ABSTRACT
Rice false smut caused by Ustilaginoidea virens is becoming one of the most devastating diseases in rice production areas in the world. Revealing U. virens potential pathogenic mechanisms provides ideas for formulating more effective prevention and control strategies. Sucrose non-fermenting 1 (Snf1) protein kinase plays a critical role in activating transcription and suppressing gene expression, as well as in cellular response to various stresses, such as nutrient limitation. In our study, we identified the Snf1 homolog UvSnf1 and analyzed its biological functions in U. virens. The expression level of UvSnf1 was dramatically up-regulated during invasion, indicating that UvSnf1 may participate in infection. Phenotypic analyses of UvSnf1 deletion mutants revealed that UvSnf1 is necessary for hyphae growth, spore production, and virulence in U. virens. Moreover, UvSnf1 promotes U. virens to use unfavorable carbon sources when the sucrose is insufficient. In addition, deletion of UvSnf1 down-regulates the expression of the cell wall-degrading enzymes (CWDEs) genes under sucrose limitation conditions in U. virens. Further analyses showed that CWDEs (UvCut1 and UvXyp1) are not only involved in growth, spore production, and virulence but are also required for the utilization of carbon sources. In conclusion, this study demonstrates that UvSnf1 plays vital roles in virulence and carbon source utilization in U. virens, and one of the possible mechanisms is playing a role in regulating the expression of CWDE genes.
Introduction
Ustilaginoidea virens (teleomorph form: Villosiclava virens) is a heterothallic ascomycetous pathogenic fungus, which is a critical agent that incurs false smut disease. In recent years, false smut disease has become a destructive disease in the rice growing regions worldwide [Citation1,Citation2]. The formation of false smut balls covered by powdery chlamydospores is the representative phenotype of false smut disease [Citation1]. The invasion of U. virens not only interrupts rice grain filling, causes production decrease, but also endangers food safety on account of the production of mycotoxins, including ustilaginoidins and ustiloxins [Citation3,Citation4]. Recently, the functions of various U. virens genes, including UvHog1, UvPro1, SCRE6, UvCBP1, UvATF21, UvSMEK1 and so on, which participate in the tolerance to various stresses and virulence, have been analyzed [Citation5–10]. However, the molecular mechanism of virulence in U. virens still needs to be in-depth studied.
The false smut balls are produced through the process of energy hijacking to continuously absorb nutrients from rice [Citation11]. In the process of invasive growth, U. virens needs to coordinate nutrient perception, obtain nutrients from rice cells, regulate gene expression, and evade plant immunity to facilitate infection. To date, multiple pathways related to nutrient metabolism in various fungi have been characterized. Among them, the following three pathways have been extensively studied, including TOR (target of rapamycin), cAMP-PKA (cyclic-monophosphate (AMP) dependent protein kinase A) and AMPK (adenosine 5’-AMP activated protein kinase) [Citation12,Citation13]. Among them, the AMPK pathway mainly takes part in carbon metabolism [Citation14]. In Saccharomyces cerevisiae, AMPK, which is also known as Snf1 (sucrose non-fermenting protein kinase 1), functions as a heterotrimer made up of an α-catalytic subunit, a β-regulatory subunit, and a γ subunit, wherein the β subunit includes Sip1, Gal83, or Sip2 [Citation15]. Snf1 is the central element of glucose repression signaling, and plays a main role in activating transcription and suppressing gene expression [Citation16]. The Snf1 homologs have been identified in various pathogenic fungi with crucial functions, including Magnaporthe oryzae, Verticillium dahliae, Fusarium graminearum, Fusarium oxysporum, Cochliobolus carbonum, Leptosphaeria maculans, Ustilago maydis, Colletotrichum fructicola, and Botrytis cinerea [Citation14,Citation17–24]. Disruption of Snf1 gene led to reduced virulence in several fungal pathogens, such as F. oxysporum on green cabbage seedlings, Alternaria alternata on tangerine leaves and Penicillium digitatum on citrus fruits [Citation20,Citation25,Citation26]. On the other hand, deletion of Snf1 in U. maydis did not profoundly affect the virulence. Moreover, abnormal spore morphology and reduction of conidiation were observed in the Snf1 mutants of Podospora anserina, Cordyceps militaris, and so on [Citation27,Citation28]. In S. cerevisiae, Snf1 is a crucial regulatory factor during glucose repression, enabling yeast cells to utilize alternative or unfavorable carbon sources, such as sucrose and ethanol [Citation29]. In addition, Snf1 plays a role in lipid metabolism [Citation30]. In M. oryzae, the knockout of Snf1 gene led to a decrease in the number of peroxisomes, indicating that Snf1 is necessary for peroxisomal maintenance and lipid metabolism [Citation30]. In summary, Snf1 performs diverse functions in different fungi, and its biological functions in plant-pathogenic fungi still need to be studied.
The plant cell wall is the first barrier for plant pathogenic fungi to successfully infect and colonize hosts. During the process of long-term coevolution, fungi have formed a series of weapons to attack the cell wall of plants, including the generation of mechanical forces or the secretion of extracellular hydrolase [Citation31]. For instance, various plant fungal pathogens produce CWDEs, which are known as biochemical weapons [Citation23,Citation32]. C. carbonum, a corn leaves blight fungi, secretes CWDEs to disrupt the host cell walls, permitting the fungus to infect and spread in the host [Citation21]. In V. dahliae, a soilborne fungus, the activities of hydrolytic CWDEs are required for virulence [Citation18]. CWDEs in fungi are often subject to carbon catabolize repression at the transcriptional level. The expression levels of CWDEs, along with many other genes, are down-regulated when glucose is sufficient, and one of the main regulators controlling this process is Snf1 [Citation33]. In phytopathogenic fungi, Snf1 is demanded to relieve this repression and to regulate the expression of CWDEs during infection process [Citation13,Citation17,Citation26]. However, the relationship between CWDEs and UvSnf1 and their effect on the virulence of U. virens are not clear.
In this study, we identified the Snf1 homolog UvSnf1 in U. virens. We found that the UvSnf1 gene expression level was remarkably up-regulated during invasion. Further analyses suggested that UvSnf1 is involved in vegetative hyphae growth, spore production, and pathogenicity of the U. virens. Moreover, we found that UvSnf1 helps U. virens to use unfavorable carbon sources, when the sucrose is insufficient. In addition, UvSnf1 regulates the expression of CWDE genes, including UvCut1 and UvXyp1, under sucrose limited conditions. Furthermore, disruption of UvCut1 and UvXyp1 led to the reduction of mycelial growth, spore production, and pathogenicity.
Materials and methods
Fungal strains and culture media
The wild-type (WT) U. virens strain HWD-2 provided by Prof. Junbin Huang of Huazhong Agriculture University (China) was used in this study [Citation34]. The potato sucrose agar medium (PSA, sucrose 20 g, potato 200 g and agar 20 g, add water to 1 L) was used for culturing the U. virens strains at 28°C. To assess the effects of deletion genes on spore production, six mycelial plugs within the potato sucrose medium (PS) were shaken for 7 days at 28°C to determine the number of spores. To test the sensitivity to various carbon sources, mycelial plugs of indicated strains were inoculated on PA with tween 80, sucrose, olive oil, glucose, triolein, maltose, raffinose, trehalose, or glycerol at a concentration of 1% (m/v). Carbon sources other than sucrose are considered unfavorable carbon sources and used for subsequent experiments.
Infection assay of U. virens
The U. virens artificial inoculation was conducted as described previously [Citation35]. To be specific, mycelial plugs were cultured in the PS medium for 7 days at 28°C. The resulting spores and mycelia were cursed and adjusted to a concentration of 10 [Citation6]/mL. The obtained mixture of hyphae and spores of the WT, deletion mutants, and complemented strains were injection-inoculated with W × 98(Wanxian 98, Oryza sativa subsp. indica) at the booting stage. The numbers of false smut balls were counted and imaged at about 30 dpi (days post inoculation).
Construction knockout and complemented strains
The deletion mutants of UvSnf1 (Uv8b_04520), UvCut1 (Uv8b_03824) and UvXyp1 (Uv8b_02447) were performed by one-step gene replacement strategy. The flanking sequences (about 1 kb) of these genes were amplified using 2×Hieff® PCR Master Mix (Yeasen, 10102ES03). The obtained fragments and vector pFGL821 (addgene, ID: 58223) were digested with restriction enzymes at the same time. After recovering the digested fragments and vectors, the ClonExpress II One Step Cloning Kit (Vazyme, C112–01) was used to ligate the gene fragments and vector to construct the recombinant vector. The amplification primers have been listed in Table S1. The sequenced vectors were transformed into Agrobacterium tumefaciens strain AGL1 using a freeze–thaw method and subsequently introduced into the WT strain. The transformation of U. virens mediated by A. tumefaciens was performed as described [Citation36]. Briefly, after shaking A. tumefaciens in LB medium containing kanamycin for 12 h, the OD600 value was measured and adjusted to 0.15 using LB medium and IM medium (0.008 mM KH2PO4, 0.03% MgSO4, 0.03% NaCl, 0.001% CaCl2, 0.2% glucose, 0.5% glycerol, 0.05% NH4NO3, 0.0001% FeSO4, 0.1 g/L MES, and 4 mM ACS). The liquid culture of the corresponding U. virens strain was filtered through miracloth, and spores were collected by centrifugation. The spores (adjusted to 10 [Citation6] spores/mL) were mixed with an equal volume of Agrobacterium and spread onto each Co-IM medium (add 2% agar based on IM medium) plates with cellophanes on the top surface. After 4 days, the cellophanes were transferred to YT medium (10 g/L glucose, 1 g/L yeast extract, 1 g/L tryptone, and 20 g/L agar) with selection antibiotic for screening. In this way, the native gene was replaced with the hygromycin (HYG) gene via A. tumefaciens-mediated transformation (ATMT) [Citation37]. To construct the complemented strain, the full-length genomic sequence of the target genes, including the 2 kb promoter and 500 bp downstream flanking regions, was amplified and then inserted into pFGL823 [Citation38]. The resultant constructs were transformed into mutants, respectively. The required primers are listed in Supplementary Table S1.
Southern blot and quantitative real-time polymerase chain reaction (qRT-PCR) assays were used to test whether the knockout and complementation transformants were correct. For the Southern blot assay, genomic DNA was extracted from the WT and deletion mutant strains and digested by selected restriction enzyme XhoI. Subsequently, the resultant fragments were separated by electrophoresis using 0.8% agarose gel and transferred to a positively charged nylon membrane (GE Healthcare, Buckinghamshire, UK). Then, the restriction enzymes digested genomic DNA were used to hybridize with the DIG-labeled probe. The probes were amplified from the genome DNA, and then labeled with a DIG High Prime DNA Labeling and Detection Starter Kit I following their manufacturer’s instructions (Roche, Germany, Cat. No. 11745832910). The probe primers are listed in Table S1. The hybridization process refers to the manufacturing instructions of Amersham™ AlkPhos Direct Labeling Reagents (GE Healthcare).
QRt-PCR analysis
The mycelia of the corresponding strains were cultured in the PS medium at 28°C for 7 days. The process of collecting mycelia involved filtering the shaken liquid culture through gauze, and then using paper towels to dry the remaining moisture. After grinding the collected mycelia into a fine powder with liquid nitrogen, RNA extraction was performed using Trizol (Sango Biotech, B511311) reagent according to the instructions. Reverse transcription of total RNA was conducted with the PrimeScript™ RT reagent Kit with gDNA Eraser (TAKARA, RR047A). SYBR Green qPCR Master Mix (Yeason, 11201ES03) was used to conduct the qRT-PCR assay. The relative expression levels of genes were evaluated by the 2−ΔΔCt method with the tubulin gene (Uv8b_900) as an endogenous reference. In the supplementary Table S1, the required primers are listed. All assays were independently conducted with three technical replicates and two biological replicates.
Live cell imaging and image processing
To construct the plasmid expressing the green fluorescent protein (GFP) and UvSnf1 fusion protein, the RP27 promoter and open reading frame (ORF) of UvSnf1 were cloned with primers and ligated into the XhoI/KpnI sites of pFGL821-GFP vector. The pFGL821-GFP vector was modified from the pFGL821 vector by insertion of EGFP into KpnI/BamHI sites. The resultant construct GFP-UvSnf1 was introduced into the WT strain via ATMT method. The mycelia of the correct transformants were inoculated in the PS medium for 7 days at 28°C. The live cell imaging assay was conducted by a Zeiss LSM 700 inverted confocal microscope (Carl Zeiss, Inc) with a Plan-Apochromat 63 (NA 1.40) oil immersion lens. The fluorescence of EGFP is excited at 488 nm (Em. 505–530 nm). The images were processed using the ImageJ program (http://rsb.info.nih.gov/) and combined with Adobe Illustrator 2020.
Results
Characterization of UvSnf1 in U. virens
To identify the Snf1 homolog in U. virens, the amino acid sequence of M. oryzae Snf1 (GenBank MGG_00803.5) was used as a reference sequence for search homolog on NCBI database with a BlastP tool. The XP_042997952.1 was obtained as the Snf1 homolog in U. virens (65% coverage and 62% similarity) (Fig. S1A). A phylogenetic analysis using the neighbor-joining method showed that UvSnf1 and MbSnf1 (M. brunneum) share the closest relationship among the Snf1 homologs from F. graminearum, A. niger, S. sclerotiorum, B. cinerea, U. maydis and M. oryzae (Fig. S1B). All these Snf1 homologs were predicted to harbor an S_TKc (Serine/Threonine protein kinases, catalytic) domain on their N-side predicted by the SMART website (http://smart.embl-heidelberg.de) (Fig. S1B and C). Although the similarities between the N-terminal and C-terminal are low, the activation domain, which is phosphorylated and activated by upstream kinases under glucose limitation and other stresses, shares 100% identity in the Snf1 homologs (Fig. S1A and B) [Citation20,Citation39]. These analyses suggested that Snf1 is conserved in several fungi, and UvSnf1 may play an important role in U. virens.
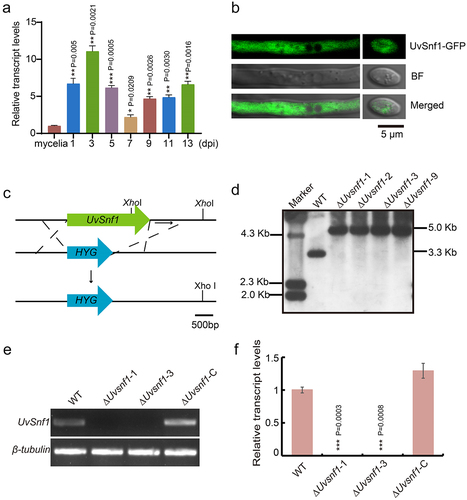
To investigate whether UvSnf1 is involved in the infection of U. virens, its expression levels were examined during invasive growth stage, including 1, 3, 5, 7, 9, 11, and 13 dpi, by qRT-PCR assay. Compared with the mycelial stage, the expression level of UvSnf1 was remarkably up-regulated at the stage of invasive hyphae extended to floral organs (three dpi) (), implicating that UvSnf1 may be regarded as the process of U. virens infection. To determine the subcellular localization of UvSnf1, a GFP was fused to the C-terminus of UvSnf1 and introduced into the WT strain. To verify whether UvSnf1 is properly expressed in the UvSnf1-GFP strain, we conducted qRT-PCR and western blot analyses. The experimental results are presented in Figure S2. Fluorescence observation showed that the GFP signal was present throughout the cytoplasm of hyphae and conidia (). Furthermore, UvSnf1 entered the nucleus under glucose-deprived conditions (Fig. S3). These results indicated that when the U. virens is in an environment lacking sucrose, UvSnf1 is activated and enters the nucleus to promote the utilization of other carbon sources, in order to maintain the balance of cell metabolism and the energy supply required for growth.
Figure 1. Characterization of UvSnf1 in U. virens. a, Relative expression levels of UvSnf1 during vegetative growth and invasive growth stages. Six mycelial plugs within the PS medium were shaken for 7 days at 28°C to extract RNA for determining the expression level of UvSnf1. The expression levels of UvSnf1 in the mycelia were normalized to 1. b, Subcellular localization of UvSnf1-GFP. Vegetative hyphae and spores of the transformants expressing UvSnf1-GFP were imaged using confocal microscope. Scale bar = 5 μm. BF, bright field. c, Deletion strategy of the UvSnf1 gene and the restriction enzyme sites for southern blot assay. The open reading frame of UvSnf1 was replaced by hygromycin (HYG) gene through homologous recombination. Scale bar = 500 bp. d, Southern blot assay of the WT and ΔUvsnf1 mutants. The XhoI was chose to digested genomic DNA of the WT and ΔUvsnf1 strains. The 1 Kb downstream sequence of UvSnf1 served as the probe for Southern blot assay. e, RT-PCR analyses of the WT, ΔUvsnf1-1, ΔUvsnf1-3 and ΔUvsnf1-C strains. f, Abundance of UvSnf1 transcript in mycelia of the WT, ΔUvsnf1-1, −3 and ΔUvsnf1-C strains. The relative expression levels were determined by Qrt-PCR with the tubulin gene as reference. The mean ± SD was calculated by the data of three independent replicates. P values were generated using the student’s t-test. Asterisks indicated remarkable differences compared with mycelia of the WT strain (*, ** and *** represent P < 0.05, P < 0.01 and P < 0.001).
Deletion of UvSnf1 and complementation assays
To investigate the functions of UvSnf1, UvSnf1 knock-out transformants were obtained by targeted gene replacement in the HWD strain. A total of five transformants with similar colony phenotypes were acquired (). Two independent deletion mutants, ΔUvsnf1-1, and ΔUvsnf1-3 were chosen to confirm the correct UvSnf1 gene knock-out event by PCR and qRT-PCR analyses (). Subsequently, the mutants were examined to exclude ectopic integrations by Southern blot assay (). The results showed that a band at approximately 3.3 Kb was detected in the WT strain, while an expected band was observed at 5.0 Kb in the ΔUvsnf1 mutants.
To further investigate whether the phenotypic differences in colonies were related to the deletion of UvSnf1 gene, a complementation assay was performed by transforming the pFGL823-UvSnf1 vector containing UvSnf1 full-length DNA copy and its native promoter into the UvSnf1 mutant. RT-PCR and qRT-PCR assays showed that the UvSnf1 gene expression levels in the ΔUvsnf1-C transformants were equivalent to that of the WT (). Furthermore, the phenotype of the resultant ΔUvsnf1-C strain was restored, and there was no difference compared with the WT strain. In general, these results demonstrated that the gene replacement event of UvSnf1 is correct, and that the reintroduction of UvSnf1 functionally restores the defects in the ∆snf1 mutant.
Deletion of UvSnf1 compromises growth, spore production, and pathogenicity in U. virens
To investigate whether deletion of UvSnf1 gene affects the vegetative growth of hyphae, the growth of the WT, ΔUvsnf1 mutants, and complemented strains was determined in the PSA medium for 14 days. Compared with WT, the mycelial growth of the ΔUvsnf1 mutants was dramatically reduced by approximately 18% in vitro. In contrast, the phenotype was restored after complementation (). These results showed that UvSnf1 plays a crucial role in fungal growth in U. virens.
Figure 2. Deletion of UvSnf1 compromised growth, spore production and pathogenicity of U. virens. a, Colonies of the WT, ΔUvsnf1-1, −3 and ΔUvsnf1-C strains. The mycelial plugs of indicated strains were cultured on PSA plates for 14 days at 28°C to take pictures. b, Deletion of UvSnf1 reduced the colony diameters. The colony diameters of the WT, ΔUvsnf1-1, −3 and ΔUvsnf1-C strains were presented with mean ± SD. c and d, Deletion of UvSnf1 reduced spore production. Six mycelial plugs of the WT, ΔUvsnf1-1, −3 and ΔUvsnf1-C strains were cultured in the same volume of liquid PS medium for 7 days at 28°C. The pictures of conidia were taken under a bright field microscope. We conducted three technical replicates and two biological replicates for this experiment. Bar = 10 μm. e, Infection assay of WT, ΔUvsnf1-1, −3 and ΔUvsnf1-C strains. The mixture of hyphae and spores of the WT, deletion mutants and complemented strains were injection-inoculated with W×98(Wanxian 98, Oryza sativa subsp. indica) at booting stage. The photos were taken at 30 dpi. f, the false smut balls formed on rice panicles inoculated with the ΔUvsnf1 mutants were fewer than those of the WT and complemented strains. Three independent biological experiments were performed with at least 30 inoculated panicles of W×98cultivar each time. Significant differences were indicated by asterisks (** represent P < 0.01).
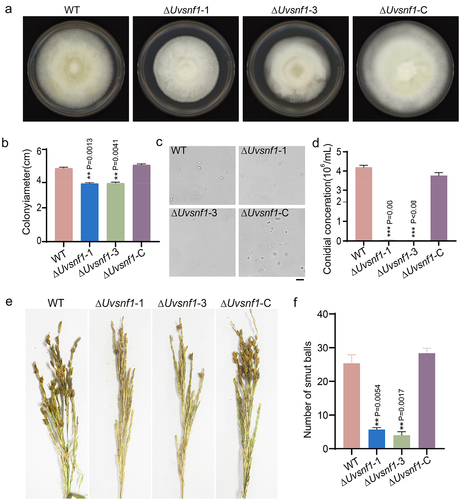
To reveal the function of UvSnf1 in spore production, the same quantity of plugs of WT, ΔUvsnf1-1, ΔUvsnf1-3 and ΔUvsnf1-C strains were cultured in an equal volume PS medium for 7 days. The observation and quantity statistics of spores revealed that knockout of UvSnf1 resulted in approximately 95% reduction of spore production compared with the conidial data of the WT strain in vitro experiments (), suggesting that UvSnf1 participates in spore production in U. virens.
To determine whether UvSnf1 is involved in the virulence of U. virens, the rice panicles at the booting stage of cultivar Wanxian 98 were inoculated with the mixture of hyphae and spores of the WT, ΔUvsnf1-1, −3, and ΔUvsnf1-C strains, respectively. The pathogenicity evaluation results showed that the panicles inoculated with the ΔUvsnf1 mutants formed much fewer (approximately 78%) smut balls than those of the WT and ΔUvsnf1-C strains (), suggesting that UvSnf1 is involved in fungal virulence in U. virens.
UvSnf1 contributes to various carbon source utilization
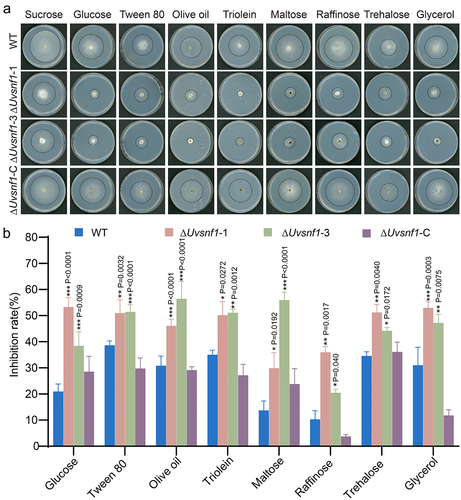
In S. cerevisiae, the essential regulator Snf1 allows yeast cells to utilize alternative or even unfavorable carbon sources when glucose is deficient [Citation40]. To investigate the role of UvSnf1 in the utilization of carbon sources in U. virens, the indicated strains were cultured on PA medium with sugar or lipid reagents, such as sucrose, glucose, tween 80, olive oil, triolein, maltose, raffinose, trehalose, and glycerol, for 14 days. Potato medium containing sucrose (PSA) is usually used to cultivate U. virens, so PSA medium was used as a control. Compared with the inhibition rates of the WT and complemented strains of about 10%–40%, the growth inhibition rates of the ΔUvsnf1-1, and −3 strains increased to around 30%–60% on these unfavorable media in vitro culture experiments (). These results demonstrated that UvSnf1 contributes to the use of unfavorable carbon sources for growth when the sucrose was insufficient.
Figure 3. UvSnf1 participates in various carbon source utilization. a, Radial growth of the WT, ΔUvsnf1-1, −3 and ΔUvsnf1-C strains on the indicated medium for 14 days at 28°C. Basal PA (potato agar, potato 200 g and agar 20 g, add water to 1 L) medium was supplemented with various carbon source, including sucrose, glucose, tween 80, olive oil, triolein, maltose, raffinose, trehalose or glycerol at 1% (w/v). b, Inhibition rates of colony growth by various carbon source in the WT, ΔUvsnf1 mutants and complemented strains. The relative inhibition rate was calculated as follow: growth inhibition rate = (diameters of indicated strain on the PSA – diameters of strain on the PA medium with alternative carbon source)/diameters of the indicated strain on the PSA × 100%. The data were obtained from three repeats. Bars indicate the mean ± SD. *, ** and *** represent significant differences between the inhibition rates of mutants and WT at P < 0.05, P < 0.01 and P < 0.001 levels.
Deletion of UvSnf1 down- regulated the expression of cell wall-degrading enzymes genes under sucrose limitation condition
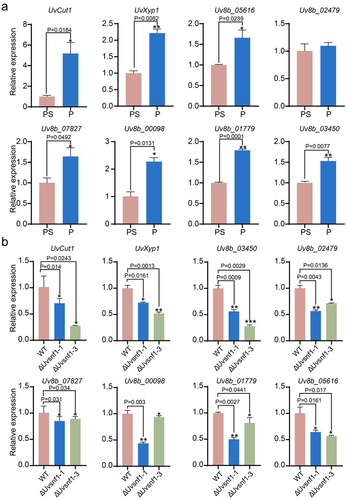
The premise of successful infection of plant pathogenic fungi is to depolymerize the cell wall by exerting mechanical pressure or secreting extracellular hydrolase, such as CWDEs. CWDEs in fungi are usually repressed by carbon catabolism at the transcription level, and when the carbon source is limited, CWDEs are derepressed [Citation41]. In yeast, one of the main players controlling the derepression process is Snf1 [Citation33]. To clarify whether the amount of carbon source had an effect on the expression of the CWDEs in U. virens, qRT-PCR assays were performed using PS medium with or without sucrose-cultured mycelia. The results showed that the expression levels of various CWDEs, including endo-β-1,4-xylanase genes (Uv8b_05616 and Uv8b_01779), cutinase gene (Uv8b_03824, UvCut1), acetyl xylanesterase gene (Uv8b_00098), β-xylosidase gene (Uv8b_02447, UvXyp1), exopolygalacturonase gene (Uv8b_03450) and glucanases genes (Uv8b_07827 and Uv8b_02479), were up-regulated significantly when sucrose was insufficient (). Subsequently, whether UvSnf1 participates in the regulation of CWDEs expression under sucrose limitation condition was investigated by culturing the ΔUvsnf1 mutants and WT strains in PS medium without sucrose. The results revealed that the expression levels of CWDEs were lower in the ΔUvsnf1 mutants than those of the WT when sucrose was limited (), indicating that UvSnf1 regulates the expression of CWDEs. We performed one biological replicate for these two experiments, and the results are shown in Figure S4. Taken together, in U. virens, deletion of UvSnf1 prevented the derepression of CWDEs when the sucrose was insufficient. Moreover, two of these CWDEs, UvCut1 and UvXyp1, were chosen for the following characterization.
Figure 4. Deletion of UvSnf1 down-regulated the expression of cell wall-degrading enzymes genes under sucrose limited condition. a, the relative expression levels of CWDEs in the WT strain. The hyphae of WT strain were cultured by PA or PSA medium for 7 days at 28°C. b, the relative expression of CWDEs in the WT, ΔUvsnf1-1 and − 3 strains when sucrose is limited. The plugs of strains were inoculated in the indicated medium for 7 days at 28°C. PS medium is composed of 200 g potato and 20 g sucrose per L. P represents the medium containing potato 200 g per L. Quantitative experiments for each gene were performed with three technical replicates and two biological replicates. Error bars represent standard variations of three independent replicates experiments. Asterisks represent significant differences of the expression of CWDEs (*, ** and *** represent P < 0.05, P < 0.01 and P < 0.001).
Disruption of UvCut1 and UvXyp1 affects growth, spore production and virulence in U. virens
To reveal the function of UvCut1 and UvXyp1 in U. virens, the deletion mutants of UvCut1 and UvXyp1 genes were generated by the homologous recombination method. Phenotypic analyses revealed that compared with the WT and complemented strains, disruption of UvCut1 led to an increase in colony diameters on the PSA medium for 14 days by around 8%. In contrast, the deletion of UvXyp1 resulted in the reduction of mycelial density by approximately 26% in vitro (). These results demonstrated that the CWDE genes UvCut1 and UvXyp1 play key roles in the hyphae growth in U. virens. In addition, spore production of the ΔUvcut1 and ΔUvxyp1 mutants were reduced by about 93% and 98%, respectively (), suggesting that UvCut1 and UvXyp1 participate in asexual development in U. virens.
Figure 5. Deletion of UvCut1 and UvXyp1 reduced mycelial growth, spore production and virulence in U. virens. a, Colonies of the WT, ΔUvcut1, ΔUvxyp1 and complemented strains. The tested strains were cultured on the PSA plates for 14 days at 28°C. b, the colony diameters of the WT, ΔUvcut1, ΔUvxyp1 and complemented strains. The data were presented with mean ± SD. c, Spore production was reduced in the ΔUvcut1 and ΔUvxyp1 mutants. These strains were inoculated in PS medium at 28°C with 180 rpm for 7 days prior observation. Three independent repetitions were conducted with similar results obtained. d, Infection assays of the WT, ΔUvcut1, ΔUvxyp1 and complemented strains. e, the average number of false smut balls on the rice panicles inoculated with the mixture of hyphae and spores of corresponding strains. Three independent biological experiments were performed with at least 30 inoculated panicles of W×98cultivar each time. *, ** and *** represent significant differences between diameter, conidiation or smut balls number of WT and mutant strains at P < 0.05, P < 0.01 and P < 0.001 levels.
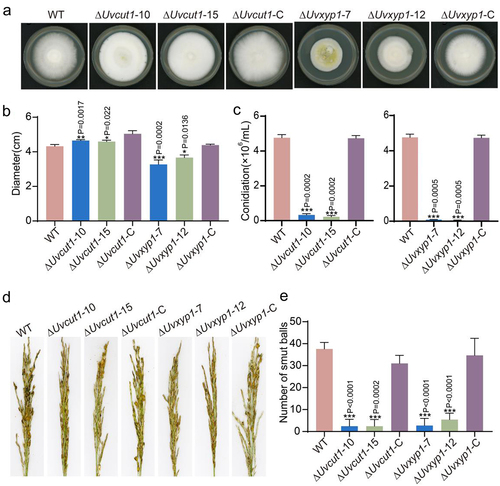
To reveal whether CWDE genes, including UvCut1 and UvXyp1, contribute to the virulence of U. virens, the Wanxian 98 panicles at the booting stage were inoculated with the mixture of mycelia and spores of the indicated strains, respectively. At 30 dpi, rice spikelets inoculated with mutants failed to form smut balls, whereas there were various typical smut balls on the panicles inoculated with the WT and complementation strains (), indicating that UvCut1 and UvXyp1 are required for the virulence in U. virens.
UvCut1 and UvXyp1 are involved in carbon source utilization
Because deletion of UvSnf1 down-regulated the expression of the CWDE genes, we attempted to determine whether UvCut1 and UvXyp1 were implicated in carbon source utilization in U. virens. Therefore, a growth assay was performed with PSA medium as the control. Among all tested, the colonies of ΔUvcut1 and Uvxyp1 mutants cultured on glucose were partially impaired compared to that of the WT strain. In addition, we found that the growth of mutants was also reduced when cultured on the unfavorable carbon source, including tween 80, glycerol, raffinose, and trehalose. Compared with the inhibition rates of the WT and complemented strains of about 7%–20% , the growth inhibition rates of the mutant strains increased to around 13%–32% on these unfavorable carbon media in vitro experiments (Fig. S5). The inhibition rates were lower compared to ΔUvSnf1 strains, indicating that UvCut1 and UvXyp1 genes may be involved in UvSnf1-mediated carbon source utilization in U. virens.
Discussion
Ustilaginoidea virens has becoming one of the most destructive rice fungal pathogens in the world. U. virens infects rice spikelets at the booting stage to form false smut balls. During infection, U. virens has to adapt to the host environment and hijack nutrients from host spikelets for pathogen growth [Citation1,Citation42]. Snf1 is a protein kinase that plays important roles in carbon catabolite derepression and managing energy status [Citation43,Citation44]. In this study, we characterized the function of Snf1 homolog in U. virens. Our results revealed that UvSnf1 is responsible for vegetative growth, spore production, and virulence in U. virens. Furthermore, deletion of UvSnf1 not only reduces the use of unfavorable carbon sources but also down-regulates the expression of CWDE genes when sucrose is limited ().
Figure 6. Proposed model for the role of UvSnf1 in U. virens. UvSnf1 is inactivated under sucrose sufficient condition. Under sucrose insufficient condition, UvSnf1 is activated and the transcriptional expression of downstream CWDE genes is derepressed. Subsequently, CWDEs degrade the cell wall and U. virens could utilize carbon source contributing to hyphae growth, spore production and infection in the host rice cells.
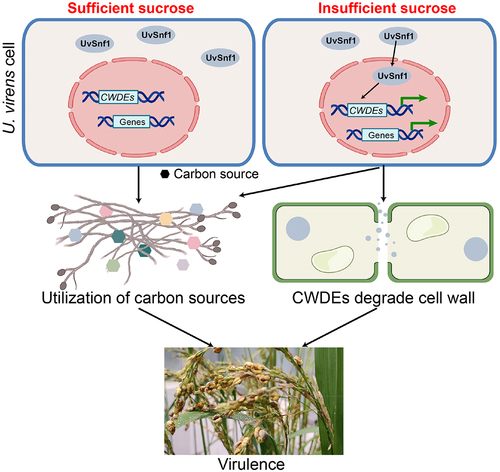
With the identification and characterization of multiple pathogenic genes, U. virens infection mechanisms have been depicted gradually, but it is still not clear. In our study, the UvSnf1 gene was revealed to be required for the virulence of U. virens. First, the radial growth of UvSnf1 mutants was reduced which may result in defects of U. virens invasive growth (). The deletion of UvSnf1 gene also inhibited the production of spores, which is one of the reasons for its significantly reduced pathogenicity (). Similar phenomena have been observed in the deletion mutants of Snf1 genes in C. fructicola, B. bassiana, G. zeae, L. maculans, P. anserina and M. acridum [Citation14,Citation19,Citation22,Citation27,Citation29,Citation45]. In addition, it is noted that the expression of UvSnf1 is increased significantly during the early stage of infection (). Consistent with these results, the expression of SsSnf1 (S. sclerotiorum), CgSnf1 (Colletotrichum gloeosporoides f. sp. malvae) and LmSnf1 (L. maculans) are up-regulated during infection process [Citation22,Citation46,Citation47]. Therefore, it is possible that UvSnf1 is involved in the colonization of U. virens in rice. Due to its important role in the utilization of carbon source, when UvSnf1 was knocked out, U. virens cannot use the nutrients properly in the plant, which restricts the growth and ultimately leads to a decrease in pathogenicity. Although UvSnf1 plays an important role in the pathogenesis of U. virens (), disruption of the Snf1 gene in U. maydis only exhibited a slight reduction in virulence, suggesting that the functions of Snf1 during infection in different pathogenic fungi may be different [Citation23].
It has been well known that Snf1 plays vital roles in the energy utilization in yeast [Citation48]. Snf1 has crucial roles in the alleviation of glucose repression, which contributes to the utilization of alternative carbon sources apart from glucose, ensuring nutrition availability and cell survival in S. cerevisiae [Citation49–51]. Snf1 regulates glucose metabolism in a concentration-dependent manner. When glucose is limited, Snf1 is activated to promote transcription of downstream genes repressed by glucose [Citation52]. In contrast, under the condition of high glucose concentration, Snf1 cannot be activated, and energy is maintained by gluconeogenesis, respiration, and alternate use of carbon sources [Citation48]. In S. cerevisiae, knockout of the Snf1 gene led to slow growth and defects in the use of sucrose, galactose, maltose, and several non-fermentable carbon source mutants [Citation40]. In this study, we found that knockout of UvSnf1 reduced the growth on sucrose-containing medium by around 25%. Similarly, the radial growth of UvSnf1 mutants was impaired as in several fungi, including C. militaris, M. oryzae, and P. digitatum, on glucose-rich media [Citation17,Citation21,Citation26,Citation28]. In contrast, the growth of CmSnf1, UmSnf1, and VdSnf1 mutants showed no significant change compared to the WT strain on sucrose media. In addition, the colony size of the ΔUvsnf1 mutants decreased by 30%–60%, on the medium with tween 80, olive oil, triolein, maltose, raffinose, trehalose, or glycerol as the primary carbon source (). These differences of Snf1 mutant in carbon utilization suggested that Snf1 is involved in distinct carbon utilization in a fungi-specific way [Citation18,Citation53].
In response to glucose limitation or growth on non-fermentable carbon sources, S. cerevisiae Snf1 protein kinase associated with the beta subunit Gal83 accumulates in the nucleus. In the nucleus, Snf1 binds with certain nuclear transcription factors, chromatin, and transcriptional apparatus to regulate the expression of a wide array of genes in adaptation to glucose limitation and cellular stresses. These genes include CWDEs which are subject to carbon catabolize repression at the transcriptional level when glucose is sufficient [Citation19,Citation20,Citation41]. In this study, we found that the expression levels of CWDEs in the UvSnf1 mutants downregulated significantly under sucrose-limited conditions, suggesting that the expression of CWDEs may be regulated by UvSnf1 in U. virens (). A similar phenomenon was observed in the Snf1 mutants in F. oxysporum, F. virguliforme, G. zeae, L. maculans and U. maydis [Citation19,Citation20,Citation22,Citation23,Citation53]. However, in M. oryzae, the expression levels of CWDE-encoding genes were down-regulated when glucose repression but not regulated by MoSnf1, suggesting that the function of Snf1 in regulating the expression of CWDEs may be not conserved [Citation17].
It is well known that CWDEs are important virulence factors in plant pathogenic fungi [Citation53]. We tried to knock out all the differentially expressed CWDE genes, but only the correct mutants of UvCut1 and UvXyp1 genes were obtained due to the low homologous recombination efficiency of U. virens. In our study, we found that knockout of the two CWDEs, UvCut1 and UvXyp1, resulted in reduced utilization of unfavorable carbon sources, including glucose, tween 80, glycerol, raffinose, and trehalose (Fig. S2). The deficiency of xylanases, cutinases, and glucanases produced by the cell wall-degrading enzymes may be one of the reasons for the reduced virulence of the UvSnf1 mutants. Moreover, many fungi overcome the obstacle of plant cell walls by secreting cell wall-degrading enzymes during infection. During infection, U. virens extends intracellularly and the host cell wall is degraded, which was observed in light micrograph assays [Citation54]. Consistent with these studies, our results showed that knock-out of the cell wall-degrading enzymes encoding genes UvCut1 and UvXyp1 led to a significant reduction in pathogenicity. Therefore, we inferred that CWDEs play important roles in the host cell wall degradation process in U. virens.
In conclusion, all these evidences indicated that UvSnf1 has crucial roles in vegetative growth, spore production, and virulence in U. virens. Furthermore, UvSnf1 promotes the U. virens to use unfavorable carbon sources when the sucrose is insufficient. In addition, UvSnf1 regulates the expression of CWDEs, which promote the utilization of sugar and the degradation of plant cell walls to contribute to pathogenesis.
Supplemental Material
Download Zip (1.3 MB)Acknowledgements
This project was supported by the National Natural Science Foundation of China (32100161 to JQ), Zhejiang Provincial Natural Science Foundation of China, grant number “LY22C140006,” key R&D project of the China National Rice Research Institute, grant number “CNRRI-2020-04,” and Zhejiang Science and Technology Major Program on Rice New Variety Breeding (2021C02063-3). This project was also supported by the Chinese Academy of Agricultural Sciences under the “Elite Youth” Program and the Agricultural Sciences and Technologies Innovation Program. We thank the Rice–Pathogen Interaction group of China National Rice Research Institute for helpful discussion and suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
All data generated or analyzed during the present study can be found within the manuscript and its supporting materials.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2023.2235460.
Additional information
Funding
References
- Sun W, Fan J, Fang A, et al. Ustilaginoidea virens: insights into an emerging rice pathogen. Annu Review Phytopathol. 2020;58:363–385. doi: 10.1146/annurev-phyto-010820-012908
- Qiu J, Meng S, Deng Y, et al. Ustilaginoidea virens: a fungus infects rice flower and threats world rice production. Rice Sci. 2019;26(4):199–14. doi: 10.1016/j.rsci.2018.10.007
- Guo X, Li Y, Fan J, et al. Progress in the study of false smut disease in rice. J Agric Sci Technol. 2012;2:1211–1217.
- Zhang Y, Zhang K, Fang A, et al. Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat Commun. 2014;5(1). doi: 10.1038/ncomms4849
- Li G-B, He J-X, Wu J-L, et al. Enhanced production of OsRACK1A, an effector-targeted scaffold protein that promotes OsRBOHB-mediated ROS production, confers rice floral resistance to false smut disease without yield penalty. Mol Plant. 2022;15(11):1790–1806. doi: 10.1016/j.molp.2022.10.009
- Zheng X, Fang A, Qiu S, et al. Ustilaginoidea virens secretes a family of phosphatases that stabilize the negative immune regulator OsMPK6 and suppress plant immunity. Plant Cell. 2022;34(8):3088–3109. doi: 10.1093/plcell/koac154
- Yu J, Yu M, Song T, et al. UvSMEK1, a suppressor of MEK null, regulates pathogenicity, conidiation and conidial germination in rice false smut fungus Ustilaginoidea virens. Rice Sci. 2021;28(5):457–465. doi: 10.1016/j.rsci.2021.07.006
- Liu Y, Qu J, Wang Y, et al. bZIP transcription factor UvATF21 mediates vegetative growth, conidiation, stress tolerance and is required for full virulence of rice false smut fungus Ustilaginoidea virens. Rice Sci. 2023;30(1):50–57. doi: 10.1016/j.rsci.2022.12.001
- Zheng D, Wang Y, Han Y, et al. UvHOG1 is important for hyphal growth and stress responses in the rice false smut fungus Ustilaginoidea virens. Sci Rep. 2016;6(1):6. doi: 10.1038/srep24824
- Lv B, Zheng L, Liu H, et al. Use of random T-DNA mutagenesis in identification of gene UvPRO1, a regulator of conidiation, stress response, and virulence in Ustilaginoidea virens. Front Microbiol. 2016;7. doi: 10.3389/fmicb.2016.02086
- Song J-H, Wei W, Lv B, et al. Rice false smut fungus hijacks the rice nutrients supply by blocking and mimicking the fertilization of rice ovary. Environ Microbiol. 2016;18(11):3840–3849. doi: 10.1111/1462-2920.13343
- Conrad M, Schothorst J, Kankipati HN, et al. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 2014;38(2):254–299. doi: 10.1111/1574-6976.12065
- Shashkova S, Welkenhuysen N, Hohmann S. Molecular communication: crosstalk between the Snf1 and other signaling pathways. FEMS Yeast Res. 2015;15(4):15. doi: 10.1093/femsyr/fov026
- Zhang S, Guo Y, Li S, et al. Functional analysis of CfSnf1 in the development and pathogenicity of anthracnose fungus Colletotrichum fructicola on tea-oil tree. BMC Genet. 2019;20(1):20. doi: 10.1186/s12863-019-0796-y
- Amodeo GA, Rudolph MJ, Tong L. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature. 2007;449(7161):492–U13. doi: 10.1038/nature06127
- Adnan M, Zheng W, Islam W, et al. Carbon catabolite repression in filamentous fungi. Int J Mol Sci. 2018;19(1):48. doi: 10.3390/ijms19010048
- Yi M, Park J-H, Ahn J-H, et al. MoSNF1 regulates sporulation and pathogenicity in the rice blast fungus Magnaporthe oryzae. Fungal Genet Biol. 2008;45(8):1172–1181. doi: 10.1016/j.fgb.2008.05.003
- Tzima AK, Paplomatas EJ, Rauyaree P, et al. VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell-wall degradation. Mol Plant-Microbe Interact. 2011;24(1):129–142. doi: 10.1094/MPMI-09-09-0217
- Lee S-H, Lee J, Lee S, et al. GzSNF1 is required for normal sexual and asexual development in the Ascomycete Gibberella zeae. Eukaryot Cell. 2009;8(1):116–127. doi: 10.1128/EC.00176-08
- Ospina-Giraldo MD, Mullins E, Kang S. Loss of function of the Fusarium oxysporum SNF1 gene reduces virulence on cabbage and Arabidopsis. Curr Genet. 2003;44(1):49–57. doi: 10.1007/s00294-003-0419-y
- Tonukari NJ, Scott-Craig JS, Walton JD. The cochliobolus carbonum SNF1 gene is required for cell wall–degrading enzyme expression and virulence on maize. Plant Cell. 2000;12(2):237–247. doi: 10.1105/tpc.12.2.237
- Feng J, Zhang H, Strelkov SE, et al. The LmSNF1 gene is required for pathogenicity in the canola blackleg pathogen Leptosphaeria maculans. PLoS One. 2014;9(3):e92503. doi: 10.1371/journal.pone.0092503
- Nadal M, Garcia-Pedrajas MD, Gold SE. The snf1 gene of Ustilago maydis acts as a dual regulator of cell wall degrading enzymes. Phytopathology®. 2010;100(12):1364–1372. doi: 10.1094/PHYTO-01-10-0011
- Lengyel S, Rascle C, Poussereau N, et al. Snf1 kinase differentially regulates botrytis cinerea pathogenicity according to the plant host. Microorganisms. 2022;10(2):10. doi: 10.3390/microorganisms10020444
- Tang K, Lv W, Zhang Q, et al. Coding the α-subunit of SNF1 kinase, Snf1 is required for the conidiogenesis and pathogenicity of the Alternaria alternata tangerine pathotype. Fungal Biol. 2020;124(6):562–570. doi: 10.1016/j.funbio.2020.02.008
- Zhang T, Sun X, Xu Q, et al. PdSNF1, a sucrose non-fermenting protein kinase gene, is required for penicillium digitatum conidiation and virulence. Appl Microbiol Biotechnol. 2013;97(12):5433–5445. doi: 10.1007/s00253-012-4593-z
- Li Y, Yan P, Lu X, et al. Involvement of PaSNF1 in fungal development, sterigmatocystin biosynthesis, and lignocellulosic degradation in the filamentous fungus podospora anserina. Front Microbiol. 2020;11:11. doi: 10.3389/fmicb.2020.01038
- Wang Y, Wang R, Li Y, et al. Diverse function and regulation of CmSnf1 in entomopathogenic fungus cordyceps militaris. Fungal Genet Biol. 2020;142:142. doi: 10.1016/j.fgb.2020.103415
- Wang X-X, He P-H, Feng M-G, et al. BbSNF1 contributes to cell differentiation, extracellular acidification, and virulence in Beauveria bassiana, a filamentous entomopathogenic fungus. Appl Microbiol Biotechnol. 2014;98(20):8657–8673. doi: 10.1007/s00253-014-5907-0
- Zeng X-Q, Chen G-Q, Liu X-H, et al. Crosstalk between SNF1 pathway and the peroxisome-mediated lipid metabolism in Magnaporthe oryzae. PLoS One. 2014;9(8):e103124. doi: 10.1371/journal.pone.0103124
- Hamer JE, Talbot NJ. Infection-related development in the rice blast fungus Magnaporthe grisea. Curr Opin Microbiol. 1998;1(6):693–697. doi: 10.1016/S1369-5274(98)80117-3
- Walton JD. Deconstructing the cell wall. Plant Physiol. 1994;104(4):1113–1118. doi: 10.1104/pp.104.4.1113
- Ruijter GJG, Visser J. Carbon repression in aspergilli. FEMS Microbiol Lett. 1997;151(2):103–114. doi: 10.1111/j.1574-6968.1997.tb12557.x
- Meng S, Qiu J, Xiong M, et al. UvWhi2 is required for stress response and pathogenicity in Ustilaginoidea virens. Rice Sci. 2022;29(1):47–54. doi: 10.1016/j.rsci.2021.12.004
- Yang J, Zhang N, Wang J, et al. SnRK1A-mediated phosphorylation of a cytosolic ATPase positively regulates rice innate immunity and is inhibited by Ustilaginoidea virens effector SCRE1. New Phytol. 2022;236(4):1422–1440. doi: 10.1111/nph.18460
- Zhang N, Yang J, Fang A, et al. The essential effector SCRE1 in Ustilaginoidea virens suppresses rice immunity via a small peptide region. Mol Plant Pathol. 2020;21(4):445–459. doi: 10.1111/mpp.12894
- Meng S, Xiong M, Jagernath JS, et al. UvAtg8-mediated autophagy regulates fungal growth, stress responses, conidiation, and pathogenesis in Ustilaginoidea virens. Rice. 2020;13(1):56. doi: 10.1186/s12284-020-00418-z
- Xiong M, Meng S, Qiu J, et al. Putative phosphatase UvPsr1 is required for mycelial growth, conidiation, stress response and pathogenicity in Ustilaginonidea virens. Rice Sci. 2020;27(6):529–536. doi: 10.1016/j.rsci.2020.09.009
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Revi Biochem. 1998;67(1):821–855. doi: 10.1146/annurev.biochem.67.1.821
- Carlson M, Osmond BC, Botstein D. Mutants of yeast defective in sucrose utilization. Genetics. 1981;98(1):25–40. doi: 10.1093/genetics/98.1.25
- Aro N, Pakula T, Penttila M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev. 2005;29(4):719–739. doi: 10.1016/j.femsre.2004.11.006
- Chen L-Q, Hou B-H, Lalonde S, et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468(7323):527–U199. doi: 10.1038/nature09606
- Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233(4769):1175–1180. doi: 10.1126/science.3526554
- Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci Landmark. 2008;13(13):2408–2420. doi: 10.2741/2854
- Ming Y, Wei Q, Jin K, et al. MaSnf1, a sucrose non-fermenting protein kinase gene, is involved in carbon source utilization, stress tolerance, and virulence in Metarhizium acridum. Appl Microbiol Biotechnol. 2014;98(24):10153–10164. doi: 10.1007/s00253-014-6066-z
- Goodwin PH, Chen GY. High expression of a sucrose non-fermenting (SNF1)-related protein kinase from colletotrichum gloeosporoides f. sp. malvae is associated with penetration of Malva pusilla. FEMS Microbiol Lett. 2002;215(2):169–174. doi: 10.1111/j.1574-6968.2002.tb11387.x
- Vacher S, Cotton P, Fevre M. Characterization of a SNF1 homologue from the phytopathogenic fungus Sclerotinia sclerotiorum. Gene. 2003;310:113–121. doi: 10.1016/S0378-1119(03)00525-0
- Coccetti P, Nicastro R, Tripodi F. Conventional and emerging roles of the energy sensor Snf1/AMPK in Saccharomyces cerevisiae. Microb Cell. 2018;5(11):482–494. doi: 10.15698/mic2018.11.655
- Backhaus K, Rippert D, Heilmann CJ, et al. Mutations in SNF1 complex genes affect yeast cell wall strength. Eur J Cell Biol. 2013;92(12):383–395. doi: 10.1016/j.ejcb.2014.01.001
- Zhang J, Vaga S, Chumnanpuen P, et al. Mapping the interaction of Snf1 with TORC1 in Saccharomyces cerevisiae. Mol Syst Biol. 2011;7(1). doi: 10.1038/msb.2011.80
- Hong S-P, Carlson M. Regulation of Snf1 protein kinase in response to environmental stress. J Biol Chem. 2007;282(23):16838–16845. doi: 10.1074/jbc.M700146200
- Lin X. The regulation of Saccharomyces cerevisiae Snf1 protein kinase on glucose utilization is in a glucose-dependent manner. Curr Genet. 2021;67(2):245–248. doi: 10.1007/s00294-020-01137-0
- Islam KT, Bond JP, Fakhoury AM. FvSNF1, the sucrose non-fermenting protein kinase gene of Fusarium virguliforme, is required for cell-wall-degrading enzymes expression and sudden death syndrome development in soybean. Curr Genet. 2017;63(4):723–738. doi: 10.1007/s00294-017-0676-9
- Tang YX, Jin J, Hu DW, et al. Elucidation of the infection process of Ustilaginoidea virens (teleomorph: Villosiclava virens) in rice spikelets. Plant Pathology. 2013;62(1):1–8. doi: 10.1111/j.1365-3059.2012.02629.x
