ABSTRACT
Human Respiratory syncytial virus (hRSV) and human metapneumovirus (hMPV) are the two major etiological viral agents of lower respiratory tract diseases, affecting mainly infants, young children and the elderly. Although the infection of both viruses trigger an antiviral immune response that mediate viral clearance and disease resolution in immunocompetent individuals, the promotion of long-term immunity appears to be deficient and reinfection are common throughout life. A possible explanation for this phenomenon is that hRSV and hMPV, can induce aberrant T cell responses, which leads to exacerbated lung inflammation and poor T and B cell memory immunity. The modulation of immune response exerted by both viruses include different strategies such as, impairment of immunological synapse mediated by viral proteins or soluble factors, and the induction of pro-inflammatory cytokines by epithelial cells, among others. All these viral strategies contribute to the alteration of the adaptive immunity in order to increase the susceptibility to reinfections.
In this review, we discuss current research related to the mechanisms underlying the impairment of T and B cell immune responses induced by hRSV and hMPV infection. In addition, we described the role each virulence factor involved in immune modulation caused by these viruses.
Introduction
The human Respiratory syncytial virus (hRSV) and human metapneumovirus (hMPV), which belong to the Pneumoviridae family,Citation1 are the main viral etiological agents of severe lower respiratory tract infection (LRTI), especially in infants, children and the elderly.Citation2,3 HRSV and hMPV can lead to bronchiolitis and pneumonia, and have also been implicated in the development of recurrent wheezing and asthma.Citation2-6
HRSV is the second most common etiological pathogen for pneumonia after influenza virus around the world.Citation7 Furthermore, it has been reported worldwide between 66,000 to 239,000 deaths per year of children less than five years of age, who suffered LRTI caused by hRSV.Citation5,8
HRSV was first isolated in 1956 from a colony of chimpanzees that presented various symptoms, including coughing, sneezing, and purulent nasal discharge.Citation9,10 The illness quickly spread from sick chimpanzees to other monkeys, indicating the presence of a highly contagious pathogen that was originally denominated the chimpanzee coryza agent.Citation10 In 1957, Chanock and Finberg isolated a similar agent from throat swab samples of infants with a severe respiratory disease,Citation11,12 which was found to be identical to the one reported by Blount et al in chimpanzees.Citation10 The isolated pathogen induced syncytia formation, via the virus fusion protein (F) on permissive cell types in cultures.Citation12,13 Therefore, this pathogen was renamed as a hRSV.
On the other hand, hMPV was first isolated in 2001 from young Dutch children with a respiratory tract disease.Citation14 HMPV is a member of the Pneumoviridae family and Metapneumovirus genus.Citation1 Genomic and phylogenic analyses suggest that hMPV diverged from avian MPV, a virus that causes serious respiratory diseases in chickens.Citation15-17 Infection caused by hMPV presents a similar symptomatology as other respiratory viruses such as hRSV, parainfluenza virus and influenza,Citation18 whereby the diagnosis is difficult. Symptoms of hMPV include rhinorrhea, cough, acute otitis media, fever, and, less frequently, conjunctivitis, rash, diarrhea and vomiting.Citation19 Frequently, risk population, including young children and the elderly, infected with hMPV require hospitalization.Citation20 Moreover, mortality as a result of hMPV can reach up to 10% in the elderly.Citation3,20
Recurrent infections with hRSV or hMPV are common in childrenCitation21,22 and adults.Citation21-24 Studies in healthy young adults subjected to experimental challenge or natural infection, showed mild upper respiratory symptoms for both viruses infection.Citation23,25 However, reports performed in humans and mouse dendritic cells (DCs) have described that both viruses infect these cells affecting DCs capacity of promote an adequate immunological memory due to their interference with naïve T cell priming.Citation26-29
The ineffectiveness of the natural infection to induce long-term immunity has hampered vaccine generation and currently there is no licensed vaccine available to prevent the bronchiolitis and pneumonia caused either by hRSV or hMPV.Citation30-34 However, several candidate vaccines are in different stages of development for preventing the diseases caused by these viral agents. Candidate vaccines for hRSV and hMPV employ different approaches, including a chimeric virus (hMPV),Citation35 live attenuated virus (hRSV),Citation36 and purified proteins (hRSV),Citation37 among others. Furthermore, recombinant Bacillus Calmette-Guérin (rBCG) vaccines have been developed using hRSV and hMPV antigens to provide a protective TH1 response in mice.Citation31,38,39 In addition, antibody-mediated immunity is relevant for protection against both viruses.Citation40,41 For example, Palivizumab, an IgG1 humanized anti-F monoclonal antibody, is safe and effective when given in a prophylactic manner to children at risk of severe hRSV infection.Citation42 Thus, the ideal vaccine for either hRSV or hMPV should provide both antibody and T cell-mediated immune protection.
In this review, we describe the most recent findings relative to the mechanisms employed by hRSV and hMPV to evade the host immune system. Further, we will discuss the virulence factors of each virus that contribute at modulating the adaptive immune response.
Adaptive immunity triggered by hRSV
The Humoral response against hRSV infection
The humoral immune response plays a major role in protecting humans from hRSV infections.Citation43-45 Indeed, high titers of pre-existent mucosal IgG are correlated with reduced viral loads in hRSV-infected infants.Citation46 Similar correlation have been obtained for nasal IgA levels in naturally infection and in experimental challenges in healthy adults.Citation47,48 On the other hand, it has been described in mice that antibodies play a major role during reinfection, even more than in the first infection, giving a principal role to T cells in the clearance of the virus during the first hRSV challenge.Citation49
Interestingly, prophylactic treatment with Palivizumab reduces severe hRSV-mediated LRTI,Citation42,50 and consequently diminish hRSV-associated hospitalization of premature infants, children with congenital heart disease (CHD) and children with cystic fibrosis (CF),Citation50 suggesting that a neutralizing antibody of high affinity and titer is enough to confer a clinical protection against hRSV disease. However, in most adults antibody titers are underneath the levels needed to reach a complete airway protection, despite repeated infection occurring throughout life.Citation51 Experimental challenges in healthy adults have shown that serum and nasal IgA titers increase after infection but those levels are poorly maintained.Citation47 Similar results have been reported for neutralizing antibodies levels in a birth cohort followed-up over three hRSV epidemics.Citation52 These antecedents suggest that acute production of short-life antibody-secreting cells (ACS) is not impaired. However, a defect occurs in long-lived plasma cells that arise from the ASC population, which normally should migrate to bone marrow and respiratory mucosa.Citation47 This phenomenon might explain, at least in part, as to why the specific hRSV-IgA generated humoral response is not sufficient to provide protection upon reinfection.
On other hand, several pieces of evidences suggest that the production of virus-specific antibodies plays an important role in the regulation of hRSV-specific T cell responses.Citation53 The interaction between antibodies and T cell responses was associated with the ratio of neutralizing and non-neutralizing antibodies. Wherein, for hRSV infection, a higher ratio of neutralizing versus non-neutralizing antibodies enhanced the balance of CD4+/CD8+ T cells in vitro that respond specifically to the virus in humans PBMC, as well as in vivo assays in mice model.Citation54 Interestingly, the infection of mice with hRSV immune-complexes increase the immune response against the virus, particularly promoting a TH1 response by CD4+ T cells and IgG2c response by B cells.Citation55 Higher amounts of non-neutralizing antibodies might enhance infection and could cause immune complex deposition, leading to enhanced respiratory disease.Citation56 Considering the whole body of data described above, it is possible to hypothesize that hRSV infection can modulate the humoral response to impair recurrent reinfection and indirectly affect T cell activation.
The cellular immune response against hRSV infection
Both memory CD4+ and CD8+ T cells contribute significantly at achieving protective immunity upon hRSV infection.Citation57-59 This applies especially in children with defective T cell responses, who exhibit severe hRSV infection and prolonged virus shedding.Citation60 Supporting this observation, T cell depletion assays in BALB/c mice results in higher hRSV replication upon infection, while the adoptive transfer of virus-specific memory T cells enhances virus clearance in recipient mice.Citation61 Furthermore, it has been demonstrated that transfer of hRSV-N-specific T cells also contribute to reduce viral immunopathology.Citation38,39 Moreover, memory T cells appear to be clinically important in protecting from severe diseases caused by hRSV reinfections. This notion is supported by the fact that minor symptoms are observed in populations of older children and young adults infected with hRSV, despite of defective responses in IgA B cell memory and in hRSV-specific serum.Citation47,62
Recently, it has been demonstrated that tissue-resident memory (Trm) T cells are relevant to the capacity of the host to rapidly limiting the spread of pathogens in tissues.Citation63,64 Thus, hRSV-specific CD4+ and CD8+ Trm T cells could provide immediate immunological protection against hRSV infections. In fact, analyses of hRSV-specific CD8+ memory T cells have shown that these cells mostly remain in lungs and a minority of these cells circulates in peripheral blood from healthy individuals.Citation65,66 Moreover, increased activated hRSV-specific airway Trm T cell frequencies were observed in bronchoalveolar lavage fluid (BALF) from healthy adults inoculated with hRSV, which coincided with a reduction in the viral load.Citation59
hRSV-mediated lung pathology in mice is not completely dissected and primary reports attributed this effect to T cells, specially CD8+ T67,68 but in humans, it has mostly been associated with a large influx of neutrophils in the lungs of patients with bronchiolitis, as well as in fatal cases of infants.Citation69-71 It is suggested that neutrophils recruitment induced by hRSV infection promote lung damage through the generation of reactive oxygen species and extracellular traps (NETs).Citation72,73
Nevertheless, a recent study using experimental hRSV infection of adults in which a 65% of individuals presented inflammation symptoms, has shown that the virus replicate in the lower respiratory tract, inducing cellular infiltration of CD8+ T cells to the airways.Citation59 Consistent with this notion, there is evidence that CD8+ T cells can cause immunopathology in infants when a high amount of CD8+ T cell encounter a large number of hRSV particles in the tissue.Citation74 However, the drawback of these studies is that no other cell types were evaluated, therefore it is not possible to rule out the neutrophils contribution to the pathology. In addition, another study showed that T cell responses are reduced or absent in exacerbated lungs of fatal cases of infants infected with hRSV, who had a severe LRTI caused by this virus.Citation71 In these tissues a positive staining for macrophages and neutrophils was observed.Citation71 Thus, in more severe cases of infantile viral LRTI caused by hRSV infection, lung inflammation appears to be due to a pronounced infiltration of neutrophils and macrophages.
CD4+ T cell response against hRSV and mechanisms of evasion used by the virus
An adequate CD4+ T cell response can efficiently aid at reducing viral load upon hRSV infection.Citation39 Indeed, it has been reported that adoptive transfer of CD4+ T cells from immunized mice with a prototype vaccine consisting in a recombinant rBCG expressing hRSV N protein (rBCG-N-hRSV), resulted in a significant reduced viral load in the lungs after infection in recipient mice, thus providing a protective TH1 antiviral response.Citation38 These data suggest that CD4+ T cells itself stimulated with the proper antigens in can significantly contribute to hRSV clearance.
However, in infants and children naturally infections, an inefficient adaptive immune response occurs, which is characterized by 1) a skewed TH2 immune response;.Citation75,76 2) a deficient anti-viral TH1 response and 3) a low secretion of IFN-γ and TNF-α in peripheral blood mononuclear cellsCitation77 and in nasopharyngeal aspirates,Citation78 respectively. Nevertheless, hRSV-infected infants suffering from bronchiolitis present higher levels of TNF-α in BALF at day 1 of intubation, as compared with controls. This observation suggest that a mixed TH1/TH2 response is generated at the first stage of the disease.Citation79 Consistently with this observation, a mixed TH1/TH2 response was also observed in the lung of hRSV-infected mice, although with a significant increase of IL-13, which is known to induce airway hyperreactivity.Citation80,81
The predominant TH2 immune response observed in infants and children could be due to the capacity of hRSV to polarize the adaptive immune response from a protective TH1 phenotype to a TH2-type response.Citation76 However, whether a pathogenic TH2 immune response significantly contributes to disease in at-risk human groups remains to be demonstrated.
Importantly, in vitro studies have described that hRSV-infected mouse DCs are unable to properly activate T cells,Citation27 due to an impairment of immunological synapse formation. This study evaluated the formation and functionality of the immunological synapse between OT-II CD4+ T cells with hRSV-infected DCs, pulsed with OVA peptide,Citation27 showing a lack of sustained immunological synapse as well as reduced secretion of cytokines from OT-II cells, as compared with uninfected controls. This phenomenon was observed by using naïve T cells, in contrast, it has been described that memory/effector T cells have different responses, which is less affected by hRSV-infected DCs.Citation82 This phenomenon, could explain why healthy adults infected with hRSV can clear the virus and not in young children who have no memory T cells.
TH2 polarization also is induced by Tymic-stromal lymphoprotein (TSLP), an epithelial cell-derived cytokine that signals through the TSLP receptor (TSLPR).Citation83 This cytokine potently activates myeloid DCs (mDCs), since these cells are known to express high levels of the TSLPR.Citation84 Then, TSLP-stimulated DCs upregulate OX40 ligand (L) cell surface expression and produce TH2 cell-attracting chemokines, including CCL17 and CCL22.Citation84 Indeed, TSLP from in vitro hRSV-stimulated rat AECs induced the functional maturation of mDCs and enhanced the surface expression of the thymus-activation-regulated chemokine (TARC) and OX40L on DCs,Citation85 which induce mainly inflammatory TH2-polarized immune responses.Citation86 Experiments performed in BALB/c mice infected by hRSV shown elevated levels of TSLP protein in lungs compare with uninfected control.Citation80 The role of TSLP in the promotion of Th2 response under hRSV infection was evaluated using a TSLPR-deficient (TSLPR−/−) mice. These studies shown a decrease hRSV-mediated immunopathology in this model after infection. In addition, analysis of supernatant fluids of re-stimulated mediastinal lymph nodes (MLN) of TSLPR−/− mice infected with hRSV show a significant decrease of IL-13 and IL-5 production, as compare with WT mice, but both mice produced equivalent levels of IFN-γ and IL-17A. These results support, the notion that TSLP is required to TH2 polarization.Citation80 In other studies, it has been shown that the TSLP-OX40L –OX40 axis contributes to the hRSV-induced airway hyperresponsiveness (AHR) and inflammation after reinfection of mice that were initially infected as neonates. Further, administration of an anti-OX 40L antibody treatment during primary infection as neonates prevented the enhancement of the AHR upon reinfection 5 weeks later.Citation86 Moreover, treatment with the anti-OX40L during primary infection in newborn BALB/c mice reduced the TH2 cytokines IL-5 and IL-13 in BALF upon reinfection.Citation86
The pathogenic role of CD4+ T during re-infection with hRSV in adult mice, after being infected as neonates, can be explained by the induction of an exaggerated TH2 response, demonstrated by the cytokine profile observed in these animals.Citation87 This could be explained, at least in part, by the upregulation of the IL-4Rα. Specific deletion of the IL-4Rα. gene in CD4+ T cells abolished hRSV-induced airway AHR and lung damage upon reinfection with hRSV.Citation87 However, these results are controversial because several reports have shown that CD4+ T cells are required for the clearance of the virus and to promote antibody response against hRSV.Citation60,88-90 In this sense, it seems that dysregulation of CD4+ T cells promote harmful Th profiles but these cells are still required for hRSV clearance.
Additionally, an IL-17-mediated TH17 response is a possible third type of immune response associated with the respiratory pathogenesis induced by CD4+ T cells during hRSV infection.Citation91,92 Indeed, binding of IL-17 to its receptor promotes an inflammatory response and increased viral loads in the lungs of infected BALB/c mice.Citation92 Stimulation of T cells with hRSV-infected human bronchial epithelial cells (HBEC) induced the production of IFN-γ, IL-4, and IL-17, suggesting that hRSV can activate these three TH cell subsets.Citation93 Consistent with the latter, stimulation of PBMC from healthy donors with hRSV infected A549 cells induced the production of IFN-γ and IL-4.Citation94 In other studies, the concentration of IL-17 in nasopharyngeal aspirates of children during hRSV infection was higher at the moment of discharge of the hospital.Citation95 However, the data are controversial since the latter was only valid for infants that do not require ventilation.Citation95
In addition to these mechanisms used by hRSV to avoid the CD4+ T other studies have demonstrated that IL-25 and IL-17RB are expressed in lungs of hRSV-infected BALB/c mice and their expressions correlate with potentially pathogenic cytokines, such as IL-13 (TH2), IL-5 (TH2) and IFN-γ (TH1), promoting the hRSV-mediated lung disease.Citation91
Finally, using a mouse model, it has been demonstrated that antibody-mediated depletion of neutrophils decreased the number of IL-13 producing CD4+ T cells, as well as TNF-α and mucin production, when compare with the isotype-control treated group upon hRSV infection, suggesting that an interaction of neutrophils and CD4+ T cells occurs during hRSV infection.Citation96 On the other hand, has been described that neutrophils can have a APC-like phenotype expressing costimulatory molecules and activating CD4+ T cells polarizing to a TH1/TH17 phenotype in inflammatory mouse model, but for hRSV infections this phenomenon has not been demonstrated yet.Citation97,98 This could be an important unexplored area because has been described that hRSV can infect human neutrophils. Indeed, it has been possible to measure F, G and N proteins and viral RNA on those cells,Citation99 suggesting that neutrophils can uptake virus in the airways of infected children and then present antigens to T cells, but further evidence is necessary to support this idea.
Taken together, data derived from in vivo and in vitro studies suggest that hRSV infection in the lung can induce different mechanisms to prevent an efficient CD4+ T cell proliferation and differentiation into protective antiviral memory or effector cells of the host.
CD8+ T cell response against hRSV and mechanisms of evasion used by the virus
It is well documented that CD8+ T cells are pivotal at controlling respiratory viral infections, such as the one caused by hRSV.Citation57 Indeed, data from an adoptive transfer model demonstrated that transfused hRSV H2-Kd-restricted Cytotoxic T lymphocytes (CTLs) specific for the M282–90 (KdM282) epitope, the most predominant one of the M2 protein recognized in vivo (dominant epitope)Citation100 rapidly clear the virus from the lungs of recipient BALB/c mice. However, a significant lung pathology was also observed.Citation67 Consistent with that observation, BALB/c mice immunized with a DNA vaccine expressing the KdM282 epitope linked to human β2-microglobulin (β2m) developed an enhanced pulmonary inflammatory response after hRSV challenge, characterized by enhanced weight loss.Citation101 Likewise, intranasal administration of the KdM282 epitope combined with Escherichia coli heat-labile toxin (LT)/LTK63 elicited a strong antiviral CD8+ T-cell response in BALB/c mice, but also enhanced lung pathology.Citation102 This data indicates that the KdM282 epitope-specific CD8+ response is associated with enhanced disease. In addition, Ruckwardt et al.Citation103 demonstrated that infection of CB6F1/J mice with a recombinant hRSV containing a mutation in the dominant KdM282 epitope resulted in an increased response of CD8+ T cells specific for the subdominant epitope DbM187 (M187-195) with significantly less clinical disease. In contrast, hRSV containing mutations in this subdominant epitope induced an augmented KdM282-specific CD8+ T cell response and increased severity of illness.Citation103 Consistent with the latter, in CB6F1 hybrid mice, which recognize multiple MHC class I-restricted epitopes, it was described that DbM187 specific CD8+ T cells control hRSV replication more efficiently with less pulmonary inflammation and illness than the KdM282 specific CD8+ T cells.Citation104 In addition, another study has shown that after immunization with a recombinant PR8 influenza virus carrying the subdominant hRSV KdF85 epitope, KdF85-specific CTLs were induced in BALB/c mice with a significant reduction of the viral load in the lungs upon hRSV challenge,Citation105 indicating a protective effect against this virus. Taken together, these results from the mouse models suggest that a subdominant epitope-specific CD8+ T cell response could be more beneficial to the host by promoting an effective anti-viral immune response and reduced lung disease, than the dominant ones, which promote a robust anti-viral immune response but also an enhanced lung pathology upon hRSV infection.
Likewise, when CD8+ T cells from rBCG-hRSV-N immunized-BALB/c mice are stimulated with hRSV N peptides, these cells produce significant amounts of IFN-γ, partially protecting recipient mice of hRSV infection.Citation38 The efficiency of the CD8+ T cell-mediated virus control could depend on how the hRSV antigen is presented by DCs, since less IFN-γ secretion was observed when BALB/c mice were immunized with purified hRSV N or M2 in alum, compare with when mice immunized with rBCG-N-hRSV and rBCG-M2-hRSV.Citation38 Furthermore, CD8+ T cell-mediated virus control and immunopathology is dependent on IFN-γ production during early infection.Citation67
In human, has been described lesser about single epitope immune-modulation, in fact the majority only describe hRSV-derived peptides that induce IFN-γ secretion by stimulated T cells,Citation59,106,107 but they do not address their possible role during pathology. , summarizes the hRSV epitopes described so far for HLAs. It is possible that these epitopes are implicated in CD8+ T cells response but further studies are required to demonstrate if these specific T cells response is favorable or harmful for an infected person.
Table 1. hRSV epitopes described for HLA.
The impairment of CTL function appears to be another immune evasion mechanism evolved by hRSV.Citation57 How specific hRSV proteins affect these cells will be discussed below, but here additional mechanisms that have not been related yet to specific hRSV proteins. For instance, the upregulation of the programmed-death ligand 1 (PD-L1), which binds PD-1 on CD8+ T cells in human bronchial epithelial cells (BECs), occurs after hRSV infection and it has been shown to cause a functional impairment of CD8+ T cells.Citation150 Indeed, blocking PD-L1 with a specific-antibody in hRSV-infected BECs, co-cultured with CD8+ T cells, enhances CD8+ T cell effector functions and decreases hRSV gene expression in BECs.Citation108
In addition, CD8+ T cells may not only be directly influenced by the impairment of the T cell priming by DCs, but also be indirectly affected by the modulation of cytokine environment, as NK cell-derived IFN-γ production precedes lung CD8+ T cell recruitment,Citation109 in a mechanism similar to the Influenza A virus.Citation110 Also, temporally association has been made between CD8+ T cells and neutrophils, where an important influx of neutrophils to the airways in infants occurs before CD8+ T cells activation. This initial neutrophil influx correlates to the expression of the most severe hRSV symptoms suggesting that the inflammatory environmental potentiated by neutrophil influx could modulate CD8+ T cell response.Citation111
In summary, CD8+ T cell function appears to be impaired by hRSV through different mechanisms. Nonetheless, these different strategies used by this virus in targeting the CD8+ T cell response may not be that relevant in populations of older children and young adults, since most of them have mild symptoms or are asymptomatic when undergoing hRSV viral infection.
Role of hRSV Proteins in Immunomodulation
The hRSV genome consists of a 15.2 kb long, negative sense RNA, which contains 10 genes encoding for 11 proteins, with two overlapping open reading frames encoding two proteins: M2-1 and M2-2 ().Citation112,113 Several hRSV proteins play a role in evading the immune system of the host (see ). The hRSV glycoproteins, located in the viral envelope, that have been involved in interfering the immune response of the host are:
Figure 1. Viral Structure and Genome Organization of hRSV and hMPV. Schematic representations of hRSV and hMPV structures are shown. Both are negative single-stranded RNA (3′ to 5′), enveloped viruses that mainly differ in the number and order of genes in their genomes. These genes encode for P, N, SH, G, F, L, M, and M2 proteins, which are similar for both viruses. The M2 gene has an open reading frame that encodes for the M2-1 and M2-2 proteins. The hRSV genome also contains the non-structural proteins NS1 and NS2, which are absent in hMPV.
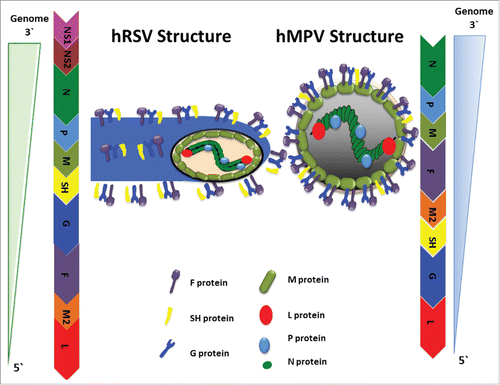
Table 2. Function of hRSV and hMPV proteins and their roles modulation of immune response.
HRSV Glycoprotein protein (G): this protein is involved in virus attachmentCitation114 and it also exists as a secreted form, which prevents opsonization and neutralization of hRSV by anti-G specific antibodies.Citation115 Moreover, the secreted form of the G protein has a chemokine-like motif (CX3C) that competes with fractalkine (CX3CL1) in binding to its receptor CX3CR1, thus reducing the CX3CR1+ T cells response.Citation116
HRSV Fusion (F) protein: this viral protein is required for the fusion of viral particle with the host cellsCitation4 and also play a direct role in the ability of hRSV to decrease the proliferation of these cells by contact.Citation117 In fact, when Vero cells express the F protein or are infected with a version of hRSV that only have F protein on its surface, they reduce the proliferation and response to mitogen stimulus.Citation117
HRSV small hydrophobic (SH) protein: it is thought that this protein works as an important viroporin during hRSV pathogenesis.Citation118 This protein also inhibits apoptosis in order to promote viral replication, as recombinant hRSV lacking the SH protein induced a significant cytopathic effect in different cell lines, compare with WT hRSV.119 In addition, the hRSV SH has been shown to inhibit the NF-κβ pathway through a decrease in the TNF-α production in mouse fibroblastic cells.Citation119
Another studies have demonstrated that glycoproteins affect the innate and adaptive immune response to hRSV. In this study, immunization of BALB/c mice with a recombinant strain of hRSV lacking both G and SH (CP52) increased the number of pulmonary natural killer (NK) cells, as well as the levels of IFN-γ and TNF-α at day 3 p.i. with a concordant reduced expression of the TH2 related cytokines (IL-4 and IL-6) when compare with the parental RSV strain (B1).Citation120 However, in the same study it was also shown that during primary infection, RSV-specific MHC II CTL precursor frequencies were delayed in CP52-immunized mice compare with B1-immunized mice in BALF, cervical lymph nodes and spleen at day 5 p.i.Citation120 Furthermore, during secondary infection, both RSV-specific MHC I and MHC II CTL precursor frequencies were delayed in spleen at day 3 p.i in CP52- immunized mice, as compare with mice immunized with B1 or control Vero cell lysate.Citation120 Altogether these data suggest that the hRSV G and/or SH proteins play a role in: a) downregulation of specific NK cell response, as mutants lacking these proteins increase pulmonary NK cells; b) polarization toward a TH2 immune response, as RSV strains deficient in these proteins decrease TH2 related cytokines.
Beside glycoproteins, hRSV also expresses other proteins involved in the impairment of the host's immune response, which are described below.
HRSV Non-structural (NS) proteins 1 and 2: these proteins impair the type I IFN pathway by targeting the interferon-regulatory factor 3 (IRF3), thus inhibiting the IFN-α/β antiviral signaling.Citation121 Additionally, these NS proteins suppress human DC maturation by downregulation of the type I IFN production; inhibit apoptosis; control protein stability; and regulate host cell mRNA.Citation122-125 Moreover, the NS2 protein induces cell rounding and shedding in vivo in hRSV-infected human ciliated cells in the large airways,Citation124 promoting the reduction of viral titers in the airway mucosa and initiating the obstruction of distal airways.Citation124 In studies using co-cultures of WT or mutant hRSV-infected human DCs with autologous CD4+ T cells, it has been demonstrated that NS1 protein promotes CD4+ T cell proliferation with a TH2 immune response.Citation126 In addition, NS1 was shown to suppress the CD8+ T cell anti-viral response, as there were increased levels of IFN-γ in the supernatant of lymphocytes co-cultured with human DCs infected with hRSV ΔNS1 as compare with lymphocytes co-cultured with DCs infected with WT RSV.Citation126 In the same study, it was also shown that NS1 suppresses the CD8+ CD103+ T cell activation and proliferation, which is required to accomplish the cytolytic function of these cells at the mucosal epithelium of the respiratory tract.Citation126 Therefore, the hRSV NS1 protein can impair the efficient anti-viral function of CD8+ T cell in different manners. Further, in vivo suppression of the CTL response by hRSV is mediated by NS2, as BALB/c mice infected with a hRSV mutant deficient in the NS2 gene (ΔNS2) or both the NS1 and NS2 genes (ΔNS1/ΔNS2) showed increased pulmonary hRSV-specific CTL responses compare with those of mice infected with WT hRSV or with the virus lacking NS1 (ΔNS1).Citation127
HRSV M2–1 protein: This is a transcription anti-termination factor important for the efficient synthesis of full-length viral mRNAs.Citation112 However, M2–1 has also been shown to activate the NFκβ pathway as demonstrated by translocation to the nuclei of the NFκβ factor in A549 cell transfected with a M2–1 encoding vector.Citation128
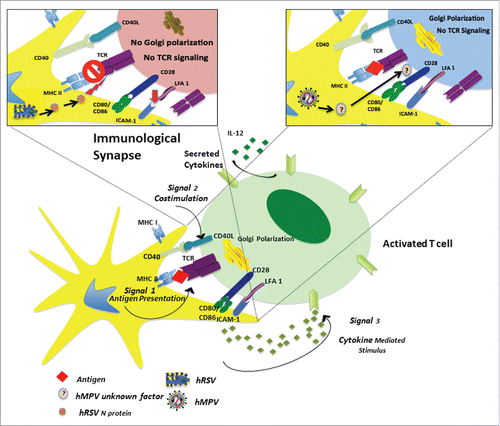
HRSV N protein: This protein is critical for the assembly of the hRSV nucleocapsid and protection of the viral RNA ().Citation129 Furthermore, the hRSV N protein interfere with the type I IFN pathway by targeting the mitochondrial antiviral signaling protein (MAVS), thus inhibiting the MAVS-dependent antiviral pathway.Citation130 Moreover, hRSV-infected DCs are unable to activate naïve CD4+ T cells in vitro probably due to the impairment of the immunological synapse assembly by the hRSV N protein expressed at the host cell membrane (, upper left box), rendering T cells unresponsive to subsequent TCR engagement..Citation27,93,131 Specifically, the Golgi apparatus polarization within T cells, an event needed for a proper immunological synapse assembly, is barely detectable in T cells co-cultured with hRSV-infected DCs, in contrast to T cells co-cultured with mock-inoculated DCs.Citation27 Moreover, impairing this signaling event significantly decreases tyrosine phosphorylation of TCR-associated CD3z-chain tyrosine-based activation motifs (ITAM) by LCK,Citation132 in naïve T cells stimulated with a cognate antigen.Citation27 Furthermore, immune synapse assembly inhibition is accompanied by a reduced binding of ICAM-1, suggesting that the N protein interferes with receptor-ligand interactions at the immunological synapse, reducing the TCR clusters that are usually observed within a mature immunological synapse.Citation131 A putative viral mechanism of this inhibitory effect could be the interaction of the hRSV N protein with an element of the TCR complex, since central clustering of this protein occurs alongside the TCR, even in the absence of pMHC.Citation131 Further studies are required to define the molecular mechanisms underlying the immune synapse inhibition by RSV infection.
Figure 2. Effect of hRSV and hMPV Infections on T cell Activation. To induce proper T cell activation by DCs presenting a specific antigen, the following three signals are required to establish a mature immunological synapse: i) interaction between MHC molecules (class I or II) and the TCR molecule; ii) co-stimulation of molecules, including CD80, CD86, and CD28; and iii) T cell-polarizing molecules, either soluble or membrane-bound. Additionally, adhesion molecules, such as the intracellular adhesion molecule 1, are also involved. In the amplified diagrams, the possible mechanisms by which hRSV or hMPV are able to impair antigen presentation are shown. In the case of hRSV (upper left box), the virus interferes with the immunological synapse through its N protein, which impairs Golgi polarization and TCR signaling. This inhibitory effect is accompanied by reduced intracellular adhesion molecule 1 binding to the lymphocyte-function associated antigen 1 (red arrow). In the case of hMPV (upper right box), the virus impairs TCR signaling and, consequently, T cell activation through an unknown soluble factor, which does not affect the immunological synapse itself.
Adaptive Immunity Triggered by hMPV
The humoral response against hMPV infection
It has been described that hMPV-specific antibodies are produced after hMPV infection in the childhood. The most predominant antibodies detected are anti-F antibodies after the first infection and can be detected over the time at least for 20 y evidenced by the fact that 95% of 20 y old individuals are seropositive for the F protein.Citation133 Likewise, it has been described that individuals with low levels of anti-hMPV antibodies are more susceptible to hMPV infection.Citation134 Studies in BALB/c mice have demonstrated that passive transfer of hyper-immune hMPV-specific mouse sera to naïve mice decreased virus titer, seven days post-infection, suggesting that hMPV-specific antibodies provide a level of protection from viral challenges.Citation135 However, the hMPV-specific antibody response appears to be inefficient in mediating viral clearance, since hMPV persists in lungs of infected mice despite the presence of neutralizing antibodies.Citation136 In fact, it has been observed that in humans hMPV can persist in immunocompromised patients, suggesting a principal role of immune response for control hMPV infection.Citation137,138 This could be explained by a waning effect on protective hMPV-specific antibodies over time, or the levels of these antibodies may not be sufficient to protect from a re-exposure to hMPV infection.Citation134 Moreover, in hMPV-infected BALB/c mice, seroconversion and the development of neutralizing anti-hMPV specific antibodies (IgG, IgG1, and IgG2a) are observed post-challenge, but these antibodies do not prevent the persistence of infectious hMPV.Citation136 In humans, from 257 hMPV-positive individuals, only a 25% remained asymptomatic, whereas 75% presented symptoms, despite their severity.Citation134 hMPV-infected individuals presented low IgA and IgG titer compare with non-infected individuals, and also lower neutralizing capacity, consistent to what is observed in mice.Citation134 Therefore, the humoral responses raised against hMPV following natural infection might not be sufficient to reduce re-infection episodes.Citation22
The cellular response against hMPV infection
For hMPV, it was recently shown that the presence of virus-specific T cells in airways and in lungs of BALB/c mice, are associated with an effective anti-viral immune response.Citation139 These data suggest that Trm T cells could also be important for controlling hMPV infection. However, further studies are still required, especially in humans.
CD4+ and CD8+ T cells are required for the clearance of hMPV from infected lungs in hMPV-infected BALB/c mice.Citation140 Likewise, an efficient anti-viral immune response based on IFN-γ-secreting CD4+ and CD8+ effector and memory T cells is necessary for preventing the spreading of hMPV in airways and to avoiding the development of bronchiolitis and pneumonitis. This is supported by a study, that used a candidate vaccine based on rBCG expressing hMPV antigens, where vaccination with this hMPV vaccine expressing the M2–1 or P protein protects BALB/c mice from hMPV-mediated lung pathology and reduced viral load in lungs.Citation31 However, this virus also evades these effective anti-viral immune responses.Citation26 Moreover, naïve BALB/c mice were shown to develop a biphasic immune response in vivo after hMPV infection.Citation135 During the first week, a TH1 response initially controls virus replication before polarizing toward a TH2 immune response that facilitates viral persistence.Citation135,136 A recent in vivo study, though, showed mixed TH1 and TH2 responses during the first week of hMPV infection in BALB/c mice.Citation141 Consistent with the notion observed in the mouse model, in adults undergoing hMPV infection, it has been described that PBMCs from these patients presented cytolitic activity, as evaluated by a Chrome-release assay, which suggest a TH1 response.Citation142 However, PBMCs, from healthy adults, stimulated with heat-inactivated hMPV promotes the induction of high levels IL-6 (a TH2 polarizing cytokine that prevents TH1 differentiationCitation143) and low levels of IFN-γ and CCR5 (TH1 cytokines) when compare with hRSV-stimulated PBMCs, thus suggesting a polarization toward a TH2 response.Citation144 In contrast, other studies demonstrate that infants undergoing hMPV infection presented lower levels of pro-inflammatory cytokines compare with infants undergoing hRSV or influenza infection, including TNF-α and IL-1β, two cytokines related to the chemotaxis of neutrophils in lungs, as well as IL-12, IL-6 and IL-8.Citation145 Furthermore, in infants undergoing hMPV infection it was observed that a predominant TH1 response is generated, since it was detected an increase in the IFN-γ/ IL-4 ratio in nasal airway secretions. Taken together, this data indicate that hMPV induces a complex immune response, which might include a mixed TH1/TH2 response, similar to what was observed in mice, though further studies are required to evaluate the T cell response in humans upon hMPV infection.
CD4+ T cell response against hMPV and mechanisms of evasion used by the virus
HMPV has also evasion mechanisms to interfere specifically with CD4+ T cell function that do not promote an efficient antiviral immune response and enhances lung pathology. Specifically, depletion of CD4+ T cells in hMPV-infected BALB/c mice reduces lung pathology and airway obstruction, without affecting viral loads.Citation140 These data demonstrate that the subset of CD4+ T cells contributes to lung pathology but they are not critical for viral clearance.
As has been mentioned above, hMPV can induce a mixed TH1/TH2 response and this type of response is dependent of activation of TSLP pathway, which favors TH2 response over TH1 response that is known is necessary for hMPV clearance. Related to this, hMPV infection has been proven: to promote TSLP expression in both human AECs and mouse lungs; to stimulate OX40L+CD11b+DCs lung infiltration; and to increase the levels of pro-inflammatory TH2 cytokines-producing T cells, including TARC, IL-5 and IL-13, but also TNF-α in BALB/c mice,Citation141 as previously reported for OX40L on TSLP-activated DCs.Citation86 Moreover, TSLPR−/− mice showed decreased lung inflammation and hMPV replication, as well as a higher frequency of CD8+ and CD4+ T cells.Citation141 These findings highlight the possibility that a repertoire of virus-specific TH and CTLs may incompletely eliminate infected cells within the airways following primary infection, leading to an exacerbated inflammatory response, mainly mediated by the TSLP pathway, thus inducing an aberrant T cell response.Citation141
Moreover, hMPV-infected DCs, similarly to hRSV-infected DCs, also impair the activation of CD4+ T cells. Upon stimulation with hMPV-infected and antigen loaded DCs, naïve antigen-specific CD4+ T cells displayed significantly reduced proliferation, expression of surface activation markers, such as CD25 (IL-2 receptor/IL-2Rα), CD69 and CD71 (transferrin receptor) and IL-2 secretion, as compare with T cells stimulated with uninfected control DCs.Citation26 However, this inefficiency is not due to a deterioration of the immune synapse assembly since both TCR cluster formation and Golgi polarization still occur (, upper left box).Citation26 This impairment may contribute to a delayed TH1 response. Indeed, CD4+ T cells elicit a poor IFN-γ response when activated with hMPV-infected human peripheral blood mononuclear cells in vitro,Citation144 suggesting that these hMPV-infected cells can also inhibit the function of CD4+ T cells to stimulate an efficient antiviral TH1 immune response in humans. The impairment of T cell immunity could be the result of impaired DC functions by hMPV, possibly through of a soluble factor derived from hMPV-infected DCs, as supernatants from these infected cells impairs T cells activation when stimulated by plate-bound anti-CD3ϵ and anti-CD28.Citation26 Thus, hMPV impairs the TCR signaling, without disturbing the immune synapse formation and Golgi polarization in T cellsCitation26 (, upper right box). Consistent with the latter, the SH and/or G proteins reduced CD4+ T cell proliferation in a co-culture assay of hMPV-infected MDDC with CD4+ T cells when compare with T cells co-cultured with ΔSH/G hMPV-infected DCs.Citation146 Likewise, in vitro studies show that human and mouse hMPV-infected DCs lose the capacity to activate and expand naïve T cells, although to a lesser degree than hRSV-infected DCs.Citation26,146-148
Furthermore, the neutralizing antibodies detected in mice after hMPV infection, appear to be dependent on CD4+ T cells, because no detectable neutralizing antibodies were observed in hMPV-infected mice depleted of CD4+ T cells.Citation140
CD8+ T cell response against hMPV and mechanisms of evasion used by the virus
In a similar manner as for hRSV, CD8+ T cell response specific to hMPV antigens is critical for an effective viral clearance, as demonstrated in studies using vaccine candidates against hMPV, such as virus-like particles harboring hMPV F or hMPV M antigens.Citation149 Supporting this notion, hMPV-specific effector CD8+ T cells, in BALB/c mice that lack protective anti-hMPV antibodies and in the absence of CD4+ T cells, no detection of virus load and reduction of lung disease were found upon hMPV infection, suggesting that the cytotoxic activity of CD8+ T cells alone can confer protection against hMPVCitation140 and highlighting the importance of CD8+ T cells in controlling hMPV infection. Moreover, when specific hMPV M2 CTLs were transferred into RAG-1−/− mice, these lymphocytes protected the host of hMPV challenge.Citation150 Furthermore, hMPV-specific IFN-γ-producing CD8+ T cells can be found in the mucosa of the airways and in lungs, but not in the lymph nodes or in spleen of hMPV-infected BALB/c mice at 7 d p.i, suggesting the activation of the Trm T cells specific for hMPV epitopes.Citation139 Nevertheless, hMPV-virus specific CTLs were also induced 21 d p.i in spleen.Citation139 These data underscore the importance of Trm CD8+ T cells in controlling rapidly hMPV infections. Recently, in human has been described that memory CD8+ T cells are reactive to the majority of hMPV proteins (M, F, G, M2-1, N and SH) and particularly, CD8+ T cells that recognize M and F protein can secrete IFN-γ after 21 months post-infection.Citation142 Nevertheless, further studies are required in humans to clarify if this CD8+ T cells populations are protective or not, because independently of the presence of this population, hMPV keeps its capacity to generate re-infection episodes.
Recent studies associate the lack of the type I IFN pathway with CD8+ T cell impairment.Citation151 In fact, hMPV infected-IFNAR−/− mice had a higher peak of early viral replication, less airway dysfunction and lung inflammation, but cleared the virus with the same kinetics as observed in WT mice.Citation151 Likewise, CD8+ T cells from IFNRA−/− mice expressed similar levels of PD-L1 when compare with CD8+ T cells from WT mice. However, these cells showed an upregulation of the inhibitory receptor TIM-3, thus impairing the CD8+ T cell function.Citation151 Additionally, CD8+ T cells can be impaired by hMPV in a PD-1 dependent manner, similar to hRSV, as lung CD8+ T cells are impaired in HLA B7.2 transgenic (B7tg) mice and had upregulated PD-1.Citation152 Conversely, blocking of PD-1 by administration of monoclonal specific antibodies in B7tg mice prevented the CD8+ T cells impairment. Similarly, impairment of CD8+ T cells was prevented on hMPV-infected PD-1−/− mice.Citation152
In other studies, an impairment of lung hMPV-specific memory CD8+ T cells was observed in μMT mice, which lack B-cells and are used as a model for hMPV reinfection, which suggest the importance of memory CD8+ T cells in viral clearance during a second infection.Citation153 Specifically, during reinfection, CD8+ T cells had upregulated several inhibitory receptors, including PD-1.Citation153 Similarly to it is observed in B7tg mice, blockade of PD-1 in μMT mice restored lung CD8+ T cell effector functions (i.e., degranulation and cytokine production) and enhanced viral clearance.Citation153 In other studies, immunization of μMT mice with virus-like particles encoding the hMPV F and M proteins, generates hMPV F-specific and M-specific CD8+ T cells in lungs, but their function is impaired, as inhibitory receptors are upregulated on these cells, similar to what is seen in WT C57BL/6 mice under a second hMPV infection.Citation149
On the other hand, the depletion of CD8+ T cells in hMPV-infected BALB/c mice resulted in a lower lung pathological score, although to a lesser degree than depletion of only CD4+ T cells.Citation140 By other part, mice that was depleted of neutrophils showed a significant reduction of TNF-α and IL-13 secreted by CD8+ T cells suggesting that neutrophils modulate the production of these cytokines by CD8+ T cells, Citation141In this manner, CD8+ T cells that infiltrate in the airways and secrete TNF-α and IL13 contribute to the lung pathology triggered by hMPV infection in mice.Citation140,141
Taken together, CD8+ T cells confer protection against hMPV infection through cytotoxic activities, but these cells may also contribute to lung pathology.Citation140 Furthermore, the function of these cells can be hampered by evasion mechanisms of the virus. However, further studies in humans are still required.
HMPV molecular characteristics and the role of its viral proteins in immunomodulation
The hMPV genome is comprised of a negative, 13 kb single-stranded RNA with the following eight genes: N-P-M-F-M2-SH-G-L (3′ to 5′).Citation18 Furthermore, the hMPV mRNA transcribed by the M2 gene contains two overlapping open reading frames that give rise to the M2–1 and M2–2 proteins, in similar manner than RSV ().Citation18 In contrast to RSV, hMPV lacks the NS1 and NS2 genes, thus the inhibition of the type I IFN pathway is less robust than the one is observed in RSV.Citation154 The G protein, a glycoprotein involved in the attachment of the viral particle,Citation155 inhibits the type I IFN pathway by targeting the retinoic-inducible gene 1 (RIG-I), thus it contributes in inhibiting the antiviral innate response of the host.Citation156 Likewise, the SH protein, a type 2 transmembrane protein with properties as a viroporin, has been shown to inhibit the NFκβ pathway, as infection with a recombinant virus lacking the SH gene increased the NFκβ-dependent transcription pathway in BALB/c mice.Citation157 The M protein, which participates in virus assembly and packaging,Citation158 stimulates the inflammatory response in vitro by inducing the maturation of monocyte-derived DCs and the secretion of inflammatory cytokines by these cells, including IL-8, IL-6, IL-1β and TNF-α ().Citation159
In addition, the M2–1 protein is critical for hMPV replication and pathogenesis through its Zinc binding activity, since a recombinant hMPV carrying mutations in the zinc binging motif was highly attenuated in cotton rats.Citation160 This is consistent with studies that demonstrate that hMPV lacking the M2–1 gene could not replicate in hamsters.Citation161 However, is not essential for hMPV infectivity and growth in vitro as deletion of the ORF of the M2–1 protein is dispensable for viral replication in VERO cells.Citation161 In contrast, the hMPV M2–2 protein, defined as a terminator factor, since it inhibits viral replication and transcription,Citation162 interact with MAVS,Citation163 a protein that links the cytoplasmic viral sensors RIG-I and the melanoma differentiation associated protein 5 (MDA5) to the downstream TNF receptor-associated factors (TRAFs) and IκB kinases (IKKs), which are known to induce the type I IFN-pathway through the activation of IRF-3 and NFκB.Citation163 Thus, hMPV M2–2 protein contributes in decreasing efficiently the anti-viral response.
Concluding remarks
Adequate T cell priming is critical for establishing effective anti-viral immune responses.Citation164,165 Due to the importance of this process several respiratory viruses, including hRSVCitation27 and hMPVCitation26 have evolved different mechanisms to impair T cell activation. Indeed, both of these viruses impair the T cell response, causing, at least in part, an aberrant adaptive immune response and poor immunological memory against hRSVCitation71,166 and hMPV.Citation136 However, in both cases, memory T cells are produced after infection but the role of these cells are still controversial because reinfection episodes are recurrent in both viruses. By the other hand, the viral mechanism underlying the inhibition of T cell functions for both viruses is different.Citation26,131 hRSV impairs T cell activation by preventing a mature immune synapse assembly, possibly through the hRSV N protein at the DC plasma membrane.Citation131 Conversely, hMPV prevents T cell activation,Citation26 likely though a soluble factor(s) without interfering with the formation of a mature immune synapse and Golgi polarization in the T cell, as the inhibition of the TCR signaling is induced with the supernatants of hMPV-infected DCs.Citation26
Interference at the immune synapse level by hRSV and T cell activation by hMPV prevents the TH1 polarization required to induce an efficient antiviral response.Citation26,27 Additionally, hRSV infection triggers detrimental inflammation in the airways that is characterized by an exacerbated TH2 response, thus preventing adequate viral clearance.Citation167 This exacerbated TH2-type immune response to hRSV could be mediated by TSLP through activation of the OX40/OX40L interaction, which consequently stimulates an inappropriate subset of T cells.Citation80 In addition, IL-25, detected in lungs of infected hRSV mice, can also contribute to the hRSV-mediated lung pathology.Citation91
Similarly to hRSV, hMPV activates the TSLP pathway to induce a mixture of TH2- and TH1-type responses, most likely through OX40/OX40L interactions.Citation141 This mixed induction causes pathology and promotes viral replication in mice lungs.Citation141
Similarly, to T cells response, antibody response is not effective to reduce reinfection and probably this is mediated by the interaction between B and T cells during infection. Where T cells that present a polarization to Th2 in case of hRSV or Th1/Th2 in case of hMPV, modulate B cell response generating non-protective antibodies. Contrary, has been showed that neutralizing antibodies are produced after infection with these viruses but the proportion between neutralizing and non-neutralizing could be mediate a better outcome.
The respective evasion mechanisms induced by each virus could synergistically act to prevent the activation of an effective anti-viral T and B cell response during infection periods. In this sense, is important to advance in human studies with these two viruses and the mechanism behind its immune modulation.
Abbreviations
| AEC | = | Airway epithelial cell |
| BEC | = | bronchial epithelial cell |
| BCG | = | Bacillus Calmette-Guérin |
| CTL | = | cytotoxic T lymphocyte |
| DC | = | Dendritic cell |
| hMPV | = | Human metapneumovirus |
| IFN | = | interferon |
| M | = | matrix |
| mDC | = | myeloid dendritic cell |
| N | = | nucleoprotein |
| NS | = | non-structural |
| pMHC | = | peptide major histocompatibility complex |
| hRSV | = | human Respiratory Syncytial Virus |
| SH | = | small hydrophobic |
| SV | = | Sendai virus |
| TCR | = | T cell receptor |
| TSLP | = | Thymic stromal lymphopoietin |
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Acknowledgments
The authors acknowledge Ms. Virna Salazar for critically reading the manuscript.
Funding
Funding awarded by Grants NO 1158262 and 3150559, from the National Fund for Scientific and Technological Development (FONDECYT) program, Ministry of Education, Chile; Grant NO D11/1080, from the Fund for the Promotion of Scientific and Technological Development (FONDEF), Ministry of Education, Chile; and Grant P09/P016-F, from the Millennium Institute of Immunology and Immunotherapy, Ministry of Economy, Chile.
References
- Afonso CL, Amarasinghe GK, Banyai K, Bao Y, Basler CF, Bavari S, Bejerman N, Blasdell KR, Briand FX, Briese T, et al. Taxonomy of the order Mononegavirales: update 2016. Arch Virol 2016; 161:2351-60; PMID:27216929; http://dx.doi.org/10.1007/s00705-016-2880-1
- Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev 1999; 12:298-309; PMID:10194461
- Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, Peret TC, Erdman DD, Anderson LJ. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis 2002; 186:1330-4; PMID:12402203; http://dx.doi.org/10.1086/344319
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 2008; 82:2040-55; PMID:17928346; http://dx.doi.org/10.1128/JVI.01625-07
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545-55; PMID:20399493; http://dx.doi.org/10.1016/S0140-6736(10)60206-1
- Hansbro PM, Starkey MR, Mattes J, Horvat JC. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc 2014; 11 Suppl 5:S297-302; PMID:25525736; http://dx.doi.org/10.1513/AnnalsATS.201402-086AW
- Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, Lukšić I, Fischer Walker CL, Black RE, Campbell H, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 2013; 3:010401; PMID:23826505; http://dx.doi.org/10.7189/jogh.03.010101
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095-128; PMID:23245604; http://dx.doi.org/10.1016/S0140-6736(12)61728-0
- Beem M, Wright FH, Hamre D, Egerer R, Oehme M. Association of the chimpanzee coryza agent with acute respiratory disease in children. N Engl J Med 1960; 263:523-30; PMID:13798226; http://dx.doi.org/10.1056/NEJM196009152631101
- Blount RE, Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med 1956; 92:544-9; PMID:13359460; http://dx.doi.org/10.3181/00379727-92-22538
- Chanock R, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. Am J Hyg 1957; 66:291-300; PMID:13478579
- Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg 1957; 66:281-90; PMID:13478578
- Walsh EE, Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol 1983; 47:171-7; PMID:6345804
- Kahn JS. Human metapneumovirus, a newly emerging respiratory virus. Pediatr Infect Dis J 2003; 22:923-4; PMID:14551495; http://dx.doi.org/10.1097/01.inf.0000091347.27554.ff
- Collins MS, Gough RE. Characterization of a virus associated with turkey rhinotracheitis. J Gen Virol 1988; 69(Pt 4):909-16; PMID:3356981; http://dx.doi.org/10.1099/0022-1317-69-4-909
- Cook JK. Avian pneumovirus infections of turkeys and chickens. Vet J 2000; 160:118-25; PMID:10985803; http://dx.doi.org/10.1016/S1090-0233(00)90486-6
- de Graaf M, Osterhaus AD, Fouchier RA, Holmes EC. Evolutionary dynamics of human and avian metapneumoviruses. J Gen Virol 2008; 89:2933-42; PMID:19008378; http://dx.doi.org/10.1099/vir.0.2008/006957-0
- Schildgen V, van den Hoogen B, Fouchier R, Tripp RA, Alvarez R, Manoha C, Williams J, Schildgen O. Human Metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev 2011; 24:734-54; PMID:21976607; http://dx.doi.org/10.1128/CMR.00015-11
- Feuillet F, Lina B, Rosa-Calatrava M, Boivin G. Ten years of human metapneumovirus research. J Clin Virol 2012; 53:97-105; PMID:22074934; http://dx.doi.org/10.1016/j.jcv.2011.10.002
- Boivin G, De Serres G, Hamelin ME, Cote S, Argouin M, Tremblay G, Maranda-Aubut R, Sauvageau C, Ouakki M, Boulianne N, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis 2007; 44:1152-8; PMID:17407031; http://dx.doi.org/10.1086/513204
- Bont L, Versteegh J, Swelsen WT, Heijnen CJ, Kavelaars A, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, et al. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr Res 2002; 52:363-7; PMID:12193668; http://dx.doi.org/10.1203/00006450-200209000-00009
- Pavlin JA, Hickey AC, Ulbrandt N, Chan YP, Endy TP, Boukhvalova MS, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, et al. Human metapneumovirus reinfection among children in Thailand determined by ELISA using purified soluble fusion protein. J Infect Dis 2008; 198:836-42; PMID:18680407; http://dx.doi.org/10.1086/591186
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000; 13:371-84; PMID:10885982; http://dx.doi.org/10.1128/CMR.13.3.371-384.2000
- Haas LEM, Thijsen SFT, van Elden L, Heemstra KA. Human Metapneumovirus in Adults. Viruses 2013; 5:87-110; PMID:23299785; http://dx.doi.org/10.3390/v5010087
- Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med 2008; 168:2489-96; PMID:19064834; http://dx.doi.org/10.1001/archinte.168.22.2489
- Cespedes PF, Gonzalez PA, Kalergis AM. Human metapneumovirus keeps dendritic cells from priming antigen-specific naive T cells. Immunology 2013; 139:366-76; PMID:23374037; http://dx.doi.org/10.1111/imm.12083
- Gonzalez PA, Prado CE, Leiva ED, Carreno LJ, Bueno SM, Riedel CA, Kalergis AM. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A 2008; 105:14999-5004; PMID:18818306; http://dx.doi.org/10.1073/pnas.0802555105
- de Graaff PMA, de Jong EC, van Capel TM, van Dijk MEA, Roholl PJM, Boes J, Luytjes W, Kimpen JL, van Bleek GM. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol 2005; 175:5904-11; PMID:16237083; http://dx.doi.org/10.4049/jimmunol.175.9.5904
- Le Nouën C, Hillyer P, Brock LG, Winter CC, Rabin RL, Collins PL, Buchholz UJ. Human metapneumovirus SH and G glycoproteins inhibit macropinocytosis-mediated entry into human dendritic cells and reduce CD4(+) T cell activation. J Virol 2014; 88:6453-69; http://dx.doi.org/10.1128/JVI.03261-13
- Deffrasnes C, Hamelin ME, Prince GA, Boivin G. Identification and evaluation of a highly effective fusion inhibitor for human metapneumovirus. Antimicrob Agents Chemother 2008; 52:279-87; PMID:17967906; http://dx.doi.org/10.1128/AAC.00793-07
- Palavecino CE, Cespedes PF, Gomez RS, Kalergis AM, Bueno SM. Immunization with a Recombinant Bacillus Calmette-Guerin Strain Confers Protective Th1 Immunity against the Human Metapneumovirus. J Immunol 2014; 192:214-23; http://dx.doi.org/10.4049/jimmunol.1300118
- Mazur NI, Martinon-Torres F, Baraldi E, Fauroux B, Greenough A, Heikkinen T, Manzoni P, Mejias A, Nair H, Papadopoulos NG, et al. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med 2015; 3(11):888-900; PMID:26411809; http://dx.doi.org/10.1016/S2213-2600(15)00255-6
- Sun Z, Wang Q, Jia R, Xia S, Li Y, Liu Q, Xu W, Xu J, Du L, Lu L, et al. Intranasal administration of maleic anhydride-modified human serum albumin for pre-exposure prophylaxis of respiratory syncytial virus infection. Viruses 2015; 7:798-819; PMID:25690799; http://dx.doi.org/10.3390/v7020798
- Schickli JH, Whitacre DC, Tang RS, Kaur J, Lawlor H, Peters CJ, Jones JE, Peterson DL, McCarthy MP, Van Nest G, et al. Palivizumab epitope-displaying virus-like particles protect rodents from RSV challenge. J Clin Invest 2015; 125:1637-47; PMID:25751145; http://dx.doi.org/10.1172/JCI78450
- Pham QN, Biacchesi S, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Chimeric recombinant human metapneumoviruses with the nucleoprotein or phosphoprotein open reading frame replaced by that of avian metapneumovirus exhibit improved growth in vitro and attenuation in vivo. J Virol 2005; 79:15114-22; PMID:16306583; http://dx.doi.org/10.1128/JVI.79.24.15114-15122.2005
- Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr Infect Dis J 2009; 28:655-8; PMID:19483659; http://dx.doi.org/10.1097/INF.0b013e318199c3b1
- Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 2003; 21:3465-7; PMID:12850361; http://dx.doi.org/10.1016/S0264-410X(03)00352-9
- Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guerin promotes virus clearance and protects from infection. J Immunol 2010; 185:7633-45; PMID:21084664; http://dx.doi.org/10.4049/jimmunol.0903452
- Bueno SM, Gonzalez PA, Cautivo KM, Mora JE, Leiva ED, Tobar HE, Fennelly GJ, Eugenin EA, Jacobs WR, Jr, Riedel CA, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci U S A 2008; 105:20822-7; PMID:19075247; http://dx.doi.org/10.1073/pnas.0806244105
- Ma X, Endo R, Ebihara T, Ishiguro N, Ishiko H, Kikuta H. Production and characterization of neutralizing monoclonal antibodies against human metapneumovirus F protein. Hybridoma (Larchmt) 2005; 24:201-5; PMID:16120026; http://dx.doi.org/10.1089/hyb.2005.24.201
- Mejias A, Chavez-Bueno S, Rios AM, Aten MF, Raynor B, Peromingo E, Soni P, Olsen KD, Kiener PA, Gómez AM, et al. Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency. Antimicrob Agents Chemother 2005; 49:4700-7; PMID:16251314; http://dx.doi.org/10.1128/AAC.49.11.4700-4707.2005
- Subramanian KN, Weisman LE, Rhodes T, Ariagno R, Sanchez PJ, Steichen J, Givner LB, Jennings TL, Top FH, Jr, Carlin D, et al. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group. Pediatr Infect Dis J 1998; 17:110-5; http://dx.doi.org/10.1097/00006454-199802000-00006
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749-59; PMID:15858184; http://dx.doi.org/10.1056/NEJMoa043951
- Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol 2006; 78:1493-7; PMID:16998887; http://dx.doi.org/10.1002/jmv.20724
- Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 2004; 190:373-8; PMID:15216475; http://dx.doi.org/10.1086/421524
- Vissers M, Ahout IM, de Jonge MI, Ferwerda G. Mucosal IgG levels correlate better with respiratory syncytial virus load and inflammation than plasma IgG levels. Clin Vaccine Immunol 2015; 23:243-5; PMID:26656116; http://dx.doi.org/10.1128/CVI.00590-15
- Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, de Haan CA, Wrammert J, Openshaw PJ, Chiu C. Impaired Antibody-mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am J Respir Crit Care Med 2015; 191:1040-9; PMID:25730467; http://dx.doi.org/10.1164/rccm.201412-2256OC
- Walsh E, Falsey AR. Humoral and Mucosal immunity in protection from Natural Respiratory Syncytial virus infection in adults. J Infect Dis 2004; 190:373-8; PMID:15216475; http://dx.doi.org/10.1086/421524
- Graham BS, Bunton LA, Rowland J, Wright PF, Karzon DT. Respiratory syncytial virus infection in anti-mu-treated mice. J Virol 1991; 65:4936-42; PMID:1908028
- Homaira N, Rawlinson W, Snelling TL, Jaffe A. Effectiveness of Palivizumab in Preventing RSV hospitalization in high risk children: A real-world perspective. Int J Pediatr 2014; 2014:571609; PMID:25548575; http://dx.doi.org/10.1155/2014/571609
- Lambert L, Sagfors AM, Openshaw PJM, Culley FJ. Immunity to RSV in Early-Life. Frontiers Immunol 2014; 5:466; PMID:25324843; http://dx.doi.org/10.3389/fimmu.2014.00466
- Sande CJ, Mutunga MN, Okiro EA, Medley GF, Cane PA, Nokes DJ. Kinetics of the neutralizing antibody response to respiratory syncytial virus infections in a birth cohort. J Medical Virol 2013; 85:2020-5; PMID:23983183; http://dx.doi.org/10.1002/jmv.23696
- Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJ, Verbeek JS, Ossendorp F. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J Immunol 2002; 168:2240-6; PMID:11859111; http://dx.doi.org/10.4049/jimmunol.168.5.2240
- Kruijsen D, Bakkers MJ, van Uden NO, Viveen MC, van der Sluis TC, Kimpen JL, Leusen JH, Coenjaerts FE, van Bleek GM. Serum antibodies critically affect virus-specific CD4+/CD8+ T cell balance during respiratory syncytial virus infections. J Immunol 2010; 185:6489-98; PMID:20971927; http://dx.doi.org/10.4049/jimmunol.1002645
- Kruijsen D, Einarsdottir HK, Schijf MA, Coenjaerts FE, van der Schoot EC, Vidarsson G, van Bleek GM. Intranasal administration of antibody-bound respiratory syncytial virus particles efficiently primes virus-specific immune responses in mice. J Virol 2013; 87:7550-7; PMID:23637394; http://dx.doi.org/10.1128/JVI.00493-13
- Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H, Lirman DD, Rabold R, Hoffman SJ, Karp CL, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. The J Exp Med 2002; 196:859-65; PMID:12235218; http://dx.doi.org/10.1084/jem.20020781
- Rossey I, Sedeyn K, De Baets S, Schepens B, Saelens X. CD8+ T cell immunity against human respiratory syncytial virus. Vaccine 2014; 32:6130-7; PMID:25223272; http://dx.doi.org/10.1016/j.vaccine.2014.08.063
- Christiaansen AF, Knudson CJ, Weiss KA, Varga SM. The CD4 T cell response to respiratory syncytial virus infection. Immunol Res 2014; 59:109-17; PMID:24838148; http://dx.doi.org/10.1007/s12026-014-8540-1
- Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EH, Sykes A, Maybeno M, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun 2015; 6:10224; PMID:26687547; http://dx.doi.org/10.1038/ncomms10224
- Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, Cohen HJ. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med 1986; 315:77-81; PMID:3724802; http://dx.doi.org/10.1056/NEJM198607103150201
- Cannon MJ, Stott EJ, Taylor G, Askonas BA. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology 1987; 62:133-8; PMID:3498683
- Bagga B, Cehelsky JE, Vaishnaw A, Wilkinson T, Meyers R, Harrison LM, Roddam PL, Walsh EE, DeVincenzo JP. Effect of preexisting serum and mucosal antibody on experimental Respiratory Syncytial Virus (RSV) challenge and infection of adults. J Infect Dis 2015; 212:1719-25; PMID:25977264; http://dx.doi.org/10.1093/infdis/jiv281
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012; 483:227-31; PMID:22388819; http://dx.doi.org/10.1038/nature10851
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 2013; 14:509-13; PMID:23542740; http://dx.doi.org/10.1038/ni.2568
- Oshansky CM, Zhang W, Moore E, Tripp RA. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol 2009; 4:279-97; PMID:19327115; http://dx.doi.org/10.2217/fmb.09.1
- de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med 2005; 202:1433-42; PMID:16301748; http://dx.doi.org/10.1084/jem.20051365
- Ostler T, Davidson W, Ehl S. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur J Immunol 2002; 32:2117-23; PMID:12209623; http://dx.doi.org/10.1002/1521-4141(200208)32:8%3c2117::AID-IMMU2117%3e3.0.CO;2-C
- Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med 1988; 168:1163-8; PMID:3262705; http://dx.doi.org/10.1084/jem.168.3.1163
- Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 1994; 71:428-32; PMID:7826113; http://dx.doi.org/10.1136/adc.71.5.428
- McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child 2003; 88:922-6; PMID:14500316; http://dx.doi.org/10.1136/adc.88.10.922
- Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 2007; 195:1126-36; PMID:17357048; http://dx.doi.org/10.1086/512615
- Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, Garofalo RP. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2011; 183:1550-60; PMID:21471094; http://dx.doi.org/10.1164/rccm.201010-1755OC
- Funchal GA, Jaeger N, Czepielewski RS, Machado MS, Muraro SP, Stein RT, Bonorino CB, Porto BN. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One 2015; 10:e0124082; PMID:25856628; http://dx.doi.org/10.1371/journal.pone.0124082
- El Saleeby CM, Suzich J, Conley ME, DeVincenzo JP. Quantitative effects of palivizumab and donor-derived T cells on chronic respiratory syncytial virus infection, lung disease, and fusion glycoprotein amino acid sequences in a patient before and after bone marrow transplantation. Clin Infect Dis 2004; 39:e17-20; PMID:15307047; http://dx.doi.org/10.1086/421779
- Roman M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, Gaggero A, Díaz PV. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med 1997; 156:190-5; PMID:9230746; http://dx.doi.org/10.1164/ajrccm.156.1.9611050
- Bermejo-Martin JF, Garcia-Arevalo MC, De Lejarazu RO, Ardura J, Eiros JM, Alonso A, Matías V, Pino M, Bernardo D, Arranz E, et al. Predominance of Th2 cytokines, CXC chemokines and innate immunity mediators at the mucosal level during severe respiratory syncytial virus infection in children. Eur Cytokine Netw 2007; 18:162-7; PMID:17823085
- Aberle JH, Aberle SW, Dworzak MN, Mandl CW, Rebhandl W, Vollnhofer G, Kundi M, Popow-Kraupp T. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med 1999; 160:1263-8; PMID:10508817; http://dx.doi.org/10.1164/ajrccm.160.4.9812025
- Sung RY, Hui SH, Wong CK, Lam CW, Yin J. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur J Pediatr 2001; 160:117-22; PMID:11271383; http://dx.doi.org/10.1007/s004310000676
- McNamara PS, Flanagan BF, Selby AM, Hart CA, Smyth RL. Pro- and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur Respir J 2004; 23:106-12; PMID:14738241; http://dx.doi.org/10.1183/09031936.03.00048103
- Lee HC, Headley MB, Loo YM, Berlin A, Gale M, Jr, Debley JS, Lukacs NW, Ziegler SF. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol 2012; 130:1187-96 e5; http://dx.doi.org/10.1016/j.jaci.2012.07.031
- Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff H, Lincoln P, Maassab H. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol 2001; 167:1060-5; PMID:11441116; http://dx.doi.org/10.4049/jimmunol.167.2.1060
- Rothoeft T, Fischer K, Zawatzki S, Schulz V, Schauer U, Korner Rettberg C. Differential response of human naive and memory/effector T cells to dendritic cells infected by respiratory syncytial virus. Clin Exp Immunol 2007; 150:263-73; PMID:17892510; http://dx.doi.org/10.1111/j.1365-2249.2007.03497.x
- He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci 2010; 1183:13-24; PMID:20146705; http://dx.doi.org/10.1111/j.1749-6632.2009.05128.x
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3:673-80; PMID:12055625; http://dx.doi.org/10.1038/nrm910
- Qiao J, Li A, Jin X. TSLP from RSV-stimulated rat airway epithelial cells activates myeloid dendritic cells. Immunol Cell Biol 2011; 89:231-8; PMID:20603637; http://dx.doi.org/10.1038/icb.2010.85
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202:1213-23; PMID:16275760; http://dx.doi.org/10.1084/jem.20051135
- You D, Marr N, Saravia J, Shrestha B, Lee GI, Turvey SE, Brombacher F, Herbert DR, Cormier SA. IL-4Ralpha on CD4+ T cells plays a pathogenic role in respiratory syncytial virus reinfection in mice infected initially as neonates. J Leukoc Biol 2013; 93:933-42; PMID:23543769; http://dx.doi.org/10.1189/jlb.1012498
- Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 1991; 88:1026-33; PMID:1909350; http://dx.doi.org/10.1172/JCI115362
- Cherukuri A, Patton K, Gasser RA, Jr, Zuo F, Woo J, Esser MT, Tang RS. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol 2013; 20:239-47; PMID:23239796; http://dx.doi.org/10.1128/CVI.00580-12
- Openshaw PJ, Chiu C. Protective and dysregulated T cell immunity in RSV infection. Curr Opin Virol 2013; 3:468-74; PMID:23806514; http://dx.doi.org/10.1016/j.coviro.2013.05.005
- Petersen BC, Dolgachev V, Rasky A, Lukacs NW. IL-17E (IL-25) and IL-17RB promote respiratory syncytial virus-induced pulmonary disease. J Leukoc Biol 2014; 95(5):809-815; http://dx.doi.org/10.1189/jlb.0913482
- Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol 2011; 179:248-58; PMID:21703407; http://dx.doi.org/10.1016/j.ajpath.2011.03.003
- Qin L, Hu CP, Feng JT, Xia Q. Activation of lymphocytes induced by bronchial epithelial cells with prolonged RSV infection. PLoS One 2011; 6:e27113; PMID:22216085; http://dx.doi.org/10.1371/journal.pone.0027113
- Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, Anderson LJ. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol 2013; 87:13466-79; PMID:24089561; http://dx.doi.org/10.1128/JVI.01741-13
- Faber TE, Groen H, Welfing M, Jansen KJ, Bont LJ. Specific increase in local IL-17 production during recovery from primary RSV bronchiolitis. J Med Virol 2012; 84:1084-8; PMID:22585726; http://dx.doi.org/10.1002/jmv.23291
- Stokes KL, Currier MG, Sakamoto K, Lee S, Collins PL, Plemper RK, Moore ML. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol 2013; 87:10070-82; PMID:23843644; http://dx.doi.org/10.1128/JVI.01347-13
- Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Page K, Blumenthal RM, Ward NL, Miyazaki T, Takashima A. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood 2013; 121:1690-700; PMID:23305733; http://dx.doi.org/10.1182/blood-2012-07-445197
- Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N, Kotol PF, Chojnacki BJ, Miyazaki T, Gallo RL, et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 2013; 121:1677-89; PMID:23305731; http://dx.doi.org/10.1182/blood-2012-07-445189
- Halfhide CP, Flanagan BF, Brearey SP, Hunt JA, Fonceca AM, McNamara PS, Howarth D, Edwards S, Smyth RL. Respiratory syncytial virus binds and undergoes transcription in neutrophils from the blood and airways of infants with severe bronchiolitis. J Infect Dis 2011; 204:451-8; PMID:21742845; http://dx.doi.org/10.1093/infdis/jir280
- Kulkarni AB, Morse HC, Bennink JR, Yewdell JW, Murphy BR. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol 1993; 67:4086-92; PMID:7685408
- Bartholdy C, Olszewska W, Stryhn A, Thomsen AR, Openshaw PJ. Gene-gun DNA vaccination aggravates respiratory syncytial virus-induced pneumonitis. J Gen Virol 2004; 85:3017-26; PMID:15448365; http://dx.doi.org/10.1099/vir.0.80098-0
- Simmons CP, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J Immunol 2001; 166:1106-13; PMID:11145691; http://dx.doi.org/10.4049/jimmunol.166.2.1106
- Ruckwardt TJ, Luongo C, Malloy AM, Liu J, Chen M, Collins PL, Graham BS. Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope. J Immunol 2010; 185:4673-80; PMID:20833834; http://dx.doi.org/10.4049/jimmunol.1001606
- Liu J, Haddad EK, Marceau J, Morabito KM, Rao SS, Filali-Mouhim A, Sekaly RP, Graham BS. A Numerically Subdominant CD8 T Cell response to matrix protein of respiratory syncytial virus controls infection with limited immunopathology. PLoS Pathog 2016; 12:e1005486; PMID:26943673; http://dx.doi.org/10.1371/journal.ppat.1005486
- De Baets S, Schepens B, Sedeyn K, Schotsaert M, Roose K, Bogaert P, Fiers W, Saelens X. Recombinant influenza virus carrying the respiratory syncytial virus (RSV) F85-93 CTL epitope reduces RSV replication in mice. J Virol 2013; 87:3314-23; PMID:23302879; http://dx.doi.org/10.1128/JVI.03019-12
- Venter M, Rock M, Puren AJ, Tiemessen CT, Crowe JE, Jr. Respiratory syncytial virus nucleoprotein-specific cytotoxic T-cell epitopes in a South African population of diverse HLA types are conserved in circulating field strains. J Virol 2003; 77:7319-29; PMID:12805430; http://dx.doi.org/10.1128/JVI.77.13.7319-7329.2003
- Heidema J, de Bree GJ, De Graaff PM, van Maren WW, Hoogerhout P, Out TA, Kimpen JL, van Bleek GM. Human CD8(+) T cell responses against five newly identified respiratory syncytial virus-derived epitopes. J Gen Virol 2004; 85:2365-74; PMID:15269378; http://dx.doi.org/10.1099/vir.0.80131-0
- Telcian AG, Laza-Stanca V, Edwards MR, Harker JA, Wang H, Bartlett NW, Mallia P, Zdrenghea MT, Kebadze T, Coyle AJ, et al. RSV-induced bronchial epithelial cell PD-L1 expression inhibits CD8+ T cell nonspecific antiviral activity. J Infect Dis 2011; 203:85-94; PMID:21148500; http://dx.doi.org/10.1093/infdis/jiq020
- Hussell T, Openshaw PJ. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol 1998; 79(Pt 11):2593-601; PMID:9820134; http://dx.doi.org/10.1099/0022-1317-79-11-2593
- Ge MQ, Ho AW, Tang Y, Wong KH, Chua BY, Gasser S, Kemeny DM. NK cells regulate CD8+ T cell priming and dendritic cell migration during influenza A infection by IFN-gamma and perforin-dependent mechanisms. J Immunol 2012; 189:2099-109; PMID:22869906; http://dx.doi.org/10.4049/jimmunol.1103474
- Lukens MV, van de Pol AC, Coenjaerts FE, Jansen NJ, Kamp VM, Kimpen JL, Rossen JW, Ulfman LH, Tacke CE, Viveen MC, et al. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol 2010; 84:2374-83; PMID:20015982; http://dx.doi.org/10.1128/JVI.01807-09
- Fearns R, Collins PL. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J Virol 1999; 73:5852-64; PMID:10364337
- Jin H, Cheng X, Zhou HZ, Li S, Seddiqui A. Respiratory syncytial virus that lacks open reading frame 2 of the M2 gene (M2-2) has altered growth characteristics and is attenuated in rodents. J Virol 2000; 74:74-82; PMID:10590093; http://dx.doi.org/10.1128/JVI.74.1.74-82.2000
- McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol 2013; 372:83-104; PMID:24362685
- Bukreyev A, Yang L, Fricke J, Cheng L, Ward JM, Murphy BR, Collins PL. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol 2008; 82:12191-204; PMID:18842713; http://dx.doi.org/10.1128/JVI.01604-08
- Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 2006; 176:1600-8; PMID:16424189; http://dx.doi.org/10.4049/jimmunol.176.3.1600
- Schlender J, Walliser G, Fricke J, Conzelmann KK. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J Virol 2002; 76:1163-70; PMID:11773392; http://dx.doi.org/10.1128/JVI.76.3.1163-1170.2002
- Gan SW, Tan E, Lin X, Yu D, Wang J, Tan GM, Vararattanavech A, Yeo CY, Soon CH, Soong TW, et al. The small hydrophobic protein of the human respiratory syncytial virus forms pentameric ion channels. J Biol Chem 2012; 287:24671-89; PMID:22621926; http://dx.doi.org/10.1074/jbc.M111.332791
- Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol 2007; 81:8361-6; PMID:17494063; http://dx.doi.org/10.1128/JVI.02717-06
- Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol 1999; 73:7099-107; PMID:10438795
- Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol 2005; 79:5353-62; PMID:15827150; http://dx.doi.org/10.1128/JVI.79.9.5353-5362.2005
- Barik S. Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr Top Microbiol Immunol 2013; 372:173-91; PMID:24362690
- Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, Buick R, Stevenson NJ, Touzelet O, Gadina M, et al. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J Virol 2007; 81:3428-36; PMID:17251292; http://dx.doi.org/10.1128/JVI.02303-06
- Liesman RM, Buchholz UJ, Luongo CL, Yang L, Proia AD, DeVincenzo JP, Collins PL, Pickles RJ. RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction. J Clin Invest 2014; 124:2219-33; PMID:24713657; http://dx.doi.org/10.1172/JCI72948
- Munir S, Le Nouen C, Luongo C, Buchholz UJ, Collins PL, Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J Virol 2008; 82:8780-96; PMID:18562519; http://dx.doi.org/10.1128/JVI.00630-08
- Munir S, Hillyer P, Le Nouen C, Buchholz UJ, Rabin RL, Collins PL, Bukreyev A. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog 2011; 7:e1001336; PMID:21533073; http://dx.doi.org/10.1371/journal.ppat.1001336
- Kotelkin A, Belyakov IM, Yang L, Berzofsky JA, Collins PL, Bukreyev A. The NS2 protein of human respiratory syncytial virus suppresses the cytotoxic T-cell response as a consequence of suppressing the type I interferon response. J Virol 2006; 80:5958-67; PMID:16731934; http://dx.doi.org/10.1128/JVI.00181-06
- Reimers K, Buchholz K, Werchau H. Respiratory syncytial virus M2-1 protein induces the activation of nuclear factor kappa B. Virology 2005; 331:260-8; PMID:15629770; http://dx.doi.org/10.1016/j.virol.2004.10.031
- Bakker SE, Duquerroy S, Galloux M, Loney C, Conner E, Eleouet JF, Rey FA, Bhella D. The respiratory syncytial virus nucleoprotein-RNA complex forms a left-handed helical nucleocapsid. J Gen Virol 2013; 94:1734-8; PMID:23677789; http://dx.doi.org/10.1099/vir.0.053025-0
- Lifland AW, Jung J, Alonas E, Zurla C, Crowe JE, Jr, Santangelo PJ. Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS. J Virol 2012; 86:8245-58; PMID:22623778; http://dx.doi.org/10.1128/JVI.00215-12
- Cespedes PF, Bueno SM, Ramirez BA, Gomez RS, Riquelme SA, Palavecino CE, Mackern-Oberti JP, Mora JE, Depoil D, Sacristán C, et al. Surface expression of the hRSV nucleoprotein impairs immunological synapse formation with T cells. Proc Natl Acad Sci U S A 2014; 111:E3214-23; PMID:25056968; http://dx.doi.org/10.1073/pnas.1400760111
- Osman N, Lucas S, Cantrell D. The role of tyrosine phosphorylation in the interaction of cellular tyrosine kinases with the T cell receptor zeta chain tyrosine-based activation motif. Eur J Immunol 1995; 25:2863-9; PMID:7589084; http://dx.doi.org/10.1002/eji.1830251023
- Leung J, Esper F, Weibel C, Kahn JS. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol 2005; 43:1213-9; PMID:15750086; http://dx.doi.org/10.1128/JCM.43.3.1213-1219.2005
- Falsey AR, Hennessey PA, Formica MA, Criddle MM, Biear JM, Walsh EE. Humoral immunity to human metapneumovirus infection in adults. Vaccine 2010; 28:1477-80; PMID:20003919; http://dx.doi.org/10.1016/j.vaccine.2009.11.063
- Alvarez R, Tripp RA. The immune response to human metapneumovirus is associated with aberrant immunity and impaired virus clearance in BALB/c mice. J Virol 2005; 79:5971-8; PMID:15857983; http://dx.doi.org/10.1128/JVI.79.10.5971-5978.2005
- Alvarez R, Harrod KS, Shieh WJ, Zaki S, Tripp RA. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J Virol 2004; 78:14003-11; PMID:15564507; http://dx.doi.org/10.1128/JVI.78.24.14003-14011.2004
- Debiaggi M, Canducci F, Sampaolo M, Marinozzi MC, Parea M, Terulla C, Colombo AA, Alessandrino EP, Bragotti LZ, Arghittu M, et al. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis 2006; 194:474-8; PMID:16845630; http://dx.doi.org/10.1086/505881
- Abed Y, Boivin G. Human metapneumovirus infection in immunocompromised child. Emerg Infect Dis 2008; 14:854-6; PMID:18439384; http://dx.doi.org/10.3201/eid1405.071459
- Herd KA, Nelson M, Mahalingam S, Tindle RW. Pulmonary infection of mice with human metapneumovirus induces local cytotoxic T-cell and immunoregulatory cytokine responses similar to those seen with human respiratory syncytial virus. J Gen Virol 2010; 91:1302-10; PMID:20053825; http://dx.doi.org/10.1099/vir.0.015396-0
- Kolli D, Bataki EL, Spetch L, Guerrero-Plata A, Jewell AM, Piedra PA, Milligan GN, Garofalo RP, Casola A. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J Virol 2008; 82:8560-9; PMID:18562525; http://dx.doi.org/10.1128/JVI.00699-08
- Lay MK, Cespedes PF, Palavecino CE, Leon MA, Diaz RA, Salazar FJ, Méndez GP, Bueno SM, Kalergis AM. Human metapneumovirus infection activates the TSLP pathway that drives excessive pulmonary inflammation and viral replication in mice. Eur J Immunol 2015; 45(6):1680-95; PMID:25763996; http://dx.doi.org/10.1002/eji.201445021
- Herd KA, Nissen MD, Hopkins PM, Sloots TP, Tindle RW. Major histocompatibility complex class I cytotoxic T lymphocyte immunity to human metapneumovirus (hMPV) in individuals with previous hMPV infection and respiratory disease. J Infect Dis 2008; 197:584-92; PMID:18240952; http://dx.doi.org/10.1086/526536
- Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 2002; 39:531-6; PMID:12431386; http://dx.doi.org/10.1016/S0161-5890(02)00210-9
- Douville RN, Bastien N, Li Y, Pochard P, Simons FE, HayGlass KT. Human metapneumovirus elicits weak IFN-gamma memory responses compared with respiratory syncytial virus. J Immunol 2006; 176:5848-55; PMID:16670291; http://dx.doi.org/10.4049/jimmunol.176.10.5848
- Laham FR, Israele V, Casellas JM, Garcia AM, Lac Prugent CM, Hoffman SJ, Hauer D, Thumar B, Name MI, Pascual A, et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis 2004; 189:2047-56; PMID:15143472; http://dx.doi.org/10.1086/383350
- Le Nouen C, Hillyer P, Brock LG, Winter CC, Rabin RL, Collins PL, Buchholz UJ. Human metapneumovirus SH and G glycoproteins inhibit macropinocytosis-mediated entry into human dendritic cells and reduce CD4+ T cell activation. J Virol 2014; 88:6453-69; PMID:24672038; http://dx.doi.org/10.1128/JVI.03261-13
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo R. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol 2006; 34:320-9; PMID:16284360; http://dx.doi.org/10.1165/rcmb.2005-0287OC
- Le Nouen C, Hillyer P, Munir S, Winter CC, McCarty T, Bukreyev A, Collins PL, Rabin RL, Buchholz UJ. Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells. PLoS One 2010; 5:e15017; PMID:21124776; http://dx.doi.org/10.1371/journal.pone.0015017
- Wen SC, Schuster JE, Gilchuk P, Boyd KL, Joyce S, Williams JV. Lung CD8+ T cell impairment occurs during human metapneumovirus infection despite virus-like particle (VLP) induction of functional CD8+ T cells. J Virol 2015; 89(17):8713-26; http://dx.doi.org/10.1128/JVI.00670-15
- Melendi GA, Zavala F, Buchholz UJ, Boivin G, Collins PL, Kleeberger SR, Polack FP. Mapping and characterization of the primary and anamnestic H-2(d)-restricted cytotoxic T-lymphocyte response in mice against human metapneumovirus. J Virol 2007; 81:11461-7; PMID:17670840; http://dx.doi.org/10.1128/JVI.02423-06
- Hastings AK, Erickson JJ, Schuster JE, Boyd KL, Tollefson SJ, Johnson M, Gilchuk P, Joyce S, Williams JV. Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication. J Virol 2015; 89:4405-20; PMID:25653440; http://dx.doi.org/10.1128/JVI.03275-14
- Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest 2012; 122:2967-82; PMID:22797302; http://dx.doi.org/10.1172/JCI62860
- Erickson JJ, Rogers MC, Hastings AK, Tollefson SJ, Williams JV. Programmed death-1 impairs secondary effector lung CD8(+) T cells during respiratory virus reinfection. J Immunol 2014; 193:5108-17; PMID:25339663; http://dx.doi.org/10.4049/jimmunol.1302208
- Deffrasnes C, Hamelin ME, Boivin G. Human metapneumovirus. Semin Respir Crit Care Med 2007; 28:213-21; PMID:17458775; http://dx.doi.org/10.1055/s-2007-976493
- Ishiguro N, Ebihara T, Endo R, Ma X, Kikuta H, Ishiko H, et al. High genetic diversity of the attachment (G) protein of human metapneumovirus. J Clin Microbiol 2004; 42:3406-14; PMID:15297475; http://dx.doi.org/10.1128/JCM.42.8.3406-3414.2004
- Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog 2008; 4:e1000077; PMID:18516301; http://dx.doi.org/10.1371/journal.ppat.1000077
- Bao X, Kolli D, Liu T, Shan Y, Garofalo RP, Casola A. Human metapneumovirus small hydrophobic protein inhibits NF-kappaB transcriptional activity. J Virol 2008; 82:8224-9; PMID:18550666; http://dx.doi.org/10.1128/JVI.02584-07
- Takimoto T, Portner A. Molecular mechanism of paramyxovirus budding. Virus Res 2004; 106:133-45; PMID:15567493; http://dx.doi.org/10.1016/j.virusres.2004.08.010
- Bagnaud-Baule A, Reynard O, Perret M, Berland JL, Maache M, Peyrefitte C, Vernet G, Volchkov V, Paranhos-Baccalà G. The human metapneumovirus matrix protein stimulates the inflammatory immune response in vitro. PLoS One 2011; 6:e17818; PMID:21412439; http://dx.doi.org/10.1371/journal.pone.0017818
- Cai H, Zhang Y, Ma Y, Sun J, Liang X, Li J. Zinc binding activity of human metapneumovirus M2-1 protein is indispensable for viral replication and pathogenesis in vivo. J Virol 2015; 89:6391-405; PMID:25855728; http://dx.doi.org/10.1128/JVI.03488-14
- Buchholz UJ, Biacchesi S, Pham QN, Tran KC, Yang L, Luongo CL, Skiadopoulos MH, Murphy BR, Collins PL. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: effects on RNA synthesis, attenuation, and immunogenicity. J Virol 2005; 79:6588-97; PMID:15890897; http://dx.doi.org/10.1128/JVI.79.11.6588-6597.2005
- Kitagawa Y, Zhou M, Yamaguchi M, Komatsu T, Takeuchi K, Itoh M, Gotoh B. Human metapneumovirus M2-2 protein inhibits viral transcription and replication. Microbes Infect 2010; 12:135-45; PMID:19913636; http://dx.doi.org/10.1016/j.micinf.2009.11.002
- Ren J, Wang Q, Kolli D, Prusak DJ, Tseng CT, Chen ZJ, Li K, Wood TG, Bao X. Human metapneumovirus M2-2 protein inhibits innate cellular signaling by targeting MAVS. J Virol 2012; 86:13049-61; PMID:23015697; http://dx.doi.org/10.1128/JVI.01248-12
- Busche A, Jirmo AC, Welten SP, Zischke J, Noack J, Constabel H, Gatzke AK, Keyser KA, Arens R, Behrens GM, et al. Priming of CD8+ T cells against cytomegalovirus-encoded antigens is dominated by cross-presentation. J Immunol 2013; 190:2767-77; PMID:23390296; http://dx.doi.org/10.4049/jimmunol.1200966
- Cox RG, Erickson JJ, Hastings AK, Becker JC, Johnson M, Craven RE, Tollefson SJ, Boyd KL, Williams JV. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J Virol 2014; 88:6368-79; PMID:24672031; http://dx.doi.org/10.1128/JVI.00332-14
- Singleton R, Etchart N, Hou S, Hyland L. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J Virol 2003; 77:11303-11; PMID:14557616; http://dx.doi.org/10.1128/JVI.77.21.11303-11311.2003
- Hussell T, Spender LC, Georgiou A, O'Garra A, Openshaw PJ. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol 1996; 77(Pt 10):2447-55; PMID:8887477; http://dx.doi.org/10.1099/0022-1317-77-10-2447
