ABSTRACT
Staphylococcus aureus is the leading cause of infective endocarditis (IE). While the role of S. aureus cell-wall associated protein clumping factor A (ClfA) in promoting IE has been already demonstrated, that of the secreted plasma-clotting factors staphylocoagulase (Coa) and von Willebrand factor-binding protein (vWbp) has not yet been elucidated. We investigated the role of Coa and vWbp in IE initiation in rats with catheter-induced aortic vegetations, using Lactococcus lactis expressing coa, vWbp, clfA or vWbp/clfA, and S. aureus Newman Δcoa, ΔvWbp, ΔclfA or Δcoa/ΔvWbp/ΔclfA mutants. vWbp-expression increased L. lactis valve infection compared to parent and coa-expressing strains (incidence: 62%, versus 0% and 13%, respectively; P < 0.01). Likewise, expression of clfA increased L. lactis infectivity (incidence: 80%), which was not further affected by co-expression of vWbp. In symmetry, deletion of the coa or vWbp genes in S. aureus did not decrease infectivity (incidence: 68 and 64%, respectively) whereas deletion of clfA did decrease valve infection (incidence: 45%; P = 0.03 versus parent), which was not further affected by the triple deletion Δcoa/ΔvWbp/ΔclfA (incidence: 36%; P > 0.05 versus ΔclfA mutant). Coa does not support the initial colonization of IE (in L. lactis) without other key virulence factors and vWbp contributes to initiation of IE (in L. lactis) but is marginal in the present of ClfA.
Introduction
Staphylococcus aureus is the most common etiological agent of infective endocarditis (IE) owing to its propensity to adhere to fibrin-platelet clots on damaged cardiac valves, known as vegetations [Citation1,Citation2]. Several structures in the S. aureus envelope, including teichoic acids and surface-associated proteins, have been implicated in ligand-receptor interactions of circulating bacteria to cardiac vegetations and contributed to the pathogenesis of IE [Citation3–Citation5].
The study of gene inactivation in S. aureus has greatly contributed to elucidate the specific role of individual virulence factors in the pathogenesis of IE [Citation6]. This has been more recently complemented by the use of the poorly pathogenic Lactococcus lactis, used as a surrogate carrier, to investigate the effects of heterologous gene expression on infectivity [Citation7]. Using these methods cooperatively, it was shown that fibrinogen-binding protein clumping factor A (ClfA) and fibronectin-binding protein A (FnBPA, which also binds fibrinogen) were major determinants of S. aureus valve colonization [Citation4,Citation6,Citation8–Citation10]. For instance, while S. aureus mutants defective in clfA became less pathogenic, non-virulent L. lactis expressing recombinant clfA demonstrated increased infectivity in rats with experimental IE, due, amongst others, to the acquisition of the ability to adhere to fibrin deposited on damaged valve tissues [Citation6,Citation7].
In addition to cell-wall anchored adhesins, S. aureus also produces two procoagulant factors, namely staphylocoagulase (Coa) [Citation11] and von Willebrand factor-binding protein (vWbp) [Citation12], that can activate prothrombin in a non-proteolytic manner [Citation13]. These proteins trigger the polymerization of fibrinogen into fibrin and promote platelet aggregation and blood clotting [Citation13–Citation15].
Contemporary studies showed that Coa and vWbp might contribute to the pathogenesis of S. aureus in murine and rabbit models of abscess formation, blood stream infection and endovascular colonization [Citation16–Citation19]. Nevertheless, their role in the pathogenesis of S. aureus IE remains unclear. On the one hand, two pioneer studies performed in rats with catheter-induced aortic vegetations reported that Coa was not involved in the early colonization of damaged valves [Citation6,Citation20] but, since at the time the presence of vWbp was still unknown [Citation12], the lack of Coa activity in coa-defective mutants might have been compensated for by that of vWbp. Moreover, one study using heterologous expression of coa in Streptococcus gordonii suggested that Coa did not contribute to infectivity, further supporting the notion that Coa was not implicated in the initiation of IE [Citation21].
On the other hand, taking the two procoagulant factors into account, Panizzi and co-workers demonstrated that knocking out both of them in S. aureus significantly increased mice survival in a model of S. aureus IE [Citation22]. However, this study did not investigate the ability of the bacteria to initiate IE.
The aim of the present work was to provide new insights into the specific roles of Coa and vWbp in the initiation of experimental IE due to S. aureus in the rat model of catheter-induced aortic vegetations. To this end we used a series of knock-in and knock-out mutants including (i) L. lactis heterologously expressing S. aureus coa, vWbp, coa plus vWbp, clfA, and vWbp plus clfA, and (ii) S. aureus Newman mutants inactivated in the coa, vWbp, clfA, and coa/vWbp/clfA genes.
Results
Recombinant L. lactis strains produce functional Coa and vWbp
In order to verify that the recombinant L. lactis strains produced functional procoagulant S. aureus Coa and vWbp proteins, we assessed both their abilities to induce blood clotting and to trigger platelet aggregation.
As shown in ), all L. lactis strains expressing the S. aureus pro-coagulant factors, but not the parent L. lactis pIL253 strain, exhibited blood clotting activity. Consistently, dabigatran prevented blood clot formation by recombinant L. lactis strains ()). Moreover, it took 100-times less lactococci producing Coa than vWbp to trigger coagulation within 24 h ()).
Figure 1. Functional analysis of recombinant L. lactis Coa and L. lactis vWbp. The activity of Coa and vWbp was evaluated by blood clotting, time required to induce plasma clotting and platelet aggregation. For blood clotting, citrated rat blood without (a) and with (b) dabigatran was infected with 107 CFU of either parent L. lactis pIL253, L. lactis Coa, L. lactis vWbp or L. lactis Coa/vWbp cells from an overnight culture and incubated 24 h at 37°C. Tubes were tilted to display blood coagulation. Data are representative of three independent experiments. To assess the time required to induce plasma clotting (c), 105 to 108 CFU of parent L. lactis pIL253, recombinant L. lactis Coa or L. lactis vWbp were incubated at 37°C with rat plasma. Tubes were tilted to assess plasma coagulation at 4, 8, 12, 24 and 36 h. Clotting values of 36 h, the maximal time of measurement, indicate that there was no clotting. Data are representative of three independent experiments. For platelet aggregation, cell-free supernatants (d) or pelleted cells (e) of parent L. lactis pIL253, or recombinant L. lactis Coa or L. lactis vWbp from overnight cultures were mixed with platelet rich plasma. Platelet aggregation was monitored by aggregometry and the maximum values of light transmission (maximal aggregation) reached over 20 min incubation were recorded. Data are expressed as mean ± standard deviation (SD) of three independent assays.
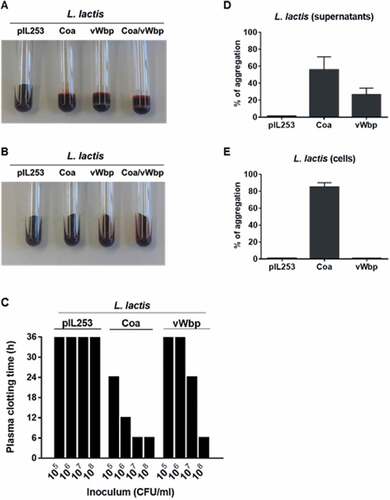
Since both S. aureus Coa and vWbp are able to induce platelet aggregation [Citation13,Citation23], the capacity of Coa and vWbp L. lactis recombinants to trigger platelet aggregation was also tested. Moreover, since a previous study by McDevitt and collaborators showed that a fraction of Coa was retained on the surface of S. aureus [Citation24], the ability of the L. lactis Coa and L. lactis vWbp cell-free supernatants and cells to induce platelet aggregation was also tested to verify whether Coa and vWbp were also partially associated to the surface of lactococci. As shown in ), platelet aggregation was observed upon addition of the cell-free supernatants of L. lactis expressing coa and vWbp, but not upon the addition of the supernatants of the parent L. lactis pIL253. When cells were used, only Coa-positive lactococcal cells exhibited platelet aggregation activity ()).
Collectively, these results indicate that the recombinant L. lactis strains produced and secreted functional S. aureus Coa and vWbp and that part of Coa, but not vWbp, was attached to the surface of L. lactis.
Expression of vWbp but not Coa supports L. lactis infection of aortic vegetations in rats
To study the effect of coa and vWbp expression on lactococcal infectivity, we titrated the ability of parent L. lactis pIL253 and recombinant L. lactis Coa, L. lactis vWbp and L. lactis Coa/vWbp to induce experimental IE in rats. As shown in , 24 h after challenge, in animals inoculated with 106 CFU, the percentage of vegetations infected with L. lactis vWbp was significantly higher (62%) than that infected with the parent L. lactis pIL253 (0%; P < 0.01) and L. lactis Coa (13%; P = 0.007). Not indicated in the Figure, the percentage of vegetations infected with L. lactis expressing both coa and vWbp was similar (62%) to that of L. lactis vWbp.
Figure 2. vWbp but not coa confers L. lactis the ability to infect pre-existing sterile valve vegetations. Infectivity titration of the L. lactis test organisms in the rat model of experimental endocarditis. The rats were challenged with bacterial inocula of gradually increasing sizes. The columns indicate the percentage of positive vegetations 24 h after bacterial challenge. The number of infected/total number of animals per group is indicated at the top of the columns. *, P < 0.05 compared with L. lactis Coa; #, P < 0.05 compared with L. lactis pIL253, as determined by the Fisher’s exact test with the Yates correction.
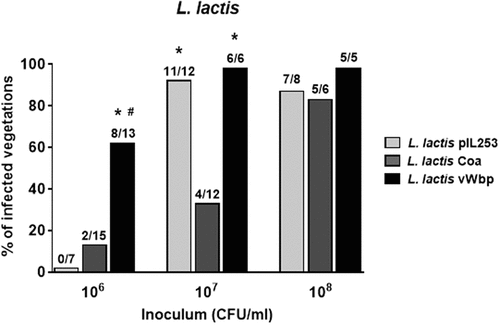
When animals were inoculated with 10-times more bacterial cells (107 CFU), L. lactis Coa remained surprisingly poorly infective (33% of endocarditis), while L. lactis pIL253 and L. lactis vWbp were able to infect ≥ 90 % of vegetations.
Of note, the mean bacterial densities ± standard deviation (SD) in the animals that developed endocarditis (3.58 ± 0.71 log10 CFU/g) in animals challenged with L. lactis Coa were significantly lower (P < 0.001; one-way ANOVA) from that of L. lactis pIL253 (5.45 ± 0.78 log10 CFU/g) and L. lactis vWbp (7.70 ± 0.40 log10 CFU/g). At higher bacterial inocula (108 CFU) all L. lactis strains were able to infect ≥ 80% of vegetations. In animals that developed endocarditis, the mean bacterial densities in the vegetations with L. lactis Coa (5.92 ± 0.67 log10 CFU/g) were similar to that with L. lactis pIL253 (5.46 ± 0.82 log10 CFU/g) but significantly lower (P < 0.01; one-way ANOVA) to that with L. lactis vWbp (7.75 ± 0.44 log10 CFU/g).
In sum, these results showed that vWbp but not Coa increased the infectivity of L. lactis, supporting a role of vWbp in the initiation of IE. On the contrary, Coa production paradoxically decreased the ability of lactococci to colonize aortic vegetations.
Coa promotes the clearance of L. lactis from the vegetations
Previous work by Bayer et al. [Citation25] showed that the hyperproduction of alpha-toxin by S. aureus resulted in a paradoxically reduced virulence in experimental endocarditis. Moreover, they demonstrated that the reduced in vivo virulence of the alpha-toxin-producer variant was not due to differences in initial adherence to sterile vegetations compared to the parental strain, and suggested a role of platelet-induced bacterial killing in the vegetations [Citation25]. We investigated whether the same scenario could apply to the lower infectivity rate observed with L. lactis Coa in animals challenged with the 107 CFU inoculum. Thus, in a second set of experiments we followed disease progression by infecting rats with 107 CFU of each lactococcal recombinant, and assessed the evolution of valve infection in groups of 6–10 rats sequentially sacrificed over a period of 24 h. As shown in , h after bacterial challenge all the rats were infected and had similar vegetation bacterial titers irrespective of the infecting organism, indicating that all the lactococcal recombinants were comparably able to colonize the vegetations. However, the disease evolved differently later on. While the vegetation density of parent L. lactis pIL253 remained stable over time, the vegetation density of L. lactis vWbp recombinants increased whereas the vegetation density of Coa-positive lactococci decreased until eradication at 24 h. These results are in line with those obtained in rats infected with 107 CFU and killed at 24 h post-challenge, where most of the vegetations were sterile (see ). Thus, Coa production by lactococci attached to the vegetations promoted active bacterial clearance from the fibrin-platelet clots.
Figure 3. Evolution of vegetation infection after challenge with parent L. lactis pIL253 and recombinant L. lactis Coa or L. lactis vWbp (a) and in vitro killing by platelets (b). (a) Groups of 6–10 rats were inoculated with of 107 CFU and sacrificed at various times after challenge. Data represent mean ± SD of vegetation bacterial titres. *, P < 0.01 vs L. lactis pIL253 and L. lactis vWbp; # P < 0.01 vs L. lactis pIL253, as compared by the one-way ANOVA followed by Tukey’s multiple comparisons test. (b) Killing of L. lactis by platelets after 4 and 24 h of exposure. *, P < 0.05 by one-way ANOVA followed by Tukey’s multiple comparisons test.
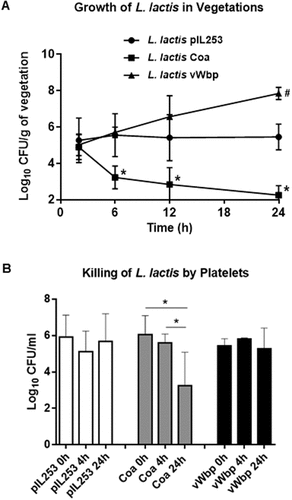
To support the hypothesis of the platelet-mediated clearance of L. lactis Coa in the vegetations, freshly-prepared platelet-rich plasma (PRP) from rats was incubated with lactococcal cells and the number of viable cells was assessed after 4 and 24 h of incubation at 37°C. As shown in ), while viability of L. lactis piL253 and L. lactis vWbp did not change over time, numbers of L. lactis Coa were significantly reduced after 24 h incubation, supporting the notion that Coa triggers platelet-induced bacterial killing in the in the platelet-fibrin network.
vWbp does not increase L. lactis IE in the presence of ClfA
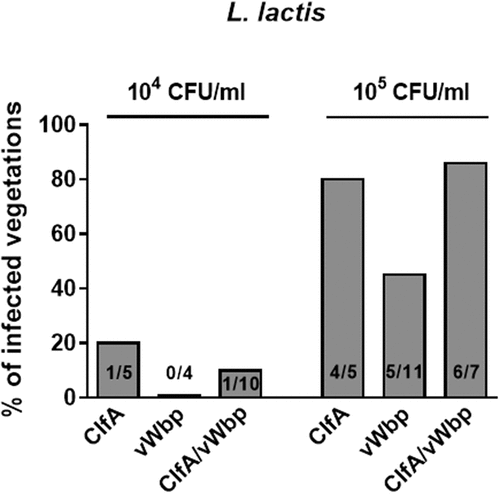
Previous studies using recombinant L. lactis expressing ClfA or ClfA-defective S. aureus have demonstrated that ClfA plays a pivotal role in the initiation of experimental IE [Citation6,Citation7]. To further investigate the role of vWbp in the presence of ClfA, we generated a double vWbp/clfA recombinant L. lactis by inserting vWbp in L. lactis ClfA [Citation6] and assessed its ability to induce IE. As shown in , when rats were challenged with inocula containing 104 CFU, L. lactis ClfA exhibited a poor valve infection rate, but co-expression of vWbp did not increase L. lactis valve infection. At higher inoculum (105 CFU), L. lactis expressing clfA and L. lactis expressing both clfA and vWbp were more prone to induce experimental IE than L. lactis expressing vWbp alone (P = 0.19 and P = 0.08, respectively; Chi-squared test). However, due to the high infectivity of L. lactis ClfA (> 80% of vegetations), it was impossible to investigate whether the concomitant production of vWBP had a cooperative effect on promoting IE initiation.
Figure 4. vWbp does not increase L. lactis IE in the presence of clfA. Experimental endocarditis induced by inoculation of parent L. lactis pIL253 and recombinant L. lactis expressing clfA or vWbp alone or in combination. Rats with catheter-induced aortic vegetations were challenged with 104 or 105 CFU of the indicated strains. The columns express the percentage of infected vegetations 24 h after inoculation. The number of infected/total number of vegetations per group is indicated at the bottom of the columns. No significant differences (P > 0.05) were observed when infection rates and bacterial burden in vegetations were compared.
All animals that developed endocarditis when inoculated with 105 CFU/ml had similar vegetation bacterial densities, i.e. 5.73 ± 0.97 log10 CFU/g (L. lactis pIL253), 6.00 ± 1.05 log10 CFU/g (L. lactis Coa) and 5.59 ± 0.46 log10 CFU/g (L. lactis vWbp); (P > 0.05; one-way ANOVA).
Neither Coa nor vWbp are necessary for the establishment of S. aureus IE when ClfA is present
In the light of the results obtained using the L. lactis surrogate expression system, we further investigated the ability of Coa and vWbp to colonize valve vegetations in the S. aureus background, in the presence or absence of ClfA. To this end, S. aureus Newman and isogenic mutants devoid of coa, vWbp or/and clfA, individually or in combination, were used. The defects in blood clotting of these mutants have already been described [Citation16].
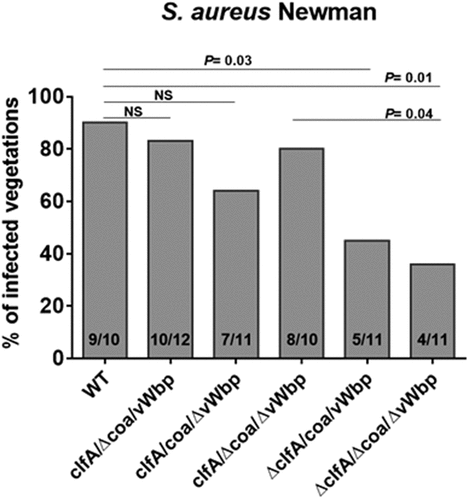
Rats were challenged with different inocula of S. aureus Newman allowing quantification of infectivity [Citation6]. Valve infection was evaluated 24 h after challenge as described above. As shown in , deletion of either the coa or vWbp gene did not significantly decrease infectivity (83% and 64% infected vegetations, respectively). In contrast, the absence of clfA (S. aureus ΔclfA/coa/vWbp) led to a significant decrease in S. aureus infectivity (45% infected vegetations; P = 0.03 compared to parent), in line with the results reported in a previous work [Citation6]. Moreover, inactivation of coa and vWbp, in addition to that of clfA (S. aureus ΔclfA/Δcoa/ΔvWbp), resulted in a slight but insignificant decrease of S. aureus infectivity (36% infected vegetations) as compared to that of the single clfA-defective mutant (45% infected vegetations). Of note, the bacterial densities in the vegetations of the animals that developed endocarditis were not significantly different, ranging from 7.24 to 8.89 log10 CFU/g. These results confirm that ClfA is the major determinant in the establishment S. aureus IE and suggest that its presence might outshine other virulence factors, such as vWbp in this particular animal model.
Figure 5. Neither coa nor vWbp significantly affects S. aureus Newman infectivity. Experimental endocarditis induced by inoculation of parent S. aureus Newman and isogenic mutant strains lacking coa, vwbp or clfA alone or in combination. Rats with catheter-induced aortic vegetations were challenged with 104 CFU of the indicated S. aureus Newman strains. The columns express the percentage of infected vegetations 24 h after bacterial challenge. The number of infected/total number of vegetations per group is indicated at the bottom of the columns. Statistical comparisons were determined by the Chi-square test. NS, not significant.
Discussion
We here examined the role of the two S. aureus procoagulant factors (Coa and vWbp) in the initiation of IE. This study was based on the assumption that by triggering fibrin formation and platelet aggregation, two essential events in the vegetation development, Coa and vWbp might contribute to the establishment of IE. To test this hypothesis, we used the poorly pathogenic L. lactis as a surrogate vector and investigated the effects of heterologous expression of the S. aureus procoagulant factors on its ability to colonize heart valve vegetations, using a rat model of catheter-induced experimental endocarditis. We generated recombinant L. lactis strains and showed that they could express and efficiently secrete the S. aureus coagulases. Moreover, consistent with the work of McDevitt and others, who showed that Coa was partly retained on S. aureus surface [Citation24], we observed that Coa, but not vWbp, is partially retained on L. lactis cell wall.
Unexpectedly, the expression of the S. aureus coagulases produced contrasting effects on the abilities of recombinant lactococci to infect damaged valves. On the one hand, we observed that Coa did not confer lactococci an increased ability to induce IE in rats, as assessed 24 h after challenge. These results are in agreement with previous studies showing that Coa is not involved in the initiation of S. aureus IE [Citation6,Citation20,Citation21]. However, at that time the presence of vWbp was unknown and its presence might have compensated for and thus outshined the loss of Coa function. Remarkably, we also found that Coa not only failed to promote IE, but even made lactococci less infective. In fact, following the evolution of IE over 24 h we observed three profiles for the parent and the vWbp and coa recombinants. While vegetation densities of the parent remained stable, those of the vWbp recombinant increased whereas those of the coa recombinant decreased down to infection eradication. Yet, this effect was not due to reduced capability of Coa-positive lactococci to colonize the valves, as titers comparable to those of the parent lactococci and L. lactis vWbp were found in the vegetations 2 h after challenge.
Although a precise mechanism of L. lactis Coa clearing remains to be determined, one plausible hypothesis is that Coa could induce a progressive killing of lactococci by platelets present in the vegetations. Consistent to this notion, it was previously shown that platelets can release microbicidal proteins that kill bacteria within vegetations [Citation25–Citation27]. Moreover, it was also demonstrated that, during the early phase of infection, the induction of the coagulation cascade by bacteria leads to their entrapment and killing within the clot, likely by the generation of additional host antimicrobial peptides [Citation28,Citation29]. We observed that the ability of L. lactis Coa cells to coagulate plasma was much faster than that of L. lactis vWbp cells, requiring 100-times less bacteria to induce clot formation after 24 h (supplementary Figure S1). This, together with the fact that L. lactis Coa displayed a greater ability to aggregate platelets and to coagulate plasma than L. lactis vWbp supports the killing hypothesis of the L. lactis Coa strain in the platelet-fibrin vegetation meshwork.
The detrimental effects of Coa expression in the early steps of IE may explain the need for tight regulation in S. aureus. In this organism, coagulase is only transiently produced at the beginning of the exponential growth phase [Citation30], a limited period of time that could be enough to promote fibrinogen-fibrin binding while avoiding overwhelming activation of platelets. This would be consistent with the observation by Panizzi et al [Citation22], who showed that Coa production was restricted to the growing edge of mature vegetations at the interface with the blood stream in mice infected with S. aureus Newman, where bacteria are not yet clustered in stationary phase-like, or biofilm-like, conditions.
On the other hand, the present results show for the first time that the expression of vWbp in L. lactis might contribute to the establishment of IE. Recently, S. aureus and Staphylococcus lugdunensis were shown to bind directly to the blood glycoprotein von Willebrand factor (VWF) [Citation31,Citation32]. In case of S. aureus, binding to VWF is mediated by secreted vWbp, which in turn binds to S. aureus via cell-wall anchored ClfA [Citation31,Citation32]. Thus, VWF present in the vegetations may act as local ligand to trap circulating bacteria.
However, this scenario was unlikely in vWbp-producing lactococci, because recombinant vWbp was secreted and not attached to the bacterial surface. Therefore, the increased infectivity of these recombinants was more likely due to the vWbp procoagulant activity alone, via binding to VWF present in vegetations and entrapping the organisms within the valve lesions [Citation33,Citation34]. Moreover, the fact that vWbp lactococci were less pro-coagulant than their Coa-producing counter parts, plus the fact that vWbp was not attached to the bacterial wall, might explain a lower platelet proximity and a lower platelet-induced lethality.
In this study we also tested the role of ClfA in these constructs. ClfA binds to fibrinogen-fibrin and also promotes platelet activation. This is why it increases infectivity when produced in lactococci [Citation4,Citation7]. However ClfA also binds vWbp and bridges lactococci to VWF, as in S. aureus [Citation31]. We asked whether this process would affect infectivity of either cflA or vWbp lactococcal recombinants, or S. aureus deleted in various vWbp, coa or clfA gene combinations. Using S. aureus Newman, a strain that does not express a functional FnbpA [Citation35], we found that ClfA was the major determinant of infectivity, and that vWbp or Coa did not add any advantage in this particular model of IE. Thus, while vWbp had indeed some effect in promoting infectivity in lactococcal recombinants, it became dispensable in the presence of ClfA.
In sum, vWbp appears to play a marginal role in the initiation of S. aureus IE. However, while this assumption is true for the present IE experimental model, where pre-existing physical valve damage is the nidus of infection, it might be different in other scenarios, such as minimal endothelial lesions, which are associated with the presence of high VWF levels. In that case the role of vWbp, together with ClfA to bridge the bacterium to the inflamed endothelium, might become more prominent. Whether vWbp becomes the predominant player of S. aureus infection in such circumstances needs yet to be clarified in vivo.
Materials and methods
Bacterial strains and growth conditions
The bacterial strains used in this study are listed in . L. lactis were cultivated at 30°C in M17 broth (Becton Dickinson, Sparks, MD) or on M17 agar plates supplemented with 0.5% glucose. S. aureus were grown under static condition in tryptic soy broth or on tryptic soy agar (Becton Dickinson). Escherichia coli were grown at 37°C in Luria-Bertani medium (Becton Dickinson). When required, antibiotics were added to the media at the following concentrations: erythromycin 5 µg/ml for L. lactis and 500 µg/ml for E. coli, chloramphenicol 10 µg/ml for L. lactis and 30 µg/ml for E. coli and ampicillin 100 µg/ml for E. coli.
Table 1. Bacterial strains used in this study.
Plasmids and plasmid constructions
All the plasmids and primers used in this work are listed in supplementary Table S1. S. aureus genomic DNA was extracted as previously described [Citation36]. S. aureus Newman coa and vWbp were PCR amplified using the forward primers coa-fw and vWbp-fw and the reverse primers coa-rv and vWbp-rv. The resulting PCR products were digested with SalI and PstI and ligated into the pori23 vector, also digested with the same enzymes. The resulting constructs pori23-coa and pori23-vWbp containing coa and vWbp under the control of the lactococcal P23 constitutive promoter [Citation37] were amplified in the E. coli DH5α intermediate host and purified using the Wizard® Plus SV Minipreps DNA Purification System (Promega, Madison, WI). The absence of mutations in the inserts was verified by commercial sequencing. The constructs were finally electroporated into L. lactis subsp. cremoris MG1363 cells using a protocol described previously [Citation38].
Characterization of the recombinant L. lactis strains
The functionality of recombinant L. lactis Coa and L. lactis vWbp was verified by assessing blood coagulation. Briefly, 100 μl containing 107 CFU of L. lactis cells were added to 900 μl of citrated rat blood in polystyrene tubes. The tubes were incubated at 37°C for 24 h and blood coagulation was verified by tipping the tubes at a 45° angle [Citation16]. For the clot inhibition tests, the direct thrombin inhibitor dabigatran (150 ng/ml, final concentration) [Citation39] was added to citrated rat blood prior to addition of the bacteria.
Quantification of platelet aggregation
The ability of L. lactis cells and cell-free supernatants to induce platelet aggregation in vitro by means of secreted and surface-associated coagulases was measured by light transmission aggregometry. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared as previously described [Citation40]. Twenty microliters containing either L. lactis cells (107 CFU) or cell-free supernatants of the same cultures were added to 180 μl of PRP in siliconized flat-bottom cuvettes. The light transmission of PRP without added bacteria and the light transmission of PPP were defined as 0% and 100% light transmission, respectively. Platelets were also tested with 10 μM ADP, used as positive control. Aggregation was recorded for 20 min. Three independent assays were performed.
Titration of plasma clotting activity of L. lactis
To titrate the plasma clotting activity over time, 100 μl of different inoculum sizes (from 105 to 108 CFU) of L. lactis cells (serially diluted in PBS) were mixed with 100 μl of rat plasma in polystyrene tubes. After gently mixing, the tubes were incubated at 37°C and observed at different times (4, 8, 12, 24 and 36 h) until clot formation.
Ethics statement
Animal experiments were carried out in accordance with the recommendations of the Swiss Federal Act on Animal Protection. All animal protocols were reviewed and approved by the Cantonal Committee on Animal Experiments of the State of Vaud (Permit Number: 879.9).
Animal model of endocarditis
Catheter-induced sterile aortic vegetations were produced in female Wistar rats (180–200 g) as previously described [Citation41]. Twenty-four hours after catheterization, animals were inoculated intravenously (i.v.) with increasing numbers of either L. lactis (ranging from 104 to 108 CFU/ml) or S. aureus (104 CFU/ml). The catheter was left in place throughout the entire experiment. The cardiac vegetations were sterilely removed, weighed, homogenized and plated on M17 agar plates containing the appropriate antibiotic marker. Vegetation infection was evaluated 24 h later [Citation6,Citation7] and expressed as log10 CFU/g of vegetation. Aortic valve vegetations were considered positive when they contained > 2 log10 CFU/g, which corresponds to the lower limit of detection of growth. In certain experiments, designed to measure diseases progression, rats were sacrificed at different time points after i.v. challenge with high inoculum sizes (107 CFU of L. lactis). Bacterial densities in the vegetations were measured as above.
In vitro killing of L. lactis by platelets
PRP were prepared from anticoagulated rat blood as described above. L. lactis suspensions containing 105–106 CFU/ml were mixed with PRP at a 1:1 ratio in polypropylene tubes. To monitor killing activity of platelets, viable bacterial cells were enumerated after 4 and 24 h incubation at 37°C. In order to accurately count viable bacteria, samples were vortexed and mixed by pipetting up and down before plating to disrupt any visible aggregate. Experiments were performed in triplicate in two independent occasions.
Statistical analysis
The incidences of valve infection were compared by the Fisher’s exact test or the Chi-squared test. Bacteria CFUs were compared by the one-way ANOVA followed by Tukey’s multiple comparisons test. A value of P < 0.05 was considered significant by using two-tailed significance levels. All statistical analyses were performed with the GraphPad Prism 6.0 program (www.graphpad.com).
Supplemental Material
Download MS Word (52.8 KB)Acknowledgments
We thank Christiane Gerschheimer, from the Service and Central Laboratory of Hematology, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, for their help with aggregometry.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
Additional information
Funding
References
- Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8:322–336.
- Holland TL, Baddour LM, Bayer AS, et al. Infective endocarditis. Nature Rev Dis Prim. 2016;2:16059.
- Weidenmaier C, Peschel A, Xiong YQ, et al. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J Infect Dis. 2005;191:1771–1777.
- Que YA, Haefliger JA, Piroth L, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med. 2005;201:1627–1635.
- Widmer E, Que YA, Entenza JM, et al. New concepts in the pathophysiology of infective endocarditis. Curr Infect Dis Rep. 2006;8:271–279.
- Moreillon P, Entenza JM, Francioli P, et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743.
- Que YA, Francois P, Haefliger JA, et al. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect Immun. 2001;69:6296–6302.
- Sinha B, Francois P, Que YA, et al. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect Immun. 2000;68:6871–6878.
- Heying R, van de Gevel J, Que YA, et al. Fibronectin-binding proteins and clumping factor A in Staphylococcus aureus experimental endocarditis: fnBPA is sufficient to activate human endothelial cells. Thromb Haaemost. 2007;97:617–626.
- Piroth L, Que YA, Widmer E, et al. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein A synergistically promote endothelial invasion and experimental endocarditis. Infect Immun. 2008;76:3824–3831.
- Friedrich R, Panizzi P, Fuentes-Prior P, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–539.
- Bjerketorp J, Jacobsson K, Frykberg L. The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol Lett. 2004;234:309–314.
- Vanassche T, Kauskot A, Verhaegen J, et al. Fibrin formation by staphylothrombin facilitates Staphylococcus aureus-induced platelet aggregation. Thromb Haemost. 2012;107:1107–1121.
- McAdow M, Missiakas DM, Schneewind O. Staphylococcus aureus secretes coagulase and von Willebrand factor binding protein to modify the coagulation cascade and establish host infections. J Innate Immun. 2012;4:141–148.
- Thomer L, Schneewind O, Missiakas D. Multiple ligands of von Willebrand factor-binding protein (vWbp) promote Staphylococcus aureus clot formation in human plasma. J Biol Chem. 2013;288:28283–28292.
- Cheng AG, McAdow M, Kim HK, et al. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathogens. 2010;6:e1001036.
- Vanassche T, Verhaegen J, Peetermans WE, et al. Inhibition of staphylothrombin by dabigatran reduces Staphylococcus aureus virulence. J Thromb Haemost. 2011;9:2436–2446.
- Claes J, Vanassche T, Peetermans M, et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood. 2014;124:1669–1676.
- Loof TG, Goldmann O, Naudin C, et al. Staphylococcus aureus-induced clotting of plasma is an immune evasion mechanism for persistence within the fibrin network. Microbiology. 2015;161:621–627.
- Baddour LM, Tayidi MM, Walker E, et al. Virulence of coagulase-deficient mutants of Staphylococcus aureus in experimental endocarditis. J Med Microbiol. 1994;41:259–263.
- Stutzmann Meier P, Entenza JM, Vaudaux P, et al. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infect Immun. 2001;69:657–664.
- Panizzi P, Nahrendorf M, Figueiredo JL, et al. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nature Med. 2011;17:1142–1146.
- Heilmann C, Herrmann M, Kehrel BE, et al. Platelet-binding domains in 2 fibrinogen-binding proteins of Staphylococcus aureus identified by phage display. J Infect Dis. 2002;186:32–39.
- McDevitt D, Vaudaux P, Foster TJ. Genetic evidence that bound coagulase of Staphylococcus aureus is not clumping factor. Infect Immun. 1992;60:1514–1523.
- Bayer AS, Ramos MD, Menzies BE, et al. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun. 1997;65:4652–4660.
- Kupferwasser LI, Yeaman MR, Shapiro SM, et al. In vitro susceptibility to thrombin-induced platelet microbicidal protein is associated with reduced disease progression and complication rates in experimental Staphylococcus aureus endocarditis: microbiological, histopathologic, and echocardiographic analyses. Circulation. 2002;105:746–752.
- Mercier RC, Dietz RM, Mazzola JL, et al. Beneficial influence of platelets on antibiotic efficacy in an in vitro model of Staphylococcus aureus-induced endocarditis. Antimicrob Agents Chemother. 2004;48:2551–2557.
- Loof TG, Morgelin M, Johansson L, et al. Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood. 2011;118:2589–2598.
- Papareddy P, Rydengard V, Pasupuleti M, et al. Proteolysis of human thrombin generates novel host defense peptides. PLoS Pathogens. 2010;6:e1000857.
- Lebeau C, Vandenesch F, Greenland T, et al. Coagulase expression in Staphylococcus aureus is positively and negatively modulated by an agr-dependent mechanism. J Bacteriol. 1994;176:5534–5536.
- Claes J, Liesenborghs L, Peetermans M, et al. Clumping factor A, von Willebrand factor-binding protein and von Willebrand factor anchor Staphylococcus aureus to the vessel wall. J Thromb Haemost. 2017;15:1009–1016.
- Liesenborghs L, Peetermans M, Claes J, et al. Shear-resistant binding to von Willebrand factor allows Staphylococcus lugdunensis to adhere to the cardiac valves and initiate endocarditis. J Infect Dis. 2016;213:1148–1156.
- Keuren JF, Baruch D, Legendre P, et al. von Willebrand factor C1C2 domain is involved in platelet adhesion to polymerized fibrin at high shear rate. Blood. 2004;103:1741–1746.
- Miszta A, Pelkmans L, Lindhout T, et al. Thrombin-dependent Incorporation of von Willebrand Factor into a Fibrin Network. J Biol Chem. 2014;289:35979–35986.
- Grundmeier M, Hussain M, Becker P, et al. Truncation of fibronectin-binding proteins in Staphylococcus aureus strain Newman leads to deficient adherence and host cell invasion due to loss of the cell wall anchor function. Infect Immun. 2004;72:7155–7163.
- Stojanov M, Sakwinska O, Moreillon P. Expression of SCCmec cassette chromosome recombinases in methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 2013;68:749–757.
- Que YA, Haefliger JA, Francioli P, et al. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect Immun. 2000;68:3516–3522.
- Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123.
- Vanassche T, Verhaegen J, Peetermans WE, et al. Dabigatran inhibits Staphylococcus aureus coagulase activity. J Clin Microbiol. 2010;48:4248–4250.
- Veloso TR, Que YA, Chaouch A, et al. Prophylaxis of experimental endocarditis with antiplatelet and antithrombin agents: a role for long-term prevention of infective endocarditis in humans? J Infect Dis. 2015;211:72–79.
- Heraief E, Glauser MP, Freedman LR. Natural history of aortic valve endocarditis in rats. Infect Immun. 1982;37:127–131.
