Abstract
Fungal infections are becoming increasingly prevalent in the human population and contribute to morbidity and mortality in healthy and immunocompromised individuals respectively. Candida albicans is the most commonly encountered fungal pathogen of humans, and is frequently found on the mucosal surfaces of the body. Host defense against C. albicans is dependent upon a finely tuned implementation of innate and adaptive immune responses, enabling the host to neutralise the invading fungus. Central to this protection are the adaptive Th1 and Th17 cellular responses, which are considered paramount to successful immune defense against C. albicans infections, and enable tissue homeostasis to be maintained in the presence of colonising fungi. This review will highlight the recent advances in our understanding of adaptive immunity to Candida albicans infections.
Introduction
The human body is a complex environment which is colonised both internally and externally by a huge number of different microbial species including bacteria, fungi and viruses. The maintenance of tissue homeostasis in the face of such overwhelming microbial diversity is critical to health and is dependent upon effective immune surveillance. Candida albicans is a polymorphic fungus found in the digestive tract and on other mucosal surfaces of the body (e.g. oral cavity and vagina). The fungus is considered to be a normal constituent of the microflora in ∼50% of the human populationCitation1 and is the cause of superficial mucosal infections such as oral and vaginal thrush which can occur following perturbations in the localized mucosal environment. C. albicans is also capable of causing life threatening illness and accounts for significant rates of mortality (40%) in the immunocompromised and those receiving immunosuppressive therapies.Citation2 The dichotomy between harmless carriage and the onset of potentially life threatening infection stems from 2 critically important factors, namely the presence or absence of an effective immune response and the ability of the fungus to alter its morphology.
C. albicans can grow in a number of distinct physical forms including unicellular yeast, pseudohyphae and hyphae.Citation1 The morphological transition(s) which occur during growth are reversible,Citation3-5 and such physical plasticity is believed to facilitate pathogenicity. Growth of C. albicans as unicellular yeast is typically associated with harmless colonisation (commensalism) whereas pseudohyphal and hyphal growth is more closely associated with infection. However, it is important to emphasize that this mutually exclusive view of morphology during health or disease is somewhat oversimplified, and may not accurately reflect the true situation during clinical pathogenesis where multiple morphologies are often encountered simultaneously. While infections of the mucosal surfaces are predominantly associated with the hyphal form of the fungus, widespread dissemination throughout the body is facilitated by the yeast morphology where cells bud-off from pre-established hyphae and transit to remote tissues and organs such as the kidneys.
A complex and dynamic relationship exists between C. albicans and the human host, the balance of which is influenced greatly by the immune system. Indeed, the pathogenic potential of C. albicans is primarily determined by the effectiveness of the host immune response. A state of relative co-existence is maintained between host and fungus in healthy individuals, whereby growth is restricted to the harmless commensal form. However, the morphological restrictions imposed upon fungal growth during health are removed in the absence of effective immune surveillance, which allow fungal burdens to increase as growth continues unchecked. The hyphae of C. albicans can breach mucosal surfaces causing infection. Hyphal growth causes damage to the underlying tissue and if it progresses to the point where access to the host vasculature is enabled, the fungus can disseminate throughout the body. Furthermore, many fungal infections also result from the use of indwelling medical devices including intravenous lines, catheters and drains, which bypass the physical barrier provided by the mucosal surface, facilitating access to the bloodstream. Such deep-seated systemic infections are a significant threat to life, and patients suffering from HIV/AIDS and those receiving immunosuppressive therapy are particularly susceptible.
Defense against microbial infection is provided by an exquisite interplay between the innate and adaptive arms of the host immune system which function together to eliminate pathogens from the body. In this review we will summarise the basic features of the adaptive immune response to C. albicans infection, describing recent advances in our understanding of adaptive immunity to this medically important fungus.
Host Recognition of Candida albicans: Dendritic Cells
Dendritic cells (DCs) are specialized antigen presenting cells (APCs) that play a central role in immune defense against pathogens, serving as a critical conduit between the innate and adaptive immune responses. DCs are vital for the initiation of adaptive T-cell-mediated immune protection against C. albicans. Immature DCs patrol the peripheral tissues beneath mucosal surfaces and are recruited to the site of infection in response to chemokines and antimicrobial peptides (e.g., CCL20Citation6-8 and β-defensin 2Citation9,10 respectively), secreted by epithelial cells in response to microbial infection. Once recruited, recognition of C. albicans by DCs occurs through interactions between pattern recognition receptors (PRRs) expressed on the surface of the DC and pathogen-associated molecular patterns (PAMPs) present on the fungal cell wall.Citation11
PRRs including C-type lectin receptors (CLRs)Citation12 such as dectin-1, dectin-2 and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), mannose receptor (MR), Mincle, Galectin-3, toll-like receptors (TLRs)Citation13 (such as TLR2 and TLR4), and complement receptor 3 (CR3) have all been associated with recognition of fungi. PRRs involved in fungal detection recognize conserved structural motifs present as part of the fungal cell wall, such as N-linkedCitation14 and O-linked mannans and β-glucans.Citation15 Although this initial interaction between host and fungus is driven primarily through innate rather than adaptive recognition, it is nevertheless a crucial event required for the initiation of an adaptive immune response. Once detected, the fungal cells are phagocytosed by DCs and degraded,Citation16 providing a source of exogenous protein which is processed into antigenic peptides within acidified vesicles. These fungal peptide antigens are assembled onto class II molecules of the major histocompatibility complex (MHC II) and subsequently transported to the surface of the activated DC. Acquired fungal antigens are presented to memory T-cells present in the local environment, and this process is accompanied by the migration of the DCs to the draining lymph nodes, where the antigens are presented to naive T-lymphocytes.
Interestingly, the activation of DCs during C. albicans infection results in different antigen-specific immune responses. Presentation of C. albicans antigens by Langerhans cells is required to elicit Th17 responses but does not promote the development of CD8+ cytotoxic T-lymphocyte (CTL) responses.Citation17 In contrast, Langerin+ dermal DCs stimulate both Th1 and CTL responses while simultaneously inhibiting the development of the Th17 response. Induction of a CTL response indicates that antigens derived from phagocytosis of fungal cells were cross-presented through the MHC I pathway for display to CD8+ T-lymphocytes. Thus, mixed populations of DCs have non-redundant functionality and can drive CD4+ and CD8+ T-cell responses against C. albicans.
T-lymphocytes sample the antigen presented to them using T-cell receptors (TCRs) expressed on their surface. Specificity to a potentially unlimited number of antigenic molecules is enabled by random variations in the amino acid sequence (and hence structure) of the antigen binding region of the TCR. Binding of the TCR to either MHC I or MHC II molecules displaying processed fungal antigen is facilitated by the expression of the co-receptors CD8+ and CD4+ respectively, and authenticated though interactions between CD28 expressed on the T-cell and CD80/CD86 on the APC. Recognition of antigen is accompanied by the secretion of cytokines which drive the activation and differentiation of the naive T-lymphocyte into one of a number of different possible T-helper (Th) subsets.
Signaling Events in DCs that Drive Th Subset Differentiation
The recognition of PAMPs by PRRs results in the activation of signaling pathways within the DC which ultimately results in the induction of a specific adaptive cellular immune response. The signaling events that transpire following fungal recognition by the DC and the induction of cellular immunity are both dynamic and complex, and have yet to be characterized in full.
The CLR dectin-1 is expressed on the surface of APCs and plays a central role in the orchestration of responses to C. albicans. Dectin-1 is comprised of an extracellular carbohydrate recognition domain and a partial immunoreceptor tyrosine-based activation (ITAM) motif and is the receptor for fungal cell wall β-glucan.Citation18 The recognition of fungal β-glucan by dectin-1 triggers receptor activation through phosphorylation of the cytoplasmic domainCitation19,20 which in turn leads to recruitment and activation of the spleen tyrosine kinase (SYK)Citation21 (). Association of phosphorylated dectin-1 with Syk triggers the assembly of the caspase recruitment domain (CARD) complex consisting of CARD9, Bcl-10 and MALT1.Citation19,20,Citation22 The importance of this process in adaptive immune responses to C. albicans can be demonstrated as CARD9−/− mice are unable to mount Th17 responses to oral infection.Citation23
Figure 1. Overview of adaptive T-cell responses to Candida albicans infection. A fungal PAMP (β-glucan) engages with the PRR dectin-1 (A), stimulating receptor phosphorylation and recruitment of the spleen tyrosine kinase (SYK). The association of dectin-1 with SYK activates assembly of the CARD complex (CARD9, BCL-10 and MALT-1), which stimulates nuclear translocation of the transcription factor NF-κB (B). The NF-κB transcription factor drives the expression of pro-inflammatory cytokines and co-stimulatory molecules required during antigen presentation. As well as signaling and gene transcription, activation of dectin-1 and recruitment of SYK triggers phagocytosis of C. albicans (C). The phagocytosed fungus is degraded in the phagocytic compartment and fungal antigens are loaded onto MHC II molecules for presentation to naive CD4+ T-cells. Recognition of antigen by a T-cell receptor (TCR) in the presence of co-stimulation from CD28 and CD80/86 (D) is followed by cytokine-directed polarization to one of the 4 known Th subsets (E). Th1 and Th17 cellular responses confer immune protection, whereas Th2 responses are considered refractory to fungal clearance.
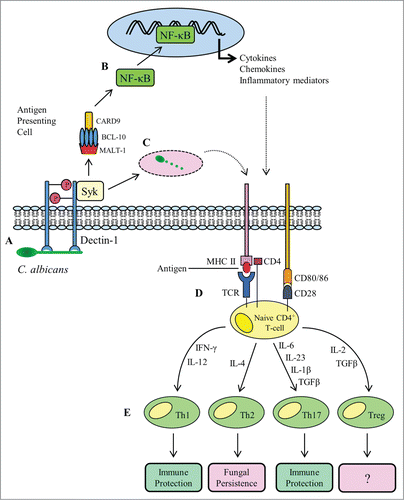
Assembly of the CARD complex leads to activation of the IκB kinase complex which promotes activation and nuclear translocation of the transcription factor NF-κBCitation22 (). Gene transcription mediated via NF-κB signaling results in secretion of pro-inflammatory cytokines and the concomitant upregulation of co-stimulatory molecules on the APC surface (), the net effect being the induction and differentiation of naive T-cells into distinct Th lineages (Th1, Th2, Th17, Treg). In addition to the “classical” signaling mediated through Syk and p65, dectin-1 can also signal through the non-cannonical NF-κB subunit RelB and importantly, through a second, Syk-independent pathway dependent upon Raf-124. All these pathways converge on NF-κB to drive Th1 and Th17 polarization, and subsequent adaptive immunity against C. albicans.Citation24
Other CLRs have also been reported as playing a role in driving adaptive immune responses to C. albicans, including dectin-2.Citation25 However in contrast to dectin-1 which, during canonical signaling, interacts with Syk directly, dectin-2 induces signaling through Syk indirectly by association with the FcRγ chain.Citation26 Blockade of dectin-2 expressed on DCs has been reported to reduce Th17 responses during systemic infection with C. albicans.Citation25
T-cell Responses to Candida albicans
T-lymphocytes (T-cells) are an integral component of the host adaptive immune response to C. albicans infection and provide direct and indirect means of controlling fungal proliferation. Both CD4+ (T helper cells) and CD8+ (CTL) T-cells have been shown to play a role in anti-fungal immunity, and their activation is controlled by dendritic cell populations. Although CTLs have been shown to inhibit the growth of C. albicans hyphae in vitro,Citation27 the principal mechanism of adaptive immune priming employed by DCs occurs through the presentation of fungal antigen to naive CD4+ T-cells, generating a T-helper (Th) response. The CD4+ Th cell response is the predominant cell-mediated adaptive immune response to C. albicans infection at mucosal surfaces. Unlike CTLs, CD4+ T-cells do not possess direct cytolytic activity but nevertheless play a crucial role in the cellular adaptive response to fungal infection. The importance of the Th cellular response in driving protective immunity against C. albicans is highlighted by the prevalence of oropharangeal candidiasis (OPC) in HIV+/AIDS patients where the CD4+ T-cell count is depleted.Citation28,29 Indeed, the correlation between HIV/AIDS and C. albicans infection in the oral cavity is now such that the presence of OPC is now widely regarded as a reliable predictor of low CD4+ cell count.Citation28,29
There are 4 different Th subsets (Th1, Th2, Th17 and Treg), and the development of each specific subset is dictated by the cytokines and microenvironment present at the instance of naive CD4+ T-cell priming by DCs. The cytokines that drive the differentiation of each particular Th phenotype are inhibitory to the development of the others, thereby maximising the potential that only one type of Th response is initiated at any one time. This so called “polarization” of Th differentiation has a profound impact on the outcome of the adaptive response and is heavily dependent upon the prevailing cytokine milieu. Th1 responses were historically regarded as being the predominant defensive cellular response to C. albicans, resulting in fungal clearance from the oral cavity and gastrointestinal tract. However, this view of the importance and effective contribution of the Th1 phenotype to protection against C. albicans infection at mucosal surfaces has since been superseded by the Th17 response. In contrast to the protective Th17/Th1 phenotypes, induction of a Th2 phenotype is more closely associated with increased growth and dissemination of the fungus.
DCs that phagocytose C. albicans yeast cells are stimulated to produce interleukin-12 (IL-12), which drives polarization to the Th1 subset. Upon stimulation with IL-12, Th1 cells initiate autocrine signaling via secretion of interferon-gamma (IFN-γ) which serves to upregulate the expression of the IL-12Rβ2 receptor rendering the cells increasingly sensitive to IL-12 stimulation, thereby perpetuating differentiation to the Th1 phenotype.Citation30 In contrast, polarization to the Th2 phenotype is driven by IL-4 and is accompanied by further secretion of IL-4.
IL-17 secreting T-lymphocytes (Th17 cells) are an additional subset of Th cell which express the chemokine receptors CCR4 and CCR6 on their surfaceCitation31 and are developmentally distinct from the Th1 and Th2 lineages.Citation32 Th17 cells secrete numerous cytokines including IL-17A, IL-17F and IL-22 and are critically important for immune protection against C. albicans at the majority of mucosal sites in the body. Indeed, such is the involvement of Th17 cells in the immune response to oral and dermal candidiasis they are now regarded as the predominant cell type that confer protection against C. albicans at these locations.Citation33,34 Interestingly, IL-17 and IL-22 do not contribute to immune protection in vaginal mucosa,Citation35 highlighting the subtle differences in immune response requirements at different mucosal sites.Citation33,34 IL-17 plays a key role in recruiting and activating neutrophils,Citation36 while IL-22 enhances epithelial barrier function.Citation37 Differentiation of naive CD4+ T-cells to the Th17 phenotype is driven initially by IL-1β,Citation38,39 while maturation and terminal differentiation is dependent upon IL-23 signaling.Citation40 Th17 differentiation is further influenced by IL-6, which has been shown to be produced by epithelial cells in response to C. albicans infection.Citation41 Notably, the production of both IL-6 and IL-23 from antigen presenting cells results from recognition of C. albicans mannan.Citation42
More recently, an additional class of natural Th17 (nTh17) cells have been described that are phenotypically distinct from conventional CD4+ Th17 cells.Citation43-45 nTh17 cells function as innate sentinels in the oral mucosa and together with γδ T cells, secrete IL-17 in response to C. albicans.Citation45 Notably, γδ T cells produce large quantities of IL-17,Citation45 yet again highlighting the close relationship between innate recognition of C. albicans and downstream adaptive immune responses. It is also important when considering immune responses to C. albicans to be aware that many of the cytokines now thought to be important in these responses (e.g. IL-17A, IL-22) can also be produced by innate lymphoid cells (ILCs) such as the aforementioned nTh17 cells, γδ T cells, as well as ILC3 cells. Further, many of the in vivo models of mucosal C. albicans infection are skewed toward assaying innate rather than adaptive immune responses given the timescales over which these models are carried out.
The importance of the Th17 phenotype cytokines in anti-Candida responses is most vividly portrayed by data from both knockout mice and human patients. Mice unable to produce IL-23 are highly susceptible to OPC,Citation46 while mice lacking IL-17 receptor-A (IL-17RA−/-) or IL-23p19 (IL-23p19−/-) have increased susceptibility to both OPC and systemic candidiasis.Citation36,47 Moreover, IL-17RC−/- mice (deficient in the second IL-17 receptor chain) are also susceptible to OPC.Citation48 Both IL-17 and IL-23 are essential in preventing fungal skin infectionsCitation33 and Th17 cells secrete IL-22 which limits fungal growth.Citation37
In addition to acquired disorders of CD4+ T-cell immunity that predispose to C. albicans infection (HIV/AIDS), the critical nature of the protection provided by IL-17/Th17 cells is further emphasized when one considers the impact of inherited genetic mutations which affect the efficacy of IL-17/Th17 responses in otherwise healthy individuals.
Patients with inherited disorders in Th17-mediated anti-fungal immunity frequently present with chronic mucocutaneous candidiasis (CMC), which manifests as severe infection of the nails, skin and upper gastrointestinal tract. IL-12 and IL-23 are important cytokines for the development of Th1 and Th17 responses respectively. The human IL12RB1 gene encodes for an integral component of the receptor to IL-12 and IL-23, and approximately 25% of patients deficient in IL-12Rβ1 are prone to CMC.Citation49,50 In patients with autosomal dominant Hyper-IgE syndrome, the differentiation, development and number of circulating Th17 cells is significantly reducedCitation51-53 due to mutations in Signal Transducer and Activator of Transcription 3 (STAT3), and CMC is a key phenotype in these individuals.Citation54,55 Further, Th17 development and associated anti-fungal activity is also impaired by gain-of-function mutations in STAT1.Citation56
A marked reduction in the number of IL-17 secreting T-cells is observed in patients carrying the dominant-negative autosomal recessive Q258X mutation in caspase recruiting domain-containing protein 9 (CARD9)Citation57 and this mutation has been associated with increased susceptibility to fungal infection.Citation58 Furthermore, individuals with autosomal recessive autoimmune polyendocrinopathy syndrome 1 (APS-1), caused by mutations in the gene encoding the human autoimmune regulator protein (AIRE)Citation59,60 produce neutralising antibodies against IL-17A, IL-17F and IL-22. The continual depletion of these key Th17 cytokines results in an associated CMC.Citation61,62 Importantly, the susceptibility of APS-1 patients to opportunistic pathogens is seemingly restricted to C. albicans alone, highlighting the specific and essential contribution of IL-17 to anti-C. albicans immunity. Moreover, both an autosomal recessive mutation of glutamine 284 to a premature termination codon in the receptor for IL-17A (IL-17 RA) and an autosomal dominant mutation (S65L) in IL-17F also predispose individuals to CMC.Citation63 A T536I mis-sense mutation in ACT1 (an adaptor protein involved in IL-17 signaling) is reported to reduce activity and impaired IL-17A and IL-17F-mediated immunity. Patients harbouring the ACT1 mutation have T-cells which are unresponsive to IL-17E and an increased prevalence of CMC.Citation64
One of the classical hallmarks of adaptive immunity is the establishment of permanent immunological memory against a specific antigen, which can be brought to bear against a pathogen in the face of a secondary immune challenge. Long-term adaptive immunity against C. albicans has been observed in mice challenged and then re-challenged with the fungus. In this system, a stable and robust antigen-specific adaptive Th17 immune response against C. albicans was reported,Citation34 but the establishment of bona fide immunological memory remains to be fully demonstrated. Taken collectively, these studies clearly demonstrate the essential role that Th17 cells, their associated cytokines and by extension, adaptive immunity, play in combating C. albicans infections.
Antibody Responses to Candida albicans
Endogenous antibody responses to C. albicans infection in humans are regarded as playing a relatively minor role in immune protection against the fungus, and are widely considered to be significantly less effective than cellular (Th17/Th1) responses. Due to their accessibility, molecules displayed on the cell surface of C. albicans provide ideal targets for antibody-mediated immune protection. Mannoproteins with complex O- and N-linked mannose polysaccharides are an integral component of the C. albicans cell wallCitation65 and are a major target for anti-Candida antibodies. The binding of antibodies to exposed cell surface components of the infecting fungus may serve to hinder or prevent biological function. Indeed, monoclonal antibodies generated against mannoprotein interfere with fungal adhesion to host substrates and germ tube formation,Citation66 while patient-derived antibodies against a 58 kDa cell surface mannoprotein of the fungus prolonged survival following systemic infection in mice.Citation67
Antibodies generated in mice against the surface mannan of C. albicans can confer varying degrees of protection, depending upon whether the subsequent fungal infection is mucosal or systemic.Citation68 Vaccination with C. albicans mannan rendered mice less susceptible to disseminated candidiasis and polyclonal serum from vaccinated animals conferred protection to both naive mice and those with severe combined immune-deficiency (SCID).Citation69 Administration of a recombinant human monoclonal antibody against C. albicans mannan to mice enabled prolonged survival following an otherwise lethal inoculum of the fungus.Citation70
The agglutinin-like sequence proteins of C. albicans also reside at the cell surface, and monoclonal antibodies which bind to Als3p interfere with adhesion to epithelial surfaces, filamentation, acquisition of iron, and also possess fungicidal activity.Citation71,72 Interestingly, antibody-mediated protection against C. albicans is not restricted to cell surface molecules alone. Antibody-mediated inhibition of secreted aspartyl proteinase (SAP) activity was reported to bestow increased protection against vaginal infection in rats,Citation73 while human antibodies specific for C. albicans heat shock protein 90 (Hsp90) protected against systemic candidiasis in mice.Citation74
Despite these observations, there is the paradox that B-cell deficiency in mice does not cause increased susceptibility to C. albicans infection,Citation75-77 highlighting the lack of robust antibody-mediated protection and emphasizing the predominance of adaptive cellular responses.
Given the extremely modest levels of protection conferred by antibodies in response to C. albicans infection, it could be argued that the inherent immunogenicity of C. albicans antigens is limited in their natural context when presented to B-cells. However, it is also clear that purified antigens, when presented to the immune system in conjunction with a suitable carrier protein and adjuvant are capable of eliciting the production of antigen-specific antibodies that can confer some, albeit limited, protection (see Vaccines to Candida albicans).
Subversion of adaptive immune responses by Candida albicans
As with most pathogens, C. albicans has developed mechanisms for avoiding immune responses. This can include evasion of recognition events or even subversion of normal immune responses. The mechanisms by which C. albicans manages this are varied. For example, the cellular immune response driven during infection can be influenced by fungal morphology. Monocytes that phagocytose C. albicans yeast cells or germ tubes were unable to differentiate into DCs, and internalisation of germ tubes was reported to render cells incapable of inducing Th polarization.Citation78 Further, the yeast and hyphal forms of C. albicans exert opposing effects on DCs, skewing Th polarization induced both in vitro and in vivo.Citation79,80 Such a polarization of the cytokine response may function to subvert Th subset differentiation to those which enable fungal persistence within the host. DCs which phagocytose C. albicans yeast cells or those pulsed with yeast cell RNA promote development of Th1 responses, leading to fungal clearance, whereas DCs that internalise hyphae or those which receive hyphal RNA generate Th2 responses leading to fungal perpetuation.Citation79,80 Importantly, DCs pulsed ex vivo with yeast but not hyphae were reported to confer anti-fungal protection when adoptively transferred into mice.Citation79 In addition to morphological influences, C. albicans can secrete a soluble factor which reduces IL-17 production from human peripheral blood mononuclear cells in vitro,Citation81 suggesting that the fungus may have the potential to dampen host Th17 responses. Furthermore, it has also been suggested that the presence of IL-17A may facilitate fungal adaptation to the host environment.Citation82
T-regulatory (Treg) cells are known to play a central role in the regulation of cellular immune responses to microbial infection. The induction of disseminated candidiasis in mice was reported to drive the expansion of a population of splenic Foxp3+ Treg cells resulting in the exacerbation of disease pathology. Interestingly, subsets of Foxp3+ Treg cells were observed to induce Th17 responses, albeit to the detriment of the host.Citation83 In contrast to these findings is the observation that Treg cells consume IL-2 (thereby preventing IL-2-mediated inhibition of Th17 polarization), assisting the generation of protective Th17 responses mounted during murine OPC.Citation84 Clearly, the roles(s) played by Treg cells in response to C. albicans infection have yet to be characterized in full.
Taken together it appears probable, whether intentional or otherwise, that C. albicans is able to influence the outcome of the host immune response in some circumstances, possibly to circumvent immune clearance and facilitate persistence.
The role of the inflammasome in the adaptive immune response to Candida albicans
The complex interplay between innate and adaptive immune responses that enables fungal clearance is further demonstrated by the involvement of the inflammasome in response to fungal challenge. Inflammasomes are cytosolic multi-protein complexes consisting of a PRR, an adaptor protein (e.g., ASC) and caspase-1, whose assembly is triggered via innate recognition of PAMPs by intracellular nucleotide-binding domain and leucine-rich repeat-containing (NLR) proteins or AIM2-like receptors (ALRs).Citation85 It is becoming increasingly clear that these structures play an important functional role in the adaptive immune response to fungal infection. The end point of inflammasome activation is the production of fully mature inflammatory cytokines IL-1β and IL-18. IL-1β and IL-18 are produced as inactive precursors that undergo caspase-mediated cleavage to yield biologically active molecules. Inflammasome activity is required to drive caspase-dependent maturation of IL-1β and IL-18, with subsequent effects on adaptive Th1 and Th17 cellular responses.Citation38,86,87 Accordingly, while the initiation of inflammasome activity is associated with innate immunity, the production of functionally mature IL-1β and IL-18 nevertheless influence the outcome of the adaptive immune response.
A number of different inflammasomes are known to be involved in the response to C. albicans infection. By far the best characterized of these is the NLRP3 inflammasome which consists of Nlrp3 complexed with apoptosis-associated speck-like protein with caspase recruitment domain (ASC) and caspase-1. The ability to produce mature IL-1β and IL-18 through the NLRP3 inflammasome is crucial for adaptive cellular protection against C. albicans. Activation of the NLRP3 inflammasome is triggered by C. albicans hyphae,Citation88 and has an impact in signaling through other PRRs involved in anti-Candida immunity. For example, NLRP3/ASC caspase-1 activity is a critical factor in SYK/CARD9 signaling induced by TLR2 and dectin-1.Citation89,90 Further, secretion of IL-1β from DCs stimulated with fungal β-glucan required NLRP3 inflammasome activity and cells deficient in either Nlrp3 or ASC were impaired in their ability to secrete IL-1β.Citation91 The importance of the NLRP3 inflammasome in anti-Candida responses can be seen in studies using knock-out mice. Mice lacking in either TLR2, dectin-1, caspase-1 or Nlrp3 are highly susceptible to systemic C. albicans infection,Citation88-90 while mice unable to produce caspase-1 or ASC were more susceptible to disseminated candidiasis and exhibited reduced Th1/Th17 reactivity concomitant with increased fungal burden in the kidneys.Citation92 These inflammasome effects are important for more than cell-mediated immunity, with studies showing that NLRP3 activity was required for the generation of antigen-specific antibody mediated immune protection against C. albicans in vivo.Citation91 In addition to NLRP3-mediated protection, immune defense against C. albicans infection can also be orchestrated through the NLRC4 inflammasome which functions to regulate resistance to infection in the oral cavity and limits early systemic dissemination following oral infection in vivo.Citation93
NLRP10 is another member of the NLR family which contributes to adaptive immune protection against disseminated candidiasis in vivoCitation94 and is expressed on DCs and CD4+, but not CD8+ T-cells. Mice lacking NLRP10 exhibited impaired Th1 and Th17 responses to C. albicans infection concomitant with an increased presence of both yeast and hyphae in the renal cortex and medulla.Citation94 Importantly, while adaptive cellular responses were negatively affected by the absence of NLRP10, activation of the NLRP3 inflammasome and secretion of IL-1β were not affected, indicating that while both NLRP3 and NLRP10 contribute to adaptive protection against C. albicans infection, they do so by different pathways.Citation94 As can be seen, the exact role of inflammasome activation in adaptive immune responses is difficult to isolate, given the parallel role these complexes play in innate immunity. The reality is likely to be a multifaceted interplay between the different inflammasome complexes and fungal PAMPs, resulting in complementary activation of both innate and adaptive immunity. It should be noted that the cytokine cocktail produced by inflammasome activity (IL-1β and IL-18) will help drive the development of a Th17 phenotype in naïve T-cells, thus the innate immune activation of inflammasomes can still have an impact on adaptive immune responses.
Since NLRs are intracellular receptors, the activation of inflammasome assembly typically proceeds following phagocytosis/internalisation of the infecting fungus. However, recognition of C. albicans by DCs expressing the extracellular PRR dectin-1 can also trigger the assembly of a non-canonical inflammasome complex consisting of CARD9, Bcl-10, MALT1, ASC and caspase-8, rather than caspase-195. Assembly of this multi-protein complex enables processing of inactive IL-1β to the active form in a caspase-8 dependent manner. Intriguingly, while activation of the NLRP3/caspase-1 inflammasome by C. albicans is dependent upon internalisation of the fungus, blocking the internalisation of C. albicans does not affect caspase-8-dependent maturation of IL-1β from the non-canonical inflammasome complex.Citation95 Hence, induction of protective Th17-based immunity against C. albicans can be triggered following intracellular and extracellular recognition of the fungus in a manner dependent upon the activity of classical and non-canonical inflammasomes respectively.
Compartmentalisation of Immunity
C. albicans is known to infect multiple mucosal sites including the oral and vaginal cavities in particular. Each of these surfaces has their own immune mechanisms and susceptibilities to infection. For example, while oral thrush is generally associated with significant underlying changes, such as denture use or immune deficiencies (AIDS, transplantation therapy, cancer treatments), vulvovaginal candidiasis (VVC) is a much more common occurrence in the general population. Indeed, ∼75% of women of fertile age will experience at least one incidence of VVCCitation96,97 and it has been estimated that 5–10% of women will suffer from repeated or chronic episodes of VVC.Citation98,99 It is therefore highly likely that there are differences in the immunity generated at each of these different sites. Interestingly, adaptive Th17 responses appear to play no role in vaginal protection to C. albicans, with no change in the vaginal fungal burden of IL-23p19−/-, IL-17RA−/- and IL-22−/- mice compared with wild type controls.Citation100 This highlights the fact that although there are common anti-fungal mucosal immune responses, it is also highly likely that there is a varying degree of specificity for each of these mucosal surfaces to the fungus. In particular, it is noteworthy that responses to systemic infection differ from responses at mucosal surfaces. Systemic responses for example, are still regarded as being predominantly Th1, while mucosal responses are now known to be predominantly Th17 in nature.
The contribution of adaptive immune responses against C. albicans during vaginal infection remains somewhat unclear. Despite participating in adaptive immune protection at a number of different sites in the body,Citation101-103 Th1 cells play no significant role in protection against C. albicans in the vaginal environment,Citation104 and a precise biological role for this so called “compartmentalisation of immunity” has yet to be unequivocally established.
In an experimental model of VVC, DCs were detected in the draining lymph nodes of the surrounding vaginal tissue. However, the predominant subset of DC found within the nodes were plasmacytoid dendritic cells (pDCs),Citation105 which are associated with immunological tolerance and poor induction of T-cell proliferation,Citation106 rather than myeloid DCs which promote inflammatory responses and fungal clearance. Importantly, the pDCs were detected prior to, and throughout the entire course of infection and did not upregulate expression of MHC II, CD80 or CD86,Citation105 consistent with a localized, tissue-specific tolerance to the fungus.
The involvement of Th17 responses to C. albicans infection in the vagina have yet to be fully elucidated. Induction of VVC in mice stimulated the production of both IL-17 and IL-23, together with a potent influx of neutrophils.Citation107 However, despite the role played by IL-17/Th17 in driving neutrophil recruitment to the sites of infection, the presence of neutrophils was ineffectual and the severity of infection was not diminished.Citation107 Interestingly, the secretion of IL-17 was noted to influence the production of the anti-microbial peptides β-defensin 2 and β-defensin 3. Reducing the level of IL-17 exacerbated VVC severity while simultaneously reducing the level of β-defensin 2, whereas production of β-defensin 2 was increased following addition of recombinant IL-17.Citation107
In contrast to the above study, recruitment of neutrophils during vaginal infection was observed in response to the presence of S100A8 and S100A9 alarmins,Citation35 and no role for the involvement of either IL-17 or the Th17 pathway was demonstrated.Citation100 Given the involvement of the S100 alarmins and β-defensins, defense against C. albicans in the vaginal environment thus appears to be mediated through innate rather than adaptive means in murine models of VVC. Indeed, the PRRs TLR4 and SIGNR1 have been implicated in S100 alarmin signaling in vitro, although the situation appears to be more complex in vivo.Citation108 Importantly, and in keeping with the observations made above, the symptoms caused by intravaginal challenge with live, unattenuated C. albicans in human subjects was attributed to potent innate rather than adaptive responses.Citation109
Vaccines to Candida Albicans
Much attention is now being given to the development of vaccines to establish long-lived immunological memory against C. albicans such that a robust and targeted adaptive immune response can be rapidly invoked upon secondary fungal challenge. The proteins selected for inclusion into anti C. albicans vaccines are predominantly (but not exclusively) those factors considered to be a requirement for virulence. One such class of molecules are the agglutinin-like sequence (Als) adhesins, which are required for the attachment of C. albicans to host surfaces during infection.Citation110,111
By far the most well studied candidate for an anti C. albicans vaccine is Als3p (amino acids 17–432, referred to as NDV-3). Subcutaneous injection of NDV-3 in the presence of an adjuvant stimulated adaptive Th1/Th17 immune protection against C. albicans bloodstream infection in mice, resulting in the recruitment of activated phagocytes which facilitated fungal clearance from infected tissues.Citation112 Consistent with the differences in immune responses at different mucosal surfaces, vaccination of mice with NDV-3 results in the production of anti-Als3p IgG and IgA antibodies and reduced fungal burdens in vaginal tissues in a manner dependent upon both T- and B-lymphocytes, rather than the purely cell-mediated protection seen in bloodstream infections.Citation113 NDV-3 was also reported as being highly efficacious in a mouse model of OPC ,Citation114 has proven itself to be safe and immunogenic in human subjects, and is now being assessed in clinical trials.Citation115
Mice vaccinated with an N-terminal region of Als1p are protected against a lethal innoculum of C. albicans,Citation116 with vaccination resulting in reduced fungal burdens in tissue following infection with C. albicansCitation117 and other Candida species.Citation118 Importantly, protection afforded by this vaccine is mediated via cellular rather than humoral means, as vaccination is still successful in B-cell deficient mice.Citation116 An MHC II-bound peptide fragment corresponding to a conserved region of ALS family proteins has been isolated from DCs infected with C. albicans.Citation119 The isolated peptide has been reported to act as a Th17 epitope and was observed to protect mice from fatal systemic candidiasis.Citation119
Other families of C. albicans virulence factors have also shown promise as potential vaccine candidates, including the secreted aspartyl proteinases (Saps).Citation120 Immunisation of mice with Sap2p confers protective immunity in the face of an otherwise lethal systemic challenge of C. albicans.Citation121 Rats receiving intravaginal immunisation with amino acids 77–400 of Sap2p were protected against subsequent vaginal challenge with C. albicans in a manner dependent upon the generation of anti-Sap2p IgG and IgA.Citation122 As well as virulence factor vaccines, other formulations have been designed based on structural motifs. A vaccine containing the algal β-glucan laminarin has been reported to improve immune protection againstCitation123 both vaginal (mucosal) and systemic C. albicans infection in mice.Citation123 Despite this progress, success remains limited. No vaccines to C. albicans are clinically available at the time of writing. However, given the prevalence of fungal infections, particularly in the face of increasing resistance to commonly used anti-fungal therapies, the field of C. albicans vaccine design will no doubt continue to flourish and remain an area of intense study.
Concluding Remarks
Adaptive immune responses to C. albicans are crucial to the successful eradication of infecting fungus. Despite tremendous advances in our understanding of the molecular events that underpin adaptive immunity to this opportunistic fungal pathogen, there is still much to be discovered. It is worth noting here that much of our understanding of the adaptive immune responses to C. albicans is based on in vitro studies. However, these studies have so far correlated with in vivo findings and have provided us with a map of events in adaptive immune responses to this medically important fungal pathogen. Critically, as our understanding of the complex relationship between innate and adaptive immunity to C. albicans continues to evolve, and in vivo data are continually refined, future developments will no doubt enable the provision of improved medical outcomes for those who suffer from C. albicans infections.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Odds FC. Candida and Candidosis. London: Baillière Tindall, 1988.
- Giusiano G, Mangiaterra M, Garcia Saito V, Rojas F, Gomez V, Diaz MC. Fluconazole and itraconazole resistance of yeasts isolated from the bloodstream and catheters of hospitalized pediatric patients. Chemotherapy 2006; 52:254-9; PMID:16899974; http://dx.doi.org/10.1159/000094867
- Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 1997; 277:105-9; PMID:9204892; http://dx.doi.org/10.1126/science.277.5322.105
- Braun BR, Kadosh D, Johnson AD. NRG1, a repressor of filamentous growth in C.albicans, is down-regulated during filament induction. EMBO J 2001; 20:4753-61; PMID:11532939; http://dx.doi.org/10.1093/emboj/20.17.4753
- Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, MacCallum D, Schnell N, Talibi D, Marechal D, Tekaia F, et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J 2001; 20:4742-52; PMID:11532938; http://dx.doi.org/10.1093/emboj/20.17.4742
- Thorley AJ, Goldstraw P, Young A, Tetley TD. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol 2005; 32:262-7; PMID:15618437; http://dx.doi.org/10.1165/rcmb.2004-0196OC
- Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci U S A 2001; 98:13722-7; PMID:11717433; http://dx.doi.org/10.1073/pnas.241308598
- Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med 2000; 192:705-18; PMID:10974036; http://dx.doi.org/10.1084/jem.192.5.705
- Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dental Res 2005; 84:445-50; PMID:15840781; http://dx.doi.org/10.1177/154405910508400509
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999; 286:525-8; PMID:10521347; http://dx.doi.org/10.1126/science.286.5439.525
- Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol 2010; 31:346-53; PMID:20705510; http://dx.doi.org/10.1016/j.it.2010.06.007
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 2012; 13:817-22; PMID:22910394; http://dx.doi.org/10.1038/ni.2369
- Bourgeois C, Kuchler K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Front Cell Infect Microbiol 2012; 2:142; PMID:23189270; http://dx.doi.org/10.3389/fcimb.2012.00142
- Pietrella D, Bistoni G, Corbucci C, Perito S, Vecchiarelli A. Candida albicans mannoprotein influences the biological function of dendritic cells. Cell Microbiol 2006; 8:602-12; PMID:16548886; http://dx.doi.org/10.1111/j.1462-5822.2005.00651.x
- Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 2002; 196:407-12; PMID:12163569; http://dx.doi.org/10.1084/jem.20020470
- Newman SL, Holly A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect Immun 2001; 69:6813-22; PMID:11598054; http://dx.doi.org/10.1128/IAI.69.11.6813-6822.2001
- Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 2011; 35:260-72; PMID:21782478; http://dx.doi.org/10.1016/j.immuni.2011.06.005
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007; 8:31-8; PMID:17159984; http://dx.doi.org/10.1038/ni1408
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005; 22:507-17; PMID:15845454; http://dx.doi.org/10.1016/j.immuni.2005.03.004
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005; 106:2543-50; PMID:15956283; http://dx.doi.org/10.1182/blood-2005-03-1239
- Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 2006; 6:33-43; PMID:16341139; http://dx.doi.org/10.1038/nri1745
- Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Förster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006; 442:651-6; PMID:16862125; http://dx.doi.org/10.1038/nature04926
- Bishu S, Hernandez-Santos N, Simpson-Abelson MR, Huppler AR, Conti HR, Ghilardi N, Mamo AJ, Gaffen SL. The adaptor CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect Immun 2014; 82:1173-80; PMID:24379290; http://dx.doi.org/10.1128/IAI.01335-13
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol 2009; 10:203-13; PMID:19122653; http://dx.doi.org/10.1038/ni.1692
- Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 2009; 206:2037-51; PMID:19703985; http://dx.doi.org/10.1084/jem.20082818
- Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD Jr, Ariizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 2006; 281:38854-66; PMID:17050534; http://dx.doi.org/10.1074/jbc.M606542200
- Beno DW, Stover AG, Mathews HL. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J Immunol 1995; 154:5273-81; PMID:7730631
- de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev 2004; 17:729-59, table of contents; PMID:15489345; http://dx.doi.org/10.1128/CMR.17.4.729-759.2004
- Fidel PL, Jr. Candida-host interactions in HIV disease: implications for oropharyngeal candidiasis. Adv Dental Res 2011; 23:45-9; PMID:21441480; http://dx.doi.org/10.1177/0022034511399284
- Smeltz RB, Chen J, Ehrhardt R, Shevach EM. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J Immunol 2002; 168:6165-72; PMID:12055229; http://dx.doi.org/10.4049/jimmunol.168.12.6165
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007; 8:639-46; PMID:17486092; http://dx.doi.org/10.1038/ni1467
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6:1123-32; PMID:16200070; http://dx.doi.org/10.1038/ni1254
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol 2010; 185:5453-62; PMID:20921529; http://dx.doi.org/10.4049/jimmunol.1001153
- Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol 2013; 6:900-10; PMID:23250275; http://dx.doi.org/10.1038/mi.2012.128
- Yano J, Lilly E, Barousse M, Fidel PL, Jr. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun 2010; 78:5126-37; PMID:20823201; http://dx.doi.org/10.1128/IAI.00388-10
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 2004; 190:624-31; PMID:15243941; http://dx.doi.org/10.1086/422329
- De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 2010; 3:361-73; PMID:20445503; http://dx.doi.org/10.1038/mi.2010.22
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009; 30:576-87; PMID:19362022; http://dx.doi.org/10.1016/j.immuni.2009.02.007
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 2012; 484:514-8; PMID:22466287; http://dx.doi.org/10.1038/nature10957
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 2009; 10:314-24; PMID:19182808; http://dx.doi.org/10.1038/ni.1698
- Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010; 8:225-35; PMID:20833374; http://dx.doi.org/10.1016/j.chom.2010.08.002
- Smeekens SP, van de Veerdonk FL, van der Meer JW, Kullberg BJ, Joosten LA, Netea MG. The Candida Th17 response is dependent on mannan- and beta-glucan-induced prostaglandin E2. Int Immunol 2010; 22:889-95; PMID:21059767; http://dx.doi.org/10.1093/intimm/dxq442
- Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol 2009; 10:1125-32; PMID:19734905; http://dx.doi.org/10.1038/ni.1783
- Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, Kubo M. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol 2009; 183:7523-30; PMID:19890042; http://dx.doi.org/10.4049/jimmunol.0803828
- Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med 2014; PMID:25200028
- Farah CS, Hu Y, Riminton S, Ashman RB. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol Immunol 2006; 21:252-5; PMID:16842510; http://dx.doi.org/10.1111/j.1399-302X.2006.00288.x
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 2009; 206:299-311; PMID:19204111; http://dx.doi.org/10.1084/jem.20081463
- Ho AW, Shen F, Conti HR, Patel N, Childs EE, Peterson AC, Hernández-Santos N, Kolls JK, Kane LP, Ouyang W, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol 2010; 185:1063-70; PMID:20554964; http://dx.doi.org/10.4049/jimmunol.0903739
- Ouederni M, Sanal O, Ikinciogullari A, Tezcan I, Dogu F, Sologuren I, Pedraza-Sánchez S, Keser M, Tanir G, Nieuwhof C, et al. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor beta1 deficiency. Clin Infect Dis 2014; 58:204-13; PMID:24186907; http://dx.doi.org/10.1093/cid/cit722
- de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Jannière L, Rose Y, de Suremain M, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010; 89:381-402; PMID:21057261; http://dx.doi.org/10.1097/MD.0b013e3181fdd832
- de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Jannière L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 2008; 205:1543-50; PMID:18591412; http://dx.doi.org/10.1084/jem.20080321
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 2008; 205:1551-7; PMID:18591410; http://dx.doi.org/10.1084/jem.20080218
- Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, Zhu Q, Jansson AF, Barboza J, Schimke LF, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol 2008; 122:181-7; PMID:18602572; http://dx.doi.org/10.1016/j.jaci.2008.04.037
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007; 448:1058-62; PMID:17676033; http://dx.doi.org/10.1038/nature06096
- Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 2007; 357:1608-19; PMID:17881745; http://dx.doi.org/10.1056/NEJMoa073687
- Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 2011; 208:1635-48; PMID:21727188; http://dx.doi.org/10.1084/jem.20110958
- Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 2009; 361:1727-35; PMID:19864672; http://dx.doi.org/10.1056/NEJMoa0810719
- Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 2013; 121:2385-92; PMID:23335372; http://dx.doi.org/10.1182/blood-2012-08-450551
- Aaltonen J, Horelli-Kuitunen N, Fan JB, Bjorses P, Perheentupa J, Myers R, Palotie A, Peltonen L. High-resolution physical and transcriptional mapping of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy locus on chromosome 21q22.3 by FISH. Genome Res 1997; 7:820-9; PMID:9267805
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Positional cloning of the APECED gene. Nat Genet 1997; 17:393-8; PMID:9398839; http://dx.doi.org/10.1038/ng1297-393
- Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachée-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 2010; 207:291-7; PMID:20123958; http://dx.doi.org/10.1084/jem.20091983
- Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 2010; 207:299-308; PMID:20123959; http://dx.doi.org/10.1084/jem.20091669
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011; 332:65-8; PMID:21350122; http://dx.doi.org/10.1126/science.1200439
- Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 2013; 39:676-86; PMID:24120361; http://dx.doi.org/10.1016/j.immuni.2013.09.002
- Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev 2008; 72:495-544; PMID:18772287; http://dx.doi.org/10.1128/MMBR.00032-07
- Moragues MD, Omaetxebarria MJ, Elguezabal N, Sevilla MJ, Conti S, Polonelli L, Pontón J. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect Immun 2003; 71:5273-9; PMID:12933874; http://dx.doi.org/10.1128/IAI.71.9.5273-5279.2003
- Viudes A, Lazzell A, Perea S, Kirkpatrick WR, Peman J, Patterson TF, Martinez JP, López-Ribot JL. The C-terminal antibody binding domain of Candida albicans mp58 represents a protective epitope during candidiasis. FEMS Microbiol Lett 2004; 232:133-8; PMID:15033231; http://dx.doi.org/10.1016/S0378-1097(04)00042-4
- Han Y, Morrison RP, Cutler JE. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun 1998; 66:5771-6; PMID:9826353
- Han Y, Cutler JE. Antibody response that protects against disseminated candidiasis. Infect Immun 1995; 63:2714-9; PMID:7790089
- Zhang MX, Bohlman MC, Itatani C, Burton DR, Parren PW, St Jeor SC, Kozel TR. Human recombinant antimannan immunoglobulin G1 antibody confers resistance to hematogenously disseminated candidiasis in mice. Infect Immun 2006; 74:362-9; PMID:16368991; http://dx.doi.org/10.1128/IAI.74.1.362-369.2006
- Brena S, Omaetxebarria MJ, Elguezabal N, Cabezas J, Moragues MD, Ponton J. Fungicidal monoclonal antibody C7 binds to Candida albicans Als3. Infect Immun 2007; 75:3680-2; PMID:17452471; http://dx.doi.org/10.1128/IAI.01840-06
- Brena S, Cabezas-Olcoz J, Moragues MD, Fernandez de Larrinoa I, Dominguez A, Quindos G, Pontón J. Fungicidal monoclonal antibody C7 interferes with iron acquisition in Candida albicans. Antimicrob Agents Chemother 2011; 55:3156-63; PMID:21518848; http://dx.doi.org/10.1128/AAC.00892-10
- De Bernardis F, Boccanera M, Adriani D, Spreghini E, Santoni G, Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun 1997; 65:3399-405; PMID:9234804
- Matthews RC, Burnie JP, Howat D, Rowland T, Walton F. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology 1991; 74:20-4; PMID:1718852
- Carrow EW, Hector RF, Domer JE. Immunodeficient CBA/N mice respond effectively to Candida albicans. Clin Immunol Immunopathol 1984; 33:371-80; PMID:6388927; http://dx.doi.org/10.1016/0090-1229(84)90308-8
- Jensen J, Warner T, Balish E. Resistance of SCID mice to Candida albicans administered intravenously or colonizing the gut: role of polymorphonuclear leukocytes and macrophages. J Infect Dis 1993; 167:912-9; PMID:8383723; http://dx.doi.org/10.1093/infdis/167.4.912
- Jensen J, Warner T, Balish E. The role of phagocytic cells in resistance to disseminated candidiasis in granulocytopenic mice. J Infect Dis 1994; 170:900-5; PMID:7930734; http://dx.doi.org/10.1093/infdis/170.4.900
- Torosantucci A, Romagnoli G, Chiani P, Stringaro A, Crateri P, Mariotti S, Teloni R, Arancia G, Cassone A, Nisini R. Candida albicans yeast and germ tube forms interfere differently with human monocyte differentiation into dendritic cells: a novel dimorphism-dependent mechanism to escape the host's immune response. Infect Immun 2004; 72:833-43; PMID:14742527; http://dx.doi.org/10.1128/IAI.72.2.833-843.2004
- d'Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med 2000; 191:1661-74; PMID:10811860; http://dx.doi.org/10.1084/jem.191.10.1661
- Bacci A, Montagnoli C, Perruccio K, Bozza S, Gaziano R, Pitzurra L, Velardi A, d'Ostiani CF, Cutler JE, Romani L. Dendritic cells pulsed with fungal RNA induce protective immunity to Candida albicans in hematopoietic transplantation. J Immunol 2002; 168:2904-13; PMID:11884461; http://dx.doi.org/10.4049/jimmunol.168.6.2904
- Cheng SC, van de Veerdonk F, Smeekens S, Joosten LA, van der Meer JW, Kullberg BJ, Netea MG. Candida albicans dampens host defense by downregulating IL-17 production. J Immunol 2010; 185:2450-7; PMID:20624941; http://dx.doi.org/10.4049/jimmunol.1000756
- Zelante T, Iannitti RG, De Luca A, Arroyo J, Blanco N, Servillo G, Sanglard D, Reichard U, Palmer GE, Latgè JP, et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat Commun 2012; 3:683; PMID:22353714; http://dx.doi.org/10.1038/ncomms1685
- Whibley N, Maccallum DM, Vickers MA, Zafreen S, Waldmann H, Hori S, Gaffen SL, Gow NA, Barker RN, Hall AM. Expansion of Foxp3(+) T-cell populations by Candida albicans enhances both Th17-cell responses and fungal dissemination after intravenous challenge. Eur J Immunol 2014; 44:1069-83; PMID:24435677; http://dx.doi.org/10.1002/eji.201343604
- Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity 2011; 34:422-34; PMID:21435589; http://dx.doi.org/10.1016/j.immuni.2011.03.002
- Lamkanfi M, Dixit Vishva M. Mechanisms and Functions of Inflammasomes. Cell 2014; 157:1013-22; PMID:24855941; http://dx.doi.org/10.1016/j.cell.2014.04.007
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31:331-41; PMID:19682929; http://dx.doi.org/10.1016/j.immuni.2009.08.001
- Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol 2011; 186:5738-48; PMID:21471445; http://dx.doi.org/10.4049/jimmunol.1003597
- Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 2009; 183:3578-81; PMID:19684085; http://dx.doi.org/10.4049/jimmunol.0901323
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 2009; 5:487-97; PMID:19454352; http://dx.doi.org/10.1016/j.chom.2009.05.002
- Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009; 459:433-6; PMID:19339971; http://dx.doi.org/10.1038/nature07965
- Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol 2009; 183:8061-7; PMID:20007575; http://dx.doi.org/10.4049/jimmunol.0902477
- van de Veerdonk FL, Joosten LA, Shaw PJ, Smeekens SP, Malireddi RK, van der Meer JW, Kullberg BJ, Netea MG, Kanneganti TD. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol 2011; 41:2260-8; PMID:21681738; http://dx.doi.org/10.1002/eji.201041226
- Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 2011; 7:e1002379
- Joly S, Eisenbarth SC, Olivier AK, Williams A, Kaplan DH, Cassel SL, Flavell RA, Sutterwala FS. Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. J Immunol 2012; 189:4713-7; PMID:23071280; http://dx.doi.org/10.4049/jimmunol.1201715
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 2012; 13:246-54; PMID:22267217; http://dx.doi.org/10.1038/ni.2222
- Sobel JD. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci 1988; 544:547-57; PMID:3063184; http://dx.doi.org/10.1111/j.1749-6632.1988.tb40450.x
- Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis 1992; 14 Suppl 1:S148-53; PMID:1562688; http://dx.doi.org/10.1093/clinids/14.Supplement_1.S148
- Foxman B, Muraglia R, Dietz JP, Sobel JD, Wagner J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. J Lower Genital Tract Dis 2013; 17:340-5; PMID:23486072; http://dx.doi.org/10.1097/LGT.0b013e318273e8cf
- Fidel PL, Jr. Immunity in vaginal candidiasis. Curr Opin Infect Dis 2005; 18:107-11; PMID:15735412; http://dx.doi.org/10.1097/01.qco.0000160897.74492.a3
- Yano J, Kolls JK, Happel KI, Wormley F, Wozniak KL, Fidel PL, Jr. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS One 2012; 7:e46311
- Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle KH, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis 1995; 171:1279-88; PMID:7751704; http://dx.doi.org/10.1093/infdis/171.5.1279
- Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol 1993; 150:925-31; PMID:8093707
- Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun 1991; 59:4647-54; PMID:1682265
- Fidel PL, Jr. History and update on host defense against vaginal candidiasis. Am J Reproduct Immunol 2007; 57:2-12; PMID:17156186; http://dx.doi.org/10.1111/j.1600-0897.2006.00450.x
- LeBlanc DM, Barousse MM, Fidel PL, Jr. Role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect Immun 2006; 74:3213-21; PMID:16714548; http://dx.doi.org/10.1128/IAI.01824-05
- Oriss TB, Ostroukhova M, Seguin-Devaux C, Dixon-McCarthy B, Stolz DB, Watkins SC, Pillemer B, Ray P, Ray A. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J Immunol 2005; 174:854-63; PMID:15634907; http://dx.doi.org/10.4049/jimmunol.174.2.854
- Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d'Enfert C, Vecchiarelli A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One 2011; 6:e22770; PMID:21818387; http://dx.doi.org/10.1371/journal.pone.0022770
- Yano J, Palmer GE, Eberle KE, Peters BM, Vogl T, McKenzie AN, Fidel PL Jr. Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect Immun 2014; 82:783-92; PMID:24478092; http://dx.doi.org/10.1128/IAI.00861-13
- Fidel PL, Jr., Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, Quayle AJ, Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun 2004; 72:2939-46; PMID:15102806; http://dx.doi.org/10.1128/IAI.72.5.2939-2946.2004
- Hoyer LL, Green CB, Oh SH, Zhao X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family–a sticky pursuit. Med Mycol 2008; 46:1-15; PMID:17852717; http://dx.doi.org/10.1080/13693780701435317
- de Groot PW, Bader O, de Boer AD, Weig M, Chauhan N. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell 2013; 12:470-81; PMID:23397570; http://dx.doi.org/10.1128/EC.00364-12
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr, Spellberg B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009; 5:e1000703
- Ibrahim AS, Luo G, Gebremariam T, Lee H, Schmidt CS, Hennessey JP, Jr., French SW, Yeaman MR, Filler SG, Edwards JE Jr. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 2013; 31:5549-56; PMID:24063977; http://dx.doi.org/10.1016/j.vaccine.2013.09.016
- Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, Filler SG, Yeaman MR, Edwards JE Jr. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis 2006; 194:256-60; PMID:16779733; http://dx.doi.org/10.1086/504691
- Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, Edwards JE Jr, Hennessey JP Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012; 30:7594-600; PMID:23099329; http://dx.doi.org/10.1016/j.vaccine.2012.10.038
- Ibrahim AS, Spellberg BJ, Avenissian V, Fu Y, Filler SG, Edwards JE, Jr. Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect Immun 2005; 73:999-1005; PMID:15664943; http://dx.doi.org/10.1128/IAI.73.2.999-1005.2005
- Spellberg BJ, Ibrahim AS, Avenissian V, Filler SG, Myers CL, Fu Y, Edwards JE Jr. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect Immun 2005; 73:6191-3; PMID:16113347; http://dx.doi.org/10.1128/IAI.73.9.6191-6193.2005
- Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JE, Jr. The anti-Candida vaccine based on the recombinant N-terminal domain of Als1p is broadly active against disseminated candidiasis. Infect Immun 2006; 74:3039-41; PMID:16622247; http://dx.doi.org/10.1128/IAI.74.5.3039-3041.2006
- Bar E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, LeibundGut-Landmann S. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol 2012; 188:5636-43; PMID:22529294; http://dx.doi.org/10.4049/jimmunol.1200594
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 2003; 67:400-28, table of contents; PMID:12966142; http://dx.doi.org/10.1128/MMBR.67.3.400-428.2003
- Vilanova M, Teixeira L, Caramalho I, Torrado E, Marques A, Madureira P, Ribeiro A, Ferreira P, Gama M, Demengeot J. Protection against systemic candidiasis in mice immunized with secreted aspartic proteinase 2. Immunology 2004; 111:334-42; PMID:15009435; http://dx.doi.org/10.1111/j.1365-2567.2004.01819.x
- Sandini S, La Valle R, Deaglio S, Malavasi F, Cassone A, De Bernardis F. A highly immunogenic recombinant and truncated protein of the secreted aspartic proteases family (rSap2t) of Candida albicans as a mucosal anticandidal vaccine. FEMS Immunol Med Microbiol 2011; 62:215-24; PMID:21535228; http://dx.doi.org/10.1111/j.1574-695X.2011.00802.x
- Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 2005; 202:597-606; PMID:16147975; http://dx.doi.org/10.1084/jem.20050749
