?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Parchment is one of the most abundant resources in archives across the world and is a unique time-sensitive material through which centuries of livestock economies, trade and craft can be explored. We examine the impact of structural and chemical modifications during production to δ13C and δ15N values in the skin, particularly the removal of cutaneous keratins and lipids and the conversion of amide functional groups into carboxyl groups via alkaline hydrolysis. Through the manufacture of 51 parchment skins (sheep, goat, calf and pig) using both historic and modern manufacturing techniques, we found production resulted in a small enrichment in 13C (average +0.12‰) and 15N (+0.26‰). Our results pave the way for the isotopic analysis of parchment in paleodietary and paleoenvironmental studies for the historic period and establish the acceptable C:N ratios in deamidated collagenous tissues.
KEYWORDS:
1. Introduction
Palaeodietary and zooarchaeological stable isotope analysis has traditionally focussed on the analysis of bone collagen and dentine due to their preservation and ubiquity in the archaeological record. While abundant, these materials are constrained by their archaeological phasing or radiocarbon date, which at best assigns the material to a single century. Historic parchment, in contrast, is both numerous and typically dated to the year of use and, as with unprocessed skin (White and Schwarcz Citation1994; Iacumin et al. Citation1996; Iacumin et al. Citation1998; Finucane Citation2007; Corr et al. Citation2009; Basha et al. Citation2016; Lamb Citation2015), offers the possibility of a time-sensitive analysis of dietary and husbandry trends from the weeks and months prior to the animals’ death.
While the isotope analysis of parchment has been conducted (Campana et al. Citation2010; Pollard and Brock Citation2011), the impact of production on measured values in the skin has not been explored. These previous studies have observed δ15N values in modern and historic parchment far higher than those expected from terrestrial herbivores, with values >10‰ suggesting isotopic fractionation as a result of the structural and chemical modifications, the skin undergoes during processing, particularly amide sidechain hydrolysis during liming (Campana et al. Citation2010; Pollard and Brock Citation2011). To address this, we present the isotopic analysis of 51 paired skin and parchment samples from a range of species to assess the impact of production on collagen isotope values and elemental composition, paving the way for the future analysis of the historical documents using this technique.
2. Parchment production
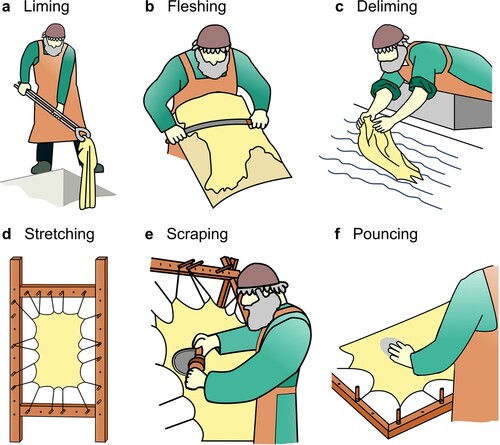
Parchment production transforms wet, perishable animal skin into a dry, durable sheet, suitable for writing purposes. Despite numerous manufacturing “recipes”, the basic principles have remained largely unchanged since the eighth century (Ryder Citation2009; Reed Citation1973; Haines Citation1990) (). Once removed from the animal, the skin is limed, exposing it to a highly alkaline solution, typically calcium hydroxide (Ca(OH)2), sodium sulfide (Na2S) or sodium hydroxide (NaOH). This process results in, (a) breaking the disulfide bonds in the keratinous hair and epidermis, facilitating their removal (Bieńkiewicz Citation1983; Covington Citation2009); (b) hydrolysis of triglyceride esters (saponification), removing around 50% of cutaneous lipids (Koppenhoefer Citation1938; Koppenhoefer Citation1939), improving the whiteness of resulting parchment and the absorption of inks; and (c) hydrolysis of some amide groups attached to aspartic and glutamic acid residues (deamidation), lowering the collagen’s iso-electric point and “opening up” the collagen fibre network, enabling greater penetration of the solution and the removal of non-collagenous proteins, purifying the collagen substrate (Covington Citation2009; Menderes et al. Citation1999).
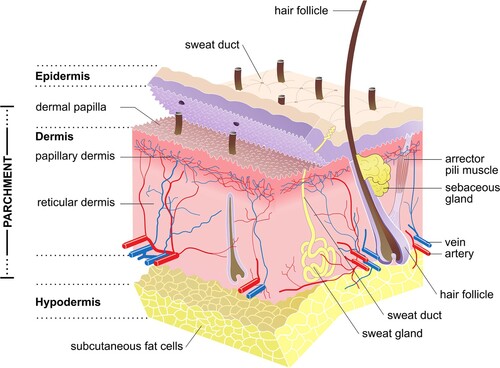
The skin is then fleshed with a double-handled knife, removing both the epidermis and adhering adipose tissue, leaving the collagen-rich dermis layer (). Following this, the skin is delimed, typically in water, to lower the pH prior to tensioning. The skin is then stretched under great pressure, where it is shaved with a lunellum (half-moon blade) to smooth the surface and remove any remaining epidermal of adipose tissue. The skin is finally allowed to dry under tension, after which the surface may be pounced with a mildly abrasive tool (often a pumice stone) to produce a uniform surface.
3. Material and methods
3.1. Experimental parchment production
Fresh skins were obtained from 42 sheep (Ovis aries) (E. Yorks. UK; Leics. UK; Notts. UK; Prague, CZ; Kansas, US), 2 goats (Capra aegagrus hircus) (Prague, CZ), 2 calves (Bos taurus) (Vienna, AU) and 5 pigs (Sus scrofa domesticus) (Notts. UK). The animals were flayed shortly after death and their skins salted and stored at 5°C prior to analysis.
To maximise this study’s comparability with both mediaeval and post-mediaeval parchment, two production methods were used. Method 1 followed traditional pre-nineteenth century European techniques as detailed in historic recipes (Ryder Citation2009; Reed Citation1973; Saxl Citation1954), with the skins unhaired in a straight lime solution, and delimed in water alone. Method 2 used industrial techniques through the addition of chemical depilatory agents during unhairing and acids during deliming (Covington Citation2009).
3.1.1. Method 1 (Historical method)
Each skin was washed and rehydrated in water at 8°C (pH 7.5) for 48 h. The water was replenished every 8 h and adhering foreign material was removed. The unsplit skins were submerged in a 3.5% calcium hydroxide solution (pH 13.5) in 220 L HDPE drums at room temperature for 6–18 days and agitated three times per day to ensure an even exposure across the skin. The ability to remove the fibre was appraised daily, and deemed sufficiently limed when it could be removed at the root by hand with ease (Covington Citation2009). Once unhaired, the skins were fleshed on the beam, with the epidermis and subcutaneous tissue removed with a double-handled knife. The skins were subsequently returned to the lime for a further 12 h, allowing for a uniform penetration of the solution, which may have been inhibited by hair or fat. The skin was then placed on the beam and mechanically squeezed with the knife to force out as much liquid as possible (“scudding”). To neutralise the alkalinity, each skin was washed vigorously for 30 min in running water and allowed to soak for 48 h, with the water replaced every 8 h. Each skin was then tightly stretched with ropes on a wooden frame, and allowed to dry under tension. While still wet and then again once dry, each side was shaved with a sharp knife to remove further layers of the dermis and produce a clean even surface.
3.1.2. Method 2 (Modern method)
Each skin was washed and rehydrated as in Method 1. The unsplit skins were treated in a 0.1% sodium hydrosulfide (NaHS) and 3% calcium hydroxide solution in large rotating tanning drums at room temperature for 30 min and agitated throughout. After this, an additional 3% sodium hydrosulfide and 0.3% sodium hydroxide were then added. The drums were agitated intermittently for 1 h and then left to stand for 18 h, during which time the hair had completely dissolved. The skins were then fleshed on the beam and neutralised in water and 0.75% formic acid (HCOOH) for 6 h. The skins were then stretched, shaved and left to dry under tension as in Method 1.
3.2. Sample preparation and analysis
Due to the limited isotopic analysis of parchment, there is currently no standard method of sample preparation. Parchment is a complex material in which the chemistry and integrity of the collagen is altered during manufacture (and potentially during conservational treatments), and can degrade via oxidation, hydrolysis and biological attack (Hedges et al. Citation1989; Badea et al. Citation2012; Brock Citation2013). Surface contaminants, including inks, glues and chalk are common, as well as areas of localised gelatinisation and deterioration (Brock Citation2013). It is often essential that only a small physical sample is taken to minimise the aesthetic change to the manuscript. As such, careful sampling and pretreatment is required to minimise and remove sources of contamination and ensure accurate analysis.
There is currently no widely accepted method for checking the integrity of collagen from skin or parchment (Lamb Citation2015); however, C:N ratios (an integrity standard for bone collagen and dentine (Ambrose Citation1990; van Klinken Citation1999))have been widely used. Modern collagen (Col1) has an atypically low C:N ratio of 3.11 due to the abundance of glycine (C:N 2:1) (). Ratios higher than this in ancient collagen may reflect the loss of nitrogen during deamidation and the conversion of amide functional groups of asparagine (Asn) and glutamine (Gln) to carboxyl groups, and the loss of nitrogen through the citrullination of arginine (Arg). Based upon the atomic elemental composition of collagen, in the unlikely circumstance of all asparagine amino acids being converted to aspartic acid (Asp), we calculate a C:N ratio of 3.14, and if the complete conversion of glutamine to glutamic acid (Glu) occurs, a C:N ratio of 3.19. We calculate a C:N ratio of 3.25 with the complete citrullination of arginine to citrulline (Cit), although there is nominal conversation during liming (Bowes and Kenten Citation1948). Therefore, if all amide groups (Asn, Gln) and guanidino groups (Arg) undergo hydrolysis (i.e. deamidated to carboxylic acids, and citrullinated to an intermediate metabolic amino acid), collagen would have a C:N ratio of 3.36. Ratios higher than this may indicate the presence of other proteins and lipids (van Klinken Citation1999; Schoeninger and DeNiro Citation1984; Kiljunen et al. Citation2006), such as elastin which has a C:N ratio of 5.8.
Table 1. Atomic elemental composition of bovine type I collagen (Col1a1, P02453 and Col1a2, P02465).
Brock (Citation2013) examined the impact of different pretreatment protocols on C:N ratios in parchment for carbon-14 dating. The highest C:N ratios were observed in untreated parchment and the lowest in those treated with strong acids or alkalis, the former highlighting the necessity for some sample preparation. The most consistently acceptable ratios (3.3) were produced from samples that had undergone lipid extraction followed by collagen extraction (demineralisation, gelatinisation, filtration and freeze-drying). This is consistent with the results from other analyses of parchment and mummified skin, where those that have not undergone collagen extraction have been shown to produce high C:N ratios, some in excess of 4.0 (Iacumin et al. Citation1996; Iacumin et al. Citation1998; Basha et al. Citation2016), while those that have undergone lipid and collagen extraction average around 3.3 (Finucane Citation2007; Pollard and Brock Citation2011; Brock Citation2013; Kiljunen et al. Citation2006).
Endogenous and exogenous lipids are often present in parchment in significant quantities (Ghioni et al. Citation2005; Strlič et al. Citation2009; Možir et al. Citation2014) and must be removed due to the different isotopic compositions of collagen and lipids (Liden, Takahashi, and Nelson Citation1995). Lipids have more negative δ13C values than other biochemical compounds due to kinetic isotope effects that occur during lipid synthesis (DeNiro and Epstein Citation1977; Logan et al. Citation2008). Variability in tissue lipid content can, therefore, alter bulk δ13C values, although, as they are composed mainly of carbon they have little impact on δ15N values (Logan et al. Citation2008). In this study, lipids were removed from both skin and parchment samples with DCM/MeOH, a solvent mixture commonly used in tissues where triglycerides dominate (Ferraz et al. Citation2004; Colonese et al. Citation2015; Guiry et al. Citation2016). Due to the potential for residual calcium carbonate/hydroxide to remain in the skin from liming, a brief demineralisation step was included to remove this. Following the results of Brock (Brock Citation2013), samples were then gelatinised, filtered and freeze-dried to purify the collagen substrate for analysis. The process for each sample type is outlined in full below.
3.2.1. Unprocessed skin
Samples were taken from the belly region after soaking, but prior to liming. Adhering hair and fat deposits were removed with a scalpel to leave a dermis-rich sample, which was freeze-dried for 48 h, and ground to a coarse powder using a ball mill (Retsch MM400). In line with published analyses of modern and archaeological skins (Finucane Citation2007; Browning et al. Citation2014; Bergamo, Botta, and Copertino Citation2016) samples underwent lipid and collagen extraction prior to analysis. Samples were defatted via solvent extraction, DCM/MeOH (2:1 v/v), by ultrasonication for 1hr, with the supernatant removed and solvent mixture replaced every 15 min. The samples were briefly demineralised in 0.6 M HCI at 4°C for 1 h, rinsed with distilled water and gelatinised in 0.001 M pH 3 HCI at 80°C for 48 h. The supernatant containing the collagen was filtered (60–90 μm Ezee-Filter™, Elkay Laboratories, UK), frozen and freeze-dried.
3.2.2. Parchment
Samples were cut from the parchment adjacent to the location sampled for the fresh skin. The lipids were solvent extracted following the same procedure used for skin. Samples were subsequently demineralised in 0.6 M HCI at 4°C for 6 h to remove residual calcium carbonate/hydroxide, rinsed with distilled water, and gelatinised in 0.001 M pH 3 HCI at 80°C for 48 h. The supernatant was then filtered, frozen, and freeze-dried.
3.3. Stable isotope analysis
Prepared collagen (0.9–1.1 mg) was weighed out in duplicate in 5 × 3.5 mm tin capsules (Elemental Microanalysis, Okehampton, UK) for carbon and nitrogen isotope analysis. Samples prepared under Method 1 were analysed at the Natural Environment Research Council Life Sciences Mass Spectrometry Facility (NERC LSMSF) in East Kilbride, where isotope ratio determinations were carried out on a ThermoElectron DeltaPlusXP (Thermo Fisher Scientific, Bremen, Germany) with an Elementar Pyrocube elemental analyser (Elementar, Langenselbold, Germany). Stable carbon and nitrogen isotopic compositions were calibrated relative to VPDB and AIR using USG40 (glutamic acid) and ratios reported permil (‰). Measurement uncertainty was monitored using three in-house standards with well characterised isotopic compositions: 13C-enriched alanine (δ13C: −8.36 ± 0.13‰, δ15N: 2.08 ± 0.06‰), 15N-enriched glycine (δ13C: −38.58 ± 0.09‰, δ15N: 23.54 ± 0.08‰) and gelatine (Sigma-Aldrich, US. δ13C: −20.31 ± 0.18‰, δ15N: 5.54 ± 0.08‰). Following the calculations outlined in Szpak, Metcalfe, and MacDonald (Citation2017), the total analytical uncertainty was estimated to be ± 0.18‰ for δ13C and ± 0.20‰ for δ15N.
Samples manufactured following Method 2 were analysed at the University of York where determinations were carried out on a Sercon GLS analyser coupled to a Sercon 20–22 Mass Spectrometer (Sercon, Crewe, UK). Stable carbon and nitrogen isotopic compositions were calibrated relative to the VPDB and AIR using IAEA-600 (caffeine) and IAEA-N2 (ammonium sulphate), with ratios reported permill (‰). Measurement uncertainty was monitored using standards of cane sugar (IA-R006 – Iso-Analytical Ltd, UK. δ13C: −11.64 ± 0.03‰) and fish gelatine (Sigma-Aldrich, US. δ13C: −15.36 ± 0.05‰, δ15N: 15.2 ± 0.05‰). The total analytical uncertainty was estimated to be ± 0.19‰ for δ13C and ± 0.11‰ for δ15N. Slight inter-lab variations in isotopic values are expected, although as the pairwise analysis was conducted at a single location, it is likely insignificant to interpretation (Pestle, Crowley, and Weirauch Citation2014). Reproducibility between samples was better than ± 0.2‰ (1σ) for both δ13C and δ15N in skin, and parchment.
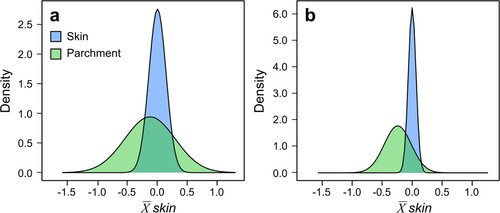
Statistical analysis was carried out using the IBM SPSS Statistic 22.0 software package (IBM 2013). Shapiro–Wilks tests for normality showed that the distribution of δ15N values in skin and parchment were normal, but that δ13C values were not (p < 0.01). As such, the statistical significance of differences between skin and parchment values was determined using a Wilcoxon signed-rank test for paired samples. In order to assess the difference and account for analytical uncertainty, for each paired skin and parchment sample, the isotopic data were normalised such that x̄skin = 0, and all replicates (skin and parchment) were reported as the difference relative to this value. The difference between these two groups was determined using an Independent Samples t-test. The difference between Δ(parchment-skin) values produced following Method 1 and Method 2 was analysed using a Mann–Whitney U test.
4. Results
Isotopic and elemental composition results are reported in and presented in (Method 1) and (Method 2). Results of statistical tests are presented in and .
Figure 3. Comparison of stable isotope values from skin and parchment produced using Method 1, (a) δ13C, (b) δ15N. Solid line = Linear trend line; Dashed line = 1:1
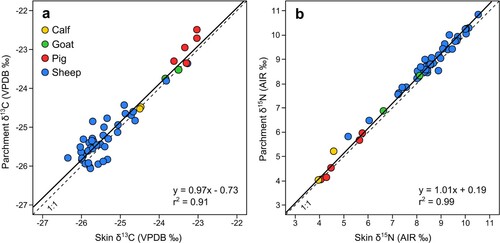
Figure 4. Comparison of stable isotope values from skin and parchment produced using Method 2, (a) δ13C, (b) δ15N. Solid line = Linear trend line; Dashed line = 1:1
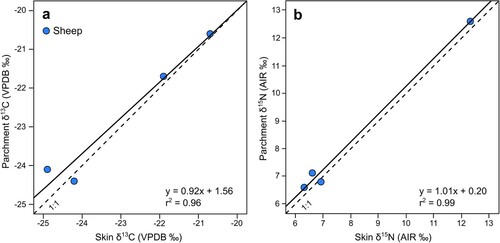
Table 2. δ13C and δ15N isotope and elemental composition of skin and parchment samples.
Table 3. Significant differences in isotope values between skin and parchment (Wilcoxon signed-rank test for paired samples).
Table 4. Significant differences in parchment to skin offset in sheep between Method 1 (Historic) and Method 2 (Modern) (Mann–Whitney U for two independent samples).
4.1. Collagen quality indicators in skin and parchment
Average collagen yields of 70% and 63% were obtained from skin and parchment, respectively, consistent with collagen constituting around 90% of the protein fraction in skin (Wenstrup, Murad, and Pinnell Citation1991). These are comparable with those reported by Brock (Brock Citation2013) from historic parchment and far greater than the 2–4% threshold applied to bone to identify problematic samples (van Klinken Citation1999; DeNiro and Weiner Citation1988). While acknowledging the varying sample sizes between species, average collagen yields in skin were highest in goats (82%), then calves (78%) and sheep (70%) and lowest in pigs (63%); a trend that matches the decreasing density of the dermal fibre network and increasing proportion of cutaneous lipids in these species (Reed Citation1973; Covington Citation2009).
Collagen yields were on average 7% lower in parchment than in skin. This is surprising as the removal of non-structural proteins during liming is thought to result in parchment being around 95% collagen (Kennedy and Wess Citation2008). Skin is heterogeneous and minor differences are to be expected between various locations, but this reduction may indicate a degree of collagen loss and damage associated with sample preparation. The greatest differences were seen in a stillborn lamb (SH09, 22% lower) and a 2-day old lamb (SH10, 32% lower) which may have been more susceptible to damage due to the higher proportion of finer type III collagen fibres in foetal skin (Epstein and Munderloh Citation1978). Brock (Citation2013) similarly observed that collagen extraction resulted in the lowest yields of any pretreatment method (47–71%), although produced consistently good C:N ratios.
In skin, %C ranged from 41.4 to 46.1, and 41.6 to 44.9 in parchment, and %N ranged from 15.1 to 16.8 in skin and 15.6 to 16.1 in parchment, consistent with those reported in modern type I collagen dominated tissues (Ambrose Citation1990; DeNiro Citation1985). Samples produced C:N ratios ranging from 2.9 to 3.5, with an average of 3.2 (2.9–3.4) in skin, and 3.2 (3.2–3.4) in parchment, with 16 (31%) skins showing higher ratios after processing, 12 (24%) lower, and 23 (45%) showing no difference. Of those that showed an increased ratio, it was often accompanied by an enrichment in 15N. A number of samples presented C:N ratios greater than 3.2, a ratio indicative of where all asparagine and glutamine amino acids have deamidated. Due to the improbability of this scenario, it may point to the presence of additional proteins (particularly elastin) or residual lipids.
4.2. Impact of production
The processing of skin to parchment resulted in a mean enrichment of 0.26‰ in 15N across both production methods (standard deviation of 0.19, and standard error of 0.03), which was statistically significant (P ≤ 0.001) (). Of the four species processed following Method 1, calfskin displayed the greatest enrichment (mean: +0.30‰), followed by sheepskin (mean: +0.29‰) and goatskin (mean: +0.20‰) with a negligible difference in pigskins (mean: +0.04‰). Of these, only the difference in sheepskin was statistically significant (P ≤ 0.001). There is no significant difference in the Δ15N(parchment-skin) offset between sheepskin processed following Method 1 or 2 (P = 0.98) ().
The impact of production on carbon values was more variable, although resulted in a mean enrichment of 0.12‰ in 13C across both production methods (standard deviation of 0.31, and standard error of 0.04), which was statistically significant (P = 0.005). Within the species manufactured following Method 1, pig (mean: +0.28‰) and sheepskin (mean: +0.12‰) showed a mean enrichment with processing, while goat (mean: −0.04‰) and calfskin (mean: −0.03‰) showed a negligible mean difference. As with nitrogen values, only the difference in sheepskin is statistically significant (P = 0.038). There is no significant difference in the Δ13C(parchment-skin) offset between sheepskin processed following Method 1 or 2 (P = 0.76).
The mean difference between skin and parchment carbon values is smaller than the estimated analytical uncertainty, while the difference in nitrogen values is only slightly greater. To incorporate this uncertainty within statistical testing, replicates were normalised to the mean value of the corresponding skin () and the difference between these groups analysed through an Independent Samples t-test. Despite the small mean differences, skin and parchment carbon (t = 2.84, df = 124.18, P = 0.005) and nitrogen values (t = 10.34, df = 117.19, P ≤ 0.001) differ significantly from each other. As with the paired samples, this difference is only significant within sheepskins (carbon: t = 3.73, df = 101.01, P ≤ 0.001; nitrogen: t = 9.15, df = 88.27, P ≤ 0.001).
5. Discussion
The observed variation between skin and parchment δ13C and δ15N values, as well as C:N ratios is likely the result of a range of factors resulting from changes made to the structure and chemistry of the skin during parchment production.
5.1. Impact of amide side chain hydrolysis
Forty-five of the 51 skins (88%) showed a small, but consistent 15N-enrichment after processing, 24 (49%) of which were greater than analytical error (0.2‰). This is surprising, given that the most probable mechanism for elevated δ15N values in parchment is the loss of the side chain-N. Asn side chain-N is significantly enriched in 15N relative to peptide-N (average Δside-peptide = +11‰) although is less significant for Gln (average Δside-peptide = +3‰) (Sacks and Brenna Citation2005) reflecting its central role in N metabolism. Thirty-one skins (61%) showed a 13C-enrichment, of which nineteen (37%) were greater than experimental error. These results are suggestive of the kinetic isotope effect associated with peptide bond hydrolysis, preferentially eliminating the isotopically lighter nitrogenous compounds.
During liming, the amide functional groups of asparagine (Asp) and glutamine (Gln) are converted into carboxyl groups through alkaline hydrolysis, producing, respectively, aspartic acid:
and glutamic acid:
liberating carbon dioxide and ammonia. This deamidation reaction forms part of the controlled damage collagen undergoes during processing, and is essential in lowering the iso-electric point of collagen, swelling the skin and aiding the removal of non-collagenous proteins (Covington Citation2009). The rate of hydrolysis increases with prolonged liming as the rigidity of the collagen backbone decreases, making the partially deamidated collagen susceptible to further damage (van Duin and Collins Citation1998; Collins, Waite, and van Duin Citation1999). In less than 24 h of liming, around 50% of all available side chains are hydrolysed (Menderes et al. Citation1999). Cleavage of the carbon–nitrogen bond during hydrolysis has been shown to favour peptide bonds containing the lighter isotopes 12C and 14N, leading to a retention and enrichment of the heavier isotopes (Macko et al. Citation1986; Macko, Fogel-Estep, and Hare Citation1987; Bada, Schoeninger, and Schimmelmann Citation1989; Silfer, Engel, and Macko Citation1992; McClelland and Montoya Citation2002; Chikaraishi et al. Citation2007; Chikaraishi et al. Citation2009; Miura and Goto Citation2012). Bada, Schoeninger, and Schimmelmann (Citation1989), for example, observed a ∼7‰ 15N-enrichment on bovine collagen after 30% hydrolysis. This enrichment has been observed in deamidation associated with the transfer of amino acids from diet to consumer (Chikaraishi et al. Citation2007; Chikaraishi et al. Citation2009; Miura and Goto Citation2012; Hare et al. Citation1991; Popp et al. Citation2007) and in the archaeological degradation of proteins (Dent, Forbes, and Stuart Citation2004; von Holstein Citation2014). Sheepskins processed using Method 2 were only in lime for 24 h, but the parchment to skin offset is not significantly different from sheepskins processed using Method 1 which had been in lime for at least 6 days (). Further 15N-enrichment may occur during the hydrolysis of arginine residues which has shown a preferential bias for releasing the lighter isotope during the urea cycle (Ambrose Citation2002), although during liming <3% of arginine residues are likely to be converted (Bowes and Kenten Citation1948; Jones Citation2004).
5.2. Removal of keratinous hair and epidermis
During production, the keratinous hair, wool and epidermis layer of the skin are removed chemically and mechanically. This has the potential to influence the isotopic value of the resulting parchment due to the isotopic disparity between keratin and collagen (Tieszen and Fagre Citation1993; O’Connell et al. Citation2012; von Holstein et al. Citation2013). Hair and wool fibres are made predominantly from keratin, which relative to collagen contains less glycine and proline and higher levels of cystine and tyrosine (Robbins Citation2012). This high cystine content results in an abundance of disulfide bonds, producing a “hard” keratin, as in nails and horn. The epidermis is composed predominantly of “soft” epithelial keratins, which have lower cystine and higher methionine and glycine content than hair keratin (Bieńkiewicz Citation1983; Fuchs Citation1983). Keratin constitutes <2% of the total composition of the skin (excluding the hair/wool), and in principle is entirely removed during production due to the different behaviour of collagen and keratin during liming (Bieńkiewicz Citation1983). At high pH, values the disulfide bonds undergo hydrolysis, dissolving the prekeratinised base of the hair, so that it is held by friction alone, and also weakens the epidermis (Covington Citation2009). This chemical attack is followed by the mechanical removal of the hair and epidermis during dehairing and shaving, with the resulting parchment made predominantly from the collagen-rich dermal/corium layer. The opening-up of the collagen fibres during liming, further results in the removal of non-collagenous components of the skin (such as non-structural proteins and lipids), increasing the relative proportion of collagen in parchment.
The isotopic relationship between collagen and keratin has been examined in a number of studies (O’Connell et al. Citation2012; von Holstein et al. Citation2013; DeNiro and Epstein Citation1978; O’Connell et al. Citation2001; Codron et al. Citation2012). Both are typically enriched over diet, with collagen enriched over keratin in both δ13C (0–4‰) and δ15N (0–2‰). In sheep, von Holstein et al. (Citation2013) noted that collagen was enriched in 13C over keratin by 2–2.7‰, but δ15N differences were within experimental error. Isotopic variation between the two tissues is largely due to differences in the routing and composition of amino acids (von Holstein et al. Citation2013; O’Connell et al. Citation2001). Previous analysis has, however, been conducted on “hard” keratins, and due to the higher glycine content of epithelial keratins, it is likely that this isotopic variation between “soft” keratin and collagen is less pronounced. All visible signs of keratinous tissue were removed from the skin sample prior to analysis, but it is possible that further removal during parchment production contributed to the observed enrichment.
5.3. Removal of lipids and non-collagenous proteins
The saponification of lipids during liming reduces the amount of 13C-depleted lipids, potentially causing a 13C-enrichment of parchment δ13C values. Lipids are isotopically lighter in δ13C than the protein component of animal tissue, and in ecological studies are typically removed through lipid extraction prior to analysis due to their impact on bulk isotope ratios (Guiry et al. Citation2016; Medeiros et al. Citation2015; Elliott, Roth, and Crook Citation2017). During liming, fatty acid esters undergo hydrolysis and are leached out into the lime solution, with as much as 50% of the total lipid content of skin removed (Koppenhoefer Citation1938; Koppenhoefer Citation1939). Lipids are likely to be lost during further deliming and shaving; however, sheepskin parchment can still retain high levels of lipids (Ghioni et al. Citation2005). Pollard and Brock (Pollard and Brock Citation2011) noted an enrichment in 13C in defatted parchment relative to that which had not undergone lipid extraction, although in this analysis lipids were extracted from both the fresh skin and parchment prior to analysis, reducing the influence they may have. Non-collagenous proteins are also removed during processing, reducing the amount of basic amino acids (arginine, lysine, and histidine), resulting in a proportional increase in glycine, which is enriched in 13C relative to other amino acids (McMahon et al. Citation2015). Therefore, purification of the collagen substrate during processing may too influence the resulting bulk isotope values.
5.4. C:N ratios
The hydrolysis of asparagine and glutamine residues during liming and the subsequent loss of nitrogen is the likely cause of elevated C:N ratios seen in 16 skins after processing. Of those with ratio >3.2, deamidation is likely the most important factor as the complete conversion of Gln and Asn is likely to result in a ratio of 3.22, although may be due in part to the presence of carbon-rich keratin or lipids. These results caution the interpretation of results from skin with values >4 (Iacumin et al. Citation1996; Iacumin et al. Citation1998; Badea et al. Citation2012) and may indicate the presence of resins, waxes or oils applied during mummification (Cockitt, Lamb, and Metcalfe Citation2020).
6. Conclusion
The structural and chemical modifications made to skin during parchment production typically results in a small enrichment in both 13C and 15N, although below the commonly cited 1‰ level of variation likely to impact interpretation (Sealy et al. Citation2014). These results confirm Campana et al. (Citation2010) and Pollard and Brock (Citation2011) hypothesis that production alters the isotopic value of the skin, although is unlikely to be the cause of the high δ15N values observed in both of these studies. Measuring the amino acid composition of paired samples could clarify the factors driving this mean enrichment, although it is likely a combination of factors, including the deamidation preferentially removing the lighter 12C and 14N isotopes, saponification removing 13C-depleted lipids, and removal of the relatively depleted keratin component during liming and shaving.
This study confirms the potential for parchment to provide valuable time-sensitive insights for paleodietary (baselines) and paleoenvironmental studies for the historic period. With no statistically significant difference in the offset from skin to parchment manufactured using historic or modern techniques, parchment from the eighth to twenty-first century offers an exceptional resource for isotopically exploring historic animal/land management, craft and trade.
Acknowledgments
Thanks go to Cluny Chapman and Sue Hatton for supplying skins, and to Jesse Meyer, Pergamena, for supplying the skin and parchment used in Method 2. SD and MJC conceived and designed the analysis. SD and JV manufactured the parchment. SD undertook the collagen extractions. SD and JN performed the stable isotope analysis. SD, MMA and MJC analysed the data. SD wrote the paper with revisions, contributions and comments from all other authors. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Sean Doherty
Sean Doherty is a Postdoctoral Research Fellow at the Department of Archaeology, University of Exeter. He specialises in examining human-animal-environment interactions through the synthesis of biomolecular, zooarchaeological and historical research.
Michelle M. Alexander
Michelle Alexander is a Senior Lecturer in Bioarchaeology at the University of York. She specialises in the application of bioarchaeological techniques to understand major socio-cultural/economic transitions and human-animal dynamics in the historical period.
Jiří Vnouček
Jiří Vnouček is a conservator at the Royal Library, Copenhagen, and previously the Head of Conservation at The National Library in Prague, Czech Republic. He holds a PhD from the University of York in the visual assessment of parchment, which drew upon his 20 years’ experience in conservation and parchment production.
Jason Newton
Jason Newton is head of NERC Life Sciences Mass Spectrometry Facility (LSMSF) in East Kilbride. He specialises in the ecological and environmental applications of stable isotope analysis, particular how stable isotopes may be used to elucidate food webs.
Matthew J. Collins
Matthew Collins is the Niels Bohr Professor of Palaeoproteomics at the Globe Institute’s Section for Evogenomics at the University of Copenhagen, and the McDonald Professor of Palaeoproteomics at the McDonald Institute for Archaeological Research of the University of Cambridge. He specialises in the persistence of proteins in ancient samples, using modelling to explore the racemization of amino acids and thermal history to predict the survival of DNA and other molecules.
References
- Ambrose, S. H. 1990. “Preparation and Characterization of Bone and Tooth Collagen for Isotopic Analysis.” Journal of Archaeological Science 17: 431–451.
- Ambrose, S. H. 2002. “Controlled Diet and Climate Experiments on Nitrogen Isotope Ratios of Rats.” In Biogeochemical Approaches to Paleodietary Analysis, edited by S. H. Ambrose and M. A. Katzenberg, 243–259. Boston, MA: Springer.
- Bada, J. L., M. J. Schoeninger, and A. Schimmelmann. 1989. “Isotopic Fractionation During Peptide Bond Hydrolysis.” Geochimica et Cosmochimica Acta 53: 3337–3341.
- Badea, E., D. V. Poulsen Sommer, K. Mühlen Axelsson, R. Larsen, A. Kurysheva, L. Miu, and G. Della Gatta. 2012. “Damage Ranking of Historic Parchment: From Microscopic Studies of Fibre Structure to Collagen Denaturation Assessment by Micro DSC.” Preservation Science 9: 97–109.
- Basha, W. A., A. T. Chamberlain, M. E. Zaki, W. A. Kandeel, and N. H. Fares. 2016. Diet Reconstruction Through Stable Isotope Analysis of Ancient Mummified Soft Tissues from Kulubnarti (Sudanese nubia).” Journal of Archaeological Science: Reports 5: 71–79.
- Bergamo, T. F., S. Botta, and M. Copertino. 2016. “Lipid Extraction in Stable Isotope Analyses of Juvenile Sea Turtle Skin and Muscle.” Aquatic Biology 25: 1–6.
- Bieńkiewicz, J. K. 1983. Physical Chemistry of Leather Making. Malabar, FL: Krieger.
- Bowes, J. H., and R. H. Kenten. 1948. “The Effect of Alkalis on Collagen.” Biochemical Journal 43: 365–372.
- Brock, F. 2013. “Radiocarbon Dating of Historical Parchments.” Radiocarbon 55 (2): 353–363.
- Browning, N. E., C. Dold, J. I-Fan, and G. A. J. Worthy. 2014. “Isotope Turnover Rates and Diet-Tissue Discrimination in Skin of Ex Situ Bottlenose Dolphins (Tursiops truncatus).” Journal of Experimental Biology 217: 214–221.
- Burjanadze, T. V. 1982. “Evidence for the Role of 4-Hydroxyproline Localized in the Third Position of the Triplet (Gly-X-Y) in Adaptational Changes of Thermostability of a Collagen Molecule and Collagen Fibrils.” Biopolymers 21: 1489–1501.
- Campana, M. G., M. A. Bower, M. J. Bailey, F. Stock, T. C. O’Connell, C. J. Edwards, C. Checkley-Scott, B. Knight, M. Spencer, and C. J. Howe. 2010. “A Flock of Sheep, Goats and Cattle: Ancient DNA Analysis Reveals Complexities of Historical Parchment Manufacture.” Journal of Archaeological Science 37 (6): 1317–1325.
- Chikaraishi, Y., Y. Kashiyama, N. O. Ogawa, H. Kitazato, and N. Ohkouchi. 2007. “Metabolic Control of Nitrogen Isotope Composition of Amino Acids in Macroalgae and Gastropods: Implications for Aquatic Food Web Studies.” Marine Ecology Programme Series 342: 85–90.
- Chikaraishi, Y., N. O. Ogawa, Y. Kashiyama, Y. Takano, H. Suga, A. Tomitani, H. Miyashita, H. Kitazato, and N. Ohkouchi. 2009. “Determination of Aquatic Food-web Structure Based on Compound-Specific Nitrogen Isotopic Composition of Amino Acids.” Limnology and Oceanography: Methods 7 (11): 740–750.
- Cockitt, J., A. Lamb, and R. Metcalfe. 2020. “An Ideal Solution? Optimising Pretreatment Methods for Artificially Mummified Ancient Egyptian Tissues.” Rapid Communications in Mass Spectrometry 34: e8686.
- Codron, D., M. Sponheimer, J. Codron, I. Newton, J. L. Lanham, and M. Clauss. 2012. “The Confounding Effects of Source Isotopic Heterogeneity on Consumer-Diet and Tissue-Tissue Stable Isotope Relationships.” Oecologia 169: 939–953.
- Collins, M. J., E. R. Waite, and A. C. van Duin. 1999. “Predicting Protein Decomposition: The Case of Aspartic-Acid Racemization Kinetics.” Philosophical Transactions of the Royal Society B: Biological Sciences 354: 51–64.
- Colonese, A. C., T. Farrell, A. Lucquin, D. Firth, S. Charlton, H. K. Robson, M. A. Alexander, and O. E. Craig. 2015. “Archaeological Bone Lipids as Palaeodietary Markers.” Rapid Communications in Mass Spectrometry 29: 611–618.
- Corr, L. T., M. P. Richards, C. Grier, A. Mackie, O. Beattie, and R. P. Evershed. 2009. “Probing Dietary Change of the Kwädąy Dän Ts’ìnchį Individual, an Ancient Glacier Body from British Columbia: II. Deconvoluting Whole Skin and Bone Collagen δ13C Values via Carbon Isotope Analysis of Individual Amino Acids.” Journal of Archaeological Science 36: 12–18.
- Covington, A. D. 2009. Tanning Chemistry: The Science of Leather. London: Royal Society of Chemistry.
- DeNiro, M. J. 1985. “Postmortem Preservation and Alteration of in Vivo Bone Collagen Isotope Ratios in Relation to Palaeodietary Reconstruction.” Nature 317: 806–809.
- DeNiro, M. J., and S. Epstein. 1977. “Mechanism of Carbon Isotope Fractionation Associated with Lipid Synthesis.” Science 197: 261–263.
- DeNiro, M. J., and S. Epstein. 1978. “Influence of Diet on the Distribution of Carbon Isotopes in Animals.” Geochimica et Cosmochimica Acta 42 (5): 495–506.
- DeNiro, M. J., and S. Weiner. 1988. “Chemical, Enzymatic and Spectroscopic Characterization of “Collagen” and Other Organic Fractions from Prehistoric Bones.” Geochimica et Cosmochimica Acta 52: 2197–2206.
- Dent, B. B., S. L. Forbes, and B. H. Stuart. 2004. “Review of Human Decomposition Processes in Soil.” Environmental Geology 45: 576–585.
- Elliott, K. H., J. D. Roth, and K. Crook. 2017. “Lipid Extraction Techniques for Stable Isotope Analysis and Ecological Assays.” Methods in Molecular Biology 1609: 9–24.
- Epstein, E. H., and N. H. Munderloh. 1978. “Human Skin Collagen. Presence of Type I and Type III at all Levels of the Dermis.” Journal of Biological Chemistry 253: 1336–1337.
- Ferraz, T. P. L., M. C. Fiúza, M. L. A. Dos Santos, L. Pontes De Carvalho, and N. M. Soares. 2004. “Comparison of Six Methods for the Extraction of Lipids from Serum in Terms of Effectiveness and Protein Preservation.” Journal of Biochemical and Biophysical Methods 58: 187–193.
- Finucane, B. C. 2007. “Mummies, Maize, and Manure: Multi-Tissue Stable Isotope Analysis of Late Prehistoric Human Remains from the Ayacucho Valley, Perú.” Journal of Archaeological Science 34 (12): 2115–2124.
- Fuchs, E. 1983. “Evolution and Complexity of the Genes Encoding the Keratins of Human Epidermal Cells.” Journal of Investigative Dermatology 81: 141–144.
- Ghioni, C., J. C. Hiller, C. J. Kennedy, A. E. Aliev, M. Odlyha, M. Boulton, and T. Wess. 2005. “Evidence of a Distinct Lipid Fraction in Historical Parchments: A Potential Role in Degradation?” Journal of Lipid Research 46: 2726–2734.
- Guiry, E. J., S. Needs-Howarth, K. D. Friedland, A. L. Hawkins, P. Szpak, R. Macdonald, M. Courtemanche, E. Holm, and M. P. Richards. 2016. “Lake Ontario Salmon (Salmo salar) Were Not Migratory: A Long-Standing Historical Debate Solved Through Stable Isotope Analysis.” Scientific Reports 6: 36249.
- Haines, B. M. 1990. Parchment: the Physical and Chemical Characteristics of Parchment and the Materials Used in its Conservation. Northampton: Leather Conservation Centre.
- Hare, P., M. L. Fogel, T. W. Stafford, A. D. Mitchell, and T. C. Hoering. 1991. “The Isotopic Composition of Carbon and Nitrogen in Individual Amino Acids Isolated from Modern and Fossil Proteins.” Journal of Archaeological Science 18: 277–292.
- Hedges, R. E. M., I. A. Law, C. R. Bronk, and R. A. Housley. 1989. “The Oxford Accelerator Mass Spectrometry Facility: Technical Developments in Routine Dating.” Archaeometry 31: 99–113.
- Iacumin, P., H. Bocherens, L. Chaix, and A. Marioth. 1998. Stable Carbon and Nitrogen Isotopes as Dietary Indicators of Ancient Nubian Populations (Northern Sudan).” Journal of Archaeological Science 25 (4): 293–301.
- Iacumin, P., H. Bocherens, A. Mariotti, and A. Longinelli. 1996. “An Isotopic Palaeoenvironmental Study of Human Skeletal Remains from the Nile Valley.” Palaeogeography, Palaeoclimatology, Palaeoecology 126: 15–30.
- Jones, B. E. 2004. “Gelatin: Manufacture and Physico-Chemical Properties.” In Pharmaceutical Capsules, edited by F. Podczeck and B. E. Jones, 23–60. London: Pharmaceutical Press.
- Kennedy, C. J., and T. J. Wess. 2008. “The Structure of Collagen Within Parchment – A Review.” Restaurator: International Journal for the Preservation of Library and Archival Material 24: 61–80.
- Kiljunen, M., J. Grey, T. Sinisalo, C. Harrod, H. Immonen, and R. I. Jones. 2006. “A Revised Model for Lipid-Normalizing δ13C Values from Aquatic Organisms, with Implications for Isotope Mixing Models: Revised Lipid-Normalization Model for C Isotope Analysis.” Journal of Applied Ecology 43: 1213–1222.
- Koppenhoefer, R. M. 1938. “The Lipids of Sheepskins. I. Lipid of Fresh Sheepskin.” Journal of the American Leather Chemists Association 33: 203–215.
- Koppenhoefer, R. M. 1939. “The Lipids of Sheepskins. II. The Effect of Pullery Processes on the Lipids of Sheep Skin.” Journal of the American Leather Chemists Association 34: 100–112.
- Lamb, A. L. 2015. “Stable Isotope Analysis of Soft Tissues from Mummified Human Remains.” Environmental Archaeology 21: 271–284.
- Liden, K., C. Takahashi, and D. E. Nelson. 1995. “The Effects of Lipids in Stable Carbon Isotope Analysis and the Effects of NaOH Treatment on the Composition of Extracted Bone Collagen.” Journal of Archaeological Science 22: 321–326.
- Logan, J. M., T. D. Jardine, T. J. Miller, S. E. Bunn, R. A. Cunjak, and M. E. Lutcavage. 2008. “Lipid Corrections in Carbon and Nitrogen Stable Isotope Analyses: Comparison of Chemical Extraction and Modelling Methods.” Journal of Animal Ecology 77: 838–846.
- Macko, S. A., M. L. Fogel-Estep, M. H. Engel, and P. E. Hare. 1986. “Kinetic Fractionation of Stable Isotopes During Amino Acid Transamination.” Geochimica et Cosmochimica Acta 50: 2143–2146.
- Macko, S. A., M. L. Fogel-Estep, and P. E. Hare. 1987. “Isotopic Fractionation of Nitrogen and Carbon in the Synthesis of Amino Acids by Microorganisms.” Geography (Sheffield, England) 65: 79–92.
- McClelland, J. W., and J. P. Montoya. 2002. “Trophic Relationships and the Nitrogen Isotopic Composition of Amino Acids in Plankton.” Ecology 83: 2173–2180.
- McMahon, K. W., M. J. Polito, S. Abel, M. D. McCarthy, and S. R. Thorrold. 2015. “Carbon and Nitrogen Isotope Fractionation of Amino Acids in an Avian Marine Predator, the Gentoo Penguin (Pygoscelis papua).” Ecology and Evolution 5: 1278–1290.
- Medeiros, L., D. da Silveira Monteiro, R. Petitet, and L. Bugoni. 2015. “Effects of Lipid Extraction on the Isotopic Values of Sea Turtle Bone Collagen.” Aquatic Biology 23: 191–199.
- Menashi, S., A. Finch, P. J. Gardner, and D. A. Ledward. 1976. “Enthalpy Changes Associated with the Denaturation of Collagens of Different Imino Acid Content.” Biochimica et Biophysica Acta 444: 623–625.
- Menderes, O., A. D. Covington, E. R. Waite, and M. J. Collins. 1999. “The Mechanism and Effects of Collagen Amide Group Hydrolysis During Liming.” Journal of the Society of Leather Technologists and Chemists 83: 107–110.
- Miura, K., and A. S. Goto. 2012. “Stable Nitrogen Isotopic Fractionation Associated with Transamination of Glutamic Acid to Aspartic Acid: Implications for Understanding 15N Trophic Enrichment in Ecological Food Webs.” Research in Organic Geochemistry 28: 13–17.
- Možir, A., I. K. Cigić, M. Marinšek, and M. Strlič. 2014. “Material Properties of Historic Parchment: A Reference Collection Survey.” Studies in Conservation 59 (3): 136–149.
- O’Connell, T. C., R. E. M. Hedges, M. A. Healey, and A. H. R. W. Simpson. 2001. “Isotopic Comparison of Hair, Nail and Bone: Modern Analyses.” Journal of Archaeological Science 28: 1247–1255.
- O’Connell, T. C., C. J. Kneale, N. Tasevska, and G. G. C. Kuhnle. 2012. “The Diet-Body Offset in Human Nitrogen Isotopic Values: A Controlled Dietary Study.” American Journal of Physical Anthropology 149: 426–434.
- Pestle, W. J., B. E. Crowley, and M. T. Weirauch. 2014. “Quantifying Inter-Laboratory Variability in Stable Isotope Analysis of Ancient Skeletal Remains.” PLoS One 9: e102844.
- Pollard, A. M., and F. Brock. 2011. “Provenancing Parchment, Leather and Paper Using Stable Isotopes.” In The Technological Study of Books and Manuscripts as Artefacts, edited by S. Neate, D. Howell, R. Ovenden, and A. M. Pollard, 85–90. London: BAR International Series.
- Popp, B. N., B. S. Graham, R. J. Olson, C. C. S. Hannides, M. J. Lott, G. A. López-Ibarra, F. Galván-Magaña, and B. Fry. 2007. “Insight Into the Trophic Ecology of Yellowfin Tuna, Thunnus Albacares, from Compound-Specific Nitrogen Isotope Analysis of Proteinaceous Amino Acids.” In Stable Isotopes as Indicators of Ecological Change, edited by T. E. Dawson and R. T. W. Siegwolf, 173–190. Amsterdam: Elsevier.
- Reed, R. 1973. Ancient Skins, Parchments and Leather. London: Seminar Press.
- Robbins, C. R. 2012. Chemical and Physical Behaviour of Human Hair. 5th ed. New York: Springer.
- Ryder, M. L. 2009. “Parchment – Its History, Manufacture and Composition.” Journal of the Society of Archivists 2 (9): 391–399.
- Sacks, G. L., and J. T. Brenna. 2005. “15N/14 N Position-Specific Isotopic Analyses of Polynitrogenous Amino Acids.” Analytical Chemistry 77: 1013–1019.
- Saxl, H. 1954. “An Investigation of the Qualities, the Methods of Manufacture and the Preservation of Historic Parchment and Vellum with a View to Identifying the Animal Species Used.” MSc thesis, University of Leeds.
- Schoeninger, M. J., and M. J. DeNiro. 1984. “Nitrogen and Carbon Isotopic Composition of Bone Collagen from Marine and Terrestrial Animals.” Geochimica et Cosmochimica Acta 48: 625–639.
- Sealy, J., M. Johnson, M. Richards, and O. Nehlich. 2014. “Comparison of two Methods of Extracting Bone Collagen for Stable Carbon and Nitrogen Isotope Analysis: Comparing Whole Bone Demineralization with Gelatinization and Ultrafiltration.” Journal of Archaeological Science 47: 64–69.
- Silfer, J. A., M. H. Engel, and S. A. Macko. 1992. Kinetic Fractionation of Stable Carbon and Nitrogen Isotopes During Peptide Bond Hydrolysis: Experimental Evidence and Geochemical Implications.” Chemical Geology: Isotope Geoscience Section 101 (3–4): 211–221.
- Strlič, M., I. K. Cigić, I. Rabin, J. Kolar, B. Pihlar, and M. Cassar. 2009. “Autoxidation of Lipids in Parchment.” Polymer Degradation and Stability 94 (6): 886–890.
- Szpak, P., J. Z. Metcalfe, and R. A. MacDonald. 2017. “Best Practices for Calibrating and Reporting Stable Isotope Measurements in Archaeology.” Journal of Archaeological Science: Reports 13: 609–616.
- Terajima, M., I. Perdivara, M. Sricholpech, Y. Deguchi, N. Pleshko, K. B. Tomer, and M. Yamauchi. 2014. “Glycosylation and Cross-Linking in Bone Type I Collagen.” Journal of Biological Chemistry 289: 22636–22647.
- Tieszen, L. L., and T. Fagre. 1993. “Effect of Diet Quality and Composition on the Isotopic Composition of Respiratory CO2, Bone Collagen, Bioapatite, and Soft Tissues.” In Prehistoric Human Bone: Archaeology at the Molecular Level, edited by J. B. Lambert, and G. Grupe, 121–155. Berlin/Heidelberg: Springer.
- van Duin, A. C. T., and M. J. Collins. 1998. “The Effects of Conformational Constraints on Aspartic Acid Racemisation.” Organic Geochemistry 29: 1227–1232.
- van Klinken, G. J. 1999. “Bone Collagen Quality Indicators for Palaeodietary and Radiocarbon Measurements.” Journal of Archaeological Science 26: 687–695.
- von Holstein, I. 2014. “A Light Stable Isotope (C, N, H, O) Approach to Identifying Movement of Medieval Textiles in North West Europe.” PhD thesis, University of York.
- von Holstein, I. C. C., J. Hamilton, O. E. Craig, J. Newton, and M. J. Collins. 2013. “Comparison of Isotopic Variability in Proteinaceous Tissues of a Domesticated Herbivore: a Baseline for Zooarchaeological Investigation.” Rapid Communications in Mass Spectrometry 27: 2601–2615.
- Wenstrup, R. J., S. Murad, and S. R. Pinnell. 1991. “Collagen.” In Physiology, Biochemistry, and Molecular Biology of the Skin, edited by L. A. Goldsmith, 481–508. Oxford: Oxford University Press.
- White, C. D., and H. P. Schwarcz. 1994. “Temporal Trends in Stable Isotopes for Nubian Mummy Tissues.” American Journal of Physical Anthropology 93: 165–187.