During the last decade the invertebrate Galleria mellonella has successfully been used as a powerful and reliable model host for the analysis of bacterial pathogenesis as it offers an inexpensive and ethically acceptable alternative to the usage of e.g. rodents in infection research.Citation1 In this context, Galleria mellonella is of special importance for biomedical research with focus on innate immune activation in inflammation and infection. Recently, Galleria mellonella transcriptome analysis revealed the presence/identified proteins related to immune signaling which are homologuous to their vertebrate counterparts.Citation2 The presence of these highly conserved innate immune mediators makes G. mellonella a good model for the investigation of innate immune system activation by microbe associated molecular pattern (MAMP) which mediate the initial interaction of microbes with the host. Therefore, MAMPs play a key role in activation of the innate immune system in vertebrates as well as in invertebrates. One of the most frequently studied MAMPs in insect immunity is LPS being a main compound of the Gram negative bacterial cell wall. LPS is often used as an immune elicitor in virulence studies or in gene expression studies using Galleria mellonella.Citation2,Citation3 In addition, LPS is used as a standard positive control in many assays focusing on activation of the innate immune system, first to facilitate the interpretation of the data and, second, to ensure the technical validity. However, when using LPS, one must be aware that different bacterial species express different LPS with different bioactivity,Citation4 and second, that distinct commercially available LPS dramatically differs in purity. This is an important point since only LPS labeled as “ultrapure” is almost completely free from contaminants such as components of the gram negative cell wall other than LPS, finally inducing a specific and defined LPS-mediated immune response in experimental settings. However, “standard” or “crude” commercially available LPS contains other components of Gram negative bacteria, including i.e. peptidoglycans or lipopeptides, finally resulting in a mixed and non-standardized immune response due to an undefined stimulus. In many publications, unfortunately, the information on the LPS used is inaccurate or missing. Often, the origin species or the degree of purity of the LPS used are not indicated, a fact that unnecessarily decreases the reliability of published experiments. In our work, we provide evidence that these informations are absolutely essential as distinct commercially available LPS achieve significantly different results.
We used “ultrapure” (UP LPS, tlrl-peklps) or “standard” (ST LPS, tlrl-eklps) Escherichia coli K12 LPS of the same manufacturer (Invivogen, Toulouse, France). Standard pure LPS is manufactured via phenol extraction and contains TLR2 and TLR4 activating compounds while ultrapure LPS is manufactured using additional enzymatic treatments and purified by the phenol-TEA-DOC extraction protocol.Citation5,Citation6 The purity was verified using human embryonic kidney cells overexpressing murine TLR2 or TLR4-MD2-CD14 receptor complex. UP LPS only activated TLR4-dependent signaling, while ST LPS activated both, TLR2- and TLR4-dependent signaling (data not shown). This clearly indicates that ST LPS contains additional co-components, like peptidoglycanes or lipopeptides, other than a defined TLR4 ligand. Therefore, this ST LPS is an inappropriate tool to investigate a defined TLR4-dependent signal.
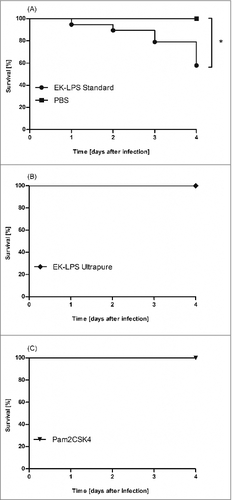
This finding is also of utmost relevance to G. mellonella based investigations. Galleria mellonella larvae were purchased from fauna topics (Germany) and reared several weeks to last instar on artificial diet (22% maizemeal, 22% wheat germ, 11% dry yeast, 17.5% bee wax, 11% honey, 11% glycerin) at 30°C in darkness. Only larvae providing a weight between 120 mg and 180 mg were used for experiments. We could show that injection of ST LPS but not of UP LPS into the hemolymph led to an enhanced lethality of the G. mellonella larvae (). None of the larvae which were injected with 100 µg UP LPS died but injection of the same amount of ST LPS resulted in exitus of 42% of the larvae within the observation time period of 4 d. This clearly demonstrates that the purity of the used LPS decides on life or death of the LPS-injected larvae.
Figure 1. G. mellonella larvae (n ≥ 19) were injected with 100 µg standard pure (ST LPS) (A), ultrapure LPS (UP LPS) (B) or synthetic TLR2 ligand Pam2CSK4 (C) dissolved in 10 µl of PBS. As negative control, larvae were injected with the same volume PBS. Survival was analyzed over 4 d. The figure clearly shows that ST LPS injected larvae but not the UP LPS or PBS injected larvae die upon injection. Shown data are mean values ± SD. ## = P ≤ 0.01, Log-rank (Mantel-Cox) Test
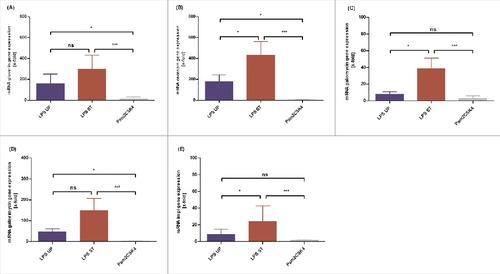
The activation of the innate immune system of G. mellonella results in expression and release of various antimicrobial molecules such as antimicrobial peptides (AMP) and opsonins or the activation of phenoloxidase. AMPs are rapid in killing bacteria and their selective toxicity combined with effectiveness against a broad range of microbes underlines their importance.Citation7 To elucidate the effects of injection of distinct LPS on these invertebrate innate immune responses, Galleria mellonella larvae were injected with 1 µg UP LPS, 1 µg ST LPS or PBS as a negative control. Larvae were incubated for 4h at 37°C. Subsequently, the expression of genes encoding for AMPs of the whole body of larvae (gloverin, cecropin, galiomycin, gallerimycin) and the inducible metalloproteinase inhibitor (impi) was analyzed by qRT- PCR and normalized to the PBS-injected control. As expected, the negative control PBS did not influence the expression of the target genes. The application of both, UP LPS as well as ST LPS resulted in an upregulation of gloverin, galimycin, gallerimycin, impi and cecropin mRNA expression. However and in line with the enhanced mortality of larvae upon injection of ST LPS, we detected a significantly increased mRNA expression of all target mRNAs in larvae treated with ST LPS as compared with UP LPS injected G. mellonella larvae (). This observation also points to the presence of further immunostimulatory molecules besides LPS in the used ST LPS preparations which are probably responsible for an additional undefined immune response.
Figure 2. G. mellonella larvae (n ≥ 8) were injected with 1 µg ultrapure or standard LPS or Pam2CSK4 were dissolved in 10 µl of PBS. As negative control larvae were injected with the same volume PBS. Expression of gloverin (A), cecropin (B), galliomyin (C), gallerimycin (D) and impi (E) mRNA was analyzed using qRT PCR. Shown data are mean values ± SD. Not significant (ns) = P>0.05; # = P ≤ 0.05; ## = P ≤ 0.01; ### = P ≤ 0.001; #### = P ≤ 0.0001, One-way analysis of variance and Tukey's Multiple Comparison Test or if values are not Gauss distributed Kruskal-Wallis test and Dunn's Multiple Comparison Test
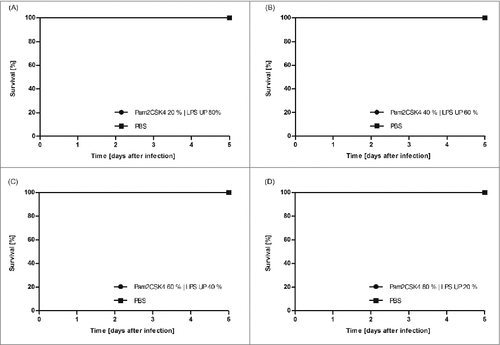
As ST LPS proved to also activate HEK TLR2 reporter cells we injected G. mellonella larvae with the synthetic TLR2 ligand Pam2CSK4. Interestingly, this defined TLR2-ligand neither induced mortality in the larvae () nor the expression of antimicrobial peptides or impi (). Additionally, the stimulation of larvae with a combination of UP LPS and Pam2CSK4, which was intended to mimic ST LPS, showed no effect on the mortality of the larvae (). As an additional finding this might indicate that G. mellonella does not express a TLR2 homolog.
Figure 3. G. mellonella larvae (n ≥ 20) were injected with 100 µg Pam2CSK4 | UP LPS mixture in various ratios (Pam2CSK4 20% | LPS UP 80% (A), Pam2CSK4 40% | LPS UP 60% (B), Pam2CSK4 60% | LPS UP 40% (C), Pam2CSK4 80% | LPS UP 20% (D)) dissolved in 10 µl of PBS. As negative control larvae were injected with the same volume PBS. Survival was monitored over 5 d. The figure clearly demonstrates that all Pam2CSK4 | UP LPS mixtures injected larvae or PBS injected larvae survived
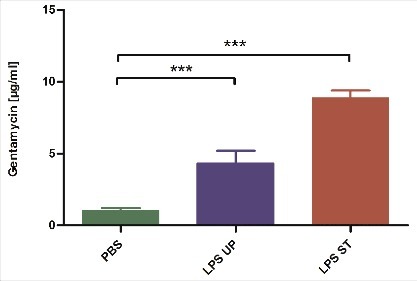
To assess the different expression of AMPs on protein level we tested the antimicrobial activity of the hemolymph of G. mellonella larvae treated with either ST LPS or UP LPS. Therefore, G. mellonella larvae were injected with 1 µg of both LPS and incubated for 18 h. Subsequently, 5 µl hemolymph of the stimulated larvae were transferred on Geobacillus stearothermophilus spore plates. Since AMPs inhibit bacterial growth on such plates, the presence of AMPs in the hemolymph should result in formation of inhibition zones. In fact and in line with the AMP gene expression levels (), the hemolymph taken from ST LPS treated larvae provided the strongest inhibitory effect indicating that this hemolymph contains higher AMP concentrations; injection of UP LPS resulted in a weaker antimicrobial activity of the hemolymph (). PBS treated larvae were used as negative control and induced an only slight growth inhibition most likely induced by the pricking injury during injection. All inhibition zones were standardized to the antimicrobial activity of Gentamicin ().
Figure 4. G. mellonella larvae (n ≥ 6) were treated with either 1 µg standard or ultrapure LPS dissolved in 10 µl of PBS. 18 h after injection 5 µl of hemolymph were transferred onto Geobacillus stearothermophilus spore plates. After 24 h the diameter of inhibition zones was measured and normalized to a gentamicin standard curve. Shown data are mean values ± SD. Not significant (ns) = P>0.05; # = P ≤ 0.05; ## = P ≤ 0.01; ### = P ≤ 0.001; #### = P ≤ 0.0001, One-way analysis of variance, Tukey's Multiple Comparison Test
Up to this point, our investigations allow the following conclusions:
| (i) | Systemic application of “standard” or “crude” LPS (ST LPS) induces high expression of genes encoding for e.g., antimicrobial peptides and leads to death of the larvae. | ||||
| (ii) | Systemic application of defined “ultrapure” LPS (UP LPS) induces an intermediate expression of genes encoding for e.g., antimicrobial peptides but does not result in death of the larvae. | ||||
| (iii) | The purity of the LPS used determines the quality of the immune response. Thus the statement about the purity of the used LPS is an essential information. | ||||
To achieve reproducibility, to produce data of high quality and meaningfulness and to make quality assurance possible, we believe it is of the utmost importance for all studies performed with G. mellonella to clearly state the purity of the used LPS, as well as the bacterial species the LPS originates from. In addition we believe that it is essential to use ultrapure LPS in studies that intent to analyze a defined LPS specific immune response.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Funding was provided by the Deutsche Forschungsgemeinschaft (grant no. GRK 1708).
References
- Mukherjee K, Vilcinskas A. Development and immunity-related microRNAs of the lepidopteran model host Galleria mellonella. BMC Genomics. 2014;15:705. https://doi.org/https://doi.org/10.1186/1471-2164-15-705. PMID:25149864
- Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol. 2010;76:310-7. https://doi.org/https://doi.org/10.1128/AEM.01301-09. PMID:19897755
- Altincicek B, Stotzel S, Wygrecka M, Preissner KT, Vilcinskas A. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol. 2008;181:2705-12. https://doi.org/https://doi.org/10.4049/jimmunol.181.4.2705. PMID:18684961
- Steimle A, Autenrieth IB, Frick JS. Structure and function: Lipid A modifications in commensals and pathogens. Int J Med Microbiol. 2016;306:290-301. https://doi.org/https://doi.org/10.1016/j.ijmm.2016.03.001. PMID:27009633
- Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618-22. https://doi.org/https://doi.org/10.4049/jimmunol.165.2.618. PMID:10878331
- Tirsoaga A, Novikov A, Adib-Conquy M, Werts C, Fitting C, Cavaillon JM, Caroff M. Simple method for repurification of endotoxins for biological use. Appl Environ Microbiol. 2007;73:1803-8. https://doi.org/https://doi.org/10.1128/AEM.02452-06. PMID:17261511
- Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1999;1462:1-10. https://doi.org/https://doi.org/10.1016/S0005-2736(99)00197-2. PMID:10590299
