Abstract
Paracoccidioidomycosis is a systemic mycosis, endemic in Latin America. The etiologic agents of this mycosis are composed of 2 species: Paracoccidioides brasiliensis and P. lutzii. Murine animal models are the gold standard for in vivo studies; however, ethical, economical and logistical considerations limit their use. Galleria mellonella is a suitable model for in vivo studies of fungal infections. In this study, we compared the virulence of P. brasiliensis and P. lutzii in G. mellonella model. The deaths of larvae infected with P. brasiliensis or P. lutzii were similar, and both species were able to reduce the number of hemocytes, which were estimated by microscopy and flow cytometer. Additionally, the phagocytosis percentage was similar for both species, but when we analyze hemocyte-Paracoccidioides spp. interaction using flow cytometer, P. lutzii showed higher interactions with hemocytes. The gene expression of gp43 as well as this protein was higher for P. lutzii, and this expression may contribute to a greater adherence to hemocytes. These results helped us evaluate the behavior of Paracoccidioides spp in G. mellonella, which is a convenient model for investigating the host-Paracoccidioides spp. interaction.
Introduction
Paracoccidioidomycosis (PCM) is a systemic mycosis that is caused by thermally dimorphic fungi from the genus Paracoccidioides. This infection is endemic in Latin America and has higher incidences in Brazil, Argentina, Colombia and Venezuela.Citation1 In Brazil, the annual incidence reaches 30 per million inhabitants, and the mortality rate is 1.4 per million per year.Citation2 This mycosis has many clinical presentations, and retrospective studies have demonstrated that some factors, such as the host's immune response, age, gender and ethnicity, may influence the clinical presentation.Citation3 The incidence of chronic infection in the lungs and the upper respiratory tract is more frequent in men older than 30. The sub-acute form is commonly found in children and young adults and in afro-descendant women older than 20 years.Citation3,4 The Paracoccidioides spp mycelial form is found in nature and its propagules are the infective forms of the disease. The infection occurs upon inhalation of the propagules, and during this process, the fungus converts into a yeast form. The temperature is essential for this transformation.Citation1,5
Genetic studies have revealed the existence of 3 different phylogenetic species in P. brasiliensis called S1, PS2 and PS3.Citation5 A new species was detected by using molecular approaches; the species started as Pb01-like and has been renamed P. lutzii .Citation6,7 In Brazil, a higher incidence of P. brasiliensis is found in the south and southeast region and P. lutzii predominantly occurs in the western-central part of Brazil.Citation8
The Paracoccidioides spp. virulence mechanism involves dimorphism,Citation9 metalloproteins,Citation10 adhesins, biofilm formation,Citation11,12 and melanin production,Citation13 which play important roles in the infection. Fungal-cell interaction is an essential factor in the establishment of the infection, and for this action, the fungus recognizes specific ligands (carbohydrates and proteins) found in the extracellular matrix, thereby initiating infection and its subsequent spread.Citation14 In Paracoccidioides spp, many proteins known as adhesins mediate this process,Citation11 and those with multiple functions are known as 'moonlighting' proteins.Citation15
Murine models are considered to be the gold standard for the study of fungal pathogenesis and for analyzing the efficacy of antifungal treatments. In 1959, initial studies were conducted in which mice were used as infection models for paracoccidioidomycosis.Citation16 At present, mice are the standard models for mimicking what occurs in the human disease. Many groups use infection by inhalation routes; however, some studies have described a lack of reproducibility caused by the difficulties the yeast have in reaching the lungs.Citation17-19 Another important aspect to consider is long-term survival curves when using mice as models; specifically, P. brasiliensis (Pb18) could require 2 to 8 months for each experiment.Citation20 Together with economical, logistical and ethical considerations, these challenges limit the use of mammals in infection experiments.Citation21,22 In this context, the use of alternative animal models or “non-vertebrate mini-hosts” such as Galleria mellonella appears to be an option for studying fungal or bacterial disease.Citation23-26
The Galleria mellonella model is inexpensive to purchase and does not require specialized facilities for maintenance. The relatively large size of the larvae facilitates both the easy handling and injection of defined inoculums and the use of a large number of samples. Furthermore, in contrast to other invertebrate hosts, G. mellonella larvae can be maintained at temperatures of 37°C, which is equivalent to the temperature of mammalian hosts. This model presents structural and functional similarities to the innate immune response of mammals. Additionally, the G. mellonella innate immune system has 6 types of cells, including phagocytic cells, and the hemocyte density reflects the pathogenicity of the fungi.Citation21,27,28 Galleria mellonella also possesses genes for pathogen-associated molecular pattern recognition, such as toll-like receptors and the transcription factor nuclear factor-κB, in addition to antimicrobial peptides and proteins, most from the families moricin-like protein genes.Citation29
The study of the virulence of different fungi such as Cryptococcus neoformans,Citation30,31 Fusarium spp,Citation32 Candida spp,Citation33,34 Histoplasma capsulatum and P. lutzii.Citation35 has been performed in G. mellonella successfully. P. lutzii was able to kill G. mellonella, however was not possible to establish a correlation between cell concentration and larvae death. Besides that, the infection caused larvae melanization and granuloma-like formation in a yeast concentration-dependent manner.Citation35 However, this model was only evaluated in P. lutzii but not in P. brasiliensis. The discovery of the P. lutzii species leads to new questions about virulence, clinical manifestations and diagnosis.Citation36,37 Moreover, because of the recent classification of 2 distinct species (P. brasiliensis and P. lutzii), few studies have compared the virulence and host interactions among them. Given this outlook, the aim of this study was to evaluate the virulence of P. brasiliensis and compare it with P. lutzii in the G. mellonella model. In addition, the hemocyte response to the infection was determined by measuring the hemocytic density, phagocytosis, hemocyte-fungal interactions as well as adhesins genes enolase, gp43, 14-3-3, malate synthase, and triosephosphate isomerase.
Results
Virulence of P. brasiliensis and P. lutzii in G. mellonella
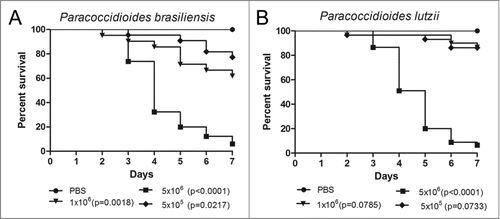
This study provides the first description of a P. brasiliensis and P. lutzii virulence comparison in G. mellonella. The larvae were infected with 3 different concentrations of both species (5×105, 1×106 and 5×106 cells/larva) with the objective of finding the concentration that was able to kill them within 7 days of starting the experiment (). This time is considered appropriate for the use of the G. mellonella model because of the length of the insect's life cycle. The 1×106 cells/larva concentration of P. brasiliensis was able to kill more larvae than the same concentration for P. lutzii but in the end of the experiment almost 60% of the larvae were alive. The 5×106 cells/larva concentration was selected because the majority of larvae were dead in within the observation period. An analysis of the survival curve for both Paracoccidioides species, for the 5×106 cells/larva concentration, showed that the time necessary to reach 50% killing (TD50%) ranged from 3 to 4 days for P. brasiliensis, and the TD50% ranged from 4 to 5 days for P. lutzii. However, the survival curve profiles were very similar, which suggested that P. brasiliensis and P. lutzii have similar virulence levels (p = 0.3051) in this model.
Histopathology analysis
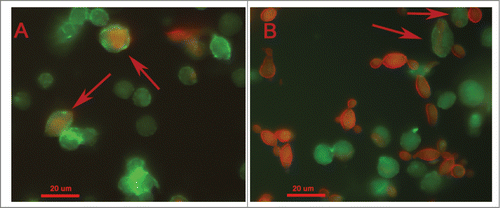
The histopathology of uninfected and infected Paracoccidioides spp. larvae was also performed to study the infection by this yeast. Larvae were collected after one hour and 4 days of infection and stained with periodic acid Schiff (PAS). After one hour of infection, P. brasiliensis () and P. lutzii () were visualized in the periphery of the G. mellonella tissues. After 4 days of infection, G. mellonella tissues of larvae infected with P. brasiliensis () and P. lutzii () were destroyed when compared with uninfected larvae (), and both fungal species were able to multiply inside the larvae. The cellular response to the invasion, encapsulation and granuloma, in addition to the humoral phenomenon, that is, the melanization, were observed in the histopathology ().
Figure 2. Histology of G. mellonella stained with PAS. Uninfected larvae (A, B); larva infected with P. brasiliensis after 1 hour (C) and after 4 days (E); larva infected with P. lutzii after 1 hour (D) and after 4 days (F). Amplification 1000x. Arrows indicates P. brasiliensis or P. lutzii. Structures annotated: (a) cuticle; (b) adipose bodies; (f) fungal cells.
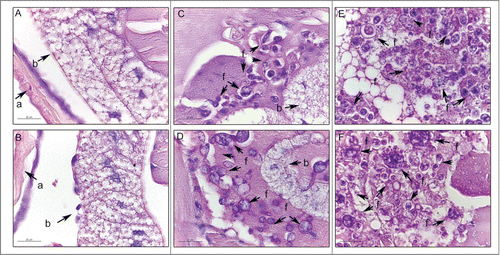
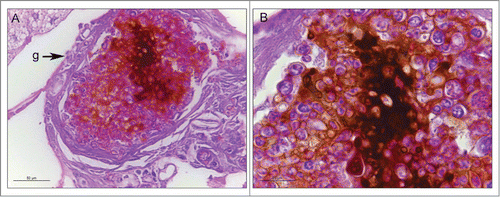
Figure 3. The cellular and humoral response to infection with P. brasiliensis after 3 days. Granuloma structure, amplification 400X (A); melanization and encapsulation process, amplification 1000x. (B). Similar structures were observed during P. lutzii infection. Structure annotated: (g) granuloma-like structure.
Hemocyte density after infection by Paracoccidioides spp
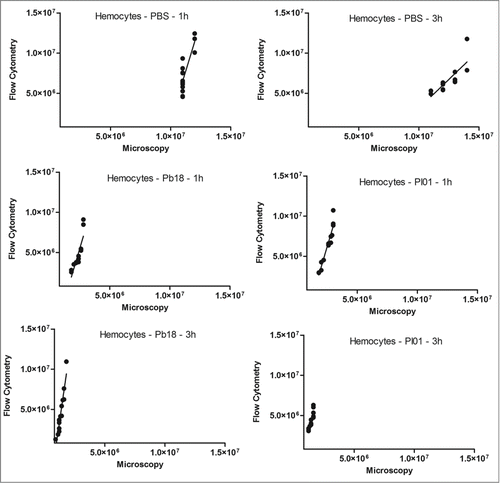
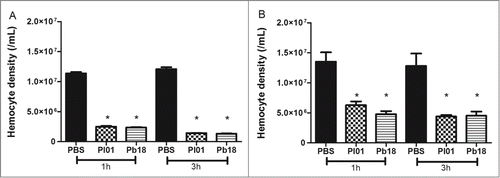
Changes in the hemocyte density are one important factor to observe in the larvae response when analyzing virulence. Larvae were infected with 5×106 cells/larvae and incubated at 37°C for one and 3 hours and measure the hemocyte density of G. mellonella after these periods of infection by Paracoccidioides species by using 2 methodologies, namely microscopy and flow cytometry (). For microscope estimation was used a hemocytometer under a brightfield microscope. According to the microscopy analyses, there was a hemocyte reduction of 5 and 8 times in both species at 1 and 3 h after infection, respectively (). In the flow cytometry analyses, the hemocyte density after infection with P. brasiliensis or P. lutzii decreased 3 times for both incubation times (). The results indicate that infections with 5×106 cells/larva of P. brasiliensis and P. lutzii reduced the density of hemocytes compared with the non-infected larvae during the analyzed time points. Additionally, the hemocyte density in the larvae infected with P. brasiliensis was similar to that with P. lutzii.
Figure 4. Hemocyte density, as obtained by microscopy (A) and flow cytometry (B), in G. mellonella larvae infected with P. brasiliensis (Pb18) and P. lutzii (Pl01) when assessed after 1 and 3 h. The asterisks indicate statistical significance (p < 0.05) relative to the PBS control.
As described above, hemocyte estimation was evaluated by using a traditional hemocytometer under a brightfield microscope and compared with the flow cytometer results. For this, Paracoccidioides spp suspension of 5×108 cells/mL was stained with 100 µM CFDA-SE for 30 minutes at 37°C and hemocytes samples of infected and uninfected larvae were stained with 0.165 µM Alexa Fluor® 647 phalloidin conjugates. The development of the flow cytometry assay for hemocyte estimation provide faster results and the possibility of analyze a greater amount of samples, moreover, because of the double stain the differentiation of fungi or hemocyte cells are precise. Both methodologies were comparable by Pearson coefficient (). Correlation coefficients for the hemocyte density in G. mellonella that were injected with PBS were 0.83 at 1 h (p=0.0002) and 0.80 at 3 h (p=0.0006), and those injected with P. brasiliensis were 0.86 for 1 h (p<0.0001) and 0.95 at 3 h (p<0.0001); those injected with P. lutzii were 0.96 for 1 h (p<0.0001) and 0.90 at 3 h (p<0.0001). This result suggested that a good correlation was found between the hemocyte density obtained by flow cytometry and by the standard method of counting cells on a hemocytometer (microscopy) in larvae that were infected with PBS or Paracoccidioides species at the different time points.
Paracoccidioides spp. - hemocyte interactions
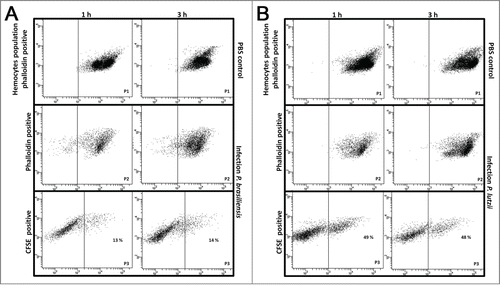
The hemocyte-fungal interactions were evaluated by flow cytometry methodology. Paracoccidioides spp suspension of 5×108 cells/mL was stained with 100 µM CFDA-SE as described above and larvae were infected. After 1 and 3 h of infection, hemocytes samples of infected and uninfected larvae were stained with 0.165 µM Alexa Fluor® 647 phalloidin conjugates. The double-stained population (the CFDA-SE-positive and phalloidin-positive population) was examined for its hemocyte-fungal interactions using flow cytometry, and the percentage of double-stained cells in the population is shown in . An important difference was observed in the hemocyte-fungal interaction profile between the fungal species. The P. brasiliensis-hemocyte interactions were 13 % and 14 % after 1 and 3 h, respectively (); however, the P. lutzii interaction was 49 % and 48 % after 1 and 3 h, respectively (). To evaluate the efficiency of the innate immune system against these species and consequentially the ability to escape from them, hemocytes phagocytosis was evaluated. The Paracoccidioides species were stained with 10 µg/mL Calcofluor white and larvae were infected with stained cells at 5×106 cells/larvae and incubated for 3 h at 37°C. After the infection hemocytes samples of infected larvae were stained with 0.165 µM Alexa Fluor→ 647 phalloidin conjugates and images were taken and a percentage of hemocytes containing yeasts was calculated using fluorescence microscopy. In this assay, 5 % of the hemocyte cells from G. mellonella were able to phagocytize P. brasiliensis or P. lutzii (), these results demonstrated that innate immune system of G. mellonella recognize equally these species.
Figure 6. Hemocyte-fungal interaction obtained by flow cytometry after infection with P. brasiliensis (A) and P. lutzii (B). In gate P1, the hemocyte population is phalloidin positive with non-infected larvae; in gate P2, the hemocyte population is phalloidin-positive with infected larvae; and gate P3 holds the doubly stained population (hemocyte-stained phalloidin and fungal-stained CFDA-SE) that are considered hemocyte-fungal interactions that were obtained by flow cytometry after infection with P. brasiliensis (A) and P. lutzii (B). Differences in the hemocyte-fungal interaction between the fungal species (p < 0.05).
Paracoccidioides spp. adhesin gene expression
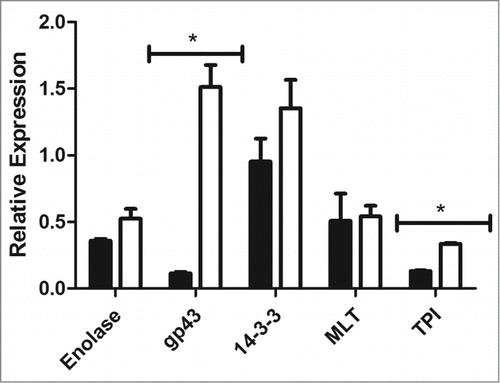
Adhesion is an important factor in establishing an infection. The gene expression of important adhesins in Paracoccidioides spp, namely enolase, gp43, 14-3-3, malate synthase, and triosephosphate isomerase, was analyzed after the infection in G. mellonella. For this, after one hour of infection the hemolymph from infected and uninfected larvae was collected and Paracoccidioides spp. cells were recovered by centrifugation, RNA was extracted and real-time PCR was performed. The P. lutzii expression of gp43 and triosephosphate isomerase were higher than they were in P. brasiliensis (13-folds (p = 0.0011) and 2.5-folds (p = 0.0018), respectively). This higher gene expression could explain the potential adhesion by P. lutzii to the hemocytes. For enolase, 14-3-3 and malate synthase, the expressions for both species were similar ().
Gp43 importance during Paracoccidioides spp- hemocyte interaction
Because of the importance of gp43 in the pathogenicity of Paracoccidioides spp, a Western blot assay was performed to verify the presence of this adhesin and compare it with the gene expression results. For this purpose, larvae protein extracts were obtained 3 days after infection with P. brasiliensis, or P. lutzii and control proteins were collected from Paracoccidioides spp., uninfected G. mellonella, and purified gp43. The presence of a 43 kDa fraction was observed in samples taken from G. mellonella that had been infected with P. lutzii and P. brasiliensis (). The antibody used in this work was obtained against the Paracoccidioides cells, and thus the test could detect other proteins in addition to gp43. In extracts from uninfected G. mellonella the presence of this protein was not detected.
Figure 9. Western blot of G. mellonella infected with P. brasiliensis or P. lutzii. 1) molecular weight marker; 2) P. lutzii; 3) G. mellonella infected with P. lutzii; 4) P. brasiliensis; 5) G. mellonella infected with P. brasiliensis; 6) G. mellonella extract infection; and 7) purified gp43. The arrow indicates the gp43.

Discussion
Ethical questions are restricting the use of experimental vertebrate animals.Citation38 In this context, the use of alternative animal models such as amoebae (Acanthamoeba castellanii and Dictyostelium discoideum), nematodes (Caenorhabditis elegans), insects (Drosophila melanogaster and Galleria mellonella) and recently embryonated chicken eggs has emerged in recent years, and they provide good alternatives for studying fungal pathogenesis.Citation39-43
In the last decade, G. mellonella has become more accepted as an alternative animal model of infection.Citation22,44 The structural and functional similarities between the immune responses of insects and mammals permits the insects to be used to predict the likely response of mammals to a variety of pathogenic microorganisms.Citation45 The hemolymph has analogous functions to blood and contains cells called hemocytes. There are at least 6 types of hemocytes and plasmatocytes, and granulocytes are the most abundant phagocytic cell types. Furthermore, antimicrobial peptides are also responsible for killing invading microorganisms.Citation46,47
In this study, we used G. mellonella to establish a comparison between the virulence of 2 Paracoccidioides species. The survival curve profiles show that Paracoccidioides spp caused the concentration-dependent death of G. mellonella. Likewise, a correlation between the yeast cell number of P. lutzii and the time to death of G. mellonella was observed in a previous study, although the tested concentrations (101 to 106 cells/larva) were different from those in the present study.Citation35 However, the death time of larvae infected with 106 yeast cells was lower than that in our study. One explanation for this finding may be related to the different preparations of the inoculums. The cells of the fungus are very irregular in size and shape, and we believe that the use of inoculums with standard cell sizes was fundamental for the experiment. Paracoccidioides brasiliensis caused larval death faster (3-4 days) than P. lutzii (4-5 days). Furthermore, the histopathology of infected larvae at 1 hour and 4 days after the infection showed that both species were able to multiply inside the larvae and destroy the G. mellonella tissue. Moreover, encapsulation, melanization and granuloma-like structures were observed in the histopathology. The presence of granulomas were described during the paracoccidioidomycosis in murine model affecting the lung and spleen.Citation48 The effect of melanization on the elimination of pathogens is widely discussed, and the activation of the pro-phenol oxidase cascade produces substances that are very toxic to microorganisms and also helps in phagocytosis, aiding in the survival of invertebrates.Citation49 The encapsulation occurred when the cells were unable to phagocytize and thus tried to control the invasion by creating a cell barrier.Citation50
By using 2 techniques, we demonstrated that the infection of Paracoccidioides spp. causes a decrease in the hemocyte density. After just one hour of infection, a significant reduction in the hemocyte density was observed in the infection with P. brasiliensis or P. lutzii compared with the control. The same reduction was observed after 2 more hours of incubation. Moreover, no significant difference was observed between the 2 tested species. Numerical differences between the techniques (flow cytometry and hemocytometer count) were observed; however, the hemocyte reduction profile remained the same. The hemocyte density is an important factor for evaluating virulence because low survival after infection is related to low hemocyte density.Citation51 In addition, the hemocyte density can vary during the infection, and a decrease was observed during the first hours of the infection.Citation52
Another important aspect of this work is the standardization of hemocyte counting. We developed a practical flow cytometry protocol with double staining to determine the hemocyte density and the percentage of hemocyte-fungal interaction. The statistical analyses show a good correlation between the traditional hemocytometer estimation and the results obtained by flow cytometry. Thus, flow cytometry may be considered a reliable method for hemocyte counting because it is faster, more practical and gives precise results.
Phagocytosis was evaluated during the early stages of infection (3h), for both species, 5% phagocytosis was found, however, P. brasiliensis showed lower interactions with hemocytes when compared with the P. lutzii infection, which would indicate a better escape mechanism by this species from phagocytosis. The choice for 3h of post-infection time for phagocytosis assay is because of the significant reduction of hemocyte density, phagocytosis could be difficult to observe after more hours of infection. Other studies have demonstrated in vitro phagocytosis of P. brasiliensis around 7% after 6 h of infection and 6-12% after 24 h of infection depending on the type of macrophage studied.Citation53,54 Even though, in this model we have similar phagocytosis index. Additionally, the literature has been shown the absence of digestive and killing capacities of PMNs from paracoccidioidomycosis patients (susceptible individuals) in unambiguous contrast of cells from healthy (resistant) individuals.Citation55
Studies showed significant similarity between the rates of in vitro phagocytosis of opsonized C. albicans by neutrophils and hemocytes. In the same way that it occurs for neutrophils, phagocytosis by hemocytes also stimulates the respiratory burst pathway involving NADPH oxidase activity and superoxide production for efficient microbial killing.Citation56 Other similarities between neutrophils and hemocytes were demonstrated by treating the hemocytes with cytochalasin b and nocodazole, leading to an increase in the susceptibility of the G. mellonella larvae to the infection caused by a disruption of hemocyte function, which is the same way that they affect mammalian neutrophils. Earlier studies have described noticeable neutrophil infiltrates in Paracoccidioides lesions of experimental animal models and also in tissue samples from patients. Neutrophils were present in P. brasiliensis microabscesses and in loose granulomas from oral lesions.Citation57 Nevertheless, they are usually not sufficient to eliminate the fungus cells completely.
The hemocyte fungal interaction was studied in this work as an important key to discuss the difference between the virulence of both fungi. Differences in the virulence between Paracoccidioides spp strains have been reported, and they are likely related to antigenic differences and the adhesion process.Citation58,59 In this study, we observe that P. lutzii is able to interact with hemocytes 3.7-folds more than P. brasiliensis.
Different proteins with roles in fungal-cell adhesion are well-described for P. brasiliensis, and most of them participate in the glycolytic pathway, tricarboxylic acid cycle and glyoxylate cycle, and because of their multiple functions, these proteins are known as 'moonlighting' proteins.Citation15 In this work, we analyze the gene expression of 5 important adhesins from Paracoccidioides spp, namely enolase, gp43, 14-3-3, triosephosphate isomerase and malate synthase. A significant difference of expression for gp43 and triosephosphate isomerase was observed in P. lutzii after the infection of G. mellonella. Because of the importance of gp43, we investigated its expression by Western blot assay during the G. mellonella infection, and we tested if its presence can explain the higher hemocyte adhesion found for this species. Although gp43 production is detected in vitro within less than one hour of incubation,Citation60 we use 3 days of infection to detect this protein because of the low amount of yeast present in the sample. The 43 kDa glycoprotein was the first to be described as an adhesin and to be able to bind to laminin,Citation61,62 and it was more recently shown to bind both fibronectin and laminin. In Paracoccidioides spp., certain additional adhesins have also been described and may play an important role in pathogenesis.Citation59,63-67
The importance of gp43 was studied.Citation68 by silencing the PbGP43 gene. No morphology, vitality or fungal growth was affected by the reduction in expression of gp43 gene. However, the expression of this protein was reduced in vitro and thus affected the recovery of yeasts from macrophages that were adhered to or phagocytized; and it was reduced in vivo from the lungs of infected mice. In addition to being described as an adhesin, the 43 kDa glycoprotein is the primary diagnostic antigen of P. brasiliensis.Citation69 Recent studies have demonstrated that gp43 is highly specific for PCM caused by P. brasiliensis and false negative for P. lutzii.Citation70,71 By analyzing the peptide sequence of gp43 protein in both species, an 81 % similarity was found; however, the molecular models show differences between them. Specifically, the N-glycosylation site is absent, and the glucanase site and its enzymatic activity are preserved in gp43 from P. lutzii.Citation36 The Galleria model has the same behavior as the in vivo model, and gp43 from P. lutzii may be expressed in the same way as it is in human disease. Moreover, gp43 appears to play a role in adhesion processes during the first hours of infection.
Triosephosphate isomerase (TPI) is a glycolytic enzyme that is present in the cytoplasm, and it is responsible for the conversion of dihydroxy acetone phosphate to glyceraldehyde-3-phosphate. Its gene was also more expressed in the G. mellonella P. lutzii infection. The importance of triosephosphate isomerase in paracoccidioidomycosis was first described when it was determined to be a protein that can react with the sera of PCM patients.Citation72 Moreover, when P. lutzii cells were treated with anti-TPI polyclonal antibody, an inhibition of the interaction with epithelial cells was observed. TPI plays an important role in host cell binding and could be considered an adhesion.Citation66 Triosephosphate isomerase is also one of the molecules responsible for interactions between other microorganisms. This binding may occur between the TPI of S. aureus and the mannotriose present in the capsule of C. neoformans, and the consequence of the binding is the death of C. neoformans.Citation73 Both microorganisms could be found in the nasopharynx, with S. aureus in healthy individuals. The hypothesis is that the presence of S. aureus in human nasal cavities may prevent the entry of C. neoformans,Citation74 and immunoelectron microscopy studies confirm that this cytoplasmic enzyme was localized on the cell surface and interacted with the mannotriose of the C. neoformans.Citation75 More studies are necessary to explain the differences between both species in the G. mellonella model as well as in human infections.
When studying virulence, it is necessary to consider that multifactorial events belong to the host and to the microbe; moreover, the likely phenomena of latency, colonization, commensalism and death could occur during host-microbe interactions.Citation76,77 In this study, we evaluated the virulence of P. brasiliensis and P. lutzii in a G. mellonella model by using survival curves, hemocyte density, phagocytosis, hemocyte interaction with Paracoccidioides spp yeast cells and adhesin expression. The parameter that differentiates the virulence of both species was yeast-hemocyte interaction, in which we found that P. lutzii has better abilities to adhere to the hemocytes than P. brasiliensis, and we attributed this capacity to the higher expression of important adhesins (gp43 and triosephosphate isomerase). However, some studies have addressed the expression of gp43 during P. lutzii infection in humans,Citation36 and in G. mellonella, it seems to be important to the adhesion process. These results were very important to understand the differences and similarities in both species better. Furthermore, G. mellonella was demonstrated to be a suitable model to study this fungus because of the ethical issues, cost, easy rearing and manipulation of larvae and reduced time, and the parameters analyzed in this study could be useful in the evaluation of different in vivo assays.
Material and Methods
Strains and growth conditions
Paracoccidioides brasiliensis (São Paulo, Brazil) (Pb18) and P. lutzii Pb01-like strain 01 (ATCC MYA-826/ Goiania, Brazil) (Pl01) in the yeast phase were used. Both species were maintained in Fava-Netto medium at 37°C and subcultured every 4-5 days. After this period, the fungal mass was transferred to a Brain Heart Infusion (BHI) broth supplemented with 1 % glucose (BHI suppl).Citation78 for 3-4 days at 37°C at 150 RPM to obtain exponential-phase cells.Citation79,80 The Paracoccidioides spp strain stock was recently reisolated from cell culture.
Inoculum standardization
The Paracoccidioides spp growths were washed 3 times with PBS buffer after their cell clumps were separated by repeated passages through an 18-gauge needle (Becton Dickinson, Brazil) into a 5 mL syringe (Becton Dickinson, Brazil). The suspension was filtered through 40 µm nylon cell strainers (Corning, USA) to obtain cells of homogeneous sizes. The cell concentrations were estimated by using a hemocytometer. The inoculum was prepared in PBS plus 20 mg/L of ampicillin to prevent bacterial contamination.
Galleria mellonella rearing and larvae manipulation
The larvae were fed with wax and pollen and maintained at 25°C.Citation81 until reaching 150-200 mg in weight. Larvae without color alterations and with adequate weights were selected, placed in Petri plates and incubated at 37°C overnight under protection from light. The insect pro-leg regions were cleaned with 70% ethanol before the experiments. Using a syringe (Hamilton, USA), 10 µL of inoculum was injected through the last left pro-leg of the larvae. Each experiment was repeated at least 3 times.
Survival assay
Sixteen larvae per group were inoculated with different inoculum concentrations of Paracoccidioides spp. (5×105, 1×106 and 5×106 cells/larva) for the survival assay. The control groups were larvae that were injected with PBS and untouched larvae. The larvae were incubated at 37°C, and this death was monitored daily for 7 days by visual inspection, which was done by checking for a lack of movement after touching the larvae with forceps.
Histopathology analysis
Uninfected and infected larvae were collected after one hour and 4 days of infection and processed as described by Scorzoni el al, 2013.Citation82 for a periodic acid Schiff (PAS) stain. Three tissue sections were observed per larva with a Leica DMI3000 microscope.
Determination of hemocyte density by flow cytometry
A Paracoccidioides spp suspension of 5×108 cells/mL was stained with 100 µM 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE/Sigma Aldrich, USA) for 30 minutes at 37°C and washed 3 times with PBS at 5000 rpm for 5 minutes. Galleria mellonella larvae were infected with the previously stained suspension and incubated at 37°C for one and 3 hours. A control group was set up by inoculating PBS. After the infection period, hemolymph samples (10 µL) were collected and stained with 50 µL of 0.165 µM Alexa Fluor® 647 phalloidin conjugates (Invitrogen) diluted in cold 4 % paraformaldehyde (Sigma Aldrich, USA) plus 0.25 % Triton X-100 (Amersham Biosciences, USA) and incubated for 20 minutes. The hemocyte density was determined by flow cytometry (BD FACSCanto) by acquiring 60 seconds on medium acquisition mode. In this mode, 60 µL was acquired, and the correlation was made in milliliters. A dot plot SSC-A (side scatter) by phalloidin (APC) was made to analyze the hemocyte number in control PBS (gate P1) and after the infection (gate P2). The hemocytes from larvae injected with PBS were compared with the infected ones.
Determination of hemocyte density using a hemocytometer
Larvae were infected with 5×106 cells/larvae and incubated at 37°C for one and 3 hours. After the infection period, hemolymph samples were collected and diluted at 1:20. A control group was set up by inoculating with PBS. The hemocyte density was estimated by using a hemocytometer under a brightfield microscope. The control was compared with PBS injected larvae.
Hemocyte-fungal interaction assay by flow cytometry
The hemocyte-fungal interactions were evaluated by flow cytometry methodology. Double-positive fluorescent-stained cells (with phalloidin and CFDA-SE) were used to observe fungal cells that adhered to or phagocytosed the hemocytes. Stain control was used on the fungal cells and hemocytes alone, under unlabeled and labeled conditions, with both stains and one stain alone. All cytometry data were analyzed using the BD FACS Diva software.
Phagocytosis assay
The yeast cells were stained with 10 µg/mL Calcofluor white (Sigma Aldrich, USA) for 30 min at 37°C. After that, 10 larvae were infected with stained cells at 5×106 cells/larvae and incubated for 3 h at 37°C. The hemolymph samples were collected and the hemocytes were stained as described for the flow cytometry assay. The hemolymph samples of each larva were placed in a 96-well plate (made of a plate glass bottom and black borders; Glass Bottom/GE), and the images were acquired with In Cell Analyzer 2000-GE at an amplification of 60x. One hundred hemocytes from each larva were counted and the percentage of hemocytes containing yeasts was calculated.
Paracoccidioides spp gene expression of adhesins by real-time PCR
Genes encoding for enolase, gp43, 14-3-3, triosephosphate isomerase and a malate synthase protein described as adhesin in Paracoccidioides spp. were analyzed by real-time PCR. For this analysis, 60 larvae were infected with 5×106 cells/larvae of P. brasiliensis or P. lutzii and incubated for one hour at 37°C, and uninfected groups of larvae and P. brasiliensis or P. lutzii without pass-through infection were used as controls. The hemolymph from each group was collected, and the Paracoccidioides spp cells were recovered by centrifugation and washed twice with PBS. RNA was extracted with RNeasy (Qiagen). First-strand cDNA synthesis was performed with reverse transcriptase (RevertAidTM H Minus Reverse Transcriptase, Fermentas, Canada). Real-time PCR was performed with Maxima® SYBR Green/ROX qPCR Master Mix (2X) (Fermentas, Canada) using Applied Biosystems 7500 cycler equipment. The relative expression was calculated by using 2−ΔΔCT values with 60S ribosomal L34 as the housekeeping gene; this constitutive gene is widely described as a control in different studies on paracoccidioidomycosis.Citation83,84
Protein Extraction and Western Blot assay
To perform a Western blot, 16 G. mellonella larvae at 3 days of infection were used with Paracoccidioides spp., along with 16 larvae that were not infected and Paracoccidioides cells. The protein extraction was performed first, which involved adding 3 mL of 10 mM Tris-HCl pH 6.8 and protease inhibitor mix to each sample. The samples were then lysed with glass beads and vortexed. The material was centrifuged at 5000 rpm for 1 hour at 4°C. After centrifugation, the supernatant was collected and the proteins were precipitated with 10 % TCA in 90 % acetone. The precipitated proteins were diluted in 10 mM Tris-HCl pH 6.8 and quantified by Bradford assay. One hundred micrograms of each sample was applied to an SDS-PAGE gel to separate the proteins. The proteins were subsequently transferred to a nitrocellulose membrane. The membrane was incubated with a polyclonal antibody that was obtained against a total P. brasiliensis extract Citation85 and the secondary antibody was rabbit anti-mouse IgG conjugated to peroxidase. The reaction was developed with chromogen: diaminobenzidine (DAB) substrate diluted in PBS that was added to hydrogen peroxide. For controls, we used the protein extract of uninfected G. mellonella, purified gp43 and Paracoccidioides spp protein.
Statistical Analysis
Graphs and statistical analyses were performed with GraphPad Prism 5 (La Jolla CA, USA). Survival curves were analyzed by Log-rank (Mantel-Cox) test. The hemocyte density data were analyzed by ANOVA with Tukey's post-test. A comparison of the hemocyte density by using a flow cytometry and a hemocytometer was performed with a Pearson correlation, and the real-time PCR was analyzed by t-test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Carlos Eduardo Winter for providing the G. mellonella eggs to initiate the culture of this insect in our laboratory.
Funding
This work was supported by the following Brazilian organizations: the Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) 2013/10917-9 and 2015/03700-9, Rede Nacional de Métodos Alternativos - Conselho Nacional de Desenvolvimento Científico e Tecnológico (RENAMA-CNPq) 403586/2012-7 and Programa de Apoio ao Desenvolvimento Científico d Faculdade de Ciências Farmacêuticas da UNESP (PADC/FCF).
References
- Shikanai-Yasuda MA, Telles Filho FeQ, Mendes RP, Colombo AL, Moretti ML. Guidelines in paracoccidioidomycosis. Rev Soc Bras Med Trop 2006; 39:297-310; PMID:16906260; http://dx.doi.org/10.1590/S0037-86822006000300017
- Magalhães EM, Ribeiro CeF, Dâmaso CS, Coelho LF, Silva RR, Ferreira EB, Rodrigues MR, Camargo ZP, Velloso TR, Malaquias LC. Prevalence of paracoccidioidomycosis infection by intradermal reaction in rural areas in Alfenas, Minas Gerais, Brazil. Rev Inst Med Trop Sao Paulo 2014; 56:281-5; http://dx.doi.org/10.1590/S0036-46652014000400002
- Bellissimo-Rodrigues F, Bollela VR, Da Fonseca BA, Martinez R. Endemic paracoccidioidomycosis: relationship between clinical presentation and patients' demographic features. Med Mycol 2013; 51:313-8; PMID:22928923; http://dx.doi.org/10.3109/13693786.2012.714529
- Benard G, Mendes-Giannini MJS. Textbook of Pediatric Infectious Diseases. Paracoccidioidomycosis: Philadelphia: Elsevier Science, 2014:2780-95
- Matute DR, McEwen JG, Puccia R, Montes BA, San-Blas G, Bagagli E, Rauscher JT, Restrepo A, Morais F, Niño-Vega G, et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol 2006; 23:65-73; PMID:16151188; http://dx.doi.org/10.1093/molbev/msj008
- Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E, San-Blas G, Felipe MS. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol 2009; 52:273-83; PMID:19376249; http://dx.doi.org/10.1016/j.ympev.2009.04.005
- Carrero LL, Niño-Vega G, Teixeira MM, Carvalho MJ, Soares CM, Pereira M, Jesuino RS, McEwen JG, Mendoza L, Taylor JW. New Paracoccidioides brasiliensis isolate reveals unexpected genomic variability in this human pathogen. Fungal Genet Biol 2008; 45:605-12; PMID:18364259; http://dx.doi.org/10.1016/j.fgb.2008.02.002
- Theodoro RC, Teixeira MeM, Felipe MS, Paduan KoS, Ribolla PM, San-Blas G, Bagagli E. Genus Paracoccidioides: Species recognition and biogeographic aspects. PLoS One 2012; 7:e37694; PMID:22666382; http://dx.doi.org/10.1371/journal.pone.0037694
- Brummer E, Castaneda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev 1993; 6:89-117; PMID:8472249
- Tristão GB, Assunção LoP, Dos Santos LP, Borges CL, Silva-Bailão MG, Soares CM, Cavallaro G, Bailão AM. Predicting copper-, iron-, and zinc-binding proteins in pathogenic species of the Paracoccidioides genus. Front Microbiol 2014; 5:761
- Mendes-Giannini MJ, Monteiro da Silva JL, de Fátima da Silva JL, Donofrio FC, Miranda ET, Andreotti PF, Soares CP. Interactions of Paracoccidioides brasiliensis with host cells: recent advances. Mycopathologia 2008; 165:237-48; PMID:17940851; http://dx.doi.org/10.1007/s11046-007-9074-z
- Sardi JC, Pitangui NS, Voltan AR, Braz JD, Machado MP, Fusco-Almeida AM, Mendes Giannini MJ. In vitro Paracoccidioides brasiliensis biofilms and gene expression of adhesins and hydrolytic enzymes. Virulence 2014; 6:663-72.
- Taborda CP, da Silva MB, Nosanchuk JD, Travassos LR. Melanin as a virulence factor of Paracoccidioides brasiliensis and other dimorphic pathogenic fungi: a minireview. Mycopathologia 2008; 165:331-9; PMID:18777637; http://dx.doi.org/10.1007/s11046-007-9061-4
- Tronchin G, Pihet M, Lopes-Bezerra LM, Bouchara JP. Adherence mechanisms in human pathogenic fungi. Med Mycol 2008; 46:749-72; PMID:18651303; http://dx.doi.org/10.1080/13693780802206435
- Marcos CM, de Oliveira HC, da Silva Jde F, Assato PA, Fusco-Almeida AM, Mendes-Giannini MJ. The multifaceted roles of metabolic enzymes in the Paracoccidioides species complex. Front Microbiol 2014; 5:719; PMID:25566229; http://dx.doi.org/10.3389/fmicb.2014.00719
- Mackinnon JE. Pathogenesis of South American blastomycosis. Trans R Soc Trop Med Hyg 1959; 53:487-94; http://dx.doi.org/10.1016/0035-9203(59)90025-2
- McEwen JG, Bedoya V, Patiño MM, Salazar ME, Restrepo A. Experimental murine paracoccidiodomycosis induced by the inhalation of conidia. J Med Vet Mycol 1987; 25:165-75; PMID:3612432; http://dx.doi.org/10.1080/02681218780000231
- Restrepo S, Tobon A, Trujillo J, Restrepo A. Development of pulmonary fibrosis in mice during infection with Paracoccidioides brasiliensis conidia. J Med Vet Mycol 1992; 30:173-84; PMID:1517956; http://dx.doi.org/10.1080/02681219280000241
- Kipnis APJ. Contribuição dos modelos murinos para a Paracoccidioidomicose. Revista de Patologia Tropical 1999; 28:14-25.
- Kashino SS, Singer-Vermes LM, Calich VL, Burger E. Alterations in the pathogenicity of one Paracoccidioides brasiliensis isolate do not correlative with its in vitro growth. Mycopathologia 1990; 111:173-80; PMID:2233986; http://dx.doi.org/10.1007/BF02282801
- Jacobsen ID. Galleria mellonella as a model host to study virulence of Candida. Virulence 2014; 5:237-9; PMID:24384470; http://dx.doi.org/10.4161/viru.27434
- Fuchs BB, O'Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010; 1:475-82; PMID:21178491; http://dx.doi.org/10.4161/viru.1.6.12985
- Mylonakis E, Aballay A. Worms and flies as genetically tractable animal models to study host-pathogen interactions. Infect Immun 2005; 73:3833-41; PMID:15972468; http://dx.doi.org/10.1128/IAI.73.7.3833-3841.2005
- Chamilos G, Lionakis MS, Lewis RE, Kontoyiannis DP. Role of mini-host models in the study of medically important fungi. Lancet Infect Dis 2007; 7:42-55; PMID:17182343; http://dx.doi.org/10.1016/S1473-3099(06)70686-7
- Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC, Mylonakis E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 2009; 53:2605-9; PMID:19332683; http://dx.doi.org/10.1128/AAC.01533-08
- Lionakis MS. Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011; 2:521-7; PMID:22186764; http://dx.doi.org/10.4161/viru.2.6.18520
- Fuchs BB, Mylonakis E. Using non-mammalian hosts to study fungal virulence and host defense. Curr Opin Microbiol 2006; 9:346-51; PMID:16814595; http://dx.doi.org/10.1016/j.mib.2006.06.004
- Desalermos A, Fuchs BB, Mylonakis E. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathog 2012; 8:e1002451; PMID:22319439; http://dx.doi.org/10.1371/journal.ppat.1002451
- Vogel H, Altincicek B, Glöckner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics 2011; 12:308; PMID:21663692; http://dx.doi.org/10.1186/1471-2164-12-308
- García-Rodas R, Casadevall A, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS One 2011; 6:e24485; http://dx.doi.org/10.1371/journal.pone.0024485
- Trevijano-Contador N, Herrero-Fernández I, García-Barbazán I, Scorzoni L, Rueda C, Rossi SA, García-Rodas R, Zaragoza O. Cryptococcus neoformans induces antimicrobial responses and behaves as a facultative intracellular pathogen in the non mammalian model Galleria mellonella. Virulence 2014; 6(1):66-74
- Coleman JJ, Muhammed M, Kasperkovitz PV, Vyas JM, Mylonakis E. Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol 2011; 115:1279-89; PMID:22115447; http://dx.doi.org/10.1016/j.funbio.2011.09.005
- Mesa-Arango AC, Forastiero A, Bernal-Martínez L, Cuenca-Estrella M, Mellado E, Zaragoza O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol 2013; 51:461-72; PMID:23170962; http://dx.doi.org/10.3109/13693786.2012.737031
- Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 2002; 34:153-7; PMID:12381467; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00617.x
- Thomaz L, García-Rodas R, Guimarães AJ, Taborda CP, Zaragoza O, Nosanchuk JD. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence 2013; 4:139-46; PMID:23302787; http://dx.doi.org/10.4161/viru.23047
- Leitão NP, Vallejo MC, Conceição PM, Camargo ZP, Hahn R, Puccia R. Paracoccidioides lutzii Plp43 Is an Active Glucanase with Partial Antigenic Identity with P. brasiliensis gp43. PLoS Negl Trop Dis 2014; 8:e3111; http://dx.doi.org/10.1371/journal.pntd.0003111
- Teixeira MeM, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, Bagagli E, San-Blas G, Soares Felipe MS. Paracoccidioides lutzii sp nov.: biological and clinical implications. Med Mycol 2014; 52:19-28; PMID:23768243
- Arora T, Mehta AK, Joshi V, Mehta KD, Rathor N, Mediratta PK, Sharma KK. Substitute of Animals in Drug Research: An Approach Towards Fulfillment of 4R's. Indian J Pharm Sci 2011; 73:1-6; PMID:22131615; http://dx.doi.org/10.4103/0250-474X.89750
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 2001; 98:15245-50; PMID:11742090; http://dx.doi.org/10.1073/pnas.261418798
- Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect Immun 2003; 71:4862-72; PMID:12933827; http://dx.doi.org/10.1128/IAI.71.9.4862-4872.2003
- Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci U S A 2002; 99:15675-80; PMID:12438649; http://dx.doi.org/10.1073/pnas.232568599
- Glavis-Bloom J, Muhammed M, Mylonakis E. Of model hosts and man: using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv Exp Med Biol 2012; 710:11-7; PMID:22127881; http://dx.doi.org/10.1007/978-1-4419-5638-5_2
- Jacobsen ID, Grosse K, Hube B. Embryonated chicken eggs as alternative infection model for pathogenic fungi. Methods Mol Biol 2012; 845:487-96; PMID:22328397; http://dx.doi.org/10.1007/978-1-61779-539-8_34
- Mylonakis E, Casadevall A, Ausubel FM. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog 2007; 3:e101; PMID:17676994; http://dx.doi.org/10.1371/journal.ppat.0030101
- Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 2004; 28:101-12; PMID:14975532; http://dx.doi.org/10.1016/j.femsre.2003.09.002
- Price CD, Ratcliffe NA. A reappraisal of insect haemocyte classification by the examination of blood from fifteen insect orders. Z Zellforsch Mikrosk Anat 1974; 147:537-49; PMID:4407658; http://dx.doi.org/10.1007/BF00307254
- Arvanitis M, Glavis-Bloom J, Mylonakis E. Invertebrate models of fungal infection. Biochim Biophys Acta 2013; 1832:1378-83; PMID:23517918; http://dx.doi.org/10.1016/j.bbadis.2013.03.008
- Soares AM, Peraçoli MT, Dos Santos RR. Correlation among immune response, morphogenesis of the granulomatous reaction and spleen lymphoid structure in murine experimental paracoccidioidomycosis. Med Mycol 2000; 38:371-7; PMID:11092384; http://dx.doi.org/10.1080/714030964
- Binggeli O, Neyen C, Poidevin M, Lemaitre B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog 2014; 10:e1004067; PMID:24788090; http://dx.doi.org/10.1371/journal.ppat.1004067
- Jiravanichpaisal P, Lee BL, Söderhäll K. Cell-mediated immunity in arthropods: hematopoiesis, coagulation, melanization and opsonization. Immunobiol 2006; 211:213-36; http://dx.doi.org/10.1016/j.imbio.2005.10.015
- Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect 2003; 5:1389-95; PMID:14670452; http://dx.doi.org/10.1016/j.micinf.2003.09.019
- Matha V, Mracek Z. Changes in haemocyte counts in Galleria mellonella (L) (Lepidoptera: Galleriidae) larvae infectedwith Steinernema sp. (Nematoda: steinernematidae). Nematology 1984; 30:86-9; http://dx.doi.org/10.1163/187529284X00482
- Soares DA, de Andrade RV, Silva SS, Bocca AL, Soares Felipe SM, Petrofeza S. Extracellular Paracoccidioides brasiliensis phospholipase B involvement in alveolar macrophage interaction. BMC Microbiol 2010; 10:241; PMID:20843362; http://dx.doi.org/10.1186/1471-2180-10-241
- da Silva MB, Marques AF, Nosanchuk JD, Casadevall A, Travassos LR, Taborda CP. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect 2006; 8:197-205; PMID:16213179; http://dx.doi.org/10.1016/j.micinf.2005.06.018
- Goihman-Yahr M, Essenfeld-Yahr E, de Albornoz MC, Yarzábal L, de Gómez MH, San Martín B, Ocanto A, Gil F, Convit J. Defect of in vitro digestive ability of polymorphonuclear leukocytes in paracoccidioidomycosis. Infect Immun 1980; 28:557-66; PMID:6995312
- Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun 2005; 73:4161-70; PMID:15972506; http://dx.doi.org/10.1128/IAI.73.7.4161-4170.2005
- Araújo VC, Demasi AP, Soares AB, Passador-Santos F, Napimoga MH, Martinez EF, Freitas NS, Araújo NS. Neutrophils in oral paracoccidioidomycosis and the involvement of Nrf2. PLoS One 2013; 8:e76976; PMID:24204715; http://dx.doi.org/10.1371/journal.pone.0076976
- Singer-Vermes LM, Burger E, Franco MF, Di-Bacchi MM, Mendes-Giannini MJ, Calich VL. Evaluation of the pathogenicity and immunogenicity of seven Paracoccidioides brasiliensis isolates in susceptible inbred mice. J Med Vet Mycol 1989; 27:71-82; PMID:2746437; http://dx.doi.org/10.1080/02681218980000111
- Mendes-Giannini MJ, Andreotti PF, Vincenzi LR, da Silva JL, Lenzi HL, Benard G, Zancopé-Oliveira R, de Matos Guedes HL, Soares CP. Binding of extracellular matrix proteins to Paracoccidioides brasiliensis. Microbes Infect 2006; 8:1550-9; PMID:16698299; http://dx.doi.org/10.1016/j.micinf.2006.01.012
- Stambuk BU, Puccia R, de Almeida ML, Travassos LR, Schenkman S. Secretion of the 43 kDa glycoprotein antigen by Paracoccidioides brasiliensis. J Med Vet Mycol 1988; 26:367-73; PMID:3246625; http://dx.doi.org/10.1080/02681218880000521
- Hanna SA, Monteiro da Silva JL, Giannini MJ. Adherence and intracellular parasitism of Paracoccidioides brasiliensis in Vero cells. Microbes Infect 2000; 2:877-84; PMID:10962270; http://dx.doi.org/10.1016/S1286-4579(00)00390-7
- Vicentini AP, Gesztesi JL, Franco MF, de Souza W, de Moraes JZ, Travassos LR, Lopes JD. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun 1994; 62:1465-9; PMID:8132354
- Andreotti PF, Monteiro da Silva JL, Bailão AM, Soares CM, Benard G, Soares CP, Mendes-Giannini MJ. Isolation and partial characterization of a 30 kDa adhesin from Paracoccidioides brasiliensis. Microbes Infect 2005; 7:875-81; PMID:15862780; http://dx.doi.org/10.1016/j.micinf.2005.02.005
- González A, Gómez BL, Diez S, Hernández O, Restrepo A, Hamilton AJ, Cano LE. Purification and partial characterization of a Paracoccidioides brasiliensis protein with capacity to bind to extracellular matrix proteins. Infect Immun 2005; 73:2486-95; http://dx.doi.org/10.1128/IAI.73.4.2486-2495.2005
- Barbosa MS, Báo SN, Andreotti PF, de Faria FP, Felipe MS, dos Santos Feitosa L, Mendes-Giannini MJ, Soares CM. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect Immun 2006; 74:382-9; PMID:16368993; http://dx.doi.org/10.1128/IAI.74.1.382-389.2006
- Pereira LA, Báo SN, Barbosa MS, da Silva JL, Felipe MS, de Santana JM, Mendes-Giannini MJ, de Almeida Soares CM. Analysis of the Paracoccidioides brasiliensis triosephosphate isomerase suggests the potential for adhesin function. FEMS Yeast Res 2007; 7:1381-8; PMID:17714474; http://dx.doi.org/10.1111/j.1567-1364.2007.00292.x
- Donofrio FC, Calil AC, Miranda ET, Almeida AM, Benard G, Soares CP, Veloso SN, Soares CM, Mendes Giannini MJ. Enolase from Paracoccidioides brasiliensis: isolation and identification as a fibronectin-binding protein. J Med Microbiol 2009; 58:706-13; PMID:19429745; http://dx.doi.org/10.1099/jmm.0.003830-0
- Torres I, Hernandez O, Tamayo D, Muñoz JF, Leitão NP, García AM, Restrepo A, Puccia R, McEwen JG. Inhibition of PbGP43 Expression May Suggest that gp43 is a Virulence Factor in Paracoccidioides brasiliensis. PLoS One 2013; 8:e68434; PMID:23874627; http://dx.doi.org/10.1371/journal.pone.0068434
- Puccia R, Travassos LR. 43-kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J Clin Microbiol 1991; 29:1610-5; PMID:1722220
- Gegembauer G, Araujo LM, Pereira EF, Rodrigues AM, Paniago AM, Hahn RC, de Camargo ZP. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis 2014; 8:e2986; PMID:25032829; http://dx.doi.org/10.1371/journal.pntd.0002986
- Queiroz Júnior LeP, de Camargo ZP, Tadano T, Rodrigues AM, Takarara DT, Gegembauer G, Araujo LM, Hahn RC. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp from Central Western Brazil. Mycoses 2014; 57:466-72; PMID:24635832; http://dx.doi.org/10.1111/myc.12183
- da Fonseca CA, Jesuino RS, Felipe MS, Cunha DA, Brito WA, Soares CM. Two-dimensional electrophoresis and characterization of antigens from Paracoccidioides brasiliensis. Microbes Infect 2001; 3:535-42; PMID:11418327; http://dx.doi.org/10.1016/S1286-4579(01)01409-5
- Furuya H, Ikeda R. Interaction of triosephosphate isomerase from the cell surface of Staphylococcus aureus and α-(1->3)-mannooligosaccharides derived from glucuronoxylomannan of Cryptococcus neoformans. Microbiology 2009; 155:2707-13; PMID:19423633; http://dx.doi.org/10.1099/mic.0.028068-0
- Saito F, Ikeda R. Killing of Cryptococcus neoformans by Staphylococcus aureus: the role of cryptococcal capsular polysaccharide in the fungal-bacteria interaction. Med Mycol 2005; 43:603-12; PMID:16396245; http://dx.doi.org/10.1080/13693780500078417
- Yamaguchi M, Ikeda R, Nishimura M, Kawamoto S. Localization by scanning immunoelectron microscopy of triosephosphate isomerase, the molecules responsible for contact-mediated killing of Cryptococcus, on the surface of Staphylococcus. Microbiol Immunol 2010; 54:368-70; PMID:20536736; http://dx.doi.org/10.1111/j.1348-0421.2010.00225.x
- Casadevall A, Pirofski L. Host-pathogen interactions: the attributes of virulence. J Infect Dis 2001; 184:337-44; PMID:11443560; http://dx.doi.org/10.1086/322044
- Casadevall A, Pirofski LA. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun 2000; 68:6511-8; PMID:11083759; http://dx.doi.org/10.1128/IAI.68.12.6511-6518.2000
- Kurita N, Sano A, Coelho KI, Takeo K, Nishimura K, Miyaji M. An improved culture medium for detecting live yeast phase cells of Paracoccidioides brasiliensis. J Med Vet Mycol 1993; 31:201-5; PMID:8360811; http://dx.doi.org/10.1080/02681219380000251
- Restrepo A, Jiménez BE. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J Clin Microbiol 1980; 12:279-81; PMID:7229010
- McGowan KL, Buckley HR. Growth characteristics of the yeast phase of Paracoccidioides brasiliensis in a chemically defined medium. J Gen Microbiol 1984; 130:2797-801; PMID:6527125
- Ramarao N, Nielsen-Leroux C, Lereclus D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J Vis Exp 2012; 11(70):e4392; PMID:23271509
- Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS One 2013; 8:e60047; PMID:23555877; http://dx.doi.org/10.1371/journal.pone.0060047
- Parente AF, Bailão AM, Borges CL, Parente JA, Magalhães AD, Ricart CA, Soares CM. Proteomic analysis reveals that iron availability alters the metabolic status of the pathogenic fungus Paracoccidioides brasiliensis. PLoS One 2011; 6:e22810; PMID:21829521; http://dx.doi.org/10.1371/journal.pone.0022810
- Peres da Silva R, Matsumoto MT, Braz JD, Voltan AR, de Oliveira HC, Soares CP, Mendes Giannini MJ. Differential gene expression analysis of Paracoccidioides brasiliensis during keratinocyte infection. J Med Microbiol 2011; 60:269-80; PMID:21071542; http://dx.doi.org/10.1099/jmm.0.022467-0
- Salina MA, Shikanai-Yasuda MA, Mendes RP, Barraviera B, Mendes Giannini MJ. Detection of circulating Paracoccidioides brasiliensis antigen in urine of paracoccidioidomycosis patients before and during treatment. J Clin Microbiol 1998; 36:1723-8; PMID:9620407