ABSTRACT
Hepatitis E virus (HEV) infection is a major cause of acute hepatitis but also provokes chronic infection in immunocompromised patients. Although the pathogenesis and treatment outcome involve complex interplay between the virus and host, the nature of adaptive responses of HEV to the host immune system remain obscure at best. In this study, we used large-scale proteomic bioinformatics to profile characteristic mutations in human HEV isolates associated to ribavirin treatment failure, chronic hepatitis, hepatic failure or altered immunoreactivity. The prevalence of specific mutations was examined in a large number of protein sequences of ORF1 and ORF2 regions of the 3 major human-derived HEV genotypes (1, 3 and 4). By analyzing potential B, CD4+ and CD8+ T cell epitopes, we found that many of these mutations overlap with the predicted epitopes and are frequently present among the 3 HEV genotypes. These overlapping mutations mediate reduced antigenicity. Finally, by delineation of diversification and evolution of the underlying epitopes, we observe that most of these variants apparently evolved earlier in genotype 1 when compared with genotypes 3 and 4. These results indicate that HEV is under substantial evolutionary pressure to develop mutations enabling evasion of the host immune response and resistance to antiviral treatment. This indicates the existence of an ongoing evolutionary arms race between human immunity, antiviral medication and HEV.
Introduction
Hepatitis E virus (HEV) is a non-enveloped, single-stranded positive sense RNA virus which mainly infects the liver.Citation1 It causes over 3 million acute cases and 57,000 deaths every year.Citation2 Although 8 HEV genotypes are now recognized, there are 4 well-defined genotypes infecting humans, including genotype 1, 2, 3 and 4. Genotypes 1 and 2 are found only in humans and are responsible for most cases of infection in the developing countries. Genotypes 3 and 4 circulate in several animals (e.g. pigs, wild boars and deer) and are the main cause of sporadic infection in the developed countries.Citation3,4 Although no FDA-approved treatment is available, pegylated interferon-α (PEG-IFN-α) or ribavirin has been used as off-label treatment of some cases of HEV infection.Citation5,6
As an RNA virus, HEV possesses a high mutation rate. It was indirectly estimated from clinical isolates that the mutation rate of HEV was ∼1.5 base substitutions per site per year, which is quite similar to that reported for hepatitis C virus (HCV).Citation7 The viral RNA-dependent RNA polymerase (RdRp), which lacks the proof-reading capacity, is an important factor contributing to the high rate of mutations in the HEV genome.Citation8 Furthermore, the selection pressure imposed by the host immune responses may also contribute to the variability of HEV genome. Recently, studies have hinted at the acquisition by the HEV genome of certain mutations associated with ribavirin treatment failure, chronic hepatitis, hepatic failure and reduced immunoreactivity.Citation9 The observation that different mutations change the HEV proteome with respect to not only the therapeutic response but also the immunoreactivity highlights the importance of studying HEV mutations and their effects on the host immune responses.
Only limited knowledge is available on the contribution of HEV genome variants toward susceptibility, pathogenesis and therapeutic responses. To elucidate these processes, we have used a large-scale proteomic bioinformatics to profile human HEV characteristic mutations. In the present study, we have comprehensively investigated the proteomic variation of the open reading frame 1 (ORF1) and ORF2 regions among the major HEV genotypes 1, 3 and 4, by retrieving a large data set of HEV sequences. We first mapped the presence and abundance of the mutations related to ribavirin treatment failure, chronic hepatitis, hepatic failure or reduced immunoreactivity. Furthermore, we examined the overlap of these mutations with predicted B cell, CD4+ and CD8+ T cell epitopes, and assessed their antigenicity. Interestingly, we have observed that these overlapped mutations evolved earlier in genotype 1 when compared with genotype 3 and 4. Thus, the acquisition and evolution of these characteristic mutations may help the virus to evade host immune response, develop resistance to antiviral treatment, and facilitate its adaptation in human population.
Results
Identification of HEV characteristic mutations
Several HEV variants were previously reported both in vitro and in vivo. Using a combination of phrases/keywords in PubMed (Table S1), we explored clinical and in vitro data reporting mutations within the ORF1 and ORF2 of HEV. In our analysis, 20 mutations (Table S2) were identified in these regions, 17 in ORF1 and 3 in ORF2 (). Among these mutations, one was related to chronic hepatitis, 9 with hepatic failure, 8 with ribavirin treatment failure, and 2 with altered immunoreactivity (Table S2).
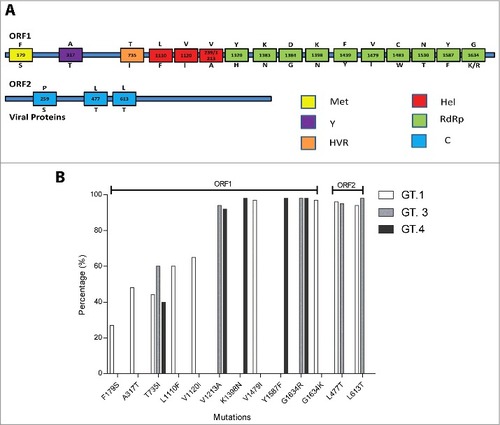
Figure 1. (A). Identified mutations of ORF1 and ORF2 regions of HEV. The number within the box represents the amino acid position; the letter(s) above the box refer to the wild type amino acid, and the letter below the box are relevant mutations reported in previous studies. (Met: methyltransferase; Y: Y-domain; HVR: hypervariable regions; Hel: RNA helicase; RdRP: RNA-dependent RNA polymerase; C: capsid protein) (B). The prevalence of mutations within HEV genotype 1 (n = 81), 3 (n = 182) and 4 (n = 143). Amino acid diversity was measured as the proportion of sequences that varies from the consensus sequence.
Frequency of mutations within ORF1 and ORF2
To evaluate the relevance of these reported characteristic mutations, we first evaluated their presence in the circulating strains based on the HEV sequences deposited in the GenBank. We have searched the 4 main genotypes identified from the human host. However, limited sequences are available for genotype 2. Thus, we retrieved 57 ORF1, 51 ORF2 (genotype 1), 131 ORF1, 131 ORF2 (genotype 3) and 99 ORF1, 96 ORF2 (genotype 4) of HEV sequences (retrieved in January 2017). The selected sequences represent all major genotypes (1, 3 and 4). The full-length sequences used in this study based on each ORF and genotype are provided in Table S3.
After removal of closely related and redundant sequences, 406 full length ORF1 and ORF2 sequences were finally selected for further analysis. , Table S2 and show the number of all possible and experimentally confirmed amino acid variants and frequency of their variation for each HEV genotype. The number of variants found in ORF1 region was higher (17 variants) than that of ORF2 region (3 variants). Out of 17 variants in ORF1, 8 were related to hepatic failure; one with chronic hepatitis; and 8 with ribavirin treatment failure. While in ORF2, one variation was associated with hepatic failure and 2 with immunoreactivity. When analyzing HEV genotypes, it was revealed that genotype 1 possesses a higher number of mutations (8) with considerable frequency when compared with genotype 3 and 4 which possesses only 6 and 3 mutations, respectively ( and ). This observation further suggests the high level of polymorphism in HEV genotype 1.
Table 1. All possible and experimentally confirmed (in vivo and in vitro) mutations and their prevalence in the major HEV genotypes (1, 3, 4). Different colors indicate the mutations related to ribavirin treatment failure (yellow), chronic hepatitis (purple), hepatic failure (red) or altered immunoreactivity (blue).
Mutations within genotype 1, 3 and 4
In genotype 1, 9 out of 17 reported mutations (V1213A, Y1320H, K1383N, D1384G, K1398N, C1483W, N1530T, Y1587F, G1634R) in ORF1 and one (P259S) out of 3 in ORF2, were found to be conserved (i.e. not present in our analyzed sequences), while others have shown considerable variations (). Among these variable mutations, T735I and G1634R/K were observed to be frequent among all selected genotype (1, 3 and 4). Another mutation (A317T) was found in 2 genotypes (1 and 3). Four mutations (ORF1 = 2; ORF2 = 2) reached the frequency of > 90% (, ). Among these, 2 mutations were related to ribavirin treatment failure [G1634K/R (ORF1; genotype 1, 3, 4) and Y1587F (ORF1; genotype 4)] and 2 were associated with altered immunoreactivity [L477T (ORF1; genotype 1, 3) and L613T (ORF1; genotype 1, 3)].
In genotype 3, we found that the reported mutation sites [13 in ORF1 (F179S, L1110F, V1120I, Y1320H, K1383N, D1384G, K1398N, F1439Y, V1479I, C1483W, N1530T, Y1587F, G1634K) and one (P259S) in ORF2] were conserved (i.e., not present in our analyzed sequences) (, ). Mutations of ORF1, i.e., V1213A and G1634R, were found with a considerable frequency in genotypes 3 and 4. The most important variants were A317T and V1213A which showed a high prevalence in genotype 3. Four mutations approached a frequency of > 90%, where one was related to chronic hepatitis (V1213A), one with ribavirin treatment failure (G1634R) and 2 with immunoreactivity (L477T, L613T) (, ).
In genotype 4, 13 sites in ORF1 (F179S, A317T, L1110F, V1120I, V1213A, Y1320H, K1383N, K1398N, D1384G, F1439Y, V1479I, C1483W, N1530T) and 3 in ORF2 (P259S, L477T, L613T) were found to be conserved. Mutation Y1587F was frequent in genotype 4. Two mutations reached the frequency of > 90% (Y1587F, G1634R) (, ). Both mutations have been reported to be related to ribavirin treatment failure (Y1587F, G1634R).
Mutations as a missense SNPs and their effects on protein structural stability
In our analysis, 4 in silico SNP prediction algorithms were used to predict the selected mutations as neutral or deleterious. According to predicted results, most of the selected mutations with high prevalence in HEV infected papulation are deleterious (Table S5). Next, most of these mutations are predicted to cause destabilization of the protein as calculated using 3 web servers (Table S6), suggesting the importance of these mutations. Because the crystal structure of HEV ORF2 (2ZTN-amino acid 129–606) is available (). We have modeled the effect of 2 mutations that are located within this region on structural stability of the protein. We found that both P259S () and L477T () are predicted to cause destabilization of ORF2 protein.
Overlap of characteristic mutations within the predicted B cell, CD4+ and CD8+ T cell epitopes
The consensus sequence of ORF1 and ORF2 of genotypes 1, 3 and 4 (respectively) was used to predict B cells, major histocompatibility complex class I (MHCI) and class II (MHCII) T cell epitopes using IEDB, ProPred-1 and ProPred, respectively.Citation10-12 In case of MHCI and II, epitope's binding to maximum number of alleles and binding capacity of < 500 mM were selected. The predicted epitopes were further confirmed by BLASTpCitation13 to avoid considering the epitopes that have a homology with human proteins.
The predicted epitopes were then evaluated for the presence or absence of the reported mutations (). We found that many of these characteristic mutations reported in ORF1 and ORF2 regions overlap with the predicted epitopes and were also frequently observed among selected HEV genotypes (1, 3 and 4) ( and ). In our analysis, all epitopes possessing mutations that are present in our analyzed HEV sequences were considered. and show mutations in the predicted epitopes of ORF1 and ORF2 (consensus genotype 1, 3 and 4) against B cells, MHC-I and MHC-II T cells. It was observed that ORF1 and ORF2 of genotype 1 possess more epitopes with reported mutations when compared with genotype 3 and 4 ( and ). Most of these overlapped mutations were related to hepatic failure (HF), followed by ribavirin treatment failure (RTF), altered immunoreactivity (AI) and chronic hepatitis (CH) (HF>RTF>AI>CH).
Table 2. Antigenicity evaluation of wild-type and mutated epitopes. The effect of mutations on the antigenicity (threshold level = 0.4) of T cell predicted epitopes (MHCI and MHCII). Bold letters (one-letter amino acid code) represent mutations within the predicted epitopes.
Table 3. Antigenicity evaluation of wild-type and mutated epitopes. The effect of mutations on the antigenicity (threshold level = 0.4) of predicted B cells epitopes. Bold letters (one-letter amino acid code) represent mutations within the predicted epitopes.
Alteration of epitope antigenicity by characteristic mutations
We hypothesize that HEV may alter its epitopes by acquiring mutations to evade the immune recognition by both B and T cells.Citation14 The online tool VaxiJenCitation15 was used to detect the effect of each mutation on the antigenicity of the epitopes (wild type and mutation containing epitopes) ( and ). Interestingly, in many cases, mutated epitopes have a reduced antigenicity when compared with the wild-type epitope (, , and ). Some mutated epitopes have a sustained antigenicity, while only few mutated epitopes have an increased antigenicity. These findings suggest that most of the reported mutations within predicted epitopes have a reduced antigenicity. Consequently, they are less recognized by both B and T cells and thus, decreasing the effective roles of the adaptive immune cells to clear the infections.
Figure 2. Antigenicity difference between wild-type and mutated T cell (MHCI and MHCII) (A) and B cell epitopes (B).
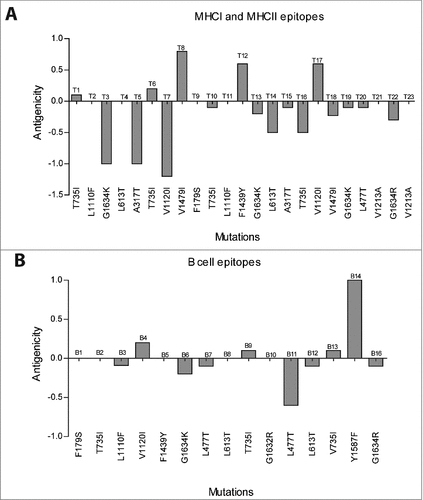
Figure 3. The effects of mutations (P259S, L477T) on the structural stability of ORF2 region of HEV predicted by DUET web server. Mutations are shown in blue color ribbon. (A) Crystal struture without mutation of ORF2 (2ZTN-amino acid 129–606). (B). P259S; feature: distabilizing; secondary structure: loop or irregular. (C). L477T; feature: distabilizing; secondary structure: extended β-strand.
Evolution of the characteristic mutations
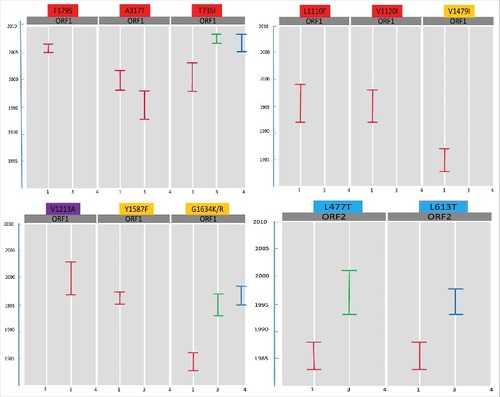
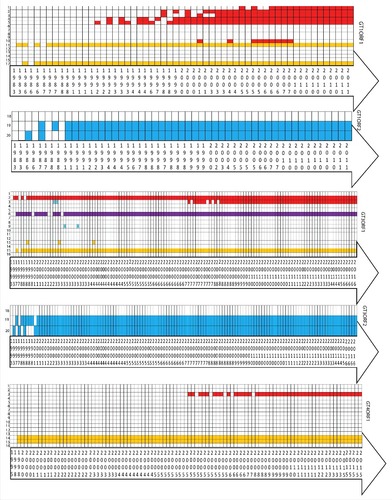
To visualize the mutation evolution within the predicted epitopes of each genotype, a heat map was generated for each reported mutation (). The concept of evolving mutations among the HEV genotypes within antigenic area will be helpful in determining their role in both immune and therapeutic responses. Different trends of evolving mutations have been observed in our analysis. The mutations A317T, T735I, L1110F, V1120I, V1479I and G1634K which were frequently observed in genotype 1 ORF1 have an evolving period of 3 y (1998–2001), 6 y (1998–2003), 8 y (1992–1999) 7 y (1992–1998), 5 y (1983–1987) and 4 y (1983–1986), respectively ( and ). A commonly observed variation in all selected genotypes (1, 3 and 4), i.e., T735I, was found to be evolved earlier in ORF1 of genotype 1 (1998–2003) when compared with genotype 3 and 4. Another common variation G1634K/R evolved earlier in ORF1 of genotype 1 (1983–1986) as compared with genotype 3 (1993–1997) and genotype 4 (1995–1998) ( and ). Similarly, the mutations in ORF2 (L477T and L613T) appear to evolve earlier in genotype 1 compared with genotype 3 ( and ). These data collectively indicate an earlier acquisition of several characteristic mutations in genotype 1 as compared with genotype 3 and 4.
Figure 4. A heat map showing the evolvment of mutations with years. Mutations 1 = F179S, 2 = A317T, 3 = T735I, 4 = L1110F, 5 = V1120I, 6 = V1213A, 7 = Y1320H, 8 = K1383N, 9 = D1384G, 10 = K1398N, 11 = F1439Y, 12 = V1479I, 13 = C1483W, 14 = N1530T, 15 = Y1587F, 16 = G1634R, 17 = G1634K, 18 = P259S, 19 = L477T, 20 = L613T. Each box represents the sequence of ORF 1 or ORF2, from genotype 1, 3 or 4 in a particular year. Red colored boxes represent the mutations related to hepatic failure mutations; purple to chronic hepatitis; yellow to ribavirin treatment failure; and blue to altered immunoreactivity. The year of deposition of each sequence is mentioned in the arrow below the heat map.
Discussion
Ribavirin monotherapy has been widely used for treatment of chronic hepatitis E.Citation5 It is in general very effective. However, for a subset of patients, ribavirin treatment fails.Citation16,17 Mutations in the viral polymerase have been noted before or during therapy in these patients.Citation18 Furthermore, many mutations related to hepatic failure, chronic hepatitis and immunoreactivity have also been reported, conferring the importance of these mutations in HEV pathogenesis and treatment responses. In support of this, several studies have shown that proteomic variations in certain epitopes that are associated with these mutations can critically influence the outcome of the immune responses.Citation19 These antigenic variations have been observed among HEV strains using genotype- and strain-specific monoclonal antibodies.Citation20 Even though several studies have reported frequencies of mutations in the HEV genome, the global prevalence of these characteristic mutations has not been comprehensively studied. Thus, this study has evaluated the HEV ORF1 and ORF2 variability in major genotypes (1, 3 and 4) by a systematic retrieval of human-derived HEV sequences. Furthermore, important co-occurrence of B and T cell (CD4+ and CD8+) epitope mutations was revealed, suggesting adaptation of viruses to escape immune surveillance.
By exploring the intra-genotypic diversity from representative human HEV genotypes, we have demonstrated that genotype 1 produces the largest number of intra-genotypic variants (). These variants were found to be evolved with a period of on average ∼4 y but varied from 3 to 5 y in the human population ( and ). Surprisingly, some reported mutations, including Y1320H, K1383N, D1384G and K1398N (related to ribavirin treatment failure) and C1483W, N1530T and P259S (related to hepatic failure), were hardly present in our retrieved large set of sequences, suggesting their insignificance. In contrast, 2 mutations T735I (related to hepatic failure) and G1634R (related to ribavirin treatment failure) were found in all selected genotypes (1, 3 and 4) with a considerably high frequency. Among these mutations, G1634R was identified in patients as a baseline mutation that effects ribavirin treatment response.Citation21 This mutation was also observed in a patient with chronic hepatitis E, experiencing ribavirin treatment failure with a completely resistant phenotype.Citation16 However, the exact clinical relevance of such mutations in ribavirin treatment failure is uncertain since findings from in vitro studies show that some of these mutations facilitate HEV replication but paradoxically seem to increase ribavirin sensitivity,Citation16 thus requiring further investigation. Furthermore, effective new antiviral therapy is needed for HEV patients with ribavirin treatment failure.Citation22 Two immunoreactivity-related mutations (L477T and L613T) were mainly found in genotype 1 and 3 highlighting the role of such mutations in affecting host immune response ().
To investigate the implication of these characteristic mutations in the host immune response, we have profiled their overlap with predicted B and T cell epitopes. We found that the hotspot sites where mutations and predicted epitopes overlap are frequently present among many HEV genotypes ( and ). The subsequent analysis of these mutations showed that this overlap mostly decreases and in few cases, sustains or enhances the antigenicity of the mutated epitopes (, and ). Experimental studies have demonstrated that only antibodies recognizing conformational epitopes are neutralizing, and the aa residues Leu477 and Leu613 in the capsid protein are important in forming a neutralization-sensitive epitope representing the importance of these mutations in epitope deformation.Citation23 In patients, HEV-specific T cells target relatively conserved HEV peptides, and those are predominantly located in the ORF2 capsid protein. The T-cell responses persist over years after resolution of HEV infection, suggesting the role in both clearance of primary infection and protective immune response against secondary infection.Citation24 Based on our study, we propose a model of immune evasion by HEV (). In this model, mutated epitopes will result in escape recognition by B and T cells. Furthermore, we have mapped the evolution of these mutations in all selected genotypes. We found that most of the overlapped mutations are more abundantly present in genotype 1 as compared with genotype 3 and 4. Interestingly, the common mutations evolved earlier in genotype 1 than genotype 3 and 4.
Figure 6. Possible mechanisms of immune evasion by hepatitis E virus. Main routes by which HEV mutations may result in evasion of the host immune responses. Mutated epitopes presented by antigen presenting cells, B and T cells will results in escape recognition of the epitopes.
In summary, our study represents a comprehensive analysis of the characteristic mutations in the major HEV genotypes. Some of these mutations overlap with predicted B and T cell epitopes that are expected to affect the antigenicity. We further revealed the evolution of these mutations among the 3 major genotypes. These results indicate that HEV is under substantial evolutionary pressure to develop mutations enabling evasion of the host immune response and resistance to antiviral treatment. This indicates the existence of an ongoing evolutionary arms race between human immunity, antiviral medication and the HEV.
Material and methods
Data collection
A database on reported mutations within the ORF1 and ORF2 regions of HEV is currently not available. We reviewed earlier studies (through January 2017), using a combination of the keywords that are listed in Table S1, and evaluated mutations in HEV-infected individuals, as well as in HEV replicons. These studies were searched from the PubMed (ncbi.nlm.nih.gov/pubmed), EMBASE, and Cochrane Library databases.
Retrieval of sequences
We retrieved 57 ORF1, 51 ORF2 (genotype 1), 131 ORF1, 131 ORF2 (genotype 3) and 99 ORF1, 96 ORF2 (genotype 4) of HEV protein sequences from the GenBankCitation25 (accessed on January 2017). The selected protein sequences represent all major genotypes (1, 3 and 4). The GenBank accession numbers of HEV protein sequences used in this study against each ORF and genotype are provided in Table S3. The sequences were trimmed manually and analyzed using the reference protein sequences of selected genotypes [AF185822 (genotype 1), AB291960 (genotype 3), AB200239 (genotype 4)]. Different quality control measures were performed for the sequences, and many sequences were disqualified for further analysis based on the following 2 conditions: (a) if the sequence derived from a non-human host; and (b) if the sequence was a clonal sequence from the same patient. Sequences were also annotated by the year of sampling. In some cases where the source did not provide the sampling year, we used the submission date to the GenBank as the sampling year.
Consensus sequence and mutations analysis
The HEV ORF1 and ORF2 sequences were aligned using ClustalW, BioEdit and CLC Workbench 7 (http://www.clcbio.com). A consensus analysis for each HEV genotype was performed to observe the presence or absence of mutation at each site. These mutations were selected on the basis of published data reporting all experimentally proven mutations (in vitro and in vivo). The prevalence of each mutation was then measured within the selected regions (ORF1 and ORF2) of each genotype (1, 3 and 4).
Analysis of mutations as a missense SNPs and their effects on structural stability
To validate selected mutations as missense SNPs in ORF1 and ORF2 regions of HEV genotype 1, 3 and 4, computational analysis was performed using 4 tools PROVEAN (Protein Variation Effect Analyzer),Citation26 nsSNP analyzer,Citation27 SNPs & GO and PMUT.Citation28 These tools describe missense SNPs as damaging or neutral to function and structure. To predict the change in protein stability due to these SNPs, DUET,Citation29 I-Mutant version 2.0,Citation30 and STRUMCitation31 web servers were used. As an input in I-Mutant and STRUM servers, FASTA sequences of ORF1, ORF2 regions of selected genotypes were used. PDB files of 3D structures (2ZTN) of ORF2 were used as an input in DUET web server.
Prediction of the B and T cell epitopes and comparison with the host proteome
Nine-mer B cell epitopes were predicted against HEV genotype (1, 3 and 4) from their consensus sequence by using online tool, the Immune Epitope Database (IEDB).Citation32 Similarly, 9-mer T cell epitopes (MHC class I and II), following the same criteria as B cell epitopes, were predicted by online T cell epitope prediction tools, including ProPred-I (MHCI) and ProPred (MHCII).Citation33,34 ProPred-I and ProPred identify and predict 47 types of MHCI and 57 types of MHCII allele specific binding peptides in a provided protein, respectively.Citation33,34 The predicted T cell epitopes were also confirmed by IEDB tool.Citation35 The selected epitopes were analyzed for comparisons with the human proteome using the Protein Blast program (BLASTp).Citation13 This was performed to validate that these epitopes will not trigger an autoimmune response.
Overlapping sites of mutations and predicted epitopes
A comprehensive exploration was performed to find out any reported mutations positioned in a predicted epitope. Predicted B cells and T cells epitopes were mapped to ORF1 and ORF2 regions that contain most of the reported mutation sites. Finally, the incidence of these overlapped mutations was determined among the selected genotypes (1, 3 and 4) by using percentage formula.
Prediction of antigenicity of epitopes
To investigate the antigenic properties of epitopes before and after mutations, VaxiJen tool was used.Citation15 The analysis was performed to find out whether these mutations lead to decreased, enhanced or sustained antigenicity of the specific epitopes.
Evolutionary analysis of mutations
To visualize the evolution of amino acid mutation related to chronic hepatitis, hepatic failure, ribavirin treatment failure or immunoreactivity within each genotype, a heat map was developed by GraphPad Prism version 7 (GraphPad Software, La Jolla California, USA) to calculate the number of amino acid and the isolation year differences between 3 individual genotypes. Isolation years were extracted from the strain-annotated information. The difference values were added into a matrix where the y-axis represents the isolation year differences and the x-axis represents the amino acid differences.
Abbreviations
| AI | = | Altered immunoreactivity |
| CH | = | Chronic hepatitis |
| HCV | = | Hepatitis C virus (HCV) |
| HEV | = | Hepatitis E virus |
| HF | = | Hepatic failure |
| MHCI | = | Major histocompatibility complex class I |
| MHCII | = | Major histocompatibility complex class II |
| ORF | = | Open reading frame |
| PEG-IFN-α | = | Pegylated interferon-α |
| RdRp | = | RNA-dependent RNA polymerase |
| RNA | = | Ribonucleic acid |
| RTF | = | Ribavirin treatment failure |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KVIR_S_1358349.zip
Download Zip (315.4 KB)Funding
The authors thank to the Higher Education Commission of Pakistan for financial support to A. Ikram; the Indonesia Endowment Fund for Education (LPDP) for PhD scholarship to Mohamad S. Hakim; the China Scholarship Council for funding PhD fellowship to W. Wang (201303250056); the Dutch Digestive Foundation (MLDS) for a career development grant (No. CDG 1304), the Daniel den Hoed Foundation for a Centennial Award fellowship and the Erasmus MC Mrace grant to Q. Pan.
References
- Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012; 379:2477-88. http://doi.org/10.1016/S0140-6736(11)61849-7. PMID:22549046
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-128. http://doi.org/10.1016/S0140-6736(12)61728-0. PMID:23245604
- Teshale EH, Hu DJ. Hepatitis E: Epidemiology and prevention. World J Hepatol. 2011;3:285-91. http://doi.org/10.4254/wjh.v3.i12.285. PMID:22216368
- Hakim MS, Wang W, Bramer WM, Geng J, Huang F, de Man RA, Peppelenbosch MP, Pan Q. The global burden of hepatitis E outbreaks: A systematic review. Liver Int. 2017;37:19-31. http://doi.org/10.1111/liv.13237. PMID:27542764
- Kamar N, Mallet V, Izopet J. Ribavirin for chronic hepatitis E virus infection. N Engl J Med. 2014;370:2447-8. http://doi.org/10.1056/NEJMoa1215246. PMID:24941183
- Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology. 2012;142:1388-97 e1. http://doi.org/10.1053/j.gastro.2012.02.014. PMID:22537448
- Takahashi K, Toyota J, Karino Y, Kang JH, Maekubo H, Abe N, Mishiro S. Estimation of the mutation rate of hepatitis E virus based on a set of closely related 7.5-year-apart isolates from Sapporo, Japan. Hepatol Res. 2004;29:212-5. http://doi.org/10.1016/j.hepres.2004.04.004. PMID:15288013
- Lhomme S, Garrouste C, Kamar N, Saune K, Abravanel F, Mansuy JM, Dubois M, Rostaing L, Izopet J. Influence of polyproline region and macro domain genetic heterogeneity on HEV persistence in immunocompromised patients. J Infect Dis. 2014;209:300-3. http://doi.org/10.1093/infdis/jit438. PMID:23964111
- van Tong H, Hoan NX, Wang B, Wedemeyer H, Bock CT, Velavan TP. Hepatitis E virus mutations: Functional and clinical relevance. EBioMedicine. 2016;11:31-42. http://doi.org/10.1016/j.ebiom.2016.07.039. PMID:27528267
- Singh R, Kaul R, Kaul A, Khan K. A comparative review of HLA associations with hepatitis B and C viral infections across global populations. World J Gastroenterol. 2007;13:1770-87. http://doi.org/10.3748/wjg.v13.i12.1770. PMID:17465466
- Sturniolo T, Bono E, Ding J, Raddrizzani L, Tuereci O, Sahin U, Braxenthaler M, Gallazzi F, Protti MP, Sinigaglia F, et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17:555-61. http://doi.org/10.1038/9858. PMID:10385319
- Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR, Wheeler DK, Gabbard JL, Hix D, Sette A, et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 2015;43:D405-12. http://doi.org/10.1093/nar/gku938. PMID:25300482
- Lavigne R, Seto D, Mahadevan P, Ackermann HW, Kropinski AM. Unifying classical and molecular taxonomic classification: Analysis of the Podoviridae using BLASTP-based tools. Res Microbiol. 2008;159:406-14. http://doi.org/10.1016/j.resmic.2008.03.005. PMID:18555669
- Gu Y, Tang X, Zhang X, Song C, Zheng M, Wang K, Zhang J, Ng MH, Hew CL, Li S, et al. Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody. Cell Res. 2015;25:604-20. http://doi.org/10.1038/cr.2015.34. PMID:25793314
- Doytchinova IA, Flower DR. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics. 2007;8:4. http://doi.org/10.1186/1471-2105-8-4. PMID:17207271
- Debing Y, Ramiere C, Dallmeier K, Piorkowski G, Trabaud MA, Lebosse F, Scholtès C, Roche M, Legras-Lachuer C, de Lamballerie X, et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J Hepatol. 2016;65:499-508. http://doi.org/10.1016/j.jhep.2016.05.002. PMID:27174035
- Debing Y, Gisa A, Dallmeier K, Pischke S, Bremer B, Manns M, Wedemeyer H, Suneetha PV, Neyts J. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology. 2014;147:1008-11 e7. quiz e15-6. http://doi.org/10.1053/j.gastro.2014.08.040. PMID:25181691
- Todt D, Walter S, Brown RJ, Steinmann E. Mutagenic effects of ribavirin on hepatitis E virus-viral extinction versus selection of fitness-enhancing mutations. Viruses. 2016; 8(10): 283-297. http://doi.org/10.3390/v8100283. PMID:27754363.
- Liang JH, Dai X, Dong C, Meng JH. A single amino acid substitution changes antigenicity of ORF2-encoded proteins of hepatitis E virus. Int J Mol Sci. 2010;11:2962-75. http://doi.org/10.3390/ijms11082962. PMID:21152284
- Liang J-H, Dai X, Dong C, Meng J-H. A single amino acid substitution changes antigenicity of ORF2-encoded proteins of hepatitis E virus. Int J Mol Sci. 2010;11:2962-75. http://doi.org/10.3390/ijms11082962. PMID:21152284
- Lhomme S, Kamar N, Nicot F, Ducos J, Bismuth M, Garrigue V, Petitjean-Lecherbonnier J, Ollivier I, Alessandri-Gradt E, Goria O, et al. Mutation in the hepatitis E virus polymerase and outcome of ribavirin therapy. Antimicrob Agents Chemother. 2016;60:1608-14. http://doi.org/10.1128/AAC.02496-15.
- Kamar N, Wang W, Dalton HR, Pan Q. Direct-acting antiviral therapy for hepatitis E virus? Lancet Gastroenterol Hepatol. 2017;2:154-5. http://doi.org/10.1016/S2468-1253(16)30242-4. PMID:28404127
- Zhang H, Dai X, Shan X, Meng J. The Leu477 and Leu613 of ORF2-encoded protein are critical in forming neutralization antigenic epitope of hepatitis E virus genotype 4. Cell Mol Immunol. 2008;5:447-56. http://doi.org/10.1038/cmi.2008.56. PMID:19118511
- Brown A, Halliday JS, Swadling L, Madden RG, Bendall R, Hunter JG, Maggs J, Simmonds P, Smith DB, Vine L, et al. Characterization of the specificity, functionality, and durability of host T-Cell responses against the full-length hepatitis E virus. Hepatology. 2016;64:1934-50. http://doi.org/10.1002/hep.28819. PMID:27631819
- Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2013;41:D36-42. http://doi.org/10.1093/nar/gks1195. PMID:23193287.
- Choi Y, Chan AP. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31:2745-7. http://doi.org/10.1093/bioinformatics/btv195. PMID:25851949
- Bao L, Zhou M, Cui Y. nsSNPAnalyzer: Identifying disease-associated nonsynonymous single nucleotide polymorphisms. Nucleic Acids Res. 2005;33:W480-W2. http://doi.org/10.1093/nar/gki372. PMID:15980516
- Ferrer-Costa C, Gelpí JL, Zamakola L, Parraga I, De La Cruz X, Orozco M. PMUT: A web-based tool for the annotation of pathological mutations on proteins. Bioinformatics. 2005;21:3176-8. http://doi.org/10.1093/bioinformatics/bti486. PMID:15879453
- Pires DE, Ascher DB, Blundell TL. DUET: A server for predicting effects of mutations on protein stability using an integrated computational approach. Nucleic Acids Res. 2014;42:W314-9. http://doi.org/10.1093/nar/gku411. PMID:24829462
- Capriotti E, Fariselli P, Casadio R. I-Mutant2. 0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33:W306-W10. http://doi.org/10.1093/nar/gki375. PMID:15980478
- Quan L, Lv Q, Zhang Y. STRUM: Structure-based prediction of protein stability changes upon single-point mutation. Bioinformatics. 2016;32:2936-46. http://doi.org/10.1093/bioinformatics/btw361. PMID:27318206
- Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, Damle R, Sette A, Peters B. The immune epitope database 2.0. Nucleic Acids Res. 2010;38:D854-62. http://doi.org/10.1093/nar/gkp1004. PMID:19906713
- Singh H, Raghava GP. ProPred: Prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236-7. http://doi.org/10.1093/bioinformatics/17.12.1236. PMID:11751237
- Singh H, Raghava GP. ProPred1: Prediction of promiscuous MHC Class-I binding sites. Bioinformatics. 2003;19:1009-14. http://doi.org/10.1093/bioinformatics/btg108. PMID:12761064
- Kim Y, Ponomarenko J, Zhu Z, Tamang D, Wang P, Greenbaum J, Lundegaard C, Sette A, Lund O, Bourne PE, et al. Immune epitope database analysis resource. Nucleic Acids Res. 2012;40:W525-30. http://doi.org/10.1093/nar/gks438. PMID:22610854