ABSTRACT
Dental calculus (calcified dental plaque) is a cross-cultural biological matrix that is emerging as a critical source of information for anthropologists and oral health professionals. It contains a multitude of diverse biomolecules, providing information about an individual’s culture, diet, ancestry, and health. Most researchers who study archaeological dental calculus use genomic or proteomic approaches, although a wide range of other techniques are now available. However, few studies have utilized efficient multiomic protocols. This lack of integration is problematic, as such approaches in other fields have proven to improve results and strengthen interpretations. Our review discusses three multiomic approaches: (1) interactions between the metaproteome and metagenome; (2) relationships between the host genome and oral metagenome; and, (3) associations between the epigenome and metagenome. We draw from multiomic studies on soil, plant, gut, and modern oral microbiomes to demonstrate how such integration can provide insights that are not attainable with single-omic approaches.
Introduction
Dental calculus is calcified dental plaque that includes host, microbial, and dietary DNA and proteins, dietary fibers, airborne and waterborne pollutants, gingival crevicular fluid, and calcium phosphate minerals (reviewed in Warinner and Lewis Citation2015; Weyrich, Dobney, and Cooper Citation2015). Many factors can impact its bacterial composition, including personal hygiene, host genetics, diet, and environmental factors (Arensburg Citation1996; Lieverse Citation1999; Al-Zahrani, Borawski, and Bissada Citation2004; Weyrich, Dobney, and Cooper Citation2015). Archaeological and epidemiological evidence show human populations worldwide develop dental calculus – the former indicating that its incidence rises with the advent of agriculture (Aufderheide, Rodríguez-Martín, and Langsjoen Citation1998), while the latter shows lower incidence with modern Industrialized oral hygiene habits (e.g. daily tooth brushing and annual teeth cleanings from a dental hygienist) (White Citation1997). The study of dental calculus has become increasingly important to archaeologists to study the past (Warinner, Speller, and Collins Citation2015). While several different types of well-preserved, high-quality biomolecules exist in dental calculus, approaches that recover all of them in tandem have yet to be established.
Technological advancements have drastically improved the ability to extract DNA, proteins, and metabolites from dental calculus. Such efforts are reshaping our understanding of past diet, behavior, ancestry, and even the health of past individuals and populations, providing significant new insights and answers to questions relevant to both archaeology and anthropology. While these achievements are noteworthy, the directions and challenges for archaeological dental calculus research in the past and present need to be addressed.
Historical overview
The study of archaeological dental calculus can point to 1975 as its beginning, when plant phytoliths were extracted from deposits found on the surface of cattle teeth and concluded that the biomaterial may have the potential for reconstructing ancient diets (Armitage Citation1975). In the following decade, scholars decalcified dental calculus deposits from ancient humans and animals, revealing that the food particles trapped within it were well-preserved (Dobney and Brothwell Citation1986). Using a scanning electron microscope (SEM), the same authors also found that microbiota were preserved within the calculus matrix (Dobney and Brothwell Citation1988), which was later supported by further evidence in other samples (Dobney Citation1994; Vandermeersch et al. Citation1994; Pap et al. Citation1995). Although these SEM studies revealed dental calculus as a potential source for studying ancient microbial communities, their techniques could not determine whether these microbes were endogenous or contaminants.
Conducting an immunohistochemical analysis with microscopy, Linossier, Gajardo, and Olavarria (Citation1996) described multiple Gram-positive and Gram-negative species preserved within dental calculus, and although their results indicated dental calculus retained ancient oral microbes, they could not confirm if their DNA remained intact. This would change, however, when a gold-labelled antibody transmission electron microscopy suggested that microbial DNA survives in archaeological samples (Preus et al. Citation2011). Further support arrived when targeted PCR-based genetic approaches recovered ancient DNA (aDNA) of S. mutans and other oral taxa (De La Fuente, Flores, and Moraga Citation2013). These early studies laid the foundations for metagenomic research on archaeological dental calculus and afforded the first glimpse into its potential explanatory power.
The PCR-based technique employed by De La Fuente and colleagues were only able to target five oral species (a tiny proportion of the at least 700 species that can inhabit the oral cavity) (Deo and Deshmukh Citation2019; Zhao et al. Citation2017). Fortunately, the advent of high-throughput sequencing (HTS) techniques (also referred to as next-generation sequencing) has enabled the recovery of millions of DNA fragments from thousands of different microbes in-parallel, overcoming the key technological limitations associated with the methods used by De La Fuente, Flores, and Moraga (Citation2013) (see Adler et al. Citation2013 and Warinner et al. Citation2017).
The first study to employ an HTS-based approach on archaeological dental calculus was undertaken by Adler et al. (Citation2013). Using 16S rRNA amplicon sequencing (), they recovered an extant oral taxonomic profile that, unlike the PCR-based methods used by De La Fuente, Flores, and Moraga (Citation2013), amplified the aDNA of thousands microbial taxa. Their groundbreaking approach illustrated that the types of oral microbial communities in individuals, dating from the Mesolithic to the present, radically shifted during the advent of agriculture and the onset of the Industrial Revolution (Adler et al. Citation2013). During the following year, another innovative study applied shotgun metagenomic sequencing to archaeological dental calculus samples from Germany (Warinner, Rodrigues, et al. Citation2014), enabling the characterization of ancient oral microbiomes, the recovery of opportunistic pathogens (and their putative antibiotic resistance genes), and the reconstruction of a draft pathogen genome associated with periodontal disease. Both studies demonstrated that it was possible to recover the DNA of ancient oral microbes from dental calculus. Since these two publications, HTS techniques applied to archaeological dental calculus have yielded significant and novel insights into paleodemography (Ozga et al. Citation2016; Eisenhofer et al. Citation2019), Neanderthal behavior and diet (Weyrich et al. Citation2017), animal oral microbiomes (Brealey et al. Citation2020), and preservation biases in ancient dental calculus (Velsko et al. Citation2019; Mann et al. Citation2018; Ziesemer et al. Citation2015; Farrer et al. Citation2018). While HTS techniques remain the most widely used omic strategy for the study of archaeological dental calculus, the number of studies applying alternative and cutting-edge omic strategies (e.g. proteomics and metabolomics) are increasing. As these technologies are deciphering new biological properties and the anthropological associations that drive their variation within the dental calculus matrix, the focus of future endeavors should be towards developing a general framework that integrates these diverse and complex lines of evidence.
Table 1. Key concepts and definitions in archaeological dental calculus analysis.
Employing multiomic strategies in ancient dental calculus research
With the number of technologies available to archaeologists multiplying, selecting which omic strategy to use becomes a critical first step. Generally speaking, only tens of milligrams of dental calculus exist on an individual, limiting the number of approaches that can be applied (Fagernäs et al. Citation2020). This amount decreases even further considering that oral geography (i.e. the type of tooth and where on the crown the dental calculus is located) influences microbial composition, and therefore, limits general comparisons between individuals (Simón-Soro et al. Citation2013; Farrer Citation2017). Furthermore, each technology has limitations and trade-offs in terms of the kind of information it can supply. For instance, PCR and HTS can only provide the study of correlations between the potential of microbial functions and their genetic variants. This limitation is not trivial, as a particular genetic variant can lead to multiple phenotypic outcomes, depending on the social and environmental context (Hasin, Seldin, and Lusis Citation2017; Org et al. Citation2015). As a result, some of these phenotypic outcomes can only be assessed by using alternative omic strategies, such as proteomics and epigenomics.
To advance the field, researchers should consider employing multiomic strategies when conducting any destructive analysis on archaeological dental calculus (). Multiomic approaches can find “hidden” biological regularities that are undetectable when employing a single omic strategy (Ebrahim et al. Citation2016). For instance, multiple research teams studied a Swedish twin cohort using 16S sequencing (Willing et al. Citation2010); metagenomics and metaproteomics (Rinke et al. Citation2013); and metabolomics (Jansson et al. Citation2009) to assess the effect of irritable bowel syndrome (IBS) on the gut microbiome. Synthesizing these omic datasets revealed differences between diseased and non-diseased patients that would not have been possible if only one approach had been used (Jansson and Baker Citation2016). This process led to the generation of new hypotheses and has led other researchers to apply similar strategies in gut (Peters et al. Citation2019), soil (Jansson and Hofmockel Citation2018), and modern oral microbiome research (Tang et al. Citation2019). While currently rare, such multiomic strategies on dental calculus have already increased the amount of information derived from a single biological sample (Fotakis et al. Citation2020).
Figure 1. Schematic illustrating a multiomic framework for the study of archaeological dental calculus.
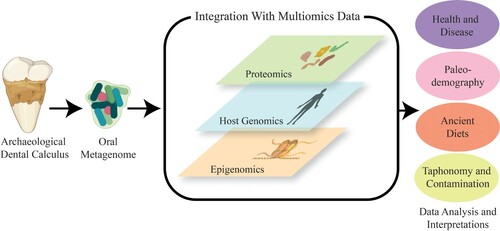
A multiomic framework for dental calculus research will encounter more issues than the fields previously mentioned. For example, multiple extractions of a single dental calculus sample has been rare, in part because many samples are too small to be subsampled (<10 mg). Commonly used protocols for the retrieval of aDNA and proteins from dental calculus are also currently incompatible (e.g. Yates et al. Citation2020; Moore and Weyrich Citation2021). DNA extraction protocols require an enzyme proteinase K which destroys many of the proteins in a sample (e.g. Dabney et al. Citation2013), whilst protein extraction protocols require heat temperatures that destroy DNA (e.g. Cappellini et al. Citation2012). In attempt to resolve this issue, two recent studies aimed to develop efficient protocols to generate both proteomic and genomic data from archaeological dental calculus specimens (Mackie et al. Citation2017; Fagernäs et al. Citation2020). The most recent study generated both genomic and proteomic data from individual samples, but their protocol resulted in 43% less DNA yield when compared to a genomic-only approach (Fagernäs et al. Citation2020).
Despite these current technological challenges, the field would greatly benefit by working towards a multiomic framework. Here, we discuss the possible insights that can come from employing multiomic strategies on human dental calculus from archaeological contexts, although it is important to note that there are dental calculus studies focusing on animal specimens (Brealey et al. Citation2020). We review the current strategies to study archaeological dental calculus, as well as draw upon the findings of multiomic approaches in other fields to explore how such efforts could improve the field. We suggest that future multiomic studies should (1) combine findings from metagenomic and metaproteomic approaches; (2) examine the relationships between the host genome and oral microbiome, and finally, (3) discuss research that has compared epigenome and microbiome data. Additional tools may be used for multiomic studies on ancient samples in the future, such as the study of microbial functions using metabolomics (Velsko et al. Citation2017); however, the application of such tools on ancient specimens is currently in its infancy and is outside the scope of this manuscript. We conclude by outlining considerations researchers should contemplate before proceeding with any destructive analysis on archaeological dental calculus specimens.
The relationships between the metaproteome and metagenome of the oral microbiome
Applying metagenomic sequencing strategies to archaeological dental calculus can retrieve the aDNA of microbial species, as well as their functional potential. However, metagenomics cannot yield information on their functional output, such as which bacteria and metabolic pathways were employed, or how the microbial networks might have modulated their environment. While RNA sequencing is used in modern contexts to explore these functions, RNA rarely survives in archaeological samples, making such analysis difficult. However, metaproteomics can be used to identify and quantify this type of information and have offered new targets and directions for dental calculus research not attainable with metagenomic data.
Within the last decade, researchers are realizing the potential of using metaproteomic approaches to study archaeological dental calculus. Metaproteomics assesses the functional activity of a microbiome sample by using mass spectrometry analysis (e.g. MS/MS). Such approaches have elucidated the relationship between the immune system of a host and their microbiome (Warinner, Rodrigues, et al. Citation2014). Metaproteomics can additionally identify whether a population consumed milk (Wilkin et al. Citation2020); which species were used for milk consumption (Warinner, Hendy, et al. Citation2014; Charlton et al. Citation2019; Jeong et al. Citation2018; Mays et al. Citation2018); the immune proteins linked to infection (Warinner, Rodrigues, et al. Citation2014); and specific peptides associated with oral health (Jersie-Christensen et al. Citation2018; Velsko et al. Citation2019). Researchers have deployed both metaproteomic and metagenomic approaches in tandem. For example, Fotakis et al. (Citation2020) have shown how integrating metagenomic and metaproteomic data can provide a novel approach to detect and authenticate the presence of specific microorganisms, including ones that are hard to detect when there are no skeletal lesions. Applying HTS and MS/MS, they were able to recover both DNA and peptides specific to Mycobacterium leprae in dental calculus.
Modern dental calculus studies further reveal how proteomic datasets can complement their genomic counterparts. For example, a metaproteomic study found that periodontal patients preferentially had the herpes virus 2 and the methylmalonyl-CoA mutase proteins (Bostanci et al. Citation2010). Overmyer et al. (Citation2020) also found that the dental plaque of periodontally diseased patients contains more host-derived proteins than their periodontally healthy control group, while Grassl et al. (Citation2016) observed that the proteins in saliva can change according to the dental hygiene practices of an individual. Such studies highlight the potential correlations researchers could investigate when applying MS strategies to archaeological dental calculus.
While the findings in Fotakis et al. (Citation2020) are promising, streamlining metaproteomic and metagenomic approaches to archaeological dental calculus is needed. Empirical evidence from the examination of both microbial proteomes and metagenomes in other biological and environmental samples (e.g. soil and plants) have reported the benefits when using both approaches. A study on the microbiome of dajiang-meju, a traditionally fermented soybean product in Northeast China, found that bacteria with high relative abundances in the metagenomic analyses, accounted for few of the proteins in the metaproteomic analyses (Xie et al. Citation2019). The metaproteomic analyses, however, indicated that most of the expressed proteins were attributed to bacteria with the lowest relative abundances in the metagenomic analyses. By integrating their separate datasets, the study provides a more detailed understanding of the microbiological processes occurring during the fermentation of dajiang-meju, affording compelling evidence for the necessity of integrating metagenomic and metaproteomic datasets.
There are several benefits to using metaproteomics, but they incur obstacles that are different from metagenomics. Metaproteomic studies face at least three major challenges (Muth et al. Citation2013): (1) Storing proteomic data can be a financial obstacle for many research groups as they require enormous amounts of computing resources (as much as two terabytes of data can be generated in a single MS/MS run) (Tariq et al. Citation2020; Heyer et al. Citation2017). This problem is compounded for multiomic projects, as HTS technologies can also generate as much as two terabytes of data in a single sequencing run (Schmidt and Hildebrandt Citation2017). With the number of dental calculus studies continuing to grow at an exponential rate, researchers will need petabytes for storage and analyses in order to perform global comparisons. (2) Protein identification and taxonomic composition estimates are difficult to compare as there remains a lack of standardization and guidelines for metaproteomic studies on the human microbiome (Zhang and Figeys Citation2019). (3) The databases used for identifying microbial proteins still need significant improvements. Identical peptides belonging to homologous proteins can be shared across multiple bacteria or archaea, which cause redundant protein identification (Herbst et al. Citation2016). For example, a peptide associated with lactate dehydrogenase (EC. 1.1.1.27) (1.1.1.27) belongs to different members of the genus Lactobacillus that ferment sugars to lactate; this peptide also belongs to some representatives in the order Clostridiales, which ferment lactate to acetate (Kohrs et al. Citation2014). In addition, the methodological incompatibility in the laboratory between metagenomic and metaproteomic approaches, such as the ones recently reported by Fagernäs et al. (Citation2020), inhibit the comparison of the two.
In summary, the integration of metagenomic and metaproteomic strategies will offer archaeological dental calculus research a wealth of information. A major goal for the field moving forward should be to continue developing laboratory approaches that can maximize the amount of DNA and proteins from a single dental calculus sample, as well as design bioinformatic tools that can integrate and analyze both datasets. Integrating metagenomic and metaproteomic profiles together may provide a more robust framework for studying the microbes that underpin oral pathologies, such as periodontitis and dental caries, in the archaeological record.
The interactions between the host genome and oral metagenome
Although many studies focus on the microbial DNA within the dental calculus matrix, few have explored the host DNA preserved within it. Host genomics refers to the study of whole genomes of organisms. Host genomics use DNA sequencing methods, and bioinformatic applications, such as assembly and statistical analysis, to analyze the structure and function of host genomes. To date, at least two studies have examined the host genome within archaeological dental calculus (Ozga et al. Citation2016; Ziesemer et al. Citation2019). Utilizing an in-solution and enrichment technique with subsequent HTS, Ozga et al. (Citation2016) reconstructed the mitochondrial genomes of pre-Columbian Native American individuals dating to circa 700 years old by sequencing DNA in their dental calculus. Applying a similar method to dental calculus samples from six spatially and temporally diverse sites, Ziesemer et al. (Citation2019) analyzed mtDNA haplogroups and sex chromosomes. Both studies report that the endogenous human DNA in these dental calculus samples accounted for a considerably small proportion (∼0.002–0.35%) of the total DNA, which is noteworthy considering the cost and applicability of these approaches. One study has sequenced paired dental calculus and tooth samples (Mann et al. Citation2018). However, their analyses focused on distinguishing DNA preservation between the two sample types, rather than investigating the links between host-genome and oral microbiome variation. Such an approach will not be applicable for all contexts because of either preservation or ethical concerns. Nevertheless, studying host genomes and oral microbiomes as covariates in appropriate contexts could increase the statistical power of dental calculus research.
Research investigating the relationships between oral microbiota and the human genome of living populations indicate that the two are highly interconnected. For example, studies have shown that host genetic factors, such as amylase, MUC7, histatins, IgA, and taste receptor genes, play a role in oral microbiome composition (Davenport Citation2017). Amylase, an enzyme that breaks down starches, exists in variable copy numbers across human populations and is connected to several bacterial species in the oral microbiome (Oppenheim et al. Citation2007; Perry et al. Citation2007), and a high copy number of salivary amylase (AMY1) is correlated with high levels of Porphyromonas (Poole et al. Citation2019). Notably, periodontitis is correlated with several members of the genus Porphyromonas (Socransky et al. Citation1998; Park et al. Citation2015; Colombo et al. Citation2012; Wade Citation2013), an interesting observation since the presence of Porphyromonas gingivalis in dental calculus has been interpreted as a biomarker for periodontal disease in ancient (Warinner, Rodrigues, et al. Citation2014) and modern individuals (Fiorillo et al. Citation2019). The mucin gene, MUC7, may also play a role in affecting oral microbiome composition, as it is linked to Neisseria abundance in supragingival plaque (Xu et al. Citation2017).
Other research supports the notion that host genetic factors can modulate susceptibility and severity of oral diseases (Nibali et al. Citation2017). Several studies report that the Vitamin D Receptor (VDR) (Shimizu et al. Citation2015; Rhodin et al. Citation2014), Fc-γRIIA, and the Interleukin-10 (IL10) (Song and Lee Citation2013) genes play a role in periodontal disease; however, the association for each gene varies among ethnic groups. The same authors also describe how other studies found that the AQP5 gene (Vieira, Hilands, and Braun Citation2015; Vieira et al. Citation2015), the estrogen related receptor beta (ESRRB) gene (Weber et al. Citation2014), and the rs7791001 SNP in the 7q22.3 gene (Sofer et al. Citation2016) may play a role in individuals developing dental caries.
Given the evidence for host-genome and microbiome interactions in living individuals, the lack of investigation into both the human and microbial DNA of respective individuals/samples may be obscuring further important information. For example, Adler et al. (Citation2013) linked apparent significant shifts over the past 7,500 years in the composition of the human oral microbiome to changes in diet, without taking into account the possible significant role that admixture and population replacement events in these European populations may have contributed (Haak et al. Citation2015; Olalde et al. Citation2018; Haak et al. Citation2005; Mathieson et al. Citation2015). Furthermore, gut microbiome studies indicate diet consistently accounts for only a small amount of microbiome variation (< 20%) (Johnson et al. Citation2019; Falony et al. Citation2016; Rothschild et al. Citation2018). Assessing whether this is the case in the oral microbiome is difficult as more studies have yet to determine whether dietary preferences and nutrients have a similar impact on composition (Ulloa, van der Veen, and Krom Citation2019). While diet is likely a significant factor, studies on living populations indicate that additional factors, such as ancestry and host genetic variants, are also important to consider and include in the analysis (Gomez et al. Citation2017; Razzouk and Termechi Citation2013).
The studies outlined above demonstrate the utility of studying host genomes along with their associated oral microbiomes. Unlike the case with integrating metaproteomic and metagenomic datasets, there are fewer issues linked with this approach since HTS can recover human and microbial DNA in tandem. While HTS can recover both types of DNA in dental calculus, it is likely that researchers will have to undertake two sets of destructive sampling – one for the dental calculus and one for the paired tooth from the same individual – in order to recover relevant data. As such, this will not only require additional destructive sampling to an individual but will also necessitate further ethical (and in some cases community) approval. However, by including human genetic variation into the analysis, additional key information will be gleaned regarding the coevolutionary relationship between oral microbes and human variation.
The associations between the epigenome and oral microbiome
No studies involving archaeological dental calculus have yet investigated the host or microbial epigenomes. Epigenetics refers to the study of the level of expression of genes that can be influenced by genetic and environmental factors. Epigenetic alterations in microbes can generate a panoply of strategies to adapt when exposed to various environmental settings (Billmyre and Heitman Citation2017). For instance, the etiological agent of malaria, Plasmodium falciparum, is able to grow in multiple host environments by epigenetically silencing portions of redundant gene sets in a process called clonally variant gene expression (Scherf et al. Citation1998). In eukaryotes, epigenetics can provide insights into how a host responds to disease (Feinberg Citation2018), nutrition (Tiffon Citation2018), and stress (Thayer and Non Citation2015). For example, epigenetic modifications associated with animal morphology (Waterland and Jirtle Citation2003; Waterland et al. Citation2006), have been linked to climate fluctuations (Guthrie Citation2003; Martin, Mead, and Barboza Citation2018). The two major factors for epigenetic modifications observed in eukaryotic cells are DNA methylation – the process where a methyl group is added to cytosines within CPG regions (Sanders Citation2006) – and histone deacetylation – the process where the removal of an acetyl group leads to the alteration of DNA wrapping around histones (Shaw Citation2006). Epigenome alterations can be induced by several factors that can include age, sex, ethnicity, lifestyle, diabetes, and obesity (Razzouk and Termechi Citation2013; Mathers, Strathdee, and Relton Citation2010), all of which can lead to or are the result of phenotypic changes (Jablonka Citation2013). Both DNA methylation and histone acetylation can regulate the gene expression of inflammatory mediators for a host (Fitzpatrick and Wilson Citation2003; McCall et al. Citation2010; Reiner Citation2005; Viana et al. Citation2011; De Oliveira et al. Citation2011; Zhang et al. Citation2010) and are detectable in the archaeological record (Liu, Weyrich, and Llamas Citation2020).
Current paleoepigenetic studies have explored epigenetic markers within several human biomaterials, including hair, bone, and teeth (Orlando, Gilbert, and Willerslev Citation2015; Orlando and Willerslev Citation2014; Pedersen et al. Citation2014; Briggs et al. Citation2010); none have systematically tested whether it is possible to explore bacterial epigenetic profiles within dental calculus. Microbial epigenomes are equally worth pursuing, as the study of the relationships between epigenomes and microbiomes in living populations have yielded fruitful insights (Allen and Sears Citation2019; Chen, Sun, and Zhang Citation2017; Fofanova, Petrosino, and Kellermayer Citation2016). The first archaeological epigenetic study analyzed the remains of a Pleistocene bison (Llamas et al. Citation2012). More recent archaeological epigenetic studies have focused on the analysis of DNA methylation from Native American skeletal remains (Smith, Monroe, and Bolnick Citation2015), archaeological barley (Smith et al. Citation2015), and other hominids (e.g. Neanderthals and Denisovans; Gokhman et al. Citation2014). Epigenetic data have also provided a novel way to infer the age of skeletal individuals (Giuliani et al. Citation2016). While the number of ancient epigenetic profiles has continued to grow (Hanghøj et al. Citation2019; Hanghøj et al. Citation2016), there are still untapped biological tissues that have yet been examined for paleoepigenetic analysis.
While none have yet studied the epigenetic signatures of ancient microbes, researchers have begun to study them in modern contexts. For instance, forms of DNA methylation patterns in bacterial and archaea genomes include 6-methyladennosine (m6A), 4-methylcytosine (m4C), and 5-methylcytosine (m5C) (Blow et al. Citation2016). The problem with identifying these types of epigenetic patterns in ancient contexts is that they have currently and only been identified with Single Molecule, Real-Time (SMRT) sequencing, which sequences DNA fragments of at least 1,000 bp, which are much too long for ancient contexts. Determining whether these epigenetic markers are still present in dental calculus and whether they are detectable with short-read sequencing should be explored as such modifications can play a role in virulence (Casadesús and Low Citation2006; Blow et al. Citation2016). A case in point has shown that epigenetic switches in S. pneumoniae can impact its phenotype and, as a result, its ability to infect a host (Li et al. Citation2016) – the outcome deriving from certain loci in the S. pnuemoniae genome undergoing extensive DNA inversions among three homologous DNA methyltransferase genes. These recombinations result in various phenotypic outcomes, such as colonizing abilities, which can potentially alter microbiome diversity and host health status (De Filippo et al. Citation2010; Jones Citation2012; Cui et al. Citation2013).
Other epigenetic work has shown that the oral microbe P. gingivalis has the potential to inactivate tumor suppressor genes within a host, as well as lead changes to human cell lines (see Gholizadeh et al. Citation2016). Fusobacterium nucleatum can equally modulate host cell pathways, promoting tumor progression since the bacterium can induce NF-κB signaling in the tongue epithelium of mice (Gallimidi et al. Citation2015). Furthermore, human behaviors, such as smoking, are known to be able to impact the epigenome of a host (Gao et al. Citation2017), as well as their oral microbiome (Wu et al. Citation2016; Kato et al. Citation2016).
Researchers have started to explore the interactions between host epigenomes and microbiomes of living populations. For instance, Krautkramer et al. (Citation2016) report that a “Western-type” diet characterized by processed foods and high sugar drinks reduce the number of short-chain fatty acids produced by microbes. This process prevents several microbiota-dependent events from occurring, leading to alterations in hepatic gene expression of the host. As a result, several negative health outcomes, such as diabetes, are associated with this diet. Concerted efforts have discovered that the products of microbes affect histone deacetylases (Maslowski and Mackay Citation2011), and that specific diet-gut microbiota-epigenetic interactions cause IBS (Aleksandrova, Romero-Mosquera, and Hernandez Citation2017). These findings suggest that even if researchers are unable to recover ancient microbial epigenomes in dental calculus, it may still be worth exploring the correlates between oral microbiome composition and the epigenetic profiles within the host’s tooth or bone tissues.
Despite obvious and appealing scientific benefits of examining microbial and human epigenomes from dental calculus, the realization of such studies is likely to be limited. Few archaeological contexts afford the environmental conditions that are conducive for methylation preservation (Gokhman, Meshorer, and Carmel Citation2016). Epigenomic analysis of aDNA also requires high coverage (15×) data and highly deaminated sites, which are rarely available (Orlando and Willerslev Citation2014). In addition, some protocols for reconstructing aDNA methylation involve UDG treatment (Briggs et al. Citation2010) which may impact the utility of authentication tools, such as MapDamage (Ginolhac et al. Citation2011; Jónsson et al. Citation2013). Furthermore, epigenetic markers are also tissue-specific, and therefore, would need comparison data from similar sample types which are currently scant (Kurushima et al. Citation2019).
Understanding epigenome and microbiome interactions can yield insights into how epigenomic reprogramming affects oral microbiota composition and its relationship with health and disease outcomes. Exploring the epigenetic mechanisms within dental calculus could provide an additional layer of analysis to understand the relationships between ancient microbes and their host.
How multiomic approaches provide a better model for the study of ancient health
Currently, the number of multiomic studies on the oral microbiome of living populations remains small (Califf et al. Citation2017) and are even more limited for archaeological populations. Each type of omics data, on its own, can aid in detecting corollaries of disease phenotypes, revealing upstream and downstream interactions within biological networks, and identifying host adaptations to environmental stimulants. However, it is clear that promoting a multiomic approach could have significant interpretive value. Such analyses can determine whether the most abundant microbes are active in a specific sample (i.e. producing protein); whether certain host genetic variants or host proteins are correlated with disease severity; and whether particular mutations or epigenetic patterns either in the microbial or human DNA are associated with disease outcomes. Below we outline how multiomic approaches is an area wide open for revealing stronger associations in two oral diseases.
Periodontal disease
Periodontal disease is a chronic infection that causes inflammation and destruction within the oral cavity (Listgarten Citation1986), whereby the host inflammatory response can lead to alveolar bone and tooth loss (Villotte, Ogden, and Trinkaus Citation2018). As a multifactorial disease, microbial components, host genetics, and environmental factors impact the progression rates for periodontitis (Kornman Citation2008). Several etiologies have been identified that influence the development and severity of periodontitis; these include age (Slots and Ting Citation1999), sex (Shiau and Reynolds Citation2010; DeWitte Citation2012), and microbial composition (Liu et al. Citation2012; Mira, Simon-Soro, and Curtis Citation2017). Contemporary and archaeological evidence also indicate that carbohydrate-enriched diets increase the incidental rates of periodontal disease and antemortem tooth loss (Clarke et al. Citation1986; Donnelly et al. Citation1977; Hunter and Arbona Citation1995; Ronderos, Pihlstrom, and Hodges Citation2001; Walker, Sugiyama, and Chacon Citation1998). The identification of periodontitis in past populations has been of major interest in bioarchaeology and bioanthropology (Hildebolt and Molnar Citation1991; DeWitte and Bekvalac Citation2011).
Bioarchaeologists have identified periodontitis in multiple human skeletal assemblages that include, Mycenean (Tsilivakos et al. Citation2002), Roman (Raitapuro-Murray, Molleson, and Hughes Citation2014) and medieval Scottish populations (DeWitte and Bekvalac Citation2010). However, bioarchaeologists use a variety of methods to identify and recognize periodontitis in the archaeological record (e.g. Kerr Citation1988; Wasterlain, Cunha, and Hillson Citation2011; DeWitte Citation2012), and although these varying methods have been independently tested and have shown to provide similar results (Tomczyk et al. Citation2017), they provide macro-scale information that cannot provide insights into the evolutionary and ecological histories for the disease. Metagenomic analyses can provide quantitative data regarding periodontal status in past individuals and populations. Metagenomic sequencing of archaeological dental calculus have specifically identified DNA of “red complex” bacteria – P. gingivalis, Tannerella forsythia, and Treponema denticola – which have been taken as indicators for individuals having periodontal disease in ancient populations (Warinner, Rodrigues, et al. Citation2014; Weyrich et al. Citation2017) because of similar diagnostic practices in modern contexts (Curtis, Diaz, and Van Dyke Citation2020). The problem with linking these microbial species to periodontal disease in ancient populations is that they have also been identified in living, orally healthy individuals (Hajishengallis and Lamont Citation2012; Mayanagi et al. Citation2004; Diaz et al. Citation2006), challenging previous assumptions about the pathogenesis for periodontal disease (e.g. Weyrich et al. Citation2017).
Multiomic measures could address these gaps in our current knowledge about periodontitis in the past, by understanding the specific underpinnings for the pathophysiology of periodontal disease. For instance, multiomics could not only lead to novel strategies for pathogen detection, as demonstrated by Fotakis et al. (Citation2020), but also lead to new understandings about the pathogenesis and evolution of periodontal disease in specific cultural contexts. Combining omic approaches could, for example, aid in understanding disease progression, as demonstrating whether the most abundance of red complex strains are also correlated with the most proteins in periodontal samples. This approach could be effective in investigating the socioecological pressures that underpin the biological and pathophysiological processes for this disease, all of which could facilitate a significant advance in treating this disease in living populations.
Dental caries
Dental caries is perhaps one of the most prevalent human diseases, affecting as much as 80–90% of the global population (Petersen Citation2004). Bacterial fermentation of dietary carbohydrates (i.e. sugars and starches) produce organic acids which demineralize the crowns and roots of teeth. This demineralization subsequently develops into small pits that increase in size and can extend into the pulp chamber of a tooth.
Carious lesions are well-documented in past human populations deriving from a vast range of archaeological contexts, representing temporally, geographically, and culturally diverse groups with different subsistence practices (Hillson Citation2008; Larsen Citation2015). Broad comparisons of archaeological populations have consistently revealed a higher (but not universal) incidence of caries in agriculturalists compared with foragers (e.g. Turner Citation1979; Hillson Citation2008; Larsen Citation1991; Milner Citation1984; Lukacs Citation2012). While the comparisons link elevated caries prevalence with consumption of domesticated plant carbohydrates, not all domesticated plants are equally cariogenic. For example, the incidence rate of dental caries for maize consumers in the New World was higher (Cucina et al. Citation2011; Lambert Citation2000; Larsen Citation1991; Milner Citation1984; Steckel and Rose Citation2002) than it was for prehistoric rice farmers in East Asia (Domett and Tayles Citation2007; Oxenham, Nguyen, and Nguyen Citation2006; Pietrusewsky and Ikehara-Quebral Citation2006). Furthermore, the prevalence of dental caries in agriculturalist populations in Western Asia and European contexts is lower than those in North America (Eshed, Gopher, and Hershkovitz Citation2006; Lubell et al. Citation1994). A multiomic framework could aid in understanding the varying incidence rates across temporal and spatial landscapes as it could determine whether certain populations had genetic predispositions or epigenetic modulations for developing dental caries, as well as provide new information about the etiology for the disease.
For decades, it has been believed that the different incidence rates of dental caries within and between populations was tied to levels of Streptococcus mutans. A wealth of studies analyzing diverse, living subject groups and study designs have linked S. mutans as the etiological agent of dental caries (Burne Citation1998; Banas and Drake Citation2018; Loesche Citation1986; Tanzer, Livingston, and Thompson Citation2001). S. mutans possesses the properties to initiate the development of enamel demineralization and, as a result, can be the major determinant that is responsible for the pathogenesis of caries. However, S. mutans may not be the universal etiological agent. Recent studies have found that S. mutans is absent in several populations with active dental caries (Johansson et al. Citation2016; Gross et al. Citation2012), while also present in populations without active dental caries (Bradshaw and Lynch Citation2013). Further studies have identified a complex of species more commonly associated with carious lesions and active caries, that include Veiononella, Lactobacillus, and Streptococcus species (Simón-Soro and Mira Citation2015; Nyvad et al. Citation2013; Badet and Thebaud Citation2008). These findings have not found their way into the archaeological and anthropological literature, as many researchers in these fields still view S. mutans as the primary agent for caries (see Mackie, Radini, and Speller Citation2017). In light of such confounding evidence, aDNA researchers should reconsider referring to S. mutans as the sole etiological agent for dental caries in past and present populations. Such discrepancies in assessing etiology could be resolved with converging lines of evidence from metagenomic, metaproteomic, host genome, and epigenetic data.
Further considerations before conducting multiomic approaches
Despite a wealth of challenges in metagenomic analyses of dental calculus, three key issues also require further consideration before multiomic work can be undertaken: taphonomy, contamination, and study design.
As is the case with all archaeological research, taphonomic processes significantly impact the biomolecular preservation of dental calculus. While research indicates that dental calculus retains more endogenous microbial DNA than dentin (Mann et al. Citation2018), there are some notable considerations. High GC content, wherein a large number of guanines and cytosines are present in the genomes of certain microorganisms, has been shown to influence the recovery and reconstruction of ancient microbiota profiles (Ziesemer et al. Citation2015) and can bias estimates of microbial diversity (Weyrich et al. Citation2017). While proteins are known to survive for thousands of years longer (Demarchi et al. Citation2016) than DNA (Orlando et al. Citation2013), future investigations are still needed to understand how taphonomy impacts the proteins in the dental calculus matrix while also accounting for and evaluating their degradation. Systematic studies of this “micro-taphonomy” are, therefore, of high priority if multiomic approaches are to reach their full potential.
Even though most ancient DNA laboratories follow standard protocols that mitigate the risk of contamination, it remains a major concern in ancient dental calculus research. Several steps in dental calculus sample preparation and extraction protocols are effective at minimizing its impact (Warinner et al. Citation2017; Eisenhofer, Minich et al. Citation2019; Farrer et al. Citation2020), but they cannot entirely eliminate all contamination. Microbes can infiltrate biological samples from numerous sources, including sampling and laboratory environments, researchers themselves, plastic consumables, nucleic acid extraction kits, laboratory reagents (including PCR mastermixes), and cross-contamination with other samples and sequencing runs within a study (Eisenhofer et al. Citation2018). Studies have found that blank controls, laboratories, and DNA extraction methods share several contaminant taxa (Salter et al. Citation2014). These findings raise a concern for both modern and ancient microbiome research and have initiated a debate and calls for a reevaluation of previous findings. For example, claims that a unique microbiome exists in the human placenta (Aagaard et al. Citation2014) were later challenged because they failed to publish sequencing data for any controls (Kliman Citation2014); additional data that clearly showed placental samples do not harbor a distinct microbial community from extractions blanks (Lauder et al. Citation2016). Similar concerns have also been voiced in ancient DNA research (Santiago-Rodriguez et al. Citation2016; Eisenhofer, Cooper, and Weyrich Citation2018; Santiago-Rodriguez and Toranzos Citation2018) and serve as a constant reminder that researchers should include extraction and library blank controls as part of their study design so that they can monitor contamination. Researchers should even consider collecting soil samples around their burials as such measures can be a useful resource for assessing contamination. Future analyses will need to examine how contamination impact the proteomic, host genomic, and epigenomic profiles in dental calculus.
Lastly, the study design of a multiomic projects is crucial. Research teams should as a matter of principle develop a detailed plan prior to collecting samples. Every omic strategy discussed here involves the removal and destruction of dental calculus from once-living individuals, and in most cases ancestors of living communities. Collaborations or agreements with Indigenous communities are not always uniformly or legally required for destructive analysis, as some institutions do not view dental calculus as “human tissue”. Dental calculus has been viewed as an alternative source for destructive analysis (Bardill et al. Citation2018), but not all Indigenous communities may share this perspective. Whatever the circumstances, it is important that researchers who wish to sample and analyze dental calculus communicate with and involve as many potential stakeholders as possible prior to any sampling or destructive analyses for a multiomic study.
Recommended guidelines before destructive analysis for multiomic applications of dental calculus research
Because archaeological dental calculus is a finite resource, we recommend considering the following before proceeding with any destructive analyses:
Identify your scientific questions and consider whether there are alternative (non-destructive) analyses that can address them.
Discuss and agree the potential research with all relevant stakeholders that may be impacted by either destructive analyses and/or by the results. There should be communication and advance agreement about how the samples (as well as the data generated) will be stored, curated, protected, and/or repatriated after the study is completed. In addition, dialogue should cover which omic strategy/strategies will be deployed. If human DNA is also to be examined, discuss the risks and benefits of the analysis to all stakeholders involved.
Rather than collecting as many samples as possible from a collection, work with collaborators to determine the number of samples for destructive analysis. Since the biomolecular integrity of archaeological samples depends on environmental conditions (Kistler et al. Citation2017), it is unlikely all samples will have equivalent preservation. It may be best to perform omic analyses on 5–10 samples to evaluate the biomolecular preservation of a site before performing destructive analyses on a larger dataset. While more statistical inference modeling for ancient samples needs to occur, models analyzing modern microbiome datasets suggest 10 or 20 well-preserved samples per group may afford adequate statistical power, depending on the distance metric being used (Kelly et al. Citation2015). These values are suggestive as the number of samples to sufficiently power a study will vary (Quince et al. Citation2017).
Assess whether performing a single-omic destructive analysis in the present outweighs a potentially powerful multiomic analysis in the future. Performing destructive analyses on limited/rare samples now may minimize the potential information that can be gained in the future.
Identify potential collaborative colleagues/groups with specific/significant expertise in omic fields who can complement those of your own. Pooling expertise insights and facilities will add value, broaden the perspective, and deepen the understanding of dental calculus.
Conclusion
The lack of a multiomic framework leaves many anthropological and archaeological questions unanswered, regarding ancient population movements, oral health, social stratification, and environmental adaptation – questions that can now be examined using insights provided by dental calculus. The wide prevalence of dental calculus in the archaeological record and its rich biomolecule preservation provide researchers an unprecedented opportunity to examine these questions at a globally and temporally diverse scale. By combining these biomolecular methods with radiocarbon dating, chemical isotopic signatures, microscopy, osteological, and other archaeological analyses, dental calculus will help unravel the lived experiences of ancient individuals that were embedded within dynamic social and environmental networks. Promoting a multiomic framework will significantly enhance our knowledge about the past and present. For these reasons, researchers should systematically take full advantage of the various and complex biomolecules within dental calculus.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Sterling L. Wright
Sterling L. Wright is a PhD student in the Department of Anthropology at Penn State University. He specializes in examining ancient oral microbiomes and how they interact with past diets, migrations, and behaviors.
Keith Dobney
Keith Dobney is Head of the School of Philosophical and Historical Inquiry at the University of Sydney and Professor in the Department of Archaeology there. He also holds a position as Research Professor of Human Palaeoecology in the Department of Archaeology, Classics and Egyptology at the University of Liverpool, UK, as well as adjunct positions in the departments of archaeology at the University of Aberdeen and Simon Fraser University. He is interested in the biology of the past and how that interacts with and influences the evolution of human behaviour and culture, using a multitude of archaeological methods.
Laura S. Weyrich
Laura S. Weyrich is an Associate Professor in the Department of Anthropology and the Huck Institutes of the Life Sciences at Penn State University, directing the Penn State Ancient Biomolecules Research Environment (PSABRE) and the microARCH research group. She also holds an Affiliate Associate Professor position at the University of Adelaide in the Department of Biological Sciences. Her research is focused on understanding the evolution of the human microbiome and exploring how ancient microorganisms can provide new information into processes of the past.
References
- Aagaard, K., J. Ma, K. M. Antony, R. Ganu, J. Petrosino, and J. Versalovic. 2014. “The Placenta Harbors a Unique Microbiome.” Science Translational Medicine 6 (237): 237ra65–237ra65.
- Adler, Christina J., Keith Dobney, Laura S. Weyrich, John Kaidonis, Alan W. Walker, Wolfgang Haak, Corey J. A. Bradshaw, et al. 2013. “Sequencing Ancient Calcified Dental Plaque Shows Changes in Oral Microbiota with Dietary Shifts of the Neolithic and Industrial Revolutions.” Nature Genetics 45 (4): 450–455.
- Al-Zahrani, Mohammad S., Elaine A. Borawski, and Nabil F. Bissada. 2004. “Poor Overall Diet Quality as a Possible Contributor to Calculus Formation.” Oral Health & Preventive Dentistry 2 (4): 345–349.
- Aleksandrova, Krasimira, Beatriz Romero-Mosquera, and Vicent Hernandez. 2017. “Diet, Gut Microbiome and Epigenetics: Emerging Links with Inflammatory Bowel Diseases and Prospects for Management and Prevention.” Nutrients 9 (9): 962.
- Allen, Jawara, and Cynthia L. Sears. 2019. “Impact of the Gut Microbiome on the Genome and Epigenome of Colon Epithelial Cells: Contributions to Colorectal Cancer Development.” Genome Medicine 11 (1): 1–18.
- Arensburg, Baruch. 1996. “Ancient Dental Calculus and Diet.” Human Evolution 11 (2): 139–145. Springer.
- Armitage, Philip L. 1975. “The Extraction and Identification of Opal Phytoliths from the Teeth of Ungulates.” Journal of Archaeological Science 2 (3): 187–197.
- Aufderheide, Arthur C., Conrado Rodríguez-Martín, and Odin Langsjoen. 1998. The Cambridge Encyclopedia of Human Paleopathology. Vol. 478. Cambridge: University Press Cambridge.
- Badet, C., and N. B. Thebaud. 2008. “Ecology of Lactobacilli in the Oral Cavity: A Review of Literature.” The Open Microbiology Journal 2 (1): 38–48. Bentham Science Publishers.
- Banas, Jeffrey A., and David R. Drake. 2018. “Are the Mutans Streptococci Still Considered Relevant to Understanding the Microbial Etiology of Dental Caries?” BMC Oral Health 18 (1): 129.
- Bardill, Jessica, Alyssa C. Bader, Nanibaa’ A. Garrison, Deborah A. Bolnick, Jennifer A. Raff, Alexa Walker, and Ripan S. Malhi. 2018. “Advancing the Ethics of Paleogenomics.” Science 360 (6387): 384–385. American Association for the Advancement of Science.
- Billmyre, R. Blake, and Joseph Heitman. 2017. “Genetic and Epigenetic Engines of Diversity in Pathogenic Microbes.” PLOS Pathogens 13 (9): 1–7.
- Blow, Matthew J., Tyson A. Clark, Chris G. Daum, Adam M. Deutschbauer, Alexey Fomenkov, Roxanne Fries, Jeff Froula, et al. 2016. “The Epigenomic Landscape of Prokaryotes.” PLoS Genetics 12 (2): e1005854.
- Bostanci, N., W. Heywood, K. Mills, M. Parkar, L. Nibali, and N. Donos. 2010. “Application of Label-Free Absolute Quantitative Proteomics in Human Gingival Crevicular Fluid by LC/MSE(Gingival Exudatome).” Journal of Proteome Research 9 (5): 2191–2199. ACS Publications.
- Bradshaw, David J., and Richard J. M. Lynch. 2013. “Diet and the Microbial Aetiology of Dental Caries: New Paradigms.” International Dental Journal 63 (s2): 64–72.
- Brealey, Jaelle C., Henrique G. Leitão, Tom van der Valk, Wenbo Xu, Katia Bougiouri, Love Dalén, and Katerina Guschanski. 2020. “Dental Calculus as a Tool to Study the Evolution of the Mammalian Oral Microbiome.” Molecular Biology and Evolution 37 (10): 3003–3022.
- Briggs, Adrian W., Udo Stenzel, Matthias Meyer, Johannes Krause, Martin Kircher, and Svante Pääbo. 2010. “Removal of Deaminated Cytosines and Detection of in Vivo Methylation in Ancient DNA.” Nucleic Acids Research 38 (6): e87–e87.
- Burne, R. A. 1998. “Oral Streptococci … Products of Their Environment.” Journal of Dental Research 77 (3): 445–452.
- Califf, Katy J., Karen Schwarzberg-Lipson, Neha Garg, Sean M. Gibbons, J. Gregory Caporaso, Jørgen Slots, Chloe Cohen, Pieter C. Dorrestein, and Scott T. Kelley. 2017. “Multi-Omics Analysis of Periodontal Pocket Microbial Communities Pre- and Posttreatment.” MSystems 2 (3): e00016–17. Am Soc Microbiol.
- Cappellini, Enrico, Lars J. Jensen, Damian Szklarczyk, Aurélien Ginolhac, Rute A. R. da Fonseca, Thomas W. Stafford, Steven R. Holen, et al. 2012. “Proteomic Analysis of a Pleistocene Mammoth Femur Reveals More than One Hundred Ancient Bone Proteins.” Journal of Proteome Research 11 (2): 917–926.
- Casadesús, Josep, and David Low. 2006. “Epigenetic Gene Regulation in the Bacterial World.” Microbiology and Molecular Biology Reviews 70 (3): 830–856.
- Charlton, Sophy, Abigail Ramsøe, Matthew Collins, Oliver E. Craig, Roman Fischer, Michelle Alexander, and Camilla F. Speller. 2019. “New Insights into Neolithic Milk Consumption through Proteomic Analysis of Dental Calculus.” Archaeological and Anthropological Sciences. doi:https://doi.org/10.1007/s12520-019-00911-7.
- Chen, Beidi, Luxi Sun, and Xuan Zhang. 2017. “Integration of Microbiome and Epigenome to Decipher the Pathogenesis of Autoimmune Diseases.” Journal of Autoimmunity 83: 31–42. Elsevier.
- Clarke, Nigel G., S. E. Carey, W. Srikandi, Robert S. Hirsch, and P. I. Leppard. 1986. “Periodontal Disease in Ancient Populations.” American Journal of Physical Anthropology 71 (2): 173–183.
- Colombo, Ana Paula V., Susan Bennet, Sean L. Cotton, J. Max Goodson, Ralph Kent, Anne D. Haffajee, Sigmund S. Socransky, et al. 2012. “Impact of Periodontal Therapy on the Subgingival Microbiota of Severe Periodontitis: Comparison Between Good Responders and Individuals with Refractory Periodontitis Using the Human Oral Microbe Identification Microarray.” Journal of Periodontology 83 (10): 1279–1287. Wiley Online Library.
- Cucina, Andrea, Cristina Perera Cantillo, Thelma Sierra Sosa, and Vera Tiesler. 2011. “Carious Lesions and Maize Consumption among the Prehispanic Maya: An Analysis of a Coastal Community in Northern Yucatan.” American Journal of Physical Anthropology 145 (4): 560–567. Wiley Online Library.
- Cui, Y., C. Yu, Y. Yan, D. Li, Y. Li, T. Jombart, L. A. Weinert, et al. 2013. “Historical Variations in Mutation Rate in an Epidemic Pathogen, Yersinia Pestis.” Proceedings of the National Academy of Sciences 110 (2): 577–582. National Acad Sciences.
- Curtis, Mike A., Patricia I. Diaz, and Thomas E. Van Dyke. 2020. “The Role of the Microbiota in Periodontal Disease.” Periodontology 2000 83 (1): 14–25.
- Dabney, Jesse, Michael Knapp, Isabelle Glocke, Marie-Theres Gansauge, Antje Weihmann, Birgit Nickel, Cristina Valdiosera, et al. 2013. “Complete Mitochondrial Genome Sequence of a Middle Pleistocene Cave Bear Reconstructed from Ultrashort DNA Fragments.” Proceedings of the National Academy of Sciences 110 (39): 15758–15763.
- Davenport, Emily R. 2017. “Tooth Be Told, Genetics Influences Oral Microbiome.” Cell Host & Microbe 22 (3): 251–253.
- De Filippo, C., D. Cavalieri, M. Di Paola, M. Ramazzotti, J. B. Poullet, S. Massart, S. Collini, G. Pieraccini, and P. Lionetti. 2010. “Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa.” Proceedings of the National Academy of Sciences 107 (33): 14691–14696. National Acad Sciences.
- De La Fuente, C., S. Flores, and M. Moraga. 2013. “DNA from Human Ancient Bacteria: A Novel Source of Genetic Evidence from Archaeological Dental Calculus.” Archaeometry 55 (4): 767–778.
- Demarchi, Beatrice, Shaun Hall, Teresa Roncal-Herrero, Colin L. Freeman, Jos Woolley, Molly K. Crisp, Julie Wilson, et al. 2016. “Protein Sequences Bound to Mineral Surfaces Persist into Deep Time.” eLife 5: e17092. eLife Sciences Publications.
- Deo, Priya Nimish, and Revati Deshmukh. 2019. “Oral Microbiome: Unveiling the Fundamentals.” Journal of Oral and Maxillofacial Pathology: JOMFP 23 (1): 122–128.
- De Oliveira, Naila Francis Paulo, Denise Carleto Andia, Aline Cristiane Planello, Silvana Pasetto, Marcelo Rocha Marques, Francisco Humberto Nociti Jr, Sérgio Roberto Peres Line, and Ana Paula De Souza. 2011. “TLR2 and TLR4 Gene Promoter Methylation Status During Chronic Periodontitis.” Journal of Clinical Periodontology 38 (11): 975–983.
- DeWitte, Sharon N. 2012. “Sex Differences in Periodontal Disease in Catastrophic and Attritional Assemblages from Medieval London.” American Journal of Physical Anthropology 149 (3): 405–416.
- DeWitte, Sharon N., and Jelena Bekvalac. 2010. “Oral Health and Frailty in the Medieval English Cemetery of St Mary Graces.” American Journal of Physical Anthropology 142 (3): 341–354.
- DeWitte, Sharon N., and Jelena Bekvalac. 2011. “The Association Between Periodontal Disease and Periosteal Lesions in the St. Mary Graces Cemetery, London, England A.D. 1350–1538.” American Journal of Physical Anthropology 146 (4): 609–618.
- Diaz, Patricia I., Natalia I. Chalmers, Alexander H. Rickard, Colin Kong, Craig L. Milburn, Robert J. Palmer, and Paul E. Kolenbrander. 2006. “Molecular Characterization of Subject-Specific Oral Microflora During Initial Colonization of Enamel.” Applied and Environmental Microbiology 72 (4): 2837–2848. Am Soc Microbiol.
- Dobney, K. 1994. “Study of the Dental Calculus.” In The Jewish Burial Ground at Jewbury, 507–521. York, UK: York Archaeological Trust, Council for British Archaeology.
- Dobney, Keith, and Don Brothwell. 1986. “Dental Calculus: Its Relevance to Ancient Diet and Oral Ecology.” In Teeth and Anthropology 291, 55–81. Oxford: BAR Publishing.
- Dobney, Keith, and Don Brothwell. 1988. “A Scanning Electron Microscope Study of Archaeological Dental Calculus.” In Scanning Electron Microscopy in Archaeology, edited by Sandra Olsen, 372–385. Oxford: British Archaeological Report.
- Domett, Kate, and Nancy Tayles. 2007. “Population Health from the Bronze to the Iron Age in the Mun River Valley, Northeastern Thailand.” In Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification, edited by Mark Nathan and M. M. Gillian, 286–299. Gainesville, FL: University Press of Florida.
- Donnelly, C. J., L. A. Thomson, H. M. Stiles, C. Brewer, J. V. Neel, and J. A. Brunelle. 1977. “Plaque, Caries, Periodontal Diseases, and Acculturation among Yanomamo Indians, Venezuela.” Community Dentistry and Oral Epidemiology 5 (1): 30–39.
- Ebrahim, Ali, Elizabeth Brunk, Justin Tan, Edward J. O’Brien, Donghyuk Kim, Richard Szubin, Joshua A. Lerman, et al. 2016. “Multi-Omic Data Integration Enables Discovery of Hidden Biological Regularities.” Nature Communications 7: 13091.
- Eisenhofer, Raphael, Atholl Anderson, Keith Dobney, Alan Cooper, and Laura S. Weyrich. 2019. “Ancient Microbial DNA in Dental Calculus: A New Method for Studying Rapid Human Migration Events.” The Journal of Island and Coastal Archaeology 14 (2): 149–162.
- Eisenhofer, Raphael, Alan Cooper, and Laura S Weyrich. 2018. “Reply to Santiago-Rodriguez et al.: Proper Authentication of Ancient DNA Is Essential.” FEMS Microbiology Ecology 93 (5): 122.
- Eisenhofer, Raphael, Jeremiah J. Minich, Clarisse Marotz, Alan Cooper, Rob Knight, and Laura S. Weyrich. 2019. “Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations.” Trends in Microbiology 27 (2): 105–117.
- Eshed, Vered, Avi Gopher, and Israel Hershkovitz. 2006. “Tooth Wear and Dental Pathology at the Advent of Agriculture: New Evidence from the Levant.” American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists 130 (2): 145–159.
- Fagernäs, Zandra, Maite I. García-Collado, Jessica Hendy, Courtney A. Hofman, Camilla Speller, Irina Velsko, and Christina Warinner. 2020. “A Unified Protocol for Simultaneous Extraction of DNA and Proteins from Archaeological Dental Calculus.” Journal of Archaeological Science 118: 105135.
- Falony, Gwen, Marie Joossens, Sara Vieira-Silva, Jun Wang, Youssef Darzi, Karoline Faust, Alexander Kurilshikov, et al. 2016. “Population-Level Analysis of Gut Microbiome Variation.” Science 352 (6285): 560–564. American Association for the Advancement of Science.
- Farrer, Andrew Graham. 2017. “Ancient DNA Studies of Dental Calculus” Accessed April 30, 2020. https://digital.library.adelaide.edu.au/dspace/handle/2440/114482.
- Farrer, Andrew G., Jelena Bekvalac, Rebecca Redfern, N. Gully, K. Dobney, A. Cooper, and L. S. Weyrich. 2018. “Biological and Cultural Drivers of Oral Microbiota in Medieval and Post-Medieval London, UK.” BioRxiv. Cold Spring Harbor Laboratory: 343889.
- Farrer, Andrew, Sterling Wright, Emily Skelly, Raphael Eisenhofer, Keith Dobney, and Laura Weyrich. 2020. “Effectiveness of Decontamination protocols when Analyzing Ancient DNA Preserved in Dental Calculus.” Research Square. doi:https://doi.org/10.21203/rs.3.rs-136836/v1:1-22.
- Feinberg, Andrew P. 2018. “The Key Role of Epigenetics in Human Disease Prevention and Mitigation.” New England Journal of Medicine 378 (14): 1323–1334. Mass Medical Soc.
- Fiorillo, Luca, Gabriele Cervino, Luigi Laino, Cesare D’Amico, Rodolfo Mauceri, Tolga Fikret Tozum, Michele Gaeta, and Marco Cicciù. 2019. “Porphyromonas Gingivalis, Periodontal and Systemic Implications: A Systematic Review.” Dentistry Journal 7 (4): 114. Multidisciplinary Digital Publishing Institute.
- Fitzpatrick, David R., and Christopher B. Wilson. 2003. “Methylation and Demethylation in the Regulation of Genes, Cells, and Responses in the Immune System.” Clinical Immunology 109 (1): 37–45.
- Fofanova, Tatiana Y., Joseph F. Petrosino, and Richard Kellermayer. 2016. “Microbiome-Epigenome Interactions and the Environmental Origins of Inflammatory Bowel Diseases.” Journal of Pediatric Gastroenterology and Nutrition 62 (2): 208. NIH Public Access.
- Fotakis, Anna K., Sean D. Denham, Meaghan Mackie, Miren Iraeta Orbegozo, Dorothea Mylopotamitaki, Shyam Gopalakrishnan, Thomas Sicheritz-Ponten, et al. 2020. “Multi-Omic Detection of Mycobacterium Leprae in Archaeological Human Dental Calculus.” Philosophical Transactions of the Royal Society B: Biological Sciences 375 (1812): 1–10.
- Gallimidi, Adi Binder, Stuart Fischman, Brurya Revach, Raanan Bulvik, Alina Maliutina, Ariel M. Rubinstein, Gabriel Nussbaum, et al. 2015. “Periodontal Pathogens Porphyromonas Gingivalis and Fusobacterium Nucleatum Promote Tumor Progression in an Oral-Specific Chemical Carcinogenesis Model.” Oncotarget 6 (26): 22613–22623.
- Gao, Xu, Xīn Gào, Yan Zhang, Lutz Philipp Breitling, Ben Schöttker, and Hermann Brenner. 2017. “Associations of Self-Reported Smoking, Cotinine Levels and Epigenetic Smoking Indicators with Oxidative Stress among Older Adults: A Population-Based Study.” European Journal of Epidemiology 32 (5): 443–456. Springer.
- Gholizadeh, Pourya, Hosein Eslami, Mehdi Yousefi, Mohammad Asgharzadeh, Mohammad Aghazadeh, and Hossein Samadi Kafil. 2016. “Role of Oral Microbiome on Oral Cancers, a Review.” Biomedicine & Pharmacotherapy 84: 552–558.
- Ginolhac, Aurelien, Morten Rasmussen, M. Thomas P. Gilbert, Eske Willerslev, and Ludovic Orlando. 2011. “MapDamage: Testing for Damage Patterns in Ancient DNA Sequences.” Bioinformatics (Oxford, England) 27 (15): 2153–2155. Oxford University Press.
- Giuliani, Cristina, Elisabetta Cilli, Maria Giulia Bacalini, Chiara Pirazzini, Marco Sazzini, Giorgio Gruppioni, Claudio Franceschi, Paolo Garagnani, and Donata Luiselli. 2016. “Inferring Chronological Age from DNA Methylation Patterns of Human Teeth.” American Journal of Physical Anthropology 159 (4): 585–595.
- Gokhman, D., E. Lavi, K. Prufer, M. F. Fraga, J. A. Riancho, J. Kelso, S. Paabo, E. Meshorer, and L. Carmel. 2014. “Reconstructing the DNA Methylation Maps of the Neandertal and the Denisovan.” Science 344 (6183): 523–527.
- Gokhman, David, Eran Meshorer, and Liran Carmel. 2016. “Epigenetics: It’s Getting Old. Past Meets Future in Paleoepigenetics.” Trends in Ecology & Evolution 31 (4): 290–300.
- Gomez, Andres, Josh L. Espinoza, Derek M. Harkins, Pamela Leong, Richard Saffery, Michelle Bockmann, Manolito Torralba, et al. 2017. “Host Genetic Control of the Oral Microbiome in Health and Disease.” Cell Host & Microbe 22 (3): 269–278.e3.
- Grassl, Niklas, Nils Alexander Kulak, Garwin Pichler, Philipp Emanuel Geyer, Jette Jung, Sören Schubert, Pavel Sinitcyn, Juergen Cox, and Matthias Mann. 2016. “Ultra-Deep and Quantitative Saliva Proteome Reveals Dynamics of the Oral Microbiome.” Genome Medicine 8 (1): 44.
- Gross, Erin L., Clifford J. Beall, Stacey R. Kutsch, Noah D. Firestone, Eugene J. Leys, and Ann L. Griffen. 2012. “Beyond Streptococcus Mutans: Dental Caries Onset Linked to Multiple Species by 16S RRNA Community Analysis.” PloS One 7 (10): e47722. Public Library of Science.
- Guthrie, R Dale. 2003. “Rapid Body Size Decline in Alaskan Pleistocene Horses Before Extinction.” Nature 426 (6963): 169–171. Nature Publishing Group.
- Haak, Wolfgang, Peter Forster, Barbara Bramanti, Shuichi Matsumura, Guido Brandt, Marc Tanzer, Richard Villems, et al. 2005. “Ancient DNA from the First European Farmers in 7500-Year-Old Neolithic Sites.” Science 310 (5750): 1016–1018.
- Haak, Wolfgang, Iosif Lazaridis, Nick Patterson, Nadin Rohland, Swapan Mallick, Bastien Llamas, Guido Brandt, et al. 2015. “Massive Migration from the Steppe was a Source for Indo-European Languages in Europe.” Nature 522 (7555): 207–211. Nature Publishing Group.
- Hajishengallis, G., and R. J. Lamont. 2012. “Beyond the Red Complex and into More Complexity: The Polymicrobial Synergy and Dysbiosis (PSD) Model of Periodontal Disease Etiology.” Molecular Oral Microbiology 27 (6): 409–419.
- Hanghøj, Kristian, Gabriel Renaud, Anders Albrechtsen, and Ludovic Orlando. 2019. “DamMet: Ancient Methylome Mapping Accounting for Errors, True Variants, and Post-Mortem DNA Damage.” GigaScience 8. doi:https://doi.org/10.1093/gigascience/giz025.
- Hanghøj, Kristian, Andaine Seguin-Orlando, Mikkel Schubert, Tobias Madsen, Jakob Skou Pedersen, Eske Willerslev, and Ludovic Orlando. 2016. “Fast, Accurate and Automatic Ancient Nucleosome and Methylation Maps with EpiPALEOMIX.” Molecular Biology and Evolution 33 (12): 3284–3298.
- Hasin, Yehudit, Marcus Seldin, and Aldons Lusis. 2017. “Multi-Omics Approaches to Disease.” Genome Biology 18 (1): 83.
- Herbst, Florian-Alexander, Vanessa Lünsmann, Henrik Kjeldal, Nico Jehmlich, Andreas Tholey, Martin von Bergen, Jeppe Lund Nielsen, Robert L. Hettich, Jana Seifert, and Per Halkjaer Nielsen. 2016. “Enhancing Metaproteomics – the Value of Models and Defined Environmental Microbial Systems.” Proteomics 16 (5): 783–798. Wiley Online Library.
- Heyer Robert, Schallert Kay, Zoun Roman, Becher Beatrice, Saake Gunter, and Benndorf Dirk 2017. “Challenges and Perspectives of Metaproteomic Data Analysis.” Journal of Biotechnology 261: 24–36.
- Hildebolt, Charles F, and Stephen Molnar. 1991. “Measurement and Description of Periodontal Disease in Anthropological Studies.” In Advances in Dental Anthropology, edited by M. Kelley and C. Larsen, 225–240. New York: Wiley-Liss.
- Hillson, Simon. 2008. “The Current State of Dental Decay.” Cambridge Studies In Biological And Evolutionary Anthropology 53: 111.
- Hunter, John M., and Sonia I. Arbona. 1995. “The Tooth as a Marker of Developing World Quality of Life: A Field Study in Guatemala.” Social Science & Medicine 41 (9): 1217–1240.
- Jablonka, Eva. 2013. “Epigenetic Inheritance and Plasticity: The Responsive Germline.” Progress in Biophysics and Molecular Biology 111 (2–3): 99–107.
- Jansson, Janet K., and Erin S. Baker. 2016. “A Multi-Omic Future for Microbiome Studies.” Nature Microbiology 1 (5): 10–1038.
- Jansson, Janet K., and Kirsten S. Hofmockel. 2018. “The Soil Microbiome – from Metagenomics to Metaphenomics.” Current Opinion in Microbiology 43: 162–168. Environmental Microbiology * The New Microscopy.
- Jansson, Janet, Ben Willing, Marianna Lucio, Ages Fekete, Johan Dicksved, Jonas Halfvarson, Curt Tysk, Philippe Schmitt-Kopplin, and Stefan Bereswill. 2009. “Metabolomics Reveals Metabolic Biomarkers of Crohn’s Disease.” PloS One 4 (7): e6386.
- Jeong, Choongwon, Shevan Wilkin, Tsend Amgalantugs, Abigail S. Bouwman, William Timothy Treal Taylor, Richard W. Hagan, Sabri Bromage, et al. 2018. “Bronze Age Population Dynamics and the Rise of Dairy Pastoralism on the Eastern Eurasian Steppe.” Proceedings of the National Academy of Sciences 115 (48): E11248–E11255. National Acad Sciences.
- Jersie-Christensen, Rosa R., Liam T. Lanigan, David Lyon, Meaghan Mackie, Daniel Belstrøm, Christian D. Kelstrup, Anna K. Fotakis, et al. 2018. “Quantitative Metaproteomics of Medieval Dental Calculus Reveals Individual Oral Health Status.” Nature Communications 9 (1): 4744.
- Johansson, I., E. Witkowska, B. Kaveh, P. Lif Holgerson, and A. C. R. Tanner. 2016. “The Microbiome in Populations with a Low and High Prevalence of Caries.” Journal of Dental Research 95 (1): 80–86.
- Johnson, Abigail J., Pajau Vangay, Gabriel A. Al-Ghalith, Benjamin M. Hillmann, Tonya L. Ward, Robin R. Shields-Cutler, Austin D. Kim, et al. 2019. “Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans.” Cell Host & Microbe 25 (6): 789–802.e5.
- Jones, Peter A. 2012. “Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond.” Nature Reviews Genetics 13 (7): 484–492. Nature Publishing Group.
- Jónsson, Hákon, Aurélien Ginolhac, Mikkel Schubert, Philip L. F. Johnson, and Ludovic Orlando. 2013. “mapDamage2.0: Fast Approximate Bayesian Estimates of Ancient DNA Damage Parameters.” Bioinformatics (Oxford, England) 29 (13): 1682–1684. Oxford University Press.
- Kato, Ikuko, Adrian A Vasquez, Gregory Moyerbrailean, Susan Land, Jun Sun, Ho-Sheng Lin, and Jeffrey L. Ram. 2016. “Oral Microbiome and History of Smoking and Colorectal Cancer.” Journal of Epidemiological Research 2 (2): 92. NIH Public Access.
- Kelly, Brendan J., Robert Gross, Kyle Bittinger, Scott Sherrill-Mix, James D. Lewis, Ronald G. Collman, Frederic D. Bushman, and Hongzhe Li. 2015. “Power and Sample-Size Estimation for Microbiome Studies Using Pairwise Distances and PERMANOVA.” Bioinformatics (Oxford, England) 31 (15): 2461–2468. Oxford Academic.
- Kerr, Neill W. 1988. “A Method of Assessing Periodontal Status in Archaeologically Derived Skeletal Material.” Journal of Paleopathology 2 (2): 67–78.
- Kistler, Logan, Roselyn Ware, Oliver Smith, Matthew Collins, and Robin G. Allaby. 2017. “A New Model for Ancient DNA Decay Based on Paleogenomic Meta-Analysis.” Nucleic Acids Research 45 (11): 6310–6320. Oxford University Press.
- Kliman, Harvey J. 2014. “Comment on ‘The Placenta Harbors a Unique Microbiome’.” Science Translational Medicine 6 (254): 254le4–254le4. American Association for the Advancement of Science.
- Kohrs, F., R. Heyer, A. Magnussen, D. Benndorf, T. Muth, A. Behne, E. Rapp, et al. 2014. “Sample Prefractionation with Liquid Isoelectric Focusing Enables in Depth Microbial Metaproteome Analysis of Mesophilic and Thermophilic Biogas Plants.” Anaerobe 29: 59–67. Elsevier.
- Kornman, Kenneth S. 2008. “Mapping the Pathogenesis of Periodontitis: A New Look.” Journal of Periodontology 79: 1560–1568.
- Krautkramer, Kimberly A., Julia H. Kreznar, Kymberleigh A. Romano, Eugenio I. Vivas, Gregory A. Barrett-Wilt, Mary E. Rabaglia, Mark P. Keller, Alan D. Attie, Federico E. Rey, and John M. Denu. 2016. “Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues.” Molecular Cell 64 (5): 982–992.
- Kurushima, Yuko, Pei-Chien Tsai, Juan Castillo-Fernandez, Alexessander Couto Alves, Julia Sarah El-Sayed Moustafa, Caroline Le Roy, Tim D. Spector, et al. 2019. “Epigenetic Findings in Periodontitis in UK Twins: A Cross-Sectional Study.” Clinical Epigenetics 11 (1): 27.
- Lambert, Patricia M. 2000. “Life on the Periphery: Health in Farming Communities of Interior North Carolina and Virginia.” Chapter 10 in Bioarchaeological Studies of Life in the Age of Agriculture: A View from the Southeast, 168–194. Tuscaloosa, AL: The University of Alabama Press.
- Larsen, Clark Spencer.. 1991. “Dental Caries Evidence for Dietary Change: An Archaeological Context.” Chapter 10 in Advances in Dental Anthropology, edited by Marc Kelley and Clark Spencer Larsen, 179–202. New York, NY: Wiley-Liss.
- Larsen, C. S. 2015. Bioarchaeology: Interpreting Behavior from the Human Skeleton. Cambridge, UK: Cambridge Univ Press.
- Lauder, Abigail P., Aoife M. Roche, Scott Sherrill-Mix, Aubrey Bailey, Alice L. Laughlin, Kyle Bittinger, Rita Leite, Michal A. Elovitz, Samuel Parry, and Frederic D. Bushman. 2016. “Comparison of Placenta Samples with Contamination Controls Does Not Provide Evidence for a Distinct Placenta Microbiota.” Microbiome 4 (1): 29.
- Li, Jing, Jing-Wen Li, Zhixing Feng, Juanjuan Wang, Haoran An, Yanni Liu, Yang Wang, et al. 2016. “Epigenetic Switch Driven by DNA Inversions Dictates Phase Variation in Streptococcus Pneumoniae.” PLOS Pathogens 12 (7): e1005762. Public Library of Science.
- Lieverse, Angela R. 1999. “Diet and the Aetiology of Dental Calculus.” International Journal of Osteoarchaeology 9 (4): 219–232.
- Linossier, A., M. Gajardo, and J. Olavarria. 1996. “Paleomicrobiological Study in Dental Calculus: Streptococcus Mutans.” Scanning Microscopy 10 (4): 1005–1013; discussion 1014.
- Listgarten, Max A. 1986. “Pathogenesis of Periodontitis.” Journal of Clinical Periodontology 13 (5): 418–425.
- Liu, Bo, Lina L. Faller, Niels Klitgord, Varun Mazumdar, Mohammad Ghodsi, Daniel D. Sommer, Theodore R. Gibbons, et al. 2012. “Deep Sequencing of the Oral Microbiome Reveals Signatures of Periodontal Disease.” PloS One 7 (6): e37919.
- Liu, Yichen, Laura S. Weyrich, and Bastien Llamas. 2020. “More Arrows in the Ancient DNA Quiver: Use of Paleoepigenomes and Paleomicrobiomes to Investigate Animal Adaptation to Environment.” Molecular Biology and Evolution 37 (2): 307–319. Oxford Academic.
- Llamas, Bastien, Michelle L. Holland, Kefei Chen, Jennifer E. Cropley, Alan Cooper, Catherine M. Suter, and Carles Lalueza-Fox. 2012. “High-Resolution Analysis of Cytosine Methylation in Ancient DNA.” PloS One 7 (1): e30226.
- Loesche, Walter J. 1986. “Role of Streptococcus Mutans in Human Dental Decay.” Microbiological Reviews 50 (4): 353–380. American Society for Microbiology (ASM).
- Lubell, David, Mary Jackes, Henry Schwarcz, Martin Knyf, and Christopher Meiklejohn. 1994. “The Mesolithic-Neolithic Transition in Portugal: Isotopic and Dental Evidence of Diet.” Journal of Archaeological Science 21 (2): 201–216. Elsevier.
- Lukacs, John R. 2012. Oral Health in Past Populations: Context, Concepts and Controversies. A Companion to Paleopathology, 553–581. Chichester: Wiley-Blackwell. Wiley Online Library.
- Mackie, Meaghan, Jessica Hendy, Abigail D. Lowe, Alessandra Sperduti, Malin Holst, Matthew Collins, and Camilla F. Speller. 2017. “Preservation of the Metaproteome: Variability of Protein Preservation in Ancient Dental Calculus.” STAR: Science & Technology of Archaeological Research 3 (1): 58–70.
- Mackie, Meaghan, Anita Radini, and Camilla Filomena Speller, and Camilla Filomena. 2017. The Sustainability of Dental Calculus for Archaeological Research. In Shallow Pasts, Endless Horizons: Sustainability & Archaeology: Proceedings of the 48th Annual Chacmool Archaeology Conference, edited by Favreau, Julien and Patalano, Robert, 74–81.
- Mann, Allison E., Susanna Sabin, Kirsten Ziesemer, Ashild J. Vagene, Hannes Schroeder, Andrew Ozga, Krithivasan Sankaranarayanan, et al. 2018. “Differential Preservation of Endogenous Human and Microbial DNA in Dental Calculus and Dentin.” Scientific Reports 8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6026117/.
- Martin, Jeff M., Jim I. Mead, and Perry S. Barboza. 2018. “Bison Body Size and Climate Change.” Ecology and Evolution 8 (9): 4564–4574. Wiley Online Library.
- Maslowski, Kendle M., and Charles R. Mackay. 2011. “Diet, Gut Microbiota and Immune Responses.” Nature Immunology 12 (1): 5–9.
- Mathers, John C., Gordon Strathdee, and Caroline L. Relton. 2010. “Induction of Epigenetic Alterations by Dietary and Other Environmental Factors.” In Advances in Genetics, edited by Zdenko Herceg and Ushijima Toshikazu, 3–39. San Diego: Elsevier.
- Mathieson, Iain, Iosif Lazaridis, Nadin Rohland, Swapan Mallick, Nick Patterson, Songül Alpaslan Roodenberg, Eadaoin Harney, et al. 2015. “Genome-Wide Patterns of Selection in 230 Ancient Eurasians.” Nature 528 (7583): 499–503.
- Mayanagi, G., T. Sato, H. Shimauchi, and Nobuhiro Takahashi. 2004. “Detection Frequency of Periodontitis-Associated Bacteria by Polymerase Chain Reaction in Subgingival and Supragingival Plaque of Periodontitis and Healthy Subjects.” Oral Microbiology and Immunology 19 (6): 379–385. Wiley Online Library.
- Mays, S., D. Roberts, P. Marshall, A. W. G. Pike, V. van Heekeren, C. Bronk Ramsey, E. Dunbar, et al. 2018. “Lives Before and After Stonehenge: An Osteobiographical Study of Four Prehistoric Burials Recently Excavated from the Stonehenge World Heritage Site.” Journal of Archaeological Science: Reports 20: 692–710. Elsevier.
- McCall, Charles E., Barbara Yoza, Tiefu Liu, and Mohamed El Gazzar. 2010. “Gene-Specific Epigenetic Regulation in Serious Infections with Systemic Inflammation.” Journal of Innate Immunity 2 (5): 395–405.
- Milner, George R. 1984. “Dental-Caries in the Permanent Dentition of a Mississippian Period Population from the American Midwest.” Collegium Antropologicum 8 (1): 77–91.
- Mira, Alex, A. Simon-Soro, and M. A. Curtis. 2017. “Role of Microbial Communities in the Pathogenesis of Periodontal Diseases and Caries.” Journal of Clinical Periodontology 44 (S18): S23–S38.
- Moore, Nicole, and Laura Weyrich. 2021. A Standardized Approach for Shotgun Metagenomic Analysis of Ancient Dental Calculus. Oral Microbiome: Methods and Protocols. London: Springer.
- Muth, Thilo, Dirk Benndorf, Udo Reichl, Erdmann Rapp, and Lennart Martens. 2013. “Searching for a Needle in a Stack of Needles: Challenges in Metaproteomics Data Analysis.” Molecular BioSystems 9 (4): 578–585. The Royal Society of Chemistry.
- Nibali, Luigi, Anna Di Iorio, Yu-Kang Tu, and Alexandre R. Vieira. 2017. “Host Genetics Role in the Pathogenesis of Periodontal Disease and Caries.” Journal of Clinical Periodontology 44 (S18): S52–S78.
- Nyvad, Bente, Wim Crielaard, Alex Mira, Nobuhiro Takahashi, and David Beighton. 2013. “Dental Caries from a Molecular Microbiological Perspective.” Caries Research 47 (2): 89–102. Karger Publishers.
- Olalde, Iñigo, Selina Brace, Morten E. Allentoft, Ian Armit, Kristian Kristiansen, Thomas Booth, Nadin Rohland, et al. 2018. “The Beaker Phenomenon and the Genomic Transformation of Northwest Europe.” Nature 555 (7695): 190–196. Nature Publishing Group.
- Oppenheim, Frank G., Erdjan Salih, Walter L. Siqueira, Weimin Zhang, and Eva J. Helmerhorst. 2007. “Salivary Proteome and Its Genetic Polymorphisms.” Annals of the New York Academy of Sciences 1098 (1): 22–50. Malden, USA: Blackwell Publishing.
- Org, Elin, Brian W. Parks, Jong Wha J. Joo, Benjamin Emert, William Schwartzman, Eun Yong Kang, Margarete Mehrabian, et al. 2015. “Genetic and Environmental Control of Host-Gut Microbiota Interactions.” Genome Research 25 (10): 1558–1569. Cold Spring Harbor Lab.
- Orlando, Ludovic, M. Thomas P. Gilbert, and Eske Willerslev. 2015. “Reconstructing Ancient Genomes and Epigenomes.” Nature Reviews Genetics 16 (7): 395–408.
- Orlando, Ludovic, Aurélien Ginolhac, Guojie Zhang, Duane Froese, Anders Albrechtsen, Mathias Stiller, Mikkel Schubert, et al. 2013. “Recalibrating Equus Evolution Using the Genome Sequence of an Early Middle Pleistocene Horse.” Nature 499 (7456): 74–78. Nature Publishing Group.
- Orlando, Ludovic, and Eske Willerslev. 2014. “An Epigenetic Window into the Past?” Science 345 (6196): 511–512. American Association for the Advancement of Science.
- Overmyer, Katherine A., Timothy Rhoads, Anna E. Merrill, Zhan Ye, Michael S. Westphall, Amit Acharya, Sanjay K. Shukla, et al. 2020. “Proteomics, Lipidomics, Metabolomics and 16S RDNA Sequencing of Dental Plaque from Patients with Diabetes and Periodontal Disease.” BioRxiv. Cold Spring Harbor Laboratory.
- Oxenham, Marc, Lan C. Nguyen, and Kim Thuy Nguyen. 2006. “The Oral Health Consequences of the Adoption and Intensification of Agriculture in Southeast Asia.” In Bioarchaeology of Southeast Asia, edited by Marc Oxenham and Nancy Tayles, 263–289. Cambridge: Cambridge University Press.
- Ozga, Andrew T., Maria A. Nieves-Colón, Tanvi P. Honap, Krithivasan Sankaranarayanan, Courtney A. Hofman, George R. Milner, Cecil M. Lewis, Anne C. Stone, and Christina Warinner. 2016. “Successful Enrichment and Recovery of Whole Mitochondrial Genomes from Ancient Human Dental Calculus.” American Journal of Physical Anthropology 160 (2): 220–228.
- Pap, Ildiko, Anne-Marie Tillier, Baruch Arensburg, Steve Weiner, and Mario Chech. 1995. “First Scanning Electron Microscope Analysis of Dental Calculus from European Neanderthals: Subalyuk, (Middle Paleolithic, Hungary). Preliminary Report.” Bulletins et Mémoires de la Société d'anthropologie de Paris 7 (1): 69–72. Société d’Anthropologie de Paris.
- Park, O.-J., H. Yi, J. H. Jeon, S.-S. Kang, K.-T. Koo, K.-Y. Kum, J. Chun, C.-H. Yun, and S. H. Han. 2015. “Pyrosequencing Analysis of Subgingival Microbiota in Distinct Periodontal Conditions.” Journal of Dental Research 94 (7): 921–927. Los Angeles, CA: SAGE Publications Sage CA.
- Pedersen, J. S., E. Valen, A. M. V. Velazquez, B. J. Parker, M. Rasmussen, S. Lindgreen, B. Lilje, et al. 2014. “Genome-Wide Nucleosome Map and Cytosine Methylation Levels of an Ancient Human Genome.” Genome Research 24 (3): 454–466.
- Perry, George H., Nathaniel J. Dominy, Katrina G. Claw, Arthur S. Lee, Heike Fiegler, Richard Redon, John Werner, et al. 2007. “Diet and the Evolution of Human Amylase Gene Copy Number Variation.” Nature Genetics 39 (10): 1256–1260. Nature Publishing Group.
- Peters, Danielle L., Wenju Wang, Xu Zhang, Zhibin Ning, Janice Mayne, and Daniel Figeys. 2019. “Metaproteomic and Metabolomic Approaches for Characterizing the Gut Microbiome.” Proteomics 19 (16): 1800363. Wiley Online Library.
- Petersen, Poul Erik. 2004. “Challenges to Improvement of Oral Health in the Twenty-First Century – the Approach of the WHO Global Oral Health Programme.” International Dental Journal 54 (S6): 329–343. Wiley Online Library.
- Pietrusewsky, Michael, and Rona Ikehara-Quebral. 2006. “The Bioarchaeology of the Vat Komnou Cemetery, Angkor Borei, Cambodia.” Bulletin of the Indo-Pacific Prehistory Association 26: 86–97.
- Poole, Angela C., Julia K. Goodrich, Nicholas D. Youngblut, Guillermo G. Luque, Albane Ruaud, Jessica L. Sutter, Jillian L. Waters, et al. 2019. “Human Salivary Amylase Gene Copy Number Impacts Oral and Gut Microbiomes.” Cell Host & Microbe 25 (4): 553–564.e7. Elsevier.
- Preus, Hans R., Ole J. Marvik, Knut A. Selvig, and Pia Bennike. 2011. “Ancient Bacterial DNA (ADNA) in Dental Calculus from Archaeological Human Remains.” Journal of Archaeological Science 38 (8): 1827–1831.
- Quince, Christopher, Alan W. Walker, Jared T. Simpson, Nicholas J. Loman, and Nicola Segata. 2017. “Shotgun Metagenomics, from Sampling to Analysis.” Nature Biotechnology 35 (9): 833–844. Nature Publishing Group.
- Raitapuro-Murray, T., T. I. Molleson, and F. J. Hughes. 2014. “The Prevalence of Periodontal Disease in a Romano-British Population c. 200-400 AD.” British Dental Journal 217 (8): 459.
- Razzouk, Sleiman, and Omid Termechi. 2013. “Host Genome, Epigenome, and Oral Microbiome Interactions: Toward Personalized Periodontal Therapy.” Journal of Periodontology 84 (9): 1266–1271.
- Reiner, Steven L. 2005. “Epigenetic Control in the Immune Response.” Human Molecular Genetics 14 (suppl_1): R41–R46.
- Rhodin, K., K. Divaris, K. E. North, S. P. Barros, K. Moss, J. D. Beck, and S. Offenbacher. 2014. “Chronic Periodontitis Genome-Wide Association Studies.” Journal of Dental Research 93 (9): 882–890. Los Angeles, CA: SAGE Publications Sage CA.
- Rinke, Christian, Patrick Schwientek, Alexander Sczyrba, Natalia N. Ivanova, Iain J. Anderson, Jan-Fang Cheng, Aaron Darling, et al. 2013. “Insights into the Phylogeny and Coding Potential of Microbial Dark Matter.” Nature 499 (7459): 431–437.
- Ronderos, Mauricio, Bruce L. Pihlstrom, and James S. Hodges. 2001. “Periodontal Disease among Indigenous People in the Amazon Rain Forest.” Journal of Clinical Periodontology 28 (11): 995–1003.
- Rothschild, Daphna, Omer Weissbrod, Elad Barkan, Alexander Kurilshikov, Tal Korem, David Zeevi, Paul I. Costea, et al. 2018. “Environment Dominates Over Host Genetics in Shaping Human Gut Microbiota.” Nature 555 (7695): 210–215. Nature Publishing Group.
- Salter, Susannah J., Michael J. Cox, Elena M. Turek, Szymon T. Calus, William O. Cookson, Miriam F. Moffatt, Paul Turner, Julian Parkhill, Nicholas J. Loman, and Alan W. Walker. 2014. “Reagent and Laboratory Contamination Can Critically Impact Sequence-Based Microbiome Analyses.” BMC Biology 12 (1): 87.
- Sanders, Virginia M. 2006. “Epigenetic Regulation of Th1 and Th2 Cell Development.” Brain, Behavior, and Immunity 20 (4): 317–324.
- Santiago-Rodriguez, Tasha M., Gino Fornaciari, Stefania Luciani, Scot E. Dowd, Gary A. Toranzos, Isolina Marota, Raul J. Cano. 2016. “Taxonomic and Predicted Metabolic Profiles of the Human Gut Microbiome in Pre-Columbian Mummies.” FEMS Microbiology Ecology 92 (11). Accessed October 12, 2020. https://academic.oup.com/femsec/article/92/11/fiw182/2403164. Oxford Academic.
- Santiago-Rodriguez, Tasha M., and Gary A. Toranzos. 2018. “On Controls in Ancient Microbiome Studies, and Microbial Resilience in Ancient Samples.” Genes 9 (10): 471. Multidisciplinary Digital Publishing Institute.
- Scherf, A., R. Hernandez-Rivas, P. Buffet, E. Bottius, C. Benatar, B. Pouvelle, J. Gysin, M. Lanzer, et al. 1998. “Antigenic Variation in Malaria: In Situ Switching, Relaxed and Mutually Exclusive Transcription of Var Genes During Intra-Erythrocytic Development in Plasmodium Falciparum.” The EMBO Journal 17 (18): 5418–5426. Wiley.
- Schmidt, Bertil, and Andreas Hildebrandt. 2017. “Next-Generation Sequencing: Big Data Meets High Performance Computing.” Drug Discovery Today 22 (4): 712–717.
- Shaw, Richard. 2006. “The Epigenetics of Oral Cancer.” International Journal of Oral and Maxillofacial Surgery 35 (2): 101–108.
- Shiau, Harlan J., and Mark A. Reynolds. 2010. “Sex Differences in Destructive Periodontal Disease: A Systematic Review.” Journal of Periodontology 81 (10): 1379–1389.
- Shimizu, S., Y. Momozawa, A. Takahashi, T. Nagasawa, K. Ashikawa, Y. Terada, Y. Izumi, et al. 2015. “A Genome-Wide Association Study of Periodontitis in a Japanese Population.” Journal of Dental Research 94 (4): 555–561. Los Angeles, CA: SAGE Publications Sage CA.
- Simón-Soro, Aurea, and Alex Mira. 2015. “Solving the Etiology of Dental Caries.” Trends in Microbiology 23 (2): 76–82. Elsevier.
- Simón-Soro, Á., I. Tomás, R. Cabrera-Rubio, M. D. Catalan, B. Nyvad, and A. Mira. 2013. “Microbial Geography of the Oral Cavity.” Journal of Dental Research 92 (7): 616–621. SAGE Publications.
- Slots, Jørgen, and Miriam Ting. 1999. “Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis in Human Periodontal Disease: Occurrence and Treatment.” Periodontology 2000 20 (1): 82–121.
- Smith, Oliver, Alan J. Clapham, Pam Rose, Yuan Liu, Jun Wang, and Robin G. Allaby. 2015. “Genomic Methylation Patterns in Archaeological Barley Show De-Methylation as a Time-Dependent Diagenetic Process.” Scientific Reports 4: 5559. https://www.nature.com/articles/srep05559.
- Smith, Rick W. A., Cara Monroe, and Deborah A. Bolnick. 2015. “Detection of Cytosine Methylation in Ancient DNA from Five Native American Populations Using Bisulfite Sequencing.” PloS One 10 (5): e0125344.
- Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent Jr. 1998. “Microbial Complexes in Subgingival Plaque.” Journal of Clinical Periodontology 25 (2): 134–144. Wiley Online Library.
- Sofer, Tamar, John R. Shaffer, Mariaelisa Graff, Qibin Qi, Adrienne M. Stilp, Stephanie M. Gogarten, Kari E. North, Carmen R. Isasi, Cathy C. Laurie, and Adam A. Szpiro. 2016. “Meta-Analysis of Genome-Wide Association Studies with Correlated Individuals: Application to the Hispanic Community Health Study/Study of Latinos (HCHS/SOL).” Genetic Epidemiology 40 (6): 492–501. Wiley Online Library.
- Song, Gwan Gyu, and Young Ho Lee. 2013. “Associations Between FCGR2A Rs1801274, FCGR3A Rs396991, FCGR3B NA1/NA2 Polymorphisms and Periodontitis: A Meta-Analysis.” Molecular Biology Reports 40 (8): 4985–4993. Springer.
- Steckel, Richard H., and Jerome C. Rose. 2002. The Backbone of History: Health and Nutrition in the Western Hemisphere. Vol. 2. Cambridge: Cambridge University Press.
- Tang, Zheng-Zheng, Guanhua Chen, Qilin Hong, Shi Huang, Holly M. Smith, Rachana D. Shah, Matthew Scholz, and Jane F. Ferguson. 2019. “Multi-Omic Analysis of the Microbiome and Metabolome in Healthy Subjects Reveals Microbiome-Dependent Relationships Between Diet and Metabolites.” Frontiers in Genetics 10: 454. Frontiers.
- Tanzer, Jason M., Jill Livingston, and Angela M. Thompson. 2001. “The Microbiology of Primary Dental Caries in Humans.” Journal of Dental Education 65 (10): 1028–1037. Wiley Online Library.
- Tariq, Muhammad Usman, Muhammad Haseeb, Mohammed Aledhari, Rehma Razzak, Reza M. Parizi, and Fahad Saeed. 2020. “Methods for Proteogenomics Data Analysis, Challenges, and Scalability Bottlenecks: A Survey .” IEEE Access. IEEE.
- Thayer, Zaneta M., and Amy L. Non. 2015. “Anthropology Meets Epigenetics: Current and Future Directions.” American Anthropologist 117 (4): 722–735.
- Tiffon, Céline. 2018. “The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease.” International Journal of Molecular Sciences 19 (11): 3425. Multidisciplinary Digital Publishing Institute.
- Tomczyk, Jacek, A. Turska-Szybka, Marta Zalewska, and Dorota Olczak-Kowalczyk. 2017. “Reliability of the Assessment of Periodontal Disease in Historical Populations.” International Journal of Osteoarchaeology 27 (2): 206–216.
- Tsilivakos, M. G., S. K. Manolis, O. Vikatou, and M. J. Papagrigorakis. 2002. “Periodontal Disease in the Mycenean (1450–1150 BC) Population of Aghia Triada, W. Peloponnese, Greece.” International Journal of Anthropology 17 (2): 91–99.
- Turner, Christy G. 1979. “Dental Anthropological Indications of Agriculture among the Jomon People of Central Japan. X. Peopling of the Pacific.” American Journal of Physical Anthropology 51 (4): 619–635. Wiley Online Library.
- Ulloa, Pilar Cornejo, Monique H. van der Veen, and Bastiaan P. Krom. 2019. “Review: Modulation of the Oral Microbiome by the Host to Promote Ecological Balance.” Odontology 107: 437–448.
- Vandermeersch, B., B. Arensburg, A.-M. Tillier, Y. Rak, and S. Weiner. 1994. “Middle Palaeolithic Dental Bacteria from Kebara, Israel.” Comptes Rendus de l’Académie Des Sciences. Série 2. Sciences de La Terre et Des Planètes 319 (6): 727–731.
- Velsko, Irina M., James A. Fellows Yates, Franziska Aron, Richard W. Hagan, Laurent A. F. Frantz, Louise Loe, Juan Bautista Rodriguez Martinez, et al. 2019. “Microbial Differences Between Dental Plaque and Historic Dental Calculus are Related to Oral Biofilm Maturation Stage.” Microbiome 7 (1): 102.
- Velsko, Irina M., Katherine A. Overmyer, Camilla Speller, Lauren Klaus, Matthew J. Collins, Louise Loe, Laurent A. F. Frantz, et al. 2017. “The Dental Calculus Metabolome in Modern and Historic Samples.” Metabolomics 13 (11): 134. Springer.
- Viana, Michelle Beatriz, Fabiano Pereira Cardoso, Marina Gonçalves Diniz, Fernando Oliveira Costa, José Eustáquio da Costa, Ricardo Santiago Gomez, and Paula Rocha Moreira. 2011. “Methylation Pattern of IFN-γ and IL-10 Genes in Periodontal Tissues.” Immunobiology 216 (8): 936–941.
- Vieira, Alexandre R., Carolyn W. Gibson, Kathleen Deeley, Hui Xue, and Yong Li. 2015. “Weaker Dental Enamel Explains Dental Decay.” PLoS One 10 (4): e0124236. Public Library of Science.
- Vieira, Alexandre R., Katelyn M. Hilands, and Thomas W. Braun. 2015. “Saving More Teeth – a Case for Personalized Care.” Journal of Personalized Medicine 5 (1): 30–35. Multidisciplinary Digital Publishing Institute.
- Villotte, Sébastien, A. R. Ogden, and Erik Trinkaus. 2018. “Dental Abnormalities and Oral Pathology of the Pataud 1 Upper Paleolithic Human.” Bulletins et Mémoires de la Société d’Anthropologie de Paris 30 (3–4): 153–161.
- Wade, William G. 2013. “The Oral Microbiome in Health and Disease.” Pharmacological Research 69 (1): 137–143.
- Walker, Phillip, L. Sugiyama, and R. Chacon. 1998. “Diet, Dental Health, and Cultural Change among Recently Contacted South American Indian Hunter-Horticulturalists.” In Human Dental Development, Morphology, and Pathology: A Tribute to Albert A. Dahlberg. Vol. 54, edited by John R. Lukacs, 355–386. Eugene, OR: University of Oregon Anthropological Papers.
- Warinner, C., J. Hendy, C. Speller, E. Cappellini, R. Fischer, C. Trachsel, J. Arneborg, et al. 2014. “Direct Evidence of Milk Consumption from Ancient Human Dental Calculus.” Scientific Reports 4: 7104.
- Warinner, Christina, Alexander Herbig, Allison Mann, James A. Fellows Yates, Clemens L. Weiß, Hernán A. Burbano, Ludovic Orlando, and Johannes Krause. 2017. “A Robust Framework for Microbial Archaeology.” Annual Review of Genomics and Human Genetics 18: 321–356.
- Warinner, Christina, and Cecil Lewis. 2015. “Microbiome and Health in Past and Present Human Populations.” American Anthropologist 117 (4): 740–741.
- Warinner, Christina, João F. Matias Rodrigues, Rounak Vyas, Christian Trachsel, Natallia Shved, Jonas Grossmann, Anita Radini, et al. 2014. “Pathogens and Host Immunity in the Ancient Human Oral Cavity.” Nature Genetics 46 (4): 336.
- Warinner, Christina, Camilla Speller, and Matthew J. Collins. 2015. “A New Era in Palaeomicrobiology: Prospects for Ancient Dental Calculus as a Long-Term Record of the Human Oral Microbiome.” Philosophical Transactions of the Royal Society B: Biological Sciences 370 (1660): 20130376.
- Wasterlain, Sofia N., Eugénia Cunha, and Simon Hillson. 2011. “Periodontal Disease in a Portuguese Identified Skeletal Sample from the Late Nineteenth and Early Twentieth Centuries.” American Journal of Physical Anthropology 145 (1): 30–42.
- Waterland, Robert A., Dana C. Dolinoy, Juan-Ru Lin, Charlotte A. Smith, Xin Shi, and Kajal G. Tahiliani. 2006. “Maternal Methyl Supplements Increase Offspring DNA Methylation at Axin Fused.” Genesis 44 (9): 401–406. Wiley Online Library.
- Waterland, Robert A., and Randy L. Jirtle. 2003. “Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation.” Molecular and Cellular Biology 23 (15): 5293–5300. Am Soc Microbiol.
- Weber, Megan L., Hong-Yuan Hsin, Ersan Kalay, Dana Š. Brožková, Takehiko Shimizu, Merve Bayram, Kathleen Deeley, et al. 2014. “Role of Estrogen Related Receptor Beta (ESRRB) in DFN35B Hearing Impairment and Dental Decay.” BMC Medical Genetics 15 (1): 81. Springer.
- Weyrich, Laura S., Keith Dobney, and Alan Cooper. 2015. “Ancient DNA Analysis of Dental Calculus.” Journal of Human Evolution 79: 119–124. Special Issue: Ancient DNA and Human Evolution.
- Weyrich, Laura S., Sebastian Duchene, Julien Soubrier, Luis Arriola, Bastien Llamas, James Breen, Alan G. Morris, et al. 2017. “Neanderthal Behaviour, Diet, and Disease Inferred from Ancient DNA in Dental Calculus.” Nature 544 (7650): 357–361.
- White, Donald J. 1997. “Dental Calculus: Recent Insights into Occurrence, Formation, Prevention, Removal and Oral Health Effects of Supragingival and Subgingival Deposits.” European Journal of Oral Sciences 105 (5): 508–522.
- Wilkin, Shevan, Alicia Ventresca Miller, William T. T. Taylor, Bryan K. Miller, Richard W. Hagan, Madeleine Bleasdale, Ashley Scott, et al. 2020. “Dairy Pastoralism Sustained Eastern Eurasian Steppe Populations for 5,000 Years.” Nature Ecology & Evolution 4 (3): 346–355. Nature Publishing Group.
- Willing, Ben P., Johan Dicksved, Jonas Halfvarson, Anders F. Andersson, Marianna Lucio, Zongli Zheng, Gunnar Järnerot, Curt Tysk, Janet K. Jansson, and Lars Engstrand. 2010. “A Pyrosequencing Study in Twins Shows That Gastrointestinal Microbial Profiles Vary with Inflammatory Bowel Disease Phenotypes.” Gastroenterology 139 (6): 1844–1854.e1.
- Wu, Jing, Brandilyn A. Peters, Christine Dominianni, Yilong Zhang, Zhiheng Pei, Liying Yang, Yingfei Ma, et al. 2016. “Cigarette Smoking and the Oral Microbiome in a Large Study of American Adults.” The ISME Journal 10 (10): 2435–2446. Nature Publishing Group.
- Xie, Mengxi, Junrui Wu, Feiyu An, Xiqing Yue, Dongbing Tao, Rina Wu, and Yuankun Lee. 2019. “An Integrated Metagenomic/Metaproteomic Investigation of Microbiota in Dajiang-Meju, a Traditional Fermented Soybean Product in Northeast China.” Food Research International 115: 414–424.
- Xu, Duo, Pavlos Pavlidis, Recep Ozgur Taskent, Nikolaos Alachiotis, Colin Flanagan, Michael DeGiorgio, Ran Blekhman, Stefan Ruhl, and Omer Gokcumen. 2017. “Archaic Hominin Introgression in Africa Contributes to Functional Salivary MUC7 Genetic Variation.” Molecular Biology and Evolution 34 (10): 2704–2715.
- Yates, James A. Fellows, Franziska Aron, Gunnar U Neumann, Irina Velsko, Eirini Skourtanioti, Eleftheria Orfanou, Zandra Fagernas, et al. 2020. “A-Z of Ancient DNA Protocols for Shotgun Illumina Next Generation Sequencing.” Accessed January 19, 2021. https://www.protocols.io/view/a-z-of-ancient-dna-protocols-for-shotgun-illumina-bj8nkrve.
- Zhang, Shaoping, Antonino Crivello, Steven Offenbacher, Antonio Moretti, David W. Paquette, and Silvana P. Barros. 2010. “Interferon-gamma Promoter Hypomethylation and Increased Expression in Chronic Periodontitis.” Journal of Clinical Periodontology 37 (11): 953–961.
- Zhang, Xu, and Daniel Figeys. 2019. “Perspective and Guidelines for Metaproteomics in Microbiome Studies.” Journal of Proteome Research 18 (6): 2370–2380. American Chemical Society.
- Zhao, Hongsen, Min Chu, Zhengwei Huang, Xi Yang, Shujun Ran, Bin Hu, Chenping Zhang, and Jingping Liang. 2017. “Variations in Oral Microbiota Associated with Oral Cancer.” Scientific Reports 7 (1): 11773. Nature Publishing Group.
- Ziesemer, Kirsten A., Allison E. Mann, Krithivasan Sankaranarayanan, Hannes Schroeder, Andrew T. Ozga, Bernd W. Brandt, Egija Zaura, et al. 2015. “Intrinsic Challenges in Ancient Microbiome Reconstruction Using 16S RRNA Gene Amplification.” Scientific Reports 5, 16498. Nature Publishing Group.
- Ziesemer, Kirsten A., Jazmín Ramos-Madrigal, Allison E. Mann, Bernd W. Brandt, Krithivasan Sankaranarayanan, Andrew T. Ozga, Menno Hoogland, et al. 2019. “The Efficacy of Whole Human Genome Capture on Ancient Dental Calculus and Dentin.” American Journal of Physical Anthropology 168 (3): 496–509.
