ABSTRACT
Carbapenem-Resistant Enterobacteriaceae (CRE) are an emerging healthcare crisis. Infections due to CRE are associated with high morbidity and mortality, especially in critically ill patients. Due to the multi-drug resistant nature of these infections only limited treatment options are available. Antimicrobials that have been described for the treatment of CRE infections include carbapenems, polymyxins, fosfomycin, tigecycline, aminoglycosides, and ceftazidime-avibactam. Given the limited treatment options it is imperative the pharmacokinetic and pharmacodynamics (PK-PD) characteristics of these agents are considered to optimize treatment regimens. This review will focus on the PK-PD challenges of the current treatment options for CRE infections.
Carbapenem-Resistant Enterobacteriaceae (CRE) are an emerging healthcare crisis. The US. Centers for Disease Control estimates 9,000 infections per year are due to CRE and has categorized it as an urgent public health threat.Citation1 Globally, the emergence of carbapenemases including class A KPC type, class B metallo-β-lactamases (examples include VIM, IMP and NDM types), and class D OXA types continue to disseminate in Enterobacteriaceae and cause serious disease with high morbidity and mortality.Citation2
CRE are resistant to the majority of antimicrobials including almost all β-lactams leaving limited treatment options for patients with infections due to CRE. Agents often used in the treatment of CRE infections include historic antibiotics with toxicity concerns, pharmacokinetic-pharmacodynamic (PK-PD) parameters which are sometimes poorly defined or challenging to achieve, or limited data regarding clinical efficacy. Specific antimicrobials investigated for CRE infections include carbapenems, polymyxins, fosfomycin, tigecycline, aminoglycosides, and ceftazidime/avibactam. Treatment selection is also complicated by the variety of types of serious infections CRE pathogens can cause including septic shock, pneumonia, intra-abdominal infections, or urinary tract infections requiring drugs with different PK properties. In addition, it is estimated that 42–72% of patients with CRE bloodstream infections are in the intensive care unit which presents additional PK-PD challenges.Citation3-6 This review will focus on the PK-PD challenges of the current treatment options for CRE infections.
PK-PD challenges in critically Ill
Critically ill patients can have a variety of pathophysiologic changes which can complicate drug, and specifically antimicrobial, dosing. Alterations in antimicrobial volume of distribution (Vd) and clearance (Cl) are often observed in critically ill patients and may change antimicrobial concentrations at infected sites, depending on the degree of lipophilicity and renal elimination. This may in turn affect the pharmacodynamic parameters associated with antimicrobial activity.
Antimicrobial activity is generally described by one of three PK-PD parameters ().Citation7 The time the antimicrobial concentration remains above the minimum inhibitory concentration (MIC) during the dosing interval (T > MIC) is the parameter best correlated with efficacy for β-lactams including carbapenems and ceftazidime. The ratio of the maximum serum concentration to the MIC (Cmax/MIC) correlates with efficacy for many concentration-dependent antimicrobials including the aminoglycosides. Finally, the ratio of area under the curve (AUC) during a 24 hour period to MIC (AUC/MIC) is the marker associated with efficacy for a number of different antimicrobials including tigecycline, polymyxins, and fosfomycin.
Table 1. Optimal PK-PD parameter for CRE treatment options.
An understanding of both the pathophysiological changes common in critical illness that may cause altered pharmacokinetics as well as the pharmacodynamic characteristics of antimicrobials is essential to dose optimization for this patient population. This can be especially important in the treatment of CRE infections for which there is a limited armamentarium and high mortality. Hydrophilic antibiotics like carbapenems, aminoglycosides, and polymyxins generally have low Vd, low intracellular penetration, and are dependent on renal clearance. In critically ill patients with sepsis, fluid shifts to the extravascular space from capillary leak syndrome increasing the Vd of hydrophilic drugs which in turn may decrease the serum drug concentrations.Citation8 The presence of mechanical ventilation, hypoalbuminemia, extracorporeal circuits, postsurgical drains, or burn injuries can all further increase the Vd of hydrophilic drugs.Citation8 The use of higher loading doses may be considered to overcome these pharmacokinetic changes and optimize serum concentrations. The impact of hypoalbuminemia on achievement of PK-PD targets can be especially complex. For example, a decrease in percentage of protein bound drug increases free or active drug which may increase maximum serum concentrations but also increases the amount of drug available for tissue distribution and clearance.Citation9 In addition, hydrophilic antibiotics are likely to be highly renally eliminated and therefore subject to the increased cardiac output and renal perfusion that can be seen in critical illness in the absence of end-organ damage which increases Cl and reduces elimination half-lives.Citation8 The use of extended or continuous infusion antibiotics is a strategy often employed for β-lactam antibiotics to minimize the influence of augmented clearance and maximize the PK-PD parameter T > MIC. Conversely, lipophilic antimicrobials like tigecycline, typically have large Vd and minimal renal elimination and remain largely unaffected by the pathophysiological changes of critical illness. End-organ damage is common in septic shock and renal or hepatic dysfunction will decrease the clearance and increase the plasma concentrations of drugs eliminated by those routes.
Treatment options for CRE infections
Current treatment options for infections due to CRE with a focus on PK-PD considerations will be reviewed.
Carbapenems
It may sound like anathema to consider using carbapenems for CRE infections, though there are situations where they may still be useful. To improve detection and treatment of carbapenemase-producing organisms, breakpoints for antipseudomonal carbapenems for Enterobacteriaceae were lowered by the Clinical Laboratory Standards Institute (CLSI) in 2010 from 4 to 1 mg/mL (ertapenem was changed from 2 to 0.5 mg/mL); breakpoints for antipseudomonal carbapenems against Pseudomonas aeruginosa were set at 2 mg/mL.Citation10,11 Select clinical outcome studies support these changes, as higher mortality rates have been seen in infections with elevated MICs, including in the previous susceptible range.Citation12 However, carbapenems may still have utility in CRE infections.
Prolonged infusions of carbapenems
Like many classes of antimicrobials, dosing strategies that maximize the pharmacodynamics of most carbapenems were not originally employed. With the knowledge that carbapenems exert time-dependent activity that is increased by maximizing the time that the free concentration of drug is above the MIC of the organism (fT>MIC), extended- and continuous-infusion dosing strategies for carbapenems have been developed, particularly for meropenem.Citation13-16 Pharmacokinetically-optimized dosing regimens of meropenem 2 g IV over 3 hours every 8 hours can achieve a high probability of target attainment (fT > MIC of >50 %) for organisms with MICs ranging from 2–8 mg/mL.Citation15 Continuous infusions of meropenem have also been utilized to achieve a high probability of pharmacodynamic target attaintment.Citation16 Doripenem at a simulated dose of 1 or 2 g IV over 3 hours every 8 hours was able to achieve bacteriostasis in neutropenic mice infected with KPC-producing K. pneumoniae with MICs of 8 mg/mL and 16 mg/mL, respectively, and was able to achieve a 1-log kill in immunocompetant animals infected with the same strains.Citation17 It should be noted that imipenem is not suitable for prolonged infusions due to its lower stability in solution and increased CNS toxicity at higher doses;Citation16 ertapenem has both unfavorable pharmacokinetics and is the most susceptible of the agents to carbapenemases.Citation18 The “right” scenarios to employ prolonged-infusion carbapenems in CRE infections are not clear, but data suggests that they may be useful in CRE infections with MICs of 4 mg/mL or less and possibly 8 mg/mL when given with another active agent.Citation14,19 Data from a large multicenter study in Italy supports this latter scenario, where extended-infusion meropenem administered in combination with another agent significantly lowered mortality in KPC-producing K. pneumoniae infections when MICs were 8 mg/mL or lower.Citation19 Combinations of prolonged-infusion carbapenems with other active agents appear to be useful regimens for CRE infections, though the identification of optimal regimens and ability to test their efficacy in vitro to predict the successful treatment of patients remain obstacles.
Double-carbapenem therapy
Data suggest that KPC enzymes have a greater affinity for ertapenem than other carbapenems.Citation18 In vitro and animal models show than when given together with another carbapenem to a KPC-producing isolate, it appears that the preferential destruction of ertapenem KPC enzymes enhances the activity of a concomitantly administered carbapenem.Citation20 This has led to the use of double-carbapenem therapy for CRE infections caused by KPC-producing organisms, where ertapenem is given as a suicide substrate to enhance the activity of an antipseudomonal carbapenem. Currently clinical experience is limited to a few case reports and case series,Citation21-23 but the synergy between carbapenem combinations warrants further study particularly in situations where other agents either cannot be utilized or can be used in combination.
Polymyxins
Polymyxin pharmacokinetics and pharmacodynamics have been sub-optimally characterized for years. Modern pharmacokinetic studies of both colistin and polymyxin B have shown that the dosing recommendations for both drugs in their package inserts are antiquated and inaccurate.Citation24-27 A discussion of their utility for CRE infections requires mention of the lack of availability of polymyxin B in many parts of the world, which is problematic since of the 2 agents it is the one with more predictable pharmacokinetics.Citation26,28 Some countries such as Japan lack access to either polymyxin agent.
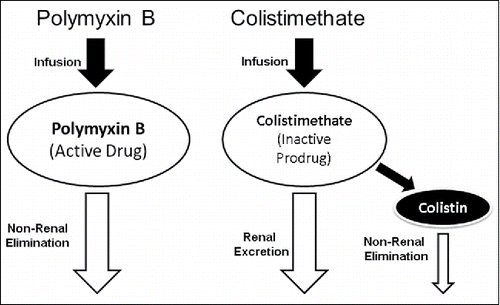
Colistin is administered intravenously as the prodrug colistimethate sodium (CMS), which is converted to colistin in vivo and in vitro in aqueous solutions such as plasma and urine.Citation27,28 This aspect leads to several difficulties with the compound. Measuring colistin concentrations is difficult and not commonly done in clinical practice, in part because CMS can convert to colistin spontaneously after collection. This also makes interpreting literature on colistin pharmacokinetics difficult since older studies using bioassays frequently have invalid results.Citation25,29 CMS itself is eliminated renally, which makes renal function a significant factor that determines the plasma concentrations of colistin.Citation24,25,27,28,30 diagrams the path from administration to elimination of the available polymyxin compounds. This conversion is highly variable between patients and makes prediction of colistin concentrations difficult.Citation24,28,31 In many countries including the US, CMS is labeled in terms of “colistin base activity” (CBA), where 80 mg of CMS is equal to approximately 30 mg of CBA, a conversion factor of about 2.7.Citation25,32,33 It is notable that this is essentially a “possible amount” of active colistin that may be achieved in vivo, since the proportion of CMS that is actually converted to colistin in vitro differs considerably between patients as they eliminate the prodrug.Citation24,25,28,29 In other parts of the world, colistin is dosed in terms of international units (IU), whereas 1 million IUs = 30 mg of CBA. These varied descriptions have led to confusion in both the clinic and the literature. Clinicians should use whichever dosing description is best understood in their country, but the use of mg of CMS should be avoided since this can lead to potentially fatal dosing errors.Citation33
CMS is efficiently removed by renal elimination via both glomerular filtration and tubular secretion.Citation24,25,27,29 Perhaps counterintuitively, high degrees of renal function are problematic with colistin use since achieving therapeutic concentrations of colistin is difficult, particularly if the patient is infected with an organism with an elevated MIC.Citation24 A commonly-used dosing recommendation equation for intravenous colistin warns against its own use for patients with a creatinine clearance (CrCl) >70 ml/min since very high doses of drug will be calculated, even though those doses may be necessary for isolates with MICs of 1 or 2 mg/mL.Citation24 Since renally-eliminated CMS is converted to colistin within the bladder, it has utility for urinary tract infections.Citation28,29
Polymyxin B is less studied, but has more predictable pharmacokinetics since it is not administered as a prodrug. It is eliminated through non-renal means, achieves low urinary concentrations, and is likely insufficient for the treatment of urinary tract infections.Citation26-28 Some recent studies have suggested that polymyxin B has less nephrotoxicity than colistin, but it is not known if the low renal elimination of polymyxin B is the reason.Citation34-36
Colistin and polymyxin B have both been subject to modern pharmacokinetic studies, though data on polymyxin B is more limited. Loading doses are recommended for both agents and are particularly important for colistin, since the achievement of a therapeutically useful concentration in the bloodstream may take 2–3 d without one.Citation26,31,37 Dosing strategies for colistin that have been derived from modern pharmacokinetic studies recommend a loading dose, followed by a fractionated daily dose that frequently exceeds the approved maximum daily dose of 300 mg of CBA (10 million IUs) that is in the US package insert.Citation24,38 Pharmacokinetic studies of polymyxin B have found that elimination is not related to renal function. In contrast with the dosing recommendations in the FDA-approved dosing regimen, these studies have found that dose adjustment in renal dysfunction is not warranted on the basis of pharmacokinetics, though nephrotoxicity is a concern.Citation26,39
Recently, both EMA and FDA have updated dosing regimens for intravenous colistimethate. To evaluate these regimens, Nation and colleagues used pharmacokinetic data from 162 adult critically ill patients in a previous study of colistin pharmacokinetics determined average colistin steady-state plasma concentrations (Css,avg) that would be achieved if each patient received FDA and EMA doses. Patient were grouped by cohorts based upon their renal function. Substantial variability was seen among the regimens in their ability to achieve targeted concentrations. In general, EMA dosing more reliably achieved targets, particularly for patients with CrCl <30 mL/min or when targeted Css,avg was ≥2 mg/L. Notably, neither set of dose recommendations reliably achieved a Css,avg ≥ 1 mg/L in the cohort of patients with CrCl Css,avg ≥ 80 mL/min, reinforcing that colistimethate is cleared too quickly in patients with good renal function for high amounts of colistin to be formed.
Tissue distribution of polymyxins is not well-studied. Since colistin is frequently used for the treatment of pneumonia, it is notable that the scant data on colistin lung concentrations is contradictory. One study that evaluated colistin concentrations in bronchoalveolar lavage fluid (BALF) did not find any detectable drug in any of 13 patients receiving a dose of 2 million units every 8 hours (approximately 60 mg of CBA).Citation40 A report of 2 patients who received the equivalent of approximately 83 mg of CBA 3 times daily found BALF concentrations 0.36 and 0.42 mg/mL 1.5 and 4 hours after infusions completed, respectively. Concentrations in alveolar lining fluid that were calculated from these values were 4.84 and 25.82 mg/mL, which would be above the MICs of pathogens considered colistin-susceptible.Citation41 While there is a history of use of intravenous colistin for pulmonary infections, these conflicting data raise the question of its suitability as monotherapy in pneumonia. A meta-analysis comparing patients with pneumonia treated with CMS to those treated with other agents did not show a difference in outcomes, though the studies included were mostly small, single-center retrospective studies with many confounders.Citation42 A methodology that could compensate for low pulmonary concentrations with intravenous CMS is the administration of aerosolized drug, and one meta-analysis showed improved outcomes when aerosolized CMS was added to intravenous CMS.Citation43 Though the designs of the included studies were again suboptimal, this therapeutic modality is of considerable interest given that the high concentrations that can be achieved in alveoli.Citation44,45
Limited data exists examining the effects of continuous renal replacement therapy (CRRT) for polymyxins. Recent data shows that colistin is efficiently removed by CRRT and that doses similar to those used in normal renal function are likely necessary.Citation24,46,47 A dose recommendation of 192 mg of CBA daily for each 1 mg/L of desired Css,avg was recommended by Garonzik and colleagues in their extensive study of colistin PK-PD, but it was based on 4 patients.Citation24 Another study of 3 patients receiving CRRT reported substantial clearance of colistin by CRRT, though total clearance was somewhat reduced.Citation47 A report of polymyxin B in 2 patients receiving CRRT showed that it was minimally removed and suggested that it should probably not be adjusted for extracorporeal elimination, though data is not definitive.Citation48
Both polymyxins exhibit concentration-dependent killing and that the pharmacodynamic parameter that is most closely associated with efficacy is the free area-under-the-curve (AUC) to MIC ratio (fAUC/MIC).Citation27,29 With colistin, maximizing the fAUC/MIC through modification of dose fractionation is likely to be ineffective since the conversion of CMS to colistin is highly variable and concentration-time curves of the 2 entities are divergent. Breakpoints for colistin differ between agencies, ranging from 2–4 mg/mL depending on the agency and organism.Citation11,49 Only EUCAST publishes a breakpoint for colistin against Enterobacteriaceae, which is 2 mg/mL.Citation49 Achieving a colistin Css,avg of 2 mg/mL may not be possible for many patients, particularly for those with a creatinine clearance >70–80 mL/min.Citation24 Only CLSI publishes standards for polymyxin B, which are the same as for colistin.Citation11 Heteroresistance is possible among species of bacteria that polymyxins are used to treat, including Klebsiella pneumoniae.Citation27,50-52 The use of polymyxin therapy can amplify the heteroresistant population, which is not detectable with typical phenotypic susceptibility testing. This amplification can result in a clinically resistant isolate even when concentrations above the MIC of the tested isolate are achieved. To combat this resistance, some investigators have suggested treating patients with combinations of polymyxins and other agents.Citation51,53,54 The variety of agents that have shown in vitro synergy with polymyxins against polymyxin-resistant isolates is broad and includes carbapenems, rifamycins, and even glycopeptides, perhaps due to a fitness cost to the organism.Citation51,54-56 The question of which agent, if any, to use clinically remains undetermined.
Fosfomycin
Fosfomycin is a broad spectrum antibiotic that is being repurposed as an agent for multi-drug resistant (MDR) Gram-negative rod infections, including those caused by CRE. Data exist showing activity against some CRE, including KPC-producing and NDM-1-producing organisms.Citation57,58 It is a small molecule that is highly distributed throughout body tissues.Citation59-61 It has negligible protein binding (∼0%) and is cleared renally. Despite this route of elimination, limited information exists on the clearance of fosfomycin in renally-impaired patients; dosage adjustment of the intravenous formulation is recommended with a CrCl <50 mL/min.Citation62 As a small, hydrophilic molecule with low protein binding, fosfomycin is extensively removed during both intermittent and continuous hemodialysis.Citation60,61 The evidence-base for dosing in critically-ill patients is small, but available data indicate an increased volume of distribution that suggests a benefit of loading doses of intravenous fosfomycin.Citation60,61
Given orally, fosfomycin has a bioavailability of 30–40% and achieves very high concentrations in the urine in both renally sufficient patients and patients with mild renal insufficiency.Citation63,64 In the United States, fosfomycin is currently only available orally and this limits its use to urinary tract infections.Citation63 The CLSI breakpoint for Enterobacteriaceae is 64 mg/mL and is based on achievable concentrations in the urine with the FDA-approved 3 g oral dose.Citation11,63 The oral availability of fosfomycin makes it a useful treatment regimen for CRE cystitis, though limited cases have been published describing this scenario.Citation65-67 There is more evidence that fosfomycin is useful in UTIs caused by other highly resistant β-lactamase producing gram-negative bacilli, including ESBL-producing organisms.Citation68 The limited bioavailability makes giving oral fosfomycin problematic for systemic CRE infections.
Intravenous fosfomycin is available in many countries but not the United States.Citation63,69 This method of administration bypasses the limited bioavailability of oral formulations and allows for considerably higher concentrations in plasma and tissues. The breakpoint set by EUCAST for fosfomycin against Enterobacteriaceae is 32 mg/mL,Citation70 which is a concentration achievable in many body tissues including the lungs and interstitial spaces.Citation70-73 There is wide variation in the dosing regimens described in the limited reports of intravenous fosfomycin for CRE infections, ranging up to 24 g of drug daily divided 3 or 4 times a day.Citation74,75 Optimal pharmacodynamic targets for fosfomycin are unclear; historically it has been considered a time-dependent agent, but some recent pharmacodynamic studies with fosfomycin against E. coli have shown the fAUC/MIC ratio to be most predictive of efficacy.Citation76,77 The pharmacodynamics of fosfomycin may differ by species.Citation76-78 The emergence of resistance to fosfomycin has been demonstrated in vitro in studies in both Enterobacteriaceae and Pseudomonas species.Citation76-78 This resistance may be suppressed by combining fosfomycin with other agents.Citation76-80 Though it is generally well tolerated, it should be noted that the intravenous formulation (fosfomycin disodium) contains 330 mg of sodium per gram of fosfomycin, which could be problematic in patients with heart failure.Citation70 A study of IV fosfomycin for UTIs for approval in the United States is currently being conducted (NCT02753946).
Tigecycline
Tigecycline is a bacteriostatic glycylcycline that is derived from minocycline and is frequently active in vitro against CRE. In a study of CRE from 18 different European countries from 2010 to 2013, the MIC50 and MIC90 for tigecycline were 0.5 mg/ml and 2 mg/ml respectively.Citation81 The percentage of isolates susceptible depended on the antimicrobial susceptibility criteria used; 88.6% were susceptible by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) susceptibility breakpoint of ≤ 1 mg/ml versus 98.6% susceptible using the Food and Drug Administration susceptibility breakpoint criteria of ≤2 mcg/ml.Citation81
The pharmacokinetic properties of tigecycline include a large Vd (7–9 L/kg), high protein binding (73–79%), long half-life (t ½) of approximately 40 hours, minimal metabolism, and low total clearance (0.2–0.3 L/h/kg).Citation82 The maximum serum concentration after multiple doses of 50 mg every 12 hours was only 0.63 mg/ml, notably below both EUCAST and CLSI breakpoints for Enterobacteriaceae. Tigecycline penetrates well into blister fluid and abdominal tissue but low epithelial lining fluid to plasma ratios were observed in patients with pneumonia.Citation83,84 Tigecycline is primarily eliminated by biliary/fecal excretion and only minimal unchanged drug is excreted in the urine. The PK-PD parameter best correlated with efficacy of tigecycline is the AUC/MIC and data from the hospital acquired pneumonia clinical trials suggested a target fAUC0–24:MIC ratio ≥ 0.9 correlated with clinical efficacy but was dependent on albumin and VAP status.Citation85
Due to elimination pathway, tigecycline is a poor option for CRE infections in the urine. In a retrospective cohort of patients with CRE bacteriuria, microbiologic clearance rates for tigecycline (43%) were significantly lower than aminoglycosides (88%) and polymyxins (64%) and similar to untreated controls (36%).Citation72 In addition, in a cohort of patients with CRE bacteriuria treated with tigecycline, the use of tigecycline was significantly associated with the development of tigecycline resistance in the same patient (OR 6.13, 95% CI 1.15–48.65, p = 0.03).Citation86 There are also some PK-PD concerns regarding the role of tigecycline in bloodstream infections given the low serum concentrations that are achieved. In patients with CRE bacteremia mortality rates ranged from 40–80% for those treated with tigecycline monotherapy compared to 0–34% for those treated with tigecycline in combination.Citation3-6
Recent data have stimulated some concern regarding the clinical efficacy of tigecycline, perhaps driven by challenges with PK-PD target attainment.Citation87 Additionally, in 2013, the FDA issued a boxed warning for tigecycline based on data from meta-analysis of 13 clinical trials showing a higher risk of death among patients receiving tigecycline compared to other antimicrobial drugs.Citation88 The greatest increased risk was in patients with ventilator-associated pneumonia (VAP), a non-FDA approved indication but a common type of CRE infection. PK-PD analysis from a hospital-acquired pneumonia study showed the mean AUC observed in patients with VAP was 15% lower and the MIC50 and MIC90 were higher compared to patients without VAP and therefore the median fAUC/MIC ratios were only 1.73 and 4.39 for patients with VAP and HAP, respectively.Citation87 A subsequent phase 2 study in patients with HAP evaluating 2 higher dose regimens of tigecycline suggested better PK-PD target attainment and clinical efficacy compared to standard doses although the small number of patients enrolled limits the interpretation of the results.Citation89
Higher dose tigecycline regimens have been evaluated in a small number of cohorts or case series of patients with carbapenem-resistant infections. In a series of 30 patients with severe intra-abdominal infections due to CRE patients treated with high-dose tigecycline (200 mg × 1, followed by 100 mg Q12) plus colistin had a lower unadjusted ICU mortality rate of 8.3% compared to standard-dose tigecycline plus colistin (61.1%, p = 0.0005).Citation90 A retrospective cohort of patients with VAP due to MDR bacteria (most commonly KPC producing K. pneumoniae and carbapenem-resistant A. baumannii) showed high dose tigecycline was an independent predictor of clinical cure (OR 6.25, 95%CI 1.59–24.57) but had no impact on ICU mortality.Citation91 A third retrospective cohort of 44 CRE infections, mostly patients with pneumonia or urinary tract infections, demonstrated no mortality benefit from higher tigecycline doses in critically ill patients.Citation92 Limited data prohibit definitive conclusions on whether or not higher dose tigecycline can overcome the PK-PD challenges, especially in the critically ill.
Aminoglycosides
Aminoglycosides, including gentamicin, tobramycin, and amikacin, exhibit some in vitro activity against CRE; however the extent of activity varies by specific aminoglycoside and type of carbapenemase. Against a collection of 25 KPC producing K. pneumoniae isolates, amikacin demonstrated a MIC50 and MIC90 of 32 mg/ml (48% susceptible by the CLSI breakpoint ≤ 16 mg/ml), gentamicin a MIC50 and MIC90 of 8 mg/ml and 16 mg/ml (44% susceptible by the CLSI breakpoint ≤ 4 mg/ml), and tobramycin a MIC50 and MIC90 of 32 mg/ml and ≥ 64 mg/ml (8% susceptible by the CLSI breakpoint ≤ 4 mg/ml).Citation93 Among 65 CRE isolates with a variety of different carbapenemase resistance mechanisms other than NDM, 75% of isolates were susceptible to amikacin (by the EUCAST breakpoint ≤ 8 mg/ml), 60% of isolates were susceptible to gentamicin (by the EUCAST breakpoint ≤ 2 mg/ml), and only 38% of isolates were susceptible to tobramycin (by the EUCAST breakpoint ≤ 2 mg/ml).Citation94 In contrast, all 17 CRE isolates expressing NDM carbapenemase were resistant to amikacin, gentamicin, and tobramycin.Citation94
Aminoglycoside pharmacokinetic properties include a small Vd (0.2–0.3 L/kg), short t ½ of 2–3 hours, low protein binding, minimal metabolism, and clearance highly dependent on renal function.Citation95 Penetration into epithelial lining fluid ranges from 32–54% of serum concentrations.Citation95 Multiple studies in critically ill patients demonstrate that aminoglycosides have increased Vd resulting in decreased Cmax concentrations.Citation96,97 Other studies show critically ill patients with burn injury or mechanical ventilation can have increased Vd and/or longer half-lives.Citation98,99 In addition, the half-life and clearance of aminoglycosides are highly dependent on glomerular filtration rate, which can be extremely variable in critical illness. Due to the variability in pharmacokinetic parameters and narrow therapeutic window of these agents, therapeutic drug monitoring is recommended.
Aminoglycosides are concentration-dependent bactericidal agents and demonstrate prolonged post-antibiotic effects. The PK-PD parameter most correlated with clinical efficacy for gram-negative infections is the Cmax/MIC ratio and the target ratio is 8–10.Citation100 Achievement of this PK-PD target can be challenging in treating infections due to CRE due to the elevated MICs. For example, the CLSI breakpoint for amikacin is 16 mg/ml and the MIC90 for CRE isolates is often at the breakpoint or above.Citation93 Therefore to achieve the PK-PD target of a peak concentration 8–10 times the MIC, the goal peak concentration would be 128–160 mg/ml. The probability of achieving these peak concentrations is very low and they would likely be unsafe for many patients.
In clinical practice, the majority of the experience with the use of aminoglycosides for the treatment of CRE infections is as part of a combination regimen. In one systematic review of 20 clinical studies of patients with CRE infections, the combination of aminoglycosides with a carbapenem had the lowest mortality rates.Citation101 However, not all data for aminoglycosides for the treatment of CRE infections is with combination therapy. Aminoglycosides concentrate in the kidneys and urine and appear to be effective, as monotherapy, in the treatment of urinary tract infections due to CRE.Citation102 In a retrospective cohort of patients with CRE bacteriuria, microbiologic clearance rates were significantly higher for aminoglycosides (88%) compared to the polymyxin B cohort (64%), tigecycline cohort (43%), and untreated controls (36%).Citation102 The multivariate analysis confirmed treatment with an aminoglycoside with in vitro activity was independently associated with microbiological clearance.Citation102 To optimize concentrations at the site of infection, aminoglycosides can be aerosolized. The use of inhaled aminoglycosides for the treatment of hospital acquired pneumonia (HAP) is limited but a few small controlled trials in patients with CRE infections demonstrated improved response rates.Citation104,107 The Society of Infectious Diseases Pharmacists consensus summary on aerosolized antibiotics states adding aerosolized antibiotics, including aminoglycosides, to systemic antibiotics may be considered in the treatment of patients with MDR HAP, which would include infections due to CRE and may be a field of future evaluation.Citation104
Plazomicin (ACHN-490), a sisomicin synthetic derivative, is a potential future treatment option currently under clinical development. This novel aminoglycoside evades aminoglycoside-modifying enzymes and displays good in vitro activity against CRE isolates (MIC90 of 1 mg/ml against KPC-producing strains) except those harboring NDM enzymes.Citation93,94 A phase 3, multicenter, randomized study is underway evaluating plazomicin vs. colistin in combination with carbapenems or tigecycline for the treatment of bloodstream infections and pneumonia due to CRE pathogens (NCT01970371).
Ceftazidime-avibactam
Ceftazidime-avibactam is a new combination antimicrobial consisting of a third generation cephalosporin and a novel non-β-lactam β-lactamase inhibitor. Avibactam inhibits many serine β-lactamases by covalently binding and inhibiting the enzymes, however, unlike currently available β-lactam inhibitors, this bond is slowly reversible via deacylation allowing some recycling of avibactam.Citation105,106 Avibactam has activity against Ambler class A, C, and many class D β-lactamases including carbapenemases.Citation107-109 This activity expands the gram-negative activity of ceftazidime to include some CRE. Against a collection of 42 K. pneumoniae isolates producing KPC carbapenemases, avibactam was effective at restoring activity to ceftazidime for all strains (MIC90 = 1 mg/ml).Citation107 In addition, ceftazidime-avibactam was active in vitro against K. pneumoniae isolates producing class D OXA-48 carbapenemases (MIC90 ≤ 0.5 mg/ml).Citation108 The FDA susceptibility breakpoint for ceftazidime-avibactam is 8/4 mg/ml. Avibactam does not inactivate Ambler class B metallo-β-lactamases or many of the other class D OXA β-lactamases therefore ceftazidime-avibactam will not have useful activity against CRE harboring these enzymes.Citation109 In addition, the first case report of a ceftazidime-avibactam resistant KPC-3-expressing K. pneumoniae isolate was recently reported; however the exact mechanism of resistance has yet to be elucidated.Citation110
Pharmacokinetic properties of ceftazidime and avibactam are very similar. The protein binding is low (<10 % ceftazidime, 8% avibactam) and the Vd is relatively small (17 L for ceftazidime, 22 L for avibactam).Citation111 The half-life is approximately 2.7 hours for both compounds and they both undergo primary renal clearance as unchanged drug (83% ceftazidime, 97% avibactam).Citation112 Ceftazidime-avibactam penetrated into epithelial lining fluid with Cmax and AUC exposure approximately 30% of plasma for both components.Citation113 Like other β-lactam antibiotics, the PK-PD parameter best correlated with efficacy for ceftazidime-avibactam is the fT > MIC. A target fT > MIC of approximately 50% predicted favorable microbiological response for ceftazidime in patients with pneumonia due to gram-negative bacilli.Citation114 Monte Carlo simulations predict >98% probability of target attainment for ceftazidime-avibactam MICs ≤ 8 mg/ml using the standard dose of 2.5 g (2 g ceftazidime, 500 mg avibactam) infused over 2 hours every 8 hours.Citation111 In critically ill patients it would be expected, based on the hydrophilicity and PK profile, that ceftazidime-avibactam might have lower serum concentrations due to the larger Vd and/or altered renal clearance.Citation8 There is no data on whether more prolonged or extended infusions of ceftazidime-avibactam beyond the approved 2 hour infusion would improve the PK-PD profile or target attainment rate in this patient population.
Limited clinical data exist evaluating the activity ceftazidime-avibactam for the treatment of infections due to CRE. In murine models of infection, ceftazidime-avibactam was effective against CRE isolates including KPC-producing K. pneumoniae.Citation115,116 Clinical data in humans is primarily limited to patients without CRE including a phase 3 randomized clinical trial for complicated intra-abdominal infections demonstrating non-inferior clinical cure rates for ceftazidime-avibactam in combination with metronidazole compared to meropenem (81.6% vs. 85.1%).Citation117 Ceftazidime-avibactam was also evaluated in a randomized, open-label trial of patients with ceftazidime-resistant Enterobacteriaceae or P. aeruginosa complicated urinary tract infections or intra-abdominal infections compared to best available therapy (which included a carbapenem in 97% of patients).Citation118 Clinical cure rates were similar with ceftazidime-avibactam (90.9%) and best available therapy (91.2%).Citation118 Additional data evaluating clinical outcomes of ceftazidime-avibactam as monotherapy and in combination for the treatment of CRE infections is greatly needed to further establish this agent's role in the treatment of CRE infections.
Conclusions
In summary, CRE represent an urgent public health threat. Infections due to CRE are associated with high morbidity and mortality, especially in critically ill patients. Pharmacodynamic optimization of antibiotic regimens may not be not critical for many types of infections, but the limited treatment options for CRE infections necessitate a firm understanding of the PK-PD of the available antimicrobials to give patients the best chance for treatment success.
Disclosure of potential conflicts of interest
E.A.N. has no potential conflicts of interest. J.C.G is a speaker and consultant for Allergan and Merck and a consultant for Pfizer.
References
- Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, 2013. Atlanta, GA: CDC. Retrieved from: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682-707; PMID:23034326; https://doi.org/10.1128/CMR.05035-11
- Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, et al. Carbapenemase-Producing Klebsiella pneumoniae bloodstream infections: Lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322-8; PMID:24514083; https://doi.org/10.1128/AAC.02166-13
- Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108-13; PMID:22252816; https://doi.org/10.1128/AAC.06268-11
- Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, et al. Predictors of mortality in bloodstream infections caused by klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943-50; PMID:22752516; https://doi.org/10.1093/cid/cis588
- Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2011; 17:1798-803
- McKinnon PS, Davis SL. Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious diseases. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 2004; 23:271-88; https://doi.org/10.1007/s10096-004-1107-7
- Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 2009; 37:840-51; quiz 859; PMID:19237886; https://doi.org/10.1097/CCM.0b013e3181961bff
- Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 2011; 50:99-110; PMID:21142293; https://doi.org/10.2165/11539220-000000000-00000
- Dudley MN. Rationale for the 2010 revised susceptibility breakpoints for cephalosporins, aztreonam, and carbapenems for enterobacteriaceae. J Pediatr Infect Dis Soc 2012; 1:166-8; https://doi.org/10.1093/jpids/pis046
- Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 26th informational supplement. CLSI document M100-S26. Clinical Laboratory Standards Institute, Wayne, PA
- Esterly JS, Wagner J, McLaughlin MM, Postelnick MJ, Qi C, Scheetz MH. Evaluation of clinical outcomes in patients with bloodstream infections due to Gram-negative bacteria according to carbapenem MIC stratification. Antimicrob Agents Chemother 2012; 56:4885-90; PMID:22777044; https://doi.org/10.1128/AAC.06365-11
- Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 2009; 64:142-50; PMID:19398460; https://doi.org/10.1093/jac/dkp139
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135-41; PMID:21635663; https://doi.org/10.1111/j.1469-0691.2011.03553.x
- Kuti JL, Dandekar PK, Nightingale CH, Nicolau DP. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J Clin Pharmacol 2003; 43:1116-23; PMID:14517194; https://doi.org/10.1177/0091270003257225
- Pea F, Viale P, Cojutti P, Furlanut M. Dosing nomograms for attaining optimum concentrations of meropenem by continuous infusion in critically Ill patients with severe gram-negative infections: a pharmacokinetics/pharmacodynamics-based approach. Antimicrob Agents Chemother 2012; 56:6343-8; PMID:23045356; https://doi.org/10.1128/AAC.01291-12
- Bulik CC, Nicolau DP. In vivo efficacy of simulated human dosing regimens of prolonged-infusion Doripenem against Carbapenemase-Producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2010; 54:4112-5; PMID:20660688; https://doi.org/10.1128/AAC.00026-10
- Anderson KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B, McDougal LK, Carey RB, Thompson A, Stocker S, Limbago B, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723-5; PMID:17581941; https://doi.org/10.1128/JCM.00015-07
- Tumbarello M, Trecarichi EM, Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Bono VD, Corcione S, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70(10):2922; dkv086; PMID:26169556; https://doi.org/10.1093/jac/dkv200
- Bulik CC, Nicolau DP. Double-Carbapenem Therapy for Carbapenemase-Producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002-4; PMID:21422205; https://doi.org/10.1128/AAC.01420-10
- Cprek JB, Gallagher JC. Ertapenem-containing double-carbapenem therapy for the treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae: a case series. Antimicrob Agents Chemother 2015; 60(1):669–73; AAC.01569–15
- Oliva A, D'Abramo A, D'Agostino C, Iannetta M, Mascellino MT, Gallinelli C, Mastroianni CM, Vullo V. Synergistic activity and effectiveness of a double-carbapenem regimen in pandrug-resistant Klebsiella pneumoniae bloodstream infections. J Antimicrob Chemother 2014; 69(6):1718-20; dku027; PMID:24521856
- Giamarellou H, Galani L, Baziaka F, Karaiskos I. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant klebsiella pneumoniae. Antimicrob Agents Chemother 2013; 57:2388-90; PMID:23439635; https://doi.org/10.1128/AAC.02399-12
- Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284-94; PMID:21555763; https://doi.org/10.1128/AAC.01733-10
- Ortwine JK, Kaye KS, Li J, Pogue JM. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy 2015; 35:11-6; PMID:25187500; https://doi.org/10.1002/phar.1484
- Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis Off Publ Infect Dis Soc Am 2013; 57:524-31; https://doi.org/10.1093/cid/cit334
- Bergen PJ, Landersdorfer CB, Zhang J, Zhao M, Lee HJ, Nation RL, Li J. Pharmacokinetics and pharmacodynamics of “old” polymyxins: what is new? Diagn Microbiol Infect Dis 2012; 74:213-23; PMID:22959816; https://doi.org/10.1016/j.diagmicrobio.2012.07.010
- Nation RL, Velkov T, Li J. Colistin and Polymyxin B: Peas in a pod, or chalk and cheese? Clin Infect Dis 2014; 59:88-94; PMID:24700659; https://doi.org/10.1093/cid/ciu213
- Bergen PJ, Li J, Nation RL. Dosing of colistin-back to basic PK/PD. Curr Opin Pharmacol 2011; 11:464-9; PMID:21835694; https://doi.org/10.1016/j.coph.2011.07.004
- Gregoire N, Mimoz O, Megarbane B, Comets E, Chatelier D, Lasocki S, Gauzit R, Balayn D, Gobin P, Marchand S, et al. New colistin population pharmacokinetic data in critically Ill patients suggesting an alternative loading dose rationale. Antimicrob Agents Chemother 2014; 58:7324-30; PMID:25267662; https://doi.org/10.1128/AAC.03508-14
- Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically Ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009; 53:3430-6; PMID:19433570; https://doi.org/10.1128/AAC.01361-08
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006; 6:589-601; PMID:16931410; https://doi.org/10.1016/S1473-3099(06)70580-1
- Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 2015; 15:225-34; PMID:25459221; https://doi.org/10.1016/S1473-3099(14)70850-3
- Rigatto MH, Oliveira MS, Perdigão-Neto LV, Levin AS, Carrilho CM, Tanita MT, Tuon FF, Cardoso DE, Lopes NT, Falci DR, et al. Multicenter prospective cohort study of renal failure in patients treated with colistin versus polymyxin B. Antimicrob Agents Chemother 2016; 60:2443-9; PMID:26856846; https://doi.org/10.1128/AAC.02634-15
- Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH. Comparison of nephrotoxicity rates associated with colistimethate versus polymyxin B therapy: in vitro assessment and a multicenter cohort study. Antimicrob Agents Chemother 2014; 58(5):2740-6; AAC.02476–13
- Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically Ill patients at a tertiary care medical center. Clin Infect Dis 2013; 57(9):1300-3; cit453; PMID:23840000; https://doi.org/10.1093/cid/cit453
- Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, et al. Application of a loading dose of colistin methanesulfonate in critically Ill patients: Population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 2012; 56:4241-9; PMID:22615285; https://doi.org/10.1128/AAC.06426-11
- Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Turnidge JD, Forrest A, Silveira FP. Updated United States and European dose recommendations for intravenous colistin: How do they perform? Clin Infect Dis 2016; 62(5):552-8; civ964; PMID:26607424
- Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. Pharmacokinetics of intravenous polymyxin B in critically Ill patients. Clin Infect Dis 2008; 47:1298-304; PMID:18840079; https://doi.org/10.1086/592577
- Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest J 2010; 138:1333-9; https://doi.org/10.1378/chest.10-0463
- Markou N, Fousteri M, Markantonis SL, Boutzouka E, Tsigou E, Baltopoulo G. Colistin penetration in the alveolar lining fluid of critically ill patients treated with IV colistimethate sodium. Chest 2011; 139:232-3; author reply 233–4; PMID:21208892; https://doi.org/10.1378/chest.10-1860
- Florescu DF, Qiu F, McCartan MA, Mindru C, Fey PD, Kalil AC. What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin Infect Dis 2012; 54:670-80; PMID:22322268; https://doi.org/10.1093/cid/cir934
- Valachis A, Samonis G, Kofteridis DP. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit Care Med 2015; 43:527-33; PMID:25493971; https://doi.org/10.1097/CCM.0000000000000771
- Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 2006; 57:306-11; PMID:16396919; https://doi.org/10.1093/jac/dki461
- Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin MH, Le Naour G, Marquette CH, et al. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med 2010; 36:1147-55; PMID:20397007; https://doi.org/10.1007/s00134-010-1879-4
- Karvanen M, Plachouras D, Friberg LE, Paramythiotou E, Papadomichelakis E, Karaiskos I, Tsangaris I, Armaganidis A, Cars O, Giamarellou H. Colistin methanesulfonate and colistin pharmacokinetics in critically Ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 2013; 57:668-71; PMID:23147733; https://doi.org/10.1128/AAC.00985-12
- Markou N, Fousteri M, Markantonis SL, Zidianakis B, Hroni D, Boutzouka E, Baltopoulos G. Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: an observational study. J Antimicrob Chemother 2012; 67:2459-62; PMID:22790220; https://doi.org/10.1093/jac/dks257
- Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Saitovitch D, Wang J, Forrest A, et al. Pharmacokinetics of polymyxin B in patients on continuous venovenous haemodialysis. J Antimicrob Chemother 2013; 68:674-7; PMID:23179561; https://doi.org/10.1093/jac/dks437
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0, 2016. http://www.eucast.org
- Jayol A, Nordmann P, Brink A, Poirel L. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob Agents Chemother 2015; 59:2780-4; PMID:25733503; https://doi.org/10.1128/AAC.05055-14
- Bergen PJ, Bulman ZP, Landersdorfer CB, Smith N, Lenhard JR, Bulitta JB, Nation RL, Li J, Tsuji BT. Optimizing polymyxin combinations against resistant gram-negative bacteria. Infect Dis Ther 2015; 4:391-415; PMID:26645096; https://doi.org/10.1007/s40121-015-0093-7
- Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist Updat 2010; 13:132-8; PMID:20843473; https://doi.org/10.1016/j.drup.2010.05.002
- Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. Systematic Review and Meta-Analysis of In Vitro Synergy of Polymyxins and Carbapenems. Antimicrob Agents Chemother 2013; 57:5104-11; PMID:23917322; https://doi.org/10.1128/AAC.01230-13
- Bergen PJ, Bulman ZP, Saju S, Bulitta JB, Landersdorfer C, Forrest A, Li J, Nation RL, Tsuji BT. Polymyxin combinations: Pharmacokinetics and pharmacodynamics for rationale use. Pharmacother J Hum Pharmacol Drug Ther 2015; 35:34-42; https://doi.org/10.1002/phar.1537
- Gordon NC, Png K, Wareham DW. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 2010; 54:5316-22; PMID:20876375; https://doi.org/10.1128/AAC.00922-10
- Lee GC, Burgess DS. Polymyxins and Doripenem combination against KPC-producing klebsiella pneumoniae. J Clin Med Res 2013; 5:97-100; PMID:23519391
- Tuon FF, Rocha JL, Formighieri MS, Sfair S, Bertoldi MB, Palmeiro JK, Dalla Costa LM. Fosfomycin susceptibility of isolates with blaKPC-2 from Brazil. J Infect 2013; 67:247-9; PMID:23639842; https://doi.org/10.1016/j.jinf.2013.04.017
- Kyle JM, Stollings JL, White KD, Noto MJ, Wheeler AP. Fosfomycin for multidrug treatment of Klebsiella pneumoniae carbapenemase bacteremia. Ann Pharmacother 2015; 49:366-7; PMID:25691478; https://doi.org/10.1177/1060028014564395
- Popovic M, Steinort D, Pillai S, Joukhadar C. Fosfomycin: an old, new friend? Eur J Clin Microbiol Infect Dis 2009; 29:127-42; PMID:19915879; https://doi.org/10.1007/s10096-009-0833-2
- Parker S, Lipman J, Koulenti D, Dimopoulos G, Roberts JA. What is the relevance of fosfomycin pharmacokinetics in the treatment of serious infections in critically ill patients? A systematic review. Int J Antimicrob Agents 2013; 42:289-93; PMID:23880170; https://doi.org/10.1016/j.ijantimicag.2013.05.018
- Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 2009; 34:506-15; PMID:19828298; https://doi.org/10.1016/j.ijantimicag.2009.08.013
- Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis 2011; 15:e732-9; PMID:21945848; https://doi.org/10.1016/j.ijid.2011.07.007
- Patel SS, Balfour JA, Bryson HM. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 1997; 53:637-56; PMID:9098664; https://doi.org/10.2165/00003495-199753040-00007
- Janknegt R, Hooymans PM, Fabius GT, Nohlmans-Paulssen MK, Machielsen C, Boogaard-van den Born J, Rang J, Smits CA, Willems-Thissen ME, Krommenhoek A. Urinary concentrations of fosfomycin after a single 3 g dose of fosfomycin to elderly nursing-home patients. Pharm World Sci PWS 1994; 16:149-53; PMID:7920366; https://doi.org/10.1007/BF01877485
- Navarro-San Francisco C, Mora-Rillo M, Romero-Gómez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gómez-Gil R, Arribas-López JR, Mingorance J, Paño-Pardo JR. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect 2013; 19:E72-9; PMID:23231088; https://doi.org/10.1111/1469-0691.12091
- Peirano G, Ahmed-Bentley J, Woodford N, Pitout JD. New Delhi metallo-β-lactamase from traveler returning to Canada. Emerg Infect Dis 2011; 17:242-4; PMID:21291595; https://doi.org/10.3201/eid1702.101313
- Kitchel B, Sundin DR, Patel JB. Regional dissemination of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2009; 53:4511-3; PMID:19687250; https://doi.org/10.1128/AAC.00784-09
- Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 2010; 10:43-50; PMID:20129148; https://doi.org/10.1016/S1473-3099(09)70325-1
- Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis 2011; 15:e732-9; PMID:21945848; https://doi.org/10.1016/j.ijid.2011.07.007
- Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant gram-negative bacterial infections. Insights from the society of infectious diseases pharmacists. Pharmacotherapy 2014; 34:845-57; PMID:24782335; https://doi.org/10.1002/phar.1434
- Matzi V, Lindenmann J, Porubsky C, Kugler SA, Maier A, Dittrich P, Smolle-Jüttner FM, Joukhadar C. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J Antimicrob Chemother 2010; 65:995-8; PMID:20228081; https://doi.org/10.1093/jac/dkq070
- Joukhadar C, Klein N, Dittrich P, Zeitlinger M, Geppert A, Skhirtladze K, Frossard M, Heinz G, Müller M. Target site penetration of fosfomycin in critically ill patients. J Antimicrob Chemother 2003; 51:1247-52; PMID:12668580; https://doi.org/10.1093/jac/dkg187
- Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 2009; 34:506-15; PMID:19828298; https://doi.org/10.1016/j.ijantimicag.2009.08.013
- Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2010; 16:184-6
- Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, Oikonomou A, Poulakou G, Roilides E, Giamarellou H. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 2014; 43:52-9; PMID:24183799; https://doi.org/10.1016/j.ijantimicag.2013.09.010
- VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, Ambrose PG. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 2015; 59:7170-7; PMID:26100706; https://doi.org/10.1128/AAC.04955-14
- Docobo-Pérez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martín V, Ballestero-Tellez M, Rodriguez-Martinez JM, Conejo MC, van Guilder M, et al. Pharmacodynamics of fosfomycin: Insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 2015; 59:5602-10; https://doi.org/10.1128/AAC.00752-15
- Walsh CC, McIntosh MP, Peleg AY, Kirkpatrick CM, Bergen PJ. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother 2015; 70:3042-50; PMID:26209311; https://doi.org/10.1093/jac/dkv221
- Albiero J, Sy SK, Mazucheli J, Caparroz-Assef SM, Costa BB, Alves JL, Gales AC, Tognim MC. Pharmacodynamic evaluation of the potential clinical utility of fosfomycin and meropenem in combination therapy against KPC-2 producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2016; 60(7):4128-39; AAC.03099–15; PMID:27139468; https://doi.org/10.1128/AAC.03099-15
- Asuphon O, Montakantikul P, Houngsaitong J, Kiratisin P, Sonthisombat P. Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int J Infect Dis 2016 Jul 11;50:23-29. PMID:27418581; https://doi.org/10.1016/j.ijd.2016.06.017. [Epub ahead of print]; Available from: http://www.sciencedirect.com/science/article/pii/S120197121631102X
- Sader HS, Castanheira M, Flamm RK, Mendes RE, Farrell DJ, Jones RN. Tigecycline activity tested against carbapenem-resistant Enterobacteriaceae from 18 European nations: results from the SENTRY surveillance program (2010-2013). Diagn Microbiol Infect Dis 2015; 83:183-6; PMID:26164275; https://doi.org/10.1016/j.diagmicrobio.2015.06.011
- Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. Pharmacokinetic/pharmacodynamic profile for tigecycline-a new glycylcycline antimicrobial agent. Diagn Microbiol Infect Dis 2005; 52:165-71; PMID:16105560; https://doi.org/10.1016/j.diagmicrobio.2005.05.006
- MacGowan AP. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemother 2008; 62(Suppl 1):i11-6; PMID:18684702; https://doi.org/10.1093/jac/dkn242
- Burkhardt O, Rauch K, Kaever V, Hadem J, Kielstein JT, Welte T. Tigecycline possibly underdosed for the treatment of pneumonia: a pharmacokinetic viewpoint. Int J Antimicrob Agents 2009; 34:101-2; PMID:19278835; https://doi.org/10.1016/j.ijantimicag.2009.01.015
- Bhavnani SM, Rubino CM, Hammel JP, Forrest A, Dartois N, Cooper CA, Korth-Bradley J, Ambrose PG. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother 2012; 56:1065-72; PMID:22143524; https://doi.org/10.1128/AAC.01615-10
- van Duin D, Cober ED, Richter SS, Perez F, Cline M, Kaye KS, Kalayjian RC, Salata RA, Evans SR, Fowler VG, et al. Tigecycline therapy for carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteriuria leads to tigecycline resistance. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2014; 20:O1117-20
- Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 2010; 68:140-51; PMID:20846586; https://doi.org/10.1016/j.diagmicrobio.2010.05.012
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning [Internet]. [cited 2016 Jul 17]; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm369580.htm
- Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013; 57:1756-62; PMID:23357775; https://doi.org/10.1128/AAC.01232-12
- Di Carlo P, Gulotta G, Casuccio A, Pantuso G, Raineri M, Farulla CA, Bonventre S, Guadagnino G, Ingrassia D, Cocorullo G, et al. KPC - 3 Klebsiella pneumoniae ST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: analysis of a case series of 30 patients. BMC Anesthesiol 2013; 13:13; PMID:23822218; https://doi.org/10.1186/1471-2253-13-13
- De Pascale G, Montini L, Pennisi M, Bernini V, Maviglia R, Bello G, Spanu T, Tumbarello M, Antonelli M. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care Lond Engl 2014; 18:R90; https://doi.org/10.1186/cc13858
- Balandin Moreno B, Fernández Simón I, Pintado García V, Sánchez Romero I, Isidoro Fernández B, Romera Ortega MA, Alcántara Carmona S, Pérez Redondo M, Galdos Anuncibay P. Tigecycline therapy for infections due to carbapenemase-producing Klebsiella pneumoniae in critically ill patients. Scand J Infect Dis 2014; 46:175-80; PMID:24354959; https://doi.org/10.3109/00365548.2013.861608
- Endimiani A, Hujer KM, Hujer AM, Armstrong ES, Choudhary Y, Aggen JB, Bonomo RA. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 2009; 53:4504-7; PMID:19770287; https://doi.org/10.1128/AAC.00641-09
- Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 2011; 66:48-53; PMID:21078604; https://doi.org/10.1093/jac/dkq408
- Craig WA. Optimizing aminoglycoside use. Crit Care Clin 2011; 27:107-21; PMID:21144989; https://doi.org/10.1016/j.ccc.2010.11.006
- Marik PE. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth Intensive Care 1993; 21:172-3; PMID:8517507
- Triginer C, Izquierdo I, Fernández R, Rello J, Torrent J, Benito S, Net A. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med 1990; 16:303-6; PMID:2212254; https://doi.org/10.1007/BF01706354
- Bracco D, Landry C, Dubois M-J, Eggimann P. Pharmacokinetic variability of extended interval tobramycin in burn patients. Burns J Int Soc Burn Inj 2008; 34:791-6; https://doi.org/10.1016/j.burns.2007.11.003
- Triginer C, Izquierdo I, Fernández R, Torrent J, Benito S, Net A, Jane F. Changes in gentamicin pharmacokinetic profiles induced by mechanical ventilation. Eur J Clin Pharmacol 1991; 40:297-302; PMID:2060568; https://doi.org/10.1007/BF00315213
- Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155:93-9; PMID:3540140; https://doi.org/10.1093/infdis/155.1.93
- Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2014; 20:862-72
- Satlin MJ, Kubin CJ, Blumenthal JS, Cohen AB, Furuya EY, Wilson SJ, Jenkins SG, Calfee DP. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893-9; PMID:21968368; https://doi.org/10.1128/AAC.00387-11
- Hallal A, Cohn SM, Namias N, Habib F, Baracco G, Manning RJ, Crookes B, Schulman CI. Aerosolized tobramycin in the treatment of ventilator-associated pneumonia: a pilot study. Surg Infect 2007; 8:73-82; https://doi.org/10.1089/sur.2006.051
- Le J, Ashley ED, Neuhauser MM, Brown J, Gentry C, Klepser ME, Marr AM, Schiller D, Schwiesow JN, Tice S, et al. Consensus summary of aerosolized antimicrobial agents: application of guideline criteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2010; 30:562-84; PMID:20500046; https://doi.org/10.1592/phco.30.6.562
- Stachyra T, Péchereau MC, Bruneau JM, Claudon M, Frère JM, Miossec C, Coleman K, Black MT. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-β-lactam β-lactamase inhibitor. Antimicrob Agents Chemother 2010; 54:5132-8; PMID:20921316; https://doi.org/10.1128/AAC.00568-10
- Ehmann DE, Jahić H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A 2012; 109:11663-8; PMID:22753474; https://doi.org/10.1073/pnas.1205073109
- Endimiani A, Choudhary Y, Bonomo RA. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother 2009; 53:3599-601; PMID:19528274; https://doi.org/10.1128/AAC.00641-09
- Aktaş Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 2012; 39:86-9; PMID:22041508; https://doi.org/10.1016/j.ijantimicag.2011.09.012
- Hackel M, Kazmierczak K, Hoban D, Biedenbach D, Bouchillon S, DeJonge BL, Stone G. An assessment of the in vitro activity of Ceftazidime-Avibactam against multi-drug resistant Klebsiella spp. Collected in the INFORM Global Surveillance Study (2012–2014). Antimicrob Agents Chemother 2016; 60(8):4677-83; PMID:27216054; https://doi.org/10.1128/AAC.02841-15
- Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. First report of Ceftazidime-Avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 2015; 59:6605-7; PMID:26195508; https://doi.org/10.1128/AAC.01165-15
- Zasowski EJ, Rybak JM, Rybak MJ. The β-Lactams strike back: Ceftazidime-Avibactam. Pharmacotherapy 2015; 35:755-70; PMID:26289307; https://doi.org/10.1002/phar.1622
- Vishwanathan K, Mair S, Gupta A, Atherton J, Clarkson-Jones J, Edeki T, Das S. Assessment of the mass balance recovery and metabolite profile of avibactam in humans and in vitro drug-drug interaction potential. Drug Metab Dispos Biol Fate Chem 2014; 42:932-42; PMID:24616266; https://doi.org/10.1124/dmd.113.055335
- Nicolau DP, Siew L, Armstrong J, Li J, Edeki T, Learoyd M, Das S. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother 2015; 70:2862-9; PMID:26133566; https://doi.org/10.1093/jac/dkv170
- MacVane SH, Kuti JL, Nicolau DP. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 2014; 58:1359-64; PMID:24342637; https://doi.org/10.1128/AAC.01463-13
- Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:82-5; PMID:21041503; https://doi.org/10.1128/AAC.01198-10
- MacVane SH, Crandon JL, Nichols WW, Nicolau DP. In vivo efficacy of humanized exposures of Ceftazidime-Avibactam in comparison with Ceftazidime against contemporary Enterobacteriaceae isolates. Antimicrob Agents Chemother 2014; 58:6913-9; PMID:25223999; https://doi.org/10.1128/AAC.03267-14
- Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection - results from a randomized, controlled, double-blind, Phase 3 program. Clin Infect Dis Off Publ Infect Dis Soc Am 2016; 62(11):1380-9; https://doi.org/10.1093/cid/ciw133
- Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman A, Gasink LB. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016; 16:661-73; PMID:27107460; https://doi.org/10.1016/S1473-3099(16)30004-4