Abstract
Glucocorticoid (GC) use is a common risk factor for invasive fungal infections. This is attributed to the complex dysregulation of immunity caused by GCs. However, studies have demonstrated increased growth with GC exposure for some molds, such as Aspergillus fumigatus and Exserohilum rostratum. No such data exist for Mucorales. Therefore, we investigated the influence of GC exposure on the growth of Rhizopus arrhizus (syn. R. oryzae) in different culture media and in different atmospheres. We measured continuous spore growth using spectrophotometry and biomass variations using XTT assay. We did not observe enhanced growth or biomass variation with any of the GCs regardless of the medium or conditions. These results support the existence of fungus-specific differences in the effect of GCs on fungal biology.
Keywords:
Corticosteroid therapy is a risk factor for invasive fungal infections and this vulnerability is attributed to the complex dysregulation of immunity caused by glucocorticoids.Citation1 Increased growth rate under corticosteroid exposure was demonstrated for some molds such as Aspergillus fumigatusCitation2 and Exserophilium rostratum,Citation3 but no similar data on Mucorales are available. To that end, we examined whether various corticosteroids directly enhance Mucorales growth and biomass in vitro.
We grew a clinical Rhizopus arrhizus (syn. R. oryzae)Citation4 isolate (Ro-696) on Yeast Extract Agar plates for 72 h at 37°C.Citation5 Spores were collected and washed twice in sterile phosphate buffer saline (PBS). The spores were counted using a haemocytometer and stored at 4°C in PBS. Dexamethasone (DEXA; Sigma-Aldrich, St Louis, MO), Methylprednisone (MPN; Tokyo Chemical Industry, Tokyo, Japan) and Hydrocortisone (HC; MP Biomedicals, Solon, Ohio) were diluted with 100% ethanol. The following pharmacological concentrationsCitation6 were tested on R. arrhizus for each corticosteroid: 80, 160, 320 and 640 μg/ml for HC and 80, 160, 320 and 640 ng/ml for DEXA and MPN.
As iron acquisition plays a key role in Mucorales's growth,Citation7 3 different liquid culture media were tested for the experiments i) RPMI with 2% glucose, which is a standard culture media, ii)Yeast Nitrogen Base (YNB), which is an iron enriched media, and iii) Yeast Extract Agar media (YAG), which is an iron depleted media.
First, we investigated the influence of corticosteroid exposure on R. arrhizus growth under standard atmosphere. A concentration of 103 spores / ml of R. arrhizus were incubated 24 h under standard atmosphere at 37°C in presence of HC, DEXA or MPN in round bottom 96 wells microtiter plates. The growth rate was assessed by spectrophotometry (600 nm absorbance) every 4 h during 24 h. No enhanced growth was observed with any of the corticosteroid tested; variation of culture media did not facilitate R. arrhizus's growth upon corticosteroid exposure ().
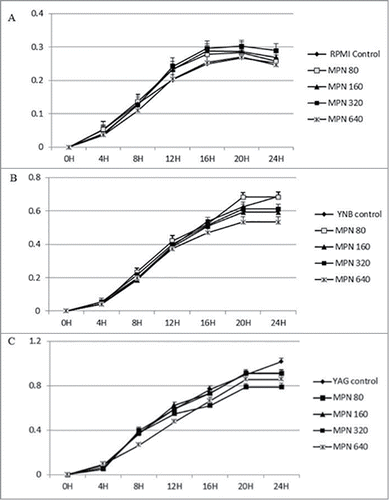
Figure 1. Continuous growth measurement of R. arrhizus in the presence of 0 (control), 80, 160, 320 and 640 ng/ml for DEXA under standard atmosphere in RPMI (A), YNB (B) and YAG (C). No promotion of growth was observed (P value > 0.05, t Student non parametric test).
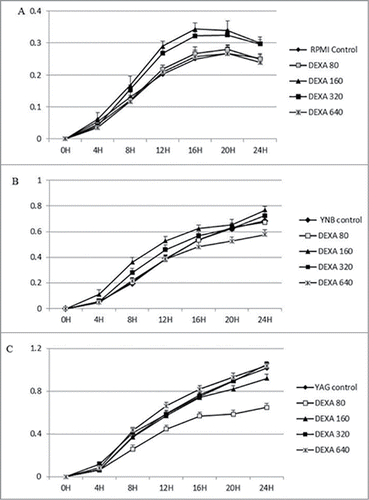
Figure 2. Continuous growth measurement of R. arrhizus in the presence of 0 (control), 80, 160, 320 and 640 ng/ml for HC under standard atmosphere in RPMI (A), YNB (B) and YAG (C). No promotion of growth was observed (P value > 0.05, t Student non parametric test).
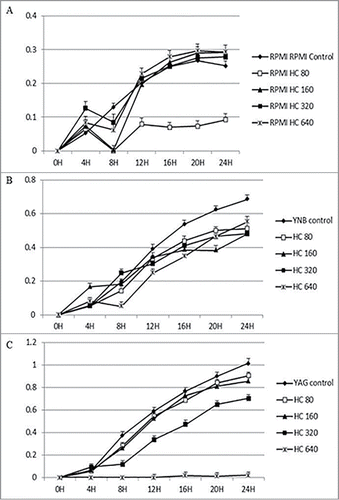
Figure 3. Continuous growth measurement of R. arrhizus in the presence of 0 (control), 80, 160, 320 and 640 ng/ml for MPN under standard atmosphere in RPMI (A), YNB (B) and YAG (C). No promotion of growth was observed (P value > 0.05, t Student non parametric test).
To verify that this result was applicable to other agents of mucormycosis, the experiment was repeated using a second clinical strain of Rhizopus sp, 2 clinical strains of Lichtheimia corymbifera (formerly Absidia corymbifera) and 2 clinical strains of Mucor sp (all the strains were isolated in cancer patients diagnosed with mucormycosis at the MD Anderson Cancer Center). Similarly we found no enhancement of growth by corticosteroid in any of the isolates tested (Fig. S1).
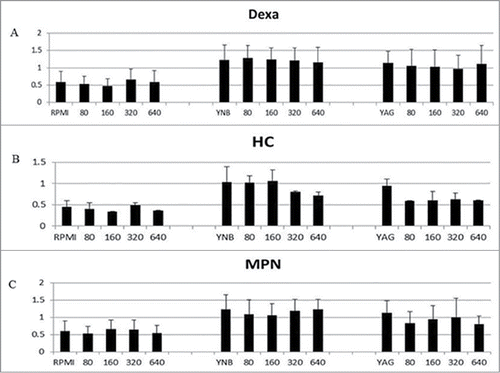
In order to examine the impact of corticosteroid exposure on R. arrhizus's biomass, we repeated the experiments but using the XTT assay which is a colorimetric method, based on the reduction of the tetrazolium salt, 3-bis[2-methyloxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-5-carboxanilide] (XTT) by mitochondrial dehydrogenases. This method quantifies biomass by measuring fungal metabolism.8 During this experiment, R. arrhizus was incubated 20 h at 37°C under 5% CO2 atmosphere, in order to see if the presence of CO2 would facilitate fungal development. Further we used that condition in an attempt to simulate Mucorales growth under hypoxic tissue condition.Citation5 Briefly, XTT (Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline at concentrations of 1 mg/ml. Menadione (Sigma-Aldrich, St. Louis, MO) was initially dissolved in acetone l at a concentration of 10 mg/ml and subsequently added to the above-mentioned XTT solutions at concentrations of 125 μM for each solution. A 104 spores/ml of R. arrhizus were incubated in RPMI 1640, Yeast Extract Medium (YAG), and Yeast Nitrogen Base Medium (YNB) at 37°C under 5% CO2 atmosphere in 96-well flat-bottom micro titration plates at a volume of 200 μl per well. After 18 h of incubation, 50 μl of one of the above-mentioned XTT-menadione solutions was added to each well and the plate was incubated at 37°C under 5% CO2 atmosphere for an additional 2 h. After 2 h of incubation, 200 μl from each test group was plate in a sterile 96-well U-bottom micro titration plate for analysis. The formazan absorbance in each well was read at 492 nm and 690 nm (plate absorbance) with the use of a micro plate spectrophotometer (Power wave Biotech Instruments, Winooski, VT). We obtained the XTT results by subtracting to the result the optical density (OD) of wells containing media alone (background). These additional assays investigating R. arrhizus's biomass under higher CO2 atmosphere in presence of GC did not show any difference upon GC exposure ().
Figure 4. R. arrhizus's biomass assessment using XTT in the presence of 0 (control), 80, 160, 320 and 640 ng/ml for DEXA (A), HC (B), MPN (C) under 5% CO2 atmosphere in RPMI, YNB and YAG. No promotion of growth was observed (P value > 0.05, t Student non parametric test).
For example, a previous report showing toxicity of steroid such as progesterone on Rhizopus nigricans suggested that the underlying mechanism was depending on G protein activation and cAMP signaling.Citation9
In conclusion, corticosteroid did not impact directly R. arrhizus's growth or biomass in vitro. These negative results support the hypothesis that the immune dysfunction induced by corticosteroid is the main reason why patients are more vulnerable to Mucormycosis. Moreover, when compared with other previous reports,Citation2,3 these negative data support the notion that there might be some fungus-specific differences regarding the effect of corticosteroids on fungal biology. The difference of impact of GCs on Aspergillus fumigatus growth in comparison with Mucorales might explain, at least in part, the higher incidence of cases of invasive aspergillosis among this patient population compared to mucormycosis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1039762_Fig_S1.pdf
Download PDF (196.7 KB)Supplemental Materia
Supplemental data for this article can be accessed on the publisher's website.
References
- Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet 2003; 362(9398):1828-38; PMID:14654323; http://dx.doi.org/10.1016/S0140-6736(03)14904-5
- Ng TTC, Robson GD, Denning DW. Hydrocortisone-emhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology 1994; 140:2475-9; PMID:7952197; http://dx.doi.org/10.1099/13500872-140-9-2475
- Farmakiotis D, Shirazi F, Zhao Y, Saad PJ, Albert ND, Roilides E, Walsh TJ, Perlin DS, Kontoyiannis DP. Methylprednisolone enhances the growth of Exserohilum rostratum in vitro, attenuates spontaneous apoptosis and increases mortality rates in immunocompetent Drosophila flies. J Infect Dis 2014; 210(9):1471-5; PMID:24837401; http://dx.doi.org/10.1093/infdis/jiu289
- Dolatabadi S, de Hoog GS, Meis JF, Walther G. Species boundaries and nomenclature of Rhizopus arrhizus (syn. R. oryzae). Mycoses 2014; 57(Suppl 3):108-27. PMID:25266947; http://dx.doi.org/10.1111/myc.12228
- Kontoyiannis DP, Chamilos G, Hassan SA, Lewis RE, Albert ND, Tarrand JJ. Increased culture recovery of Zygomycetes under physiologic temperature conditions. Am J Clin Pathol 2007; 127(2):208-12; PMID:17210526; http://dx.doi.org/10.1309/7KU5XWURYM0151YN
- Thomsom AH, Deverst MC, Wallace AM, Grant D, Campbell K, Freel M, Connell JM. Variability in hydrocortisone plasma and saliva pharmacokinetics following intravenous and oral administration to patients with adrenal insufficiency. Clin Endocrinol 2007; 66:789-96; PMID:17437510; http://dx.doi.org/10.1111/j.1365-2265.2007.02812.x
- Ibrahim AS. Host cell invasion in mucormycosis: role of iron. Curr Opin Microbiol 2011; 14(4):406-11; PMID:21807554; http://dx.doi.org/10.1016/j.mib.2011.07.004
- Antachopoulos C, Meletiadis J, Roilides E, Sein T, Sutton DA, Wickes BL, Rinaldi MG, Merz WG, Shea YR, Walsh TJ. Relationship between metabolism and biomass of medically important zygomycetes. Med Mycol 2006; 44(5):429-38; PMID:16882609; http://dx.doi.org/10.1080/13693780600644878
- Jeraj N, Lenasi H, Breskvar K. The involvement of cAMP in the growth inhibition of filamentous fungus Rhizopus nigricans by steroids. FEMS Microbiol Lett 2005; 242(1):147-54; PMID:15621431; http://dx.doi.org/10.1016/j.femsle.2004.10.051
