ABSTRACT
Complement system and dendritic cells (DCs) form – beside neutrophils and macrophages – the first line of defense to combat fungal infections. Therefore, we here studied interactions of these first immune elements with Aspergillus fumigatus lacking ß-1,3-glucans (fks1tetOnrep under repressed conditions) to mechanistically explain the mode of action of echinocandins in more detail. Echinocandins are cell wall active agents blocking β-glucan synthase, making the A. fumigatus fks1tetOn mutant a good model to study immune-modulatory actions of these drugs. We now demonstrate herein, that complement was activated to significantly higher levels by the fks1-deficient strain compared to its respective wild type. This enhanced covalent linking of complement fragments to the A. fumigatus fks1tetOnrep mutant further resulted in enhanced DC binding and internalization of the fungus. Additionally, we found that fks1tetOnrep induced a Th1-/Th17-polarizing cytokine profile program in DCs. The effect was essentially dependent on massive galactomannan shedding, since blocking of DC-SIGN significantly reduced the fks1tetOnrep-mediated induction of an inflammatory cytokine profile.
Our data demonstrate that lack of ß-1,3-glucan, also found under echinocandin therapy, results in improved recognition of Aspergillus fumigatus by complement and DCs and therefore not only directly affects the fungus by its fungistatic actions, but also is likely to exert indirect antifungal mechanisms by strengthening innate host immune mechanisms.
Abbreviations: C: complement; CR:complement receptor; DC: dendritic cell; iDC: immature dendritic cell; DC-SIGN: Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin; ERK: extracellular signal–regulated kinases; JNK : c-Jun N-terminal kinases; MAPK: mitogen-activated protein kinase; NHS: normal human serum; PRR: pattern recognition receptor; Th :T helper; TLR :Toll-like receptor; WT: wild type.
Background
Aspergillus (A.) fumigatus is one of the most prevalent fungal pathogens in immunocompromised patients [Citation1]. Together with macrophages and neutrophils the complement (C) system and dendritic cells (DCs) form a first line antifungal defense by producing inflammatory cytokines, ROS or anaphylatoxins.
A. fumigatus was demonstrated to activate the C-system via alternative or classical pathways, depending on morphology and germination status of the fungus [Citation2,Citation3]. Resting conidia represent a poorly immunogenic surface due to hydrophobins (rodlet layer), covering the fungal surface, and DHN-melanin, lying underneath, thereby preventing innate immune recognition [Citation3,Citation4]. Nevertheless resting conidia slowly activate the complement system via the alternative pathway, while swollen conidia or hyphae are mainly recognized by classical C-components [Citation2,Citation3,Citation5]. Less pathogenic Aspergillus species, e.g. A. nidulans, exhibited higher deposition of C3 molecules on their surface compared to the highly pathogenic species A. flavus and A. fumigatus [Citation5,Citation6]. Another study revealed lower C3-deposition on wild type A. fumigatus conidia when compared to conidia lacking melanin [Citation3,Citation7], thereby pointing to a role of melanin with respect to fungal evasion strategies. The lack in the early melanin enzymes Arp1 and Alb1 was demonstrated to significantly increase C-deposition on A. fumigatus, thereby modulating its virulence [Citation3,Citation8]. C3 fragments coating the surface of A. fumigatus influence binding to and internalization in phagocytes such as DCs, macrophages or neutrophils. Upon germination fungal cells expose polysaccharides, such as α-1,3–, β-1,3-glucans, galactomannan and galactosaminogalactan [Citation4,Citation9], which ligate other PRRs than CRs on DCs, i.e. TLR2, C-type lectin receptors Dectin-1 and −2 or DC-SIGN [Citation10].
Echinocandines targeting cell wall β-1,3-glucans by inhibiting the β-glucan synthase, cause changes in A. fumigatus cell wall composition [Citation11,Citation12]; when treating wild type Candida glabrata isolates with caspofungin or micafungin a significantly increased macrophage activation was detected [Citation13]. Thus, changes in cell wall composition of the fungus might also significantly contribute to differential innate and adaptive immune shaping not only via macrophages but also via DCs during echinocandin treatment.
DCs are key regulators of immunity and the most important cells in initiating adaptive fungal immunity [Citation14,Citation15]. During the acute phase of infection cytokines produced by stimulated and matured DCs polarize protective and non-protective T helper (Th) cell responses, T cell subsets providing significant protection against A. fumigatus comprise Th1 and Th17 cells, while Th2 cells are associated with a poor outcome of disease [Citation16]. Therefore, immediate secretion of Th1- or Th17-polarizing cytokines by stimulated DCs provides spontaneous triggering of protective antifungal immune responses.
This study aimed at a better understanding of how cell wall components of A. fumigatus contribute to the fungal recognition by i) complement and ii) DCs and of whether complement opsonization supports a more efficient sensing by DCs upon invasive fungal infections. Therefore, we investigated A. fumigatus wild-type strains as well as mutants devoid of DHN-melanin (∆pksP) or ß-1,3-glucan (fks1tetOn under repressed conditions, fks1tetOnrep) in their cell walls regarding complement (C) activation as well as innate sensing and cytokine up-regulation in iDCs exposed to such non- or C-opsonized fungal conidia.
Results
Lack of melanin and β-1,3-glucan favor C3 deposition on A. fumigatus conidia
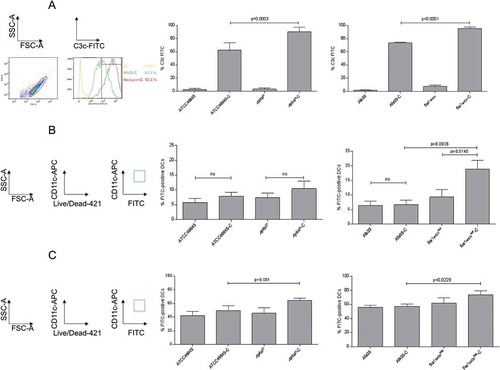
Significantly higher C3c deposition (mean 89.8% C3+ conidia, ), left) was observed for ∆pksP compared to WT ATCC46645-C (59.2% C3+ conidia, ), left), which is in agreement with published data [Citation17]. Also the fks1tetOnrep mutant showed a significantly higher complement deposition compared to its respective wild type AfS35 (94.9% vs. 76.7% C3+ conidia, ), right), while in all non-opsonized controls only background C3 deposition was analyzed ()). C3 deposition on the fks1tetOnrep was even significantly higher compared to ∆pksP (p = 0.0384). We here found that lack of either melanin or β-1,3-glucan results in significantly higher C3 coating of the fungal conidia.
Figure 1. Complement activation and DC interactions depend on cell wall composition. (a) Melanin- and β-1,3-glucan-lacking strains demonstrate significantly higher surface C3-deposition. Significantly higher covalently linked C3 levels were detected on ∆pksP (left) and fkstetOnrep (right) compared to their respective WT (46645, AfS35). Non-opsonized counterparts served as controls for background C3c-binding. A summary from 6 different experiments (n = 6) is shown for pksP opsonization, and from 10 experiments (n = 10) for fks opsonization. Binding (b) and internalization (c) of non- and C-opsonized swollen conidia by DCs. For binding (4°C) and internalization (37°C) analyzes, percentages of FITC-labelled conidia bound and/or internalized to live CD11chigh DCs from at least 5 independent experiments were assessed by multi-color flow cytometry. The gating strategy is depicted on the left. Differences were analyzed by one-way ANOVA using Bonferroni´s post test for multiple comparisons.
Complement-opsonized conidia exert improved recognition by DCs
To further characterize binding of non- and opsonized WT and mutant conidia, co-cultures of DCs and FITC-labeled conidia (MOI = 2) were incubated for 6h at 4°C [Citation18]. At 4°C, DCs just bind but do not internalize fungal pathogens – again we used FACS analyses and gated on live CD11chigh DCs. CD11chigh/FITChigh double-positive cells were used to determine percentages of conidia attached to the cells (), left). We found highest levels of fks1tetOnrep-C (mean 20% FITC-positive DCs, ), right) attached to DCs followed by ∆pksP-C (mean 10% FITC-positive DCs) (), left) and fks1tetOnrep (mean 9% FITC-positive DCs, ), right). Therefore, C-opsonization of conidia enhanced DC binding in case of both mutant strains, but not the WT conidia ()).
Regarding internalization, which was performed for 6h at 37°C, significantly higher combined binding and internalization of ∆pksP-C were detectable compared to its opsonized WT, which is due to the higher activity of the cells at this temperature (), left, 49.4% ATCC46645-C vs. 64.3% ∆pksP-C). Highest binding/internalization was measured using fks1tetOnrep-C (), right, 73.5%) but also non-opsonized fks1tetOnrep demonstrated a slightly higher, but non-significant up-take into DCs compared to its WT (), right, 59.8%). In concordance with the highest C3-deposition on fks1tetOnrep devoid of β-1,3-glucan, we here demonstrated the highest binding and internalization by DCs.
Efficient internalization of ∆pksP-C and fks1teton-C is mediated by CR3 and CR4
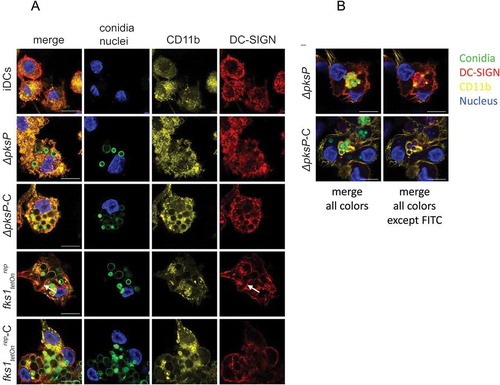
To further outline the importance of CD11b, the α-chain of CR3, and CD11c, the α-chain of CR4, in enhanced opsonized conidia uptake, we next performed confocal microscopic analyses under the same conditions as described for the internalization assay (6h, 37°C). After 6h of iDC/fungi co-culture, CD11b clearly co-localized with C-opsonized germlings (ΔpksP-C and fks1tetOnrep-C, )). In contrast, iDCs and ΔpksP-DCs demonstrated an even distribution of CD11b ()). DC-SIGN co-localized with CR3 but was mainly involved in up-take of germinating conidia as found for fks1tetOnrep (arrows, )). ) shows the receptor-dependent phagocytosis of non- and C-opsonized conidia into DCs in more detail – while non-opsonized ΔpksP were surrounded by DC-SIGN (red) after 6h incubation period, ΔpksP-C demonstrated a clear CD11b covering within DCs (yellow). Comparable to CD11b distribution, CD11c also co-localized to higher levels with opsonized fungal strains (ΔpksP-C and fks1tetOnrep-C, Suppl. ), while illustrating an even distribution after iDC exposure to the non-opsonized counterparts (ΔpksP and fks1tetOnrep, Suppl. ). DC-SIGN again recognized germinating conidia (fks1tetOnrep, arrows, Suppl. ), but also co-localized to low levels with CD11c after addition of C-opsonized mutants (ΔpksP-C and fks1tetOnrep-C, Suppl. ). In all these experiments, and also identified by flow cytometry, higher amounts of conidia were internalized by stimulated DCs when they were opsonized. Altogether we conclude that both CR3 and CR4 play a role in efficient binding and internalization of C-coated spores.
Figure 2. C-opsonized conidia show an improved up-take into DCs. After 6h of co-culture at 37°C, CD11b (yellow) in DCs co-localized with opsonized fungal conidia (green) (a and b, panels ∆pksP-C and fkstetOnrep-C), whereas DC-SIGN (red) was found to be highly involved in binding of germlings (a, arrow) and non-opsonized conidia (b, upper panel). Higher amounts of opsonized fungal conidia were bound/internalized by DCs compared to non-opsonized conidia. A more detailed analysis of the specific involvement of DC-SIGN (red) in internalizing non-opsonized and CD11b (yellow) in taking-up opsonized conidia is depicted in Figure 2(b). Left panels depict all fluorescence channels (green: conidia, red: DC-SIGN, yellow: CD11b, blue: nucleus), while in right panels the FITC channel was switched off to emphasize the involvement of DC-SIGN in internalization of non-opsonized and CD11b in up-take of opsonized fungal conidia. Data shown are representative of 3 independent experiments. Scale bar represents 10µm.
Both CR3 (CD11b/CD18) and CR4 (CD11c/CD18) contribute to phagocytosis of complement-opsonized fungi
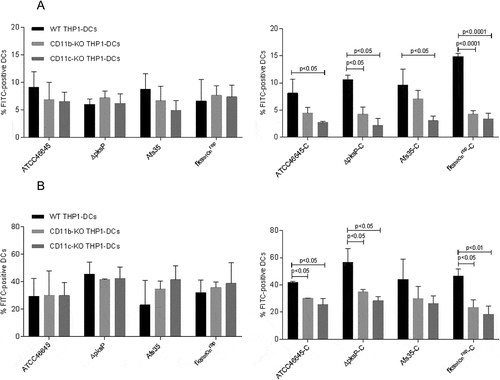
After knocking out CD11b (α-chain of CR3) and CD11c (α-chain of CR4) on THP1-DCs, binding ()) and internalization ()) of C-opsonized WT strains (ATCC46645-C, AfS35-C) and mutant strains (∆pksP-C and fks1tetOnrep-C) were significantly reduced (()). Binding and internalization of non-opsonized strains were not affected independently on the fungal surface (()). These analyses highlight that up-take of opsonized fungal spores is dependent on CR3 and CR4 and that these receptors act in a coordinated fashion with the ability to substitute each other.
Figure 3. Binding and Internalization of complement-opsonized conidia are impaired upon knock-out of either CD11b or CD11c. CD11b KO- (gray) and CD11c KO THP1-DCs (dark gray) or their respective WT THP1-DCs (black) were incubated for 6h at 4°C (a) or 37°C (b) with non-opsonized (left) or opsonized (right) fungal strains and binding (a) or internalization (b) were analyzed by flow cytometry. While all non-opsonized fungal preparations showed similar binding (a) and internalization (b) to THP1 KO and WT DCs (left), knock-out of CD11b (gray) and CD11c (dark gray) resulted in an impaired binding (a) and internalization (b) process of opsonized fungi (right). Highest impairment in binding (a) and internalization (b) were analyzed for the opsonized mutants ∆pksP-C and fkstetOnrep-C using both, CD11b KO (right, gray) and CD11c KO THP1-DCs (right, dark gray) compared to WT THP1-DCs (right, black). For both analyzes, percentages of FITC-labeled conidia bound and/or internalized by live CD11chigh DCs from 3 independent experiments were assessed by multi-color FACS and statistical significance evaluated by one way ANOVA using Bonferroni´s post test for multiple comparisons.
fks1tetOnrep induces enhanced production of pro-inflammatory cytokines from DCs
Since cytokines play an essential role in initiating and shaping T helper cell responses, we next performed multiplex PCR analyses ()) to detect expression of IL1B (A), IL6 (B), IL12A (C), IL23A (D), IL4 (E), IL10 (F) and TGFB (G) in DCs exposed to the non-/C-opsonized fungal WT and mutant strains. fks1tetOnrep significantly up-regulated IL1B (A), IL6 (B), IL12A (C), and IL23A (D) mRNA compared to the WT AfS35 and iDC control cells, thereby inducing a Th1/Th17-polarizing cytokine pattern in DCs (). IL1B (A), IL6 (B), IL12A (C), and IL23A (D) mRNA levels were elevated up to 15-fold in fks1tetOnrep-DCs (). Similar IL1B (A) mRNA levels were detected in fks1tetOnrep- and fks1tetOnrep-C-loaded DCs (). fks1tetOnrep-C-exposed DCs down-modulated IL6 (B) and IL23A (D) mRNA levels compared to fks1tetOnrep-DCs, but levels induced were still significantly higher compared to Afs35-C-DCs (). IL12A mRNA expression levels were significantly decreased in fks1tetOnrep-C-exposed DCs compared to Afs35-C- and fks1tetOnrep-DCs ()). Thus, C-opsonized fks1tetOnrep mediated a Th17-, but no Th1-polarizing cytokine profile in DCs. In contrast to the increased pro-inflammatory cytokine expression, IL-10 and TGF-β expression levels were only marginally altered in both fks1tetOnrep and fks1tetOnrep-C-DCs compared to DCs treated with the respective WT (Afs35, Afs35-C) or in immature DCs (iDCs) (()). IL-4 mRNA expression was significantly lowered by the fks1tetOnrep mutant in DCs compared to its wild type and levels were similar to iDCs ()). C-opsonization of fungi prevented up-regulation of IL-4 independent of the strain used (), 3rd panel, left, ATCC46645-C, ΔpksP-C, Afs35-C, fks1tetOnrep-C).
Figure 4. A to G Increased expression of pro-inflammatory cytokines in DCs exposed to opsonized swollen ∆pksP and fks1tetOnrep conidia. fkstetOnrep skewed DCs into polarizing inflammatory Th1 and Th17 by induction of increased IL1B (a), IL6 (b), IL12A (c) and IL23A (d) mRNA (expression and the Th1-priming capacity of DCs was abrogated in presence of complement fragments on the surface of this mutant (c). Further, we found that lack of melanin also elevated IL12A (c) and IL23A (d) mRNA expression, but showed only marginal induction of IL1B (a) or IL6 (b) mRNA expression. WT conidia induced slight but non-significant elevations of the pro-inflammatory cytokines compared to non-treated iDCs. LPS-treated DCs served as positive controls for stimulating pro-inflammatory cytokines in DCs (a to d). IL10 (f) and TGFB (g) mRNA levels were only slightly changed compared to iDCs, while IL4 (e) mRNA levels were up-regulated to some extent in DCs exposed to the WT strains, but down-modulated by ∆pksP and fkstetOnrep or complement-opsonization (e). The summary of 3 independent experiments are demonstrated. Significances were evaluated by one way ANOVA using Bonferroni´s post test for multiple comparisons.
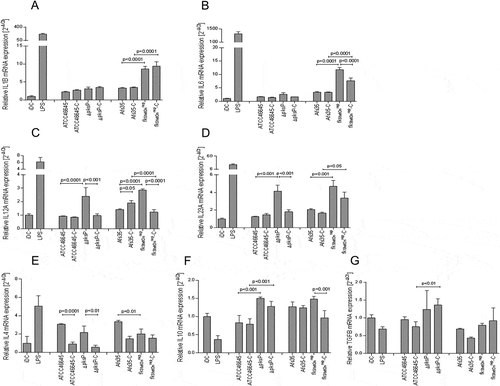
IL12A (C) and IL-23A (D) mRNA expression levels were also significantly up-regulated in DCs exposed to ΔpksP in comparison to its respective WT. As observed using C-opsonized fks1tetOnrep, ΔpksP-C significantly dampened the inflammatory response in DCs compared to their non-opsonized counterpart. IL-1B and IL-6 mRNA expression levels were slightly induced in DCs by the ΔpksP mutant compared to its WT and independent of the opsonization pattern. TGFB mRNA was slightly induced in DCs treated with the ΔpksP mutant.
These results imply a role for β-1,3-glucan to hinder the DC-mediated polarization of inflammatory Th1 and Th17 and to favor the induction of Th2- or Treg- cells. Further, we found that lack of melanin also results in elevated IL-12A and IL-23A mRNA expression, while IL-1B or IL-6 mRNA expression was not altered. These experiments emphasize the considerable impacts of cell wall components with respect to downregulation or ablation of functional antifungal T helper cell responses.
fks1tetOnrep-C activates MAPK and NFκB in DCs
Next we studied phosphorylation patterns of MAPK in DCs after exposure to non- and C-opsonized fungal WT and mutant strains. Non-stimulated iDCs were used as negative controls for expression and activation, LPS-stimulated DCs as positive controls and ERK1/2 and tubulin antibodies as controls for protein loading ()). In concordance with the results obtained by relative quantification of cytokine expression, we found that DCs exposed to fks1tetOnrep and fks1tetOnrep-C induced p38 MAPK and ERK1/2 phosphorylation to significantly higher levels compared to their counterparts (AfS35, AfS35-C; )). Using ΔpksP and ΔpksP-C-loaded DCs only modest p38 MAPK activation was detectable compared to controls, while ERK1/2 were elevated by these mutants ()). Results obtained by these immunoblot experiments are in accordance with the data obtained by real-time PCR assays. To further investigate the correlation of opsonization of fungal mutants ΔpksP and fks1tetOnrep with higher Th17-polarizing capacity, we also analyzed their influence on NFκB activation. We found that beside LPS-stimulated DCs, particularly fks1tetOnrep-C-exposed DCs showed the highest level of NFkB activation ()). These experiments clearly demonstrate the induction of various inflammatory cytokines in DCs exposed to A. fumigatus devoid of β-1,3-glucan or melanin via differential MAPK and NFκB activation profiles.
Figure 5. Differential induction of MAPKs and NFκB in DCs upon stimulation with A.fumigatus WT and mutant strains. (a) Phosphorylation patterns of ERK1/2 (42 kDa, 44 kDa), p38 MAPK (40 kDa) and NFκB in DC lysates after 1h exposure to non- and C-opsonized fungal WT and mutant conidia (ratio 1:5) were analyzed by Western Blot. Non-stimulated iDCs were used as negative, LPS-exposed DCs as positive controls for DC stimulation. We found that DCs exposed to fks1tetOnrep and fks1tetOnrep-C mediated in particular increased p38 MAPK phosphorylation compared to WTs. ERK1/2 were also activated to higher levels compared to their WT counterparts. Using ΔpksP and ΔpksP-C ERK1/2 was activated comparable to the LPS control. p38 MAPK phosphorylation was marginal. NFκB was activated in particular by C-coating of the fks1tetOnrep. Tubulin and ERK1/2 were used as controls for protein loading. One representative Western Blot out of three and histogram plots from all donors are depicted. (b) p38 MAPK phosphorylation was assessed in lysates from DCs treated with either 10µg/ml chitin (3rd lane) or 20µg/ml galactomannan (4th lane) alone, in combination (5th lane) or SNs from AfS35 WT (6th lane), fks1tetOnind (7th lane) or fks1tetOnrep (8th lane). LPS-stimulated (2nd lane) and iDCs (1st lane) served as positive or negative controls, respectively. One representative Western Blot out of three independent experiments is demonstrated, a summary of all is shown in the histogram plot. (c) Blocking DC-SIGN most significantly reduced IL23A mRNA expression upon exposure to fks1tetOnrep compared to WT- or LPS-exposed DCs. In contrast, DCs loaded with complement-coated swollen fks1tetOnrep conidia did not show any decrease in IL23A mRNA expression upon DC-SIGN blocking. The experiment was repeated three times and one representative donor is depicted.
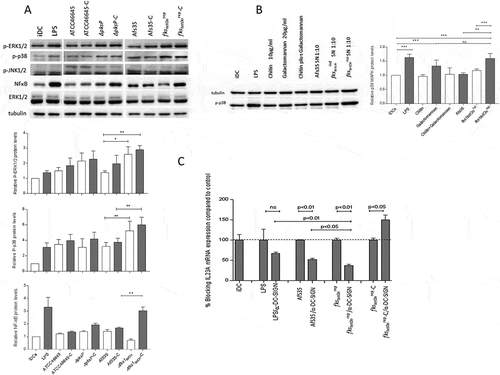
Galactomannan promotes p38 MAPK activation and induction of a pro-inflammatory cytokine profile in DCs
To examine the mechanisms underlying the significantly increased Th17-polarizing capacity of fks1tetOnrep on DCs, we further evaluated the factors responsible for spontaneous protective antifungal immune responses. We next determined p38 MAPK phosphorylation, which is activated within DCs to trigger their Th17-polarizing potential. DCs were treated with either chitin or galactomannan alone, in combination and SNs from the AfS35 WT, fks1tetOnind (under induced conditions) and fks1tetOnrep ()). Significantly higher p38 MAPK phosphorylation levels were induced using LPS, galactomannan (20µg/ml) and fks1tetOnrep SN ()).
Contrary to that, AfS35 SN, fks1tetOnind SN, chitin (10µg/ml) or a combination of chitin/galactomannan only slightly changed p38 MAPK phosphorylation compared to iDC controls ()). These experiments indicate that galactomannan contributes to the pro-inflammatory signature mediated by DCs, but not chitin or a combination of both compounds do not. To further investigate this, we applied galactomannan (20µg/ml), AfS35 and fks1tetOnrep SNs to DCs and compared their pro-inflammatory profile to chitin-treated DCs by relatively quantifying IL-1B, IL-6, IL-23A and IL-10 mRNA (Suppl. ). We found that in particular the fks1tetOnrep SN induced up-regulation of IL-1B (upper, left), IL-6 (lower, left) and IL-23A (upper, right) mRNA levels, while IL-10 (lower, right) mRNA levels were not affected compared to control cells.
Blocking DC-SIGN significantly decreases fks1tetOnrep-mediated up-regulation of pro-inflammatory mRNA expression in DCs
Previously, galactomannan was demonstrated to mainly signal via DC-SIGN [Citation19,Citation20] while β-1,3-glucan is recognized by Dectin-1 [Citation21,Citation22]. Since we suppose that the inflammatory effects induced in DCs by fks1tetOnrep are based on galactomannan-mediated mechanisms, we next hindered interactions of the various strains using a blocking anti-DC-SIGN antibody. After inhibiting DC-SIGN-mediated interactions, we found that IL-23A mRNA expression was most significantly reduced in DCs exposed to fks1tetOnrep (63% inhibition, )) compared to its AfS35 WT strain (45% inhibition, )) or LPS-exposed DCs (40% inhibition, )). Blocking DC-SIGN did not reduce IL-23A mRNA expression when DCs were exposed to C-opsonized fks1tetOn but significantly enhanced its expression ()). Blocking experiments using an anti-DC-SIGN antibody revealed galactomannan being the driving force to initiate pro-inflammatory events in DCs.
Discussion
To evaluate the effects of cell wall compounds with respect to spontaneous induction of innate immune responses, we here compared A. fumigatus WT strains and their respective mutants fks1tetOnrep and ΔpksP in their capacity to bind C3 and subsequent interactions of differentially opsonized WT and mutant conidia with human DCs.
In particular, we were interested in the conditional fks1tetOn mutant, which has the endogenous fks1 promoter replaced by the doxycycline-inducible tetON promoter [Citation11]. In A. fumigatus WT strains β-(1,3) glucan surface exposure is highest on swollen conidia and early germ tubes, and declines on hyphae, emphasizing key differences in cell wall composition among morphotypes [Citation9]. We could show that not only lack in melanin but β-1,3-glucan fosters significantly higher complement deposition on fungal conidia, indicating that polysaccharides in the DHN-melanin or β-1,3-glucan mutants are more efficiently recognized by the thioester-containing domain (TED) of complement proteins [Citation23] or that the WT strains exert higher complement-degrading or -inactivating capacities [Citation5,Citation24]. Chitin found to significantly higher levels in the cell wall of the fks1tetOnrep mutant [Citation11] was shown to activate the alternative C pathway [Citation25]. Therefore, we supposed that chitin might be the driving force for the enhanced complement deposition on the fks1tetOnrep mutant.
Increased up-take of C-opsonized swollen conidia was mediated via both, CR3 and CR4, as detected by CRISP-Cas9-deletion mutants for CD11b and CD11c. As described earlier, non-opsonized fungal strains attached to DCs preferentially via C-type lectin receptors, i.e. dectin-1 or DC-SIGN [Citation21]. DC-SIGN ligation was shown to simultaneously stimulate other PRRs, e.g. Toll-like receptor (TLR) 2, TLR4 or dectin-1. The cooperation of these intracellular pathways consequently enhanced TLR-mediated IL-12A, IL-12B and IL-6 transcription [Citation26–Citation28], thereby promoting a protective antifungal Th1 response. Not only Th1 but also Th17 cells were activated via DC-SIGN ligation, since indirectly also IL-1B is induced in DCs upon the DC-SIGN/mannose interaction domain via RAF1 activation [Citation26,Citation28]. Thereby, signaling by mannose-ligated DC-SIGN activates a pro-inflammatory cytokine program, a fact we also found in our study using the β-glucan lacking mutant fks1tetOnrep. This mutant induced significantly higher transcription of IL-1B, IL-6, and IL-12A as well as IL-23A mRNA expression compared to its corresponding WT. Not only the β-1,3-glucan- but also the melanin-lacking mutant mediated transcription of both, IL-12A and IL-23A mRNA due to their higher visibility for the immune system compared to wildtype conidia. In contrast IL-1B and IL-6 mRNAs were transcribed to lower levels by the ∆pksP mutant, thereby pointing to a role of cell wall composition with respect to the magnitude of inflammatory immune response. Dampening of in particular a Th1 polarizing cytokine pattern was noted upon opsonization of the fks1tetOnrep mutant. This implicates that the fks1tetOnrep strain is a very strong inducer of a Th1/Th17-polarizing cytokine pattern; its opsonized counterpart more likely mediates a Th17-polarizing program in DCs. We earlier showed that complement-opsonization of HIV-1 mediates polarization of naïve CD4 + T cells into Th17 cells and IL-1β, IL-6 and IL-23 mRNA – secreted protein levels correlated in this study [Citation29]. Induction of a Th17-polarizing cytokine pattern by fks1tetOnrep–C may arise from cross-talk between CR3 or CR4 with DC-SIGN as shown by confocal microscopic analyses.
In contrast to fks1tetOnrep, complement opsonization of the DHN-melanin-lacking strain ∆pksP caused dampening of both IL-12 and IL-23A mRNA levels. Dampening of pro-inflammatory immune responses by complement opsonization was described earlier in the literature [Citation14], but is also dependent on the surface composition of the fungal strain as described herein. p38 MAPK phosphorylation was shown to be activated by various cytokines as IL-1β [Citation30] and in our analyses we detected the highest p38 MAPK activation using fks1tetOnrep and fks1tetOnrep – C, which also showed the highest expression levels of IL-1B mRNA. mRNA expression of IL-1B as well as activation of p38 MAPK were significantly lower using ∆pksP. C-opsonization triggered NFκB activation particularly in DCs exposed to the C-opsonized mutants.
Since DC-mediated inflammatory responses were highest in A. fumigatus lacking β-1,3-glucan, we aimed to characterize the mechanism(s) responsible for this stronger protective antifungal immunity.
The composition of the A. fumigatus cell wall, which is built up of covalently bound β-glucan, chitin, galactomannan and α-glucan, makes recognition by pattern recognition receptors (PRRs) possible [Citation31]. The C-type lectin receptor Dectin-1 was demonstrated to be crucial for recognition of β-glucan expressed at the budding scars of pathogenic or opportunistic fungi, e.g. Candida or A. fumigatus [Citation19]. DC-SIGN has been shown to be involved in recognition of fungal PAMPs, expressed by CD1c+ DCs and inflammatory DCs and on certain macrophage subsets [Citation32]. Binding of fungal PAMPs, i.e. galactomannan, to DC-SIGN initiates simultaneous stimulation of other PRRs, including TLR2, TLR4 or dectin-1. The cooperation of these intracellular pathways consequently enhances TLR-mediated transcription of IL12A, IL12B and IL6 [Citation26–Citation28], thereby promoting a protective antifungal Th1 response [Citation32]. Not only Th1, but also Th17 cells are activated via DC-SIGN ligation, since indirectly also IL1B is induced in DCs upon the DC-SIGN/mannose interaction domain via RAF1 activation [Citation26,Citation28]. Thereby, signaling by mannose-ligated DC-SIGN activates a pro-inflammatory cytokine program, which is consistent with our studies.
Lack in β-1,3-glucan was compensated by massive galactomannan shedding, and this polysaccharide more likely induces a Th1-/Th17 signature via DC-SIGN cross-linking on inflammatory DCs [Citation19,Citation20]. We found 15-fold higher galactomannan levels in fks1tetOnrep SNs – these SNs also efficiently activated p38 MAPK. Several groups using purified cell wall molecules could show, that different receptors recognize cell wall mannan and for example DC-SIGN was able to discriminate between C. albicans and Saccharomyces cerevisiae mannans [Citation33]. In our study blocking experiments using an anti-DC-SIGN antibody revealed that DC-SIGN is the main – but not sole – target for fks1tetOnrep-mediated induction of a pro-inflammatory immune response. In contrast, AfS35 or fks1tetOnrep-C mainly signaled via receptors other than DC-SIGN.
CR3 (CD11b/CD18) as well as several scavenger receptors (CD5, CD36 and SCARF1) have also been demonstrated to bind β-1,3-glucan and to play an important role in antifungal immunity [Citation34]. These effects are supposed to be even enhanced in our study using a complement-opsonized strain.
β-1,3-glucan-lacking A.fumigatus promoted improved complement and DC activation via various mechanisms. On the one hand, complement was efficiently activated by the mutant and covalently fixed at the surface of fungal conidia, probably via the increased cell wall chitin levels. These in turn resulted in better recognition by DCs via CR3 (CD11b/CD18) and CR4 (CD11c/CD18). On the other hand, we here demonstrated DCs move towards a pro-inflammatory, Th1- and Th17-polarizing profile in a DC-SIGN-dependent manner. Our results using β-1,3-glucan-lacking A. fumigatus may explain novel immune-modulatory effects mediated by echinocandins, the newest class of antifungals used in the clinics. These drugs impairing cell wall β-1,3-glucans [Citation11,Citation12] may not only act directly on fungal pathogens, but also exert efficient complement- and DC-modulatory mechanisms due to elevated levels of cell wall chitin and elevated galactomannan release.
Methods
Ethics statement
Written informed consent was obtained from all blood donors by the Central Institute for Blood Transfusion & Immunological Department, Innsbruck, Austria. All participants were adults; no minors were enclosed in this study. The use of anonymized leftover specimens for scientific purposes was approved by the Ethics Committee of the Medical University of Innsbruck.
Generation of human monocyte-derived DCs and THP1-DCs
Monocytes were isolated from blood of healthy donors as described before [Citation35,Citation36]. Blood for the monocyte isolation was received by the Central Institute for Blood Transfusion & Immunological Department, Innsbruck, Austria. Briefly, PBMCs (peripheral blood mononuclear cells) were isolated from blood of healthy donors, obtained by a density gradient centrifugation using a Ficoll Paque Premium (GE Healthcare) gradient. After washing, CD14+ monocytes were isolated from PBMCs using anti-human CD14 Magnetic Beads (BD) – the purity of the isolated cells was at least 98%. Monocytes were stimulated by addition of IL-4 (200U/ml) and GM-CSF (300U/ml) for five days to generate immature moDCs (iDCs), which were used for all further experiments. Non-stimulated iDCs were used as controls for all experiments using moDCs. THP1-WT and KO DCs were generated from the respective THP1 cells by addition of IL-4 (200U/ml), GM-CSF (300U/ml) and TNF-α (10ng/ml).
Genome editing using CRISPR/Cas9-mediated depletion of CD11b and CD11c
For CRISPR/Cas9-mediated depletion, guide RNA (gRNA) targeting sequences for CD11b (5´- GCCGTAGGTTGGATCCAAACAGG-3´) and CD11c (5´-GTAGAGGCCACCCGTTTGGTTGG-3´) were selected using an online prediction tool (CRISPR Design; Zhang lab [Citation37]). gRNAs were cloned into a lentiCRISPRv2 vector via BsmBI restriction sites. lentiCRISPRv2 was a gift from F. Zhang (Massachusetts Institute of Technology, Cambridge, MA; Addgene plasmid 52961; [Citation38]). Lentiviral transduction was performed as described in the following paragraph. Depletion efficiency was verified via FACS and live CD11b-KO or CD11c-KO-THP1 cells were isolated by the Core Facility FACS Sorting at the Medical University of Innsbruck.
Lentiviral transduction
Lentiviral plasmids were co-transfected with Lipofectamine LTX (Invitrogen, cat 15338100) together with pVSV-G and psPAX2 into the HEK293LTV producer cell line. Supernatants containing viral particles were harvested 48h and 72h post transfection, filtered using a 0.2µm filter and directly used to transduce target THP1 cells with 5µg/ml Polybrene (Sigma-Aldrich, cat TR-1003-G). After seven days transduced cells were selected using puromycin (5µg/ml, Sigma-Aldrich, cat SBR00017) and the depletion efficiency of CD11b-KO- and CD11c-KO THP-1 cells was analyzed by flow cytometry (Suppl. Figure 3). Single-cell clones of the specific KO cells were generated after FACS sorting.
Fungal strains and growth conditions
Fungal strains comprized the clinical isolate ATCC46645 (WT) [Citation39] and its mutant ΔpksP, lacking the polyketide synthase required for DHN-melanin synthesis [Citation39]. Further we used WT AfS35 [Citation40] and its mutant fks1tetOnrep [Citation11]. Fks1 as ß-1,3-glucan synthase is an integral plasma membrane protein and provides the ß-1,3 glucan scaffold for other polysaccharides of the cell wall, e.g. galactomannan [Citation41–Citation43]. In the conditional fks1tetOnrep mutant the endogenous fks1 promoter was replaced by the doxycycline-inducible tetON promoter [Citation44]. Growth media with doxycycline (0.5µg/ml) allowed WT-like growth characteristics (induced (ind) conditions, fks1tetOnind), which is shown in Suppl. Video 1. Without doxycycline and under repressed conditions, germinated conidia display growth alterations like delayed growth, hyperbranching or cell lysis due to the lack of fks1 (Suppl. Video 2).
The strains were cultivated on Aspergillus complete medium (ACM) agar plates for 5 d at 37°C, whereas for the fks1tetOn mutant strain the ACM was supplemented with 0.5µg/ml doxycycline under induced conditions [Citation11]. Conidia were harvested with spore buffer (PBS with 0.1% Tween20), filtered through a 5µm filter (PARTEC, CellTricks) to remove hyphae, counted by a hemacytometer and suspended at a concentration of 1*107 spores per ml. For all analyzes, repressed conditions (fks1tetOnrep) were induced by pre-incubating conidia for 3h in Aspergillus minimal medium (AMM) without doxycycline prior to further treatments (opsonization, DC exposure) for another 1h to 6h.
Opsonization and FITC-labeling of conidia
Opsonization was performed by adding 10% NHS (normal human serum from healthy donors) as complement (C) source to pre-incubated swollen conidia for 30 min and another 10% for 30 min. To quantify C3c deposition by flow cytometry (FACS Verse, BD Biosciences), which was prerequisite for all other experiments, an aliquot of the conidia was used, washed and stained with anti-human C3c FITC antibody (Dako). FITC-labeled swollen non- or C-opsonized conidia were used for binding and internalization assays acquired by FACS and confocal microscopic analyzes. FITC labeling was performed as described elsewhere [Citation17]. Briefly, swollen conidia were labeled using 0.1 mg/ml FITC (Sigma) in 0.1 M Na2CO3 for 30 min at 37°C. Conidia were washed several times and re-suspended in RPMI 1640 (Gibco), supplemented with 10% FCS and 1% L-Glutamine (RPMI++) and used immediately for all further experiments.
Cell assays
DCs were incubated with swollen opsonized and non-opsonized FITC-labeled WT and mutant conidia at a MOI = 2 either on ice (binding only, since DCs do not internalize conidia at this temperatures [Citation18]) or at 37°C (binding and internalization) for 6h. Binding and internalization were analyzed by flow cytometry gating for FITC-positive conidia in DCs. For blocking experiments, DCs were pre-incubated with blocking Abs (DC-SIGN/DC-SIGNR, Novus Biologicals, 10µg/ml, cat 120612) prior addition of differentially opsonized conidia and analyzed by RT-PCR. In detail, for blocking experiments, DCs were incubated with 10µg/ml α-DC-SIGN + DC-SIGNR antibody (Novus Biologicals) for 30 minutes at 37°C. Opsonized and non-opsonized swollen conidia of the WT and fks1tetOn mutant were co-cultured with DCs at MOI of 5 and cells were lysed after 3h for RNA isolation and RT-PCR for IL23A (BioRad Laboratories) was performed, as described in detail above.
Percentages of FITC-labelled conidia bound and/or internalized to DCs from at least 5 independent experiments were assessed by multi-color flow cytometry. Differences were analyzed by one Way ANOVA using Bonferroni´s posttest for multiple comparisons.
Immunoblot analyses of phosphorylated proteins
DCs were starved in RPMI1640 containing 0.5% FCS and 1% L-Glutamine for 3h. The starving of cells was performed to set their phosphorylation to background levels. Following starvation, DCs were incubated with the differentially opsonized WT and mutant A. fumigatus strains at a MOI of 5. Cells were lysed after 1h co-incubation with RIPA Buffer (Sigma-Aldrich) containing protease and phosphates inhibitors and EDTA (Thermo-Scientific). The protein content was determined by BCA (Thermo-Scientific). Lysates were separated using 14% SDS-PAGE gels, transferred to PVDF membranes and incubated with anti-human a-tubulin or ERK1/2 as loading controls as well as anti-human phospho-p38, P-JNK1/2, NFκB and phospho-ERK1/2 (all from Cell Signaling Technology) and developed with the Lass 4000 Image Quant.
Multiplex PCR analyses
DCs were infected with the differentially opsonized WT and mutant A. fumigatus for 3h at 37°C with a MOI of 5. Cells were lysed with the RLT Buffer (Qiagen) and total RNA was purified according to the manufacturer’s advice. RNA was then measured (NanoVue) and reverse transcribed into cDNA (iSkript Reverse Transcription Supermix for RT-qPCR, BioRad). The cDNA was then used for multiplex qPCR (iQ Multiplex Powermix, BioRad) amplification, using PrimePCR™ Probes for IL-10, IL-6, IL-1B, IL12A, IL23A and TGFB (all from BioRad Laboratories). The RT-qPCR was run in the BioRad CFX96 Real Time PCR System. Data were analyzed with the BioRad CFX Manager Software and values were exported to GraphPad Prism.
Confocal microscopy
To study the interaction of swollen, opsonized and non-opsonized conidia with CRs on DCs, cells were incubated with conidia at a 1:2 ratio. Confocal slides were coated with Poly-L-Lysine (Sigma-Aldrich) for 2h at 37°C. DCs were stained with Hoechst, anti-human CD11b- or CD11c-Alexa594 and anti-human DC-SIGN Alexa633 (all from BD) and added to the PBS washed slides for at least 1h until they were adherent. FITC-labeled opsonized and non-opsonized conidia of the different strains were added to the cells for 6h to have comparable conditions as during binding/internalization experiments evaluated by flow cytometry. Pre-warmed D-PBS was used to remove unbound conidia and slides were fixed using 1% formaldehyde for 20min. Slides were mounted onto coverslips with Mowiol (Sigma) and used for microscopy. Images were acquired on a Leica SP5 (x63 Glycerin) confocal microscope at the Biooptics core facility, CCB, Innsbruck, Austria. Deconvolution was performed using Huygens Professional and images were merged using ImageJ Software.
Incubation of DCs with soluble chitin, galactomannan and fungal supernatants
Soluble polysaccharides as chitin (Sigma-Aldrich) and galactomannan (Carl-Roth) were diluted as previously described [Citation45,Citation46]. DCs were incubated with chitin (10µg/ml), galactomannan (20µg/ml) or fungal supernatants. WT and fks1tetOnrep were grown in a concentration of 25 × 108 spores in 200ml AMM for 24h. The supernatants were centrifuged and sterile-filtered prior to addition at a ratio 1:10 to the cells, which represent a MOI of 5. Cells were lysed after 1h for Immunoblot analysis and after 3h co-culture for multiplex PCR analysis, as described above in detail.
Statistical analyses
Differences were analyzed by the GraphPad prism software using the unpaired student’s t-test (2-tailed) or one-way ANOVA with Bonferroni posttest for multiple comparisons depending on the analyzes.
Supplemental Material
Download Zip (13.1 MB)Acknowledgments
We thank our technician Karolin Thurnes, Dr. Michael Blatzer, Dr. Paul Hörtnagl and Dr Annelies Mühlbacher, Central Institute for Blood Transfusion & Immunological Department, Medical University of Innsbruck, Austria, for their valuable help and support regarding this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4(1):1–23.
- Braem SG, Rooijakkers SH, van Kessel KP, et al. Effective neutrophil phagocytosis of Aspergillus fumigatus is mediated by classical pathway complement activation. J Innate Immun. 2015;7(4):364–374.
- Tsai HF, Washburn RG, Chang YC, et al. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol Microbiol. 1997;26(1):175–183.
- Latge JP. Tasting the fungal cell wall. Cell Microbiol. 2010;12(7):863–872.
- Heinekamp T, Schmidt H, Lapp K, et al. Interference of Aspergillus fumigatus with the immune response. Semin Immunopathol. 2015;37(2):141–152.
- Henwick S, Hetherington SV, Patrick CC. Complement binding to Aspergillus conidia correlates with pathogenicity. J Lab Clin Med. 1993;122(1):27–35.
- Brakhage AA, Liebmann B. Aspergillus fumigatus conidial pigment and cAMP signal transduction: significance for virulence. Med Mycol. 2005;43(Suppl 1):S75–82.
- Tsai HF, Chang YC, Washburn RG, et al. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol. 1998;180(12):3031–3038.
- Hohl TM, Van Epps HL, Rivera A, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 2005;1(3):e30.
- Lother J, Breitschopf T, Krappmann S, et al. Human dendritic cell subsets display distinct interactions with the pathogenic mould Aspergillus fumigatus. Int J Med Microbiol. 2014;304(8):1160–1168.
- Dichtl K, Samantaray S, Aimanianda V, et al. Aspergillus fumigatus devoid of cell wall β-1,3-glucan is viable, massively sheds galactomannan and is killed by septum formation inhibitors. Mol Microbiol. 2015;95(3):458–471.
- Beyda ND, Liao G, Endres BT, et al. Innate inflammatory response and immunopharmacologic activity of micafungin, caspofungin, and voriconazole against wild-type and FKS mutant Candida glabrata isolates. Antimicrob Agents Chemother. 2015;59(9):5405–5412.
- Lin J, Wester MJ, Graus MS, et al. Nanoscopic cell-wall architecture of an immunogenic ligand in Candida albicans during antifungal drug treatment. Mol Biol Cell. 2016;27(6):1002–1014.
- Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–288.
- Wuthrich M, Deepe GS Jr., Klein B. Adaptive immunity to fungi. Annu Rev Immunol. 2012;30:115–148.
- Thakur R, Anand R, Tiwari S, et al. Cytokines induce effector T-helper cells during invasive aspergillosis; what we have learned about T-helper cells? Front Microbiol. 2015;6:429.
- Mech F, Thywissen A, Guthke R, et al. Automated image analysis of the host-pathogen interaction between phagocytes and Aspergillus fumigatus. PLoS One. 2011;6(5):e19591.
- Pruenster M, Wilflingseder D, Banki Z, et al. C-type lectin-independent interaction of complement opsonized HIV with monocyte-derived dendritic cells. Eur J Immunol. 2005;35(9):2691–2698.
- Geijtenbeek TB, Gringhuis SI. C-type lectin receptors in the control of T helper cell differentiation. Nat Rev Immunol. 2016;16(7):433–448.
- van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5(4):329–340.
- Barreto-Bergter E, Figueiredo RT. Fungal glycans and the innate immune recognition. Front Cell Infect Microbiol. 2014;4:145.
- Amarsaikhan N, Templeton SP. Co-recognition of β-glucan and chitin and programming of adaptive immunity to Aspergillus fumigatus. Front Microbiol. 2015;6:344.
- Merle NS, Church SE, Fremeaux-Bacchi V, et al. Complement system Part I - molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262.
- Chotirmall SH, Mirkovic B, Lavelle GM, et al. Immunoevasive Aspergillus virulence factors. Mycopathologia. 2014;178(5–6):363–370.
- Roy RM, Paes HC, Nanjappa SG, et al. Complement component 3C3 and C3a receptor are required in chitin-dependent allergic sensitization to Aspergillus fumigatus but dispensable in chitin-induced innate allergic inflammation. MBio. 2013;4(2).
- Gringhuis SI, Den Dunnen J, Litjens M, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10(2):203–213.
- Gringhuis SI, Den Dunnen J, Litjens M, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26(5):605–616.
- Gringhuis SI, Den Dunnen J, Litjens M, et al. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10(10):1081–1088.
- Wilflingseder D, Schroll A, Hackl H, et al. Immediate T-Helper 17 polarization upon triggering CD11b/c on HIV-exposed dendritic cells. J Infect Dis. 2015;212(1):44–56.
- Raingeaud J, Gupta S, Rogers JS, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270(13):7420–7426.
- Chai LY, Vonk AG, Kullberg BJ, et al. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. 2011;13(2):151–159.
- Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9(7):465–479.
- Cambi A, Netea MG, Mora-Montes HM, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283(29):20590–20599.
- Means TK, Mylonakis E, Tampakakis E, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206(3):637–653.
- Posch W, Cardinaud S, Hamimi C, et al. Antibodies attenuate the capacity of dendritic cells to stimulate HIV-specific cytotoxic T lymphocytes. J Allergy Clin Immunol. 2012;130(6):1368–1374 e1362.
- Wilflingseder D, Banki Z, Garcia E, et al. IgG opsonization of HIV impedes provirus formation in and infection of dendritic cells and subsequent long-term transfer to T cells. J Immunol. 2007;178(12):7840–7848.
- Hsu PD, Scott DA, Weinstein JA, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–832.
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11(8):783–784.
- Langfelder K, Jahn B, Gehringer H, et al. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med Microbiol Immunol. 1998;187(2):79–89.
- Krappmann S, Sasse C, Braus GH. Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end- joining-deficient genetic background. Eukaryot Cell. 2006;5(1):212–215.
- Beauvais A, Latge JP. Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist Updat. 2001;4(1):38–49.
- Beauvais A, Bruneau JM, Mol PC, et al. Glucan synthase complex of Aspergillus fumigatus. J Bacteriol. 2001;183(7):2273–2279.
- Fontaine T, Simenel C, Dubreucq G, et al. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem. 2000;275(52):41528.
- Helmschrott C, Sasse A, Samantaray S, et al. Upgrading fungal gene expression on demand: improved systems for doxycycline-dependent silencing in Aspergillus fumigatus. Appl Environ Microbiol. 2013;79(5):1751–1754.
- Santander SP, Aoki M, Hernandez JF, et al. Galactomannan from Caesalpinia spinosa induces phenotypic and functional maturation of human dendritic cells. Int Immunopharmacol. 2011;11(6):652–660.
- Becker KL, Aimanianda V, Wang X, et al. Aspergillus cell wall chitin induces anti- and proinflammatory cytokines in human PBMCs via the Fc-gamma receptor/Syk/PI3K pathway. MBio. 2016;7(3).
