ABSTRACT
Infection is the leading cause of non-relapse mortality after allogeneic haematopoietic cell transplantation (HCT). This occurs as a result of dysfunction to the host immune system from the preparative regimen used prior to HCT, combined with a delay in reconstitution of the donor-derived immune system after HCT. In this article, we elaborate on the process of immune reconstitution post-HCT that begins with the innate system and is followed by recovery of adaptive immunity. Simultaneously, we describe how the tempo of immune reconstitution influences the risk of various infections. We explain some of the key differences in immune reconstitution and the consequent risk of infections in recipients of peripheral blood stem cell, bone marrow or umbilical cord blood grafts. Other factors that impact on immune recovery are also highlighted. Finally, we allude to various strategies that are being tested to enhance immune reconstitution post-HCT.
Introduction
Allogeneic haematopoietic cell transplantation (HCT) is a potentially curative treatment option for a variety of malignancies.Citation1 A large part of this benefit is derived from the transfer of the donor's immune system to the host, which can elicit a potent graft-vs.-tumor effect. However, the recovery of a broad, functional T- and B cell immunity is delayed following HCT. The reason for this is manifold. High dose chemotherapy and/or radiation therapy used as conditioning regimen for allogeneic HCT results in severe mucosal, humoral and cellular immune dysfunction. Moreover, the host's thymopoiesis may be blunted, even prior to transplantation, as a result of thymic toxicity induced by cytotoxic therapy or radiation, which can further delay functional immune recovery.Citation2,3 These factors collectively predispose the host to a variety of infections. In fact, despite the routine use of prophylactic antimicrobials in the peri-transplant period,Citation4,5 infections occur is about 80–85% of HCT recipients and are one of the leading causes of non-relapse mortality after allogeneic HCT, even in long-term survivors.Citation6-10
Epidemiology of infections post transplantation
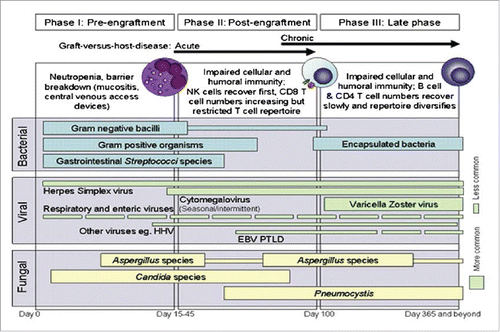
The spectrum of infections after HCT appears to correlate with the kinetics of immune recovery.Citation4,9-16 []. In the very early period post-HCT (up to day 30), conditioning regimen-related mucosal injury and severe aplasia predisposes patients to a variety of bacterial (mostly coagulase-negative Staphylococcus, Enterococcus, gram negative gastrointestinal bacteria and Clostridium difficile), fungal (mostly related to Candida species), and viral infections (mostly secondary to herpes simplex virus (HSV) reactivation). Reactivation of cytomegalovirus (CMV), Epstein-Barr virus (EBV) and infections from Pneumocystis jirovecii and Aspergillus species generally occur from engraftment until around day 100 or later, in the presence of graft-versus-host disease (GVHD) or prolonged immunosuppression. Varicella-zoster virus (VZV) reactivation usually occurs after day 100 - reflecting functional immaturity of T lymphocytes.Citation17 During the same time period, infections secondary to encapsulated bacteria (such as Streptococcus pneumoniae, Neisseria meningitides and Haemophilus influenzae) are also common due to deficient humoral immunity and impaired opsonization.Citation6,13,18,19 Risks of invasive fungal infections, community respiratory viruses and parasitic infections are evenly spread for up to 2 y post-transplantation.Citation10 Depending upon the graft source, infections account for 15–30% of deaths in the first 100 d post-transplant, and about 10–40% of deaths beyond day 100.Citation9,10 Fungal organisms are responsible for most of the infection-related mortality (50–80%), followed by bacterial causes (15–50%).Citation10
Several donor, host and transplant-related factors determine the risk of infections after HCT.Citation5 For instance, higher infection risk is associated with older age or advanced disease at the time of transplant, use of myeloablative regimens compared with reduced-intensity conditioning (RIC) regimens, use of ex vivo or in vivo T-cell depletion (TCD), delayed engraftment of neutrophils, development of GVHD, HCT with human leucocyte antigen (HLA)-mismatched grafts, use of umbilical cord blood (UCB) or bone marrow (BM) grafts compared with granulocyte-colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSC), to name a few.Citation5,6,10,11,20-25
Immune reconstitution post transplantation
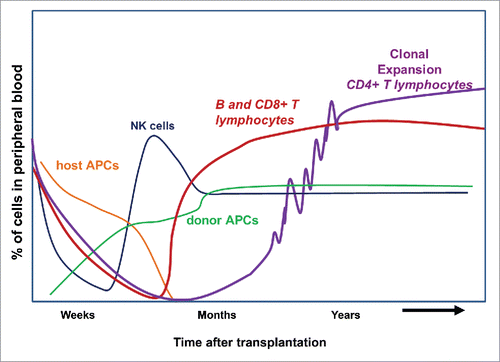
Recovery of the immune system after HCT is a highly dynamic process. It begins with resurgence of innate immunity within the first few weeks of HCT, followed by that of the adaptive immune system. The latter may take 2 y or longer to recover fully,Citation26,27 as depicted in .
The innate immune system
Recovery of mucosal injury
The conditioning regimen often results in a breach of epithelial surfaces and mucous membranes, which are the first line of defense against infections. This damage is expected to be worse with myeloablative than with RIC regimens, worse with BM compared to PBSC grafts, and is observed more frequently with matched unrelated donor (MUD) than with matched sibling donor (MSD) HCT.Citation12 Due to the diversity of the normal microbial flora that flourishes on mucous membranes, regimen-related mucosal damage can lead to an array of infections as a result of the organisms entering the blood stream. Patients with oral mucositis are especially at risk of infections by α-hemolytic streptococcal bacteria,Citation28-31 Candida speciesCitation32-35 and HSV.Citation33 Patients with gastrointestinal tract mucositis are prone to enterocolitis and systemic infections caused by colonic and opportunistic organisms, including pseudomonas and fungal organisms.Citation36,37 The recovery of damage to the intestinal epithelial barrier is mediated by the secretion of antimicrobial peptides, such as Reg3α by the Paneth cells, which regulates the normal gut microbiome and protects against GVHD. Absence of this protective mechanism leads to bacterial overgrowth, low microbial diversity, uncontrolled GVHD and high risk of non-relapse mortality after HSCT.Citation38,39 This may be partly explained by the important role of gut bacteria in the induction of colonic regulatory T cells (Tregs).Citation40,41 A recent elegantly designed murine study reported that the reduction in the microbiota-derived short-chain fatty acids, specifically the histone deacetylase inhibitor (HDACi) butyrate, in intestinal epithelial cells was associated with GVHD.Citation42 In this study, restoration of butyrate improved intestinal epithelial junctional integrity, decreased apoptosis, decreased clinical GVHD scores and improved survival. This effect was independent of intestinal Tregs or macrophages, but associated with reduced intestinal infiltration by CD4+ and CD8+ activated T cells.Citation42
It is conceivable that protection of the mucous membranes from conditioning-regimen related damage may reduce the risk of infections. A recombinant keratinocyte growth factor - Palifermin is approved by the US. Food and Drug Administration for prophylaxis and treatment of severe oral mucositis. Interestingly, it also has the potential to preserve normal thymopoiesis and enhance thymic cellularity.Citation43,44 However, the impact of palifermin on the recovery of specific immune subsets has not been studied extensively. An exploratory analysis from a double-blind placebo controlled study of palifermin in patients undergoing autologous HCT showed a lower incidence of febrile neutropenia (75% vs. 92%, P < 0.001) and a trend toward a lower incidence of blood-borne infections (15% vs. Twenty-five%) in the palifermin group.Citation45 Another retrospective study reported that in patients undergoing BEAM (carmustine, etoposide, cytarabine and melphalan) or busulfan-thiotepa conditioned autologous HCT, treatment with palifermin is associated with a lower risk of febrile neutropenia and severe infections not related to gram-positive organisms.Citation46 Similarly, oral or intravenous glutamine can reduce the severity of mucositis, but clinical evidence for its efficacy in preventing infections is lacking.Citation47,48
Without any specific interventions, mucosal damage starts to heal just before, or with the recovery of granulocytes in peripheral blood (PB),Citation49 as neutrophil recruitment to the site of mucosal injury may occur up to a week before recovery of neutrophils in PB.Citation50,51
Reconstitution of the innate cellular immune system
The appearance of granulocytes, monocytes, dendritic cells and natural killer (NK) cells in PB hallmarks the commencement of cellular recovery. With the routine use of recombinant human granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF), neutrophil engraftment occurs within 2 weeks after myeloablative HCT using PBSC, and 2–3 weeks after unmanipulated BM or UCB grafts. The use of growth factors not only hastens engraftment of granulocytes and monocytes but also enhances their function to some extent.Citation52-54 As compared with G-CSF, which primarily aids in the proliferation of neutrophils, GM-CSF also increases the number or monocytes and macrophages.Citation55 A prospective randomized trial reported that the use of GM-CSF or a combination of GM-CSF and G-CSF post-HSCT is associated with a significantly lower incidence of IFI-related mortality (1.5%) compared with G-CSF alone (12%), P = 0.034.Citation56 However, despite the routine use of growth factors, neutrophils can remain dysfunctional for up to 2 months post-HCT, and for up to a year in those who develop GVHD.Citation54,57-60 The ability of neutrophils to kill invasive fungi is regained in most patients by about 3 months post-HSCT; however, in patients who develop invasive fungal infection (IFI),neutrophil function make take 6–12 months to recover.Citation61
Polymorphisms in genes involved in the innate recognition of fungi also appear to play a critical role in determining infection outcomes. Fungal pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are recognized by pattern recognition receptors (PRRs) on various innate immune cells.Citation62-65 Among several major PRPs are the toll-like receptors, C-type lectin receptors (such as dectin 1, dectin 2) and galectin family proteins (such as galectin 3).Citation63,66 Several single nucleotide polymorphisms (SNPs) in these innate immune genes have been reported to be associated with susceptibility or resistance to fungal infections. The review of significance of these SNPs in fungal infections is outside the scope of this paper and interested readers are directed to review by Romani.Citation67
Natural killer cell immune reconstitution
NK cells influence both innate and adaptive immune responses Citation68-70 and are the first lymphocyte subset to appear in PB after HCT.Citation71-81 They have a protective role against many viral infections commonly encountered after HCT including, but not limited to, HSV, VZV, CMV and influenza virus as well as some bacterial infections.Citation82-92 The role of NK cells in antifungal immunity is controversial. In patients who develop IFI, NK cell recovery of is delayed for up to 6 months, while in those with well controlled IFI, NK cell numbers are significantly increased.Citation61 Moreover, in patients with IFI, higher number of NK cells is correlated with fewer fungal lesions.Citation61 These correlative data and some in vitro studiesCitation93,94 suggest that NK cells may have a role in the prevention of fungal infections although the exact mechanism for this protection is not yet clear. Interestingly, whereas, patients with an isolated absolute, classical or functional NK cell deficiency are prone to severe viral infections, fungal infections are much less uncommon, suggesting that in the absence of neutropenia NK cells may have a less important role in fungal protection.Citation95
Reconstitution of NK cells varies by graft source. NK cell recovery in the first few months post-HCT is more robust in BM and UCB graft recipients compared to PBSC recipients, despite the fact that PBSC grafts contain 10-fold higher absolute number of NK cells than other grafts.Citation96,74 This may be related to the relative delay in T- and B- lymphocyte recovery in BM and UCB graft recipients, as elaborated in the following sections.
NK cell recovery differs from the recovery of other lymphocytes in several aspects. Not only do NK cells appear early, they also acquire functional competence much earlier than other lymphocytes. Specifically, while B- and T-cells may take months to years to become fully functional,Citation97 NK cells can mature in about 2–4 weeks.Citation79,81,98,99 Another unique feature of NK cells is that their activity after HCT remains normal even in the presence of severe GVHD,Citation79,99 which usually suppresses the activity of other immune subsets such as T and B lymphocytes.Citation20 CMV reactivation or infection, which occurs commonly after HCT, can further augment NK cell activity.Citation79,100 The cytolytic mechanism of NK cells is also unique. In contrast to B- and T-cells, NK cells do not possess antigen specific receptors. Instead, they recognize their targets not by detection of foreign antigens, but by a failure to detect self HLA class I expression, a common antigen present on all healthy cells. This forms the basis for the ‘missing-self hypothesis’. Viruses often downregulate HLA class I molecules on the surface of cells they invade in order to avoid T cell recognition and thus, can be targeted by NK cells [reviewed by Horowitz et alCitation82]. However, viruses have also evolved mechanisms to escape NK cell recognition via upregulation of ligands that bind inhibitory NK cell receptors, and downregulation of ligands for the activating NK cell receptors.Citation101 When the innate immune system fails to eradicate an infection, the adaptive immune system is activated, which is also critical for long-term memory and protection against re-infection.
Humoral immunity
Normal development of B cells
The earliest B cells formed in BM are immature transitional cells (CD10+CD38hiCD24hiCD44lo). These cells emigrate to secondary lymphoid organs, where they differentiate into mature naïve cells. After antigen-mediated activation with the help of T helper (Th) cells, naïve cells undergo clonal expansion and either differentiate into plasma cells that secrete antibodies or form memory B cells [reviewed by Kurosaki Citation102 and Batista Citation103]. The presence or absence of IgD and CD27 delineates naïve B cells (IgD+CD27-), non-class-switched/ IgM+ memory B cells (IgD+CD27+) and class-switched memory B cells (IgD-CD27+). In healthy adult PB, transitional B cells constitute only about 2% of total B cells, naïve B cells comprise about 50%, and IgM+ memory B cells and IgM− class-switched memory B cells each comprise about 10–15%.Citation104,105
B cell reconstitution post-HCT
After HCT, the proportion of total B cells in most patients reach normal levels by 3 months, but the absolute numbers may take up to 6–12 months to normalize, which is further delayed in patients with GVHD.Citation96,106,107 Moreover, although the number of total B cells may reach levels comparable to adult controls, most of the reconstituting B cells in the first year after HCT are comprised primarily of transitional and naïve subsets, while the recovery of memory B cells occurs much later.Citation108,109 One exception is EBV reactivation, which leads to preferential expansion of IgG+ or IgA+ class-switched memory B cells.Citation108
Different graft sources have differential impacts on the tempo of B cell reconstitution. For instance, the number of total B cells, naïve and memory B cells are 10-20-fold higher in PBSC grafts compared to BM.Citation96 Consequently, these mature B cell subsets are passively transferred in the PBSC graft and can be found at higher numbers in PBSCT recipients for up to 3 months post-transplantation.Citation96,110 On the other hand, the pace of B cell recovery is steeper in BMT compared to PBSC recipients, likely due to higher numbers of progenitor B cells being infused in the BM graft.Citation96 By 3 months post-transplant, the total immunoglobulin (Ig) levels are comparable in PBSC and BM graft recipients; however, for the first year post HCT, Ig levels remain significantly lower than that seen in normal controls.Citation96 Recipients of UCB grafts achieve very rapid recovery of B cells, and have higher numbers of total B cells compared to PBSC recipients for up to 2 y post-HCT.Citation74 Recovery of immunoglobulins is also faster after UCBT compared with PBSC HCT.Citation74
Functional reconstitution of B cells
Functional recovery of B lymphocytes takes several months to years,Citation20,80,111-116 and follows that of normal ontogeny.Citation106,107,117-119 In the first few months of transplant, regenerating B cells lack proliferative and differentiative responses to antigen-specific factors, indicating their functional incompetence.Citation120 Coincident with the recovery of B cell counts after HCT, IgM production normalizes after about 3 months.Citation19,107 Isotype-switched memory B cells that produce IgG can be detected between 3 and 6 months and their ability to secrete specific IgG (in response to pokeweed antigen or Staphylococcus aureus antigen) is gradually acquired between 1–2 y post-transplantation.Citation107 However, although the levels of IgG1 and IgG3 normalize during the first year after HCT, the deficiencies of IgG2 and IgG4 persist for more than 18 months.Citation19,20,106,107,114,121-123 As lgG2 responses are protective against capsular carbohydrate antigens from gram-positive bacteria,Citation124 prolonged deficiency of IgG2 can explain the undue susceptibility of HCT recipients to late bacterial infections. The last immunoglobulin to recover is IgA, which may be undetectable for several years.Citation119 The prominent role of IgA in mucosal humoral immunity partly explains why patients remain at risk of recurrent sino-pulmonary and gastrointestinal tract infections, even years after transplantation. The deficiencies of immunoglobulins is much more pronounced and prolonged in those who develop GVHD or those who receive antithymocyte globulin (ATG).Citation19,107,121,125 However, interestingly, the functional recovery of B cells is similar after T-replete or T-deplete HCT,Citation107 and after B- and T-deplete PB graft (CD34+ selected) vs. unmanipulated grafts.Citation126
The functional immaturity of donor-derived lymphocytes, combined with a decrease in the recipient plasma cells and Ig levels over time, result in the loss of immunity against viral and bacterial pathogens attained through childhood vaccination or infection.Citation127-129 Therefore, patients need to be re-vaccinated - the precise timing of which can be challenging due to remarkable differences in functional recovery of B cells in individual patients [reviewed Pirofski and Casadevall Citation130 and Avigan et al Citation131]. In general, vaccinations are avoided in the first 3–6 months after HCT, after administration of rituximab or intravenous immunoglobulins, in the presence of GVHD, or while patients are on immunosuppressive drugs. Due to a lack of clear evidence, the recommended vaccination schedule is similar for autologous and allogeneic HCT recipients, regardless of the type of conditioning regimen or the graft source.Citation5,132,133
Cellular adaptive immunity
Normal development of T cells
The name “T” lymphocyte denotes the essential role of the thymus in the maturation of T-cells. Double negative (CD4-CD8-) precursor T cells produced from pluripotent haematopoietic stem cells in the BM migrate to the thymus, where they undergo positive and negative selection to become naïve CD4+ or CD8+ T cells. Naïve T-cells encounter foreign antigens in secondary lymphoid tissues. After a prolonged period of stimulation (about 20 hours), T-cells either proliferate to form activated effector cells, or they become memory cells of central memory (TCM) or effector memory (TEM) phenotype. TCM cells express homing receptors (CCR7 and CD62L), reside in secondary lymphoid tissues, possess little effector function but can undergo terminal differentiation upon re-stimulation with an antigen. TEM cells, on the other hand, are terminally differentiated and respond immediately to antigenic stimulation. In contrast to naïve T cells, effector T cells require a short period of stimulation (about 30 min or shorter) to trigger proliferation and immediate effector functions.Citation134,135
T cell reconstitution post-HCT
After HCT, T cell reconstitution occurs in 2 distinct phases. The initial phase is thymus-independent, antigen-driven peripheral expansion of T cells infused with the graft that possess a limited and skewed T cell receptor (TCR) repertoire. The later phase is thymus-dependent expansion of naïve T cells derived from the donor stem cells that possess a diverse TCR repertoire.Citation3,136,137 Because thymopoiesis after HCT is extremely slow, the thymus-dependent T cell recovery can remain incomplete for years.Citation3 This is further delayed in older patients due to thymic involution and those who develop GVHD as the thymic epithelial cells are damaged by alloreactive T-cells.Citation3,138 There are 2 important consequences of impaired thymopoiesis post HCT. First, thymic-independent pathway can rapidly generate CD8+, but not CD4+ T cells,Citation139 resulting in an inversion in the CD4:CD8 ratio. Second, it leads to peripheral expansion of memory (CD45+RO+/ CD45RO+CD27+) T cells, as the generation of naïve T cells (CD45+RA+/ CD45RO-CD27+) from prethymic progenitors is largely dependent on a functional thymus.Citation140-142
The inversion of CD4:CD8 ratio is one of the earliest features of T cell reconstitution after autologous or allogeneic transplantation from any graft source and can persist for up to several years after HCT.Citation2,20,73,80,81,97,115,116,143 Different graft sources, however, can impact on other aspects of T cell reconstitution. The majority of these differences are a consequence of substantially higher absolute numbers of CD4+ and CD8+ T cells infused with a PBSC (10-20-fold higher) than with a BM graft.Citation96,110,144 As a result, PBSC transplant is associated with faster T cell reconstitution compared to other graft sources. However, even after PBSC transplant, the absolute number of CD4+ T cells, including both CD4+ regulatory T cells (Treg) and conventional CD4+ cells (CD4Tcon), remain low for up to 2 y post-HCT, while normalization of CD8+ T cells and their subsets can take anywhere from 1 month to a year, depending upon the conditioning regimen and GVHD prophylaxis regimen.Citation81,97,126 Compared with other grafts, UCB contains the lowest numbers of total nucleated cells and T cells, most of which are antigen-naïve. Additionally, in contrast to other graft sources, UCBT is associated with delayed thymopoiesis; Citation2 as a result, T cell recovery after UCBT occurs primarily due to thymus-independent peripheral expansion of mature donor T cells for long time.Citation136,145,146 Moreover, many of the UCBT regimens incorporate in vivo T cell depletion with antithmyocyte globulin (ATG), which leads to a prolonged period of lymphopenia. Therefore, reconstitution of all T cell subtypes is delayed for at least 6 months after UCBT but becomes comparable to that of PBSC graft by one year and reaches normal level by 2 y.Citation74
In the setting of haploidentical HSCT with post-transplantation high dose cyclophosphamide, which selectively depletes transferred memory T cells and rapidly proliferating alloreactive T cells in vivo,Citation147 the early stages of T cell development are dominated by large numbers of memory T cells with stem-like properties (TSCM). These TSCM are derived from naive T cells in the donor graft and are responsible for the reconstitution of a T cell compartment with a diverse TCR repertoire post-HSCT.Citation148,149
Assessment of T cell receptor repertoire
The TCR is a heterodimer composed of α and β chains. Both α and β chains consist of variable (V), joining (J) and constant (C) regions, while the β chains also have an additional diversity (D) region. T cells undergo somatic V(D)J recombination during their developmental phase, producing an extensive TCR repertoire. During this rearrangement, nucleotides are added or removed at a specific region denoted as CDR3, which imparts clonality and specificity to individual T cells. This rearrangement phenomenon can be exploited to assess reconstitution of the TCR repertoire after HCT. Traditional methods employed southern blot analysis, reverse transcriptase-polymerase chain reaction (RT-PCR)Citation150 or flow cytometric techniques Citation151 to study the Vβ repertoire. Another method assesses the heterogeneity in the size of the CDR3 region within Vβ gene families using V family-specific PCR, a technique called CDR3 size spectratyping.Citation152 These techniques are, however, limited to measuring only the known exons and none of them is able to determine the frequency of individual TCRs. More recently, novel deep sequencing techniques have been employed to assess TCR diversity with very high resolution. One such technique combines the use of 5′ rapid amplification of cDNA ends (RACE)-PCR to amplify all the possible combinations of TCR α and β chains followed by next-generation sequencing of TCR.Citation153,154 Using this method, a study showed that recipients of double unit UCBT without ATG had the highest diversity of CD4+ and CD8+ TCR repertoire, a greater proportion of which were naive T cells, as compared with recipients of conventional or TCD PBPC grafts at 6- and 12-months post-transplantation.Citation154
With the deep sequencing methods, the correlation of TCR diversity with GVHD and relapse is also recognized. A study including haploidentical-UCB donors and matched related or unrelated donors using in vivo TCD showed that patients who remained in remission displayed a significantly higher TCR diversity compared with patients with relapsed disease.Citation153 It is therefore conceivable that acquisition of higher TCR diversity may be protective against disease relapse. The study also found that the diversity of TCR α and β was significantly lower in patients with GVHD (presumably related to preferential expansion of certain T cell clones) than in patients who do not develop this complication.Citation153 Similar results were seen in prior studies using CDR3 spectratyping.Citation155,156 In contrast, another study utilizing deep sequencing methods reported that grade 2–3 acute GVHD is actually associated with a higher TCR diversity than those who developed grade 0–1 acute GVHD, suggesting that GVHD did not restrict recovery of TCR repertoire.Citation154 The differences noted in these studies are a reflection of different graft and donor sources, conditioning regimens and the use of T cell depletion.
Functional reconstitution of T cells
Despite differences in the number of T cells in BM and PB grafts and the different pace of immune reconstitution after HCT, differences in the functional recovery of T cells are rather subtle.Citation96,157 For instance, the in vitro responsiveness of T cells to HSV and VZV antigens or to non-specific mitogens such as phorbol myristate acetate (PMA) is similar in PB samples collected from PBSC or BM graft recipients at all time points after transplantation.Citation96 After BM transplantation, T cell proliferative responses to specific antigens, such as candidin, tetanus toxoid, tuberculin or toxoplasma, are absent in about 20–50% of patients, even up to a year after transplantation.Citation20 Primary T cell immunity against fungi is provided by CD4+ Th1 and Th17 cells Citation158-161 as well as memory CD8+ T-cell cells.Citation160 Consequently, at 3 months or later after HSCT, fewer than a quarter of patients will have detectable functional T cell responses against the most common invasive fungal infection. In a study by Einsele's group,Citation161 T-cell response against Aspergillus fumigatus antigen was detected at higher levels in patients with invasive aspergillosis and disease regression on antifungal therapy compared to those with stable or progressive disease. This may partly explain the bimodal incidence of invasive aspergillosis seen after allogeneic HSCT. The first peak occurs within the first 2 weeks with neutropenia,Citation162 after which the incidence declines with reconstitution of the innate immunity. A second peak occurs at around 3 months,Citation16,162 coinciding with the deficient T cell immunity. In contrast to antifungal immunity, CMV-specific CD4+ and CD8+ T cells can be detected within 2–3 months after HCT.Citation11,157,161,163-166
UCB graft recipients experience remarkably delayed T cell functional recovery compared with BM or PBSC graft recipients.Citation2,73-75,80 Specifically, T cell activity against staphylococcal enterotoxin B, CMV, EBV, and adenovirus is delayed for at least 8–9 months post CBT, while activity against BK virus, influenza and respiratory syncytial virus is delayed even further.Citation80 Consequently, more than 50% of UCB recipients develop viral reactivation or infection in the first 6-months, and over 90% within a year of transplantation.Citation73,80 On the other hand, graft source does not appear to independently contribute to the risk of CMV infection; the risk of CMV reactivation, CMV disease and the probability of response to antiviral therapy is similar in UCB, PBSC and BM graft recipients.Citation167 Depletion of T cells from PBSC or BM grafts also leads to significant delay in the recovery and functional maturity of T cells.Citation168,169 As a result, around 50–70% of patients who receive TCD grafts have evidence of CMV reactivation within the first year post HCT, with a median time to reactivation of less than a month.Citation126,165,170,171
Strategies to enhance immune reconstitution
Various strategies have been attempted to augment immune recovery after HCT. The majority of these approaches are directed at the thymus, and include strategies to protect the thymic epithelium, stimulate thymopoiesis, or increase the number of T-lymphoid precursors. Approaches under investigation include administration of cytokines such as interleukin (IL)-2, IL-7 or IL-15, growth factors including insulin-like growth factor-1, recombinant human growth hormone, parathyroid hormone, sex steroid ablation using luteinizing hormone-releasing hormone agonist (goserelin), or small molecules such as keratinocyte growth factor (palifermin), the tyrosine kinase inhibitor sunitinib, and anti-CD25 antibody, to name a few [Reviewed Citation172,173]. Another approach is the use of biological or cellular therapies such as the use of Notch-based culture systems to obtain T-cell lineage committed precursor cells,Citation174 ex vivo expansion and infusion of T cell precursors, or transplantation of thymic tissue.Citation175,176 Most of these methods are either still in pre-clinical development or have failed to demonstrate a significant improvement in in immune recovery or infection risk to date. Review of these methods is beyond the scope of this paper and interested readers are referred to articles by BernsteinCitation172 and Seggewiss.Citation173 Infusion of ex vivo expanded virus-specific cytotoxic T cells, on the other hand, have resulted in impressive responses in early phase clinical trials for the treatment of specific infections [reviewed Barrett & Bollard Citation177].
Conclusion
Over the past several years, substantial knowledge has been gained on the complexity of immune reconstitution after HCT; however, the translation of these findings to the clinic has been rather disappointing. Moreover, despite the recognition that the pace of immune recovery varies considerably depending on recipient age, underlying disease, graft source, type of conditioning regimen, GVHD prophylaxis and other factors, our current approach to antimicrobial prophylaxis and vaccination post HCT is essentially non-specific. Moreover, although several techniques are being tested to enhance the recovery and speed of immune reconstitution, none has resulted in a conclusive reduction in the risk of infections. Two major factors contribute to delayed immune reconstitution after HCT—(i) preparative regimen-related damage to the host microenvironment, and (ii) the inevitable lag in the natural maturity of donor-derived immune cells. Thus, combination strategies that aim at protecting the host immune microenvironment, while facilitating recovery of donor-derived immune subsets, may be more beneficial than using either of these approaches alone.
Abbreviations
| ATG | = | antithymocyte globulin |
| BM | = | bone marrow |
| CD | = | cluster differentiation |
| CMV | = | cytomegalovirus |
| EBV | = | Epstein–Barr virus |
| G-CSF | = | granulocyte colony-stimulating factor |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| GVHD | = | graft-versus-host disease |
| HCT | = | haematopoietic cell transplantation |
| HLA | = | human leucocyte antigen |
| HSV | = | herpes simplex virus |
| IFI | = | Invasive fungal infection |
| Ig | = | immunoglobulin |
| MSD | = | matched sibling donor |
| MUD | = | matched unrelated donor |
| NK | = | natural killer |
| PBSC | = | peripheral blood stem cells |
| PHA | = | phorbol myristate acetate |
| RIC | = | reduced intensity conditioning |
| TCD | = | T cell deplete |
| TCM | = | central memory |
| TEM | = | effector memory |
| TCR | = | T cell receptor |
| Th cell | = | helper T cells |
| UCB | = | umbilical cord blood |
| UCBT | = | umbilical cord blood transplantation |
| VZV | = | Varicella-zoster virus (VZV) |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Dr. Rezvani's research in NK cell reconstitution after transplant is partially funded by the Leukemia and Lymphoma Society (LLS) (grant no. 6470-15), the HHS | NIH | National Cancer Institute (NCI) (grant no. RO1 CA061508-18), and the American Cancer Society (ACS) (grant no. RSG-15-218-01-LIB).
References
- Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, Omel JL, Orchard PJ, Palmer J, Saber W, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the american society for blood and marrow transplantation. Biol Blood Marrow Transplant 2015; 21:1863-9; PMID:26256941; http://dx.doi.org/10.1016/j.bbmt.2015.07.032
- Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, Bryan SG, Kaur I, Martin S, Wieder ED, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood 2007; 110:4543-51; PMID:17671230; http://dx.doi.org/10.1182/blood-2007-05-092130
- Ringhoffer S, Rojewski M, Dohner H, Bunjes D, Ringhoffer M. T-cell reconstitution after allogeneic stem cell transplantation: assessment by measurement of the sjTREC/betaTREC ratio and thymic naive T cells. Haematologica 2013; 98:1600-8; PMID:23585532; http://dx.doi.org/10.3324/haematol.2012.072264
- Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, Burns LJ, Chaudhri N, Davies S, Okamoto S, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18:348-71; PMID:22178693; http://dx.doi.org/10.1016/j.bbmt.2011.12.519
- Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ, Center for International B, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143-238; PMID:19747629; http://dx.doi.org/10.1016/j.bbmt.2009.06.019
- Young JH, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, Knust K, Horowitz MM, Confer DL, Dubberke ER, et al. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol Blood Marrow Transplant 2016 Feb;22(2):359-70; http://dx.doi.org/10.1016/j.bbmt.2015.09.013
- Pasquini MC, Zhu X. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2015. Available at: http://www.cibmtr.org. 2015.
- Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, Cahn JY, Passweg JR, Rowlings PA, Schouten HC, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late effects working committee of the international bone marrow transplant registry. N Engl J Med 1999; 341:14-21; PMID:10387937; http://dx.doi.org/10.1056/NEJM199907013410103
- Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, Sirvent A, Champlin RE, Chao N, Gee AP, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010; 11:653-60; PMID:20558104; http://dx.doi.org/10.1016/S1470-2045(10)70127-3
- Parody R, Martino R, Rovira M, Vazquez L, Vazquez MJ, de la Camara R, Blazquez C, Fernandez-Aviles F, Carreras E, Salavert M, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant 2006; 12:734-48; PMID:16785063; http://dx.doi.org/10.1016/j.bbmt.2006.03.007
- Sauter C, Abboud M, Jia X, Heller G, Gonzales AM, Lubin M, Hawke R, Perales MA, van den Brink MR, Giralt S, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant 2011; 17:1460-71; PMID:21310254; http://dx.doi.org/10.1016/j.bbmt.2011.02.001
- Winston DJ, Gale RP, Meyer DV, Young LS. Infectious complications of human bone marrow transplantation. Med (Baltimore) 1979; 58:1-31; PMID:368507; http://dx.doi.org/10.1097/00005792-197901000-00001
- Atkinson K, Storb R, Prentice RL, Weiden PL, Witherspoon RP, Sullivan K, Noel D, Thomas ED. Analysis of late infections in 89 long-term survivors of bone marrow transplantation. Blood 1979; 53:720-31; PMID:34408
- Meyers JD. Fungal infections in bone marrow transplant patients. Semin Oncol 1990; 17:10-3; PMID:2353204
- Peterson PK, McGlave P, Ramsay NK, Rhame F, Cohen E, Perry GS, 3rd, Goldman AI, Kersey J. A prospective study of infectious diseases following bone marrow transplantation: emergence of Aspergillus and Cytomegalovirus as the major causes of mortality. Infect Control 1983; 4:81-9; PMID:6302027
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50:1091-100; PMID:20218877; http://dx.doi.org/10.1086/651263
- Han CS, Miller W, Haake R, Weisdorf D. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant 1994; 13:277-83; PMID:8199570
- Atkinson K, Farewell V, Storb R, Tsoi MS, Sullivan KM, Witherspoon RP, Fefer A, Clift R, Goodell B, Thomas ED. Analysis of late infections after human bone marrow transplantation: role of genotypic nonidentity between marrow donor and recipient and of nonspecific suppressor cells in patients with chronic graft-versus-host disease. Blood 1982; 60:714-20; PMID:6213276
- Velardi A, Cucciaioni S, Terenzi A, Quinti I, Aversa F, Grossi CE, Grignani F, Martelli MF. Acquisition of Ig isotype diversity after bone marrow transplantation in adults. A recapitulation of normal B cell ontogeny. J Immunol 1988; 141:815-20; PMID:3294290
- Maury S, Mary JY, Rabian C, Schwarzinger M, Toubert A, Scieux C, Carmagnat M, Esperou H, Ribaud P, Devergie A, et al. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol 2001; 115:630-41; PMID:11736948; http://dx.doi.org/10.1046/j.1365-2141.2001.03135.x
- Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100:4358-66; PMID:12393425; http://dx.doi.org/10.1182/blood-2002-05-1496
- Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, Ibatici A, Del Bono V, Bacigalupo A, Viscoli C. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant 2009; 44:361-70; PMID:19308042; http://dx.doi.org/10.1038/bmt.2009.39
- Barker JN, Hough RE, van Burik JA, DeFor TE, MacMillan ML, O'Brien MR, Wagner JE. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant 2005; 11:362-70; PMID:15846290; http://dx.doi.org/10.1016/j.bbmt.2005.02.004
- Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, Wagner JE. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006; 108:2874-80; PMID:16804113; http://dx.doi.org/10.1182/blood-2006-03-011791
- Cohen J, Gandhi M, Naik P, Cubitt D, Rao K, Thaker U, Davies EG, Gaspar HB, Amrolia PJ, Veys P. Increased incidence of EBV-related disease following paediatric stem cell transplantation with reduced-intensity conditioning. Br J Haematol 2005; 129:229-39; PMID:15813851; http://dx.doi.org/10.1111/j.1365-2141.2005.05439.x
- Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, Passweg J, Roosnek E. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol 2008; 30:425-37.; PMID:18949477; http://dx.doi.org/10.1007/s00281-008-0132-5
- Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol 2012; 19:324-35.; PMID:22517587; http://dx.doi.org/10.1097/MOH.0b013e328353bc7d
- Rapoport AP, Miller Watelet LF, Linder T, Eberly S, Raubertas RF, Lipp J, Duerst R, Abboud CN, Constine L, Andrews J, et al. Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J Clin Oncol 1999; 17:2446-53; PMID:10561308
- Stiff P. Mucositis associated with stem cell transplantation: current status and innovative approaches to management. Bone Marrow Transplant 2001; 27 Suppl 2:S3-S11; http://dx.doi.org/10.1038/sj.bmt.1702863
- Ruescher TJ, Sodeifi A, Scrivani SJ, Kaban LB, Sonis ST. The impact of mucositis on α-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer 1998; 82:2275-81; PMID:9610710; http://dx.doi.org/10.1002/(SICI)1097-0142(19980601)82:11%3c2275::AID-CNCR25%3e3.0.CO;2-Q
- Donnelly JP, Muus P, Horrevorts AM, Sauerwein RW, De Pauw BE. Failure of clindamycin to influence the course of severe oromucositis associated with streptococcal bacteraemia in allogeneic bone marrow transplant recipients. Scand J Infect Dis 1993; 25:43-50; PMID:8460348; http://dx.doi.org/10.1080/00365549309169668
- Epstein JB, Hancock PJ, Nantel S. Oral candidiasis in hematopoietic cell transplantation patients: an outcome-based analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96:154-63; PMID:12931087; http://dx.doi.org/10.1016/S1079-2104(03)00296-8
- Eisen D, Essell J, Broun ER. Oral cavity complications of bone marrow transplantation. Semin Cutan Med Surg 1997; 16:265-72; PMID:9421217; http://dx.doi.org/10.1016/S1085-5629(97)80015-6
- Wingard JR. Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant 1999; 5:55-68; PMID:10371357; http://dx.doi.org/10.1053/bbmt.1999.v5.pm10371357
- Epstein JB, Ransier A, Lunn R, Chin E, Jacobson JJ, Le N, Reece D. Prophylaxis of candidiasis in patients with leukemia and bone marrow transplants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 81:291-6; PMID:8653462; http://dx.doi.org/10.1016/S1079-2104(96)80328-3
- Avigan D, Richardson P, Elias A, Demetri G, Shapiro M, Schnipper L, Wheeler C. Neutropenic enterocolitis as a complication of high dose chemotherapy with stem cell rescue in patients with solid tumors: a case series with a review of the literature. Cancer 1998; 83:409-14; PMID:9690531; http://dx.doi.org/10.1002/(SICI)1097-0142(19980801)83:3%3c409::AID-CNCR7%3e3.0.CO;2-J
- Ettinghausen SE. Collagenous colitis, eosinophilic colitis, and neutropenic colitis. Surg Clin North Am 1993; 73:993-1016; PMID:8378836
- Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124:1174-82; PMID:24939656; http://dx.doi.org/10.1182/blood-2014-02-554725
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 2012; 209:903-11; PMID:22547653; http://dx.doi.org/10.1084/jem.20112408
- Sarrabayrouse G, Bossard C, Chauvin JM, Jarry A, Meurette G, Quevrain E, Bridonneau C, Preisser L, Asehnoune K, Labarriere N, et al. CD4CD8alphaalpha lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol 2014; 12:e1001833; PMID:24714093; http://dx.doi.org/10.1371/journal.pbio.1001833
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500:232-6; PMID:23842501; http://dx.doi.org/10.1038/nature12331
- Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, Oravecz-Wilson K, Wu SR, Sun Y, Rossi C, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol 2016; 17:505-13; PMID:26998764; http://dx.doi.org/10.1038/ni.3400
- Rossi S, Blazar BR, Farrell CL, Danilenko DM, Lacey DL, Weinberg KI, Krenger W, Hollander GA. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood 2002; 100:682-91; PMID:12091365; http://dx.doi.org/10.1182/blood.V100.2.682
- Min D, Panoskaltsis-Mortari A, Kuro OM, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood 2007; 109:2529-37; PMID:17138819; http://dx.doi.org/10.1182/blood-2006-08-043794
- Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, Shea T, Yanovich S, Hansen K, Noga S, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 2004; 351:2590-8; PMID:15602019; http://dx.doi.org/10.1056/NEJMoa040125
- Milone G, Leotta S, Cupri A, Fauci AL, Spina P, Parisi M, Berritta D, Tripepi G. Palifermin reduces infection rate and hyperfibrinogenemia in patients treated with high-dose chemotherapy based on beam or BU-thiothepa. Bone Marrow Transplant 2014; 49:1193-7; PMID:25000456; http://dx.doi.org/10.1038/bmt.2014.140
- Sayles C, Hickerson SC, Bhat RR, Hall J, Garey KW, Trivedi MV. Oral glutamine in preventing Treatment-Related Mucositis in adult patients with cancer: a systematic review. Nutr Clin Pract 2016 Apr;31(2):171-9; PMID:26507188; http://dx.doi.org/10.1177/0884533615611857
- Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A, McCabe MG, Meyer S, Khalid T. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2011; 25(6):284-5:CD000978
- Lieschke GJ, Ramenghi U, O'Connor MP, Sheridan W, Szer J, Morstyn G. Studies of oral neutrophil levels in patients receiving G-CSF after autologous marrow transplantation. Br J Haematol 1992; 82:589-95; PMID:1283080; http://dx.doi.org/10.1111/j.1365-2141.1992.tb06472.x
- Cheretakis C, Dror Y, Glogauer M. A noninvasive oral rinse assay to monitor engraftment, neutrophil tissue delivery and susceptibility to infection following HSCT in pediatric patients. Bone Marrow Transplant 2005; 36:227-32; PMID:15937506
- Cheretakis C, Leung R, Sun CX, Dror Y, Glogauer M. Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in a murine bone marrow transplantation model. Blood 2006; 108:2821-6; PMID:16804110; http://dx.doi.org/10.1182/blood-2006-04-018184
- Wiltschke C, Krainer M, Nanut M, Wagner A, Linkesch W, Zielinski CC. In vivo administration of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor increases neutrophil oxidative burst activity. J Interferon Cytokine Res 1995; 15:249-53; PMID:7584671; http://dx.doi.org/10.1089/jir.1995.15.249
- Fabian I, Kletter Y, Bleiberg I, Gadish M, Naparsteck E, Slavin S. Effect of exogenous recombinant human granulocyte and granulocyte-macrophage colony-stimulating factor on neutrophil function following allogeneic bone marrow transplantation. Exp Hematol 1991; 19:868-73; PMID:1716591
- Gadish M, Kletter Y, Flidel O, Nagler A, Slavin S, Fabian I. Effects of recombinant human granulocyte and granulocyte-macrophage colony-stimulating factors on neutrophil function following autologous bone marrow transplantation. Leuk Res 1991; 15:1175-82; PMID:1722549; http://dx.doi.org/10.1016/0145-2126(91)90187-X
- Ruef C, Coleman DL. Granulocyte-macrophage colony-stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis 1990; 12:41-62; PMID:2405468; http://dx.doi.org/10.1093/clinids/12.1.41
- Wan L, Zhang Y, Lai Y, Jiang M, Song Y, Zhou J, Zhang Z, Duan X, Fu Y, Liao L, et al. Effect of Granulocyte-Macrophage Colony-Stimulating factor on prevention and treatment of invasive fungal disease in recipients of allogeneic stem-cell transplantation: A prospective multicenter randomized phase IV Trial. J Clin Oncol 2015; 33:3999-4006; PMID:26392095; http://dx.doi.org/10.1200/JCO.2014.60.5121
- Zimmerli W, Zarth A, Gratwohl A, Speck B. Neutrophil function and pyogenic infections in bone marrow transplant recipients. Blood 1991; 77:393-9; PMID:1845932
- Cayeux S, Meuer S, Pezzutto A, Korbling M, Haas R, Schulz R, Dorken B. Allogeneic mixed lymphocyte reactions during a second round of ontogeny: normal accessory cells did not restore defective interleukin-2 (IL-2) synthesis in T cells but induced responsiveness to exogeneous IL-2. Blood 1989; 74:2278-84; PMID:2478226
- Sahdev I, O'Reilly R, Black P, Heller G, Hoffmann M. Interleukin-1 production following T-cell-depleted and unmodified marrow grafts. Pediatr Hematol Oncol 1996; 13:55-67; PMID:8718503; http://dx.doi.org/10.3109/08880019609033372
- Clark RA, Johnson FL, Klebanoff SJ, Thomas ED. Defective neutrophil chemotaxis in bone marrow transplant patients. J Clin Invest 1976; 58:22-31; PMID:777029; http://dx.doi.org/10.1172/JCI108452
- Stuehler C, Kuenzli E, Jaeger VK, Baettig V, Ferracin F, Rajacic Z, Kaiser D, Bernardini C, Forrer P, Weisser M, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation and association with occurrence and outcome of invasive aspergillosis. J Infect Dis 2015; 212:959-67; PMID:25748323; http://dx.doi.org/10.1093/infdis/jiv143
- Bourgeois C, Majer O, Frohner IE, Tierney L, Kuchler K. Fungal attacks on mammalian hosts: pathogen elimination requires sensing and tasting. Curr Opin Microbiol 2010; 13:401-8; PMID:20538507; http://dx.doi.org/10.1016/j.mib.2010.05.004
- Jouault T, Sarazin A, Martinez-Esparza M, Fradin C, Sendid B, Poulain D. Host responses to a versatile commensal: PAMPs and PRRs interplay leading to tolerance or infection by Candida albicans. Cell Microbiol 2009; 11:1007-15; PMID:19388906; http://dx.doi.org/10.1111/j.1462-5822.2009.01318.x
- Moretti S, Bellocchio S, Bonifazi P, Bozza S, Zelante T, Bistoni F, Romani L. The contribution of PARs to inflammation and immunity to fungi. Mucosal Immunol 2008; 1:156-68; PMID:19079173; http://dx.doi.org/10.1038/mi.2007.13
- Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006; 442:651-6; PMID:16862125; http://dx.doi.org/10.1038/nature04926
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 2011; 29:1-21; PMID:20936972; http://dx.doi.org/10.1146/annurev-immunol-030409-101229
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275-88; PMID:21394104; http://dx.doi.org/10.1038/nri2939
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood 1990; 76:2421-38; PMID:2265240
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633-40; PMID:11698225; http://dx.doi.org/10.1016/S1471-4906(01)02060-9
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 2003; 101:3052-7; PMID:12480696; http://dx.doi.org/10.1182/blood-2002-09-2876
- Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, Collins N, Gillio A, George D, Jakubowski A, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood 1999; 93:467-80; PMID:9885208
- Thomson BG, Robertson KA, Gowan D, Heilman D, Broxmeyer HE, Emanuel D, Kotylo P, Brahmi Z, Smith FO. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood 2000; 96:2703-11; PMID:11023501
- Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J, Cayuela JM, Traineau R, Robin M, Madureira A, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis 2011; 13:456-65; PMID:21466640; http://dx.doi.org/10.1111/j.1399-3062.2011.00632.x
- Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, Herrera MI, Reynolds CG, Alyea EP, Ho VT, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012; 18:565-74; PMID:21875503; http://dx.doi.org/10.1016/j.bbmt.2011.08.018
- Somers JA, Brand A, van Hensbergen Y, Mulder A, Oudshoorn M, Sintnicolaas K, Gratama JW, Falkenburg JH, Braakman E, Cornelissen JJ. Double umbilical cord blood transplantation: a study of early engraftment kinetics in leukocyte subsets using HLA-specific monoclonal antibodies. Biol Blood Marrow Transplant 2013; 19:266-73; PMID:23041604; http://dx.doi.org/10.1016/j.bbmt.2012.09.022
- Saliba RM, Rezvani K, Leen A, Jorgensen J, Shah N, Hosing C, Parmar S, Oran B, Olson A, Rondon G, et al. General and virus-specific immune cell reconstitution after double cord blood transplantation. Biol Blood Marrow Transplant 2015; 21(7):1284-90; PMID:25708219; http://dx.doi.org/10.1016/j.bbmt.2015.02.017
- Brahmi Z, Hommel-Berrey G, Smith F, Thomson B. NK cells recover early and mediate cytotoxicity via perforin/granzyme and Fas/FasL pathways in umbilical cord blood recipients. Hum Immunol 2001; 62:782-90; PMID:11476901; http://dx.doi.org/10.1016/S0198-8859(01)00275-0
- Hercend T, Takvorian T, Nowill A, Tantravahi R, Moingeon P, Anderson KC, Murray C, Bohuon C, Ythier A, Ritz J. Characterization of natural killer cells with antileukemia activity following allogeneic bone marrow transplantation. Blood 1986; 67:722-8; PMID:3081066
- Hokland M, Jacobsen N, Ellegaard J, Hokland P. Natural killer function following allogeneic bone marrow transplantation. Very early reemergence but strong dependence of cytomegalovirus infection. Transplantation 1988; 45:1080-4; PMID:2837847; http://dx.doi.org/10.1097/00007890-198806000-00016
- Saliba RM, Rezvani K, Leen A, Jorgensen J, Shah N, Hosing C, Parmar S, Oran B, Olson A, Rondon G, et al. General and virus-specific immune cell reconstitution after double cord blood transplantation. Biol Blood Marrow Transplant 2015; 21:1284-90; PMID:25708219; http://dx.doi.org/10.1016/j.bbmt.2015.02.017
- Petersen SL, Ryder LP, Bjork P, Madsen HO, Heilmann C, Jacobsen N, Sengelov H, Vindelov LL. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant 2003; 32:65-72; PMID:12815480; http://dx.doi.org/10.1038/sj.bmt.1704084
- Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front Immunol 2011; 2:88; PMID:22566877
- Adib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol 2014; 92:256-62; PMID:24366517; http://dx.doi.org/10.1038/icb.2013.99
- Lunemann S, Malone DF, Hengst J, Port K, Grabowski J, Deterding K, Markova A, Bremer B, Schlaphoff V, Cornberg M, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis 2014; 209:1362-73; PMID:24154737; http://dx.doi.org/10.1093/infdis/jit561
- Hall LJ, Murphy CT, Hurley G, Quinlan A, Shanahan F, Nally K, Melgar S. Natural killer cells protect against mucosal and systemic infection with the enteric pathogen Citrobacter rodentium. Infect Immun 2013; 81:460-9; PMID:23208605; http://dx.doi.org/10.1128/IAI.00953-12
- Han X, Fan Y, Wang S, Jiao L, Qiu H, Yang X. NK cells contribute to intracellular bacterial infection-mediated inhibition of allergic responses. J Immunol 2008; 180:4621-8; PMID:18354185; http://dx.doi.org/10.4049/jimmunol.180.7.4621
- Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev 2008; 129:223-30; PMID:18304606; http://dx.doi.org/10.1016/j.mad.2008.01.003
- Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med 2007; 204:3027-36; PMID:18025129; http://dx.doi.org/10.1084/jem.20070695
- Harshan KV, Gangadharam PR. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun 1991; 59:2818-21; PMID:1855997
- Katz P, Yeager H, Jr, Whalen G, Evans M, Swartz RP, Roecklein J. Natural killer cell-mediated lysis of Mycobacterium-avium complex-infected monocytes. J Clin Immunol 1990; 10:71-7; PMID:2312668; http://dx.doi.org/10.1007/BF00917500
- Blanchard DK, Stewart WE, 2nd, Klein TW, Friedman H, Djeu JY. Cytolytic activity of human peripheral blood leukocytes against Legionella pneumophila-infected monocytes: characterization of the effector cell and augmentation by interleukin 2. J Immunol 1987; 139:551-6; PMID:3496384
- Klimpel GR, Niesel DW, Klimpel KD. Natural cytotoxic effector cell activity against Shigella flexneri-infected HeLa cells. J Immunol 1986; 136:1081-6; PMID:3510252
- Bouzani M, Ok M, McCormick A, Ebel F, Kurzai O, Morton CO, Einsele H, Loeffler J. Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN-gamma release. J Immunol 2011; 187:1369-76; PMID:21697457; http://dx.doi.org/10.4049/jimmunol.1003593
- Schmidt S, Tramsen L, Hanisch M, Latge JP, Huenecke S, Koehl U, Lehrnbecher T. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J Infect Dis 2011; 203:430-5; PMID:21208932; http://dx.doi.org/10.1093/infdis/jiq062
- Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol 2006; 6:399-409; PMID:17088643; http://dx.doi.org/10.1097/ACI.0b013e3280106b65
- Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA, Witherspoon RP, Bensinger W, Flowers ME, Martin P, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 2001; 97:3380-9; PMID:11369627; http://dx.doi.org/10.1182/blood.V97.11.3380
- Alho AC, Kim HT, Chammas MJ, Reynolds CG, Matos TR, Forcade E, Whangbo J, Nikiforow S, Cutler CS, Koreth J, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood 2016 Feb 4;127(5):646-57; PMID:26670634; http://dx.doi.org/1182/blood-2015-10-672345
- Vitale C, Pitto A, Benvenuto F, Ponte M, Bellomo R, Frassoni F, Mingari MC, Bacigalupo A, Moretta L. Phenotypic and functional analysis of the HLA-class I-specific inhibitory receptors of natural killer cells isolated from peripheral blood of patients undergoing bone marrow transplantation from matched unrelated donors. Hematol J 2000; 1:136-44; PMID:11920181; http://dx.doi.org/10.1038/sj.thj.6200018
- Dokhelar MC, Wiels J, Lipinski M, Tetaud C, Devergie A, Gluckman E, Tursz T. Natural killer cell activity in human bone marrow recipients: early reappearance of peripheral natural killer activity in graft-versus-host disease. Transplantation 1981; 31:61-5; PMID:7015602; http://dx.doi.org/10.1097/00007890-198101000-00014
- Della Chiesa M, Falco M, Bertaina A, Muccio L, Alicata C, Frassoni F, Locatelli F, Moretta L, Moretta A. Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C−/− umbilical cord blood. J Immunol 2014; 192:1471-9; PMID:24442432; http://dx.doi.org/10.4049/jimmunol.1302053
- Jonjic S, Babic M, Polic B, Krmpotic A. Immune evasion of natural killer cells by viruses. Curr Opin Immunol 2008; 20:30-8; PMID:18206359; http://dx.doi.org/10.1016/j.coi.2007.11.002
- Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol 2015; 15:149-59; PMID:25677494; http://dx.doi.org/10.1038/nri3802
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol 2009; 9:15-27; PMID:19079135; http://dx.doi.org/10.1038/nri2454
- Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood 2005; 105:4390-8; PMID:15701725; http://dx.doi.org/10.1182/blood-2004-11-4284
- Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 1998; 188:1679-89; PMID:9802980; http://dx.doi.org/10.1084/jem.188.9.1679
- Storek J, Ferrara S, Ku N, Giorgi JV, Champlin RE, Saxon A. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone Marrow Transplant 1993; 12:387-98; PMID:8275039
- Small TN, Keever CA, Weiner-Fedus S, Heller G, O'Reilly RJ, Flomenberg N. B-cell differentiation following autologous, conventional, or T-cell depleted bone marrow transplantation: a recapitulation of normal B-cell ontogeny. Blood 1990; 76:1647-56; PMID:1698484
- Burns DM, Tierney R, Shannon-Lowe C, Croudace J, Inman C, Abbotts B, Nagra S, Fox CP, Chaganti S, Craddock CF, et al. Memory B cell reconstitution following allogeneic haematopoietic stem cell transplantation is an EBV-associated transformation event. Blood 2015; 126(25):2665-75; PMID:26450987; http://dx.doi.org/10.1182/blood-2015-08-665000
- Marie-Cardine A, Divay F, Dutot I, Green A, Perdrix A, Boyer O, Contentin N, Tilly H, Tron F, Vannier JP, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol 2008; 127:14-25; PMID:18191619; http://dx.doi.org/10.1016/j.clim.2007.11.013
- Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood 1996; 88:2775-9; PMID:8839875
- Gale RP, Opelz G, Mickey MR, Graze PR, Saxon A. Immunodeficiency following allogeneic bone marrow transplantation. Transplant Proc 1978; 10:223-7; PMID:147534
- Elfenbein GJ, Anderson PN, Humphrey RL, Mullins GM, Sensenbrenner LL, Wands JR, Santos GW. Immune system reconstitution following allogeneic bone marrow transplantation in man: a multiparameter analysis. Transplant Proc 1976; 8:641-6; PMID:136774
- Storb R, Ochs HD, Weiden PL, Thomas ED. Immunologic reactivity in marrow graft recipients. Transplant Proc 1976; 8:637-9; PMID:793117
- Noel DR, Witherspoon RP, Storb R, Atkinson K, Doney K, Mickelson EM, Ochs HD, Warren RP, Weiden PL, Thomas ED. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood 1978; 51:1087-105; PMID:25684
- Linch DC, Knott LJ, Thomas RM, Harper P, Goldstone AH, Davis EG, Levinski RJ. T cell regeneration after allogeneic and autologous bone marrow transplantation. Br J Haematol 1983; 53:451-8; PMID:6218816; http://dx.doi.org/10.1111/j.1365-2141.1983.tb02046.x
- Ueda M, Harada M, Shiobara S, Nakao S, Kondo K, Odaka K, Matsue K, Mori T, Hattori K. T lymphocyte reconstitution in long-term survivors after allogeneic and autologous marrow transplantation. Transplantation 1984; 37:552-6; PMID:6427996; http://dx.doi.org/10.1097/00007890-198406000-00005
- Lum LG. The kinetics of immune reconstitution after human marrow transplantation. Blood 1987; 69:369-80; PMID:3542077
- Storek J, Saxon A. Reconstitution of B cell immunity following bone marrow transplantation. Bone Marrow Transplant 1992; 9:395-408; PMID:1628122
- Gerritsen EJ, van Tol MJ, Lankester AC, van der Weijden-Ragas CP, Jol-van der Zijde CM, Oudeman-Gruber NJ, Radl J, Vossen JM. Immunoglobulin levels and monoclonal gammopathies in children after bone marrow transplantation. Blood 1993; 82:3493-502; PMID:8241517
- Matsue K, Lum LG, Witherspoon RP, Storb R. Proliferative and differentiative responses of B cells from human marrow graft recipients to T cell-derived factors. Blood 1987; 69:308-15; PMID:3539229
- Perreault C, Giasson M, Gyger M, Belanger R, David M, Bonny Y, Boileau J, Barcelo R, Moquin JP. Serum immunoglobulin levels following allogeneic bone marrow transplantation. Blut 1985; 51:137-42; PMID:3896351; http://dx.doi.org/10.1007/BF00320027
- Norhagen G, Engstrom PE, Bjorkstrand B, Hammarstrom L, Smith CI, Ringden O. Salivary and serum immunoglobulins in recipients of transplanted allogeneic and autologous bone marrow. Bone Marrow Transplant 1994; 14:229-34; PMID:7994237
- Aucouturier P, Barra A, Intrator L, Cordonnier C, Schulz D, Duarte F, Vernant JP, Preud'homme JL. Long lasting IgG subclass and antibacterial polysaccharide antibody deficiency after allogeneic bone marrow transplantation. Blood 1987; 70:779-85; PMID:3304461
- Umetsu DT, Ambrosino DM, Quinti I, Siber GR, Geha RS. Recurrent sinopulmonary infection and impaired antibody response to bacterial capsular polysaccharide antigen in children with selective IgG-subclass deficiency. N Engl J Med 1985; 313:1247-51; PMID:3877240; http://dx.doi.org/10.1056/NEJM198511143132002
- Witherspoon RP, Storb R, Ochs HD, Fluornoy N, Kopecky KJ, Sullivan KM, Deeg JH, Sosa R, Noel DR, Atkinson K, et al. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood 1981; 58:360-8; PMID:6454452
- Martinez C, Urbano-Ispizua A, Rozman C, Marin P, Rovira M, Sierra J, Montfort N, Carreras E, Montserrat E. Immune reconstitution following allogeneic peripheral blood progenitor cell transplantation: comparison of recipients of positive CD34+ selected grafts with recipients of unmanipulated grafts. Exp Hematol 1999; 27:561-8; PMID:10089920; http://dx.doi.org/10.1016/S0301-472X(98)00029-0
- van Tol MJ, Gerritsen EJ, de Lange GG, van Leeuwen AM, Jol-van der Zijde CM, Oudeman-Gruber NJ, de Vries E, Radl J, Vossen JM. The origin of IgG production and homogeneous IgG components after allogeneic bone marrow transplantation. Blood 1996; 87:818-26; PMID:8555508
- Parkkali T, Ruutu T, Stenvik M, Kuronen T, Kayhty H, Hovi T, Olander RM, Volin L, Ruutu P. Loss of protective immunity to polio, diphtheria and Haemophilus influenzae type b after allogeneic bone marrow transplantation. APMIS 1996; 104:383-8; PMID:8703445; http://dx.doi.org/10.1111/j.1699-0463.1996.tb00731.x
- Gandhi MK, Egner W, Sizer L, Inman I, Zambon M, Craig JI, Marcus RE. Antibody responses to vaccinations given within the first two years after transplant are similar between autologous peripheral blood stem cell and bone marrow transplant recipients. Bone Marrow Transplant 2001; 28:775-81; PMID:11781630; http://dx.doi.org/10.1038/sj.bmt.1703239
- Pirofski LA, Casadevall A. Use of licensed vaccines for active immunization of the immunocompromised host. Clin Microbiol Rev 1998; 11:1-26; PMID:9457426
- Avigan D, Pirofski LA, Lazarus HM. Vaccination against infectious disease following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001; 7:171-83; PMID:11302551; http://dx.doi.org/10.1053/bbmt.2001.v7.pm11302551
- Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44-100; PMID:24311479; http://dx.doi.org/10.1093/cid/cit684
- CDC. Centers for Disease Control and Prevention. Vaccination of hematopoietic stem cell transplant (HSCT) recipients. 2011
- Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 2000; 290:92-7; PMID:11021806; http://dx.doi.org/10.1126/science.290.5489.92
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745-63; PMID:15032595; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104702
- Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol 1996; 156:4609-16; PMID:8648103
- Mackall CL, Hakim FT, Gress RE. T-cell regeneration: all repertoires are not created equal. Immunol Today 1997; 18:245-51; PMID:9153957; http://dx.doi.org/10.1016/S0167-5699(97)81664-7
- Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, Carmagnat M, Rocha V, Charron D, Socie G, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood 2009; 113:6477-84; PMID:19258596; http://dx.doi.org/10.1182/blood-2008-09-176594
- Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Magrath IT, Wexler LH, Dimitrov DS, Gress RE. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 1997; 89:3700-7; PMID:9160675
- Mackall CL, Granger L, Sheard MA, Cepeda R, Gress RE. T-cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood 1993; 82:2585-94; PMID:7691265
- Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995; 332:143-9; PMID:7800006; http://dx.doi.org/10.1056/NEJM199501193320303
- Fallen PR, Duarte RF, McGreavey L, Potter M, Ethell M, Prentice HG, Madrigal JA, Travers PJ. Identification of non-naive CD4+CD45RA+ T cell subsets in adult allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant 2003; 32:609-16; PMID:12953134; http://dx.doi.org/10.1038/sj.bmt.1704185
- Forman SJ, Nocker P, Gallagher M, Zaia J, Wright C, Bolen J, Mills B, Hecht T. Pattern of T cell reconstitution following allogeneic bone marrow transplantation for acute hematological malignancy. Transplantation 1982; 34:96-8; PMID:6753269; http://dx.doi.org/10.1097/00007890-198208000-00007
- Storek J, Witherspoon RP, Maloney DG, Chauncey TR, Storb R. Improved reconstitution of CD4 T cells and B cells but worsened reconstitution of serum IgG levels after allogeneic transplantation of blood stem cells instead of marrow. Blood 1997; 89:3891-3; PMID:9160701
- Garderet L, Dulphy N, Douay C, Chalumeau N, Schaeffer V, Zilber MT, Lim A, Even J, Mooney N, Gelin C, et al. The umbilical cord blood alphabeta T-cell repertoire: characteristics of a polyclonal and naive but completely formed repertoire. Blood 1998; 91:340-6; PMID:9414303
- Moretta A, Maccario R, Fagioli F, Giraldi E, Busca A, Montagna D, Miniero R, Comoli P, Giorgiani G, Zecca M, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol 2001; 29:371-9; PMID:11274766; http://dx.doi.org/10.1016/S0301-472X(00)00667-6
- Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 2010; 47:65-77; PMID:20066512; http://dx.doi.org/10.1007/s12026-009-8139-0
- Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE, Gandolfi S, Tentorio P, Sarina B, Timofeeva I, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood 2015; 125:2855-64; PMID:25742699; http://dx.doi.org/10.1182/blood-2014-11-608406
- Cieri N, Oliveira G, Greco R, Forcato M, Taccioli C, Cianciotti B, Valtolina V, Noviello M, Vago L, Bondanza A, et al. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood 2015; 125:2865-74; PMID:25736310; http://dx.doi.org/10.1182/blood-2014-11-608539
- Kneba M, Bolz I, Linke B, Bertram J, Rothaupt D, Hiddemann W. Characterization of clone-specific rearrangement T-cell receptor gamma-chain genes in lymphomas and leukemias by the polymerase chain reaction and DNA sequencing. Blood 1994; 84:574-81; PMID:8025283
- van den Beemd R, Boor PP, van Lochem EG, Hop WC, Langerak AW, Wolvers-Tettero IL, Hooijkaas H, van Dongen JJ. Flow cytometric analysis of the Vbeta repertoire in healthy controls. Cytometry 2000; 40:336-45; PMID:10918284; http://dx.doi.org/10.1002/1097-0320(20000801)40:4%3c336::AID-CYTO9%3e3.0.CO;2-0
- Gorski J, Yassai M, Zhu X, Kissela B, Kissella B, Keever C, Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol 1994; 152:5109-19; PMID:8176227
- Yew PY, Alachkar H, Yamaguchi R, Kiyotani K, Fang H, Yap KL, Liu HT, Wickrema A, Artz A, van Besien K, et al. Quantitative characterization of T-cell repertoire in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2015; 50:1227-34; PMID:26052909; http://dx.doi.org/10.1038/bmt.2015.133
- van Heijst JW, Ceberio I, Lipuma LB, Samilo DW, Wasilewski GD, Gonzales AM, Nieves JL, van den Brink MR, Perales MA, Pamer EG. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med 2013; 19:372-7; PMID:23435170; http://dx.doi.org/10.1038/nm.3100
- Du JW, Gu JY, Liu J, Cen XN, Zhang Y, Ou Y, Chu B, Zhu P. TCR spectratyping revealed T lymphocytes associated with graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma 2007; 48:1618-27; PMID:17701594; http://dx.doi.org/10.1080/10428190701474357
- Liu C, He M, Rooney B, Kepler TB, Chao NJ. Longitudinal analysis of T-cell receptor variable β chain repertoire in patients with acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2006; 12:335-45; PMID:16503503; http://dx.doi.org/10.1016/j.bbmt.2005.09.019
- Ozdemir E, St John LS, Gillespie G, Rowland-Jones S, Champlin RE, Molldrem JJ, Komanduri KV. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 2002; 100:3690-7; PMID:12393402; http://dx.doi.org/10.1182/blood-2002-05-1387
- de Oliveira LL, Coltri KC, Cardoso CR, Roque-Barreira MC, Panunto-Castelo A. T helper 1-inducing adjuvant protects against experimental paracoccidioidomycosis. PLoS Negl Trop Dis 2008; 2:e183; PMID:18335066; http://dx.doi.org/10.1371/journal.pntd.0000183
- Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol 2009; 175:2489-500; PMID:19893050; http://dx.doi.org/10.2353/ajpath.2009.090530
- Carvalho A, De Luca A, Bozza S, Cunha C, D'Angelo C, Moretti S, Perruccio K, Iannitti RG, Fallarino F, Pierini A, et al. TLR3 essentially promotes protective class I-restricted memory CD8(+) T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 2012; 119:967-77; PMID:22147891; http://dx.doi.org/10.1182/blood-2011-06-362582
- Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, Loeffler J, Monod M, Latge JP, Einsele H. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood 2002; 100:4521-8; PMID:12393638; http://dx.doi.org/10.1182/blood-2002-01-0265
- Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997; 175:1459-66; PMID:9180187; http://dx.doi.org/10.1086/516480
- Gratama JW, Brooimans RA, van der Holt B, Sintnicolaas K, van Doornum G, Niesters HG, Lowenberg B, Cornelissen JJ. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytometry B Clin Cytom 2008; 74:211-20; PMID:18454493; http://dx.doi.org/10.1002/cyto.b.20420
- Lilleri D, Gerna G, Fornara C, Lozza L, Maccario R, Locatelli F. Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplants. Blood 2006; 108:1406-12; PMID:16614242; http://dx.doi.org/10.1182/blood-2005-11-012864
- Lamba R, Carrum G, Myers GD, Bollard CM, Krance RA, Heslop HE, Brenner MK, Popat U. Cytomegalovirus (CMV) infections and CMV-specific cellular immune reconstitution following reduced intensity conditioning allogeneic stem cell transplantation with Alemtuzumab. Bone Marrow Transplant 2005; 36:797-802; PMID:16151431; http://dx.doi.org/10.1038/sj.bmt.1705121
- Tey SK, Kennedy GA, Cromer D, Davenport MP, Walker S, Jones LI, Crough T, Durrant ST, Morton JA, Butler JP, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV(R) assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One 2013; 8:e74744; PMID:24146744; http://dx.doi.org/10.1371/journal.pone.0074744
- Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant 2007; 13:1106-15; PMID:17697973; http://dx.doi.org/10.1016/j.bbmt.2007.06.006
- Keever CA, Small TN, Flomenberg N, Heller G, Pekle K, Black P, Pecora A, Gillio A, Kernan NA, O'Reilly RJ. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood 1989; 73:1340-50; PMID:2649174
- Oshrine BR, Li Y, Teachey DT, Heimall J, Barrett DM, Bunin N. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies: comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood. Biol Blood Marrow Transplant 2013; 19:1581-9; PMID:23939199; http://dx.doi.org/10.1016/j.bbmt.2013.08.003
- Chae YS, Sohn SK, Kim JG, Cho YY, Moon JH, Yang DH, Lee JJ, Kim YK, Kim HJ, Shin HJ, et al. Impact of alemtuzumab as conditioning regimen component on transplantation outcomes in case of CMV-seropositive recipients and donors. Am J Hematol 2008; 83:649-53; PMID:18528826; http://dx.doi.org/10.1002/ajh.21215
- Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P, Chakraverty R, Marshall T, Osman H, Mahendra P, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood 2002; 99:4357-63; PMID:12036862; http://dx.doi.org/10.1182/blood.V99.12.4357
- Bernstein ID, Boyd RL, van den Brink MR. Clinical strategies to enhance posttransplant immune reconstitution. Biol Blood Marrow Transplant 2008; 14:94-9; PMID:18162228; http://dx.doi.org/10.1016/j.bbmt.2007.10.003
- Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood 2010; 115:3861-8; PMID:20215642; http://dx.doi.org/10.1182/blood-2009-12-234096
- Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med 2006; 12:1039-47; PMID:16936725; http://dx.doi.org/10.1038/nm1463
- Rice HE, Skinner MA, Mahaffey SM, Oldham KT, Ing RJ, Hale LP, Markert ML. Thymic transplantation for complete DiGeorge syndrome: medical and surgical considerations. J Pediatr Surg 2004; 39:1607-15; PMID:15547821; http://dx.doi.org/10.1016/j.jpedsurg.2004.07.020
- Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol 2010; 135:236-46; PMID:20236866; http://dx.doi.org/10.1016/j.clim.2010.02.007
- Barrett AJ, Bollard CM. The coming of age of adoptive T-cell therapy for viral infection after stem cell transplantation. Ann Transl Med 2015; 3:62; PMID:25992361