In this issue, A. Papadopoulos et al.Citation1 reported that different subtypes of dendritic cells (DCs) play specific roles as sensors of Brucella. This paper introduced the diversity of DC subtypes in the understanding of host response to pathogens.
Brucellosis is a worldwide zoonosis due to Brucella sp., which is transmitted by contamination of food or airborne infectious particles. The disease evolves from an initial non-specific primary infection to chronic brucellosis.Citation2 The necessity to understand how the host fights Brucella and how new vaccines can be developed supposes a better understanding of the way the host uses bacterial signals to mount an efficient immune response against the invader.
Dendritic cells (DCs) are specialized in the processing and presentation of endogenous and exogenous antigens to T cells in a major histocompatibility complex (MHC)-I and MHC-II context. Positioned at the host periphery as sentinels, they require efficient directional migration toward the T cell zones of lymph nodes. They stimulate naïve T cells leading to an efficient anti-bacterial immune response. Conversely, the immune activity of DCs could be switched off, contributing to the regulation of the immune response.Citation3 Hence, the maturation of DCs may be inhibited by varied pathogens, preventing the mounting of a microbicidal response.Citation4
In this new paper,Citation1 the authors firstly observed that most studies are based on DCs derived from bone marrow monocytes (BMDCs) in the presence of granulocyte macrophage-colony stimulating factor (GM-CSF). This is a very reductionist model since murine DCs represent a heterogeneous population made of phenotypically and functionally distinct subsets. In addition, using only one type of pathogen is largely insufficient to analyze DC functions. Indeed, we recently reported that human DCs derived from monocytes cultured in the presence of interleukin (IL)-4 and GM-CSF and exposed to a panel of intracellular bacteria led us to change our point of view on DC maturation. Whereas it was commonly admitted that intracellular bacteria prevent the activation of DCs, our experiments showed that there is a gradient of activation from the lack of activation by Tropheryma whipplei, the agent of Whipple's disease, to the exacerbated maturation in response to Orientia tsutsugamushi, the agent of scrub typhus; Brucella abortus partly induced DC maturation.Citation5 This strategy, based on the comparison of several bacterial infections, may be useful to accurately analyze the pathways involved in DC responses to pathogens. Clearly, the reductionist model of DC maturation induced by GM-CSF cannot reflect the adaptation of DCs to the diversity of their microenvironment, as revealed by the development of polychromatic flow cytometry and highly improved extraction protocols, which enable a better assessment of the role of DCs as sensors of infection in different tissues.
To analyze in detail the responses of DCs, the authors used 4 experimental conditions. Besides the canonical model of BMDCs differentiated in the presence of GM-CSF, BMDCs were cultured in the presence of FMS-like tyrosine 3 ligand (FLT3L), FLT3L and GM-CSF or GM-CSF and IL-15 (). GM-CSF induces the generation of CD11b-expressing DCs that are close to monocyte-derived DCs produced in an inflammatory context; FLT3L favors the formation of 3 DC subsets, plasmacytoid DCs (pDCs) and the equivalent of splenic CD8+ and splenic CD11b+; combined with GM-CSF, FLT3L promotes the differentiation of equivalents of tissue-resident CD103+ DCs.
Figure 1. The figure summarizes the main findings of the paperCitation1 and introduces some findings from the literature.Citation5 The schematic dendritic cells (DC) represent murine bone marrow-derived DCs (BMDCs) differentiated into DCs in the presence of GM-CSF, FLT3L or IL-15, alone or combined, and human circulating monocytes differentiated into DCs in the presence of GM-CSF and IL-4. The figure shows the replication of B. abortus, the expression of co-stimulation molecules such as HLA-DR and the production of cytokines by murine and human DCs. Note that IL-12p40, TNF, IL-6 and CCL−2 are produced by both murine and human DCs. While GM-CSF plays a major role in B. abortus replication, FLT3L, which drives the differentiation of pDCs and classical DCs, does not create favorable conditions for B. abortus infection.
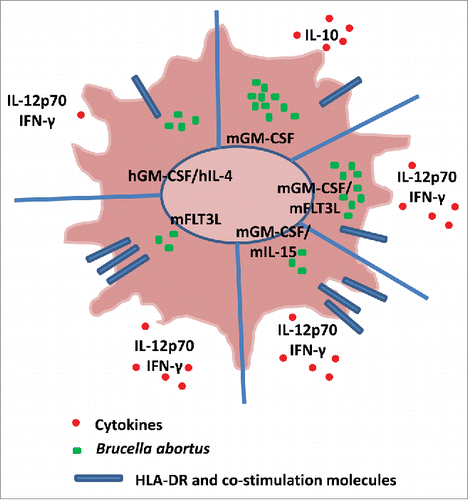
The authors show that B. abortus replicated within the 4 BMDC subtypes and that the bacterial replication was inversely related to the internalization efficiency. The nature of the vacuolar compartment in which resided the bacteria differed according to the subtype of BMDCs. Indeed, the number of ribosomes around bacterial vacuoles was higher in DCs with the highest level of bacterial replication. In addition, subtle changes in the maturation process induced by B. abortus were observed in the 4 DC subtypes, demonstrating that multiple markers are necessary to discriminate the responses of DCs to B. abortus. The study of cytokine pattern reveals several unexpected data. The combination of GM-CSF and IL-15 induced the release of high levels of inflammatory cytokines whereas Flt3L-differentiated DCs were poor producers of inflammatory cytokines. Interestingly, only GM-CSF-differentiated DCs are a source of IL-10. One can hypothesize that the IL-10 release by GM-CSF-differentiated DCs was related to their poor activation and maturation in response to B. abortus and that this poor reactivity may be a consequence of the bacterial strategy to evade from inflammatory DCs. This is emphasized by previous reports of the same group in which Brucella infection affects GM-CSF-differentiated T cells and impairs T cell proliferation.Citation6 On the other hand, the results obtained with Flt3L-differentiated DCs that represent the equivalent of splenic DCs demonstrate that Brucella is able to induce the maturation of splenic DCs.
Such findings require a reappraisal of the impact of in vivo infection of DCs by Brucella. The diversity of the DC lineage obliges us to be careful when transferring the data obtained with murine DCs to human infectious diseases, although some equivalence between human and murine DCs has been reported.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Papadopoulos A, Gagnaire A, Degos C, De Chastellier C, Gorvel JP. Brucella discriminates between dendritic cell subsets upon in vitro infection. Virulence 2015; PMID:26606688
- Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med 2005; 352:2325–36; PMID:15930423; http://dx.doi.org/10.1056/NEJMra050570
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity 2014; 40:642–56; PMID:24837101; http://dx.doi.org/10.1016/j.immuni.2014.04.016
- Papadopoulos A, Gorvel J-P. Subversion of mouse dendritic cell subset function by bacterial pathogens. Microb Pathog 2015; 89:140–9; PMID:26453826; http://dx.doi.org/10.1016/j.micpath.2015.10.004
- Gorvel L, Textoris J, Banchereau R, Ben Amara A, Tantibhedhyangkul W, von Bargen K, Ka MB, Capo C, Ghigo E, Gorvel J-P, et al. Intracellular bacteria interfere with dendritic cell functions: role of the type I interferon pathway. PloS One 2014; 9:e99420; PMID:24915541; http://dx.doi.org/10.1371/journal.pone.0099420
- Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, et al. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog 2008; 4:e21; PMID:18266466; http://dx.doi.org/10.1371/journal.ppat.0040021
