ABSTRACT
Influenza is a respiratory viral infection that causes significant morbidity and mortality worldwide. The innate immune cell response elicited during influenza A virus (IAV) infection forms the critical first line of defense, which typically is impaired as we age. As such, elderly individuals more commonly succumb to influenza-associated complications, which is reflected in most aged animal models of IAV infection. Here, we review the important roles of several major innate immune cell populations in influenza pathogenesis, some of which being deleterious to the host, and the current knowledge of how age-associated numerical, phenotypic and functional cell changes impact disease development. Further investigation into age-related modulation of innate immune cell responses, using appropriate animal models, will help reveal how immunity to IAV may be compromised by aging and inform the development of novel therapies, tailored for use in this vulnerable group.
Introduction
Influenza is a common, widespread infectious disease, estimated to be responsible for close to 400,000 annual deaths worldwide and substantial morbidity [Citation1]. Influenza A virus (IAV) infections are often asymptomatic; however, common symptoms include fever, chills and headache. Viral pneumonia is a rare complication of IAV infection and occurs mainly in immunocompromised individuals with underlying medical conditions such as chronic heart or pulmonary diseases and comorbidities including those associated with obesity and smoking [Citation2,Citation3]. Severe IAV infections in humans are characterized by hyperinflammatory responses, as well as vascular leakage and pulmonary edema; hallmark pathological features of acute respiratory distress syndrome (ARDS) [Citation4]. Additionally, IAV infection can commonly lead to subsequent secondary bacterial infection, which account for the majority of influenza-associated deaths in the elderly or during IAV pandemics [Citation5,Citation6]. Indeed, during the 2009 Swine H1N1 pandemic it was estimated that up to 55% of mortalities resulted from secondary bacterial pneumonia. With our increasing longevity, the human population is rapidly aging, with over 700 million people currently over the age of 65, a figure that is expected to double within 30 years [Citation7]. This is particularly alarming as the elderly are routinely overrepresented in influenza-related fatalities and hospitalization and the risk of death increases with advancing age [Citation1,Citation8,Citation9].
The innate immune system forms the first line of defense against IAV infection and is partially comprised of a heterogenous assortment of fast-acting immune cells, which collectively act to limit the dissemination of the virus and shape the character of the ensuing adaptive immune response. However, a dysregulated hyperinflammatory innate immune cell response can cause significant damage to the lung [Citation10,Citation11]. and IAV disease severity and successful recovery from infection is proposed to result from either efficient elimination of the virus or by tolerance of the infection and limiting the associated immunopathology [Citation12]. Irregular immune cell function (immunosenescense) and the persistent low levels of systemic sterile inflammation (inflammaging) are consequences and common features of aging and positively correlate with increased susceptibility in older individuals, (>65 years old); however, how these age-associated changes affect innate immunity to IAV remains to be fully elucidated [Citation13,Citation14]. Due to limitations on studying IAV disease in humans, animal models of acute infection are heavily relied upon to examine important aspects of the disease and the immune response to it. Most of our knowledge stems from murine models of IAV infection owing to the relative ease that mice can be genetically manipulated, in addition to the prohibitive ethical and financial barriers associated with using higher organisms such as ferrets and non-human primates, to model this disease, particularly in the context of aging. Translation of findings from animal models to the clinic can be difficult, however, particularly from these heavily utilized mouse models of IAV infection. Notable issues include differences in IAV infection susceptibility between inbred laboratory mouse strains [Citation15,Citation16], which also possess a genetic-deficiency in a key antiviral factor normally present in mice and humans that shows reduced expression in monocytes from elderly humans [Citation17,Citation18]. Specifically, most laboratory mouse strains lack functional Mx1 alleles the encode an interferon (IFN)-inducible influenza resistance factor (Mx1), which when restored can imbue mice with increased resistance to several respiratory viruses [Citation18–20]. Nevertheless, across several different mouse strains and consistent with the poorer influenza disease outcomes in elderly humans, most reports indicate that aged mice (>16 months of age) exhibit increased lung tissue damage, increased inflammation, impaired viral clearance, increased weight loss and decreased resistance to IAV infection [Citation21–30]. Similar observations have been made in IAV-infected non-human primates with increased levels of virus and proinflammatory interleukin (IL)-6 present in the lungs of older rhesus macaques compared to younger counterparts [Citation31]. Other recent studies, however, have reported that aged C57BL/6 mice (16–30 months of age) are more resistant to mouse-adapted A/PR8/8/34 (PR8; H1N1) or A/California/04/09 (2009 pandemic H1N1) infection than young adult mice (6–9 weeks of age) [Citation18,Citation32]. These studies used a relatively low volume of IAV inoculum (20 μL), a seemingly important experimental distinction as in direct comparison, aged C57BL/6 inoculated with PR8 using this volume showed no age-associated changes in survival, while the same viral dose administered in 40 μL induced increased mortality only in the older mouse cohort [Citation33]. Despite these inconsistencies, such models have provided valuable insight into the pathogenesis of influenza disease in aged organisms and as detailed below, how various facets of the innate immune system response to IAV infection are altered. Here, we review how aging affects the early stages of IAV infection as well as the innate cellular immune response it elicits and examine how these changes may contribute to the increased influenza-associated morbidity and mortality observed in the elderly human population.
Influenza virus and early infection dynamics
Influenza viruses belong to the Orthomyxoviridae family, which is comprised of type A, B and C viruses. IAV cause most influenza epidemics in humans and have the potential to cause severe pandemics due to their ability to readily mutate and as such will be the focus of this review. While type B influenza viruses also circulate in the human population, they mutate at a significantly slower rate than IAV [Citation34] and are generally associated with less severe epidemics. Type C influenza viruses induce mild disease in man and have not been associated with either epidemics or pandemics.
IAV is an enveloped virus consisting of a segmented negative-sense ribonucleic acid (RNA) genome surrounded by a lipid bilayer derived from the host cell plasma membrane. The single-stranded viral genome is organized into eight segments, each of which forms a distinct ribonucleoprotein (RNP) complex within the virion. The RNA segments encode for 11 viral proteins, 9 of which have been identified within the virion and 2 additional proteins, PB1-F2 and non-structural (NS1), that are expressed in infected cells [Citation35]. The hemagglutinin (HA) and the neuraminidase (NA) glycoproteins are embedded in the viral envelope and form the characteristic surface spikes of the virus that are visible by electron microscopy [Citation36]. IAVs are subtyped according to the serological reactivities of their HA and NA surface glycoproteins. To date, 18 avian and mammalian HA and 11 NA subtypes have been identified and while some avian subtypes (e.g. H5N1, H7N1, H7N3, H7N7 and H9N2) have sporadically infected humans, only 3 HA subtypes (H1, H2 and H3) and 2 NA subtypes (N1 and N2) are known to have circulated in the human population [Citation37].
During the early stages of IAV infection, pathogen-associated molecular patterns are detected by pattern recognition receptors (PRR), including retinoic acid inducible gene-I (RIG-I)-like receptors and toll-like receptors (TLR), leading to the induction of proinflammatory signaling pathways. For example, TLR3 and TLR7 in the endosomal compartment and RIG-I in the cytosol recognize viral single-stranded RNA (ssRNA) or the ‘panhandle” structure of the (sub)genomic RNA/double-stranded RNA (dsRNA), respectively [Citation10,Citation38], leading to the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which mediates the production of proinflammatory cytokines and chemokines, such as interleukin (IL)-6, tumor necrosis factor (TNF)α, C-C motif chemokine ligand (CCL)2, pro-IL-18, and pro-IL-1β. Additionally, interferon regulatory factor (IRF)3 and IRF7 activation results in production of type I (IFNα and IFNβ) and III (IFNλ) IFNs, which play a major role by inducing the expression of hundreds of genes encoding proteins involved in limiting IAV infection and replication, as well as influencing cell migration, proliferation, differentiation and survival [Citation39,Citation40]. Activation of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3), a cytosolic PRR, during IAV infection facilitates inflammasome complex formation and enzymatic maturation of inactive precursors pro-IL-1β and pro-IL-18 into bioactive IL-1β and IL-18 [Citation10,Citation38]. Several changes to these signaling pathways have been associated with aging, which have recently been reviewed elsewhere [Citation41] and therefore will not be the focus of this review. Rather, notable age-associated differences in the early cellular response to IAV infection and these signaling events, particularly changes in innate immune cell abundance, phenotype and function will be covered here.
Epithelial cells lining the respiratory tract and associated respiratory macrophages are the initial targets of IAV infection. Respiratory epithelial cells serve as the primary source of IAV replication, amplification and release of new virions [Citation42–44], which largely results in cell death [Citation10]. Infection is initiated via the IAV hemagglutinin (HA) protein binding to sialic acid on the cell surface of epithelial cells, which triggers endocytosis of the virion. Following fusion of viral and cellular membranes, viral RNP are released into the cytoplasm and enter the nucleus [Citation45]. Once inside the nucleus, viral RNP undergo transcription and replication and progeny viral RNP are then exported to the cytoplasm. Viral proteins are translated, assembled and transported to the plasma membrane prior to the budding and the release of newly synthesized virions from the surface of infected cells. By contrast, entry into respiratory macrophages typically leads to destruction of the virus and apoptosis of the infected cell [Citation42–44]. The early inflammatory response to IAV infection is largely determined by the spectrum of chemokines and cytokines released from respiratory epithelial cells and macrophages. IAV infection of primary respiratory epithelial cells and cell lines from various species results in the production of proinflammatory cytokines IL-6 and TNFα, type I IFNs and leukocyte-attracting chemokines CCL2, CCL5 and C-X-C chemokine ligand (CXCL)8 [Citation46–53]. Macrophages/monocytes infected with IAV are also potent producers of proinflammatory cytokines IL-6 and TNFα, type I IFNs and chemokines CCL2, CXCL8 and CXCL10 [Citation54–57]. The production of inflammatory cytokines following IAV infection is critical for the initial control of virus replication; however, aberrant cytokine responses can be detrimental to the host, with hyperinflammatory “cytokine storms” often associated with severe morbidity and mortality in infected humans [Citation58–61]. The early cytokine and chemokine production elicited by IAV-infected respiratory epithelial cells and macrophages contributes to the activation, rapid recruitment and local expansion of several innate immune cells that have demonstrated roles in defense against, and recovery from, influenza disease. This includes the infiltration of neutrophils into the lung within 24 hours post-infection followed closely by the accumulation of natural killer (NK) cells, inflammatory macrophages and dendritic cells (DC) in the airways [Citation62,Citation63] and subsequently, the activation of local type 2 innate lymphoid (ILC2) cells, that can assist lung tissue repair [Citation64–66].
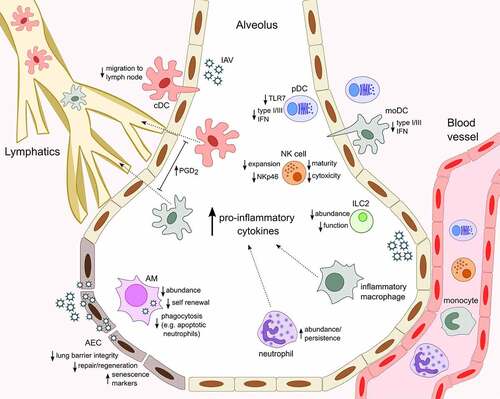
The production of high levels of cytokines during severe IAV infection is also thought to contribute to epithelial damage, leading to vascular leakage and pulmonary edema. This loss of epithelium integrity may increase the host’s susceptibility to secondary bacterial infection, which is a major complication associated with IAV infection and can be fatal, particularly in the elderly, whom may already have had reduced lung epithelial barrier function [Citation5,Citation6,Citation67]. In the lung, Club (or Clara) cells are non-ciliated, non-mucous, secretory cells, which predominate in the bronchioles, whereas two forms of epithelial cells exist within the alveoli, namely alveolar epithelial cell (AEC) type I and type II. Club cells exhibit progenitor cell properties and are thought to mediate maintenance and repair of both the bronchiolar and alveolar epithelium following IAV infection [Citation68,Citation69]. Epithelial repair is a critical process for recovery from IAV infection and aging has been shown to be associated with a functional decline in stem and progenitor cells [Citation70], potentially compromising tissue maintenance and repair. A study by Yin and colleagues suggested that there was no difference in the extent of damage and kinetics of regeneration by club cells between young (2–3 months) and aged (16–18 months) C57BL/6 mice following PR8 infection [Citation69]. However, increased damage to type I and type II AEC and a delay in the regeneration of type II cells was found in aged mice. Additionally, AEC from 18–22-month-old C57BL/6 mice have been shown to produce higher levels of neutrophil-attracting chemokines CXCL1 and CXCL2 following PR8 infection, while no differences were noted in IL-1β and TNFα production () [Citation30]. The authors proposed that this enhanced chemokine production was associated with an increased presence of senescent AEC in aged mice both before and after infection.
Figure 1. Age-associated changes in innate immune cell response to influenza infection.The heightened baseline inflammatory environment that corresponds with aging (inflammaging) influences innate immune cell responses, typically manifesting as altered activity and abundance within the infected lung and resulting in less efficient resolution and recovery from IAV infection in older organisms. With increased age, alveolar epithelial cells (AEC) appear more senescent, regenerate slower and secrete increased levels neutrophil-attracting chemokines upon IAV infection. AM are less numerous, poorly phagocytic and produce higher levels of CXCL8, favoring further recruitment and persistence of neutrophils in the airways. Similarly, elevated levels of CCL2 in the infected lungs facilitate increased entry of monocyte-derived Ly6Chi inflammatory macrophages from the circulation. Respiratory conventional and monocyte-derived dendritic cells (cDC and moDC) secrete cytokines in the lung and traffic antigen to draining lymph nodes via the lymphatics. Elevated levels of prostaglandin D2 (PGD2) in aged mice contributes to impaired cDC migration to the lymph node and T cell responses and moDC have an age-associated defect in type I and III IFN production. pDC display a similar defect in IFN production, which correlates with reduced expression of TLR7, a sensor of viral ssRNA. Aging is linked to reduced NK cell terminal differentiation, expansion, cytotoxicity and NKp46-mediated recognition of IAV-infected cells and fewer type 2 innate lymphoid cells (ILC2) populate the lungs in aged mice and are functionally impaired. In the context of IAV infection, ILC2 respond to the damage-associated molecular pattern IL-33 and can support lung tissue integrity, remodeling and repair
Macrophages
Lung-resident interstitial macrophages (IM) and alveolar macrophages (AM) are phagocytic cells that reside in the airways and respond rapidly to invading microbes, including viruses. Located in the alveoli, AM have direct contact with our environment and area critical first line of defense against inhaled pathogens. Due to their location, AM can be collected relatively easily in bronchoalveolar lavage (BAL) fluid from humans and animals for analysis [Citation42,Citation43] and are thought to have a regulatory phenotype, working to limit excessive inflammation in the airways [Citation71,Citation72]. Both IM and AM express CD169 in humans and mice, are capable of local self-renewal from progenitor populations or after IAV infection, can be replenished from circulating monocytes [Citation73–76], yet IM exist in the interstitium along with respiratory DC and are much less abundant than AM [Citation77,Citation78]. IM can be distinguished from AM by their lack of CD11c expression [Citation79] and are generally less phagocytic but more effective at stimulating T cell proliferation [Citation77,Citation80].
As sentinels of the airways, AM are a primary target of influenza infection and are susceptible to infection by most IAV [Citation81], with the highly virulent mouse-adapted PR8 strain being a notable exception [Citation44,Citation82,Citation83]. Typically, infection of AM results in early virus-specific protein synthesis [Citation84] but this is interrupted within a few hours and no infectious viral progeny are released prior to the infected AM undergoing apoptosis [Citation42–44]. As such, IAV infection of mouse and human AM is generally considered to be abortive, although particular highly pathogenic H1N1 and H5N1 IAV can infect and replicate productively to some degree in murine and human macrophages [Citation85]. As previously mentioned, human and murine macrophages respond to IAV infection via the production of an array of proinflammatory cytokines and chemokines, which act to limit virus spread and recruit inflammatory leukocytes to the lung. As IAV vary in their ability to infect AM [Citation43,Citation44], the cellular tropism of the virus could be a critical factor in determining the airway cytokine milieu during the early phase of infection, which in turn impacts the specific cell types recruited to the lung and the nature of the subsequent immune response. Additionally, the release of the damage-associated molecular pattern (DAMP) IL-33 by infected AM may be critically important for recovery from IAV infection [Citation86,Citation87]. The crucial role of respiratory macrophages in vivo following IAV infection has been illustrated in numerous depletion studies. In particular, treatment of mice with clodronate-loaded liposomes during infection with mild and virulent strains of IAV is associated with enhanced virus replication [Citation44,Citation88–92], increased inflammation, as well as alveolar leakage and pulmonary edema [Citation44]. This method of depletion also eliminates other phagocytic cells in the lung, yet similar observations were made in IAV-infected mice after conditional ablation of CD169+ AM and IM [Citation93].
IAV infection triggers the rapid recruitment of circulating leukocytes to the lung, dominated by neutrophils and monocytes, the latter of which being able to differentiate into macrophages and DC. Monocyte-derived inflammatory Ly6C+ macrophages infiltrate the lung in high numbers several days following IAV infection in response to CCL2 produced either by AEC or previously recruited inflammatory macrophages [Citation47,Citation63,Citation94–96]. This latter source of CCL2, which sustains inflammatory macrophage recruitment to the lung requires intrinsic type I IFN signaling, which also appears to regulate infiltration of other leukocytes [Citation94]. Indeed, mice lacking the type I IFN receptor (Ifnar1-/-), including strains with intact Mx1, more readily succumb to PR8 H1N1 and avian H7N7 IAV infection and possess reduced lung infiltrating inflammatory macrophages, increased numbers of neutrophils and elevated levels of the neutrophil-attracting chemokine CXCL1 in the lung [Citation94,Citation97]. Contradicting observations have been made in IAV-infected mice lacking key mediators of monocyte chemotaxis. In support of Ly6C+ inflammatory macrophages playing a protective role during severe IAV infection, another study demonstrated that PR8-infected mice deficient in CCL2 were found to be more susceptible to influenza disease [Citation98]. In contrast, recruited Ly6C+ inflammatory macrophages have also been shown to contribute to lung damage and mortality during severe IAV infection as demonstrated by several studies using CCL2- or CCR2-deficient mice, which display improved survival rates following infection with PR8 H1N1 or avian H7N9 IAV [Citation99–102].
Aging is generally accepted to result in increased basal levels of inflammatory mediators including cytokines and chemokines. IAV-infected aged mice have been shown to have elevated levels of TNFα, IL-1α, IL-6 and monocyte-attracting CCL2 while similarly, older rhesus macaques (20–24 years of age) infected with 2009 pandemic H1N1 IAV displayed significantly higher levels of IL-6 and CCL2 in their lungs compared with younger macaques (10–12 years of age) [Citation22,Citation26,Citation31,Citation103]. Interestingly, monocytes and macrophages from older individuals (65–89 years of age) produce similar amounts of IL-6 and IL-1β after in vitro infection with PR8 H1N1 compared to those from younger donors (20–30 years of age), yet type I IFN production, as well as the expression of IFN signaling molecules and IFN-stimulated genes were reduced [Citation18]. In mouse studies, Wong and colleagues demonstrated that increased age is associated with reduced numbers of AM in the lungs of 22–24- month-old C57BL/6 and BALB/c mice both prior to and on day 6 following PR8 infection compared to younger (2–4 month-old) counterparts [Citation25] (). Macrophage deletion by clodronate-loaded liposome treatment was shown to decrease survival in both young and aged mice and retention of neutrophils in the lungs, suggesting macrophages play a critical role during IAV infection independent of age [Citation25]. However, using adoptive transfer experiments and measuring lactate dehydrogenase release, the authors demonstrated that aging impairs the intrinsic ability of AM to limit lung damage during PR8 infection. Transcriptional analysis also revealed a downregulation of genes involved in cell cycle pathways such as metaphase checkpoint and chromosome separation, whereas CXCL8 and vascular endothelial growth factor signaling was enhanced with increasing age. Aging AM were also shown to exhibit a defect in phagocytosis of apoptotic neutrophils, as well as the expression of the phagocytic receptor CD204 [Citation25]. Similar in vitro phagocytic defects were observed in AM from aged (26-monthold) C57BL/6 mice [Citation104]. Significant IAV-induced depletion of AM has also been observed in young BALB/c mice after PR8 infection, yet Toapanta and colleagues reported a similar degree of macrophage accumulation in the lungs of 72–76-week-old and 12–16-week-old BALB/c mice until day 15 post-infection, at which point significantly higher numbers of lung macrophages persisted for at least a further 4 days in the older animals [Citation22,Citation105]. Upregulation of the activation marker CD40 on this unspecified lung macrophage population was delayed in these infected aged BALB/c mice, suggestive of an age-associated impact on their function [Citation22]. A recent study found that aged AM from 18–20-month-old BALB/c mice exhibit decreased indoleamine-pyrrole 2,3-dioxygenase (IDO1)-dependent tryptophan metabolism following PR8 infection [Citation26]. This deficiency correlated with increased lung viral load, tissue damage, proinflammatory IL-6 and IL-1β in aged mice, as well as reduced IL-10 and fewer regulatory T cells, that support recovery from IAV infection. Interestingly, in vivo treatment of IAV-infected young mice with an IDO1-inhibitor enhanced viral titers, inflammation and morbidity while treatment of aged mice with mitoquinol, a mitochondrial targeted antioxidant, restored tryptophan metabolism and reduced weight loss and levels of virus in the lung, demonstrating the importance of this pathway [Citation26]. Of note, the fate of murine AM following IAV infection of mice appears to be more dependent on mouse strain rather than virus strain or inoculum dose. Specifically, Califano and colleagues recently reported that PR8 and 2009 pandemic H1N1 infection causes depletion of AM in BALB/c mice in an IFNγ-dependent manner, but only diminished AM phagocytic activity in C57BL/6 mice, a discrepancy that should be considered when examining AM in aged mice in future studies [Citation106].
Finally, multiple studies have shown that AM from recently IAV-infected mice are either depleted, phenotypically altered and/or functionally paralyzed, resulting in reduced phagocytic capacity and increased susceptibility to secondary infection by opportunistic bacteria for weeks following the initial viral infection [Citation6,Citation105,Citation107]. A similarly sustained phagocytic defect was observed in human monocytes from patients with trauma-induced inflammation and those recently cured of sepsis [Citation107]. Therefore, the combination of this stunning effect of IAV infection on AM that may already be diminished in frequency and function together with the compromised lung epithelium associated with increased age, could help explain the increased susceptibility of elderly (>65 years old) IAV-infected individuals to secondary bacterial pneumonia [Citation5,Citation6,Citation67].
Dendritic cells
Dendritic cells (DCs) are a critical bridge between the innate and adaptive arms of the immune system. DC express an array of PRR such as TLR and C-type lectins, which allow detection of invading pathogens and changes in the local environment, and this information is conveyed to nearby lymphoid organs to prime CD4+ and CD8+ effector T cell responses [Citation108]. Presentation of antigen to T cells is the primary role of respiratory CD11c+ conventional (cDC) that migrate to the lung-draining mediastinal lymph node, whereas the principal function of plasmacytoid DC (pDC) is the production of type I IFNs. The main cDC subsets in the uninfected lung are the CD103+ and CD11b+ cDC, sampling environmental antigens proximal to the respiratory epithelial cells [Citation109]. pDC are a minority DC subset in the lung and are found in the large conducting airways and lung interstitium [Citation110]. These cells express high levels of endosomal TLR7, which senses virals sRNA and upon TLR7 stimulation, pDC produce vast amounts of antiviral type I IFNs (IFNα and IFNβ), which are important for recruiting leukocytes from the blood and shaping the immune response [Citation111,Citation112]. Following PR8 infection of BALB/c mice, circulating monocytes enter the lung and can differentiate into monocyte-derived DC (moDC), which together with the lung-resident CD103+ DC and CD11b+ DC can then traffic IAV antigens to the draining lymph node where they present viral antigen to naïve CD4+ and CD8+ T lymphocytes to facilitate viral clearance [Citation113]. In vivo co-culture experiments have demonstrated that CD4+ T cell expansion on IAV antigen can be driven by all three DC subsets; however, CD103+ DC are the most efficient stimulators of CD8+ T cell responses; a finding supported by other groups [Citation113–116]. Additionally, GeurtsvanKessel and colleagues illustrated that depletion of langerin+ CD11b− respiratory cDC (CD103+ DC) during HKx31 H3N2 IAV infection resulted in increased clinical disease severity and delayed viral clearance, which correlated with a sharp decrease in virus-specific CD8+ T lymphocytes [Citation117]. Following in vitro infection with A/Japan/305/57, murine CD103+ DC readily produce IL-12 and CXCL1, whereas CD11b+ DC secreted CCL5 [Citation118]. During PR8 infection, CD11b+ DC express less CCR7 and do not migrate as efficiently to lymph nodes; however, ex vivo re-stimulation of DC from infected lung with synthetic dsRNA polyinosinic-polycytidylic acid (poly I:C) shows CD11b+ DC to a be potent producers of proinflammatory TNFα [Citation116]. Respiratory cDC are also pivotal producers of antiviral type III IFN (IFNλ), which act directly upon lung epithelial cells, as evidenced by targeted depletion of CD11chigh cells in mice administered poly I:C being sufficient to completely abolish the TLR3-mediated IFNλ response [Citation119,Citation120]. In C57BL/6 mice, type III IFN were previously shown to be beneficial for viral mucosal immunity; however, more recent studies have suggested they can contribute to damage and hinder epithelium repair, which can facilitate bacterial superinfections [Citation120–124].
pDC-derived type I IFN can induce apoptosis of infected cells and activate NK cells and CD8+ T cells during IAV infection [Citation125]. Using BALB/c mice infected with respiratory syncytial virus for comparison, Jewell and colleagues observed that antibody-mediated depletion of pDC only resulted in a significant loss of type I IFN in the lungs of A/WSN/33 H1N1 IAV-infected animals [Citation48]. The authors concluded that during IAV infection, pDC and lung epithelial cells are the main sources of type I IFNs. Similarly, anti-120G8-mediated depletion of pDC in PR8-infected BALB/c mice also resulted in significantly less IFNα in the lung as well as reduced weight loss and lung viral titers [Citation126]. Interestingly, in the absence of pDC, AM, monocyte-derived macrophages and CD11b+ DC expanded in the lung with a greater proportion of each of these populations producing proinflammatory mediators TNFα and IL-6 [Citation126]. Despite their influence, mice lacking pDC have also exhibited a normal influenza disease course, comparable IFNα production and normal viral clearance kinetics following infection with HKx31 H3N2 or PR8 H1N1 IAV [Citation117,Citation127].
In a healthy aging human population (>65 years old with no comorbidities) the circulating myeloid cDC population remains stable [Citation128] with pDC reported to either decrease in frequency with age [Citation128–131] or to not diminish at all [Citation132,Citation133]. Similarly, the influence of age on pDC TLR expression is disputed, with decreased TLR7 expression reported in elderly individuals [Citation128], and comparable expression observed by others [Citation132]. Interestingly, two studies examining moDC from aged individuals (65–88 and 64–92 years old) observed a similar phenotype compared to those derived from younger donors (20–35 and 21–40 years old); however, moDC from older donors were less phagocytic, possessed impaired production of type I and III IFNs and either increased or comparable production of IL-6 and TNFα when stimulated with PR8 IAV or viral elements in vitro [Citation133,Citation134]. This age-associated expression defect was linked with epigenetic changes at IFN gene loci, whereas production of IL-6, TNFα, IL-1β, CCL2 and CXCL8 remained unchanged. Levels of IL-1β and IL-18 are decreased in the lungs of HKx31-infected elderly BALB/c mice (15 months of age), which was linked with reduced NLRP3 inflammasome activity in pulmonary DC as well as in bone marrow-derived DC after culture with the virus [Citation23]. Further, adoptive transfer of bone-marrow derived DC from young (2–4 months of age) mice into aged recipients, restored the production of these cytokines following IAV infection and significantly ameliorated IAV-induced morbidity. The elevated basal proinflammatory environment seen in aging individuals, termed inflammaging [Citation135], has been implicated in the defective migration of DC from the lung to draining lymph node during IAV infection. From the age of 6 months, both CD11b+ and CD103+ cDC display decreased ability to migrate to the lymph nodes following PR8 infection of C57BL/6 mice, which inversely correlated with increasing mouse age and susceptibility to infection [Citation24]. Elevated basal levels of prostaglandin D2 (PGD2) in BAL fluid, which can inhibit DC migration, corresponded with the increasing age of C57BL/6 mice and were maintained after IAV infection (). PGD2 antagonism restored DC migration in PR8-infected aged mice (22-month-old) and correlated both with upregulation of CCR7 on cDC and a significantly enhanced influenza-specific T cell response [Citation24]. Age-related defects in DC migration to the lymph node has also been attributed to cell-intrinsic factors such as reduced phosphoinositide 3-kinases (PI3K) signaling, a positive regulator of DC migration and phagocytosis [Citation133,Citation136].
Most studies agree that type I IFN production is impaired in pDC in older individuals, whereas the production of other inflammatory cytokines and chemokines such as IL-6, IL-10, TNFα and CXCL10 are maintained [Citation128,Citation131,Citation132]. In vitro stimulation of young (20–35 years old) compared to aged (65–90 years old) human pDC with IAV led to decreased production of type I IFN in the aged population [Citation128,Citation132]. Comparable to observations made with moDC, Sridharan and colleagues found that elderly human (65–89 years old) pDC isolated from the blood were found to have decreased production of both type I and III IFNs after in vitro PR8 IAV stimulation compared to pDC from younger individuals (20–35 years old), yet production of other cytokines such as TNFα and IL-6 was unaffected [Citation132]. The same study reported that pDC TLR expression was maintained with age, which corresponds with observations that IAV recognition and uptake is not impeded in aged pDC [Citation131,Citation132]. Instead, the authors demonstrate that this defect was likely the result of a decreased ability to phosphorylate IRF7, resulting in aged pDC being less potent stimulators of effector T cell responses following exposure to IAV [Citation132]. Overall, aging appears to result in several respiratory DC impairments, which can include decreased abundance, activity and migration, collectively leading to poorer viral clearance and influenza disease outcomes.
Neutrophils
Neutrophils are the most abundant type of granulocyte and are the chief lung infiltrators following IAV infection, initially attracted and activated by several chemokines and cytokines, particularly CXCR2 ligands, produced by lung-resident cells [Citation137]. In response to IAV infection, lung-infiltrating neutrophils have been shown to destroy infected cells by phagocytosis and to produce reactive oxygen species (ROS), lytic granules and DNA-rich neutrophil extracellular traps (NETs), as well as generate inflammation and promote further leukocyte recruitment, including CD8+ T cells and additional neutrophils [Citation92,Citation137–140]. In the context of influenza, neutrophils may be described as a “double-edged sword”, crucial for early protection and control of infectious pathogens, yet also capable of deleterious immunopathology. Depletion of neutrophils in mice prior to infection with a range of H1N1, H3N2 and H5N1 IAV of varying virulence results in worsened weight loss, survival and ARDS-like disease as well as increased viral titers and extrapulmonary viral spread [Citation30,Citation62,Citation89,Citation92,Citation95,Citation139,Citation141,Citation142]. From studies in mice, circulating neutrophils are known to enter the lungs by CXCR2-dependent chemoattraction in two waves following IAV infection with the initial arrivals promoting further neutrophil recruitment [Citation22,Citation95,Citation143]. In contrast to the increased morbidity and mortality exhibited by mice depleted of neutrophils, the complete ablation of neutrophil trafficking to the lung observed following PR8 infection of CXCR2-deficient BALB/c mice had no significant effect on IAV clearance, inflammation, lung tissue damage or survival rate [Citation143]. However, CXCR2 confers responsiveness to a series of chemokines and its expression is not unique to neutrophils, which may account for this contradictory observation [Citation144].
Neutrophils retain the greatest cytotoxic potential of the several leukocyte populations that rapidly infiltrate the lung following IAV infection; however, excessive infiltration and dysregulated activation of neutrophils is closely linked to acute lung injury and lethal disease in infected individuals and mouse models, particularly in response to infection by lethal doses of highly virulent 1918 pandemic and PR8 H1N1, as well as H5N1 IAV [Citation92,Citation95,Citation145]. PR8 IAV-infected mice lacking type I or III IFN signaling more readily succumb to infection and this correlates with increased CXCL1 production and neutrophil accumulation in the lung compared to wildtype C57BL/6 mice [Citation94,Citation121,Citation122,Citation124]. Patients with severe H1N1, H3N2 and H7N9 influenza disease have increased circulating neutrophils and higher levels of IL-6 and CXCL8 in their serum [Citation146,Citation147]. Both human blood and mouse whole lung transcriptomic analysis of IAV-infected individuals and mice, respectively, strongly linked neutrophil activation with severe disease and lethality [Citation95,Citation146,Citation148]. From their transcriptomic analysis, Tang and colleagues determined that high expression of the neutrophil-specific marker CD177 was highly predictive of patient mortality [Citation146]. NETs are an important antimicrobial defense mechanism composed of DNA and various proteins including myeloperoxidase (MPO) and NET release by neutrophils requires MPO activity and ROS generation [Citation138,Citation149,Citation150]. Excessive production of NETs has been implicated in lung injury during PR8 infection of C57BL/6 and BALB/c mice and increased NET-associated gene expression and MPO-DNA complexes have been observed in the patients with severe influenza [Citation92,Citation146–148,Citation151]. Neutrophils taken from the lungs of IAV-infected mice released NETs in response to IAV-infected LA-4 cells, a mouse respiratory epithelial cell line [Citation92] and similarly, NET-containing supernatant from neutrophil cultures of patients with severe H7N9 IAV infection disrupted lipopolysaccharide-primed epithelial cell monolayers in vitro, which was mitigated by the addition of DNase [Citation147]. Intriguingly, measured antibody-mediated reduction, but not complete depletion, of neutrophils during, or depletion subsequent to IAV infection was found to either have no profound effect on IAV-induced morbidity and mortality or actually improved IAV infection resistance [Citation30,Citation89,Citation95]. These results suggest that during influenza disease there is a delicate balance of neutrophil activity that could be redressed to limit damaging immunopathology by lung-infiltrating neutrophils, while retaining their contribution to early host defense against the virus.
In general, neutrophils from older individuals exhibit less phagocytic, microbicidal and migration ability [Citation152]; however, how these functional defects affect influenza disease outcomes have not been elucidated. Studies of IAV-infected aged mice have shown significantly higher numbers of granulocytes retained in the lung (), potentially caused by impaired clearance of the cells by AM, which also correlates with persistent high levels of IL-6, IL-1β and MPO [Citation22,Citation25,Citation26,Citation142]. The impact of age on neutrophil responses to IAV infection has more recently been thoroughly investigated in aged mice. Kulkarni and colleagues observed neutrophils were markedly more abundant in the lung and blood of older C57BL/6 mice (18–22 months of age) 6 days following PR8 infection [Citation30]. Targeted depletion of neutrophils prior to or at this point post-infection resulted in worse or improved survival during IAV infection, respectively and independent of age, suggesting that the differing roles of neutrophils at different stages of IAV infection is maintained. Although viral titers remained unchanged, proinflammatory cytokines and indicators of lung damage were reduced when neutrophil depletion was delayed. Aged mice had significantly higher levels of TNFα, IL-6, the crucial neutrophil-attracting chemokines CXCL1 and CXCL2, as well as IL-17, which reinforces the production of these chemokines by AEC [Citation30]. Aged macaques infected with H1N1 IAV had increased CXCL8 and IL-6 in the lung which, although not assessed in these studies, could facilitate greater neutrophil accumulation at the site of infection and potentially increase NET release [Citation31]. Whether excessive NET release in the lung significantly contributes to the increased influenza susceptibility observed in elderly individuals is still unclear; however, human neutrophils appear to have an age-associated impairment in NET production after in vitro stimulation with CXCL8 and in vivo deoxyribonuclease (DNase) treatment to breakdown NETs does not improve IAV-induced lethality in aged mice [Citation30,Citation153].
Natural killer cells
NK cells are bone-marrow-derived, granular lymphocytes that account for approximately 10% of circulating lymphocytes in humans. NK cells respond rapidly to viral infection by the targeted killing of infected cells via secretion of cytotoxic granules and providing an important early source of antiviral cytokines such as IFNγ, which can also skew the ensuing adaptive T cell response [Citation154,Citation155]. In mice, surface expression of killer cell lectin-like receptor subfamily G member 1 (KLRG1), CD11b and CD27 on NK cells define different maturity states and indicate distinct functionality, with terminally differentiated, highly cytolytic NK cells identified as CD11b+ CD27− KLRG1+ [Citation156–159]. The heterogenous human NK cell population can be divided similarly; however, they are commonly categorized CD56bright NK cells, which are proficient at cytokine production and the more mature, cytotoxic CD56dim NK cells, which predominate in the blood and lung [Citation156,Citation160–162]. Finally, the lung also houses a small population of NK cells that express markers of tissue residence, including CD69, CD103 and CD49a [Citation162,Citation163].
NK cells proliferate and accumulate in the lung during the first days of IAV infection where they kill IAV-infected cells, produce antiviral type II IFN (IFNγ) and promote regeneration of lung epithelium via secretion of IL-22 [Citation29,Citation164,Citation165]. This includes lung-resident CD49a+ NK cells, which can rapidly respond to IAV infection of human lung explants [Citation163]. Human and murine NK cells are susceptible to A/Hong Kong/54/98 H1N1 and PR8 H1N1 IAV infection, respectively, which leads to functional impairment of the cells and induces programmed cell death, which thwarts viral dissemination [Citation166,Citation167]. Type I and II IFN signaling is crucial for NK cell activity following PR8 infection as NK cells in Ifnar1-/- mice failed to produce IFNγ or degranulate. IFNγ administration promptly after PR8 infection of mice increased lung NK cell numbers, cytotoxicity and improved resistance to infection, which was lost when NK cells were depleted [Citation168,Citation169]. The latter observation is congruent with several additional studies using antibody-mediated NK cell depletion with either anti-NK1.1 or anti-asialo-GM1, suggesting a critical role for NK cells in early defense against IAV infection [Citation15,Citation29,Citation170–172] and corresponds with consistent observations of NK lymphopenia in severe 2009 H1N1 pandemic infections in humans [Citation173,Citation174]. Conversely, others have shown that NK cells can contribute to influenza-associated pathology and decreased survival using similar in vivo NK cell depletion methods but seemingly only during lethal PR8 infection [Citation175,Citation176]. Care must be taken when interpreting these results, however, as the common depleting antibody targets NK1.1 and asialo-GM1 are expressed on other cells such as pulmonary macrophages, NKT cells, CD8+ T cells [Citation177–179], including activated influenza-specific CD8+ T cells in the lung [Citation180]. Interestingly, others have recently reported improved survival rates and reduced immunopathology in IFNγ-deficient mice infected with 2009 pandemic H1N1 IAV [Citation106,Citation181]. Importantly, NK cells can eliminate infected cells by recognizing the IAV HA via the activation receptor NKp46 and mice lacking this receptor are more susceptible to IAV infection [Citation182–184]. The same receptor is necessary for NK-cell-mediated apoptosis of neutrophils, which may be important for the resolution of inflammation within the lung following IAV infection [Citation185].
Several age-associated changes to NK cells have been observed in both mice and humans (). At the steady state aged (22-month-old) C57BL/6 mice have reduced frequency and absolute numbers of lung NK cells [Citation186]. Furthermore, NK cells in the lung and spleen of aged mice expressed fewer markers of maturity, potentially resulting from impaired NK cell production in the bone marrow [Citation186,Citation187]. In humans, aging is associated with a profound reduction in the ratio of circulating CD56bright to CD56dim NK cells, an increase in the frequency of NK cells bearing the maturation marker CD57, either comparable or reduced NK cell activity, including decreased IFNγ and CXCL8 secretion, a slower rate of homeostatic NK cell generation and finally, fewer NK cells expressing NKp46, a key receptor in defense against IAV spread and disease resolution [Citation183,Citation185,Citation188–191]. Reduced NK cell abundance and maturation in older mice does not appear to be due to intrinsic defects but instead is a consequence of the aged environment, as donor bone marrow or splenic NK cells from both young (4–6 months) or aged (20–22 months) mice displayed improved development, proliferation and upregulation of KLRG1 when transferred into young recipients but not older ones [Citation192]. The majority of evidence of age-associated changes in NK cell responses to influenza comes from aged mouse models of infection. Although no significant differences in NK cell activity was evident in young (6–8 weeks) and aged (22 months) C57BL/6 mice at the steady state, Nogusa and colleagues observed that following PR8 infection, NK cells failed to expand in either the lung or spleen of aged mice, with the former also displaying reduced cytotoxicity [Citation29]. Interestingly, a later study from the same group observed fewer NK cells in the lungs and spleen of naïve and PR8 IAV-infected young (6 months) and aged (22 months) C57BL/6 mice, which were also less cytotoxic, less responsive to NKp46 stimulation and were comprised of more immature CD11b− and less terminally-differentiated CD11b+ CD27− NK cells [Citation193]. Age-associated NK cell impairment has also been linked to diet, with mortality and morbidity of aged C57BL/6 mice (>23 months of age) infected with PR8 found to significantly worsen when fed a caloric restricted diet, which was also associated increased viral titers and further decreased lung NK cell cytotoxicity compared to aged control mice [Citation28].
Type 2 innate lymphoid cells (ILC2)
ILCs are a heterogeneous collection of lymphocytes, including NK cells, that are classified into several subpopulations based on their cytokine expression repertoire, akin to CD4+ effector T cells. While NK cells are grouped together with IFNγ-producing type 1 ILC (ILC1), ILC2 are the predominant ILC subset resident in the mouse lung and are characterized by their ability to secrete type 2 cytokines such as IL-4, IL-5, and IL-13 in response to DAMPs such as IL-25 and IL-33 [Citation194–198]. Although ILC1, ILC2 and the IL-17-producing ILC3, can all be found within the lung [Citation199], the majority of research in the context of IAV infection has concentrated on ILC2 [Citation200,Citation201]. In mice and humans, the phenotype of lung ILC2 can vary, yet they are typically identified by a lack of common immune cell lineage markers and their expression of a combination of CD25, CD127, CD90, KLRG1 and ST2, the receptor for IL-3364, [Citation202,Citation203]. IL-33 is produced by pulmonary epithelial and endothelial cells, AM and natural killer T (NKT) cells following infection with A/WSN/1933 H1N1, as well as A/Memphis/71 H3N2 IAV and in response, ILC2 proliferate and can exacerbate airway hyperreactivity in an IL-13-dependent manner in the absence of T cells [Citation86,Citation87,Citation204].
Alternatively, ILC2 can also play a beneficial role in the context of IAV infection. Following infection of C57BL/6 mice with HKx31 H3N2, PR8 or 2009 pandemic H1N1, ILC2 expand, peaking around day 4 post-infection and persist in the lung until the later stages of infection to support lung function, tissue integrity and tissue remodeling, the latter being critical for lung repair and tissue homeostasis [Citation64–66]. Adoptive transfer of ILC2 improved influenza-associated morbidity and required IL-33/IL-33 R signaling and the growth factor, amphiregulin [Citation64]. Using IFNγ-deficient mice and anti-IFNγ neutralization, Califano and colleagues demonstrated that ILC2 promote increased resistance to 2009 pandemic H1N1 via the production of amphiregulin and IL-5 activity, which is ordinarily under IFNγ-mediated suppression [Citation65]. These findings were congruent with previous observations that ILC2 responses, including their production of IL-5 is negatively regulated by type I IFNs and IL-27 in a signal transducer and activator of transcription 1 (STAT1)-dependent manner and that IL-33-induced airway hyperreactivity was ameliorated by the IFN-γ supplementation [Citation205,Citation206]. Fewer mature ILC2 populate the lungs of aged mice (19–24 months of age) and have diminished functionality compared to younger counterparts (2–3 months of age) (), which may be partially due to changes in the lung microenvironment, as indicated by elevated levels of the proinflammatory cytokines IL-18 and IL-12 [Citation27]. This numerical disparity was sustained following 2009 pandemic H1N1 infection, which resulted in greater lung inflammation and complete lethality of the older mice [Intriguingly, only adoptive transfer of lung-resident ILC2 from IL-33-treated young mice into aged recipients, lessened infection-induced weight loss and lung inflammation and significantly improved the survival rate of older mice.
Conclusion
Aging influences the cells of the innate immune system in multiple different ways and to varying degrees. Continued examination of the intrinsic and extrinsic age-related factors that induce numerical and functional changes in innate immune cells will be crucial to inform the design of new vaccines, adjuvants, supplements and drugs to improve outcomes for the elderly, who account for the majority of influenza-associated hospitalizations and deaths. As IAV infection susceptibility is often disconnected from viral titer, this could be facilitated by exploiting elements of the immune system to bolster influenza disease tolerance and recovery in the elderly, rather than direct targeting of the virus with antiviral drugs that show limited efficacy. For example, adjusting the inflammatory environment within the IAV infected lung, which may already be dysregulated in an elderly patient, could reduce potential immunopathology, the chance of secondary bacterial infections and expedite disease resolution. In addition to the innate immune cells described above, there are multiple other immune cells with emerging roles in IAV infection that display some degree of immunosenescence or age-associated alteration that could be manipulated. For example, unconventional T cells that encompass NKT cells, gamma delta T cells and mucosal-associated invariant T cells, share features of innate and adaptive immunity, being able to respond to both antigen-specific and cytokine stimuli [Citation207]. Although the cognate antigens for these cells are not necessarily produced during IAV infections, unconventional T cells can rapidly respond to induced proinflammatory cytokines such as IL-18 and IL-12, which can be elevated with aging, and release a wide array of cytokines that can influence the succeeding adaptive immune response and therefore the course of the disease [Citation27,Citation207–210]. Therefore, unconventional T cells as well as the innate immune cells highlighted in this review that significantly contribute to IAV immunity and recovery from IAV infection, warrant further investigation in this setting.
Currently, most of our knowledge on the effects of aging on immunity to IAV stems from mouse models, which have several notable caveats highlighted throughout this review, that need to be considered when attempting to translate any findings to the clinic. The inbred laboratory mice used in most of the studies reviewed here lack functional Mx1, an IFN I and III–inducible antiviral protein that limits IAV replication. Fully IFN-competent Mx1-sufficient mice may serve as a more faithful model of the early antiviral response to IAV infection and depending on the genetic background, can exhibit greater resistance to IAV infection. As Mx1 gene expression diminishes with age, future studies on the influence of aging on IAV pathogenesis, vaccination or candidate therapeutics would ideally be conducted with aged Mx1-sufficient mice and potentially newly developed humanized mice engrafted with human immune systems, prior to extension to higher order animals and humans [Citation18]. Another potential consideration is the frequent use of the PR8 strain of IAV, which was adapted to mice by >300 sequential passages in mouse lung [Citation211]. PR8 is likely to have acquired mutations that facilitate evasion of host responses and as such PR8 poorly infects murine respiratory macrophages, the sentinels of the respiratory tract and is highly virulent [Citation43,Citation44]. Moreover, the fate of murine AM following IAV infection is seemingly dependent on mouse genetic background, therefore experimental results using PR8 may not accurately reflect human influenza pathogenesis [Citation106]. Lastly, use of less virulent IAV such as HKx31 H3N2 at varying inoculum doses to model mild and severe disease may be more informative in aged animal models of IAV infection, particularly as elderly IAV-associated mortality is highest during seasonal epidemics dominated by the H3N2 subtype [Citation1].
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Paget J, Spreeuwenberg P, Charu V, et al. Global mortality associated with seasonal influenza epidemics: new burden estimates and predictors from the GLaMOR project. J Glob Health. 2019;9(2):020421.
- Clohisey S, Baillie JK. Host susceptibility to severe influenza A virus infection. Crit Care. 2019;23(1):303.
- Sellers SA, Hagan RS, Hayden FG, et al. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372–393.
- Short KR, Kroeze E, Fouchier RAM, et al. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. 2014;14(1):57–69.
- van der Sluijs KF, Van Der Poll T, Lutter R, et al. Bench-to-bedside review: bacterial pneumonia with influenza - pathogenesis and clinical implications. Crit Care. 2010;14(2):219.
- Morris DE, Cleary DW, Clarke SC, et al. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041.
- United Nations Department of Economic and Social Affairs. World population ageing 2019: highlights (ST/ESA/SER.A/430). 2019. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
- Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54(10):1427–1436.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186.
- Laghlali G, Lawlor KE, Tate MD, et al. Die another way: interplay between influenza A virus, inflammation and cell death. Viruses. 2020;12(4):401.
- Newton AH, Cardani A, Braciale TJ, et al. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38(4):471–482.
- Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–328.
- Brandenberger C, Muhlfeld C. Mechanisms of lung aging. Cell Tissue Res. 2017;367(3):469–480.
- Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes?. Front Immunol. 2017;8:1960.
- Zhou K, Wang J, Li A, et al. Swift and strong NK cell responses protect 129 mice against high-dose influenza virus infection. J Immunol. 2016;196(4):1842–1854.
- Srivastava B, Blazejewska P, Hessmann M, et al. Host genetic background strongly influences the response to influenza a virus infections. PLoS One. 2009;4(3):e4857.
- Staeheli P, Grob R, Meier E, et al. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol Cell Biol. 1988;8(10):4518–4523.
- Pillai PS, Molony RD, Martinod K, et al. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science. 2016;352(6284):463–466.
- Tumpey TM, Szretter KJ, Van Hoeven N, et al. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J Virol. 2007;81(19):10818–10821.
- Shin DL, Hatesuer B, Bergmann S, et al. Protection from severe influenza virus infections in mice carrying the Mx1 influenza virus resistance gene strongly depends on genetic background. J Virol. 2015;89(19):9998–10009.
- Po JL, Gardner EM, Anaraki F, et al. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123(8):1167–1181.
- Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10(1):112.
- Stout-Delgado HW, Vaughan SE, Shirali AC, et al. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol. 2012;188(6):2815–2824.
- Zhao J, Zhao J, Legge K, et al. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121(12):4921–4930.
- Wong CK, Smith CA, Sakamoto K, et al. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J Immunol. 2017;199(3):1060–1068.
- Cho SJ, Hong KS, Schenck E, et al. Decreased IDO1 dependent tryptophan metabolism in aged lung during influenza. Eur Respir J. 2020;57(5):2000443.
- D’Souza SS, Shen X, Fung ITH, et al. Compartmentalized effects of aging on group 2 innate lymphoid cell development and function. Aging Cell. 2019;18(6):e13019.
- Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60(6):688–694.
- Nogusa S, Ritz BW, Kassim SH, et al. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev. 2008;129(4):223–230.
- Kulkarni U, Zemans RL, Smith CA, et al. Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol. 2019;12(2):545–554.
- Josset L, Engelmann F, Haberthur K, et al. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. J Virol. 2012;86(20):11115–11127.
- Lu J, Duan X, Zhao W, et al. Aged mice are more resistant to influenza virus infection due to reduced inflammation and lung pathology. Aging Dis. 2018;9(3):358–373.
- Smith CA, Kulkarni U, Chen J, et al. Influenza virus inoculum volume is critical to elucidate age-dependent mortality in mice. Aging Cell. 2019;18(2):e12893.
- Nobusawa E, Sato K. Comparison of the mutation rates of human influenza A and B viruses. J Virol. 2006;80(7):3675–3678.
- Samji T. Influenza A: understanding the viral life cycle. Yale J Biol Med. 2009;82(4):153–159.
- Compans RW, Klenk HD, Caliguiri LA, et al. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970;42(4):880–889.
- Belser JA, Bridges CB, Katz JM, et al. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg Infect Dis. 2009;15(6):859–865.
- Ong JD, Mansell A, Tate MD, et al. Hero turned villain: NLRP3 inflammasome-induced inflammation during influenza A virus infection. J Leukoc Biol. 2017;101(4):863–874.
- Odendall C, Kagan JC. The unique regulation and functions of type III interferons in antiviral immunity. Curr Opin Virol. 2015;12:47–52.
- Schoggins JW. Interferon-stimulated genes: what do they all do?. Annu Rev Virol. 2019;6(1):567–584.
- Molony RD, Malawista A, Montgomery RR, et al. Reduced dynamic range of antiviral innate immune responses in aging. Exp Gerontol. 2018;107:130–135.
- Thomas BJ, Porritt RA, Hertzog PJ, et al. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep. 2014;4(1):7176.
- Tate MD, Schilter HC, Brooks AG, et al. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011;24(2):77–88.
- Tate MD, Pickett DL, van Rooijen N, et al. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84(15):7569–7580.
- Rossman JS, Lamb RA. Influenza virus assembly and budding. Virology. 2011;411(2):229–236.
- Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002;76(3):1071–1076.
- Herold S, von Wulffen W, Steinmueller M, et al. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol. 2006;177(3):1817–1824.
- Jewell NA, Vaghefi N, Mertz SE, et al. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J Virol. 2007;81(18):9790–9800.
- Matsukura S, Kokubu F, Kubo H, et al. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol. 1998;18(2):255–264.
- Matsukura S, Kokubu F, Noda H, et al. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98(6):1080–1087.
- Choi AM, Jacoby DB. Influenza virus A infection induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett. 1992;309(3):327–329.
- Arndt U, Wennemuth G, Barth P, et al. Release of macrophage migration inhibitory factor and CXCL8/interleukin-8 from lung epithelial cells rendered necrotic by influenza A virus infection. J Virol. 2002;76(18):9298–9306.
- Chan MC, Cheung CY, Chui WH, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6(1):135.
- Wareing MD, Lyon AB, Lu B, et al. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76(4):886–895.
- Sprenger H, Meyer RG, Kaufmann A, et al. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J Exp Med. 1996;184(3):1191–1196.
- Wang J, Nikrad MP, Travanty EA, et al. Innate immune response of human alveolar macrophages during influenza A infection. PLoS One. 2012;7(3):e29879.
- Chan RW, Yuen KM, Yu WC, et al. Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation. PLoS One. 2010;5(1):e8713.
- Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease?. Lancet. 2002;360(9348):1831–1837.
- Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351(9101):467–471.
- To KF, Chan PK, Chan KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63(3):242–246.
- de Jong MD, Hien TT. Avian influenza A (H5N1). J Clin Virol. 2006;35(1):2–13.
- Tate MD, Ioannidis LJ, Croker B, et al. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011;6(3):e17618.
- Bawazeer A, Rosli S, Harpur CM, et al. IL-1β exacerbates disease and is a potential therapeutic target to reduce pulmonary inflammation during severe influenza A virus infection. Immunol Cell Biol. 2021. DOI:10.1111/imcb.12459.
- Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–1054.
- Califano D, Furuya Y, Roberts S, et al. IFN-γ increases susceptibility to influenza A infection through suppression of group II innate lymphoid cells. Mucosal Immunol. 2018;11(1):209–219.
- Li BWS, Mjw DB, Lukkes M, et al. T cells and ILC2s are major effector cells in influenza-induced exacerbation of allergic airway inflammation in mice. Eur J Immunol. 2019;49(1):144–156.
- Muizer K, De Vries M, Timens W, et al. The effect of age on lung epithelial barrier function. Eur Respir J. 2018(52):OA2124.
- Zheng D, Limmon GV, Yin L, et al. A cellular pathway involved in clara cell to alveolar type II cell differentiation after severe lung injury. PLoS One. 2013;8(8):e71028.
- Yin L, Zheng D, Limmon GV, et al. Aging exacerbates damage and delays repair of alveolar epithelia following influenza viral pneumonia. Respir Res. 2014;15(1):116.
- Watson JK, Sanders P, Dunmore R, et al. Distal lung epithelial progenitor cell function declines with age. Sci Rep. 2020;10(1):10490.
- Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol (1985). 1986;60(2):353–369.
- Holt PG. Inhibitory activity of unstimulated alveolar macrophages on T-Lymphocyte blastogenic response 1. Am Rev Respir Dis. 1978;118(4):791–793.
- Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804.
- Tarling JD, Lin HS, Hsu S, et al. Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. J Leukoc Biol. 1987;42(5):443–446.
- Hartnell A, Steel J, Turley H, et al. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97(1):288–296.
- Ducreux J, Crocker PR, Vanbever R, et al. Analysis of sialoadhesin expression on mouse alveolar macrophages. Immunol Lett. 2009;124:77–80.
- Bedoret D, Wallemacq H, Marichal T, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119(12):3723–3738.
- Hoffmann FM, Berger JL, Lingel I, et al. Distribution and interaction of murine pulmonary phagocytes in the naive and allergic lung. Front Immunol. 2018;9:1046.
- Lagranderie M, Nahori MA, Balazuc AM, et al. Dendritic cells recruited to the lung shortly after intranasal delivery of mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology. 2003;108(3):352–364.
- Franke-Ullmann G, Pfortner C, Walter P, et al. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol. 1996;157(7):3097–3104.
- Londrigan SL, Tate MD, Brooks AG, et al. Cell-surface receptors on macrophages and dendritic cells for attachment and entry of influenza virus. J Leukoc Biol. 2012;92(1):97–106.
- Tate MD, Brooks AG, Reading PC, et al. Inhibition of lectin-mediated innate host defences in vivo modulates disease severity during influenza virus infection. Immunol Cell Biol. 2011;89(3):482–491.
- Tate MD, Brooks AG, Reading PC, et al. Specific sites of N-linked glycosylation on the hemagglutinin of H1N1 subtype influenza A virus determine sensitivity to inhibitors of the innate immune system and virulence in mice. J Immunol. 2011;187(4):1884–1894.
- Meischel T, Villalon-Letelier F, Saunders PM, et al. Influenza A virus interactions with macrophages: lessons from epithelial cells. Cell Microbiol. 2020;22(5):e13170.
- Short KR, Brooks AG, Reading PC, et al. The fate of influenza A virus after infection of human macrophages and dendritic cells. J Gen Virol. 2012;93(11):2315–2325.
- Gorski SA, Hahn YS, Braciale TJ, et al. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS Pathog. 2013;9(9):e1003615.
- Chang YJ, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–638.
- McGill J, Van Rooijen N, Legge KL, et al. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205(7):1635–1646.
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–14944.
- Wijburg OL, DiNatale S, Vadolas J, et al. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J Virol. 1997;71(12):9450–9457.
- Murphy EA, Davis JM, McClellan JL, et al. Susceptibility to infection and inflammatory response following influenza virus (H1N1, A/PR/8/34) challenge: role of macrophages. J Interferon Cytokine Res. 2011;31(6):501–508.
- Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179(1):199–210.
- Purnama C, Ng SL, Tetlak P, et al. Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur J Immunol. 2014;44(7):2003–2012.
- Seo SU, Kwon HJ, Ko HJ, et al. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7(2):e1001304.
- Brandes M, Klauschen F, Kuchen S, et al. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154(1):197–212.
- Rosli S, Kirby FJ, Lawlor KE, et al. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br J Pharmacol. 2019;176(19):3834–3844.
- Koerner I, Kochs G, Kalinke U, et al. Protective role of beta interferon in host defense against influenza A virus. J Virol. 2007;81(4):2025–2030.
- Dessing MC, van der Sluijs KF, Florquin S, et al. Monocyte chemoattractant protein 1 contributes to an adequate immune response in influenza pneumonia. Clin Immunol. 2007;125(3):328–336.
- Dawson TC, Beck MA, Kuziel WA, et al. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156(6):1951–1959.
- Lai C, Wang K, Zhao Z, et al. C-C motif chemokine ligand 2 (CCL2) mediates acute lung injury induced by lethal influenza H7N9 virus. Front Microbiol. 2017;8:587.
- Herold S, Steinmueller M, von Wulffen W, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205(13):3065–3077.
- Lin KL, Suzuki Y, Nakano H, et al. CCR2 +Monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180(4):2562–2572.
- Hernandez-Vargas EA, Wilk E, Canini L, et al. Effects of aging on influenza virus infection dynamics. J Virol. 2014;88(8):4123–4131.
- Higashimoto Y, Fukuchi Y, Shimada Y, et al. The effects of aging on the function of alveolar macrophages in mice. Mech Ageing Dev. 1993;69(3):207–217.
- Ghoneim HE, Thomas PG, McCullers JA, et al. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol. 2013;191(3):1250–1259.
- Califano D, Furuya Y, Metzger DW, et al. Effects of influenza on alveolar macrophage viability are dependent on mouse genetic strain. J Immunol. 2018;201(1):134–144.
- Roquilly A, Jacqueline C, Davieau M, et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat Immunol. 2020;21(6):636–648.
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252.
- Sung SS, Fu SM, Rose CE Jr., et al. A major lung CD103 (αE)-β7 integrin-positive epithelial dendritic cell population expressing langerin and tight junction Proteins. J Immunol. 2006;176(4):2161–2172.
- Wikstrom ME, Stumbles PA. Mouse respiratory tract dendritic cell subsets and the immunological fate of inhaled antigens. Immunol Cell Biol. 2007;85(3):182–188.
- Diebold SS, Kaisho T, Hemmi H, et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531.
- Lund JM, Alexopoulou L, Sato A, et al. Recognition of single-stranded RNA viruses by toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101(15):5598–5603.
- Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4(1):e4204.
- Belz GT, Smith CM, Kleinert L, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101(23):8670–8675.
- Waithman J, Zanker D, Xiao K, et al. Resident CD8+ and migratory <sup>CD103+ dendritic cells control CD8 T cell immunity during acute influenza infection. PLoS One. 2013;8(6):e66136.
- Ho AW, Prabhu N, Betts RJ, et al. Lung CD103 +dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J Immunol. 2011;187(11):6011–6021.
- GeurtsvanKessel CH, Willart MA, van Rijt LS, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J Exp Med. 2008;205(7):1621–1634.
- Hao X, Kim TS, Braciale TJ, et al. Differential response of respiratory dendritic cell subsets to influenza virus infection. J Virol. 2008;82(10):4908–4919.
- Sommereyns C, Paul S, Staeheli P, et al. IFN-λ (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4(3):e1000017.
- Broggi A, Ghosh S, Sposito B, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369(6504):706–712.
- Mordstein M, Kochs G, Dumoutier L, et al. Interferon-λ contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4(9):e1000151.
- Mordstein M, Neugebauer E, Ditt V, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84(11):5670–5677.
- Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712–717.
- Galani IE, Triantafyllia V, Eleminiadou EE, et al. Interferon-λ mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity. 2017;46(5):875–90 e6.
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25(3):373–381.
- Soloff AC, Weirback HK, Ross TM, et al. Plasmacytoid dendritic cell depletion leads to an enhanced mononuclear phagocyte response in lungs of mice with lethal influenza virus infection. Comp Immunol Microbiol Infect Dis. 2012;35(4):309–317.
- Wolf AI, Buehler D, Hensley SE, et al. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. J Immunol. 2009;182(2):871–879.
- Jing Y, Shaheen E, Drake RR, et al. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol. 2009;70(10):777–784.
- Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56(5):518–521.
- Perez-Cabezas B, Naranjo-Gomez M, Fernandez MA, et al. Reduced numbers of plasmacytoid dendritic cells in aged blood donors. Exp Gerontol. 2007;42(10):1033–1038.
- Canaday DH, Amponsah NA, Jones L, et al. Influenza-induced production of interferon-α is defective in geriatric individuals. J Clin Immunol. 2010;30(3):373–383.
- Sridharan A, Esposo M, Kaushal K, et al. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age (Dordr). 2011;33(3):363–376.
- Agrawal A, Agrawal S, Cao JN, et al. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178(11):6912–6922.
- Prakash S, Agrawal S, Cao JN, et al. Impaired secretion of interferons by dendritic cells from aged subjects to influenza: role of histone modifications. Age (Dordr). 2013;35(5):1785–1797.
- Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105.
- Aksoy E, Saveanu L, Manoury B, et al. The isoform selective roles of PI3Ks in dendritic cell biology and function. Front Immunol. 2018;9:2574.
- Camp JV, Jonsson CB. A role for neutrophils in viral respiratory disease. Front Immunol. 2017;8:550.
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535.
- Lim K, Hyun YM, Lambert-Emo K, et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science. 2015;349(6252):aaa4352.
- Hashimoto Y, Moki T, Takizawa T, et al. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178(4):2448–2457.
- Tate MD, Deng YM, Jones JE, et al. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183(11):7441–7450.
- Dienz O, Rud JG, Eaton SM, et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5(3):258–266.
- Wareing MD, Shea AL, Inglis CA, et al. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol. 2007;20(3):369–378.
- Stadtmann A, Zarbock A. CXCR2: from bench to bedside. Front Immunol. 2012;3:263.
- Perrone LA, Plowden JK, Garcia-Sastre A, et al. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4(8):e1000115.
- Tang BM, Shojaei M, Teoh S, et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat Commun. 2019;10(1):3422.
- Zhu L, Liu L, Zhang Y, et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza A infection. J Infect Dis. 2018;217(3):428–437.
- Dunning J, Blankley S, Hoang LT, et al. Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza. Nat Immunol. 2018;19(6):625–635.
- Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691.
- Metzler KD, Fuchs TA, Nauseef WM, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117(3):953–959.
- Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189(6):2689–2695.
- Wenisch C, Patruta S, Daxbock F, et al. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67(1):40–45.
- Hazeldine J, Harris P, Chapple IL, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13(4):690–698.
- Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510.
- Martin-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265.
- Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–1524.
- Chiossone L, Chaix J, Fuseri N, et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–5496.
- Kim S, Iizuka K, Kang HS, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3(6):523–528.
- Huntington ND, Tabarias H, Fairfax K, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178(8):4764–4770.
- Romagnani C, Juelke K, Falco M, et al. CD56 bright CD16 − killer Ig-like receptor −NK cells display longer telomeres and acquire features of CD56 dimNK cells upon activation. J Immunol. 2007;178(8):4947–4955.
- Fu B, Wang F, Sun R, et al. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 2011;133(3):350–359.
- Marquardt N, Kekalainen E, Chen P, et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69(-)CD56(dim) cells. J Allergy Clin Immunol. 2017;139(4):1321–30 e4.
- Cooper GE, Ostridge K, Khakoo SI, et al. Human CD49a(+) lung natural killer cell cytotoxicity in response to influenza A virus. Front Immunol. 2018;9:1671.
- Kumar P, Thakar MS, Ouyang W, et al. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013;6(1):69–82.
- Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary natural killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol. 2010;84(15):7750–7759.
- Guo H, Kumar P, Moran TM, et al. The functional impairment of natural killer cells during influenza virus infection. Immunol Cell Biol. 2009;87(8):579–589.
- Mao H, Tu W, Qin G, et al. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J Virol. 2009;83(18):9215–9222.
- Hwang I, Scott JM, Kakarla T, et al. Activation mechanisms of natural killer cells during influenza virus infection. PLoS One. 2012;7(12):e51858.
- Weiss ID, Wald O, Wald H, et al. IFN-γ treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J Interferon Cytokine Res. 2010;30(6):439–449.
- Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136(4):1435–1441.
- GeurtsvanKessel CH, Bergen IM, Muskens F, et al. Both conventional and interferon killer dendritic cells have antigen-presenting capacity during influenza virus infection. PLoS One. 2009;4(9):e7187.
- Kos FJ, Engleman EG. Role of natural killer cells in the generation of influenza virus-specific cytotoxic T cells. Cell Immunol. 1996;173(1):1–6.
- Fox A, Le NM, Horby P, et al. Severe pandemic H1N1 2009 infection is associated with transient NK and T deficiency and aberrant CD8 responses. PLoS One. 2012;7(2):e31535.
- Denney L, Aitken C, Li CK, et al. Reduction of natural killer but not effector CD8 T lymphocytes in three consecutive cases of severe/lethal H1N1/09 influenza A virus infection. PLoS One. 2010;5(5):e10675.
- Abdul-Careem MF, Mian MF, Yue G, et al. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J Infect Dis. 2012;206(2):167–177.
- Zhou G, Juang SW, Kane KP, et al. NK cells exacerbate the pathology of influenza virus infection in mice. Eur J Immunol. 2013;43(4):929–938.
- Yang H, Yogeeswaran G, Bukowski JF, et al. Expression of asialo GM1 and other antigens and glycolipids on natural killer cells and spleen leukocytes in virus-infected mice. Nat Immun Cell Growth Regul. 1985;4(1):21–39.
- Moore ML, Chi MH, Goleniewska K, et al. Differential regulation of GM1 and asialo-GM1 expression by T cells and natural killer (NK) cells in respiratory syncytial virus infection. Viral Immunol. 2008;21(3):327–339.
- Slifka MK, Pagarigan RR, Whitton JL, et al. NK markers are expressed on a high percentage of virus-specific CD8 + and CD4 + T cells. J Immunol. 2000;164(4):2009–2015.
- Kambayashi T, Assarsson E, Michaelsson J, et al. Emergence of CD8 + T cells expressing NK cell receptors in influenza A virus-infected mice. J Immunol. 2000;165(9):4964–4969.
- Liu B, Bao L, Wang L, et al. Anti-IFN-γ therapy alleviates acute lung injury induced by severe influenza A (H1N1) pdm09 infection in mice. J Microbiol Immunol Infect. 2019. DOI:10.1016/j.jmii.2019.07.009.
- Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–1060.
- Gazit R, Gruda R, Elboim M, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7(5):517–523.
- Draghi M, Pashine A, Sanjanwala B, et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178(5):2688–2698.
- Thoren FB, Riise RE, Ousback J, et al. Human NK cells induce neutrophil apoptosis via an NKp46- and fas-dependent mechanism. J Immunol. 2012;188(4):1668–1674.
- Beli E, Duriancik DM, Clinthorne JF, et al. Natural killer cell development and maturation in aged mice. Mech Ageing Dev. 2014;135:33–40.
- Nair S, Fang M, Sigal LJ, et al. The natural killer cell dysfunction of aged mice is due to the bone marrow stroma and is not restored by IL-15/ IL-15Rα treatment. Aging Cell. 2015;14(2):180–190.
- Zhang Y, Wallace DL, De Lara CM, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265.
- Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 2013;12(4):1069–1078.
- Almeida-Oliveira A, Smith-Carvalho M, Porto LC, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72(4):319–329.
- Hazeldine J, Hampson P, Lord JM, et al. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell. 2012;11(5):751–759.
- Chiu BC, Martin BE, Stolberg VR, et al. The host environment is responsible for aging-related functional NK cell deficiency. J Immunol. 2013;191(9):4688–4698.
- Beli E, Clinthorne JF, Duriancik DM, et al. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech Ageing Dev. 2011;132(10):503–510.
- Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463(7280):540–544.
- Mindt BC, Fritz JH, Duerr CU, et al. Group 2 innate lymphoid cells in pulmonary immunity and tissue homeostasis. Front Immunol. 2018;9:840.
- Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370.
- Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107(25):11489–11494.
- Gasteiger G, Fan X, Dikiy S, et al. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350(6263):981–985.
- De Grove KC, Provoost S, Hendriks RW, et al. Dysregulation of type 2 innate lymphoid cells and TH2 cells impairs pollutant-induced allergic airway responses. J Allergy Clin Immunol. 2017;139(1):246–57 e4.
- Ardain A, Porterfield JZ, Kloverpris HN, et al. Type 3 ILCs in lung disease. Front Immunol. 2019;10:92.
- Lai DM, Shu Q, Fan J, et al. The origin and role of innate lymphoid cells in the lung. Mil Med Res. 2016;3:25.
- Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062.
- Entwistle LJ, Gregory LG, Oliver RA, et al. Pulmonary group 2 innate lymphoid cell phenotype is context specific: determining the effect of strain, Location, and stimuli. Front Immunol. 2019;10:3114.
- Le Goffic R, Arshad MI, Rauch M, et al. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol. 2011;45(6):1125–1132.
- Duerr CU, McCarthy CD, Mindt BC, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2016;17(1):65–75.
- Moro K, Kabata H, Tanabe M, et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 2016;17(1):76–86.
- Godfrey DI, Uldrich AP, McCluskey J, et al. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16(11):1114–1123.
- Sabbaghi A, Miri SM, Keshavarz M, et al. Role of gamma delta T cells in controlling viral infections with a focus on influenza virus: implications for designing novel therapeutic approaches. Virol J. 2020;17(1):174.
- van Wilgenburg B, Loh L, Chen Z, et al. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat Commun. 2018;9(1):4706.
- Juno JA, Keynan Y, Fowke KR, et al. Invariant NKT cells: regulation and function during viral infection. PLoS Pathog. 2012;8(8):e1002838.
- Taylor RM. Experimental infection with influenza a virus in mice: the increase in intrapulmonary virus after inoculation and the influence of various factors thereon. J Exp Med. 1941;73(1):43–55.
