ABSTRACT
It was demonstrated that specific IgG can enhance the infection with CV-B4, in vitro, in the human system. This enhancement could be involved in the pathophysiology of CV-B4 induced diseases. To investigate further the role of enhancing IgG in the infection with CV-B4 E2 in vivo, animal models are needed. Therefore, it was decided to assess whether inoculation of CV-B4 E2 to mice results in the appearance of IgG able to enhance the infection with this virus. Swiss albino mice were inoculated with CV-B4 E2 intraperitoneally. Serum samples were obtained from tail vein blood collected from day 0 to day 80 p.i. IgG were isolated by Protein G affinity chromatography. Seroneutralisation assays were carried out. In total murine spleen cells cultures inoculated with CV-B4 E2 mixed with various dilutions of serum or IgG samples, the enhancing activity was assayed through i) the antiviral activity titer of supernatants ii) the detection of intracellular viral RNA by RT-PCR iii) the level of infectious particles in supernatants.
In most serum samples (76/105), neutralizing and enhancing activities were detected peaking between days 14 and 30 p.i and were higher in sera from mice inoculated with 2.106 TCID50 units than with lower doses. The enhancing activity was due to the IgG-enriched fraction of serum from CV-B4 E2 infected animals but not from control animals.
These data show that IgG from immune mice can enhance the infection of splenocytes with CV-B4 E2 in vitro and open the way to explore whether such an enhancing activity can play a role in vivo.
Introduction
Enteroviruses (family: Picornaviridae) are small, non-enveloped, positive single-stranded RNA viruses. The Enterovirus genus classically subdivided in polioviruses (PV), echoviruses (E), types A and B coxsackieviruses (CV-A and CV-B) and non-classified enteroviruses (EV), actually encompasses 271 human serotypes distributed in 7 species Enterovirus A to D and Rhinovirus A to C.Citation1 These ubiquitous pathogens are essentially transmitted through the fecal-oral plus respiratory route. Type B Coxsackievirus infections are generally asymptomatic, but when symptomatic, the pathological specter varies from benign acute infection of the gastrointestinal tract to severe chronic infections such as chronic myocarditis, dilated cardiomyopathy, and type 1 diabetes.Citation2 Indeed epidemiological data evidenced an association between enteroviruses, especially CV-B and type 1 diabetes (T1D) in genetically predisposed individuals, and experimental studies, in vitro and in vivo in animal models, revealed several mechanisms through which CV-B infection may be involved in the pathogenesis of T1D.Citation2-5
Plasma, serum and IgG from CV-B4 E2 seropositive subjects have been shown to strongly increase the CV-B4 E2-induced production of IFNα by human peripheral blood mononuclear cells (PBMCs), in vitro.Citation6,7 That increase was the consequence of an enhancement of the infection of PBMC with CV-B4 E2 by non-neutralizing anti-CV-B4 E2 IgG.Citation8,9 The role of the anti-CV-B4 E2 enhancing activity of serum in the outcome of the infection by this virus, in vivo, remains to be determined.
To address this issue, an animal model is needed. It was reported that CV-B4 E2 could infect mice and that the diabetogenic strain CV-B4 E2 could infect outbred Swiss albino mice.Citation3,10 Therefore, we decided to investigate whether the inoculation of CV-B4 E2 to mice results in an anti-CV-B4 E2 enhancing activity of their sera.
Results
Serum from CV-B4 E2 inoculated mice can neutralize the virus in vitro
Mice were inoculated intraperitoneally with various doses of CV-B4 E2 or culture medium as described in the material and methods section. Serum was collected from day 0 (just before virus inoculation) through day 80 p.i. (day 0, 7, 14, 21, 30, 40, 60 and 80 p.i.), The anti-CV-B4 E2 neutralizing activity, in 29 out of 105 samples obtained from day 7 p.i. through day 80 p.i. was lower or equal to 20. In contrast, in most serum samples, 76 out of 105, the values were higher than 160 on day 7. In these samples, the anti-CV-B4 E2 neutralizing activity peaked between days 14 and 30 p.i, (mean titers ranging between 800 and 2,500), and decreased thereafter (mean titers ranging between 32 and 64 at day 80 p.i. (see ).
Figure 1. Anti-CV-B4 E2 neutralizing activity of serum from mice inoculated with various doses of CV-B4 E2. Serum samples collected from 76 CV-B4 E2-infected mice were tested for their neutralizing activity. Neutralizing titers of serum samples were defined as the reciprocal of the last dilution that totally inhibited the CV-B4 E2-induced CPE in HEp-2 cell cultures. The results are the means + SD of titer values of serum collected on day 0 through day 80 post-infection from 3 to 4 mice inoculated with each dose of virus.
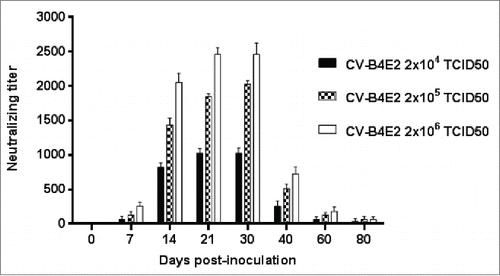
The extent of the anti-CV-B4 E2 neutralizing activity of serum was dependent on the amount of inoculated infectious particles. Indeed, higher levels of anti-CV-B4 E2 neutralizing activity were observed in serum obtained from mice inoculated with 2 × 106 TCID50 compared to those inoculated with 2 × 105, then to those inoculated with 2 × 104 TCID50 units (p=0.001, Friedman's test, ). The level of anti-CV-B4 E2 neutralizing activity was null in serum collected from mock-inoculated mice.
Serum from CV-B4 E2-inoculated mice can enhance the CV-B4 E2 induced production of antiviral mediators by spleen cells culture in vitro
Virus suspension (CV-B4 E2 at 105 TCID50/mL) was incubated in presence of culture medium or mouse serum (final dilution 1:10, 1:100, 1:500 and 1:1000), then the mixtures were added to murine spleen cells cultures. Supernatants were harvested 48 hours later and checked for their antiviral activity by using the bioassay described in the material and methods section.
In these experiments, when CV-B4 E2 was incubated in presence of immune serum diluted 1:500 and 1:1000 obtained from mice that had been inoculated with CV-B4 E2, the supernatants of spleen cells cultures protected L-929 cell cultures against the CPE induced by EMCV. When CV-B4 E2 was incubated in presence of the same immune sera diluted 1:10 and 1:100, the protective effects of supernatants were respectively null and very weak.
The enhancing activity of serum samples from 29 CV-B4 E2-inoculatd mice was weak (< 2), in agreement with the low neutralizing activity of these samples described above. The enhancing activity of serum from the 76 other CV-B4 E2-inoculatd mice was detected on day 7 p.i, peaked between days 21 and 30 p.i., and was still detectable on day 80 p.i but lower than 2 ().
Figure 2. Antiviral activity in culture supernatants of spleen cells cultures inoculated with CV-B4 E2 mixed with serum from CV-B4 E2-infected mice. Serum samples from 76 CV-B4 E2-infected mice were diluted 1:500, mixed with CV-B4 E2 then the mixture was inoculated to murine spleen cells cultures (MOI = 0.02) and supernatants were harvested after 48 hours of incubation. The antiviral activity of supernatants was tested by using a bioassay. The antiviral titers of supernatant samples were defined as the reciprocal of the last dilution that totally inhibited the EMCV-induced cytopathic effect in L-929 cell cultures. The results are the means + SD of values obtained with serum collected on day 0 through day 80 from 3 to 4 mice infected with each dose of virus.
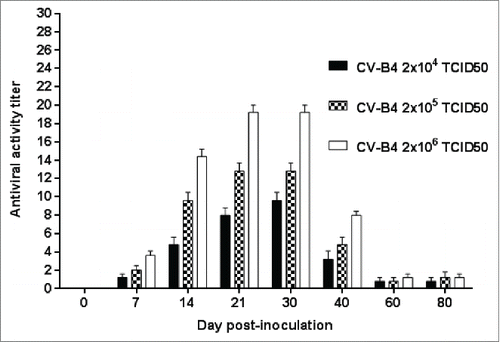
The enhancing activity of serum samples increased with the virus dose since the lowest antiviral titers in our assays were reached with sera from mice inoculated with 2 × 104 TCID50, and the highest one with sera from mice inoculated with 2 × 106 TCID50 units (p=0.001, ). Supernatants of spleen cells cultures mock-inoculated or inoculated with CV-B4 E2 or with CV-B4 E2 incubated with serum from mock-infected mice did not show any antiviral activity.
Serum from CV-B4 E2-inoculated mice enhance the in vitro infection of spleen cells cultures with CV-B4 E2
The impact of serum from CV-B4 E2-inoculated mice onto the infection of splenocytes with CV-B4 E2 has been studied. When spleen cell cultures were inoculated with CV-B4 E2 (105 TCID50/mL) mixed with CV-B4 E2-inoculated mice serum (diluted 1:500 and 1:1,000), intracellular enteroviral RNA was detectable by RT-PCR at 48 hours p.i. (). Enteroviral RNA was detectable only following a subsequent nested-PCR when spleen cells cultures were inoculated with CV-B4 E2 mixed with culture medium (). There was no enteroviral RNA detectable by RT-PCR when spleen cells cultures were inoculated with CV-B4 E2 mixed with serum of mock-inoculated mice (data not shown).
Figure 3. Detection of viral RNA in spleen cells cultures inoculated with CV-B4 E2 in vitro. Spleen cells cultures were inoculated with CV-B4 E2 mixed with serum from 4 infected mice collected day 30 p.i. (lanes 1, 2, 3 and 4) or inoculated with CV-B4 E2 (lanes 5 and 6), MOI 0.02. Agarose gel electrophoresis of amplicons resulting from RT-PCR (top) and semi-nested-RT-PCR (bottom) are presented. CV-B4: supernatant of CV-B4 E2 -infected HEp-2 cell culture, W: purified sterile water ; S: last washing supernatant.
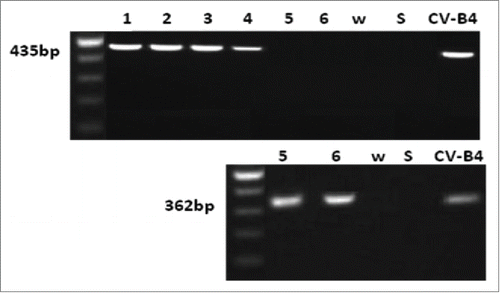
When spleen cells cultures were inoculated with CV-B4 E2 mixed with CV-B4 E2-inoculated mice serum (diluted 1:10 and 1:100), the detection of enteroviral RNA by RT-PCR was negative and revealed positive only after subsequent nested-PCR (data not shown).
Serum-derived IgG from CV-B4 E2-inoculated mice enhance the in vitro infection of spleen cells cultures with CV-B4 E2
In so far as serum from CV-B4 E2 inoculated mice but not from mock-infected mice was able to enhance the infection of splenocytes with CV-B4 E2, it was hypothesized that immune IgG were responsible for the enhancing activity. To adress this issue, serum samples from CV-B4 E2 inoculated mice and from mock-infected mice were submitted to the protein G purification method to segregate IgG-enriched and IgG-depleted fractions, as described in the materials and methods section. IgG-enriched and IgG-depleted serum fractions were incubated with CV-B4 E2, then the mixture was inoculated to spleen cells cultures.
When fractions of serum samples from CV-B4 E2-infected mice were segregated, an antiviral activity was detected in supernatants of spleen cells cultures inoculated with CV-B4 E2/IgG-enriched fraction mixtures but not in those of spleen cells cultures inoculated with CV-B4 E2/IgG-depleted fraction mixtures (). The antiviral activity level was higher when CV-B4 E2 was mixed with IgG than with serum. Along with these data, intracellular enteroviral CV-B4 E2 RNA was detectable by RT-PCR in spleen cells cultures when cultures were inoculated with CV-B4 E2/IgG-enriched serum mixtures whereas it was detected by semi-nested RT-PCR when cultures were inoculated with CV-B4 E2/IgG-depleted serum mixtures ().
Figure 4. Antiviral activity of supernatants of spleen cells cultures inoculated with CV-B4 E2 mixed with serum-derived IgG from CV-B4 E2-inoculated mice. The antiviral activity of supernatants of spleen cells cultures inoculated with CV-B4 E2, MOI 0.02, mixed with IgG-enriched or IgG-depleted fractions of serum, or serum (diluted 1:1,000) from 5 CV-B4 E2-infected mice has been determined. Serum samples were collected on days 7, 14, 21, 30 and 60 p.i. IgG-enriched and IgG-depleted fractions of serum were obtained by using Protein G affinity chromatography. The results are expressed as mean + SD (n=3) of titers obtained by using the bioassay as described in the legend.
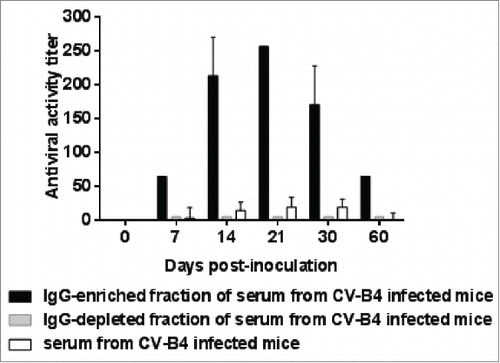
Figure 5. Enhancing activity of serum-derived IgG from CV-B4 E2-inoculated mice. Spleen cells cultures were inoculated with CV-B4 E2, CV-B4 E2 mixed with IgG-enriched or IgG-depleted fractions of serum (diluted 1:1,000) from CV-B4 E2-infected mice or mixed with serum from control mice. (A) Detection of intracellular viral RNA. Agarose gel electrophoresis of amplicons resulting from RT-PCR (top) and semi-nested-RT-PCR (bottom) are presented. Spleen cells cultures were inoculated with: CV-B4 E2, MOI=0.02, in presence of IgG-enriched (1, 2, 3, 4 and 5) or IgG-depleted (6, 7, 8, 9 and 10) serum collected on day 14 post-infection from 5 CV-B4 E2-infected mice, or CV-B4 E2 (11), or CV-B4 E2 in presence of serum from control mice (12).Supernatant of CV-B4 E2-infected HEp-2 cell culture (CV-B4), sterile water (W). (B) Levels of infectious particles in supernatants of spleen cell cultures. Spleen cells cultures were incubated with medium containing CV-B4 E2 mixed with mouse serum, IgG-enriched or IgG-depleted fractions of mouse serum (dilution 1/1000) obtained from CV-B4 E2-infected mice (black bars) or mock-infected mice (white bars) on day 14 post-infection (a) or with medium containing CV-B4 E2 (b). The MOI was 0.02. Culture supernatant samples were harvested 3 days after inoculation. IgG-enriched and IgG-depleted fractions of serum were obtained by using Protein G affinity chromatography. The results are mean + SD (n=3) of virus titers determined on HEp-2 cells by limiting dilution assay for 50% tissue culture infectious doses (TCID50).
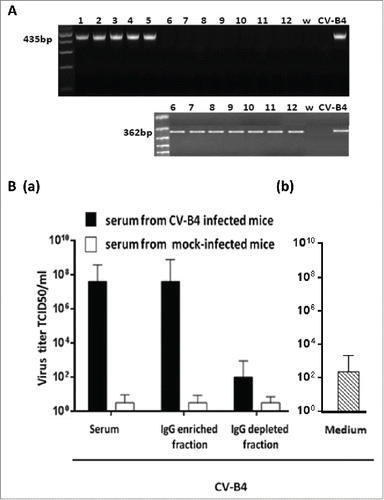
Moreover, spleen cells cultures inoculated with CV-B4 E2 mixed with serum or IgG from CV-B4 E2-infected mice but not from mock-infected mice released a high level of infectious particles, up to 6.2 × 107 +/− 1.3 TCID50/mL (see ), whereas the levels of infectious particles in supernatants of cultures inoculated with CV-B4 E2 was 3.2 × 101 +/− 1 TCID50/mL (data not shown). These levels were close to the residual infectious levels of inocula that were incubated in 96-well plates in similar conditions, 6.5 × 102 +/− 0. 8 TCID50/mL (). The levels of infectious particles in supernatants of spleen cells cultures inoculated with CV-B4 E2 mixed with the IgG-depleted fraction of serum samples from infected animals were much lower, 2.6 × 102 +/− 0.85 TCID50/mL than in supernatants of spleen cells cultures inoculated with CV-B4 E2 mixed with the IgG-enriched fraction of serum samples from infected animals.
Altogether, these data display that the enhancing activity of serum from CV-B4 E2-infected mice was supported by the IgG-enriched but not by the IgG-depleted fraction.
Discussion
The current study is different in many respects from those of other investigators, it is reported for the first time that the serum from mice inoculated with CV-B4 E2 can have an anti-CV-B4 E2 enhancing activity.
Several considerations in the present report are noteworthy. Small volume blood samples were collected from the tail vein to investigate the anti-CV-B4 E2 neutralizing and enhancing activities of serum in each sample. Screening on spleen cells cultures was performed with small volume of serum, 5 µL, in duplicate which enabled testing both activities with each serum sample obtained consecutively up to 80 days p.i.
In the human system, the anti-CV-B4 E2 non-neutralizing activity of serum was investigated in PBMC cultures.Citation6 In mice the volume of available blood being very limited it was not conceivable to use PBMC to address the issue of the biological activity of the serum. Therefore, as an alternative to PBMC, it was decided to use murine spleen cells cultures, since spleen cells cultures are more abundant, poorly permissive to CV-B4 E2 and the supernatants of these cultures have no antiviral activity in response to CV-B4 E2 as displayed by our team previously.Citation11 This system allowed us in the current study to evaluate the properties of serum from CV-B4 E2-infected mice, that has been proven to be able: i) to enhance the CV-B4 E2-induced production by spleen cells cultures of antiviral mediators contained in supernatants as shown by a bioassay already described;Citation11 ii) to enhance the CV-B4 E2 infection of spleen cells cultures as demonstrated by the detection of intracellular viral RNA by RT-PCR whereas viral RNA was detected by sn-RT-PCR in these cells inoculated with CV-B4 E2 or CV-B4 E2 mixed with serum from control mice. iii) to enhance the production of viral particles by spleen cells cultures inoculated with CV-B4 E2 mixed with serum from CV-B4-infected mice. It was shown, in the current study that the enhancing activity of serum from CV-B4 E2 infected mice was due to IgG.
Kinetics of both neutralizing and enhancing anti-CV-B4 E2 activities of serum samples from CV-B4 E2-infected mice were similar. Indeed, the neutralizing activity and the enhancement of the CV-B4 E2-induced antiviral activity in serum were detected on day 7, peaked between days 21 and 30, and were dramatically reduced on day 40 and then barely detectable. These both activities were detectable in serum from mice inoculated with 2 × 104 TCID50 and, as expected, their extent increased with the viral dose inoculated to mice. The high extent of the anti-CV-B4 E2 neutralizing activity, up to 3 × 103 can explain that the serum-dependent enhancement of CV-B4 E2-induced antiviral activity released by spleen cells cultures was masked when serum samples were diluted 1:10 and 1:100.
The enhanced CV-B4 E2-induced release of antiviral mediators by spleen cells cultures obtained with diluted serum in our assay is due to anti-CV-B4 E2 IgG as strongly suggested by the activity of serum-derived IgG from CV-B4 E2-inoculated mice but not from mock-inoculated animals. This is reminiscent of previous studies describing that human serum harbouring an anti-CV-B4 E2 neutralizing activity had an enhancing anti-CV-B4 E2 activity, due to anti-CV-B4 E2 IgG, in human PBMC or cell line cultures, provided the serum was diluted.Citation6,12,13
Intracellular viral RNA was detected by RT-PCR when spleen cells cultures were inoculated with CV-B4 E2 mixed with diluted serum or serum-derived IgG from CV-B4 E2-infected mice, whereas it was only detected by sn-RT-PCR, a more sensitive technique, when cells where inoculated with CV-B4 E2 or with CV-B4 E2 mixed with serum or serum-derived IgG from mock-infected mice. These observations suggest that the level of intracellular viral RNA was higher in the first case. The absence of viral RNA in the last supernatant washing fluids of spleen cells cultures show that the positive detection of viral RNA in our experiments was not due to remaining residual virus. This pattern of data is in agreement with previous reports of the serum/IgG-dependent enhancement of the infection of human PBMC with CV-B4 E2, which was due to non-neutralizing antibodies bound to viral particles.Citation8,9,14
In the human system it was shown that the target cells of serum/IgG-dependent enhanced CV-B4 E2 infection among PBMC were monocytes.Citation7,8 Further studies are needed to identify which cell types are infected through enhancing serum/IgG within mouse spleen cells cultures in order to determine whether macrophages are involved since these cells can be reservoirs for viruses within lymphoid organs with implications in the pathogenesis of CV-B4 E2 –induced diseases.
Serum samples from most of Swiss albino mice inoculated with CV-B4 E2 had an enhancing activity as shown by both assays: CV-B4 E2-induced antiviral activity and infection of splenocytes with CV-B4 E2. However there was no enhancing anti-CV-B4 E2 activity in both assays in serum from 29 out of 105 (18%) mice, whatever was the serum dilution, which can be explained by a limited level of specific antibodies as suggested by low anti-CV-B4 E2 neutralizing titers in those animals compared with the others. Together these data indicate, as expected, that the pattern of individual response to CV-B4 E2 and, hence, the biological properties of serum in outbred mice may be different. Moreover, it has been observed that the antiviral activity produced by spleen cells cultures inoculated with CV-B4 E2 is enhanced to a higher extent with serum-derived IgG of infected animals than with their serum. It was not due to an impact of diluted serum from CV-B4 E2-inoculated mice onto the bioassay based on the infection of L-929 with EMCV detecting the antiviral activity of spleen cells cultures supernatants (data not shown). In contrast, the levels of infectious particles in supernatants of CV-B4 E2-inoculated spleen cells cultures were enhanced to the same extent. It can be assumed that inhibitors contained in serum from infected animals can modulate the biological enhancing effect of IgG in the production of CV-B4 E2-induced antiviral mediators by spleen cells cultures, but not in the infection of these cells. Studies are needed to investigate further the mechanisms of this inhibition.
Interestingly our observations may explain previous results focusing on enhanced pathology in animal models. Indeed, heterologous challenge of mice with CV-B3 after an initial infection with CV-B2 resulted in enhanced pathologyCitation15,16 which especially can be due to the phenomenon of antibody-dependent enhancement.Citation15,17,18,19,9Moreover it has been shown that in adult mice homologous challenge with CVB4-E2 resulted in hyperglycaemia,Citation20 and that challenge of pups of dams infected during pregnancy resulted in enhanced pathology in the offspring through possibly immune-mediated mechanisms due to pre-existing immunity.Citation21
In conclusion, in vitro assays displayed that serum samples of mice inoculated with CV-B4 E2 had an enhancing effect able to increase, on the one hand, the CV-B4 E2-induced production of antiviral mediators by spleen cells cultures and, on the other hand, the infection of these cells with CV-B4 E2. This enhancing effect was due to IgG present in CV-B4 E2-infected mice sera but not in those from mock-infected animals, which strongly suggests that anti-CV-B4 E2 antibodies were involved in the process. Whether such enhancing IgG type antibodies can play a role in the outcome of the infection with CV-B4 E2, in vivo, remains to be determined. Future studies will be directed along this line in our laboratory.
Material & methods
Viruses
The diabetogenic strain CV-B4 E2 (kindly provided by J. W. Yoon, Julia McFarlane Diabetes Research Center, Calgary, Alberta, Canada), was propagated in HEp-2 cells (BioWhittaker, Walkersville, MD, USA) in Eagle's minimal essential medium (MEM; Gibco BRL, Invitrogen, Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (FCS, Sigma St Louis, MO, USA), 1% L-glutamine (Gibco BRL), 50 µg/mL streptomycin, and 50 IU/mL penicillin (BioWhittaker). Supernatants were collected 3 days after inoculation, clarified by centrifugation at 2,000 × g for 10 min, divided into aliquots, and stored at −80°C. Virus titers in stocks were determined on HEp-2 cells by limiting dilution assay for 50% tissue culture infectious doses (TCID50s) as described elsewhere.Citation11 The encephalomyocarditis virus (EMCV) EMC strain (ATCC) was propagated in L-929 cells (kindly provided by T Jouault, Lille, France) in Dulbecco's modified eagle's medium (DMEM; Gibco BRL, Invitrogen, Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (FCS, Sigma St Louis, MO, USA), 1% L-glutamine (Gibco BRL), 50 µg/mL streptomycin, and 50 IU/mL penicillin (BioWhittaker). Supernatants were collected 3 days after inoculation, clarified by centrifugation at 2,000 × g for 10 min, divided into aliquots, and stored at −80°C. Virus titers in stocks were determined on L-929 cells by limiting dilution assay for 50% tissue culture infectious doses (TCID50s) as described elsewhere.Citation11
Mice inoculation and follow-up
All experiments were conducted following the guidelines of 2010 EU directory and were approved by Faculty of Pharmacy University of Monastir (Tunisia) and by the Ethical Committee for Animal Experimentation of Nord-Pas-de-Calais (France). All animals were housed in a specific pathogen free facility and had free access to sterile pellets and water.
Three to 4 weeks old female Swiss albino mice (Institut Pasteur, Tunis) were inoculated through the intraperitoneal route with 200 µL culture medium or with CV-B4 E2 at various doses: 2 × 106, 2 × 105 or 2 × 104 TCID50 units contained in 200 µL culture medium (each group encompassed 35 mice). Blood was obtained from the tail vein of animals by using a needle on day 0, 7, 14, 21, 30, 40, 60 and 80 post-infection (p.i). Serum samples obtained following centrifugation were stored at −80°C.
IgG preparation
IgG-enriched and IgG-depleted fractions of mice serum samples were obtained by Protein G affinity chromatography by using Protein G HP columns Spintrap system (GE Healthcare Life Sciences) following manufacturer's instructions. IgG were recovered in a volume of culture medium (IgG-enriched fraction) similar to the volume of serum sample.
Antibodies titration by seroneutralization
A modified procedure for seroneutralization assay was used in this study. Suspended HEp-2 cells were first seeded at 105 cells per well in 96-well microtiter plates (Falcon, Oxnard, CA, USA). After 16 h of incubation at 37°C in a humidified incubator with 5% CO2, the medium was discarded and 50 µL of fresh MEM supplemented with 5% FCS, 1% L-glutamine, 50 µg/mL streptomycin, 50UI/mL penicillin, 1% non-essential amino-acids and 0.05% fungizone, were added in each well. Then 5 µL of MEM or mouse serum samples mixed with 45 µl of MEM (1:10 dilution to prevent cytotoxicity) were added to the first wells and 2-fold serial dilutions were performed. Each sample was tested in triplicate. Afterwards, 25 µL of CV-B4 E2 suspended in medium (103 TCID50/mL) and 25 µl of medium culture were added and the plates were incubated for 48 h at 37°C. The neutralizing titer was defined as the reciprocal of the last dilution of sample that totally inhibited the viral cytopathic effect (CPE) as observed under an inverted microscopy.
Primary culture of total spleen cells cultures
The CV-B4 E2 enhancing activity of serum/IgG from mice have been tested in murine spleen cell culture that are poorly infectable by CV-B4 E2.Citation11 Briefly, 5 week old male Swiss albino mice (Janvier Laboratories, France) were killed by cervical dislocation, and their spleens aseptically removed and used to prepare primary cultures of total spleen cells cultures as described previously.Citation22 Cells were prepared on ice, depleted of erythrocytes by hypotonic shock, then suspended in RPMI-1640 (Eurobio, Paris, France) supplemented with 10% FCS, 1% L-glutamine, 50 μg/mL streptomycin, 50 IU/mL penicillin and 10−5M β-mercaptoethanol (Sigma). Then, cells were seeded into 96-well culture plates at 5 × 105 cells per well and incubated at 37°C in a humidified atmosphere with 5% CO2.
Inoculation of splenic cell cultures
5 µL of mouse serum/IgG sample was diluted (1:10, 1:100, 1:500 and 1:1000) in duplicate in supplemented RPMI and mixed (2/1; vol/vol) with CV-B4 E2 suspension (105 TCID50/mL). The mixtures were incubated 2h at 37°C. Then 85 µL of these mixtures were inoculated in duplicate to spleen cell cultures (5 × 105 in 100 µl/well) and 100 µl of supplemented medium were added. The microplates were incubated for 48h, afterwards culture supernatants were collected, clarified and stored at −80°C for antiviral activity titration and for evaluating the levels of viral particles. Spleen cells cultures were washed 5 times with cold phosphate buffered saline (PBS) and then prepared for RNA extraction.
Bioassay for antiviral activity titration
A bioassay based on the protection of L929 cells against the cytopathic effect (CPE) induced by EMCV was used as previously described.Citation11 Briefly, 50 μL of DMEM containing 4.5 g/L glucose and sodium pyruvate (Gibco BRL), and supplemented with 5% FCS, 1% L-glutamine, 50 μg/mL streptomycin and 50 IU/mL penicillin, were first distributed into each well of a 96-well flat-bottomed microtiter plate (Falcon). 50 µL of splenic cell cultures supernatants were then added into the first wells and then serially twofold diluted. Versène trypsine (Eurobio) dissociated murine L-929 fibroblasts (kindly provided by T Jouault, Lille, France) (3 × 104 cells per well in 100 μL of the above described supplemented DMEM) were then added into each well. After 18 h of incubation at 37°C in a humidified atmosphere with 5% CO2, supernatants were removed and cells were inoculated with 100 μL per well of EMCV suspension giving 100% CPE after 18 h of incubation. Virus-induced CPE was assessed by microscopic examination. The inverse of the highest dilution providing 100% protection of the cells from virus-induced CPE was considered as the endpoint for antiviral activity.
RNA extraction
Total RNA was extracted from washed spleen cells cultures by the acid guanidium thiocyanate-phenol-chloroform extraction procedure by using Tri-Reagent (Sigma), as described by Jaidane.Citation23
Extracted RNA was then dissolved in 50 µL of nuclease-free water (Promega), quantified using the Quant-iT RiboGreen RNA assay kit (Molecular Probes, Invitrogen) according to the manufacturer's instructions, and prepared to be used in reverse transcription (RT)-PCR assays. Purified water for injection (C.O.M Lavoisier) was submitted to the same extraction procedure and served as a negative control. Supernatant of CV-B4 E2-infected HEp-2 cells served as a positive control.
One-step RT-PCR for CV-B4 E2 RNA detection
cDNA synthesis and cDNA amplification were performed in a single tube by using the Super-Script One-Step RT-PCR with Platinum Taq kit according to manufacturers' instructions, as described previously.Citation10 Sense (EV1:5′-CAAGCACTTCTGTTTCCCCGG-3′) and antisense (EV2: 5′-ATTGTCACCATAAGCAGCCA-3′) primers were selected within the 5′non-coding region of the enterovirus genome, generating a 435 bp fragment.Citation10
Both primers (EV1 and EV2) were present into the tube in which cDNA synthesis (reverse transcription) was followed by cDNA amplification (PCR). Briefly the samples were subjected to a first step of reverse transcription for 30 min at 50°C, and then to denaturation at 94°C for 2 mn. Afterwards, the products of reverse transcription were subjected to 40 cycles of amplification consisting of denaturation for 30 s at 94°C, annealing for 45 s at 55°C, and extension for 45 s at 72°C, and then a final extension step for 10 min at 72°C.
Semi-nested RT-PCR for CV-B4 E2 RNA detection
RT-PCR products were submitted to a semi-nested PCR in which anti-sense primer EV2 was replaced by an internal anti-sense primer EV3. Sense primer (EV1:5′-CAAGCACTTCTGTTTCCCCGG-3′) and antisense primer EV3: 5′- CTTGCGCGTTACGAC-3′, generated a 362 bp fragment.Citation10
For this purpose, we used the Platinum® PCR SuperMix kit (Invitrogen) according to manufacturer's instructions, as described previously.Citation10 Samples were subjected to 2 min of denaturation at 94°C, followed by 30 cycles consisting of denaturation for 30 s at 94°C, annealing for 30 s at 55°C, and extension for 30 s at 72°C, followed by a final extension step for 10 min at 72°C. All reactions were performed by using a preheated Perkin Elmer Applied GeneAmp PCR system 2400.
Detection and analysis of amplification products
The amplified RT-PCR products were analyzed by electrophoresis on a 2% agarose gel containing 0.5 mg/mL ethidium bromide (Sigma) and visualized by using a Gel Doc 2000 system (Bio-Rad). A 100-bp DNA ladder (Invitrogen) was used as a molecular mass marker.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by: the Comité Mixte de Coopération Universitaire Franco-Tunisien (CMCU 08/G808) and Egide Paris. Ministère de l'Enseignement Supérieur et de la Recherche Scientifique, (LR99ES27), Tunisia, Ministère de l'Education Nationale de la Recherche et de la Technologie, Université Lille 2 (Equipe d'Accueil 3610), France, and Center Hospitalier Régional et Universitaire de Lille, and EU FP7 (GA-261441-PEVNET: Persistent virus infection as a cause of pathogenic inflammation in type 1 diabetes - an innovative research program of biobanks and expertise).
References
- Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg M, Pallansch MA, Palmenberg AC, Simmonds P, Skern T, Stanway G, et al. 2012. Picornaviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, Eds.; Publisher: San Diego, CA, 2012; pp. 855–880.
- Jaïdane H, Sauter P, Sane F, Goffard A, Gharbi J, Hober D. Enteroviruses and type 1 diabetes: towards a better understanding of the relationship. Rev Med Virol 2010; 20:265-80; http://dx.doi.org/10.1002/rmv.647
- Jaïdane H, Sané F, Gharbi J, Aouni M, Romond MB, Hober D. Coxsackievirus B4 and type 1 diabetes pathogenesis: contribution of animal models. Diabetes Metab Res Rev 2009; 25:591-603; http://dx.doi.org/10.1002/dmrr.995
- Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol 2010; 6:279-89; PMID:20351698; http://dx.doi.org/10.1038/nrendo.2010.27
- Yeung W-CG, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 2011; 342:d35; PMID:21292721; http://dx.doi.org/10.1136/bmj.d35
- Chehadeh W, Bouzidi A, Alm G, Wattré P, Hober D. Human antibodies isolated from plasma by affinity chromatography increase the coxsackievirus B4-induced synthesis of interferon-alpha by human peripheral blood mononuclear cells in vitro. J Gen Virol 2001; 82:1899-907; PMID:11457996; http://dx.doi.org/10.1099/0022-1317-82-8-1899
- Hober D, Chehadeh W, Weill J, Hober C, Vantyghem M-C, Gronnier P, Wattré P. Circulating and cell-bound antibodies increase coxsackievirus B4-induced production of IFN-alpha by peripheral blood mononuclear cells from patients with type 1 diabetes. J Gen Virol 2002; 83:2169-76; PMID:12185270; http://dx.doi.org/10.1099/0022-1317-83-9-2169
- Hober D, Chehadeh W, Bouzidi A, Wattré P. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-alpha synthesis. J Infect Dis 2001; 184:1098-108; PMID:11598831; http://dx.doi.org/10.1086/323801
- Sauter P, Hober D. Mechanisms and results of the antibody-dependent enhancement of viral infections and role in the pathogenesis of coxsackievirus B-induced diseases. Microbes Infect 2009; 11:443-51; PMID:19399964; http://dx.doi.org/10.1016/j.micinf.2009.01.005
- Jaïdane H, Gharbi J, Lobert P-E, Lucas B, Hiar R, M'hadheb MB, Brilot F, Geenen V, Aouni M, Hober D. Prolonged viral RNA detection in blood and lymphoid tissues from coxsackievirus B4 E2 orally-inoculated Swiss mice. Microbiol Immunol 2006; 50:971-4; http://dx.doi.org/10.1111/j.1348-0421.2006.tb03874.x
- Jaïdane H, Gharbi J, Lobert P-E, Caloone D, Lucas B, Sané F, Idziorek T, Romond M-B, Aouni M, Hober D. Infection of primary cultures of murine splenic and thymic cells with coxsackievirus B4. Microbiol Immunol 2008; 52:40-6; http://dx.doi.org/10.1111/j.1348-0421.2008.00002.x
- Chehadeh W, Lobert P-E, Sauter P, Goffard A, Lucas B, Weill J, Vantyghem M-C, Alm G, Pigny P, Hober D. Viral protein VP4 is a target of human antibodies enhancing coxsackievirus B4- and B3-induced synthesis of alpha interferon. J Virol 2005; 79:13882-91; PMID:16254324; http://dx.doi.org/10.1128/JVI.79.22.13882-13891.2005
- Goffard A, Alidjinou EK, Sané F, Choteau L, Bouquillon C, Caloone D, Lobert PE, Hober D. Antibodies enhance the infection of phorbol-ester-differentiated human monocyte-like cells with coxsackievirus B4. Microbes Infect 2013; 15:18-27; PMID:23108316; http://dx.doi.org/10.1016/j.micinf.2012.10.005
- Sauter P, Lobert P-E, Lucas B, Varela-Calvino R, Alm G, Wattre P, Hober D. Role of the capsid protein VP4 in the plasma-dependent enhancement of the Coxsackievirus B4E2-infection of human peripheral blood cells. Virus Res 2007; 125:183-90; PMID:17291618; http://dx.doi.org/10.1016/j.virusres.2007.01.001
- Beck MA, Chapman NM, McManus BM, Mullican JC, Tracy S. Secondary enterovirus infection in the murine model of mycarditis. Am J Pathol 1990; 156: 669-81.
- Yu JZ, Wilson JE, Wood SM, Kandolf R, Klingel K, Yang D, McManus BM. Secondary heterotypic versus homotypic infection by Coxsackie B group viruses: impact onearly and late histopathological lesions and virus genome prominence. Cardiovasc Pathol 1999; 8:93-102; PMID:10724506; http://dx.doi.org/10.1016/S1054-8807(98)00025-8
- Girn J, Kavoosi M, Chantler J. Enhancement of coxsackievirus B3 infection by antibody to a different coxsackievirus strain. J Gen Virol 2002; 83:351-8; PMID:11807228; http://dx.doi.org/10.1099/0022-1317-83-2-351
- Kishimoto C, Kurokawa M, Ochiai H. Antibody-mediated immune enhancement in coxsackievirus B3 myocarditis. J Mol Cell Cardiol 2002; 3:1227-38; http://dx.doi.org/10.1006/jmcc.2002.2087
- Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol 2003; 13(6):387-98; PMID:14625886; http://dx.doi.org/10.1002/rmv.405
- Horwitz MS, Ilic A, Fine C, Rodriguez E, Sarvetnick N.Coxsackievirus-mediated hyperglycemia is enhanced by reinfection and this occurs independent of T cells. Virology 2003; 314(2):510-20; PMID:14554080; http://dx.doi.org/10.1016/S0042-6822(03)00462-8
- Bopegamage S, Precechtelova J, Marosova L, Stipalova D, Sojka M, Borsanyiova M, Gomolcak P, Berakova K, Galama JM. Outcome of challenge with Coxsackievirus B4 in young mice after maternal infection with thesame virus during gestation. FEMS Immunol Med Microbiol 2012; 64(2):184-90; PMID:22066931; http://dx.doi.org/10.1111/j.1574-695X.2011.00886.x
- Zamzami N, Marchetti P, Castedo M, Hirsch T, Susin SA, Masse B, Kroemer G. Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett 1996; 384:53-7; PMID:8797802; http://dx.doi.org/10.1016/0014-5793(96)00280-3
- Jaïdane H, CalooneD, Lobert PE, Sane F, Dardenne O, Naquet P, Gharbi J, Aouni M, Geenen V, Hober D. Persistent infection of thymic epithelial cells with coxsackievirus B4 results in decreased expression of type 2 insulin-like growth factor. J Virol 2012; 86(20):11151-62; http://dx.doi.org/10.1128/JVI.00726-12
