ABSTRACT
Brucella microti was isolated a decade ago from wildlife and soil in Europe. Compared to the classical Brucella species, it exhibits atypical virulence properties such as increased growth in human and murine macrophages and lethality in experimentally infected mice. A spontaneous rough (R) mutant strain, derived from the smooth reference strain CCM4915T, showed increased macrophage colonization and was non-lethal in murine infections. Whole-genome sequencing and construction of an isogenic mutant of B. microti and Brucella suis 1330 revealed that the R-phenotype was due to a deletion in a single gene, namely wbkE (BMI_I539), encoding a putative glycosyltransferase involved in lipopolysaccharide (LPS) O-polysaccharide biosynthesis. Complementation of the R-strains with the wbkE gene restored the smooth phenotype and the ability of B. microti to kill infected mice. LPS with an intact O-polysaccharide is therefore essential for lethal B. microti infections in the murine model, demonstrating its importance in pathogenesis.
Introduction
Brucellae are Gram-negative facultative intracellular coccobacilli causing brucellosis, a major bacterial zoonosis with 500,000 human cases globally reported every year [Citation1]. In the last decade, new species of Brucella, such as Brucella microti, Brucella inopinata and isolates from Australian rodents and amphibians, have been described [Citation2]. These strains are metabolically more active, acid-resistant and fast-growing when compared to the well-known classical, human-pathogenic Brucella species, which include Brucella abortus, Brucella melitensis, Brucella suis and Brucella canis [Citation2–Citation7]. Their isolation from hitherto unknown wildlife hosts and the environment raised the question whether Brucella may be transmitted from these reservoirs to livestock and humans living in officially brucellosis-free areas of the world.
B. microti was isolated from common vole, red fox, wild boar, and soil in Central Europe and, more recently, from a domestic marsh frog farm [Citation8,Citation9]. Phylogenetically, this species is closer to those pathogenic for human and livestock than to the group of newly described atypical species/strains [Citation2,Citation10]. However, in the absence of clinical reports, the pathogenic potential of B. microti remains to be verified. We were the first to describe that, unlike the classical Brucella species, B. microti is lethal in mice when injected intraperitoneally (i.p.) at a standard dose [Citation11]. The lethal phenotype in mice depends on the type IV secretion system VirB [Citation12] and is also a general unambiguous criterion to establish if a specific Brucella gene plays a role in virulence of B. microti, as wild-type bacteria kill the murine host at the infection dose of 105 CFU (colony-forming units); in contrast, in classical species virulence has been correlated to the capacity of a strain to establish or maintain various degrees of chronic infection of the spleen and/or the liver, necessitating repeated bacterial enumeration in these organs to follow up the course of infection. On the other hand, at sub-lethal doses (≤104 CFU), B. microti is rapidly cleared from infected mice, never gives rise to chronic infection and confers protection [Citation11]. Lethality in mice was later also demonstrated for B. inopinata BO1 and Brucella strain 83–210 [Citation13]. We and others assumed that the ability of these Brucella species to kill the murine host may be due to differences in surface antigens, in particular the structure of lipopolysaccharide (LPS) with a possibly higher endotoxic potential [Citation13,Citation14]. Because of its low endotoxicity, the LPS of classical Brucella species is considered as non-canonical in comparison with that of Escherichia coli and other pathogenic bacteria, enabling Brucella to establish chronic infections and evade TLR4 detection [Citation15–Citation17]. The LPS is a major component of the outer membrane and consists of three key elements: (1) the lipid A, which provides the hydrophobic LPS anchor in the outer membrane, (2) an inner and outer core composed of branched-chain oligosaccharides, and (3) an O-polysaccharide (O-PS), linked to the outer core and protruding into the extracellular environment. In Brucella, the O-PS is characterized by a homopolymeric linear chain of N-formyl-perosamine residues linked via α-1,2 and/or α-1,3 glycosidic bonds [Citation18]. Depending on the relative abundance and distribution of these bonds, the O-PS provides the A, M, and common (C) epitopes widely used for serotyping [Citation19,Citation20]. Depending on the presence or absence of the O-PS, the colony phenotype is either smooth (S) or rough (R). All Brucella species that infect humans and livestock are naturally S, except for Brucella ovis and B. canis [Citation21]. For vaccination of livestock against brucellosis, S- and R-strains have been used [Citation22].
Notably, a specific interaction between intact LPS and the lipid rafts in phagocytic cells is responsible for the selective entry of Brucella S-strains into the host cells and trafficking along the endocytic pathway [Citation23–Citation26]. In contrast, R strains do not enter the cell through the lipid rafts and are rapidly eliminated [Citation25].
In this study, we characterized a spontaneous R-mutant (BmRSM) of the B. microti reference strain CCM4915T. Its complete genome sequence helped to identify a mutation inactivating the wbkE gene, known to be involved in the synthesis of O-PS [Citation27]. To correlate this mutation with the R phenotype and virulence, we constructed a knock-out mutant (BmRΔwbkE) by allelic exchange. The fate of RSM, RΔwbkE and their complemented strains in cellular and murine infection models was studied and compared to that of the zoonotic strain B. suis 1330.
Material and methods
Bacterial strains, culture conditions and phenotypic characterization
E. coli and Brucella strains () were grown under aerobic conditions at 37°C in Luria Bertani (LB, Invitrogen) and Tryptic Soy (TS, Difco) media, respectively. When necessary, media were supplemented with kanamycin or ampicillin at 50 µg/ml, or with chloramphenicol at 25 µg/ml. All experiments with viable Brucella were performed in a BSL-3 facility. The smooth (S) and rough (R) phenotypes of Brucella were assessed by crystal violet staining [Citation28] and by agglutination tests using anti-R polyclonal antiserum and anti-A and anti-M monospecific sera (ANSES, France). Bacterial morphology was observed by atomic force microscopy (AFM).
Table 1. Bacterial strains, plasmids, and primers used in this study.
DNA analysis and mutant strains construction
Genomic DNA of B. microti was isolated using the Qiagen Mini Kit. Whole-genome shotgun sequencing of the spontaneous rough strain (BmRSM) was performed using Illumina paired-end sequencing with a library insert size of 300 bp and an average target coverage of 484 x (GATC). BmRΔwbkE of B. microti was obtained by replacing an internal portion of gene BMI_I539 with a KanR cassette. A recombinant wbkE-KanR-containing plasmid derived from pGEM®-T was created as previously described [Citation5,Citation30]. Briefly, two DNA fragments (A, 526 bp and B, 595 bp) each carrying an EcoRI restriction site in a 46-bp homology region at the 3ʹ- and 5ʹ-end, respectively, were amplified by PCR. The fragments were fused in a second PCR, to yield fragment AB (1075 bp) containing the EcoRI site in the middle and missing 608 out of 1110 bp of the target gene BMI_I539 (i.e. from positions 71 to 678; ). Following cloning of fragment AB in pGEM-T, the resulting plasmid (pGEM-T-AB; ) was digested with EcoRI and ligated with the KanR cassette (1282 bp) excised from plasmid pUC4K. The resulting plasmid (pGEM-T-ABKan, 5357 bp; ) was electroporated into B. microti. The KanR/AmpS clones arising from double-crossover were selected and verified by PCR (primers in ). Same constructions and protocols were used to obtain the ΔwbkE mutant of B. suis (BsRΔwbkE; ).
Using electroporation, the three mutant strains (BmRSM, BmRΔwbkE, BsRΔwbkE) were complemented with pBBR1MCS-wbkE vector, containing the wild-type B. microti wbkE gene and its up- and downstream regions ().
Atomic force microscopy
Bacteria were grown to stationary phase in TS, washed in PBS, fixed for 1 h in 2.5% glutaraldehyde and stored in PBS at 5 × 109 bacteria/ml. FluoroDish™ cell culture dishes (World Precision Instruments, UK) were coated overnight at 4°C with 0.1% poly-L-lysine, washed with PBS, air dried and stored at 4°C. Bacteria were diluted 20-fold in PBS and added to the functionalized dish. Images were recorded with qp-BioAC CB2 cantilevers using the quantitative imaging mode available on the NanoWizard IV AFM (JPK Instruments – Bruker). The applied force was kept at 0.3 nN, and a constant approach/retract speed of 80 µm/s (z range of 800 nm).
Macrophage infection with Brucella strains
Murine J774A.1 macrophage-like cells were infected with B. microti and B. suis strains at a multiplicity of infection (MOI) of 20 as described previously [Citation11]. All experiments were performed in triplicate.
Infection of Balb/c mice with B. microti strains
Approved animal experimentation guidelines were followed in the mouse experiments and the working protocol was approved by the CITA ethical animal experiment committee. A procedure for the assessment of pain, distress and discomfort in experimental animals, adapted from [Citation31] was followed, assigning a score to each animal regarding several variables (weight loss, appearance, spontaneous behavior, responses to external stimuli and clinical signs). If the score rose to 15–20 points prior to spontaneous death, animals were euthanized and considered as having succumbed to infection. Bacteria were inoculated i.p [Citation11]. To test the lethality of the bacterial strains, groups of six 9-weeks-old Balb/c female mice (Janvier Labs) each were infected i.p. with 105 CFU of the wild-type, BmRΔwbkE and complemented BmRΔwbkE strains or with 108 and 109 CFU of BmRSM and BmRΔwbkE mutant strains. Mice survival was monitored over a period of 25 days post-infection (d.p.i.).
To study the course of infection in mice, Balb/c were inoculated i.p. with a dose of 104 CFU of B. microti strains. Five mice per strain were sacrificed at 3, 14 and 21 d.p.i. Following mice euthanasia by CO2 asphyxiation, spleens were aseptically collected, weighed, homogenized, serially diluted and plated onto TS agar for viable counts of Brucella. The significance of differences between strains was analyzed by the Student t-test. P values ≤ 0.05 were considered significant.
Sequence accession number
The genomic DNA sequence of BmRSM has been deposited in the SRA database (NCBI) under the accession number PRJNA545613.
Results
A spontaneous rough mutant of B. microti shows increased colonization of macrophages and is avirulent in mice
Following the first plating of B. microti CCM4915T on TS agar and staining with crystal violet, a rough colony was observed. The colony was picked, subcultured three times and stained again with crystal violet: the rough phenotype remained stable for all colonies on plate. This strain was named B. microti rough spontaneous mutant and abbreviated BmRSM.
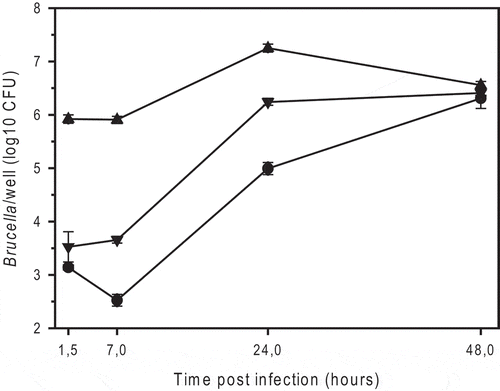
It has been reported that rough mutant strains of B. suis and B. melitensis exhibit reduced intracellular survival in infected macrophages, though entry is improved [Citation28]. BmRSM indeed entered murine J774A.1 macrophage cells approximately 100-fold better than the wild-type strain and B. suis 1330, which was used as standard reference (). In contrast to B. suis and B. melitensis rough strains [Citation28], BmRSM replicated 20-30-fold, at least up to 24 hours post-infection ().
Figure 1. Intracellular replication of smooth B. microti CCM4915T (triangle down), the spontaneous rough mutant of B. microti (BmRSM; triangle up), and smooth B. suis 1330 (circle), in murine J774A.1 macrophage-like cells. The number of colony forming units (CFU) was determined by plating serial dilutions on TS agar plates after 2 or 3 days of incubation at 37°C for B. microti and B. suis, respectively. The experiments were performed three times in triplicate each. Data are presented as mean values ± SD of one experiment (in triplicate).
To characterize the behavior of BmRSM in vivo, Balb/c mice were injected i.p. with the sublethal dose of 104 bacteria, as previously published by the authors for the B. microti wild-type strain [Citation11]. The number of bacteria recovered from spleen and liver 3 days after inoculation, corresponding to the peak of infection for B. microti [Citation11], was reduced by 3.5 and 2.2 logs (P < 0.001), respectively, compared to those previously obtained with the wild-type strain () and also confirmed in this work (see later section on “acute murine infection”). Therefore, the rough mutant colonized these organs significantly less than the wild-type, resulting in the lack of a transient acute phase of infection.
Table 2. Balb/c mice liver and spleen colonization by B. microti S and RSM strains 3 days post-inoculation.
BmRSM strain is characterized by a mutation of the glycosyltransferase-encoding gene wbkE
To identify the mutation(s) responsible for the rough phenotype in BmRSM, its genome was sequenced and compared with that of B. microti CCM4915T, accessible in the NCBI database. Out of 48 variants, 28 were assigned to SNV (Single Nucleotide Variants) and 20 to InDel (Insertion/Deletion) variants. Scrutiny of the variants resulted in retaining of 3 SNV and 4 InDels, fulfilling all the following criteria: (1) located within open reading frames or in the immediate upstream vicinity, (2) causing amino acids substitutions, frameshift- or stop-mutations in the corresponding genes, (3) not located in pseudogenes, and (4) representing the most prevalent variant according to the number of sequencing reads (≥ 90%) with respect to the reference sequence (Supplementary Table S1). Only four mutations were intragenic and affected the following genes: BMI_I525 (2 SNVs), BMI_I539 (1 InDel) and BMI_I1103 (1 SNV), encoding a transposase (ISBm1), a glycosyltransferase (wbkE) and a queuine tRNA-ribosyltransferase (tgt), respectively. The mutation found in wbkE was regarded as the most plausible cause for the rough phenotype, because the homologous gene BMEI1393 of B. melitensis, located in a highly conserved cluster of the major (wbk) genetic region of LPS synthesis, participates in O-PS biosynthesis [Citation2,Citation27]. Protein sequences of BMI_I539 and BMEI1393 are identical for 368 out of 369 amino acids. In BmRSM, deletion of a T at position 452 of wbkE causes a frameshift and the generation of a premature stop codon at position 622. The resulting protein sequence is therefore expected to be truncated at position 207.
Deletion of the B. microti wbkE gene confirms its role in smooth (S-)LPS biosynthesis and results in enhanced macrophage uptake
To confirm that the absence of a functional wbkE is responsible for the rough phenotype and the reduced virulence of BmRSM, we constructed a mutant (BmRΔwbkE) by replacing a 608-bp internal fragment of wbkE with a KanR cassette in the parental strain. Colony staining with crystal violet and agglutination with anti-R antiserum [Citation32] confirmed that BmRΔwbkE was rough as BmRSM (). In parallel, we performed a surface analysis of smooth and rough bacterial strains by atomic force microscopy (AFM). AFM has been established as a powerful imaging technique and allows characterization of the surface morphology of microbial cells at the nanoscale [Citation33]. We used a force-curve-based imaging mode where the AFM tip is pushed toward an area of the cell surface and retracted from it, generating a force vs. separation distance curve encoding information about height, adhesion or elasticity for each image pixel. Surface topography of B. microti wild-type and BmRΔwbkE revealed a uniform, smooth structure for B. microti wild-type, in contrast to a jagged, irregular structure for the BmRΔwbkE mutant, with significantly increased roughness ((a,b); ). Mapping of the adhesion forces between the tip and the bacterial surface also revealed the presence of large patches of increased adhesion at the surface of BmRΔwbkE when compared with the surface of wild-type bacteria, suggesting important differences in the molecular structure of the BmRΔwbkE mutant surface ((a,c); ).
Figure 2. Atomic Force Microscopy (AFM) images of B. microti wild-type (Bm WT), ΔwbkE mutant (Bm RΔwbkE) and the complemented ΔwbkE mutant (Bm RΔwbkE compl). (a) Each column shows from top to bottom the vertical deflection image (height) of the whole bacteria and 0.3 × 0.3 µm2 areas of the cell surface, representing roughness and adhesion recorded on the shown bacteria (blue square). Quantitative roughness (b) and adhesion (c) measurements of Bm WT, Bm RΔwbkE and complemented Bm RΔwbkE: 0.5 × 0.5 µm2 images were recorded and used for measurements of 0.25 × 0.25 µm2 areas to quantify arithmetic roughness Ra and adhesion (Peak-to-Valley). n = 9 bacteria/strain. Statistical differences were analyzed by t-test and yielded P values < 0.001 when comparing Bm WT or Bm RΔwbkE compl with Bm RΔwbkE. Image analysis was done with Gwyddion [Citation34].
![Figure 2. Atomic Force Microscopy (AFM) images of B. microti wild-type (Bm WT), ΔwbkE mutant (Bm RΔwbkE) and the complemented ΔwbkE mutant (Bm RΔwbkE compl). (a) Each column shows from top to bottom the vertical deflection image (height) of the whole bacteria and 0.3 × 0.3 µm2 areas of the cell surface, representing roughness and adhesion recorded on the shown bacteria (blue square). Quantitative roughness (b) and adhesion (c) measurements of Bm WT, Bm RΔwbkE and complemented Bm RΔwbkE: 0.5 × 0.5 µm2 images were recorded and used for measurements of 0.25 × 0.25 µm2 areas to quantify arithmetic roughness Ra and adhesion (Peak-to-Valley). n = 9 bacteria/strain. Statistical differences were analyzed by t-test and yielded P values < 0.001 when comparing Bm WT or Bm RΔwbkE compl with Bm RΔwbkE. Image analysis was done with Gwyddion [Citation34].](/cms/asset/21e5c724-6a16-4bb1-923d-9d94708bb74c/kvir_a_1682762_f0002_oc.jpg)
Table 3. Phenotypes of S and R strains of B. microti and B. suis.
Both rough mutants BmRΔwbkE and BmRSM were then complemented with an intact copy of wbkE cloned in vector pBBR1MCS, which restored the smooth phenotype, as evidenced by lack of crystal violet staining and by the agglutination with anti-M antiserum only [Citation8] (). In addition, AFM confirmed a smooth surface structure of complemented BmRΔwbkE, with roughness and adhesion forces back to wild-type levels (, ).
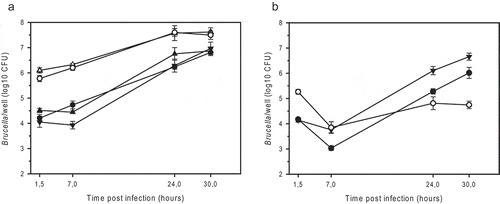
The BmRΔwbkE mutant entered J774A.1 cells to an extent similar to that of the BmRSM mutant (100 times more efficient than the parental strain) and replicated 60-fold over 30 hours ()). In contrast, BmRΔwbkE and BmRSM strains complemented with an intact copy of wbkE infected macrophages like the parental strain and replicated 400- and 250-fold, respectively ()). An isogenic wbkE mutant of B. suis 1330 (BsRΔwbkE) was also constructed in order to compare its behavior to that of other rough mutants described in the past [Citation25,Citation28]. As expected, the BsRΔwbkE mutant retained crystal violet and agglutinated with anti-R antiserum (). It colonized the macrophages 12 times more efficiently than the wild-type, but in contrast to R-strains of B. microti, a 3-fold reduction in intracellular survival was observed within a period of 30 h, resulting in 100 times lower viable counts when compared to the wild-type ()). This was very similar to the behavior of the manBcore mutant of B. suis 1330 [Citation25]. The complemented BsRΔwbkE strain agglutinated specifically with anti-A antiserum as the wild-type strain () and showed wild-type levels of intracellular infection and replication, despite a stronger transitional decrease in the early phase of infection ()).
Figure 3. Intracellular replication of smooth and rough strains of B. microti (a) and B. suis (b) in murine J774A.1 macrophage-like cells. (a) B. microti CCM4915T wild-type (filled triangle down), the spontaneous R-strain BmRSM (open triangle up), the complemented BmRSM mutant (filled triangle up), the constructed R-strain BmRΔwbkE (open circle), and the complemented BmRΔwbkE mutant (filled circle). (b) B. suis 1330 wild-type (filled triangle down), the constructed R-strain BsRΔwbkE (open circle), and the complemented BsRΔwbkE mutant (filled circle). The complemented strains expressed native wbkE cloned into the replicative plasmid pBBR1MCS. The experiments were performed three times in triplicate each. Data are presented as mean values ± SD of one experiment (in triplicate).
The B. microti wbkE gene is indispensable for acute murine infection
Infection of Balb/c with 104 CFU of B. microti strains showed a 3-logs reduction of BmRΔwbkE in the spleen at day 3 post-injection, as compared to the wbkE-complemented mutant and wild-type strains ()), confirming the incapacity of a ΔwbkE mutant strain to establish an acute phase of infection in the host (see also ). The acute infection phase observed with the wild-type and the wbkE-complemented strains was followed by an increase of the spleen weight until at least day 14, reflecting an inflammatory response ()). In contrast, mice infected with BmRΔwbkE did not gain spleen weight. A similar finding was obtained when the strain BmRSM was injected i.p. at 104 CFU, as spleen weights remained unchanged at day 3 and day 14 (0.09 ± 0.007 and 0.10 ± 0.015, respectively; P < 0.001), confirming a reduced immune response induction with the wbkE-mutant rough strains.
Figure 4. Infection of Balb/c mice with B. microti strains: growth and survival of B. microti strains in the spleen (a) and spleen weights of infected animals (b) after i.p. inoculation of 104 bacteria. The number of viable B. microti CCM4915T wild-type (black bars), BmRΔwbkE strain (open bars), and complemented BmRΔwbkE mutant (grey bars) was determined at days 3, 14, and 21 post-infection. The arrow indicates the infection dose of 104 bacteria. Five mice were sacrificed per bacterial strain and time point, and values represent means ± SD. Asterisks indicate variable significance of the differences between the R-strain and the wild-type (next to left bar) or R-strain and the complemented mutant (next to right bar), or between the R-strain and both the wild-type and the complemented mutant (above middle bar): * P < 0.05; ** P < 0.005; *** P < 0.001.
The wbkE gene is essential for the lethal character of B. microti infections in Balb/c mice
Our previous work showed that the intra-peritoneal injection of 105 CFU of B. microti CCM4915T caused the death of 83% of the Balb/c mice within four days of infection [Citation11]. To investigate the possible involvement of the wbkE gene in the lethal outcome of a B. microti infection, the susceptibility of Balb/c mice infected with BmRSM or BmRΔwbkE was compared to that observed following infection with the B. microti CCM4915T wild-type and the complemented BmRΔwbkE strains, over a monitoring period of 25 days (): 67% and 83% of the mice infected with the standard dose of 105 CFU of the wild-type or the complemented BmRΔwbkE strain, respectively, died between days 2 and 6 post-inoculation. In striking contrast, all the mice infected with 105 CFU of BmRΔwbkE survived without any symptoms. 100% survival was also observed for both R-mutant strains BmRSM and BmRΔwbkE after the injection of 108 CFU. However, when inoculated with 109 CFU of either R-strain, all mice died between days 2 and 6 post-inoculation. These results are even more notable if combined with our preliminary observation (not shown) that BmRSM in Balb/c mice provided protection against B. microti wild-type, B. abortus, B. melitensis and B. suis 1330.
Table 4. Lethality of B. microti S and R strains in Balb/c mice
Discussion
The non-canonical LPS of classical brucellae lacks endotoxicity and possesses a particular core structure helping to evade the host’s immune system [Citation35,Citation36]. The phenomenon of dissociation, resulting in the conversion of S- to R-phenotype, has been first described for Brucella in 1933 [Citation37]. More recently, B. abortus, B. melitensis and B. suis R-mutants devoid of O-PS have been studied in cellular and murine models of infection [Citation25,Citation27,Citation28,Citation38,Citation39]. These studies consistently showed the relevance of O-PS for virulence. The reduced virulence of R-mutants has been attributed to (1) high sensitivity to complement-mediated lysis in mice [Citation40,Citation41], (2) lipid raft-independent entry into macrophages resulting in enhanced phagolysosome fusion [Citation25], and (3) lack of intracellular replication due to macrophage activation [Citation28]. Monoclonal antibodies specific for common O-PS epitopes of A- or M-dominant classical strains also recognize LPS of the M-dominant B. microti reference strain [Citation14], indicating a conserved structure of O-PS. However, anti-R-LPS monoclonal antibodies do not react with B. microti LPS, suggesting structural specificities in the core-lipid A moiety of its LPS [Citation14], which may result in enhanced endotoxic properties and possibly explain the killing ability of B. microti in the murine model of infection.
Because of the lack of data available on atypical species mutants affected in O-PS biosynthesis, we investigated the virulence properties of a spontaneous R-mutant of B. microti, which was fortuitously isolated. Murine infection experiments showed a loss of lethality of this mutant, and subsequent analysis by whole-genome sequencing allowed to link this ability to the wbkE gene, encoding a glycosyltransferase located in the major O-PS biosynthesis region, as previously described for B. melitensis [Citation27]. Complementation of R-mutants restored wild-type properties including smooth character, reduced macrophage entry and murine lethality, demonstrating that the BmRSM strain was affected in a single LPS biosynthesis gene. In contrast, spontaneous R-mutants of B. abortus and B. melitensis isolated from macrophage and murine infections were often simultaneously affected in several loci [Citation42]. Cell surface structure analysis by AFM confirmed the smooth character of the wild-type and complemented BmRΔwbkE strains, whereas a distinct irregular surface was recorded for BmRΔwbkE, possibly due to exposure of outer membrane proteins and lipid A/outer core disaccharides in the absence of the O-chain [Citation27]. This exposure may also explain the increased adhesion forces observed during the interaction of the AFM tip with the R-mutant surface. Conversely, in a recent report, AFM analysis of two B. abortus strains, a wild-type and its isogenic mutant Δgmd R lacking O-chain, shows similar degrees of roughness [Citation43]. Structural differences in the LPS core-lipid A moieties of B. microti and B. abortus could be a reason for this divergence.
In the macrophage model of infection, BmRΔwbkE and BmRSM replicated to a number of intracellular bacteria higher than that observed for the wild-type, in part due to the increased rate of entry (100 times). The latter was also described previously for R-mutants of classical species, but with variations in intracellular survival, reaching at best the initial level post-phagocytosis [Citation25,Citation28,Citation44]. Interestingly, this was also confirmed for BsRΔwbkE, leading to the hypothesis that B. suis and B. microti R-mutants may use distinct intracellular trafficking pathways depending on sites of entry and resulting in specific phagolysosome fusion and escape kinetics. Measurement of NO- and TNF-α-production by infected macrophages yielded only minor differences between BsRΔwbkE and BmRΔwbkE strains, indicating that these immune mediators were not involved (not shown).
Intramacrophagic replication and high intracellular loads of bacteria are essential for the lethal phenotype of B. microti in vivo, with the major virulence factor VirB playing a crucial role [Citation12]. We assume that massive intracellular replication of the pathogen and increased cell lysis results in high concentrations of circulating bacteria, infecting new cells and leading to septic shock and murine death, possibly due to a modified core-lipid A moiety. However, intramacrophagic replication is not sufficient, as shown for the R-mutant. Lack of murine lethality after infection with R-mutants may indeed be explained by increased sensitivity to complement-mediated lysis, keeping the overall concentrations of bacteria too low to trigger an endotoxic shock and, at a sublethal dose, strongly reducing the load of infected macrophages prior to their settling in the spleen and mitigating the inflammatory reaction. The biological potential of dissociation based on enhanced dissemination of S-bacteria in the presence of R-forms inducing cytotoxicity has been discussed in the context of B. abortus and B. melitensis infections [Citation42,Citation45], but in the case of rough B. microti, final bacterial loads in the macrophage are even higher than with the wild-type, making a cytotoxic effect unlikely. Given the different natural habitats of B. microti and the observation that B. microti strains isolated from soil were rough [Citation32], we speculate that the R-phenotype either confers an advantage to this species outside the host or appears at higher rates due to the absence of selective pressure to which intracellular smooth organism may be exposed.
In surveillance and control of livestock brucellosis, the distinction between infected and vaccinated animals remains a major challenge, since field strains and the commonly used efficient vaccine strains, B. abortus S19 and B. melitensis Rev1, both possess a S-LPS, which is the relevant diagnostic antigen recognized by anti-Brucella antibodies following infection or vaccination. Therefore, O-PS mutants are interesting vaccine candidates, as they do not elicit an antibody response undistinguishable from that induced during active infection. As a matter of fact, B. abortus RB51 has been commercialized as a rough vaccine strain, despite its controversial effectiveness and drawbacks [Citation46]. Based on our preliminary data in mice, we suggest that the spontaneous, well-characterized R-strain of B. microti described in this study, or a R-mutant deletion strain devoid of the antibiotic resistance marker, might thus be exploited as a potential DIVA (Differentiating Infected from Vaccinated Animals)-vaccine candidate against animal brucellosis, with the potential advantage to confer a broad-range protection against heterologous Brucella species pathogenic for livestock.
In summary, we have described R-mutants of the atypical species B. microti defective in wkbE expression/activity in cellular and in vivo models of infection. Together with VirB, the S-LPS was identified as a virulence factor essential for lethality of B. microti in the murine model of infection. Further characterization of its LPS structure, elucidation of intracellular trafficking, and additional in vivo studies in livestock animals will show whether B. microti R-strains are suitable live vaccines against brucellosis.
Supplemental Material
Download MS Word (21.5 KB)Acknowledgments
We thank Pascale Bouhours and Magali Abrantes for technical assistance in solution and media preparation, Julien Ogier and Margot Bichon in constructing plasmids in E. coli, Dr. Anthony Boureux and the Bio2M bioinformatics and bio-markers platform of the CHU Montpellier for support in bioinformatics analysis. The authors are grateful to Dr. Véronique Jubier-Maurin (IRIM, Montpellier) for helpful discussions. This work was supported by grants from the German Federal Institute for Risk Assessment (n° 1329-562) and from the Spanish INIA (n° RTA2017-00083-00-00). LF and JdlG were recipients of a doctoral fellowship from the foundation Infectiopôle Sud, JdlG also of a doctoral fellowship from the Conacyt/Mexico, and AO received an International mobility support (Muse Explore) from the I-SITE Montpellier University of Excellence (MUSE) for visiting DDB laboratory. The atomic force microscope was funded by the French REDSAIM project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
supplemental data for this article can be accessed here.
Additional information
Funding
References
- Pappas G, Papadimitriou P, Akritidis N, et al. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99.
- Al Dahouk S, Köhler S, Occhialini A, et al. Brucella spp. of amphibians comprise genomically diverse motile strains competent for replication in macrophages and survival in mammalian hosts. Sci Rep. 2017;7:44420.
- Aujoulat F, Roger F, Bourdier A, et al. From environment to man: genome evolution and adaptation of human opportunistic bacterial pathogens. Genes (Basel). 2012;3:191–232.
- Damiano MA, Bastianelli D, Al Dahouk S, et al. Glutamate decarboxylase-dependent acid resistance in Brucella spp.: distribution and contribution to fitness under extremely acidic conditions. Appl Environ Microbiol. 2015;81:578–586.
- Freddi L, Damiano MA, Chaloin L, et al. The glutaminase-dependent system confers extreme acid resistance to new species and atypical strains of Brucella. Front Microbiol. 2017;8:2236.
- Pennacchietti E, D’Alonzo C, Freddi L, et al. The glutaminase-dependent acid resistance system: qualitative and quantitative assays and analysis of its distribution in enteric bacteria. Front Microbiol. 2018;9:2869.
- Soler-Llorens PF, Quance CR, Lawhon SD, et al. A Brucella spp. isolate from a Pac-Man frog (Ceratophrys ornata) reveals characteristics departing from classical brucellae. Front Cell Infect Microbiol. 2016;6:116.
- Scholz HC, Hubalek Z, Sedlacek I, et al. Brucella microti sp. nov., isolated from the common vole Microtus arvalis. Int J Syst Evol Microbiol. 2008;58:375–382.
- Jay M, Girault G, Perrot L, et al. Phenotypic and molecular characterization of Brucella microti-like bacteria from a domestic marsh frog (Pelophylax ridibundus). Front Vet Sci. 2018;5:283.
- Wattam AR, Inzana TJ, Williams KP, et al. Comparative genomics of early-diverging Brucella strains reveals a novel lipopolysaccharide biosynthesis pathway. MBio. 2012;3:e00246–12.
- Jiménez de Bagüés MP, Ouahrani-Bettache S, Quintana JF, et al. The new species Brucella microti replicates in macrophages and causes death in murine models of infection. J Infect Dis. 2010;202:3–10.
- Hanna N, Jiménez de Bagüés MP, Ouahrani-Bettache S, et al. The virB operon is essential for lethality of Brucella microti in the Balb/c murine model of infection. J Infect Dis. 2011;203:1129–1135.
- Jiménez de Bagüés MP, Iturralde M, Arias MA, et al. The new strains Brucella inopinata BO1 and Brucella species 83-210 behave biologically like classic infectious Brucella species and cause death in murine models of infection. J Infect Dis. 2014;210:467–472.
- Zygmunt MS, Jacques I, Bernardet N, et al. Lipopolysaccharide heterogeneity in the atypical group of novel emerging Brucella species. Clin Vaccine Immunol. 2012;19:1370–1373.
- Haag AF, Myka KK, Arnold MF, et al. Importance of Lipopolysaccharide and Cyclic beta-1,2-Glucans in Brucella-Mammalian Infections. Int J Microbiol. 2010;2010:124509.
- Lapaque N, Moriyon I, Moreno E, et al. Brucella lipopolysaccharide acts as a virulence factor. Curr Opin Microbiol. 2005;8:60–66.
- Smith JA. Brucella Lipopolysaccharide and pathogenicity: the core of the matter. Virulence. 2018;9:379–382.
- Moriyon I, Lopez-Goni I. Structure and properties of the outer membranes of Brucella abortus and Brucella melitensis. Int Microbiol. 1998;1:19–26.
- Bundle DR, Cherwonogrodzky JW, Gidney MA, et al. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun. 1989;57:2829–2836.
- Zygmunt MS, Bundle DR, Ganesh NV, et al. Monoclonal antibody-defined specific C epitope of Brucella O-polysaccharide revisited. Clin Vaccine Immunol. 2015;22:979–982.
- Hull NC, Schumaker BA. Comparisons of brucellosis between human and veterinary medicine. Infect Ecol Epidemiol. 2018;8:1500846.
- Godfroid J, Scholz HC, Barbier T, et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev Vet Med. 2011;102:118–131.
- Porte F, Liautard JP, Köhler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect Immun. 1999;67:4041–4047.
- Celli J, de Chastellier C, Franchini DM, et al. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556.
- Porte F, Naroeni A, Ouahrani-Bettache S, et al. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect Immun. 2003;71:1481–1490.
- Starr T, Ng TW, Wehrly TD, et al. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic. 2008;9:678–694.
- Gonzalez D, Grillo MJ, De Miguel MJ, et al. Brucellosis vaccines: assessment of Brucella melitensis lipopolysaccharide rough mutants defective in core and O-polysaccharide synthesis and export. PLoS One. 2008;3:e2760.
- Jiménez de Bagüés MP, Terraza A, Gross A, et al. Different responses of macrophages to smooth and rough Brucella spp.: relationship to virulence. Infect Immun. 2004;72:2429–2433.
- Kovach ME, Phillips RW, Elzer PH, et al. 2nd and Peterson KM. pBBR1MCS: a broad-host-range cloning vector. Biotechniques. 1994;16:800–802.
- Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924–932.
- Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436.
- Al Dahouk S, Hofer E, Tomaso H, et al. Intraspecies biodiversity of the genetically homologous species Brucella microti. Appl Environ Microbiol. 2012;78:1534–1543.
- Dorobantu LS, Gray MR. Application of atomic force microscopy in bacterial research. Scanning. 2010;32:74–96.
- Necas D, Klapetek P. Gwyddion: an open-source software for SPM data analysis. Central European Journal of Physics. 2012;10:181–188.
- Cardoso PG, Macedo GC, Azevedo V, et al. Brucella spp noncanonical LPS: structure, biosynthesis, and interaction with host immune system. Microb Cell Fact. 2006;5:13.
- Conde-Alvarez R, Arce-Gorvel V, Iriarte M, et al. The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog. 2012;8:e1002675.
- Henry BS. Dissociation in the Genus Brucella. J Infect Dis. 1933;53:374–402.
- Ugalde JE, Czibener C, Feldman MF, et al. Identification and characterization of the Brucella abortus phosphoglucomutase gene: role of lipopolysaccharide in virulence and intracellular multiplication. Infect Immun. 2000;68:5716–5723.
- Godfroid F, Taminiau B, Danese I, et al. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect Immun. 1998;66:5485–5493.
- Corbeil LB, Blau K, Inzana TJ, et al. Killing of Brucella abortus by bovine serum. Infect Immun. 1988;56:3251–3261.
- Fernandez-Prada CM, Nikolich M, Vemulapalli R, et al. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect Immun. 2001;69:4407–4416.
- Turse JE, Pei J, Ficht TA. Lipopolysaccharide-deficient Brucella variants arise spontaneously during infection. Front Microbiol. 2011;2:54.
- Vassen V, Valotteau C, Feuillie C, et al. Localized incorporation of outer membrane components in the pathogen Brucella abortus. Embo J. 2019;38:e100323.
- Pei J, Ficht TA. Brucella abortus rough mutants are cytopathic for macrophages in culture. Infect Immun. 2004;72:440–450.
- Pei J, Kahl-McDonagh M, Ficht TA. Brucella dissociation is essential for macrophage egress and bacterial dissemination. Front Cell Infect Microbiol. 2014;4:23.
- Moriyon I, Grillo MJ, Monreal D, et al. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet Res. 2004;35:1–38.
