ABSTRACT
Objective
To evaluate the changes in epithelial mapping using anterior segment OCT-based tomography (MS-39) after 1 week, 1, 3, and 6 months of manual versus transepithelial photorefractive keratectomy in patients with myopia and myopic astigmatism.
Methods
We designed, prospective, interventional paired eye study on 52 eyes who underwent photorefractive keratectomy to correct myopia and myopic astigmatism. Each eye of a participant was assigned to a different treatment group; 26 eyes underwent transepithelial Photorefractive keratectomy (transPRK) using Stream Light WaveLight E × 500were categorized as Group A “tPRK group” and 26 eyes underwent conventional PRK by manual epithelium removal and wavefront-optimized PRK using WaveLight E × 500excimer were categorized as Group B “mPRK group.” Epithelial mapping was evaluated for all cases preoperatively and postoperative for six months of follow-up.
Results
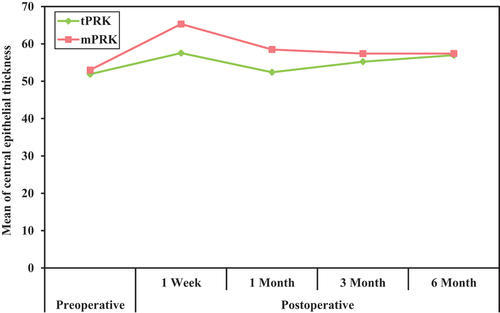
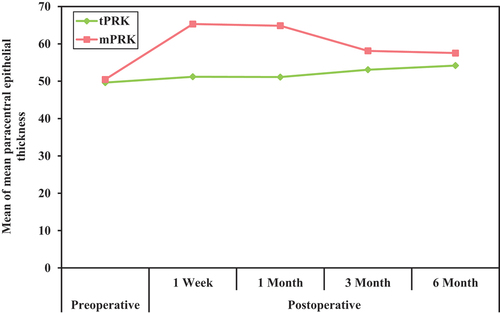
The postoperative central epithelial thickness increased in both groups; it was higher in group B (mPRK) with a statistically significant difference between both groups in the 1-week, 1-month (p < 0.001), and 3-month (p < 0.05) follow-up points with a mean of 57.54 µ,52.40 µ, and 55.23 µ in group A, respectively, and a mean of 65.31µ, 58.50 µ, and 57.41 µin group B, respectively. The postoperative paracentral epithelial thickness increased in both groups; it was higher in group B (mPRK) with a statistically significant difference between both groups at all postoperative follow-up points (p < 0.001) with a mean of 51.20µ, 51.12µ, 53.09µ, and 54.20 µ in group A while with a mean of 65.32µ,64.87µ,58.13µ, and 57.55µ in group B.
Conclusion
Epithelial thickness mapping after PRK shows epithelial hyperplasia in both mPRK and tPRK methods with a smoother and less fluctuant course in the transepithelial treated eyes, especially in the early preoperative period.
1. Introduction
The corneal epithelium is a stratified nonkeratinized squamous epithelium composed of 5–7 layers of cells. As the outermost layer of the cornea, it serves as a regenerative barrier for the eye and plays a crucial role in maximizing visual acuity by providing a smooth optical surface. On average, normal corneas have a mean epithelial thickness of approximately 53.4 ± 4.6μ at the corneal vertex. Recent studies have revealed that the thickness of the epithelium is not uniform, contrary to previous beliefs. The epithelium tends to be thicker in the inferior and nasal regions as compared to the superior and temporal regions. This observation can be attributed to the mechanism of blinking and the forces exerted by the eyelids [Citation1–3].
OCT was first used for measuring the epithelium in the early 2000s, avoiding the VHF digital ultrasound disadvantages as a non-contact method; it is important to consider that OCT images cannot distinguish the tear film from the epithelium when evaluating them. The first commercially available OCT-based instrument that provided epithelial mapping was the Optovue RT-100 (Optovue, Inc), with spectral-domain OCT technology, providing 6-mm diameter mapping, replaced by a 9-mm profile device (Avanti). The Cirrus HD (Carl Zeiss Meditec, Jena, Germany) and MS-39 (CSO, Firenze, Italy) are other spectral-domain OCT-based devices currently available for clinical use. The more recently introduced swept-source (SS) OCT-based devices like the Anterion (Heidelberg Engineering) and Casia 2 (Tomey Corporation) also feature epithelial mapping [Citation4–6].
The MS-39 (CSO, Firenze, Italy) the device used in the current study is a novel anterior segment OCT that employs hybrid technology, integrating a high-resolution SD-OCT-based tomography with twice the axial resolution of the best SS-OCT and Placido disk corneal topography. It has high specifications for capturing images, where the axial resolution of images is 3.5 µm in tissue, allowing for precise pachymetry and sublayer measurement (epithelial and stromal thickness). Good repeatability was demonstrated by MS-39 in normal eyes and those who had undergone excimer laser surgery [Citation7–9].
1.1. Epithelial remodeling after myopic refractive surgery
The corneal flattening in myopic PRK results in postoperative epithelial thickening. This epithelial hyperplasia is associated with deep stromal ablation depths and small size of ablation zones because there is a marked curvature change in the edges of the ablated area. Confocal microscopy studies have found that the central epithelial thickness after PRK returned to preoperative levels at one month. However, it continued to progressively increase during the first year, being 21% thicker at that time [Citation10,Citation11].
It has been suggested that epithelial hyperplasia can induce a reduction of the postoperative refractive effect. This refractive regression is a major factor limiting the predictability of myopic excimer laser surgery. The regression process is multifactorial and is not fully understood. However, epithelial remodeling is generally suspected to play an essential role. Evidence has shown that the corneal epithelium over the flattened region in myopic correction undergoes steady growth in the months and years after surgery. Hyperopic treatments have an even more significant amount of remodeling, potentially due to the steeper gradients used in the periphery for effective treatment. However, the contribution of these epithelial changes in the process of regression is still a point of debate [Citation12].
1.2. Photorefractive keratectomy (PRK)
PRK was introduced as the first corrective eye surgery that utilized a laser instead of a blade to remove corneal tissue in 1983 by Dr Steven Trokel and colleagues. Conventionally, the epithelial layer can be removed mechanically using a blunt hockey spatula or by alcohol-assisted debridement. However, mechanical removal tends to be a lengthy process for inexperienced surgeons, which subsequently reduces stromal hydration and increases patient anxiety; alcohol-assisted debridement has been found to cause inflammation and damage to underlying stromal keratocytes and also affect stromal hydration. Alternately, transepithelial PRK is a contactless method that involves epithelial removal through the use of an excimer laser [Citation13–15].
The transepithelial PRK concept was introduced by Alio et al. in the early 1990s and has undergone many evolutions and advancements since then. Transepithelial PRK addresses the drawbacks of conventional PRK by eliminating the need for instrument contact with the eye, decreasing intervention time, and minimizing the epithelial defect size to that required for ablation. It was initially introduced as a two-step procedure. The first step involved using excimer laser phototherapeutic keratectomy (PTK) to remove the epithelium, followed by stromal laser ablation as the second step. However, studies have shown that this technique has no significant superiority in efficacy or postoperative complications compared with mechanical PRK and that the mechanical technique tended to have better results in almost all clinical outcomes. Incorporating epithelial removal and stromal laser ablation in a single-step profile is a novel technology that overcomes the limitations of less uniform ablation of classic PTK-PRK procedure. The single-step profile has been found to offer superior clinical outcomes compared to the previous two-step procedures in terms of clinical outcomes, including reduced pain, less dehydration, no hyperopic shift, and faster re-epithelialization [Citation16–18].
With the introduction of single-step transepithelial PRK, many studies claim its smoother stromal bed and less effect on epithelial changes. In this study, we will evaluate changes in epithelial thickness after myopic PRK using two modalities of epithelial removal, the mechanical vs single-step transepithelial PRK (StreamLight™ platform) compare both groups together to study the effect of transepithelial PRK on post-operative epithelial remodeling.
2. Subjects and methods
This study was conducted as a prospective comparative interventional study.52 eyes of 26 patients who underwent photorefractive keratectomy to correct myopia and myopic astigmatism between June 2022 and June 2023 were enrolled in the study. The study adhered to the tenets of the Declaration of Helsinki. All patients signed a written informed consent to participate in the study and for publication of data before enrollment in the study.
The study was designed as a paired eye study in which each participant’s eye was assigned to a different treatment group, in paired eye study design 2 treatments are evaluated through within-participant comparison, and all participants are followed for outcomes in both eyes with 1 eye randomized to 1 treatment and the fellow eye randomized to another treatment. Twenty-six eyes underwent transepithelial Photorefractive keratectomy (tPRK) using StreamLight™ WaveLight E×500 which incorporates wavefront-optimized profile and categorized as Group A, “tPRK group,” and 26 eyes underwent conventional PRK by manual epithelium removal and wavefront-optimized PRK using WaveLight EX500, which was categorized as Group B, “mPRK group.” [Citation19]
The inclusion criteria include an age of 18 years or more, myopia and myopic astigmatism with a spherical equivalent between −2.0 and −6.0 D and a maximum astigmatism of −2.0 D, and Stable refraction for 1 year. Patients with corneal thickness at the thinnest location of less than 470 µm, a calculated residual stromal bed of less than 270 um, signs of abnormal topography suggesting keratoconus, corneal scarring, opacification, previous corneal and ocular disease, previous refractive surgery, and corneal or systemic disease known to affect wound healing, e.g. Diabetes mellitus was excluded from the study.
Preoperatively, a detailed eye examination was performed, including uncorrected and best-corrected visual acuity, refraction (manifest and cycloplegic), intraocular pressure measurement, fundus evaluation, and Pentacam analysis. Measurement of the epithelial thickness was done using the spectral domainMS-39 anterior segment OCT (Costruzione Strumenti Oftalmici, Florence, Italy).
Surgical procedure: Group A eyes underwent a single-step PRK using StreamLight™ WaveLight®EX500_Alcon, whereas group B eyes underwent conventional PRK by manual epithelium removal and wavefront-optimized PRK using the same machine. All surgeries were done by the same experienced surgeon with an optical zone diameter of 6.5 mm and a transition zone between 1 and 1.5 mm. After standard disinfection and draping, a topical anesthetic (benoxinate hydrochloride 0.4%) was applied (Benox 0.4%; EIPICO, Tenth of Ramadan City, Egypt). In Group A (right eye of each participant), tPRK was performed using the StreamLight™ platform with a depth of epithelial ablation of 55 µm. In Group B (left eye of each participant), the epithelium was removed manually using a hockey knife with a diameter of epithelial removal of 7 mm; then, a wavefront-optimized profile was used to achieve the desired emmetropia. In both groups, a topical application of mitomycin C (MMC; Zydus, Ahmedabad, Uttar Pradesh, India) 0.02% (0.2 mg/ml) diluted in balanced salt solution BSS (Alcon, Geneva, Switzerland) was applied with a microsponge placed over the ablated stroma for 20–30 s immediately after the laser ablation. The ocular surface was then irrigated vigorously with 20 ml of cold BSS. Bandage contact lenses were used for (5–6) days. Topical moxifloxacin (Vigamox; Novartis, Basel, Switzerland) and prednisolone acetate eye drops (Optipred; Jamjoom Pharma, Jeddah, Saudi Arabia) were prescribed postoperatively lubricant eye drops and gel (Systane eye drops, Alcon, Fort Worth, Texas, USA) were advised to use for the first 3 months. All patients were examined at one day, one week, one month, three, and six months postoperatively.
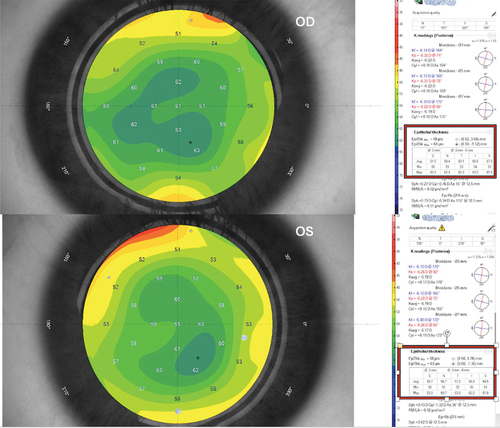
Epithelial Mapping: All patients were examined using MS-39 anterior segment OCT (Costruzione Strumenti Oftalmici, Florence, Italy) using the Pheonix v.4.0.057 software version preoperatively and postoperative at 1 week, 1, 3, and 6 months. The software measures the epithelial thickness in the central 6 mm of the cornea, dividing it into a central 3 mm and peripheral superior, inferior, nasal, and temporal 3–6 mm annulus; the mean of these paracentral quadrants was calculated. ()
Figure 1. Preoperative epithelial thickness map of the case (X) in both eyes. The associated table divides the measurement to central 3mm (C) and paracentral nasal (N), temporal (T), inferior (I), and superior (S) 3–6mm annulus. Both eyes show almost the same epithelial thickness.
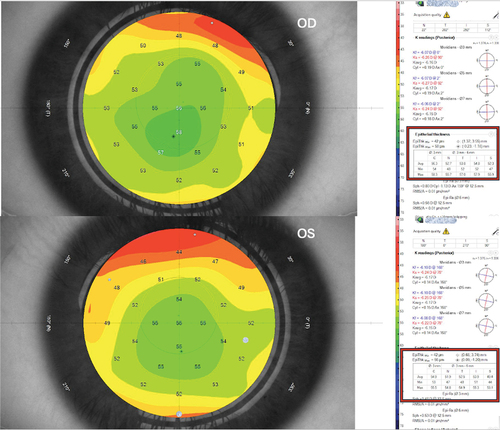
Figure 2. 1-week postoperative epithelial thickness map in both eyes for the same case. The associated table divides the measurement to central 3mm (C) and paracentral nasal (N), temporal (T), inferior (I), and superior (S) 3–6mm annulus. mPRK-treated eye shows increased central and paracentral epithelial thickness than tPRK-treated eye.
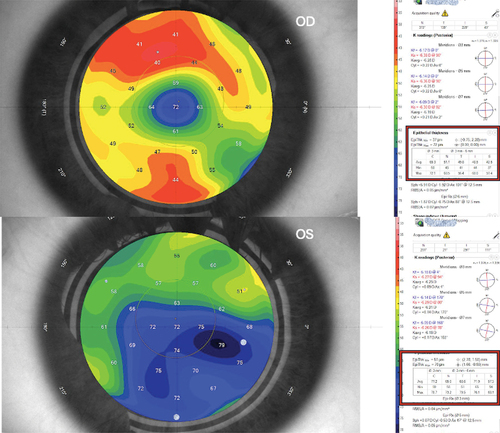
Figure 3. 6-month follow-up epithelial thickness map of the same case in both eyes. The associated table divides the measurement to central 3mm (C) and paracentral nasal (N), temporal (T), inferior (I), and superior (S) 3–6mm annulus. mPRK-treated eye shows slightly increased epithelial thickness than tPRK-treated eye.
2.1. Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The Shapiro–Wilk test was used to verify the normality of distribution. The significance of the obtained results was judged at the 5% level. Paired t-test was used to compare the two groups, tPRK and mPRK.
3. Results
The eyes included were 46 eyes of 23 female patients, “88.5%,” and 6 eyes of 3 male patients, “11.5%.” The age ranged from 18.0 to 38.0 years, with a mean of 25.42 ± 5.25.
The mean preoperative spherical equivalent of tPRK-treated eyes was −2.81 and that of mPRK-treated eyes was −2.72 D. Six months postoperative, the spherical equivalent of the tPRK group was −0.33 D, while that of the mPRK group was −0.04 D ().
Table 1. Comparison between tPRK and mPRK according to spherical equivalent.
The median preoperative UCVA was 0.79 LogMAR in tPRK-treated eyes and 0.78 LogMAR in mPRK-treated eyes. Six months postoperative the median UCVA was −0.08 LogMAR in the tPRK group and −0.04 LogMAR
in mPRK group ().
Table 2. Comparison between tPRK and mPRK according to Central epithelial thickness and mean Paracentral epithelial thickness.
The mean value of the epithelial thickness in the central 3 mm in group A was 51.89µ, while in group B, it was 53.0µin the preoperative evaluation, with no significant difference between both groups. Postoperatively, the mean value increased in both groups and was 57.54 µ in group A while 65.31 µ in group B in the first follow-up (1 week). One-month post-operative, it decreased to 52.40 µ in group A and 58.50 µin group B. Three months postoperative, it increased in group A to 55.23 µ, while it decreased in group B to 57.41 µ with a statistically significant difference between both groups in the 1-week,1-month, and 3-month postoperative follow-ups. Six months postoperative, the value was 56.97 µin group A and 57.42 µ in group B. There was no statistically significant difference between both groups at six months follow-up. () ()
The mean value of the epithelial thickness in all paracentral annulus in the preoperative evaluation in group A was 49.63 ± 3.16 µ, while in group B, it was 50.47 ± 2.81. There was no significant difference between both groups. Post-operative in group A, the value increased to 51.20 ± 7.65 µ in the first follow-up, changed to 51.12 ± 4.16 µ after one month, then continued to increase to 53.09 ± 3.03 µ in the three-month and 54.20 ± 2.88 µin the six-month follow-up. On the other hand, in group B, the mean value of the epithelial thickness in the paracentral zone dramatically increased to 65.32 ± 11.24 µ in the first follow-up visit, changed to 64.87 ± 9.68µ after one month, then decreased to 58.13 ± 5.02 µ in the three months and57.55 ± 3.53 µ in the six months follow-up visit. There was a statistically significant difference between both groups at each postoperative follow-up point. ()
The correlation between epithelial thickness and visual acuity at different follow-up point times was not significant.
4. Discussion
Applications of corneal epithelial mapping have gained a growing interest in recent years. Epithelial remodeling compensates for changes in the underlying stroma, thins over areas of steepened curvature, and thickens over flattened areas. This masking effect of epithelium makes epithelial mapping an essential tool in preoperative assessment and screening for keratoconus and the risk of ectasia [Citation20].
It has been reported that epithelial changes after refractive surgery are related to the rate of changes in curvature, diameter of the ablation zone, and stromal bed irregularity. With the introduction of single-step transepithelial PRK, many studies claim its smoother stromal bed and less effect on epithelial changes, apart from its contactless advantages and faster corneal reepithelization [Citation21,Citation22].
In this prospective comparative non-randomized study that was performed on 52 eyes of 26 patients, we evaluated changes in epithelial thickness before and after PRK for correction of myopia and myopic astigmatism using two modalities of epithelial removal, the mechanical vs single-step transepithelial PRK (StreamLight™ platform) with six months of postoperative follow-up and compared both groups together.
This study was designed as a paired eye (contralateral eye) study. When each eye of a participant is assigned to a different treatment group, the accuracy of the comparison is increased. This is because all individual-specific characteristics that may affect the measurement are perfectly balanced between groups and thus removed as sources of variability [Citation23].
Repeated thickness measures after PRK at different follow-up points showed increased epithelial thickness compared to the preoperative level in all studied zones in both groups, with a significant difference in thickness between both groups. ()
The central 3 mm zone showed epithelial hyperplasia in the 1st week of follow-up, and a marked significant difference was noticed between both groups (p < 0.001), with a mean of 57.54 µ in group A and a mean of 65.31 µ in group B. The significant difference between both groups continues to decrease to disappear after 6 months post-operative, with a mean of 56.97 µin group A and a mean of 57.42 µ in group B. ()
The paracentral 3–6 mm zone also showed epithelial hyperplasia in both groups in each quadrant (superior, inferior, nasal, and temporal) at different postoperative follow-up points. The mean of these paracentral quadrants was calculated. It showed increased epithelial thickness than the preoperative level, much noticed in the 1 week and 1-month follow-ups, with a significant difference between both groups, which is most prominent in the 1st week, with a mean of 51.20 µ in the tPRK group and a mean of 65.32 µ in the mPRK group. This difference between both groups continues to decrease but is still significant after 6 months postoperative (p < 0.001), with a mean of 54.20 µin the tPRK group and a mean of 57.55 µ in the mPRK group.
It was evident that both groups experienced a postoperative increase in epithelial thickness but with a smoother and less fluctuating course in the tPRK-treated eyes, especially in the early postoperative period (1 week-1 month), with significant hyperplasia in all studied zones in the mPRK group. However, both groups reach a comparable level at the end of the 6-month follow-up. This may be attributed to the smoother stromal bed produced in tPRK-treated eyes.
In agreement with our results, Chen et al. found in their study to evaluate the 6 mm central epithelial thickness in 46 eyes after manual PRK for myopia using spectral domain anterior segment OCT that the corneal epithelial thickness increased after PRK up to 6 months postoperatively, and this change in thickness was affected by the amount of myopia treated, treatment zone, and preoperative epithelial thickness [Citation12].
Sedaghat et al. also studied the changes in epithelial thickness in 52 eyes after myopic mechanical PRK and found a gradual epithelial thickening in the central and mid-peripheral corneal after 3 and 6 months postoperatively [Citation24].
Weng et al. evaluated in their study, which enrolled 162 eyes, the epithelial thickness and irregularity post-tPRK using the SCHWIND Amaris platform; they concluded that although spherical equivalent refraction and visual acuity achieved initial stability 1 month after t-PRK, central corneal epithelial thickness and the standard deviation of the corneal epithelial thickness took 3–6 months to progressive recovery [Citation25].
Gauthier and colleagues conducted a study to analyze the factors contributing to epithelial thickening after myopic PRK. According to their findings, small ablation zones, increased ablation depth, and a greater degree of myopic correction are the three most significant factors influencing corneal epithelial hyperplasia after PRK. They recommended using smoother ablation profiles to reduce epithelial hyperplasia [Citation26].
To our knowledge, only one publication evaluates the changes in epithelial thickness after myopic mechanical PRK vs. transepithelial PRK. In contrast to our result, they report an increase in the epithelial thickness in the tPRK group 1 week post-operative compared to the mechanical group. However, this study used the classic two-step PRK/PTK instead of single-step tPRK. The researcher did not clarify which value from the epithelial map they depended on in their evaluation, and they did not mention the difference between both groups in the following follow-ups (up to 3 months) [Citation27].
5. Limitation
The follow-up point time in this study was designed to assess the epithelial remodeling after complete healing at 1 week and 1, 3, and 6 months. Assessment of the duration needed for complete re-epithelialization was not included in this study as it requires daily post-operative follow-up for the first 5 postoperative days and this is a different follow-up schedule that was not included in our study.
6. Conclusion
Epithelial thickness mapping after PRK shows epithelial hyperplasia in both conventional and transepithelial methods with a smoother and less fluctuant course in the transepithelial-treated eyes, especially in the early preoperative period.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Gonzalez-Andrades M, Argüeso P, Gipson I. Corneal anatomy. In: Alió J, Alió del Barrio J Arnalich-Montiel F, editors. Corneal Regeneration. Essentials in ophthalmology. Cham: Springer; 2019. p. 3–12.
- Gipson IK, Stepp MA. Anatomy and cell biology of the cornea, superficial limbus, and conjunctiva. In: Albert D, Miller J, Azar D Young L, editors. Albert and Jakobiec’s Principles and Practice of Ophthalmology. Cham: Springer International Publishing; 2022. p. 3–30.
- Reinstein DZ, Archer TJ, Gobbe M, et al. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24(6):571–581.
- Wylegała E, Teper S, Nowińska AK, et al. Anterior segment imaging: Fourier-domain optical coherence tomography versus time-domain optical coherence tomography. J Cataract Refract Surg. 2009;35(8):1410–1414. doi: 10.1016/j.jcrs.2009.03.034
- Hwang ES, Schallhorn JM, Randleman JB. Utility of regional epithelial thickness measurements in corneal evaluations. SurvOphthalmol. 2020;65(2):187–204. doi: 10.1016/j.survophthal.2019.09.003
- Feng Y, Reinstein DZ, Nitter T, et al. Epithelial thickness mapping in keratoconic corneas: repeatability and agreement between CSO MS-39, Heidelberg Anterion, and Optovue Avanti OCT devices. J Refract Surg. 2023;39(7):474–480. doi: 10.3928/1081597X-20230606-01
- Elkitkat RS, Rifay Y, Gharieb HM, et al. Accuracy of the indices of MS-39 anterior segment optical coherence tomography in the diagnosis of keratoconic corneas. Eur J Ophthalmol. 2022;32(4):2116–2124. doi: 10.1177/11206721211063720
- MS-39. CSO ophtalmic, device for Anterior Segment Analysis of Eye. Available from cited 2023 Oct]. Available from: https://www.csoitalia.it/en/prodotto/info/63-ms-39
- Savini G, Schiano-Lomoriello D, Hoffer KJ. Repeatability of automatic measurements by a new anterior segment optical coherence tomographer combined with Placido topography and agreement with 2 scheimpflug cameras. J Cataract Refract Surg. 2018;44(4):471–478. doi: 10.1016/j.jcrs.2018.02.015
- Ivarsen A, Fledelius W, Hjortdal J. Three-year changes in epithelial and stromal thickness after PRK or LASIK for high myopia. Invest Ophthalmol Vis Sci. 2009;50(5):2061–2066. doi: 10.1167/iovs.08-2853
- Patel SV, Erie JC, Jw M, et al. Confocal microscopy changes in epithelial and stromal thickness up to 7 years after LASIK and photorefractive keratectomy for myopia. J Refract Surg. 2007;23(4):385–392. doi: 10.3928/1081-597X-20070401-11
- Chen X, Stojanovic A, Liu Y, et al. Postoperative changes in corneal epithelial and stromal thickness profiles after photorefractive keratectomy in treatment of myopia. J Refract Surg. 2015;31(7):446–453. doi: 10.3928/1081597X-20150623-02
- Shapira Y, Mimouni M, Levartovsky S, et al. Comparison of three epithelial removal techniques in PRK: mechanical, alcohol-assisted, and transepithelial laser. J Refract Surg. 2015;31(11):760–766. doi: 10.3928/1081597X-20151021-05
- Lee HK, Lee KS, Kim JK, et al. Epithelial healing and clinical outcomes in excimer laser photorefractive surgery following three epithelial removal techniques: mechanical, alcohol, and excimer laser. Am J Ophthalmol. 2005;139(1):56–63. doi: 10.1016/j.ajo.2004.08.049
- Sia RK, Ryan DS, Stutzman RD, et al. Alcohol versus brush PRK: visual outcomes and adverse effects. Lasers Surg Med. 2012;44(6):475–481. doi: 10.1002/lsm.22036
- Adib-Moghaddam S, Soleyman-Jahi S, Sanjari Moghaddam A, et al. Efficacy and safety of transepithelial photorefractive keratectomy. J Cataract Refract Surg. 2018;44(10):1267–1279. doi: 10.1016/j.jcrs.2018.07.021
- Gharieb HM, Allah MA, Ahmed AA, et al. Transepithelial laser versus alcohol assisted photorefractive keratectomy safety and efficacy: 1-year follow-up of a contralateral eye study. Korean J Ophthalmol. 2021;35(2):142. doi: 10.3341/kjo.2020.0105
- Clinch TE, Moshirfar M, Weis JR, et al. Comparison of mechanical and transepithelial debridement during photorefractive keratectomy. Ophthalmol. 1999;106(3):483–489. doi: 10.1016/S0161-6420(99)90135-5
- Dong R, Ying GS. Characteristics of design and analysis of ophthalmic randomized controlled trials: A review of ophthalmic papers 2020-2021. Ophthalmology Sci. [2022 Dec 31];3(2):100266. doi: 10.1016/j.xops.2022.100266
- Reinstein DZ, Archer TJ, Vida RS. Applications of epithelial thickness mapping in corneal refractive surgery. Saudi J Ophthalmol. 2022;36(1):25. doi: 10.4103/sjopt.sjopt_227_21
- Gaeckle HC. Early clinical outcomes and comparison between trans-PRK and PRK, regarding refractive outcome, wound healing, pain intensity and visual recovery time in a real-world setup. BMC Ophthalmol. 2021;21(1):181. doi: 10.1186/s12886-021-01941-3
- Abdelwahab SM, Salem MH, Elfayoumi MA. Single-step transepithelial photorefractive keratectomy in low to moderate myopia: a one-year follow-up study. Clin Ophthalmol. 2021;15:3305–3313. doi: 10.2147/OPTH.S326048
- Maguire MG. Assessing intereye symmetry and its implications for study design. Invest Ophthalmol Vis Sci. 2020;61(6):27. doi: 10.1167/iovs.61.6.27
- Sedaghat MR, Momeni-Moghaddam H, Gazanchian M, et al. Corneal epithelial thickness mapping after photorefractive keratectomy for myopia. J Refract Surg. 2019;35(10):632–642. doi: 10.3928/1081597X-20190826-03
- Weng TH, Chang YM, Lin FH, et al. Investigation of corneal epithelial thickness and irregularity by optical coherence tomography after transepithelial photorefractive keratectomy. Clin Exp Optom. 2024;107(1):23–31. doi: 10.1080/08164622.2023.2197107
- Gauthier CA, Holden BA, Epstein D, et al. Factors affecting epithelial hyperplasia after photorefractive keratectomy. J Cataract Refract Surg. 1997;23(7):1042–1050. doi: 10.1016/S0886-3350(97)80078-8
- El-Shahed AF, El-Shahed RF, Gaballah KA. Epithelial thickness mapping: mechanical photorefractive keratectomy versus transepithelial photorefractive keratectomy. Delta J Ophthalmol. 2022;23(4):226–233. doi: 10.4103/djo.djo_48_22