?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
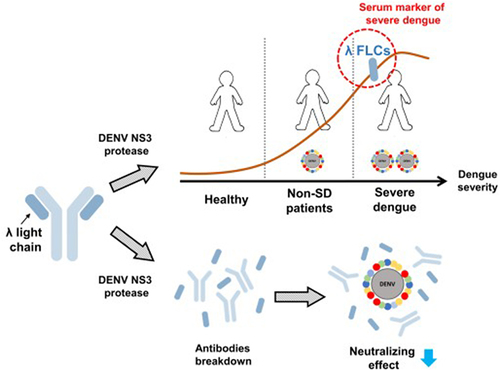
Dengue poses a significant global public health threat, with diverse clinical manifestations due to complex interactions between the host and the pathogen. Recent reports have highlighted elevated serum-free light chain (FLC) levels in viral infectious diseases. Hence, our study aimed to investigate serum FLC levels in dengue patients. The findings revealed elevated serum λ FLCs, which were associated with the severity of dengue. Receiver operating characteristic curve (ROC) analysis demonstrated that λ FLCs may serve as a serum marker for identifying dengue disease (AUC: 0.7825, sensitivity: 80, specificity: 71.43) and classifying severe dengue (AUC: 0.8102, sensitivity: 75, specificity: 79.52). The viral protease, Dengue virus (DENV) nonstructural protein 3 (NS3), acts as a protease that cleaves viral polyproteins as well as host substrates. Therefore, we proposed that antibodies might be potential targets of NS3 protease, leading to an increase in FLCs. LC/MS-MS analysis confirmed that λ FLCs were the predominant products after antibody degradation by NS3 protease. Additionally, purified NS3 protease cleaved both human IgG and DENV2-neutralizing antibodies, resulting in the presence of λ FLCs. Moreover, NS3 protease administration in vitro led to a reduction in the neutralizing efficacy of DENV2-neutralizing antibodies. In summary, the elevated serum λ FLC levels effectively differentiate dengue patients from healthy individuals and identify severe dengue. Furthermore, the elevation of serum λ FLCs is, at least in part, mediated through NS3 protease-mediated antibody cleavage. These findings provide new insights for developing diagnostic tools and understanding the pathogenesis of DENV infection.
Introduction
Dengue virus (DENV) is one of the most prevalent infectious viruses worldwide, with an estimated 390 million cases of DENV infection and 96 million individuals experiencing symptoms each year [Citation1]. Additionally, approximately 500,000 cases develop into severe diseases, leading to 22,000 deaths annually [Citation2]. The impact of DENV on public health is unquestionably significant. Clinical manifestations of DENV infection can vary from subclinical disease and mild febrile illness to classical dengue fever and severe dengue (SD), previously known as dengue haemorrhagic fever or dengue shock syndrome, due to the complex interaction between the human immune system and the virus [Citation3–5]. SD is characterized by severe plasma leakage, severe bleeding, and organ impairment, and it has a high case fatality rate without proper treatment [Citation6]. Therefore, identifying an ideal prediction factor for SD in dengue patients without warning signs is crucial for providing optimal supportive care and reducing the economic burden.
Immunoglobulin (Ig) plays a vital role in combating pathogen infections. Its structure consists of two pairs of identical heavy chains and light chains. Production of light chains occurs throughout B-cell development until light chains bind to heavy chains, forming intact Ig. Excessive light chains are then released into the bloodstream as free light chains (FLCs). In healthy individuals, small amounts of lambda (λ) and kappa (κ) light chains, which are two distinct types of human light chains, can be detected in the serum (λ FLCs: 5.7–26.3 mg/L; κ FLCs: 3.3–19.4 mg/L). The ratio of κ to λ FLCs in healthy individuals ranges from 0.26 to 1.65 [Citation7]. However, the abnormal κ to λ FLCs ratio is indicative of renal diseases and blood disorders, including intact immunoglobulin multiple myeloma, light chain multiple myeloma, and light chain amyloidosis [Citation8,Citation9]. Serum FLC levels are also affected by viral infections. Previous studies have shown that serum levels of κ and λ FLCs are significantly increased in acute hantavirus diseases [Citation10]. Additionally, notable elevations in serum κ and λ FLC levels have been observed in severe cases of SARS-CoV-2-infected patients [Citation11].
DENV is an enveloped RNA virus belonging to the family Flaviviridae, the genus Flavivirus. There are four serotypes in DENV, which are genetically diverse but share approximately 60–75% genome identity [Citation12]. The DENV genome is approximately 11 kilobases in length and consists of a positive-sense single-stranded RNA molecule. The genome is translated into a single polyprotein, which has to be properly cleaved into three structural proteins (C, prM, and E) and seven nonstructural proteins (NS1, 2A/B, 3, 4A/B, 5) by the viral NS3 protease. The NS3 protease is essential for the maturation of DENV viral particles [Citation13–16]. It is also a highly conserved multifunctional protein that acts as a serine protease and possesses RNA helicase, RNA 5′-triphosphatase (RTPase), RNA-stimulated nucleoside triphosphatase (NTPase) activities. The proteolytic activity of DENV NS3 protease for cleaving viral components requires the presence of its cofactor, NS2B, which is a conserved protein among flaviviruses. The importance of NS2B-NS3pro comes from its ability to cleave various regions in the viral polyprotein during viral replication [Citation15–17]. In addition to viral replication, recent studies have shown that NS2B-NS3pro helps DENV evade and inhibit the innate immune system. NS2B-NS3pro, for example, can inhibit the production of type I interferons in human monocyte-derived dendritic cells by degrading the human adaptor protein, stimulator of interferon genes (STING) [Citation18,Citation19]. Furthermore, recent studies suggest that NS3pro alone may possess protease activity, implying the existence of an unidentified substrate specifically targeted by NS3pro [Citation20].
Some research has unveiled that pathogens can evade human adaptive immunity by utilizing protease-mediated antibody cleavage [Citation21]. One well-known pathogen is Streptococcus pyogenes, which encodes a specific protease, designated IdeS or streptococcal Mac-1, that can specifically cleave the heavy chain of IgG [Citation22–25]. Another example is Staphylococcus aureus, which can produce serine proteases to cleave the heavy chains of IgG, IgA, and IgM [Citation26,Citation27]. Additionally, several mucosal bacteria can release specific proteases that cleave IgA1 at the hinge region, causing the loss of antibody function, such as Haemophilus influenzae, Neisseria gonorrhoeae, N. meningitidis, S. sanguis, and S. pneumoniae [Citation21,Citation28].
Based on previous literature, viral infection has been shown to impact the serum FLC levels. Therefore, our aim was to investigate the influence of dengue infection on serum FLC levels and explore the potential mechanism underlying the elevation of serum FLCs following DENV infection.
Methods
Ethics statement
The specimens used in this study were obtained from retrospective banked samples at the National Cheng Kung University Hospital during the dengue epidemic in Tainan, Taiwan, in 2015. A total of 88 dengue patients were included in the study and classified according to the severity level: dengue without warning signs, dengue with warning signs, and severe dengue, based on the 2009 World Health Organization (WHO) dengue case classification criteria [Citation29]. Additionally, 21 healthy controls were recruited from a community serosurvey conducted in late 2015. The age range of healthy donors was 22–75 years old, while dengue patients ranged from 20 to 93 years old. Written informed consent was obtained from all volunteers. All the analyses conducted in this study were approved by the Institutional Review Board of the National Cheng Kung University Hospital (IRB number: B-ER-104-178).
ELISA for free light chain (FLCs) detection
Commercial ELISA kits were used to detect λ and κ FLCs in human serum samples (Cat. No: RD194088100R, BioVendor R&D). The assays were performed and interpreted according to the manufacturer’s protocol. Briefly, serum samples were diluted 1:200 with a dilution buffer before the assay. Standards, quality controls, and samples were incubated in microplate wells pre-coated with monoclonal anti-human immunoglobulin free light chain (FLC) λ and κ antibodies. After incubation and washing, a biotin-labelled second monoclonal antibody was added to the wells and allowed to incubate with the captured antibody-FLC λ and κ complex. Subsequently, the wells were washed, and the streptavidin-HRP conjugate was added. After incubation and washing, the remaining conjugate was reacted with the TMB substrate. The reaction was stopped by adding 2 M H2SO4, and the absorbance was measured by Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher). The absorbance values were compared to the concentrations of the standards to construct a standard curve, which was then used to estimate the concentrations of the samples.
DENV NS3 ELISA
Sera were collected from 10 acute dengue patients within the first four days after fever onset to detect DENV NS3. Currently, no commercial kit is available for the NS3 ELISA assay; thus, a homemade sandwich ELISA protocol was established. Briefly, an anti-dengue NS3 polyclonal antibody (Cat. No: GTX124252, GeneTex) was used as the capture antibody and coated onto a 96-well plate. The plate was covered with plastic and incubated at 4°C overnight. The plate was washed three times with 200 μL of phosphate-buffered saline with 0.1% Tween 20 (PBST). Blocking was performed using 5% skimmed milk for 2 hours at 37°C. Then, the plate was washed three times with 200 µL of PBST. Serum from acute dengue patients (50 μL) was added to the wells and incubated for 1 hour at 37°C. Serum samples were then discarded, and the plate was washed three times with 200 μL of PBST. A Monoclonal anti-dengue NS3 antibody (Cat. No: GTX629477, GeneTex), diluted at 1:1000, was used as the detection antibody. The diluted detection antibody was added and incubated for 2 hours at room temperature (RT). The solution was discarded and the plate was washed three times with PBST. Goat anti-mouse HRP-conjugated antibody, diluted at 1:2000, was added and incubated for 1 hour. After washing, 100 μL of 3,3,’5,5’-tetramethylbenzidine (TMB) was added for a 15-minutes incubation. The reaction was terminated by adding 50 µL of 2 M H2SO4. Cell extracts from DENV-infected and uninfected Vero cells served as positive and negative controls, respectively. The optical density was read at 450 nm and calculated by subtracting the blank value (1% BSA).
Purification for NS2B-NS3pro and NS3pro
The complementary DNA (cDNA) of NS2B-NS3pro and NS3pro [Citation20] was reconstructed into the pET28a vector (Cat. No: 69-864-3, Millipore) for protein expression and purification. Escherichia coli strain Rosetta 2 was transformed with the pET28a NS2B-NS3pro and NS3pro, respectively. Various protein expression conditions were tested (S1-S2 Fig). Then, fresh transformants of Rosetta 2 cells were grown in the LB medium containing 25 μg/mL kanamycin at 37°C until the OD 600 reached 0.8. Protein expression was induced by adding 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), and the cells were grown overnight at 20°C. Cells were harvested by centrifugation at 5,000 rpm, and the cell pellet was resuspended in lysis buffer containing 20 mM Tris-HCl (pH 8.5), 50 mM NaCl, 10 mM β-mercaptoethanol, 10% glycerol, and 10 mM imidazole. The cells were lysed by sonication, and the pellet and supernatant were separated by centrifugation at 12,000 rpm for 20 minutes. For protein purification, the supernatant was incubated with Ni-NTA resin (Cat. No: 30210, QIAGEN) for 1 hour at 4°C. The resins were washed with washing buffer containing 20 mM Tris-HCl (pH 8.5), 50 mM NaCl, 10 mM β-mercaptoethanol, 10% glycerol, and 40 mM imidazole and eluted with elution buffer containing 20 mM Tris-HCl (pH 8.5), 50 mM NaCl, 10 mM β-mercaptoethanol, 10% glycerol and 1 M imidazole. To remove the glycerol and imidazole in the elution buffer, a desalting column (Cat. No: 17140801, Cytiva) was used with the running buffer containing 20 mM Tris-HCl (pH 8.5), 50 mM NaCl, and 1 mM TCEP. The purified NS2B-NS3pro and NS3pro with His-tags were concentrated to about 1.3 mg/mL by Amicon® Ultra-15 Centrifugal Filter (Cat. No: UFC903024, Millipore). Site-specific digestion of the His-tag by thrombin was used to check protein expression. As expected, both NS2B-NS3pro and NS3pro proteins could be digested by thrombin (S3 Fig).
SDS-PAGE analysis
Human IgG was purchased from Thermo Fisher (Cat. No: I 4506, Thermo Fisher). Human IgG (0.8 µg/µL) was mixed with DENV proteins, including NS2B-NS3pro (0.33 µg/µL), NS3pro (0.33 µg/µL), and NS1 protein (control) (0.33 µg/µL) (Cat. No: A2312, CTK Biotech). The DENV protein-IgG mixture was reacted in a 37°C water bath for 60 minutes. After the reaction, non-reducing sample buffer (Cat. No: JB06-F003, T-Pro Biotechnology) was added to the sample mixture, and the mixture was analysed by 12.5% SDS-PAGE at 70 V for 1 hour. Subsequently, the SDS-PAGE gel was stained by a protein assay dye reagent (Cat. No: 5000006, Bio-Rad) to visualize the protein expression patterns.
LC-MS/MS analysis
The cutting gel was de-stained first and then reduced with 10 mM dithiothreitol (Merck). Next, cysteine-blocking was performed by adding 55 mM iodoacetamide (Sigma). The gel was further digested with trypsin at 37°C for 16 hours. The reverse-phase column was used to prepare the desalted peptides. Then, the desalted peptides were separated on a homemade column by a multi-step gradient of HPLC buffer (99.9% acetonitrile/0.1% formic acid) for 70 minutes. The LC apparatus was coupled with a 2D linear ion trap mass spectrometer (Orbitrap Elite ETD; Thermo Fisher) operated using Xcalibur 2.2 software (Thermo Fisher). The full-scan MS was performed in the Orbitrap over a range of 400 to 2,000 Da and a resolution of 120,000 at m/z 400. The 20 data-dependent MS/MS scan events (10 MS2, 10 neutral-loss triggered MS3) were followed by one MS scan for the 10 most abundant precursor ions in the preview MS scan. The m/z values selected for MS/MS were dynamically excluded for 50 seconds with a relative mass window of 20 ppm. The electrospray voltage was set to 2.0 kV, and the temperature of the capillary was set to 200°C. MS and MS/MS automatic gain control was set to 1,000 ms (full scan) and 200 ms (MS/MS), or 3 × 106 ions (full scan) and 3,000 ions (MS/MS) for maximum accumulated time or ions, respectively.
Protein identification
Protein identification was performed using 1DLC, LTQ-Orbitrap MS (BIOTOOLS). The data was analysed by Proteome Discoverer software (version 1.4, Thermo Fisher). The Mascot search engine (Matrix Science, London, UK; version 2.5) was utilized against the NCBI (RefSeq) database for the MS/MS spectra searching. For peptide identification, 10 ppm mass tolerance was allowed for intact peptide masses, and 0.5 Da for CID fragment ions with allowance for two missed cleavages made from the trypsin digestion. For instance, oxidized on methionine and acetyl on protein N-terminal were considered variable modifications; carbamidomethyl on cysteine was regarded as a static modification. Peptide-spectrum matches (PSMs) were then filtered based on high confidence in peptide identification and Mascot search engine rank 1 to check the overall false discovery rate under 0.01. Proteins with single peptide hits were kept.
Western blotting
Human IgG (8 µg/mL) and DENV2 neutralizing antibody (3H5–1 clone, 16 µg/mL) (Cat. No: HB-46, ATCC) were used as the substrates to prepare western blotting samples. Substrates were added to DENV proteins, including NS2B-NS3pro, NS3pro, and NS1 control (Cat. No: A2312, CTK Biotech), in a dose manner (0.05 µg/µL−0.2 µg/µL for human IgG; 0.1 µg/µL−0.4 µg/µL for DENV2 neutralizing antibody). The mixture was further reacted at 37°C water bath for 60 minutes. Non-reducing sample buffer (Cat. No: JB06-F003, T-Pro Biotechnology) was added to the sample mixture, and the sample volume was adjusted to 40 µL. The proteins were separated using 12.5% SDS-PAGE gels in a running buffer. The gels were then transferred to the activated PVDF membranes. After that, the PVDF membrane was incubated with 5% skim milk in phosphate-buffered saline (PBS) for blocking at room temperature (RT) for at least 1 hour. For antibody detection, the following primary antibodies were used: anti-dengue NS1 primary antibody (1:2500, Cat. No: GTX124280, GeneTex) and HRP-conjugated donkey anti-rabbit secondary antibody (1:2500, Cat. No: 406401, BioLegend) for DENV NS1 detection; anti-human Ig λ light chain primary antibody (Cat. No: L65221:1000, Sigma) and HRP-conjugated donkey anti-rabbit secondary antibody (1:2500, Cat. No: 406401, BioLegend) for Ig λ light chain detection; anti-mouse Ig λ light chain (1:1000, Sigma) primary antibody and HRP-conjugated goat anti-rabbit secondary antibody (1:2500, BioLegend) for Ig λ light chain detection in DENV2 neutralizing antibody (3H5–1 clone); anti-His-tag antibody (1:5000, Cat. No: 362601, BioLegend) and HRP-conjugated goat anti-mouse secondary antibody (1:2500, Cat. No: 405306 BioLegend) for NS2B-NS3pro and NS3pro. The membrane was incubated with the primary antibody at 4℃ overnight. For the secondary antibody binding, the PVDF membrane was incubated at RT for 1 hour. After that, T-Pro LumiFast Plus Chemiluminescent Substrate Detection Kit (Cat. No: JT96-K002S, T-Pro Biotechnology) was used to detect the signal of the proteins, and the images were captured using the BioSpectrum AC Imaging system.
Focus reduction neutralization test (FRNT)
BHK21 cells (1 × 104/well) were seeded in a 96-well plate with 1 mL of DMEM containing 5% FBS and placed in a 37°C incubator for 16–18 hours. DENV2 neutralizing antibody (3H5–1 clone) was incubated with NS2B-NS3pro and NS3pro, respectively, at a 37℃ water bath for 2 hours. The working concentration of the DENV2 neutralizing antibody (3H5–1 clone) was at 0.33 μg/μL, and the concentrations of DENV proteases were 0.4 μg/μL. DENV2 (16681 strain) was diluted in DMEM containing 2% FBS to a concentration of 1,000 pfu/well. An equal volume of DENV was added to the DENV2 neutralizing antibodies (3H5–1 clone) and the protease-treated antibodies. The viruses were co-incubated with antibody-enzyme mixture in a 37°C incubator for 30 minutes, and 50 μL of the final product was added to each well. After 2–3 hours of absorption, the mixture was removed, and methylcellulose media containing 2% FBS was added to the wells and placed in the 37°C incubator for 3–4 days. Following incubation, methylcellulose media was removed; the cells were washed with PBS and then fixed with 4% paraformaldehyde at 37°C for 30 minutes. Subsequently, 50 μL of 0.5% Triton X-100 was added at RT for 10 minutes after washing with PBS. The cells were stained with anti-dengue NS1 (mAb 33D2, 5 μg/mL) at 4°C overnight. Donkey anti-mouse IgG secondary antibody (2 μg/mL) (Cat. No: A-21202, Invitrogen, Alexa Fluor™ 488) was added and incubated at RT for 1 hour in the absence of light. The cells were stained with DAPI (5 μg/mL) at RT for 10 minutes. After staining, the number of foci was counted in the entire well using a fluorescence microscope (Olympus, Tokyo, Japan) and further converted into the relative neutralization efficacy of antibodies (%) using the following equation.
Equation of relative neutralizing efficacy (%):
Statistical analysis
Graphs generated from data were shown with mean ± standard error of the mean (SEM). Statistical analyses were performed using the Mann-Whitney U test to determine significant differences between groups. Correlation tests were conducted using the Pearson correlation test to assess relationships between variables. Receiver operating characteristic (ROC) curves and area under the curves (AUC) were constructed to evaluate the diagnostic efficacy in distinguishing dengue fever and severe dengue cases. The optimal cut-off values were determined by maximizing the Youden index, which is calculated as (Sensitivity + Specificity − 1). Statistically significant differences were marked as * (p < 0.05), ** (p < 0.01), *** (p < 0.001) and **** (p < 0.0001), respectively. All statistical calculations and analyses were done using GraphPad Prism Version 9.
Results
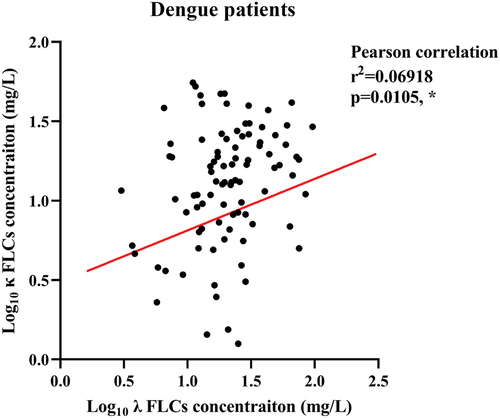
Positive correlation between serum λ and κ FLCs in dengue patients
Considering that free light chains (FLCs) have been reported to be upregulated and exhibited positive correlation in viral diseases [Citation10,Citation30], the Pearson correlation was used to study the correlation between λ and κ FLCs in DENV infection. The result revealed a weak positive correlation between serum levels of λ and κ FLCs in dengue patients (r2 = 0.06918, p = 0.0105*) ().
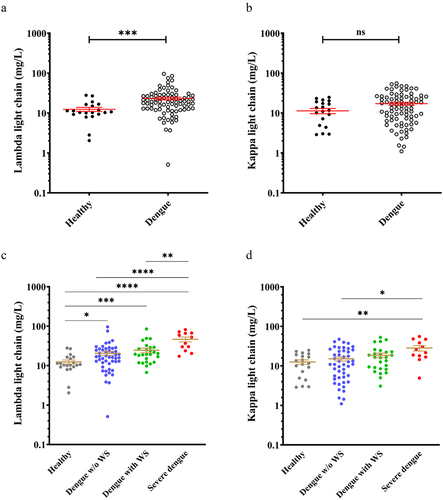
Serum λ free light chain is higher in dengue patients with varying severity
We first confirmed that the ratio of κ:λ FLCs stayed within the reference range in dengue patients (dengue patients without warning signs: 0.7256 ± 0.064; dengue patients with warning signs: 0.7537 ± 0.1272; Severe dengue 0.7260 ± 0.1437). Analysis of serum FLCs showed higher levels of λ FLCs in dengue patients compared to healthy donors (). However, there was no significant difference in κ FLCs expression between the healthy donors and dengue patients (). Further comparison of serum FLCs levels among dengue patients with varying disease severity showed serum λ FLCs levels increased following disease severity, particularly in severe dengue (SD) patients, where the levels were significantly higher compared to both the dengue patients with warning signs and without warning signs (). On the other hand, a higher level of κ light chains was only detected in SD patients when compared to healthy donors and dengue patients without warning signs (). In summary, our findings indicate that serum λ FLCs levels were higher in dengue patients compared to healthy donors and increased with disease severity. Specifically, patients with severe dengue exhibited elevated levels of both serum λ FLCs and κ light chains.
Figure 2. Higher serum λ free light chain expression in dengue patients with varying severity.
Lambda (λ) free light chain as a potential marker of dengue disease and severe dengue identification
We further assessed the possibility of elevated serum FLCs as a marker for dengue disease, using ROC curves analyses. The results demonstrated that serum λ FLCs had a larger area under the ROC curve (AUC) compared to κ FLCs, indicating better diagnostic performance (λ FLCs: AUC = 0.7825, Sensitivity = 80%, Specificity = 71.43%, p < 0.0001****; κ FLCs: AUC = 0.5911, Sensitivity = 55.67%, Specificity = 71.43%, p = 0.1918) (, ); a concentration of λ FLC >12.09 mg/L was the optimal cut-off value according to the Youden index calculation for distinguishing dengue patients from the healthy donor. Furthermore, we evaluated the ability of serum FLCs to differentiate SD patients from non-SD patients, both dengue patients with and without warning signs. The AUC for λ FLCs was also larger than that for κ FLCs and a concentration of >29.55 mg/L was identified as the optimal cut-off value based on the Youden index calculation for distinguishing SD cases from non-SD patients (λ FLCs: AUC = 0.8102, Sensitivity = 75%, Specificity = 79.52%, p = 0.0005***; κ FLCs: AUC = 0.7216, Sensitivity = 91.67%, Specificity = 50.52%, p = 0.0125*) (, ). Therefore, instead of κ FLCs, λ FLCs were revealed to be the proper marker for distinguishing dengue patients from healthy individuals and also the SD cases identification from non-severe dengue patients.
Figure 3. Lambda (λ) free light chain as a potential serum marker of dengue disease and severe dengue classification.
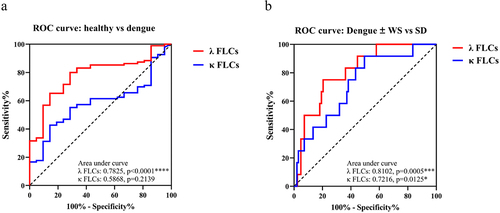
Table 1. Area under ROC curve of serum free light chains to discriminate dengue patients.
Lambda (λ) free light chain appears after IgG and DENV protease co-incubation
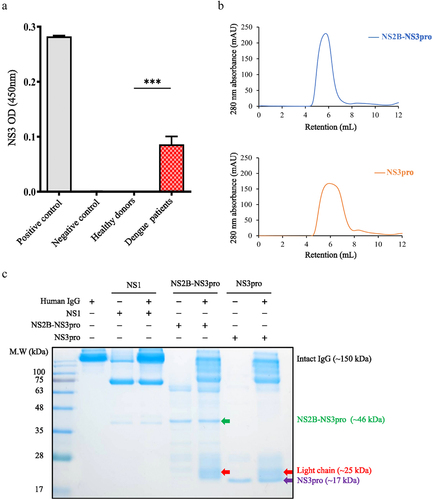
The results presented above have suggested that serum level of λ FLCs could serve as a potential marker for differentiating both dengue and SD patients. Therefore, we conducted further research to investigate the underlying mechanism behind the observed elevation of serum λ FLCs during DENV infection. It has been discovered that the dengue NS3 protein possesses protease activity, allowing it to cleave both viral polyproteins and host protein substrates [Citation17–19]. Therefore, we hypothesize that the increase of serum FLCs might be attributed to the DENV NS3 protease activity, which could potentially target antibodies as substrates. Initially, we evaluated the level of DENV NS3 protease in the serum of individuals with acute DENV infection. A homemade sandwich ELISA protocol was employed to detect DENV NS3 protease levels using sera from 10 acute dengue patients. The findings revealed the presence of DENV NS3 protease in the circulation of individuals with acute dengue infection, as compared to healthy controls (). Subsequently, we proceeded to purify DENV protease, both NS2B-NS3pro (with NS2B co-factor) () and NS3pro (without NS2B co-factor) () were purified from an E. coli expression system. Afterward, we further conducted experiments to determine whether DENV NS2B-NS3pro and NS3pro possess the ability to cleave antibodies. Human IgG was employed as a substrate and reacted with NS2B-NS3pro, NS3pro, and NS1 (without protease activity), respectively. The results demonstrated that intact IgG (150 kDa) remained unaltered in the NS1 group and control group, whereas the IgG showed a smear pattern when treated with NS2B-NS3pro and NS3pro, indicating IgG cleaved. Apparently, FLCs (approximately 25 kDa) were shown in both the NS2B-NS3pro and the NS3pro treatment groups (). These findings strongly suggest the involvement of NS2B-NS3pro and NS3pro in the cleavage of IgG.
Figure 4. Lambda (λ) free light chain appears upon co-incubation of IgG and DENV protease.
The SDS-PAGE gel containing FLCs was cut and subjected to further analysis using LC-MS/MS. The data analysis was performed utilizing Proteome Discoverer software and the MS/MS spectra were searched against the NCBI (RefSeq) database. The findings indicate that the most abundant protein identified is Ig λ-like polypeptide, the precursor of Ig λ light chain, following co-incubation of human IgG with both DENV NS2B-NS3pro () and NS3pro (). Overall, these results strongly suggest the presence of λ FLCs resulting from the cleavage of human IgG by DENV protease in vitro.
Table 2. Protein composition revealing light chain product after NS2B-NS3pro treatment.
Table 3. Protein composition revealing light chain product after NS3pro treatment.
Lambda (λ) free light chain increases in a dose-dependent manner after DENV protease cleaves human IgG
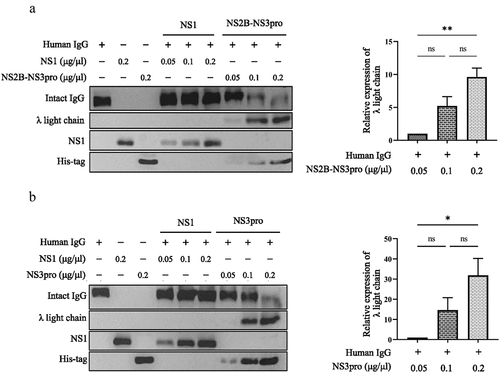
Subsequently, we performed western blotting to evaluate the proteolytic activity of NS2B-NS3pro and NS3pro on IgG, leading to the appearance of λ FLCs. Different concentrations of DENV proteases were utilized for this purpose. The results demonstrated a dose-dependent trend in the protein levels of λ FLCs, with evident cleavage observed when treated with medium (0.1 µg/µL) and higher doses (0.2 µg/µL) of both NS2B-NS3pro () and NS3pro (). Bar graphs were generated to summarize the results, confirming that the treatment with both DENV NS2B-NS3pro and NS3pro increased the protein levels of λ FLCs in a trend of dose-dependent manner. These findings suggest that both NS2B-NS3pro and NS3pro possess the ability to cleave human IgG. Additionally, the results indicate that DENV NS3pro retains its immunoglobulin cleavage activity even in the absence of the NS2B cofactor. In agreement with the results using purified human IgG, our study using plasma from dengue patients also showed an increase in the protein levels of λ FLCs in accordance with the dose-dependent pattern following the administration of both DENV NS2B-NS3pro and NS3pro (S4 Fig).
Figure 5. Lambda (λ) free light chain increases in a trend of dose-dependent manner after DENV protease cleaves human IgG.
DENV protease cleaves DENV2-neutralizing antibodies leading to λ free light chains increase and neutralizing efficacy reduction
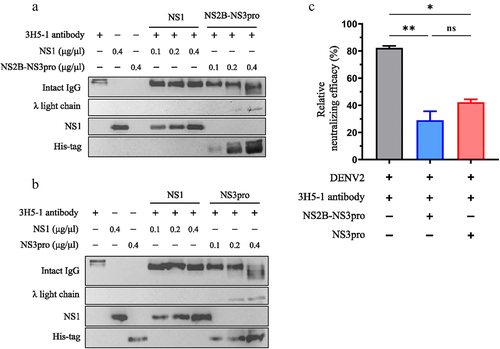
We successfully detected an increase in λ FLCs following the cleavage of human IgG by DENV protease. We hypothesized that DENV protease might also play a role in the degradation of DENV-neutralizing antibodies. To evaluate the impact on neutralizing antibodies, DENV2-neutralizing monoclonal antibodies (3H5–1 clone) [Citation31,Citation32] were treated with DENV protease. The results demonstrated that both NS2B-NS3pro () and NS3pro () cleaved DENV2 neutralizing antibodies, resulting in an increase in λ FLCs protein in a dose-dependent trend. Furthermore, the neutralizing efficacy of the DENV protease-treated antibodies was assessed using the FRNT. The results revealed a significant reduction in antibody-neutralizing efficacy after treatment with both NS2B-NS3pro and NS3pro (). Collectively, these findings indicate that DENV protease can cleave antibodies, leading to the appearance of λ FLCs and a subsequent decrease in their neutralizing efficacy.
Figure 6. DENV protease cleaves DENV2-neutralizing antibodies resulting in λ free light chain increase and neutralizing efficacy reduction.
Discussion
In this study, we observed elevated levels of serum λ FLCs in dengue patients, which further increased with disease severity. Additionally, we investigated the potential of serum λ FLCs as markers for distinguishing both dengue disease and severe dengue (SD). Furthermore, we explored a novel mechanism suggesting that the increase in λ FLCs after DENV infection might be attributed to the proteolytic activity of DENV NS3 protease, as observed in our in vitro experiments. Both human IgG and DENV2-neutralizing antibodies were identified as potential substrates of DENV protease, leading to the elevated levels of λ FLCs and subsequent reduction in the neutralizing efficacy of antibodies.
In serum from healthy individuals, a small amount of unbound κ and λ FLCs, not bound to immunoglobulin heavy chains, can be detected [Citation33]. However, excessive production of light chain products has been reported in haematological disorders characterized by immune system stimulation, such as multiple myeloma, monoclonal gammopathies, and autoimmune diseases [Citation8,Citation34,Citation35]. Recent studies have provided further evidence suggesting an association between serum FLCs levels and viral infections. These infections include hepatitis C virus, hepatitis B virus (HBV), human immunodeficiency virus (HIV), hantavirus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [Citation10,Citation11,Citation30,Citation36,Citation37]. Evidence suggests a strong association between κ FLCs and the anti-hepatitis B virus (HBV) immune response, highlighting κ FLCs as potential prognostic markers for HBV [Citation37]. Additionally, κ FLCs have been identified as diagnostic tools for distinguishing between mild/moderate and severe cases of COVID-19 (coronavirus disease 2019), with a sensitivity of 87% and specificity of 93% [Citation11]. In contrast to κ FLCs, our findings suggest that serum λ FLCs have potential as a serum marker for discriminating dengue disease (AUC = 0.7825, Sensitivity = 80%, Specificity = 71.43%) from healthy individuals and SD cases identification (AUC = 0.8102, Sensitivity = 75%, Specificity = 79.52%) from non-severe dengue patients.
Previous reports have indicated that elevated serum FLCs are associated with B cell activation, resulting in excessive FLC production by terminally-differentiated B cells and plasma cells [Citation38]. In our study, a positive correlation was found between serum levels of λ and κ FLCs in dengue patients. Additionally, we also observed elevated levels of λ FLCs in dengue patients across all disease grades, whereas κ FLCs were only higher in SD patients. Interestingly, our in vitro study revealed an alternative mechanism for serum FLCs elevation involving DENV protease targeting human antibodies. Through LC/MS-MS analysis, we identified Ig λ-like polypeptide as the most abundant peptide following co-incubation of human IgG with DENV protease (, ). Western blotting confirmed the presence of λ FLCs resulting from DENV protease-mediated antibody cleavage. Hence, the novel function of DENV protease which targeting IgG as substrates was identified in our study. Moreover, detailed investigations are necessary to elucidate the mechanism by which DENV protease cleaves human IgG and identify the specific cleavage site on the IgG molecule.
Pepsin and papain have been recognized as enzymes capable of cleaving IgG antibodies [Citation39,Citation40]. Additionally, various bacterial proteases have been identified as antibody-cleaving enzymes targeting IgM, IgG, and IgA antibodies [Citation22,Citation24,Citation26]. In our study, we have discovered that DENV protease targets antibodies circulating in the bloodstream, suggesting its role as an additional antibody-cleaving enzyme. Moreover, our in vitro assay demonstrated a decrease in the neutralizing efficacy of DENV2-neutralizing antibodies following co-incubation with DENV protease (). Further research is necessary to investigate whether antibody cleavage by DENV protease occurs in vivo and its potential implications in reducing antibody titres. It is crucial to elucidate the primary target of DENV protease within the IgG subclasses, as well as explore its potential targeting of other antibody classes such as IgM, IgE, and IgA.
The proteolytic activity of DENV NS3 protease is primarily known to occur within the cell’s cytoplasm, where it cleaves the viral polyprotein into distinct proteins to facilitate viral replication and assembly [Citation17,Citation41]. Interestingly, we detected the presence of DENV NS3 protease in the serum of acute dengue patients (), consistent with recent study reporting NS3 protein in clinical serum samples from various dengue disease grades [Citation42]. Notably, in addition to viral polyproteins, several host proteins have been identified as targets of DENV protease. For instance, the NS2B-NS3pro interacts with the innate immune adaptor protein STING, resulting in a reduction in type 1 interferon secretion [Citation19]. The NS2B-NS3pro complex has also been implicated in the cleavage of nucleoporins (Nups) and disruption of nuclear-cytoplasmic protein transport in Huh7 cells [Citation43,Citation44]. Initially, it was believed that the hydrophobic domain of NS2B interacts with NS3 protease, enabling the enzymatic activity of the NS2B-NS3pro complex for catalysing viral polyprotein cleavage [Citation45,Citation46]. However, recent evidence suggests that NS3pro alone can interact with cellular proteins, such as IκBα and IκBβ [Citation20], and can cleavage the GrpE-like 1 (GrpEL1) protein in the mitochondria [Citation47]. Consistent with these findings, our study also observed that NS3pro alone, even in the absence of the NS2B cofactor, retained the ability to cleave human IgG and DENV2-neutralizing antibodies. Therefore, future research should focus on quantifying the serum levels of DENV NS3 protease in dengue patients with varying disease severity and identifying the unknown host proteins that serve as substrates for DENV NS3 protease.
Our study was limited by a small sample size of both healthy donors and SD patients. Therefore, a well-designed study with a larger cohort of healthy donors and dengue patients is necessary to further investigate the changes in serum FLCs levels after DENV infection. To our knowledge, this is the first study to uncover the novel function of DENV protease in specifically targeting antibodies as substrates. Hence, validating DENV protease-mediated antibody cleavage in an appropriate animal model is crucial for understanding the physiological role of DENV protease during DENV infection. Additionally, future research can be conducted to explore the enzyme kinetics of dengue protease and its capacity to cleave antibodies. This investigation will enhance our understanding of antibody cleavage by dengue protease and the interactions between DENV and the host immune response.
In conclusion, our findings demonstrate elevated levels of serum FLCs in dengue patients. We specifically identified lambda (λ) FLCs as a potential serum marker for distinguishing dengue patients from healthy individuals, as well as for the classification of SD patients from dengue patients. Moreover, we investigated the mechanism behind the increase of λ FLCs, revealing the involvement of antibody cleavage by DENV NS3 protease. These novel insights may contribute to the development of diagnostic tools and enhance our understanding of the pathogenesis of DENV infection.
Author contributions
Sheng-Hsuan Wang Role: Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing; Bai-Jiun Kuo Role: Investigation, Methodology, Resources; Tzu-Chuan Ho Role: Formal analysis, Investigation, Methodology; Shu-Wen Wan Role: Methodology, Resources, Writing – review & editing; Ko-Lun Yen Role: Methodology, Resources; Po-Huei Huang Role: Methodology, Resources; Oscar Guey Chuen Perng; Role: Conceptualization, Data curation, Funding acquisition, Project administration, Resources; Po-Lin Chen Role: Resources, Supervision; Yu-Wen Chien Role: Funding acquisition, Project administration, Supervision, Writing – review & editing; Yu-Chih Lo Role: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Writing – review & editing
Supplemental Material
Download Zip (1.3 MB)Acknowledgements
We would like to thank all the volunteers for kindly support of our project. We are grateful to Professor Yee-Shin Lin for her constructive suggestions on this study. We also thank Professor Betty A. Wu-Hsieh at the Graduate Institute of Immunology, College of Medicine, National Taiwan University, for kindly providing the cDNA of DENV protease.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data Availability statement
The supporting data for this study can be obtained from the corresponding author upon reasonable request.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2023.2279355
Additional information
Funding
References
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013 Apr 25;496(7446):504–15. doi: 10.1038/nature12060
- Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010 Dec;8(12 Suppl):S7–16. doi: 10.1038/nrmicro2460
- St John AL, Rathore APS. Adaptive immune responses to primary and secondary dengue virus infections. Nat Rev Immunol. 2019 Apr;19(4):218–230. doi: 10.1038/s41577-019-0123-x
- Screaton G, Mongkolsapaya J, Yacoub S, et al. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol. 2015 Dec;15(12):745–759. doi: 10.1038/nri3916
- Bhatt P, Sabeena SP, Varma M, et al. Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021 Jan;78(1):17–32. doi: 10.1007/s00284-020-02284-w
- World Health Organization. Dengue and severe dengue. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- Bhole MV, Sadler R, Ramasamy K. Serum-free light-chain assay: clinical utility and limitations. Ann Clin Biochem. 2014 Sep;51(Pt 5):528–542. doi: 10.1177/0004563213518758
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–1473. doi: 10.1038/sj.leu.2404284
- Jenner E. Serum free light chains in clinical laboratory diagnostics. Clin Chim Acta. 2014 Jan 1;427:15–20. doi: 10.1016/j.cca.2013.08.018
- Hepojoki J, Cabrera LE, Hepojoki S, et al. Hantavirus infection-induced B cell activation elevates free light chains levels in circulation. PLOS Pathog. 2021 Aug;17(8):e1009843. doi: 10.1371/journal.ppat.1009843
- Malecka-Gieldowska M, Folta M, Wisniewska A, et al. Cell population data and serum polyclonal immunoglobulin free light chains in the assessment of COVID-19 severity. Viruses. 2021 Jul 15;13(7):1381. doi: 10.3390/v13071381
- Guzman MG, Harris E. Dengue. Lancet (London, England). 2015 Jan 31;385(9966):453–465. doi: 10.1016/S0140-6736(14)60572-9
- Perera R, Kuhn RJ. Structural proteomics of dengue virus. Curr Opin Microbiol. 2008 Aug;11(4):369–377. doi: 10.1016/j.mib.2008.06.004
- Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005 Jan;3(1):13–22. doi: 10.1038/nrmicro1067
- Yamshchikov VF, Compans RW. Formation of the flavivirus envelope: role of the viral NS2B-NS3 protease. J Virol. 1995 Apr;69(4):1995–2003. doi: 10.1128/jvi.69.4.1995-2003.1995
- Yamshchikov VF, Trent DW, Compans RW. Upregulation of signalase processing and induction of prM-E secretion by the flavivirus NS2B-NS3 protease: roles of protease components. J Virol. 1997 Jun;71(6):4364–4371. doi: 10.1128/jvi.71.6.4364-4371.1997
- Falgout B, Pethel M, Zhang YM, et al. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol. 1991 May;65(5):2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991
- Rodriguez-Madoz JR, Belicha-Villanueva A, Bernal-Rubio D, et al. Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex. J Virol. 2010 Oct;84(19):9760–9774. doi: 10.1128/JVI.01051-10
- Aguirre S, Maestre AM, Pagni S, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLOS Pathog. 2012;8(10):e1002934.
- Lin JC, Lin SC, Chen WY, et al. Dengue viral protease interaction with NF-kappaB inhibitor alpha/beta results in endothelial cell apoptosis and hemorrhage development. J Immunol. 2014 Aug 1;193(3):1258–1267. doi: 10.4049/jimmunol.1302675
- Travis J, Potempa J. Bacterial proteinases as targets for the development of second-generation antibiotics. Biochim Biophys Acta. 2000 Mar 7;1477(1–2):35–50. doi: 10.1016/S0167-4838(99)00278-2
- von Pawel-Rammingen U, Johansson BP, Tapper H, et al. Streptococcus pyogenes and phagocytic killing. Nat Med. 2002 Oct;8(10):1044–1045. author reply 1045-6. doi: 10.1038/nm1002-1044
- Wenig K, Chatwell L, von Pawel-Rammingen U, et al. Structure of the streptococcal endopeptidase IdeS, a cysteine proteinase with strict specificity for IgG. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17371–17376. doi: 10.1073/pnas.0407965101
- Vindebro R, Spoerry C, von Pawel-Rammingen U. Rapid IgG heavy chain cleavage by the streptococcal IgG endopeptidase IdeS is mediated by IdeS monomers and is not due to enzyme dimerization. FEBS Lett. 2013 Jun 19;587(12):1818–1822. doi: 10.1016/j.febslet.2013.04.039
- Leborgne C, Barbon E, Alexander JM, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med. 2020 Jul;26(7):1096–1101. doi: 10.1038/s41591-020-0911-7
- Prokesova L, Potuznikova B, Potempa J, et al. Cleavage of human immunoglobulins by serine proteinase from staphylococcus aureus. Immunol Lett. 1992 Feb 15;31(3):259–265. doi: 10.1016/0165-2478(92)90124-7
- Ryan MH, Petrone D, Nemeth JF, et al. Proteolysis of purified IgGs by human and bacterial enzymes in vitro and the detection of specific proteolytic fragments of endogenous IgG in rheumatoid synovial fluid. Mol Immunol. 2008 Apr;45(7):1837–1846. doi: 10.1016/j.molimm.2007.10.043
- Siegel SJ, Weiser JN. Mechanisms of bacterial colonization of the respiratory tract. Annu Rev Microbiol. 2015;69(1):425–444. doi: 10.1146/annurev-micro-091014-104209
- World Health O. Dengue guidelines for diagnosis, treatment, prevention and control : new edition. Geneva: World Health Organization; 2009.
- Shiels MS, Landgren O, Costello R, et al. Free light chains and the risk of AIDS-defining opportunistic infections in HIV-infected individuals. Clin Infect Dis. 2012 Nov 15;55(10):e103–8. doi: 10.1093/cid/cis692
- Shen WF, Galula JU, Liu JH, et al. Epitope resurfacing on dengue virus-like particle vaccine preparation to induce broad neutralizing antibody. Elife. [2018 Oct 18];7. doi: 10.7554/eLife.38970
- Renner M, Flanagan A, Dejnirattisai W, et al. Characterization of a potent and highly unusual minimally enhancing antibody directed against dengue virus. Nat Immunol. 2018 Nov;19(11):1248–1256. doi: 10.1038/s41590-018-0227-7
- Katzmann JA, Abraham RS, Dispenzieri A, et al. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem. 2005 May;51(5):878–881. doi: 10.1373/clinchem.2004.046870
- Aggarwal R, Sequeira W, Kokebie R, et al. Serum free light chains as biomarkers for systemic lupus erythematosus disease activity. Arthritis Care Res (Hoboken). 2011 Jun;63(6):891–898. doi: 10.1002/acr.20446
- Bettacchioli E, Le Gaffric C, Mazeas M, et al. An elevated polyclonal free light chain level reflects a strong interferon signature in patients with systemic autoimmune diseases. J Transl Autoimmun. 2021;4:100090. doi: 10.1016/j.jtauto.2021.100090
- Basile U, Napodano C, Pocino K, et al. Lack of association between vitamin D status and free light chains profile with different chronic HCV-related liver and extrahepatic disorders. Eur Rev Med Pharmacol Sci. 2019 Oct;23(19):8506–8514. doi: 10.26355/eurrev_201910_19164
- Chen B, Wang W, Xu W, et al. Serum free light chain is associated with histological activity and cirrhosis in patients with chronic hepatitis B. Int Immunopharmacol. 2021 Oct;99:107881.
- Deng X, Crowson CS, Rajkumar SV, et al. Elevation of serum immunoglobulin free light chains during the preclinical period of rheumatoid arthritis. J Rheumatol. 2015 Feb;42(2):181–187. doi: 10.3899/jrheum.140543
- Virella G, Parkhouse RM. Papain sensitivity of heavy chain sub-classes in normal human IgG and localizaton of antigenic determinants for the sub-classes. Immunochemistry. 1971 Mar;8(3):243–250. doi: 10.1016/0019-2791(71)90478-2
- Turner MW, Bennich HH, Natvig JB. Pepsin digestion of human G-myeloma proteins of different subclasses. II. Immunochemical investigations of the products of peptic digestion. Clin Exp Immunol. 1970 Nov;7(5):627–640.
- Li H, Clum S, You S, et al. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J Virol. 1999 Apr;73(4):3108–3116. doi: 10.1128/JVI.73.4.3108-3116.1999
- Gandikota C, Gandhi L, Maisnam D, et al. A novel anti-NS2BNS3pro antibody-based indirect ELISA test for the diagnosis of dengue virus infections. J Med Virol. 2021 Jun;93(6):3312–3321. doi: 10.1002/jmv.26024
- Jesús-González LA D, Cervantes-Salazar M, Reyes-Ruiz JM, et al. The nuclear pore complex: a target for NS3 protease of dengue and Zika Viruses. Viruses. 2020;12(6):583.
- Palacios-Rapalo SN, De Jesus-Gonzalez LA, Reyes-Ruiz JM, et al. Nuclear localization of non-structural protein 3 (NS3) during dengue virus infection. Arch Virol. 2021 May;166(5):1439–1446. doi: 10.1007/s00705-021-05026-w
- Khromykh AA, Varnavski AN, Sedlak PL, et al. Coupling between replication and packaging of flavivirus RNA: evidence derived from the use of DNA-based full-length cDNA clones of kunjin virus. J Virol. 2001 May;75(10):4633–4640. doi: 10.1128/JVI.75.10.4633-4640.2001
- Patkar CG, Kuhn RJ. Yellow fever virus NS3 plays an essential role in virus assembly independent of its known enzymatic functions. J Virol. 2008 Apr;82(7):3342–3352. doi: 10.1128/JVI.02447-07
- Gandikota C, Mohammed F, Gandhi L, et al. Mitochondrial import of dengue virus NS3 protease and cleavage of GrpEL1, a cochaperone of mitochondrial Hsp70. J Virol. 2020 Aug 17;94(17). doi: 10.1128/JVI.01178-20