ABSTRACT
Bovine tuberculosis is a zoonotic infectious disease caused by Mycobacterium bovis that affects cattle and can cause tuberculosis in a range of wildlife animals. A bacteriophage-based method combined with PCR (phage-PCR) has been recently used to detect and identify viable pathogenic mycobacteria in the peripheral blood mononuclear cells (PBMCs) of animals suffering from paratuberculosis. To adapt this method for the detection of M. bovis in blood, a new isothermal DNA amplification protocol using Recombinase Polymerase Amplification (RPA) was developed and was found to be able to detect M. bovis BCG within 48 h, with a limit of detection of approximately 10 cells per ml of blood for artificially inoculated blood samples. When blood samples (2 ml) from a Single Comparative Cervical Intradermal Tuberculin (SCCIT)- negative beef herd were tested, Mycobacterium tuberculosis complex (MTC) cells were not detected from any (45) of the blood samples. However when blood samples from SCCIT-positive animals were tested, viable MTC bacteria were detected in 66 % (27/41) of samples. Of these 41 animals sampled, 32 % (13) had visible lesions. In the visible lesion (VL) group, 85 % (11/13) had detectable levels of MTC whereas only 57 % (16/28) of animals which had no visible lesions (NVL) were found to have detectable mycobacteraemia. These results indicated that this simple, rapid method can be applied for the study of M. bovis infections. The frequency with which viable mycobacteria were detected in the peripheral blood of SCCIT-positive animals changes the paradigm of this disease.
Introduction
Mycobacterium bovis is the causative agent of bovine tuberculosis and forms part of Mycobacterium tuberculosis Complex group of pathogens. Bovine tuberculosis is a disease that affects primarily cattle, but can infect humans as well as a variety of other domestic and wild mammals.Citation1 Despite eradication schemes being in place since the 1950s, the UK has struggled to eradicate the disease; in fact the incidence of BTB outbreaks has increased and control measures continue to be a significant economic burden for this UK agriculture sector.Citation2 A major barrier to understanding and diagnosing M. bovis infection is that culture of these slow growing organisms is difficult, time consuming and often impracticable. This is especially true when undertaking epidemiological, infection or immunological studies compared to bacterial load.Citation3 In addition to the long incubation times required, a specific limitation of culture is that chemical decontamination used to inhibit the growth of competing bacteria also reduces the viability of the mycobacterial cells present in samples, reducing the sensitivity and reliability of culture as a method to detect and understand the disease.Citation4
Essentially due to these difficulties, an area of bovine tuberculosis pathophysiology that is underreported is the development of disseminated infection and bacteraemia. It is known that M. bovis infection in an animal can have a number of possible outcomes ranging from elimination, self-limiting infection, localized lesions or a life-threatening systemic disease. The immune response following challenge is known to be complex, with variable responses occurring during natural infections with low numbers of organisms.Citation5 Bacteraemia occurs during post-primary dissemination in humans and it has been reported, although rarely, in cattle.Citation6 Recently a study in India reported the culture of M. bovis in the blood of apparently healthy cattle, which suggested M. bovis may be circulating in the blood of sub-clinically infected animals at higher levels than expected.Citation7 The paucity of information concerning this aspect of the disease means that it is not known whether bacteraemia will ultimately lead to active infection or clearance, nor how a positive SCCIT test result relates to the potential for bacteraemia.
Bacteriophage amplification technology was developed 20 y ago as a method to rapidly detect and enumerate slow growing pathogenic mycobacteria.Citation8 In addition it can be used as a tool to rapidly detect antibiotic resistanceCitation9 and to investigate mycobacterial dormancy.Citation10 The assay detects the growth of broad host range mycobacteriophage, capable of infecting a wide range of both pathogenic and non-pathogenic mycobacteria. As the phage is specific for members of the Mycobacterium genus, and can only successfully replicate within a viable cell, a positive test result (a plaque) indicates the presence of viable mycobacterial cells in the original sample. The specificity of the detection event is then achieved by amplification of signature sequences from the plaque following the phage assay (see ;Citation11). In contrast, direct PCR will detect DNA from both viable and non-viable cells and can be inhibited by components in the blood.Citation12 The combined phage-PCR method has been shown to be able to detect and enumerate Mycobacterium avium subsp. paratuberculosis (MAP) in a range of matrices such as milk, cheese and blood,Citation11,13,Citation14 but detection of M. bovis in clinical samples using this approach has not been described before.
Figure 1. Schematic of the bacteriophage amplification assay. To perform the phage assay, the mycobacteria first need to be isolated from the sample. In all these experiments PBMCs were purified and suspended in supplemented 7H9 medium that lyses the PBMCs and promotes efficient phage infection: Step 1: Isolated mycobacteria are incubated with phage D29 for 1 h to allow virus infection of mycobacterial cells present in the sample. Step 2: Extracellular phage that have not infected bacterial cells are inactivated by virucide. Step 3: Virucide is neutralized and fast-growing M. smegmatis are added to the sample which will form the bacterial lawn. Step 4: Samples are plated in soft agar and incubated overnight permitting lawn formation by M. smegmatis+. Phage released from the infected mycobacterial cells present in the original sample are released and infect the M. smegmatis cells resulting in the formation of plaques. Step 5: Plates are inspected for plaques which indicate detection of a mycobacterial cell in the original sample. If plaques are present, DNA is extracted from the agar and presence of MTC bacteria is determined by RPA/PCR.
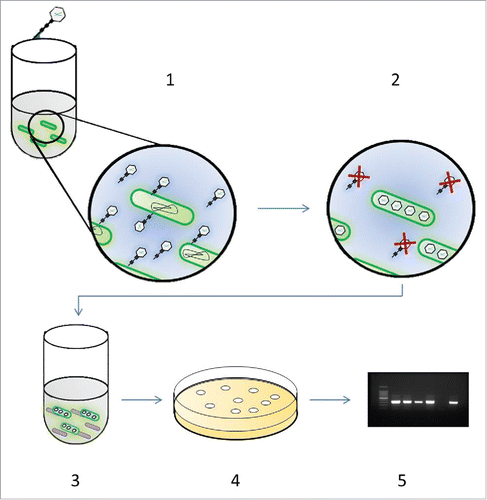
It was already known that the phage-PCR assay could be used to sensitively detect M. bovis cells from liquid culturesCitation15 and that this method can be used to detect MAP cells in clinical blood samples.Citation16 The aim of these experiments was to design and optimise a phage-PCR method to allow detection of M. bovis cells in blood and to determine whether it could detect mycobacteria in SCCIT-positive animals.
Materials and methods
Bacterial strains, bacteriophage and growth media
M. bovis BCG (Glaxo) was used to optimise the phage-PCR assay. Cells were cultured and maintained on Middlebrook 7H10 agar supplemented with OADC (Becton Dickenson, UK) without glycerol and liquid cultures were prepared in Middlebrook 7H9 media (Becton Dickenson, UK) containing OADC. When performing the phage assay the media was supplemented with CaCl2 (2 mM final concentration; Sigma, UK). All cultures were grown at 37°C without shaking to prevent clumping. For the phage assays the Mycobacterium smegmatis strain used was mc2155 (Lab21 Ltd, UK) and the bacteriophage used was D29 (Lab21 Ltd, UK).
Bacteriophage amplification assay
The phage assay () and experimental controls were carried out as previously described.Citation15,16 Briefly, samples (1 ml) were mixed with bacteriophage D29 (100 µl; 1 × 108 pfu) in supplemented 7H9 Media and incubated for 1 h to allow the phage to infect any mycobacteria present in the sample. Phage that had not infected a host cell were then inactivated using a virucide (100 µl ferrous ammonium sulfate; final concentration 10 mM; Lab21 Ltd, UK). Samples were mixed thoroughly ensuring all sides of the container are covered and incubated for 5 min at room temperature. One ml of M. smegmatis cells (to form the bacterial lawn) was added to the sample which was plated in 7H10 agar (0.75 % final agar concentration). As experimental controls, a 1 ml sample containing approx. One × 103 cfu ml−1 M. smegmatis cells or 1 ml of medium alone were used as positive and negative controls, respectively for the phage assays. Plaques formed at the end of the assay were counted and the number taken to represent the number of cells that could be detected by the phage in the sample.
Molecular identification of M. tuberculosis complex cells
The identity of the mycobacterial cell detected was determined by extracting DNA from the center of plaques (a maximum of 5) using agarose gel-DNA extraction columns (ZymoResearch, UK).Citation16 For PCR detection of MTC DNA, the IS6110 PCR assay described by EisenachCitation17 was used. In a 25 µl reaction volume, 1 µM of each primer was used with HotStarTaq Plus Master Mix Kit (Qiagen, UK). The PCR cycle conditions were 95°C for 5 min followed by 30 cycles of 95°C for 30s, 68°C for 30 s and 72°C for 1 min and a final extension at 72°C for 7 min. Either IS6110 or IS1081Citation18 was targeted for RPA detection using lyophilised reagents (TwistDx Ltd, UK). These were rehydrated using 37.5 µL TwistAmp Resuspension Buffer and 4 µL of 280 mM MgOAc. Template DNA (8.5 µL) was added to this and samples were incubated for 30 min at 39°C. DNA extracted from M. bovis BCG and sterile water were used as positive and negative controls, respectively. Amplified RPA products of approximately 220 bp were visualised using agarose gel DNA electrophoresis.
To determine the sensitivity of the RPA assay, agar extracted from M. bovis BCG plaques was mixed with agar extracted from plaques formed using the non-pathogen M. smegmatis. The number of M. bovis plaques added varied between 1 and 5, with the total number of plaques per sample being made up to 5 in each case using M. smegmatis plaques. DNA samples extracted from these mixtures were then subsequently tested using either the PCR or RPA assays described above.
Blood samples, preparation and isolation of peripheral blood mononuclear cells
For method optimisation experiments commercial heparinised sheep blood (Oxoid, UK) was used. The number of M. bovis BCG cells in laboratory cultures was enumerated using the bacteriophage assay as described above (data reported as pfu.ml−1)Citation8 and then cells diluted in PBS (105 to 10 pfu ml−1). To allow uptake of bacterial cells by leukocytes the method of Citation19 was used. Briefly 1 ml of cell suspension was added to 9 ml fresh (< 1 d old) heparinised sheep blood and samples incubated at 37°C on a rotating mixer for 4 h. After the uptake period, PBMCs were isolated from 2 ml blood using Ficoll-Paque Plus in Leucosep tubes (GE Healthcare Life Sciences, UK). The purified PBMC's were then lysed by osmotic shock by addition of supplemented 7H9 media to release any internalised bacteria. The phage assay was then performed to detect mycobacteria released from the PBMCs as described above.Citation11
Clinical blood samples from the SCCIT-positive cattle were obtained directly after slaughter by a veterinary surgeon in the abattoir. These were Holstein cows from a farm in the South West of the UK within the High Risk zone. Blood samples from the control animals were obtained from a closed Holstein beef herd in a non-TB endemic area of the UK provided as superfluous material as part of an on-going herd health screening program under the Veterinary Surgeons Act. The study protocol was approved by the University of Nottingham, School of Veterinary Medicine and Science ethical review panel prior to sample usage. Blood was collected in Vacutainer heparin tubes (Becton Dickenson) and 2 ml samples used for the isolation of the PBMCs which were tested using the phage assay using the same method described above.
Statistical analysis
Excel 2010 statistical add on package was used to initially determine whether the data were distributed normally or not, then GraphPad Prism was used for performing ANOVAs and post-hoc tests to determine significant differences in the data. Significance was determined at p < 0.05.
Results
Development of phage-PCR method for detection of M. bovis BCG in blood
An experiment was performed to determine whether the phage assay could also be used to detect M. bovis BCG cells present inside PBMCs but using a published method that allows mycobacteria to be taken up by leukocytes.Citation18,19 When using this method, if no incubation time is allowed for uptake, no mycobacteria are detected in the buffy coat layer demonstrating that the bacteria do not co-purify with the PBMCs (). When 105 M. bovis BCG cells were added to the blood, the number of plaques detected in the PBMC fraction was uncountable, indicating that the purified PBMCs contained more than 103 cells (). As the number of cells added to the blood was decreased, the number of plaques recorded in each sample also decreased until the number of cells detected in the PBMC fraction reached countable levels. This occurred when approximately 102 M. bovis BCG cells were added to the blood, at which point 65 (±13 ) plaques were recorded () indicating an efficiency of uptake of the M. bovis BCG cells of at least 50%. If less than 10 M. bovis BCG cells were added to the sample, no cells were detected using the phage assay and this is consistent with the results gained when this method was used for the internalisation of MAP.Citation19 Given the efficiency of uptake, it was assumed that the limit of detection of this method was approximately 10 cells per sample. However it could be lower than this when naturally infected samples are tested since the cells are already internalised.
Table 1. Detection of M. bovis BCG in PBMC's using the phage assay.
Molecular identification of M. tuberculosis complex DNA
Previously we have used PCR to detect the MAP signature genetic elements present in DNA extracted from individual plaques,Citation14,15 hence this approach was also applied here and the IS6110 MTC signature sequence was amplified by PCR using a published method.Citation17 When the sample was composed of DNA extracted from 5 plaques arising from the detection of M. bovis BCG cells, the PCR assay was routinely able to detect the IS6110 element (data not shown). However when the number of M. bovis BCG plaques varying between 1 and 5, was tested (), it was found that the PCR assay could not consistently detect the IS6110 genetic element when only one or 2 M. bovis BCG plaques were present in the sample, suggesting that the PCR assay was not sensitive enough for reliable detection of low amounts of DNA. This is consistent with our observations when developing an assay for MAP, where it was found that a nested PCR more consistently detected low concentrations of IS900 in samples that only contained one MAP-positive plaque.Citation16
Table 2. Performance of different amplification methods to Detect MTC signature sequences.
To improve the sensitivity of the molecular detection event, PCR amplification was replaced with DNA amplification using a published isothermal RPA method.Citation18 Using this method, both the IS6110 and IS1081 MTC signature sequences were always detected from DNA samples that contained the DNA extracted from 5 M. bovis BCG plaques. However, only the RPA IS6110 primers were able to consistently amplify MTC DNA when agar was extracted from one M. bovis BCG plaque mixed with 4 M. smegmatis plaques (). Thus the RPA method using the IS6110 primers was chosen for use for further experiments.
Detection of viable M. tuberculosis complex cells in clinical blood samples
Blood samples were obtained from 41 SCCIT-positive animals after slaughter and the optimised phage-RPA method was used to detect any viable MTC cells present in the sample. As a control 45 blood samples from cattle in herds with no previous history of M. bovis infection were also tested. From the 45 SCCIT-negative samples, 6 produced plaques (range = 1–11 per 2 ml blood; mean = 3.1), however none of these gave a positive IS6110 RPA result () indicating that no MTC DNA was detected in these samples. We have previously reported that the phage assay will produce low numbers of plaques from samples that do not give a positive result when interrogated using PCR.Citation15 However the plaque number in these samples is always low as seen here.
Table 3. Detection of viable MTC bacteria in PBMCs isolated from a SCCIT-negative herd.
Within the SCCIT-positive group, 31 produced plaques. After DNA extraction, 27 of these samples produced a positive IS6110 RPA result () indicating that DNA from MTC bacteria was present. At post mortem 32 % (13/41) of the SCCIT-positive animals had visible lesions (VL; 3 Multiple, 10 Diffuse). Of the animals in the VL group, 85 % (11/13) had detectable mycobacteraemia based on the phage-RPA results (; ). In samples from NVL animals only 57% (16/28) had detectable levels of MTC mycobacteria in their blood (). No post mortem results were available for the negative control group, as blood samples were taken from healthy animals.
Figure 2. Frequency of detection of MTC-bacteraemia in VL and NVL groups. Distribution of MTC bacteraemia-positive (Dark Gray) and MTC bacteraemia-negative (Light samples for animals classified as having visible lesions (VL) or non-visible lesions (NVL) at post mortem.
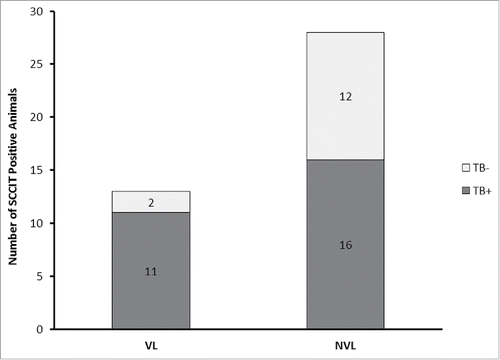
Table 4. Detection of viable MTC bacteria in PBMCs isolated from SCCIT-positive cattle.
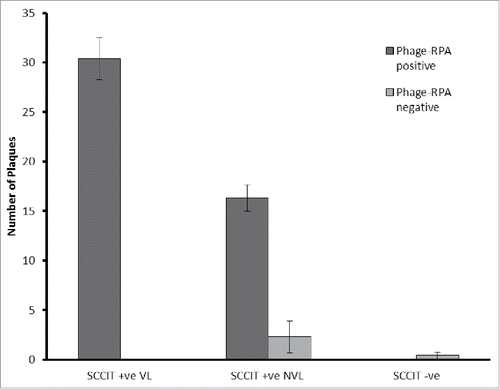
Since the phage assay detects individual mycobacterial cells, the number of plaques detected is indicative of the microbial load in the blood sample. In the SCCIT-positive VL group, the average number of cells detected in phage-RPA positive samples was 30.4 (range = 8–63 pfu per 2 ml blood; ). For the SCCIT-positive NVL group, the average number of cells detected in RPA-positive samples was 16.3 (range = 2–43 pfu per 2 ml blood; ) and this number is significantly lower than the number of mycobacterial cells detected in the VL samples (p < 0.05) suggesting that the VL animals had a higher microbial load in the blood. Within the NVL group, the average plaque number for MTC-negative samples (phage-negative or phage positive, RPA-negative) was 2.3 (range = 0–16; ) and this number was also significantly lower (p < 0.05) than the plaque number for the NVL-MTC positive samples ().
Figure 3. Average plaque number detected for SCCIT positive and negative VL and NVL samples. Average number of plaques (±SD ) formed per 2 ml blood sample for SCCIT positive animals that were classified as having visible lesions (VL; n = 13) or non-visible lesions (NVL; n = 28) at post mortem or SCCIT negative animals (n = 45). Samples are further divided into those that gave a positive IS6110-RPA result (dark gray) and those that either formed no plaques or gave a negative IS6110 RPA result (Light Gray). One-way ANOVA and Post-hoc Dunnetts-test was used to determine differences between these data sets.
Discussion
The bacteriophage-based detection method used here was originally developed as a commercial assay for the detection of human M. tuberculosis infections,Citation20 however more recently it has been shown to be a useful tool for the investigation of animal mycobacterial infections, such as Johne's disease.Citation13,16 Combining phage-based detection with PCR-based identification methods allows both live/dead differentiation (the phage can only replicate in a viable cell) and also increases the sensitivity of PCR-based detection methods because the plaques formed allow efficient targeting of the genomic DNA released from individual cells which is preserved in the agar. The IS6110 genetic element, although not specific for M. bovis, is extremely useful as a target for any DNA-based detection method, as it is present in multiple copies in the genome, allowing more sensitive detection of single cells. Although components of the PBMC fraction does not affect the phage-based assay, RPA was chosen as an alternative DNA amplification method since it is reported to be less susceptible to inhibition by blood,Citation21 and in this study the RPA detection of low concentrations of IS6110 was found to be more reproducible than PCR-based amplification of the same signature sequences.
As we found that MAP cells were primarily located in the PBMCs of MAP-infected cattle,Citation16 we hypothesized that this may also be true for cattle suffering from M. bovis infection. Hence for this study the SCCIT-positive cattle were chosen simply to determine whether mycobacteraemia could be detected in the blood of these animals using the phage-RPA method. The fact that we detected viable MTC cells in the blood of 26 of the 41 animals tested is extremely interesting and demonstrates that SCCIT reactor animals (i.e those identified as infected by M. bovis) commonly contain detectable levels of viable mycobacteria within PBMCs. Although there is a lack of other reports describing the detection of M. bovis in the blood of cattle (apart from one study in 1977Citation6), there are many publications that have demonstrated the detection of mycobacteria in blood for humans suffering from human tuberculosis by both culture and PCR.Citation22,23
In this study we have not used a signature sequence that is specific for M. bovis, rather we targeted the IS6110 element which is specific for the MTC group of which M. bovis is a member. Targeting this well-characterized genetic element allows rapid and sensitive detection which can be routinely used for a variety of applications, making the IS6110 genetic element a useful target despite the lack of specificity. Nonetheless as all the animals tested were SCCIT test positive, and M. bovis was cultured from some samples from this outbreak sent to the UK veterinary diagnostic laboratories (D Brewer, APHA pers. comm.), it is a reasonable interpretation of the results gained in this study that the cells detected in these samples were M. bovis. While this is true in the UK, other members of the MTC may be detected using this method in countries where M. bovis is not prevalent. Interestingly none of the samples from control animals gave a positive result with the IS6110 RPA assay, but it must also be noted these samples were obtained from live animals rather than at slaughter which may have influenced our results gained. Samples from 6 of these animals produce plaques and this may be due to the presence of a different type of mycobacterial cell, such as MAP, which is also widespread within UK cattle populations.Citation16 In this study we did not test the samples for the presence of other mycobacterial signature sequences, but this result emphasizes the fact that when using the phage-based detection method amplification of signature sequences to confirm the identity of the cell detected is essential for accurate interpretation of plaque results. In future studies it will be interesting to include more specific M. bovis signature sequences as well as developing multiplex DNA amplification assays such as we have previously describedCitation14 to allow simultaneous detection of different mycobacterial species.
Of the 41 SCCIT-positive samples tested, only 13 had visible lesions, suggesting these animals had an advanced stage of infection and MTC cells were not detected in blood samples from only 2 of these. This may have been because there was low bacterial load in these blood samples which was not detectable by the phage assay. However the overall pattern of results is consistent with the idea that animals with visible lesions have a more disseminated infection and therefore perhaps it is not surprising to find that M. bovis cells are actively replicating inside the PBMCs (phage D29 can only productively infect actively growing cellsCitation10). It is also known that the sensitivity of identifying lesions at post-mortem is low,Citation24 which may explain why 57% of the NVLs also had detectable levels of viable mycobacteria in their blood. Despite this, there was a significantly lower number of plaques detected in the MTC-positive NVL samples than was detected in MTC-positive VL animals indicating a lower microbial load in this group of animals. This may reflect the ability of the phage assay to detect early stages of infection in some animals, but further studies on animals in the early stages of infection are required to support this conclusion.
The level of mycobacteraemia in animals suffering from M. bovis infection is underreported due to the difficulties associated with culturing M. bovis from clinical samples, and therefore this aspect of the disease is not well understood. In human tuberculosis and in other mycobacterial diseases, mycobacteraemia is considered as one of the potentially most useful approaches to definitively diagnose tuberculosis.Citation23 However culturing these organisms is very slow, expensive and insensitive, which limits the use of culture as a Gold Standard when investigating and detecting infectious diseases.Citation25 Although, several research groups have detected mycobacteraemia using extraction of DNA and direct PCR, it is not certain whether the DNA detected came from a viable cell or whether PCR inhibitors in blood limit the detection event.Citation26 The fact that the phage-RPA method could rapidly and sensitively detect and enumerate viable mycobacterial cells in clinical blood samples, providing results within 48 h, means that it could be used to increase our understanding of M. bovis infections. Although parallel testing with culture is needed, the phage assay may also aid the confirmation of BTB infection in NVL cattle without the need for extended culture of samples. The blood from the SCCIT test positive reactor animals was obtained from animals directly after slaughter which may have impacted our finding compared to obtained samples from live animals. To this end it would be interesting to know whether intermediate or non-SCCIT reactor animals from an infected herd harbour mycobacteria in their blood in live animals, and whether this relates to the probability of their progression to SCCIT-positive status. Hence the phage-RPA provides a useful tool for monitoring levels of mycobacteraemia in relation to the animals' immune response to disease, especially during vaccine trials and will enable researchers to ask clinically important questions to further the understanding of this extremely difficult to control disease.
Conclusion
Here we show that the phage-RPA method can be applied to provide rapid, sensitive and specific detection of MTC cells in clinical blood samples. The finding that a number of SCCIT-positive animals with detectable levels of viable MTC cells present in these initial trial results were both encouraging and surprising, in particular the relationship seen between mycobacterial load in the blood and the animals' lesion status. More work is now required to develop species-specific RPA assays and to establish what sample volume is required to ensure sensitive detection of low levels of bacteria. In addition, studies specifically designed to investigate exactly how bacteraemia relates to disseminated infection are required.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Would like to thank Dr Natalie Gartin from the University of Leicester for gifting us the M. bovis BCG strains and David Brewer from APHA who was crucial in helping us obtaining the clinical blood samples.
Funding
UoN Hermes Innovation Fellowship was awarded to B. M. C. Swift for this work. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- Crawshaw T. Wildlife hosts for Mycobacterium bovis. Vet Rec 2013; 173:164-5; PMID:23956289; http://dx.doi.org/10.1136/vr.f5053
- Szmaragd C, Green LE, Medley GF, Browne WJ. Factors associated with herd restriction and de-restriction with bovine tuberculosis in British cattle herds. Prev Vet Med 2013; 111:31-41; PMID:23608481; http://dx.doi.org/10.1016/j.prevetmed.2013.03.005
- Corner LAL, Gormley E, Pfeiffer DU. Primary isolation of Mycobacterium bovis from bovine tissues: Conditions for maximising the number of positive cultures. Vet Microbiol 2012; 156:162-71; PMID:22074859; http://dx.doi.org/10.1016/j.vetmic.2011.10.016
- Ambrosio SR, de Deus Oliveira EM, Rodriguez CA, Ferreira Neto JS, Amaku M. Comparison of three decontamination methods for Mycobacterium bovis isolation. Braz J Microbiol 2008; 39:241-4; PMID:24031209; http://dx.doi.org/10.1590/S1517-83822008000200008
- Kennedy HE, Welsh MD, Bryson DG, Cassidy JP, Forster FI, Howard CJ, Collins RA, Pollock JM. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1(+) gamma delta T cells. Infect Immun 2002; 70:1488-500; PMID:11854237; http://dx.doi.org/10.1128/IAI.70.3.1488-1500.2002
- Lepper AWD, Corner LA, Pearson CW. Serological Responses in Experimental Bovine Tuberculosis. Aust Vet J 1977; 53:301-5; PMID:336017; http://dx.doi.org/10.1111/j.1751-0813.1977.tb00236.x
- Srivastava K, Chauhan DS, Gupta P, Singh HB, Sharma VD, Yadav VS, Sreekumaran , Thakral SS, Dharamdheeran JS, Nigam P, et al. Isolation of Mycobacterium bovis and Mycobacterium tuberculosis from cattle of some farms in north India - Possible relevance in human health. Indian J Med Res 2008; 128:26-31; PMID:18820355
- Rees CR, Botsaris G. The Use of Phage for Detection, Antibiotic Sensitivity Testing and Enumeration. In: Understanding Tuberculosis - Global Experiences and Innovative Approaches to the Diagnosis; Cardona P. J, ed; InTech: Rijeka, 2012; pp. 293-306.
- Albert H, Trollip A, Seaman I, Mole RJ. Simple, phage-based (FASTPlaque) technology to determine rifampicin resistance of Mycobacterium tuberculosis directly from sputum. Int J Tuberc Lung Dis 2004; 8:1114-9; PMID:15455597
- Swift BM, Gerrard ZE, Huxley JN, Rees CE. Factors Affecting Phage D29 Infection: A Tool to Investigate Different Growth States of Mycobacteria. PLoS One 2014; 9:e106690; PMID:25184428; http://dx.doi.org/10.1371/journal.pone.0106690
- Swift BM, Rees CE. Detecting mycobacteria in cattle blood. Veterinary Record 2013; 173:522-3; PMID:24293440; http://dx.doi.org/10.1136/vr.f7067
- Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors - occurrence, properties and removal. J Appl Microbiol 2012; 113:1014-26; PMID:22747964; http://dx.doi.org/10.1111/j.1365-2672.2012.05384.x
- Botsaris G, Liapi M, Kakogiannis C, Dodd CER, Rees CED. Detection of Mycobacterium avium subsp paratuberculosis in bulk tank milk by combined phage-PCR assay: Evidence that plaque number is a good predictor of MAP. Int J Food Microbiol 2013; 164:76-80; PMID:23603220; http://dx.doi.org/10.1016/j.ijfoodmicro.2013.03.023
- Botsaris G, Slana I, Liapi M, Dodd C, Economides C, Rees C, Pavlik I. Rapid detection methods for viable Mycobacterium avium subspecies paratuberculosis in milk and cheese. Int J Food Microbiol 2010; 141:S87-S90; PMID:20381185; http://dx.doi.org/10.1016/j.ijfoodmicro.2010.03.016
- Stanley EC, Mole RJ, Smith RJ, Glenn SM, Barer MR, McGowan M, Rees CE. Development of a new, combined rapid method using phage and PCR for detection and identification of viable Mycobacterium paratuberculosis bacteria within 48 hours. Appl Environ Microbiol 2007; 73:1851-7; PMID:17259362; http://dx.doi.org/10.1128/AEM.01722-06
- Swift BM, Denton EJ, Mahendran SA, Huxley JN, Rees CE. Development of a rapid phage-based method for the detection of viable Mycobacterium avium subsp. paratuberculosis in blood within 48 h. J Microbiol Methods 2013; 94:175-9; PMID:23811207; http://dx.doi.org/10.1016/j.mimet.2013.06.015
- Eisenach KD, Cave MD, Bates JH, Crawford JT. Polymerase Chain-Reaction Amplification of a Repetitive DNA-Sequence Specific for Mycobacterium tuberculosis. J Infect Dis 1990; 161:977-81; PMID:2109022; http://dx.doi.org/10.1093/infdis/161.5.977
- Boyle DS, McNerney R, Low HT, Leader BT, Perez-Osorio AC, Meyer JC, et al. Rapid Detection of Mycobacterium tuberculosis by Recombinase Polymerase Amplification. Plos One 2014; 9:e103091; http://dx.doi.org/10.1371/annotation/4fbfa4f0-cb27-4ec4-80d6-3b0ed5e9e8b8
- Bower K, Begg DJ, Whittington RJ. Optimisation of culture of Mycobacterium avium subspecies paratuberculosis from blood samples. J Microbiol Methods 2010; 80:93-9; PMID:19932719; http://dx.doi.org/10.1016/j.mimet.2009.11.005
- Albert H, Heydenrych A, Brookes R, Mole RJ, Harley B, Subotsky E, Henry R, Azevedo V. Performance of a rapid phage-based test, FASTPlaqueTB, to diagnose pulmonary tuberculosis from sputum specimens in South Africa. Int J Tuberc Lung Dis 2002; 6:529-37; PMID:12068987
- Boyle DS, Lehman DA, Lillis L, Peterson D, Singhal M, Armes N, Parker M, Piepenburg O, Overbaugh J. Rapid Detection of HIV-1 Proviral DNA for Early Infant Diagnosis Using Recombinase Polymerase Amplification. Mbio 2013; 4:pii: e00135-13; PMID:23549916; http://dx.doi.org/10.1128/mBio.00135-13
- David ST, Mukundan U, Brahmadathan KN, John TJ. Detecting mycobacteraemia for diagnosing tuberculosis. Indian J Med Res 2004; 119:259-66; PMID:15243163
- Katoch VM. Diagnostic relevance of detection of mycobacteraemia. Indian J Med Res 2004; 119:Xi-Xii; PMID:15243169
- Corner LA, Mccubbin K, Small KJ, Mccormick BS, Wood PR, Rothel JS. Efficiency of Inspection Procedures for the Detection of Tuberculous Lesions in Cattle. Aust Vet J 1990; 67:389-92; PMID:2085291; http://dx.doi.org/10.1111/j.1751-0813.1990.tb03020.x
- Stewart LD, McNair J, McCallan L, Gordon A, Grant IR. Improved Detection of Mycobacterium bovis Infection in Bovine Lymph Node Tissue Using Immunomagnetic Separation (IMS)-Based Methods. Plos One 2013; 8:e58374.
- Mishra A, Singhal A, Chauhan DS, Katoch VM, Srivastava K, Thakral SS, Bharadwaj SS, Sreenivas V, Prasad HK. Direct detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis in bovine samples by a novel nested PCR assay: Correlation with conventional techniques. J Clin Microbiol 2005; 43:5670-8; PMID:16272503; http://dx.doi.org/10.1128/JCM.43.11.5670-5678.2005
