the increase in Firmicutes (particularly the order Clostridiales) and the depletion of Bacteroidetes (particularly the order Bacteroidales) in NC-IBS compared to HC, as reported in IBS also by two systematic reviewsCitation26,Citation27, supporting the notion that these taxa may represent valid microbial signatures in the fecal microbiota of patients with IBS.
Available research data suggest that the primary bacterial metabolites of the human gut, i.e., the SCFAs, may play a crucial role IBS in pathogenesis. In particular, several mechanistic studies suggest the causal involvement of intestinal SCFAs in the pathophysiology of diarrhea-predominant IBS as a result of the direct effect of these organic acids on serotonin synthesis, colon motility, and visceral hypersensitivityCitation14,Citation28–31. As a result, it has been suggested that regulating colonic SCFA levels could serve as a potential therapeutic target for IBSCitation11,Citation17,Citation32. With this in mind, we quantified the main bacterial catabolites in the feces of the NC-IBS patients. The analysis of the obtained data revealed a lower concentration of butyrate, succinate, and valerate in their fecal samples compared to the HC subjects. Elevated succinate levels in the gut were associated with inflammationCitation33, and were reported in IBS-D patients with symptom exacerbationCitation34 and IBS-D compared to IBS-CCitation35; nonetheless little is known about succinate concentration in IBS compared to the healthy population. Valerate, shown to reduce autoimmunity by enhancing IL-10 and suppressing Th17 cellsCitation36, was reported to be positively associated with fecal type and significantly higher in patients with IBS-D than in those with IBS-CCitation12,Citation13. Fecal valerate levels were also shown to be correlated with visceral sensation (abdominal pain or visceral hypersensitivity) in IBS-D patients, but a significant difference compared to healthy control subjects was not foundCitation17,Citation37. A much broader, nonetheless partly contradicting, literature is available for butyrate. As opposite to the data presented here, in a previous publication, we reported that fecal butyrate levels, calculated as the median values of five measurements conducted over 16 weeks, were significantly higher in a smaller group of IBS-D patients (n = 11) than in IBS-C subjects (n = 12) and healthy controls(n = 23)Citation12. Also in the meta-analysis by Sun et al., fecal butyrate was shown higher in patients with IBS-D than in HCsCitation13. Nonetheless, in other studies, fecal butyrate levels in IBS-D were reported to be not dissimilar from HCCitation18,Citation38.
The available literature suggests that the subtype, particularly IBS-D versus IBS-C, may partly explain the ambiguous results regarding the differences in colonic levels of butyrate and other SCFAs between IBS patients and the healthy population. However, our findings demonstrate a wider variability in fecal SCFA levels in NC-IBS compared to HC (for instance, a σCitation2 variance of 20.5 in NC-IBS versus 6.7 in HC samples for butyrate; ). This let us speculate about the possibility of different subgroups within the NC-IBS patient population, for which SCFAs may have variable clinical relevance. To test this hypothesis, we analyzed the data of fecal SCFA levels with an algorithm already adopted for the unsupervised stratification of human individuals based on bacterial metataxonomic data of their fecal microbiota, known as “enterotyping”Citation39. The enterotype concept is a controversial theoryCitation40 suggesting that the gut microbiome can be classified into a small number of distinct community types based on the abundance of specific bacterial groups. These enterotypes have been proposed to be relatively stable and to play a role in the host’s health and disease risk, therefore potentially relevant in clinical practiceCitation41. In analogy with the enterotype concept, here we aimed to assess the hypothesis that IBS patients could be stratified in catabotypes, i.e., distinct groups of subjects based on the fecal levels of the main organic acid produced by the microbial catabolism in the human colon. The application of catabotyping permitted the stratification of the 240 IBS patients here investigated into two groups with sharply different levels of fecal SCFAs, which for one group were significantly higher (catabotype FC-H) and for the other (catabotype FC-L) significantly lower than those in HC subjects. Notably, we found that the catabotype FC-H showed higher levels of abdominal pain and fecal type compared to FC-L suggesting that an association between IBS symptoms and catabotypes may exist. Nonetheless, we cannot conclusively state the direction of the observed association between SCFAs and IBS symptoms since it can either be possible that alterations in gut motility and sensitivity lead to changes in SCFA production or vice versa. According to available literature, discrepancies are evident regarding the possible association between SCFAs and bowel symptoms. Ringel-Kulka et al. reported that total fecal SCFA levels correlated negatively with colon transit time and positively with stool frequencyCitation18. According to Tana et al., high acetate and propionate levels are associated with significantly worse abdominal symptomsCitation42. Nonetheless, Wang et al. reported that SCFA signatures were not consistently associated with IBS severity over timeCitation43. Furthermore, administering SCFAs in the ileum increased ileal motility and abdominal pain in humansCitation44 and promoted visceral hypersensitivity in ratsCitation45,Citation46. However, in the distal colon, butyrate was shown to increase and propionate to decrease the rate of colonic propulsion in guinea pigsCitation47. Exogenous administration of butyrate was also shown to increase colon motility in a mouse model of IBSCitation28. Nonetheless, recently, a prospective multicenter clinical trial involving 3000 non-hospitalized IBS patients showed that the oral administration of sodium butyrate may effectively relieve the IBS symptomsCitation16.
In this study, we demonstrated that the fecal microbiota of individuals with the FC-H catabotype, who have a greater abundance of SCFAs in their feces, differed significantly (more than FC-L) from those of HC, precisely due to the overrepresentation of multiple bacterial taxa. This finding suggests that the FC-H catabotype may represent a subset of IBS patients with a higher degree of dysbiosis. Recently, Vervier et al. used shotgun metagenomics to analyze fecal samples from 56 IBS-D and IBS-M patients before and after a low-FODMAP diet. Through unsupervised clustering, they identified two fecal microbiota subtypes with different clinical responses to the dietary therapy. In particular, they reported a significantly enhanced responsiveness in the “pathogenic-like” subtype (IBSP), enriched in Firmicutes and genes for carbohydrate metabolismCitation23. Our data suggest that the FC-H catabotype may share similarities with the IBSP subtype proposed by Vervier et al. Therefore, we hypothesize that the FC-H catabotype may represent a subgroup of NC-IBS patients who could benefit the most from a low-FODMAP diet or other dietary interventions targeting the fiber-fermenting microbial components of the gut microbiota. In substantiation of this hypothesis, our study findings indicate that adherence to the dietary guidelines provided to patients in the PROBE-IBS/2 trial, which included the avoidance of fibrous, large-leaf vegetables, legumes, fruits with high fiber content, and whole grains (further information on the recommendations on lifestyle and dietary habits are available in the Supplementary Material file), yielded a more substantial amelioration in abdominal pain and fecal consistency within the FC-H subgroup. Contextually, Eetemadi and TagkopoulosCitation48 analyzed metataxonomic data from six different low-FODMAP intervention trials and concluded that IBS patients who are likely to respond better to a low-FODMAP diet are characterized by a higher abundance of putative genes related to SCFA metabolism pathwaysCitation48. Likewise, Chumpitazi and colleagues documented that in children with IBS who responded positively to the FODMAP diet, there were elevated levels of particular microbial groups, such as Ruminococcaceae and Faecalibacterium prausnitzii, which have been linked to higher rates of saccharolytic metabolismCitation49. Furthermore, our correlation analysis revealed a significant positive association between butyrate and fecal type, and between propionate and valerate levels and abdominal pain exclusively in the FC-H catabotype. This observation supports the hypothesis that only in patients with higher concentration, fecal SCFAs may play a role in IBS pathogenesis and could represent a valid target to enhance diagnosis and guide toward potentially more effective treatment options. Specifically, we speculate that higher levels of fecal organic acids in NC-IBS patients may indicate a greater likelihood of success for therapeutic interventions targeting the microbiome. In addition to the low-FODMAP diet, which has significant limitations in long-term sustainability, this subset of IBS patients may represent the population most likely to derive substantial benefits from treatments such as antibiotics (e.g., as rifaximin), interventions involving probiotics or live biotherapeutics (e.g., Blautia hydrogenotrophica,Citation50, and fecal microbiota transplantation (FMT), the efficacy of which in IBS is controversialCitation51. Concerning FMT, we can postulate that in IBS patients where the colonic microbial ecosystem is a major contributor to symptoms (as we hypothesized here, particularly in FC-H patients), FMT may offer the most significant and potentially enduring advantages.
In conclusion, this study investigated the role of SCFAs in IBS, and specifically explored the presence of a subgroup of non-constipated patients characterized by higher levels of SCFAs (that we named catabotype FC-H). We hypothesized that the FC-H subgroup may represent a distinct clinical phenotype of IBS with specific clinical features. In order to understand if the identification of this catabotype may have implications for the diagnosis and treatment of IBS, further research efforts should be first directed toward the development of a standardized analytical protocol and the identification of a threshold for fecal organic acids that can help establish whether a non-constipated IBS patient falls into this category of patients.
Overall, our study offers novel perspectives on the intricate relationship between the gut microbiome and bowel symptoms in IBS, emphasizing the importance of tailored strategies for its treatment.
Patients and methods
Study participants and clinical assessment
The present study included a total of 240 patients diagnosed with non-constipated [i.e., IBS with predominant diarrhea (IBS-D) or IBS with mixed bowel habits (IBS-M); NC-IBS] according to Rome IV criteria, recruited in 19 Italian hospitals. These subjects were from the cohort of the PROBE-IBS/2 trial (ClinicalTrials.gov Identifier NCT03449628). In addition, we included 100 healthy controls matching the IBS patients of the PROBE-IBS/2 cohort in terms of age and sex. The characteristics of the study populations are summarized in . The standard 11-point numeric rating scale (ranging from 0, indicating no pain, to 10, indicating the worst possible pain) was used to measure abdominal pain in IBS patients at two-week intervals. Finally, we assessed stool frequency and form using the Bristol stool chart. The patients’ abdominal pain and bowel habits were monitored over 16 weeks. At recruitment, patients received the recommendations on life-style and eating habits described in the supplementary material file (Supplementary Methods).
Table 3. Demography and clinical information of subjects involved in the study. NC-IBS, cohort of non-constipated irritable bowel syndrome patients of the PROBE-IBS/2 trial. HC, healthy control subjects.
Ethics statement
The ethical approvals were obtained for trial PROBE-IBS/2 by the Committee of the coordinating center (Hospital S. Orsola Malpighi – Bologna - Approval identification number 67/2017/U/Sper on June 13, 2017) and by the Ethics Committee of each participating site. The trial was carried out in compliance with the Declaration of Helsinki and the principles of good clinical practice. A written informed consent was provided by all participants.
Organic acid quantification in fecal samples
We quantified organic acids in fecal samples obtained from 240 IBS patients of the PROBE-IBS/2 cohort at recruitment and from 100 healthy controls. The organic acids measured included acetate, butyrate, propionate, valerate, lactate, and succinate. Ultra-Performance Liquid Chromatography – High-Resolution Mass Spectrometry (UPLC-HR-MS) was used to detect and quantify these molecules in fecal samples. This technique utilized an Acquity UPLC separation module (Waters, Milford, MA) coupled with an Exactive Orbitrap MS and a HESI-II probe for electrospray ionization (Thermo Scientific, San Jose, CA), as previously describedCitation12.
Metataxonomics of fecal samples
Metataxonomic analysis was performed on fecal samples collected from all 235 patients of the PROBE-IBS/2 cohort and 100 control samples from healthy subjects by profiling the 16S rRNA gene. Fecal samples were collected by the patients and delivered to the reference hospital within 24 h with instructions to store them in a refrigerator (without freezing) before delivery. Subsequently, the samples were promptly frozen at −80°C and ultimately transferred in dry ice to the centralized laboratory at the University of Milan for analysis. Total DNA was extracted from 150 mg of feces using the QIAsymphony PowerFecal Pro DNA Kit (Qiagen, Milan, Italy) in accordance with the manufacturer’s instructions. To amplify a fragment of the 16S rRNA gene that includes the V3 and V4 variable regions, primers 341F (5’-CCT ACG GGN GGC WGC AG-3’) and 805 R (5’-GAC TAC HVG GGT ATC TAA TCC-3’) (LC Sciences, Houston, TX) were used. The obtained amplicons were sequenced using the NovaSeq 6000, 2 × 250bp (NovaSeq 6000 SP Reagent Kit, 500 cycles). The resulting sequencing reads were analyzed using the bioinformatic pipeline Quantitative Insights Into Microbial Ecology (QIIME) 2 version 2022.2, with the Greengenes database v. 13_8 for taxonomic assignment to amplicon sequence variants (ASVs) clustered at a 97% similarity (cASV) using the Divisive Amplicon Denoising Algorithm (DADA2; 27214047).
Data analysis and statistics
Statistical calculations, including partial least squares discriminant analysis (PLSDA), were carried out using R programming language (version 3.4.2). Differently abundant taxa between groups were identified using linear discriminant analysis (LDA) combined with effect size (LEfSe) algorithmCitation52; a cutoff value of LDA score (log10) above 2.0 was chosen. In addition, significantly different taxa were also identified by Mann-Whitney test on read abundances that underwent centered log ratio (CLR) transformation. Stratification based on catabotyping analysis was carried out based on the fecal concentrations of acetate, butyrate, propionate, lactate, succinate, and valerate using JSD distance and the Partitioning around Medoids (PAM) algorithm. Principal Coordinate Analysis with centroid was used to represent the samples graphically. For paired/unpaired matches with all the other variables considered in the study, paired/unpaired Student’s t-test or Mann-Whitney U and Wilcoxon Signed-Ranks tests were adopted depending on normal distribution assessed through the Shapiro-Francia test. Correlation analyses were carried out calculating Kendall’s τ rank correlation coefficient.
Declaration of generative AI and AI-assisted technologies in the writing process
While preparing this work, the authors used the AI-powered language model ChatGPT-3.5 (https://chat.openai.com/) and Grammarly (https://app.grammarly.com/) to improve readability and language. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the publication’s content.
PROBE-IBS/2 group collaborators
Bruno Annibale, Roma; Guido Basilisco, Milano; Leonilde Bonfrate, Bari; Michele Cicala, Roma; Rocco Cosintino, Roma; Antonio Di Sabatino, Pavia; Raffaella Ferraro, Vercelli; Francesca Galeazzi, Padova; Bastianello Germanà, Belluno; Giovanni Maconi, Milano; Santino Marchi, Pisa; Gerardo Nardone, Napoli; Matteo Neri, Chieti; Fabio Pace, Seriate; Piero Portincasa, Bari; Franco Radaelli, Como; Marcello Rodi, Vercelli; Giovanni Sarnelli, Napoli; Paolo Usai, Cagliari.
Supplemental Material
Download MS Word (895 KB)Acknowledgments
The authors acknowledge support from the University of Milan through the APC initiative.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Metataxonomic raw sequencing data are available as FASTQ files in the European Nucleotide Archive (ENA) of the European Bioinformatics Institute under the accession code PRJEB64301. Processed data are included in the article or uploaded as supplementary materials. All the other data are available upon request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2274128
Additional information
Funding
References
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterol. 2021;160:99–114 e3. doi:10.1053/j.gastro.2020.04.014.
- Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol. 2014;20(27):8807–17. doi:10.3748/wjg.v20.i27.8807.
- Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, Agrawal A, Aziz I, Farmer AD, Eugenicos MP, et al. British society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70(7):1214–1240. doi:10.1136/gutjnl-2021-324598.
- Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The microbiome and irritable bowel syndrome - a review on the pathophysiology current research and future therapy. Front Microbiol. 2019;10:1136. doi:10.3389/fmicb.2019.01870.
- Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, et al. Microbiota in health and diseases. Signal Transduction And Targeted Therapy. 2022;7:135. doi:10.1038/s41392-022-00974-4.
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi:10.1136/gut.28.10.1221.
- Akhtar M, Chen Y, Ma Z, Zhang X, Shi D, Khan JA, Liu H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr. 2022;8:350–360. doi:10.1016/j.aninu.2021.11.005.
- Martin-Gallausiaux C, Marinelli L, Blottiere HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49. doi:10.1017/S0029665120006916.
- Cong J, Zhou P, Zhang R. Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients. 2022;14(9):14. doi:10.3390/nu14091977.
- Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). 2020;11:25. doi:10.3389/fendo.2020.00025.
- Luo M, Zhuang X, Tian Z, Xiong L. Alterations in short-chain fatty acids and serotonin in irritable bowel syndrome: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21(1):14. doi:10.1186/s12876-020-01577-5.
- Gargari G, Taverniti V, Gardana C, Cremon C, Canducci F, Pagano I, Barbaro MR, Bellacosa L, Castellazzi AM, Valsecchi C, et al. Fecal Clostridiales distribution and short-chain fatty acids reflect bowel habits in irritable bowel syndrome. Environ Microbiol. 2018;20(9):3201–3213. doi:10.1111/1462-2920.14271.
- Sun Q, Jia Q, Song L, Duan L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: a systematic review and meta-analysis. Medicine. 2019;98(7):e14513. doi:10.1097/MD.0000000000014513.
- Fredericks E, Theunissen R, Roux S. Short chain fatty acids and monocarboxylate transporters in irritable bowel syndrome. Turk J Gastroenterol. 2020;31:840–847. doi:10.5152/tjg.2020.19856.
- Scarpellini E, Lauritano EC, Lupascu A, Petruzzellis C, Novi ML, Roccarina D, Gabrielli M, Serricchio M, Gasbarrini G, Gasbarrini A, et al. Efficacy of butyrate in the treatment of diarrhoea-predominant irritable bowel syndrome. Dig And Liver Dis Supp. 2007;1(1):19–22. doi:10.1016/S1594-5804(08)60006-6.
- Lewandowski K, Kaniewska M, Karlowicz K, Rosolowski M, Rydzewska G. The effectiveness of microencapsulated sodium butyrate at reducing symptoms in patients with irritable bowel syndrome. Prz Gastroenterol. 2022;17:28–34. doi:10.5114/pg.2021.112681.
- Farup PG, Rudi K, Hestad K. Faecal short-chain fatty acids - a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016;16(1):51. doi:10.1186/s12876-016-0446-z.
- Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA, Ringel Y. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol. 2015;110:1339–1346. doi:10.1038/ajg.2015.220.
- Shaikh SD, Sun N, Canakis A, Park WY, Weber HC. Irritable bowel syndrome and the gut microbiome: a comprehensive review. J Clin Med. 2023;12(7):2558. doi:10.3390/jcm12072558.
- Su Q, Tun HM, Liu Q, Yeoh YK, Mak JWY, Chan FKL, Ng SC. Gut microbiome signatures reflect different subtypes of irritable bowel syndrome. Gut Microbes. 2023;15:2157697. doi:10.1080/19490976.2022.2157697.
- Jacobs JP, Lagishetty V, Hauer MC, Labus JS, Dong TS, Toma R, Vuyisich M, Naliboff BD, Lackner JM, Gupta A, et al. Multi-omics profiles of the intestinal microbiome in irritable bowel syndrome and its bowel habit subtypes. Microbiome. 2023;11(1):5. doi:10.1186/s40168-022-01450-5.
- Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32. doi:10.1056/NEJMoa1004409.
- Vervier K, Moss S, Kumar N, Adoum A, Barne M, Browne H, Kaser A, Kiely CJ, Neville BA, Powell N, et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut. 2022;71(9):1821–1830. doi:10.1136/gutjnl-2021-325177.
- Rangel I, Sundin J, Fuentes S, Repsilber D, de Vos WM, Brummer RJ, de Vos WM. The relationship between faecal-associated and mucosal-associated microbiota in irritable bowel syndrome patients and healthy subjects. Aliment Pharmacol Ther. 2015;42:1211–1221. doi:10.1111/apt.13399.
- Tap J, Derrien M, Tornblom H, Brazeilles R, Cools-Portier S, Dore J, Störsrud S, Le Nevé B, Öhman L, Simrén M, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2017;152:111–23 e8. doi:10.1053/j.gastro.2016.09.049.
- Duan R, Zhu S, Wang B, Duan L. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10(2):e00012. doi:10.14309/ctg.0000000000000012.
- Wang L, Alammar N, Singh R, Nanavati J, Song Y, Chaudhary R, Mullin GE. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. J Acad Nutr Diet. 2020;120:565–586. doi:10.1016/j.jand.2019.05.015.
- Shaidullov IF, Sorokina DM, Sitdikov FG, Hermann A, Abdulkhakov SR, Sitdikova GF. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome. BMC Gastroenterol. 2021;21(1):37. doi:10.1186/s12876-021-01613-y.
- Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17(4):585–594. doi:10.1111/j.1365-2982.2005.00669.x.
- Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi:10.1096/fj.14-259598.
- BouSaba J, Zheng T, Dilmaghani S, Johnson S, Chen J, Camilleri M. Effect of rapid colonic transit on stool microbiome and short-chain fatty acids in diarrhoea-predominant irritable bowel syndrome. Gut. 2023;gutjnl-2022–329359. doi:10.1136/gutjnl-2022-329359.
- Jiang W, Wu J, Zhu S, Xin L, Yu C, Shen Z. The role of short chain fatty acids in irritable bowel syndrome. J Neurogastroenterol Motil. 2022;28(4):540–548. doi:10.5056/jnm22093.
- Connors J, Dawe N, Van Limbergen J. The role of succinate in the Regulation of intestinal inflammation. Nutrients. 2018;11(1):11. doi:10.3390/nu11010025.
- Tanaka Y, Yamashita R, Kawashima J, Mori H, Kurokawa K, Fukuda S, Gotoh Y, Nakamura K, Hayashi T, Kasahara Y, et al. Omics profiles of fecal and oral microbiota change in irritable bowel syndrome patients with diarrhea and symptom exacerbation. J Gastroenterol. 2022;57(10):748–760. doi:10.1007/s00535-022-01888-2.
- Ahluwalia B, Iribarren C, Magnusson MK, Sundin J, Clevers E, Savolainen O, Ross AB, Törnblom H, Simrén M, Öhman L, et al. A distinct faecal microbiota and metabolite profile linked to bowel habits in patients with irritable bowel syndrome. Cells. 2021;10(6):10. doi:10.3390/cells10061459.
- Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10(1):760. doi:10.1038/s41467-019-08711-2.
- Zhang WX, Zhang Y, Qin G, Li KM, Wei W, Li SY, Yao S-K. Altered profiles of fecal metabolites correlate with visceral hypersensitivity and may contribute to symptom severity of diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2019;25:6416–6429. doi:10.3748/wjg.v25.i43.6416.
- Sun QH, Liu ZJ, Zhang L, Wei H, Song LJ, Zhu SW, He MB, Duan LP. Sex-based differences in fecal short-chain fatty acid and gut microbiota in irritable bowel syndrome patients. J Dig Dis. 2021;22:246–255. doi:10.1111/1751-2980.12988.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi:10.1038/nature09944.
- Knights D, Ward TL, McKinlay CE, Miller H, Gonzalez A, McDonald D, Knight R. Rethinking “enterotypes”. Cell Host & Microbe. 2014;16:433–437. doi:10.1016/j.chom.2014.09.013.
- Costea PI, Hildebrand F, Arumugam M, Backhed F, Blaser MJ, Bushman FD, de Vos WM, Ehrlich S, Fraser CM, Hattori M, et al. Enterotypes in the landscape of gut microbial community composition. Nat microbiol. 2018;3:8–16. doi:10.1038/s41564-017-0072-8.
- Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512-9, e114–5. doi:10.1111/j.1365-2982.2009.01427.x.
- Wang T, Rijnaarts I, Hermes GDA, de Roos NM, Witteman BJM, de Wit NJW, Govers C, Smidt H, Zoetendal EG. Fecal microbiota signatures are not consistently related to symptom severity in irritable bowel syndrome. Dig Dis Sci. 2022;67:5137–5148. doi:10.1007/s10620-022-07543-3.
- Kamath PS, Phillips SF, Zinsmeister AR. Short-chain fatty acids stimulate ileal motility in humans. Gastroenterology. 1988;95(6):1496–1502. doi:10.1016/S0016-5085(88)80068-4.
- Xu D, Wu X, Grabauskas G, Owyang C. Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root ganglia. Gut. 2013;62(10):1466–1474. doi:10.1136/gutjnl-2012-302260.
- Carco C, Young W, Gearry RB, Talley NJ, McNabb WC, Roy NC. Increasing evidence that irritable bowel syndrome and functional gastrointestinal disorders have a microbial pathogenesis. Front Cell Infect Microbiol. 2020;10:10. doi:10.3389/fcimb.2020.00468.
- Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26(11):1586–1596. doi:10.1111/nmo.12425.
- Eetemadi A, Tagkopoulos I. Methane and fatty acid metabolism pathways are predictive of low-FODMAP diet efficacy for patients with irritable bowel syndrome. Clin Nutr. 2021;40:4414–4421. doi:10.1016/j.clnu.2020.12.041.
- Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, Versalovic J, Shulman RJ. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42:418–427. doi:10.1111/apt.13286.
- Quigley EMM, Markinson L, Stevenson A, Treasure FP, Lacy BE. Randomised clinical trial: efficacy and safety of the live biotherapeutic product MRx1234 in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2023;57(1):81–93. doi:10.1111/apt.17310.
- Wang M, Xie X, Zhao S, Ma X, Wang Z, Zhang Y. Fecal microbiota transplantation for irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Front Immunol. 2023;14:14. doi:10.3389/fimmu.2023.1136343.
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi:10.1186/gb-2011-12-6-r60.
