Combination antiretroviral therapy blocks HIV-1 replication, allowing the immune system to clear the virus producing cells. This reduces new HIV-1 particle production to undetectable levels. Alas, latent HIV-1 infections frequently occur in activated and memory T cells, macrophages, dendritic cells (DCs) and other susceptible cell types.Citation1,2 These latently infected cells form latent reservoirs because they are not removed via cytotoxic T cell killing due to the lack of viral antigen presentation. The reservoir in patients on therapy mostly consists of long-lived resting memory T cellsCitation3,4 as activated T cells have a very short half-life. Monocytes and DCs have long half-lives, but their presence in the latent reservoir is strongly reduced after 6 months of therapy.Citation5,6 Long-lived latently infected cells that harbor replication competent virus can be triggered to start virus production as evidenced by a rapid viral rebound when combined antiretroviral therapy is stopped.Citation5,7 Ren et al. have shed light on how this triggering might occur.Citation8 They demonstrated that dendritic cells, when matured upon T cell contact or via stimulation with bacterial compounds, are capable of purging latently infected T cells via secretion of TNFα. These results highlight that bacterial co-infections can modulate HIV-1 reservoirs via dendritic cell maturation.
It is unknown why some cells are latently infected while others allow productive virus replication. Studying the influence of drugs to purge latency by activating signal transduction cascades can provide insight in the molecular mechanisms of latency establishment and the possibility of therapeutic purging. Such drugs are known as latency reversing agents (LRAs) and were reviewed in Spina et al.Citation9 Prostratin or bryostatin activate the protein kinase C (PKC) pathway and reverse latency by increasing active NF-κB levels.Citation10 TNFα induces the MEK-ERK and IκK pathways and activate viral transcription by elevating the level of active NF-κB and c-Fos/c-Jun.Citation11 The HDAC inhibitors, romidepsin and vorinostat can remodel the chromatin structure.Citation12,13 They act as LRA by increasing the binding accessibility of transcription factors to the HIV-1 long terminal repeat (LTR) promoter region, thus boosting viral transcription. However, the strongest LRAs are T cell receptor (TCR) agonists such as phytohaemagluttinin (PHA) and CD3/CD28 antibodies. These agonists drive clonal T cell proliferation and activate multiple signaling cascades such as the MEK-ERK, PKC, IκK, PI3K-Akt and other pathways.Citation14
Tests of various LRAs on resting memory cells from patients indicated striking differences in the efficiency of latency purging.Citation9,13,15,16 Per million resting T cells, maximally 3 cells could be converted to produce virus by repeated TCR stimulation with PHA or CD3/CD28,Citation17,18 whereas other LRAs failed with the exception of PMA/ionomycin.Citation13 The estimated number of cells that contain infectious replication competent HIV-1, however, is thought to be 10-fold higher.Citation19 Thus latency reversal with drug-based LRAs is quite inefficient.
It is not known whether the rapid viral rebound is caused by reversal of latency or by ongoing virus replication in tissues with a low drug penetration. There is evidence that virus evolution still occurs during therapy, in particular with some drug cocktails or therapy non-adherence.Citation20 Other reports show ongoing virus replication without viral evolution as the HIV-1 progeny is related to the virus present at earlier time points.Citation21,22 The latter data imply that latent HIV-1 can be activated spontaneously via a natural mechanism, such as cell-cell contact or via production of latency regulating cytokines during inflammation.
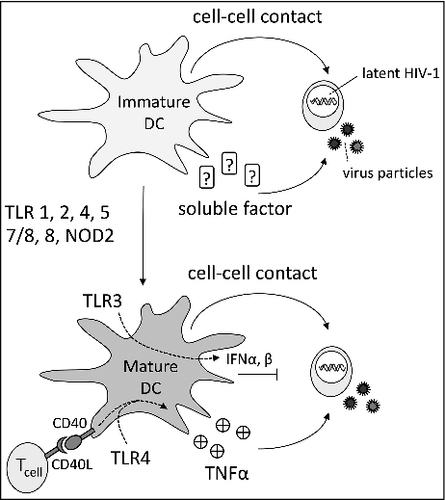
A few groups have studied reversal of HIV-1 latency mediated by cell-cell interactions.Citation23-25 Especially immature dendritic cells (DCs) were capable of undoing latency in TCR-activated latently infected primary T cells upon cell-cell contact and via secretion of an unknown soluble factor (). Macrophages were also capable of overcoming HIV-1 latency, but are not as potent as DCs, whereas B cells, T cells or monocytes did not purge latent HIV-1.Citation23 Studying the anti-latency properties of DCs derived from various tissues showed differential activities: genital tract DCs like dermal DCs and plasmacytoid DCs could not revert latency, whereas representative gut DCs purged latent HIV-1 efficiently.Citation23 Maturation of DCs with different Toll-like receptor (TLR 1, 2, 4, 5, 7/8, 8) agonists or NOD2 enabled efficient reversal of latency, with the exception of TLR3 matured DCs treated with double stranded (poly I:C) RNA. TLR3-matured DCs produce high amounts of interferons α and β, which negate the purging effect.Citation23 Thus latent HIV-1 can be purged naturally by DCs (), depending on the type of tissue and DCs present therein.
Figure 1. DC-mediated purging of latency. Immature DCs are able to revert HIV-1 latency in primary T cells upon cell-cell contact or via secretion of an unknown soluble natural LRA. Purging latent HIV-1 can also be achieved with mature DCs stimulated with TLR 1,2,4,5,7/8,8 or NOD2. TLR3 stimulated DCs probably can revert latency, but additional production of IFNα and β negate latency reversion capacities.Citation23 DCs can revert latency via secretion of TNFα. This can be achieved by stimulating DCs with TLR4 or upon contact with T cells in a CD40/CD40-ligand dependent manner.Citation8
It may not be unexpected that DCs undo HIV-1 latency in T cells as the antigen presentation and recognition processes can induce T cell receptor activation and clonal expansion. Thus DCs should have similar purging properties as the most powerful LRAs, CD3/CD28 or PHA combined with HDAC inhibitors. Since the HIV-1 reservoir in treated patients primarily consists of memory T cells recognizing highly specific antigens the chance that DCs present those antigens is slim.Citation26 Moreover, HIV-1 specific memory T cells cannot be stimulated by DCs when therapy is initiated and antigens become unavailable.
Reversion of latent HIV-1 by DCs, however, does not only work via antigen presentation pathways. Latently infected T cells can initiate virus production upon DC stimulation without antigen presentation.Citation24 In addition, DCs can secrete soluble components that have LRA activity. Now, Ren et al. identified TNFα as such a natural LRA secreted by DCsCitation8 (). They show that immature DCs increase CD40 expression and mature upon contact with T cells. The matured DC subsequently releases TNFα that can initiate virus production in the latently infected cell line. Maturation of DCs could also be induced with lipopolysaccharide or Mycobacterium bovis, and DC-supernatant could purge resting memory T cells from infected patients. This important finding confirms that cells belonging to the patient reservoir can be purged by immature DCs via cell-cell contact or upon their maturation with bacterial products, which might be regulated via release of TNFα.Citation8,23-25 Further evaluation of TNFα as LRA on primary T cells is of utmost importance. Surprisingly, in the experimental setup used by Ren et al., cell-cell contact between matured DCs and T cells did not lead to reversion of HIV-1 latency, but a diminished expression of CD40 on the DC was noticed. Possibly reduced CD40 binding to its CD40 ligand partner on the T cell decreases activation of signaling cascades or TNFα release below the purging threshold. Alternatively, matured DCs can release interferons that block reversion of latent HIV-1Citation23 or upregulate co-inhibitory molecules such as PDL1/PDL2 that might block reversal of latency.Citation27
Drug-based LRAs provided insights on how to purge latent HIV-1, but the use of these drugs to reduce the reservoir in patients is either blocked by toxicity or the drugs are not sufficiently effective to allow one to stop treatment.Citation28,29 Not much is known about natural mechanisms that can influence activation of latently infected cells and their contribution to infection reestablishment. As proven by Ren et al. and others, DCs can purge latency, either via cell-cell interactions or via release of natural LRAs.Citation8,23-25 Understanding what natural signaling pathway is required to purge latently infected cells in patients might help to develop drugs that mimic a natural purging mechanism to accelerate reservoir decay towards thresholds allowing therapy termination. Alternatively, drugs could be developed that prevent DC-mediated purging. In the end, such drugs might forever prevent HIV-1 from awakening.
HIV-1 latency research by TvM was supported by the Dutch Aidsfonds (Project number 2013021 and P-22602 http://www.aidsfonds.nl/about/organisation).
References
- Kumar A, Abbas W, Herbein G. HIV-1 latency in monocytes/macrophages. Viruses. 2014;6:1837-60. https://doi.org/https://doi.org/10.3390/v6041837 PMID:24759213
- Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology [Internet]. 2009;6:51. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2697150&tool=pmcentrez&rendertype=abstract https://doi.org/https://doi.org/10.1186/1742-4690-6-51
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893-900. https://doi.org/https://doi.org/10.1038/nm.1972 PMID:19543283
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512-7. https://doi.org/https://doi.org/10.1038/8394 PMID:10229227
- Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol. 2009;186:255-68. https://doi.org/https://doi.org/10.1083/jcb.200903070 PMID:19635843
- Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9:853-60. https://doi.org/https://doi.org/10.1038/nm0703-853 PMID:12835705
- Chun T-W, Davey RT, Engel D, Lane HC, Fauci AS. AIDS: Re-emergence of HIV after stopping therapy. Nature [Internet]. 1999;401:874-5. Available from: https://doi.org/https://doi.org/10.1038/44755
- Ren X-X, Ma L, Sun W-W, Kuang W-D, Li T-S, Jin X, Wang J-H. Dendritic cells maturated by co-culturing with HIV-1 latently infected Jurkat T cells or stimulating with AIDS-associated pathogens secrete TNF-alpha to reactivate HIV-1 from latency. Virulence 2017. https://doi.org/https://doi.org/10.1080/21505594.2017.1356535
- Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, Greene WC, Kashuba A, Lewin SR, Margolis DM, et al. An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients. PLoS Pathog. 2013;9:1-15. https://doi.org/https://doi.org/10.1371/journal.ppat.1003834
- Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Discov [Internet]. 2012;11:937-57. Available from: https://doi.org/https://doi.org/10.1038/nrd3871
- Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A [Internet]. 1989;86:2336-40. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=286907&tool=pmcentrez&rendertype=abstract https://doi.org/https://doi.org/10.1073/pnas.86.7.2336
- Gardner KE, Allis CD, Strahl BD. Operating on chromatin, a colorful language where context matters. J Mol Biol [Internet]. 2011;409:36-46. Available from: https://doi.org/https://doi.org/10.1016/j.jmb.2011.01.040
- Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med [Internet]. 2014;20:425-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3981911&tool=pmcentrez&rendertype=abstract%5Cnhttp://www.nature.com/doifinder/https://doi.org/10.1038/nm.3489 https://doi.org/https://doi.org/10.1038/nm.3489
- Smith-Garvin JE, Koretzky 1 Gary A, Jordan MS, Koretzky G. T Cell Activation. Annu Rev Immunol. 2009;27:591-619. https://doi.org/https://doi.org/10.1146/annurev.immunol.021908.132706 PMID:19132916
- Darcis G, Kula A, Bouchat S, Fujinaga K, Corazza F, Ait-Ammar A, Delacourt N, Melard A, Kabeya K, Vanhulle C, et al. An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLOS Pathog [Internet]. 2015;11:e1005063. Available from: https://doi.org/https://doi.org/10.1371/journal.ppat.1005063
- Laird GM, Bullen CK, Rosenbloom DIS, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF. Ex vivo analysis identifies effective HIV-1 latency – reversing drug combinations. The journal of clinical investigation. 2015;125:1-12.
- Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med [Internet]. 2016;22:1043-1049. doi:https://doi.org/10.1038/nm.4156. Available from: http://www.nature.com/doifinder/https://doi.org/10.1038/nm.4156
- Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DIS, Keele BF, Ho Y-C, Siliciano JD, Siliciano RF. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp Med. 2017;214:959 LP–972. https://doi.org/https://doi.org/10.1084/jem.20170193
- Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-Competent Noninduced Proviruses in the Latent Reservoir Increase Barrier to HIV-1 Cure. Cell [Internet]. 2013;155:540-51. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867413011574 https://doi.org/https://doi.org/10.1016/j.cell.2013.09.020
- Pasternak AO, De Bruin M, Jurriaans S, Bakker M, Berkhout B, Prins JM, Lukashov VV. Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J Infect Dis. 2012;206:1443-52. https://doi.org/https://doi.org/10.1093/infdis/jis502 PMID:22927449
- Joos B, Fischer M, Kuster H, Pillai SK, Wong JK, Böni J, Hirschel B, Weber R, Trkola A, Günthard HF, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci U S A 2008;105:16725-30. https://doi.org/https://doi.org/10.1073/pnas.0804192105
- Wong JK, Hezareh M, Günthard HF, Havlir D V, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291-5. https://doi.org/https://doi.org/10.1126/science.278.5341.1291 PMID:9360926
- van der Sluis RM, van Capel TMM, Speijer D, Sanders RW, Berkhout B, de Jong EC, Jeeninga RE, van Montfort T. Dendritic cell type-specific HIV-1 activation in effector T cells. Aids. 2015;29:1003-14. https://doi.org/https://doi.org/10.1097/QAD.0000000000000637
- van der Sluis RM, van Montfort T, Pollakis G, Sanders RW, Speijer D, Berkhout B, Jeeninga RE. Dendritic Cell-induced Activation of Latent HIV-1 Provirus in Actively Proliferating Primary T Lymphocytes. PLoS Pathog. 2013;9(3). https://doi.org/https://doi.org/10.1371/journal.ppat.1003259
- Marini A, Harper JM, Romerio F. An In Vitro System to Model the Establishment and Reactivation of HIV-1 Latency. J Immunol. 2008;181:7713-20. https://doi.org/https://doi.org/10.4049/jimmunol.181.11.7713
- Chun BT, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 Replication in Latently Infected CD4+ T Cells Using a Combination of Cytokines. J Exp Med. 1998;188:83-91. https://doi.org/https://doi.org/10.1084/jem.188.1.83 PMID:9653086
- Meier A, Bagchi A, Sidhu HK, Alter G, Suscovich TJ, Kavanagh DG, Streeck H, Brockman MA, LeGall S, Hellman J, et al. Up-regulation of PD-L1 on Monocytes and Dendritic Cells by HIV-1 derived TLR Ligands. AIDS. 2008;22:655-8. https://doi.org/https://doi.org/10.1097/QAD.0b013e3282f4de23
- Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, Schellekens PT, ten Berge IJ, Yong SL, Fox CH, Roos MT, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13:2405-10. https://doi.org/https://doi.org/10.1097/00002030-199912030-00012 PMID:10597782
- Delagrèverie HM, Delaugerre C, Lewin SR, Deeks SG, Li JZ. Ongoing clinical trials of human immunodeficiency virus latency-reversing and immunomodulatory agents. Open Forum Infect Dis. 2016;3. https://doi.org/https://doi.org/10.1093/ofid/ofw189
