ABSTRACT
Streptococcus suis is a zoonotic Gram-positive bacterium that causes invasive infections such as sepsis and meningitis, threatening public health worldwide. For successful establishment of infection, the bacterium should subvert the innate effectors of immune defence, including the cathelicidin family of host-defence peptides that combat pathogenic bacteria by directly disrupting cell membranes and coordinating immune responses. Here, our study shows that an extracellular endopeptidase O (PepO) of S. suis contributes to assisting the bacterium to resist cathelicidin-mediated killing, as the deletion of the pepO gene makes S. suis more sensitive to the human cathelicidin LL-37, as well as its mouse equivalent, mCRAMP. This protease targets and cleaves both LL-37 and mCRAMP, degrading them into shorter peptides with only a few amino acids, thereby abrogating their ability to kill S. suis. By cleaving LL-37 and mCRAMP, PepO impairs their chemotactic properties for neutrophil migration and undermines their anti-apoptosis activity, which is required for prolonging neutrophil lifespan. Also, PepO inhibits the ability of LL-37 and mCRAMP to promote lysosome development in macrophages. Moreover, the loss of PepO attenuates organ injury and decreases bacterial burdens in a murine model of S. suis bacteraemia. Taken together, these data provide novel insights into the role of the intrinsic proteolytic characteristics of PepO in S. suis-host interaction. Our findings demonstrate that S. suis utilizes the PepO protease to cleave cathelicidins, which is an immunosuppressive strategy adopted by this bacterium to facilitate pathogenesis.
Introduction
Streptococcus suis is a gram-positive pathogen that represents a principal health problem in the porcine industry, leading to serious economic losses worldwide [Citation1]. As an emerging zoonotic pathogen, S. suis is also responsible for serious invasive infections in humans, including meningitis, bacteraemia, arthritis, and endocarditis [Citation2–4]. There have been over 1600 human cases of S. suis infection worldwide [Citation1,Citation3]. Indeed, adult meningitis caused by S. suis is prevalent in Vietnam, Thailand, and China [Citation5,Citation6]. Although human infection with S. suis is usually sporadic, the pooled case fatality rate is up to 12.8% [Citation2]. Notably, two significant S. suis outbreaks in China in 1998 and 2005 led to 229 person infections and 52 fatalities, which sparked considerable public health concerns [Citation7–9].
Early clearance of extracellular bacteria, such as S. suis, is mainly dependent on the primary innate effectors of host immunity systems. Of these effectors, cathelicidins are a family of ubiquitous host-peptides (HDPs) found in vertebrates that serve a critical front-line role in innate immunity against invasive pathogens [Citation10]. Cathelicidins can be rapidly and strongly induced after invasive infection, and the high concentrations of these peptides at the sites of infection can effectively kill pathogens [Citation10,Citation11]. LL-37 is the sole human cathelicidin, which is mainly secreted from epithelial cells and many immune cells [Citation12]. LL-37 shows a broad spectrum of antimicrobial activity against multiple microorganisms [Citation13]. Interestingly, LL-37 effectively combats infections not only through its direct microbicidal property but also enhancing the immune responses of its host [Citation14,Citation15]. The modulation of LL-37 during immune responses is complex, including regulation of Toll-like receptor (TLR) activation, induction of cytokine production, regulation of leukocyte chemotaxis, prolongation of neutrophil lifespan, promotion of wound healing, and regulation of phagocytosis [Citation12,Citation15,Citation16]. As the functions of cathelicidins from different species are largely conserved [Citation17], mCRAMP, the mouse ortholog of LL-37, performs a similar function as LL-37 in impeding bacterial infection [Citation18,Citation19].
For successful establishment of infection, S. suis must pass through host mucosal barriers to enter the bloodstream, where it can then avoid immune system clearance and invade different organs throughout the body [Citation20,Citation21]. During this process, S. suis is exposed to a large amount of cathelicidins and must overcome cathelicidin-mediated defence, which is indispensable for S. suis survival and proliferation in its host.
S. suis produces an endopeptidase O, also known as PepO, which has been previously identified as an extracellular protein [Citation22]. PepO has been found to aid S. suis adherence to and invasion of brain microvascular endothelial cells by interacting with human fibronectin and plasminogen [Citation23,Citation24]. Although the interactions of PepO with extracellular matrix components have been reported, the possible contribution of the intrinsic proteolytic characteristic of PepO to S. suis-host interaction and, thus, to its mediated immune evasion and pathogenesis, is still unknown.
Here, we investigated the involvement of PepO in the interaction with LL-37 and mCRAMP and its contribution to the pathogenesis of S. suis. We show that S. suis PepO cleaves LL-37 and mCRAMP, thereby sabotaging the bactericidal activity of LL-37 and mCRAMP against S. suis. Moreover, the cleavage of PepO on LL-37 and mCRAMP undermines their immunomodulatory functions for neutrophil migration, prolongation of neutrophil lifespan, and lysosome formation in macrophages. We also demonstrated that the deletion of pepO attenuates the virulence of S. suis in vivo. Our data not only provide a functional explanation for the PepO-cathelicidin interaction but also demonstrate a key function of this interaction for the immune evasion and pathogenesis of S. suis.
Materials and methods
Ethics statement
The procedures of the mouse experiments were approved by the Institutional Animal Care and Use Committee of the Harbin Veterinary Research Institute (Permission No. HVRI-IACUC-210412-02).
Bacterial strains, plasmids, and culture conditions
Bacterial strains and plasmids are listed in . S. suis wild-type (WT) strain was the serotype 2 highly virulent strain 05ZYH33. S. suis strains were cultured at 37°C and 5% CO2 in tryptic soy broth (TSB; Difco) medium or tryptic soy agar (TSA). Escherichia coli strains were grown in Luria-Bertani (LB; Difco) medium with shaking. Growth media were supplemented with appropriate antibiotics: erythromycin (5 μg/ml for S. suis, 180 μg/ml for E. coli), spectinomycin (100 μg/ml for S. suis, 50 μg/ml for E. coli), and ampicillin (100 μg/ml for E. coli).
Table 1. Characteristics of bacterial strains and plasmids used in this study.
Isolation of mouse neutrophils and macrophages
Mouse neutrophils were isolated from the bone marrow of C57BL/6 mice and purified by isodensity centrifugation using Histopaque-1119 (Sigma-Aldrich, 11191) and Histopaque-1077 (Sigma-Aldrich, 10771). All neutrophils were finally suspended in RPMI-1640 medium.
Mouse peritoneal macrophages were isolated from C57BL/6 mice three days following an intraperitoneal injection of 3 mL Thioglycollate Medium (Difco). The mice were sacrificed, and peritoneal fluid was collected and centrifuged at 600 × g for 10 minutes. The obtained cells were washed and resuspended in RPMI-1640 medium. Non-adherent cells were removed with PBS after 6 hours of incubation, and adherent cells were grown at 37°C in a humidified 5% CO2 atmosphere.
Peptide synthesis and recombinant protein purification
All of the peptides, namely LL-37, mCRAMP, and their truncated peptides FR-7, IG-7, IV-8, FK-7, IK-7, LR-32, LR-21, LR-18, GL-13 and LR-11, are commercially synthesized by GL Biochem with a purity of > 95%.
The signal peptide cleavage site of S. suis PepO was predicted by SignalP 4.0 [Citation25]. The conserved domains of PepO were identified using SMART [Citation26]. Protein motif sequences were aligned using ClustalW [Citation27] and were presented by ESPript [Citation28]. Structure homology modelling of PepO was conducted by SWISS-MODEL using the 3D structure of Mycobacterium tuberculosis metalloprotease Zmp1 (PDB:3ZUK) as a template [Citation29]. The structure of PepO was presented by PyMOL (DeLano Scientific). Gene fragment encoding of the PepO residues Pro29–Trp663 (without signal peptide sequence) was amplified from the genomic DNA of S. suis 05ZYH33 by PCR using the primers pepO-F/R (). Using overlap extension PCR, a gene fragment containing the Glu513 to Ala mutation was amplified for site-directed mutagenesis. The PCR products were ligated into a NdeI and XhoI-linearized pET22b vector (Novagen). After being verified by sequencing, the recombinant vectors were introduced into competent E. coli BL21 (DE3) cells. Then, protein expression was stimulated at 16°C for 12 hours by adding isopropyl 1-thio-D-galactopyranoside (IPTG) to a final concentration of 0.5 mM. Bacterial cells were harvested by centrifugation at 6000 ×g for 10 min, washed twice, and then resuspended in 50 ml of lysis buffer (20 mM Tris-HCl, 5 mM imidazole, 500 mM NaCl, pH 8.0). After sonication on ice, the lysates were centrifuged at 12,000 ×g for 10 min. His-tagged proteins were purified using the Ni-Sepharose fast flow column (GE Healthcare, 17-5318-02). After the proteins were applied to the column and washed with 80 ml of washing buffer (20 mM Tris-HCl, 20 mM imidazole, 500 mM NaCl, pH 8.0), the recombinant protein was eluted with 30 ml of elution buffer (20 mM Tris-HCl, 250 mM imidazole, 500 mM NaCl, pH 8.0). PBS was used in place of elution buffer using a PD-10 desalination column (GE Healthcare).
Table 2. Primers used in this study. The punctuations of the primers below are incorrect. Please refer to Table 2 in our manuscript.
Proteolytic activity assays
PepO activity was assayed using MCA-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 (MCA is an abbreviation for (7-methoxycoumarin-4-yl)-acetyl, and Dpa is an abbreviation for N-3-(2,4-dinitrophenyl)-L-2,3-diaminopropionyl) as a substrate. Kinetic parameters for PepO were determined in digestion buffer (100 mM Tris-HCl, 150 mM NaCl, and 10 mM CaCl2, pH 8.0) using substrate concentrations ranging from 0.3 μM to 5 μM and the enzyme concentration of 20 nM. After incubation for 20 min at 37 °C, the fluorescence was detected with an EnVision Multilabel Reader (PerkinElmer) at 320 nm excitation and 395 nm emission.
The proteolytic activities of PepO were then measured using the substrates LL-37 and mCRAMP. Briefly, 20 μM of LL-37 or mCRAMP was incubated with 500 nM of PepO in PBS at 37°C for 3 hours. The uncleaved LL-37 and mCRAMP were used as controls. The samples were then subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) or tandem mass spectrometry analysis using an MALDI-TOF/TOF mass spectrometer (AB Sciex 5800).
Construction of the S. suis pepO mutant and complementary strain
Flanking sequences of the 1022 bp upstream region and the 1081 bp downstream region of the pepO gene were PCR-amplified with the primer pairs U-P1/P2 and D-P1/P2 (), respectively. The erythromycin-resistance (ermr) gene was PCR-amplified from pAT18 [Citation31] using the primer pairs erm-P1/P2 (). The PCR products were purified and digested by the appropriate restriction enzymes. T4 DNA ligase was used to ligate the digested DNA fragments, and then PCR was performed using the primers U-P1 and D-P2. The plasmid pSET4SpepO was obtained by cloning the resulting PCR product into the plasmid pSET4S after restriction endonuclease digestion and ligation. Then, the recombinant plasmid pSET4SpepO was electroporated into the S. suis WT strain [Citation32], and the gene-knockout mutant ΔpepO was identified with PCR and DNA sequencing.
A 2403 bp DNA fragment including the promoter and the coding sequence of PepO was PCR-amplified with the primer pairs pepOC-P1/P2 (). To create the recombinant plasmid pSET2-pepO, the PCR product was then cloned into the pSET2 vector [Citation33]. The resulting plasmid pSET2-pepO was transformed into the S. suis ΔpepO mutant to acquire the complementary strain, which was selected on TSA under Spc selective pressure and confirmed by PCR and DNA sequencing.
Bactericidal assays
The S. suis WT strain, the ΔpepO mutant, and the complementary strain CΔpepO were cultured in TSB at 37°C until reaching an optical density at 600 nm (OD600) of 0.5. Each strain was harvested from the TSB cultures, washed twice, and suspended in PBS at a concentration of 8 × 104 CFU/ml. The diluted samples were incubated with LL-37 or mCRAMP at final concentrations of 0–4 μM at 37°C for 1 hour. The samples were serially diluted and plated on TSA for CFU counting.
To compare the bactericidal capability of LL-37/mCRAMP and their proteolytic products by PepO, the bacterial suspensions of S. suis ΔpepO mutant at a concentration of 1 × 104 CFU/ml were incubated with 2 μM of LL-37, mCRAMP, the proteolytic products LL-37/PepO and mCRAMP/PepO, and the truncated peptides at 37°C for 1 hour. The samples were serially diluted and plated on TSA for CFU counting.
SYTOX green influx
Mid-logarithmic phase cultures of the S. suis ΔpepO mutant were suspended in PBS to 1 × 106 CFU/mL. SYTOX green (Invitrogen, S7020) was added to a concentration of 2 μM and incubated for 15 min. Then, the bacteria were incubated with 2 μM of LL-37, mCRAMP, the proteolytic products LL-37/PepO and mCRAMP/PepO, and the truncated peptides at 37°C for 1 hour. The fluorescence intensity of SYTOX green was measured using an EnSpire Multilabel Reader (PerkinElmer) with an excitation at 485 nm and an emission at 520 nm to determine the membrane permeabilization.
Live/Dead staining
Bacteria incubated with different treatments were assessed by live-dead staining, which could monitor the membrane integrity of bacterial populations [Citation34]. Briefly, fresh cultures of S. suis ΔpepO were washed twice and diluted to 1 × 105 CFU/ml. Then, the bacterial suspensions were incubated with 2 μM of LL-37, mCRAMP, the proteolytic products LL-37/PepO and mCRAMP/PepO, and the truncated peptides for 1 hour and stained using the LIVE/DEAD BacLight bacterial viability kit (Thermo Fisher, L13152). Fifteen minutes after staining, the samples were washed and observed under the LSM 800 Confocal Laser Scanning Microscopy (CLSM, Zeiss). The excitation and emission maxima for these dyes are 480/500 nm for SYTO9 stain and 490/635 nm for propidium iodide.
Neutrophil chemotaxis assay
Mouse neutrophils were labelled with CellTracker Green (Invitrogen, C2925) and diluted in RPMI-1640 to a concentration of 1 × 107 cells/ml. Transwell inserts (Corning Life Sciences, 3421) were then filled with 50 μl of the neutrophil suspension (5 × 105 cells). Then, 1 μM of LL-37, mCRAMP, the proteolytic products LL-37/PepO and mCRAMP/PepO, and the truncated peptides were added to the bottom chambers. 5 μM of fMLP (Sigma-Aldrich, F3506) was used as a positive control. The chambers were incubated for 40 minutes at 37°C, and the upper chambers were removed and fluorescence microscopy (Zeiss) was used to monitor migrated cells in the lower chambers. The chemotaxis index (CI) was determined by dividing the number of cells that migrated towards the chemoattractant by the number of cells that migrated towards the medium.
Measurement of neutrophil apoptosis
Freshly collected mouse neutrophils (1 × 106 cells) were incubated with 1 μM of LL-37, mCRAMP, the proteolytic products LL-37/PepO and mCRAMP/PepO, and the truncated peptides at 37°C for 30 min. Then, apoptosis was induced at 37°C for 6 hours using 30 ng/ml of tumour necrosis factor-alpha (TNF-α, R&D systems, 210-TA-020). Neutrophils only treated with TNF-α were used as a positive control. Neutrophils were stained with the Annexin-V FITC apoptosis detection kit (Sigma-Aldrich, APOAF-50TST) for 10 minutes at room temperature in the dark. Neutrophils were then analysed by flow cytometry to report the percentage of apoptotic neutrophils. The apoptotic rate was calculated by dividing the percentage of apoptotic neutrophils in the test group by the percentage of apoptotic neutrophils in the control group. Neutrophil apoptosis in the presence of TNF-α alone was designated as 100%.
Lysosome formation analysis
Mouse peritoneal macrophages (5 × 104 cells) were plated in the glass-bottom culture dish in RPMI-1640 medium, then treated with 2 μM of LL-37, mCRAMP, the proteolytic products LL-37/PepO and mCRAMP/PepO, and the truncated peptides at 37°C for 4 hours. Cells were fixed in 4% formaldehyde for 20 minutes, washed with PBS, and permeabilized for 15 minutes in 0.1% Triton X-100. Then, cells were blocked in PBS containing 3% BSA and incubated with rabbit anti-LAMP1(Abcam, ab208943) at 37°C for 1 hour. Cells were stained with the fluorescent secondary antibody goat anti-rabbit Alexa Fluor 594 for 30 minutes. Cellular nuclei were then counterstained with DAPI for 10 minutes. Images were captured using the LSM 800 CLSM (Zeiss).
The acidic degree of lysosomes in mouse peritoneal macrophage cells with different treatments was assessed by measuring the fluorescence intensity of the LysoTracker Probe, which is a dye to measure pH values in viable cells. Briefly, mouse peritoneal macrophages were cultured in black, clear bottom, 96-well plates and incubated with 2 μM of LL-37, mCRAMP, and the proteolytic products LL-37/PepO and mCRAMP/PepO for 4 hours. Lysotracker Red DND-99 (Thermo Fisher, L7528) was added at 100 nM to plate wells. After 30 minutes, the fluorescence intensity was measured using the EnSpire Multilabel Reader (PerkinElmer) with an excitation at 544 nm and an emission at 610 nm.
Mouse bacteremia model
6-week-old C57BL/6 mice were inoculated with 5 × 107 CFU of the S. suis WT strain or ΔpepO mutant by intravenous injection. One hour after inoculation, the mice were injected intraperitoneally with either LL-37 (4 mg/kg) or PBS. The mice were humanely euthanized after 24 hours of infection, and the organs and blood were obtained. Brain, liver, and lung samples were fixed in 4% formalin and embedded in paraffin. The samples were then cut into 4 μm-thick slices and stained with haematoxylin-eosin (H&E). Images were obtained using an inverted microscope (Olympus, Japan). The pathological changes in brain, liver, and lung tissue were blindly evaluated by pathologists [Citation35,Citation36]. For enumeration of bacterial load, the organs were weighed and homogenized in PBS for 15 minutes. The number of viable bacteria was calculated by plating serial dilutions of each homogenate and blood onto TSA.
Statistical analysis
The graphs and statistics were created with GraphPad Prism 9.0. Student’s t test (two-tailed, unpaired), one-way ANOVA, or two-way ANOVA were used to determine statistical significance. A P-value <0.05 was considered to be statistically significant.
Results
S. suis PepO belongs to the peptidase M13 family.
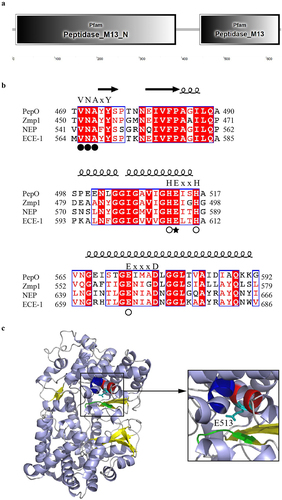
S. suis PepO is a 663-amino-acid, 74.5-kDa protein containing domains that associate with the peptidase M13 family (). SignalP predicted the PepO N-terminus to have a signal peptide domain (amino acids 1 to 28). Sequence alignment revealed that S. suis PepO shared close similarities with other M13 family proteins, such as M. tuberculosis metalloprotease Zmp1, and M13 metalloproteases neprilysin (NEP) and endothelin-converting enzyme (ECE)-1, which originated from humans. Like these M13 family proteins, PepO has three characteristic motifs that are engaged in the substrate/inhibitor interaction (VNAYY) and metal coordination (HEISH and EIMAD) (). PepO’s catalytic site is predicted to be the conserved residue Glu-513. ().
Figure 1. General features of S. suis PepO protease.(a) protein domains of PepO identified by SMART. (b) sequence alignments of three characteristic motifs of S. suis PepO with M. tuberculosis Zmp1, human NEP, and ECE-1. Spirals and arrows indicate helices and β-strands, respectively. Residues for substrate/inhibitor interaction are labeled with a black circle. Residues for metal coordination are labeled with a white circle. Catalytic Glu513 is labeled with a black star. (c) 3D model of PepO. Cartoon showing the α-helix, β-sheet, and random coil in lightblue, yellow, and grey. The VNAYY motif is depicted in green. Except for catalytic Glu513, the HEISH motif is depicted in red. The EIMAD motif is depicted in blue. Structure homology modelling was conducted by SWISS-MODEL using the 3D structure of Zmp1 (PDB:3ZUK) as a template.
S. suis PepO cleaves cathelicidins LL-37 and mCRAMP
The amino acids (AA) 29 to 663 of PepO and its variant PepOE513A (catalytic site Glu513 was mutated Ala) were respectively overexpressed in E. coli and purified with a Ni2+ affinity column. M13 peptidases often prefer substrates with Gly at position P1 and Leu at position P1.’ Thus, PepO catalytic activity was determined on the generic fluorogenic substrate MCA-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, which is specific for a family of matrix metalloproteinases with the same catalytic motif (HExxH) as the M13 family. Cleavage at the Gly-Leu bond separates the highly fluorescent MCA from the efficient Dpa quencher, resulting in an increase in fluorescence intensity. Analysis of the Michaelis-Menten kinetics of PepO revealed that its Km value was 1.568 μM and its Kcat value was 0.053S−1 ().
Figure 2. S. suis PepO protease degrades LL-37 and mCRAMP into shorter peptides. (a) proteolytic activity of PepO with MCA-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as a substrate. The Vmax and Km values were determined from the Michaelis-Menten equation. (b and c) Representative SDS-PAGE images of the proteolytic products of LL-37 (b) and mCRAMP (c) after cleavage by catalytically active PepO or inactive mutant PepOE513A. LL-37 and mCRAMP were respectively incubated with the purified PepO or PepOE513A for 3 h, the proteolytic products were analyzed using SDS-PAGE. (d-g) the MALDI-TOF/TOF mass spectrometry analyses of LL-37 (d), mCRAMP (f), the proteolytic products of LL-37 (e) and mCRAMP (g) after cleavage by the PepO. (h and i) amino acid sequences of the truncated peptides of LL-37 (h) and mCRAMP (i) after cleavage by PepO.
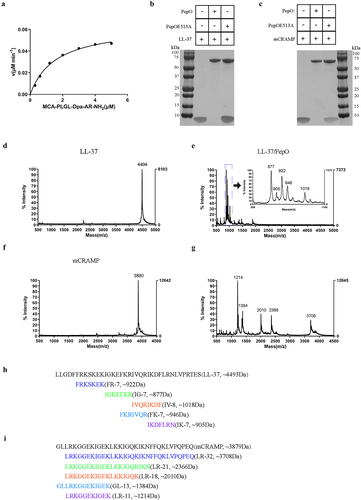
To determine the proteolytic activity of PepO on cathelicidin, LL-37 was incubated with the recombinant PepO, and the reaction mixture was analysed by SDS-PAGE. As shown in , when LL-37 was incubated with PepO, the band intensity of LL-37 disappeared, suggesting that PepO could cleave LL-37. However, the catalytic site-mutated protein PepOE513A could not cleave LL-37. As the murine ortholog of LL-37, mCRAMP was also investigated as a digestive substrate. The result showed that, similar to LL-37, mCRAMP was also cleaved by PepO ().
To further monitor cleavage, LL-37 or mCRAMP was incubated in the presence or absence of PepO, and the cleaved products were analysed by mass spectrometry. The results showed that LL-37 migrated as a single peak with a molecular weight (MW) of 4494 Da (), and when it was cleaved by PepO, five new peaks with MWs ranging from 850 to 1050 Da were generated (). A peak with a MW of 3880 Da was visible in mCRAMP (), and its cleaved product generated five new peaks with MWs ranging from 1200 to 3710 Da (). By matching the acquired fragment patterns, the amino acid sequences of the main cleaved fragments were determined. The five cleaved fragments of LL-37 were designated as FR-7, IG-7, IV-8, FK-7, and IK-7, and the cleaved fragments of mCRAMP were designated as LR-32, LR-21, LR-18, GL-13, and LR-11; these peptide sequences are shown in and .
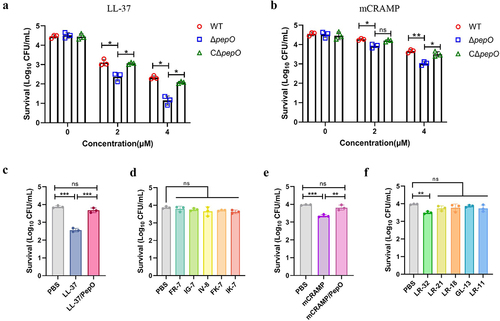
PepO-dependent cleavage of cathelicidins destroys their bactericidal activities
S. suis disseminates to the organs through the bloodstream, which is exposed to LL-37 during infection. To investigate whether PepO contributes to the LL-37 resistance of S. suis, a deletion mutant strain ΔpepO and its complementary strain CΔpepO were successfully constructed (Figure S1). The vitality of the wild-type (WT), ΔpepO, and CΔpepO strains when facing LL-37 was assessed with the bactericidal assays. When exposed to LL-37, it was observed that the ΔpepO mutant was significantly more sensitive to LL-37 compared to the WT strain (). Resistance to LL-37 was partially restored by transcomplementation of the wild-type pepO gene. These results confirmed that the PepO helped S. suis withstand the killing of LL-37. Additionally, S. suis became more sensitive to mCRAMP after the pepO gene was deleted (). Following that, we directly compared the bactericidal ability of cathelicidin peptides, their proteolytic products after PepO treatment, and the truncated peptides in the proteolytic products. When S. suis was treated with LL-37, a significant decrease in bacterial count was observed (). However, this bactericidal effect was not observed with the proteolytic product LL-37/PepO or the truncated peptides of LL-37 at the same concentrations ( and ). Similarly, although weaker than LL-37, mCRAMP also showed bactericidal activity against S. suis, but treatment with the proteolytic product mCRAMP/PepO or the truncated peptides of mCRAMP, except for LR-32, did not significantly reduce bacterial counts ( and ).
Figure 3. PepO protease protects S. suis from the bactericidal activity of LL-37 and mCRAMP. (a and b) sensitivity of S. suis strains toward LL-37 or mCRAMP. S. suis WT, ΔpepO, and CΔpepO strains were incubated with increasing concentrations of LL-37 (a) or mCRAMP (b) for 1 h. (c and d) bactericidal activities of LL-37, the proteolytic products LL-37/PepO (c), and the truncated peptides of LL-37 (d). (e and f) bactericidal activities of mCRAMP, the proteolytic products mCRAMP/PepO (e), and the truncated peptides of mCRAMP (f). The S. suis ΔpepO were exposed to different peptides or proteolytic products for 1 h. Each sample was plated on TSA to identify viable bacteria. Values represent the mean ± SD from three independent experiments. Statistical significance was calculated using two-way ANOVA tests followed by Tukey’s multiple-comparison test for panels a and b. Panels c and e were analysed via one-way ANOVA test followed by Tukey’s multiple-comparison test. Panels d and f were analysed via one-way ANOVA test followed by Dunnett’s multiple-comparison test. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
PepO-dependent cleavage of cathelicidins reduces their membrane permeabilizing activity toward S. suis
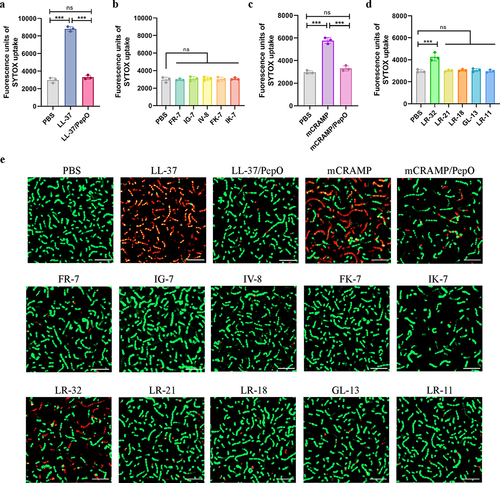
To determine the influence of PepO-dependent cleavage on the membrane permeabilizing activities of cathelicidins, the fluorescence intensity of SYTOX Green was examined after each peptide was added to the bacterial suspension of S. suis. Compared to PBS-treated bacteria, the fluorescence intensity of SYTOX Green dramatically increased after the addition of LL-37 (), while no significant increment in fluorescence intensity was observed in LL-37/PepO or the truncated peptide-treated bacteria ( and ). Similarly, mCRAMP also produced higher fluorescence than its proteolytic products mCRAMP/PepO after 1 h of incubation (). However, the fluorescence intensity of SYTOX green was not increased with the most truncated peptides of mCRAMP at the same concentrations, except LR-32 ().
Figure 4. Cleavage of cathelicidins by PepO reduces their membrane permeabilizing activity towards S. suis. (a and b) membrane permeabilizing activities of LL-37, the proteolytic products LL-37/PepO (a), and the truncated peptides of LL-37 (b). (c and d) membrane permeabilizing activities of mCRAMP, the proteolytic products mCRAMP/PepO (c), and the truncated peptides of mCRAMP (d). The S. suis ΔpepO loaded with SYTOX green were exposed to different peptides or proteolytic products for 1 h, and the fluorescence intensity was measured. (e) microscopic visualization of the viability of S. suis ΔpepO after treatment with LL-37, mCRAMP, their proteolytic products, and their truncated peptides. Bacterial cells with intact membranes are represented by green, whereas those with damaged membranes are represented by red. Scale bars = 10 μm. Values represent the mean ± SD from three independent experiments. Statistical significance was calculated using one-way ANOVA test followed by Tukey’s multiple-comparison test for panels a and c. Panels b and d were analysed via one-way ANOVA test followed by Dunnett’s multiple-comparison test. ns, not significant; ***P < 0.001.
Membrane damage of S. suis following exposure to cathelicidins, their proteolytic products, and the truncated peptides was also examined by using a LIVE/DEAD assay and observed under the CLSM. SYTO9 penetrates all of the bacterial membranes and binds nucleic acids, staining them green, whereas PI only penetrates permeabilized membranes and stains cells with a damaged cytoplasmic membrane red, thus allowing the quantification of viable and dead bacteria. After LL-37 or mCRAMP treatment, the number of dead bacteria was clearly larger than that in the PBS-treated group. Consistent with the results of the SYTOX assay, higher percentages of viable bacteria were visualized for the LL-37/PepO, mCRAMP/PepO, or each truncated peptide-treated groups compared to the LL-37 or mCRAMP-treated groups ().
PepO-dependent cleavage of cathelicidins affects their immunomodulation of neutrophils
According to cellular and environmental circumstances, cathelicidins have been found to exert various immune-modulatory functions. A remarkable characteristic of LL-37 and mCRAMP is their ability to promote the chemotaxis of neutrophils to clear invasive pathogens [Citation37]. Thus, to determine whether the PepO cleavage affects the chemotactic abilities of LL-37 and mCRAMP, we tested the chemotactic properties of LL-37, mCRAMP, their proteolytic products after PepO treatment, and their truncated peptides on neutrophils. We found that LL-37 effectively chemoattracted mouse neutrophils, while its proteolytic product, LL-37/PepO, significantly decreased the chemotaxis of mouse neutrophils (). The abilities of the truncated peptides of LL-37 to chemoattract neutrophils were markedly weaker than those of the intact LL-37 (). As expected, mCRAMP chemoattracted mouse neutrophils just as well as LL-37; however, its proteolytic product, mCRAMP/PepO, recruited neutrophils much less effectively (). Similarly, compared to mCRAMP, the truncated peptides of mCRAMP significantly reduced neutrophil chemotaxis (). These findings showed that PepO might damage the chemotactic characteristics of LL-37 and mCRAMP, which are needed for neutrophil migration.
Figure 5. Cleavage of cathelicidins by PepO affects their chemotactic ability and anti-apoptotic activity towards neutrophils. (a and b) CI of neutrophils in response to LL-37, the proteolytic products LL-37/PepO (a), and the truncated peptides of LL-37 (b). (c and d) CI of neutrophils in response to mCRAMP, the proteolytic products mCRAMP/PepO (c), and the truncated peptides of mCRAMP (d). Mouse neutrophils were seeded in the upper chamber of a 5-μm Transwell, and migration was assessed after stimulation with different peptides or proteolytic products in the lower chamber. As a positive control, 5 μM of fMLP was used. The neutrophils chemotaxis toward medium was designated as 1 to calculate the CI of the different samples. (e and f) anti-apoptotic activities of LL-37, the proteolytic products LL-37/PepO (e), and the truncated peptides of LL-37 (f) toward neutrophils. (g and h) anti-apoptotic activities of mCRAMP, the proteolytic products mCRAMP/PepO (g), and the truncated peptides of mCRAMP (h) toward neutrophils. Flow cytometry was used to measure apoptosis in mouse neutrophils stimulated by TNF-α in the presence or absence of various peptides or proteolytic products. Neutrophil apoptosis in the presence of TNF-α alone was designated as 100%. Values represent the mean ± SD from three independent experiments. Statistical significance was calculated using two-tailed, unpaired t tests for panels a, c, e, and g. Panels b, d, f, and h were analyzed via one-way ANOVA test followed by Dunnett’s multiple-comparison test. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
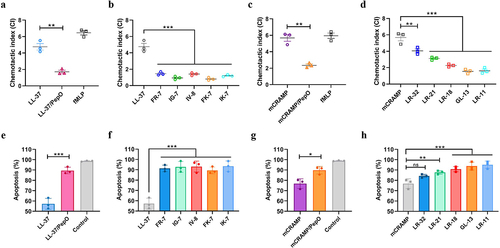
Neutrophils have a naturally short lifespan that is terminated by spontaneous apoptosis. By inhibiting the activation of caspase-3, a crucial executor of apoptosis, LL-37 may be able to prolong neutrophil lifespan [Citation38]. To examine whether the PepO cleavage affects the anti-apoptotic effect of LL-37, we used TNF-α to trigger cell apoptosis in mouse neutrophils with or without LL-37, mCRAMP, their proteolytic products, or their truncated peptides, and then counted the number of apoptotic cells. In the presence of intact LL-37, the apoptotic rate reduced to nearly 57% (), thereby confirming the anti-apoptotic activity of this peptide. However, the proteolytic product LL-37/PepO and the truncated peptides of LL-37 reduced the anti-apoptotic activity of LL-37 for mouse neutrophils ( and ). Furthermore, TNF-α-induced mouse neutrophil death was likewise postponed by mCRAMP (), although the effect was much weaker than that of LL-37. The proteolytic product mCRAMP/PepO and the truncated peptides of mCRAMP, except LR-32, reduced the anti-apoptotic effect of mCRAMP when used in the same concentration ( and ). These data imply that PepO protease undermines the protective effects of LL-37 and mCRAMP in prolonging neutrophil lifespan.
PepO-dependent cleavage of cathelicidins affects their promotion of lysosome formation in macrophages
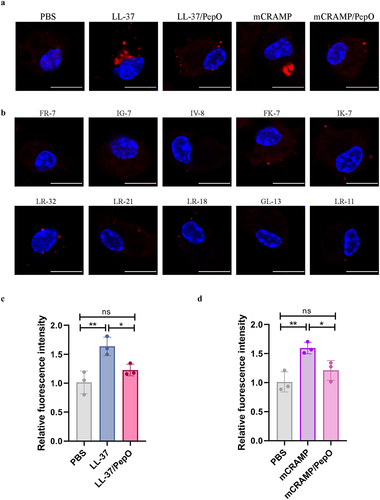
It has been found that LL-37 stimulates lysosome development in phagocytes, which is important for killing invading bacteria [Citation39,Citation40]. To investigate if the PepO cleavage affects the LL-37-induced lysosome formation, the mouse macrophages were incubated with different peptides, and lysosome formation was observed by CLSM using an antibody against the lysosomal marker LAMP1. Both LL-37 and mCRAMP facilitated lysosome formation in mouse macrophages, while the proteolytic products LL-37/PepO and mCRAMP/PepO did not affect intracellular lysosome formation (). In addition, the truncated peptides of LL-37 and mCRAMP also did not cause changes in lysosome formation (). Furthermore, the acidic degree of lysosomes in mouse macrophages was examined by measuring the fluorescence intensity of LysoTracker Red. Lysosomal acidity was significantly enhanced by LL-37 treatment, whereas this enhancement was abrogated by PepO cleavage (). Similarly, mCRAMP treatment also increased the lysosomal acidity but not the proteolytic product mCRAMP/PepO (). These results demonstrated that PepO-dependent cleavage suppressed the cathelicidin-induced increase in lysosomal formation.
Figure 6. Cleavage of cathelicidins by PepO impairs their ability to promote lysosome formation. (a and b) microscopic visualization of lysosome formation induced by LL-37, mCRAMP, and their proteolytic products after cleavage by PepO (a), or the truncated peptides of LL-37 and mCRAMP (b). Mouse peritoneal macrophages were treated with different peptides or proteolytic products for 4 h, and stained for LAMP1 in red, nuclei in blue. Scale bars = 5 μm. (c and d) the acidic degree of lysosomes in mouse peritoneal macrophages treated with LL-37 and the proteolytic products LL-37/PepO (c), or mCRAMP and the proteolytic products mCRAMP/PepO (d) was tested by quantifying the fluorescent intensities of LysoTracker Red. Values represent the mean ± SD from three independent experiments. Statistical significance was calculated using one-way ANOVA test followed by Tukey’s multiple-comparison test. ns, not significant; *P < 0.05; **P < 0.01.
Loss of PepO attenuates organ injury in mice infected with S. suis
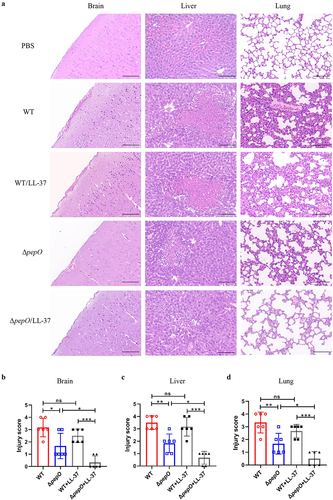
Given the importance of cathelicidins for mammalian immune defence, we hypothesized that PepO-mediated cathelicidin resistance is necessary for S. suis infection. To verify this hypothesis, a murine infection model was used to assess the changes in virulence of S. suis after pepO deletion. C57BL/6 mice were infected with the WT and ΔpepO strains by intravenous injection and subsequently treated with or without LL-37. Histopathological analysis was conducted to estimate the lesion severity of the brain, lung, and liver in mice. As shown in , mice inoculated with PBS had no notable lesions. Mice inoculated with the WT strain displayed intracerebral haemorrhage and inflammatory cell infiltration in the brain, large amounts of spotty necrosis accompanied by fatty infiltration in the liver, and severe congestion and thickened respiratory membranes accompanied by inflammatory cell infiltration in the lung. LL-37 treatment did not alleviate tissue damage in mice infected with the WT strain. By contrast, the mice infected with the ΔpepO strain showed alleviated histopathological lesions in the brain, liver, and lung. Furthermore, LL-37 treatment showed few pathological changes in mice infected with the ΔpepO mutant. These findings were fully supported by the pathological score analysis (), which showed that the mice in the ΔpepO group had lower pathological scores in their brain, lung, and liver than the mice in the WT group. LL-37 treatment did not significantly decrease the organ pathological scores of mice in the WT group. However, after LL-37 treatment, the organ pathological scores of mice in the ΔpepO group were considerably lower than those of untreated mice.
Figure 7. Loss of PepO attenuates organ injury in S. suis -infected mice. (a-d) C57BL/6 mice were inoculated with the S. suis WT strain or ΔpepO mutant by intravenous injection, and subsequently treated with or without LL-37. (a) histopathological H&E staining of brain, liver, and lung tissue sections from each group of mice. Scale bars = 100 μm. The pathological scores of the brain (b), liver (c), and lung (d) were blindly evaluated in three random fields by two independent scientists. Statistical significance was calculated using one-way ANOVA test followed by Tukey’s multiple-comparison test. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
PepO promotes bacterial survival and dissemination in S. suis-infected mice
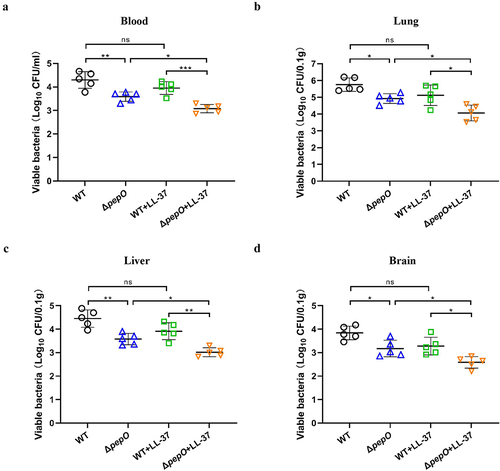
To determine whether PepO aids S. suis survival in the blood and/or dissemination to the organs, the bacterial load was examined using a colony-count assay. At 24 hours post-infection, the viable counts of S. suis were quantified using samples obtained from harvested organs and blood. In comparison to mice infected with the WT strain, mice inoculated with the ΔpepO strain exhibited much lower bacterial loads in the blood (). Additionally, high bacterial loads were found in the brain, liver, and lung of mice inoculated with the WT strain, demonstrating that S. suis had spread from the blood to different organs. (). There was a statistically significant reduction in bacterial burdens in the brain, lung, and liver from mice inoculated with the ΔpepO strain compared to the WT-infected mice ( to ). LL-37 treatment did not alter the bacterial burdens in the blood, brain, liver, and lung of WT-infected mice. In contrast, in ΔpepO-infected mice, LL-37 treatment significantly reduced the bacterial burdens ( to ). These data indicated that PepO-mediated cathelicidin resistance was critical for facilitating the survival and dissemination of S. suis.
Figure 8. PepO contributes to S. suis survival and dissemination in mice. (a-d) C57BL/6 mice were inoculated with the S. suis WT strain or ΔpepO mutant by intravenous injection, and subsequently treated with or without LL-37. After 24 h of treatment, samples were collected from blood (a), lung (b), liver (c), and brain (d) for bacterial quantification. Values represent the mean ± SD from five biological replicates contained in each experimental group. Statistical significance was calculated using one-way ANOVA test followed by Tukey’s multiple-comparison test. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
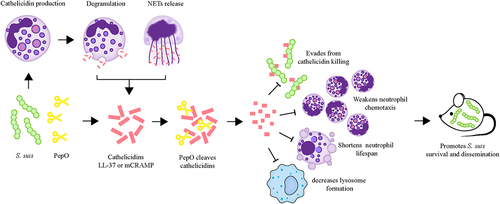
S. suis is a successful zoonotic bacterium seemingly capable of invading and surviving in many niches of the host, illustrating that this bacterium employs effective measures to circumvent immune defences. Here, we emphasize a key function of the S. suis PepO protease in the evasion of the host-defence peptide cathelicidins, thus influencing the pathogenesis of S. suis. PepO was found to cleave both human cathelicidin LL-37 and mouse cathelicidin mCRAMP, rendering them inactive against S. suis. Cleavage of LL-37 and mCRAMP by PepO also impairs their immunomodulatory effects on neutrophil migration and prolongation of neutrophil lifespan. Further, through a mouse bacteraemia model, we demonstrate that PepO is essential for S. suis pathogenicity in vivo. Our observations emphasize the importance of PepO for manipulating the host innate immune response, which may aid in the establishment and expansion of S. suis infection ().
Figure 9. Schematic representation of the role of PepO-mediated cleavage of cathelicidins in S. suis immune evasion and dissemination. At the site of infection, S. suis stimulates cathelicidin production, which is stored in neutrophil granules. The mature peptide of cathelicidin can be released during neutrophil degranulation or the formation of neutrophil extracellular traps (NETs) to combat S. suis. To escape from cathelicidin-mediated antibacterial immune responses, S. suis produces an extracellular protease PepO to degrade cathelicidins LL-37 or mCRAMP into shorter fragments, abolishing its bactericidal activity against S. suis, and undermining its immunomodulatory functions, including the promotion of neutrophil migration, anti-apoptotic activity to prolong neutrophil lifespan, and the promotion of lysosome formation in macrophages. The cleavage of cathelicidins mediated by PepO promotes S. suis dissemination and exacerbates organ injury in the mouse bacteraemia model.
Previous studies have described the functions of PepO in Streptococcus species interactions with the host. For example, S. pneumoniae PepO can bind complement component C1q to evade complement-mediated bacteriolysis [Citation41]. Moreover, S. pneumoniae PepO was shown to be a multifunctional protein that binds both plasminogen and fibronectin, thus enabling this bacterium to evade innate immunity and invade host cells [Citation42]. The interaction of PepO with plasminogen and fibronectin was also demonstrated in S. suis [Citation23,Citation24], suggesting that these interactions are a common strategy exploited by several Streptococcus species to invade the host. As S. pneumoniae PepO performs diverse functions that were affirmed by the MoonProt database [Citation43], it is reasonable to speculate that S. suis PepO may be a novel moonlighting protein, with further moonlighting features expected to be discovered in the future.
Our findings indicate for the first time the contribution of the intrinsic proteolytic feature of PepO to the S. suis-host interaction, that is, the role of PepO in cleaving cathelicidin LL-37. As a first line of innate defence, LL-37 displays formidable bactericidal activity against various microbes [Citation13]. Nevertheless, many pathogens have evolved various mechanisms to thwart this immune effector. The main strategies for pathogenic bacteria to resist HDPs are membrane alteration, modification of surface charge, capture by surface proteins, efflux pump, and inactivation by proteolytic digestion [Citation11,Citation44]. Our previous work demonstrated that S. suis had the ability to rapidly trigger massive amounts of LL-37 synthesis in human neutrophils [Citation45]. This finding corroborated the previous studies concerning the high concentrations of cathelicidins surrounding activated leukocytes after other bacterial infections [Citation37], indicating the significance of LL-37 in the host’s defence against S. suis invasion. Our previous work also found that, despite high amounts of LL-37, S. suis manoeuvred an aminopeptidase ApdS to cleave 3 AA at the N-terminal of this peptide [Citation45]. Although the bactericidal ability of the cleaved peptide was reduced compared to that of the intact peptide, it could still effectively kill S. suis. These data imply that there may be other molecules involved in the S. suis immune escape of LL-37. In this study, our analysis of the secreted protease PepO produced by S. suis demonstrated that it belonged to the peptidase family M13, which was restricted to cleave polypeptides in the MEROPS peptidase database [Citation46], suggesting that this protease could cleave HDPs. The results of mass spectrometry found that the PepO-dependent cleavage of LL-37 has occurred at multiple cleavage sites, thereby ultimately cleaving it into short peptides no more than 8AA. In contrast to the truncated peptide after ApdS cleavage, the proteolytic product of PepO has no bactericidal activity against S. suis, indicating that PepO provides better protection to S. suis from LL-37-mediated bacteriolysis. The reason why S. suis employs two proteases to resist LL-37 may be due to the different proteolytic speeds of these two proteases. Our previous work found that LL-37 was cleaved by ApdS in just a few seconds [Citation45], even more rapid than PepO. When responding to the rapidly activated LL-37, it is speculated that S. suis may first mobilize ApdS to counteract LL-37 as a “forward army,” before using PepO as the “main army” with stronger attack power to participate in this “battle.” Other pathogenic bacteria also have two sets of molecular mechanisms to cleave LL-37, such as cysteine protease SpeB and subtilisin-like protease ScpC from S. pyogenes [Citation19,Citation47], and metalloproteinase aureolysin and glutamyltranspeptidase V8 from Staphylococcus aureus [Citation48]. This synergistic effect of LL-37 cleavage mediated by different proteases may be a common mechanism exploited by several pathogens to efficiently escape LL-37-mediated antibacterial immune responses.
Of note, LL-37 has drawn the attention of the researchers because, besides its antibacterial activity, it also possesses a variety of immunomodulatory functions [Citation12,Citation37]. Furthermore, it has been reported that, compared to the direct bacteriolytic property, LL-37-mediated manipulation of the host immune system is more crucial for protection against pathogenic bacteria [Citation19]. Because neutrophils are crucial components of innate immunity against bacterial invasion and represent the primary source of LL-37 [Citation49,Citation50], we explored the effect of PepO-dependent cleavage of LL-37 on neutrophil functions. The proteolytic product LL-37/PepO and truncated peptides were found to abolish the LL-37-mediated neutrophil chemotaxis. A previous study showed that the ability of the truncated peptide (L1-V21) from the N-terminus of LL-37 to attract neutrophils was weaker than that of the two other peptides (G14-R34, F17-S37) derived from the C terminus [Citation51]. In this study, the peptides FR-7 (F6-K12) and IG-7 (I13-R19) are the main truncated peptides in the proteolytic product LL-37/PepO and are also located at the N-terminus of LL-37, which may be responsible for the abolished chemotaxis. Additionally, after cleavage of mCRAMP by PepO, the main truncated peptide LR-11 is also located at the N-terminus of mCRAMP, and its chemotactic ability is significantly reduced. Moreover, LL-37 has been demonstrated to increase the neutrophil lifespan by inhibiting spontaneous apoptosis, which could effectively promote bacterial clearance by neutrophils [Citation38]. Although not as effective as LL-37, mCRAMP is also shown here to inhibit neutrophil apoptosis. Similar to the findings of our study, mCRAMP has potential roles in preventing cardiac damage by suppressing cardiomyocyte apoptosis [Citation52]. In addition, our study showed that LL-37 and mCRAMP promoted lysosome development in mouse macrophages to the same extent, but this enhancement was abrogated by PepO cleavage. Our findings demonstrate that PepO-dependent cleavage of LL-37 and mCRAMP influences their immunomodulatory functions.
Although mCRAMP shares a high identity with LL-37, several proteases from other bacteria, such as the ScpC protease from S. pyogenes [Citation19], cannot cleave mCRAMP like LL-37. Nevertheless, our work shows that S. suis PepO cleaves mCRAMP as well as LL-37 and attenuates their bactericidal activity and immunomodulatory activity. Hence, it is feasible to use an experimental mouse model to assess the effect of PepO-dependent cleavage of cathelicidins on S. suis pathogenicity in vivo. Here, it is demonstrated that the deletion of the pepO gene attenuates organ injury, reduces inflammatory cytokines, and promotes the clearance of S. suis in the mouse bacteraemia model. Similarly, when P. aeruginosa invades the host, it uses elastase to degrade LL-37 in wound fluid, which improves bacterial survival [Citation47]. Notably, LL-37 treatment did not protect mice from the infection by the S. suis WT strain, but it protected mice from the infection by the S. suis ΔpepO mutant. In view of these findings, it is possible that the protease-dependent obstruction of HDPs is an important mechanism in bacterial virulence.
In summary, this work provides a novel understanding of the proteolytic characteristics of the PepO in S. suis-host interaction and its role in immune evasion and pathogenesis of S. suis.
Authors contribution
Mingjie Jin: Conceptualization, Methodology. Siyu Liang: Methodology, Validation, Writing – original draft. Jing Wang: Data curation. Huihui Zhang: Investigation, Resources. Yueling Zhang: Funding acquisition, Resources. Wanjiang Zhang: Supervision. Siguo Liu: Conceptualization, Project administration, Writing – review & editing. Fang Xie: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Figure S1.jpg
Download JPEG Image (104.7 KB)Acknowledgements
We thank Dr. Takamatsu for the generous donation of the plasmids pSET4S and pSET2. This research was supported by grants from the National Natural Science Foundation of China (31873016 and 32172853).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
All the data supporting the findings of this study are provided in the article and its Supplementary file. Additional data are available upon request from the corresponding authors.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2023.2283896.
Additional information
Funding
References
- Dutkiewicz J, Sroka J, Zajac V, et al. Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part I - Epidemiology. Ann Agric Environ Med. 2017;24(4):1–19. doi: 10.26444/aaem/79813
- Huong VT, Ha N, Huy NT, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis. 2014;20(7):1105–1114. doi: 10.3201/eid2007.131594
- Goyette-Desjardins G, Auger JP, Xu J, et al. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3(6):e45. doi: 10.1038/emi.2014.45
- Lun ZR, Wang QP, Chen XG, et al. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7(3):201–209. doi: 10.1016/S1473-3099(07)70001-4
- van Samkar A, Brouwer MC, Schultsz C, et al. Streptococcus suis meningitis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9(10):e0004191. doi: 10.1371/journal.pntd.0004191
- Wertheim HF, Nguyen HN, Taylor W, et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One. 2009;4(6):e5973. doi: 10.1371/journal.pone.0005973
- Tang J, Wang C, Feng Y, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLOS Med. 2006;3(5):e151. doi: 10.1371/journal.pmed.0030151
- Ye C, Zheng H, Zhang J, et al. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J Infect Dis. 2009;199(1):97–107. doi: 10.1086/594370
- Segura M. Streptococcus suis: an emerging human threat. J Infect Dis. 2009;199(1):4–6. doi: 10.1086/594371
- Alford MA, Baquir B, Santana FL, et al. Cathelicidin host defense peptides and inflammatory signaling: striking a balance. Front Microbiol. 2020;11:1902. doi: 10.3389/fmicb.2020.01902
- LaRock CN, Nizet V. Cationic antimicrobial peptide resistance mechanisms of streptococcal pathogens. Biochim Biophys Acta. 2015;1848(11 Pt B):3047–3054. doi: 10.1016/j.bbamem.2015.02.010
- Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16(5):321–334. doi: 10.1038/nri.2016.29
- Pachon-Ibanez ME, Smani Y, Pachon J, et al. Perspectives for clinical use of engineered human host defense antimicrobial peptides. FEMS Microbiol Rev. 2017;41(3):323–342. doi: 10.1093/femsre/fux012
- Mookherjee N, Anderson MA, Haagsman HP, et al. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19(5):311–332. doi: 10.1038/s41573-019-0058-8
- Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–768. doi: 10.1038/nchembio.1393
- Scheenstra MR, van Harten RM, Veldhuizen EJA, et al. Cathelicidins modulate TLR-Activation and inflammation. Front Immunol. 2020;11:1137. doi: 10.3389/fimmu.2020.01137
- Coorens M, Scheenstra MR, Veldhuizen EJ, et al. Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis. Sci Rep. 2017;7(1):40874. doi: 10.1038/srep40874
- Kurosaka K, Chen Q, Yarovinsky F, et al. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174(10):6257–6265. doi: 10.4049/jimmunol.174.10.6257
- Biswas D, Ambalavanan P, Ravins M, et al. LL-37-mediated activation of host receptors is critical for defense against group a streptococcal infection. Cell Rep. 2021;34(9):108766. doi: 10.1016/j.celrep.2021.108766
- Segura M, Fittipaldi N, Calzas C, et al. Critical Streptococcus suis virulence factors: are they all really critical? Trends Microbiol. 2017;25(7):585–599. doi: 10.1016/j.tim.2017.02.005
- Bleuze M, Gottschalk M, Segura M. Neutrophils in Streptococcus suis infection: from host defense to pathology. Microorganisms. 2021;9(11):2392. doi: 10.3390/microorganisms9112392
- Zhang W, Lu CP. Immunoproteomics of extracellular proteins of Chinese virulent strains of Streptococcus suis type 2. Proteomics. 2007;7(24):4468–4476. doi: 10.1002/pmic.200700294
- Liu F, Li J, Yan K, et al. Binding of fibronectin to SsPepO facilitates the development of Streptococcus suis meningitis. J Infect Dis. 2018;217(6):973–982. doi: 10.1093/infdis/jix523
- Zhou Y, Yan K, Sun C, et al. Binding of Plasminogen to Streptococcus suis Protein Endopeptidase O Facilitates Evasion of Innate Immunity in Streptococcus suis. Front Microbiol. 2021;12:694103. doi: 10.3389/fmicb.2021.694103
- Petersen TN, Brunak S, von Heijne G, et al. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701
- Letunic I, Khedkar S, Bork P. SMART: recent updates, new developments and status in 2020. Nucleic Acids Res. 2021;49(D1):D458–D460. doi: 10.1093/nar/gkaa937
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673
- Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42(Web Server issue):W320–W324. doi: 10.1093/nar/gku316
- Bienert S, Waterhouse A, de Beer TA, et al. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017;45(D1):D313–D319. doi: 10.1093/nar/gkw1132
- Okwumabua O, O'Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiology Letters. 2003;218(1):79–84. doi: 10.1111/j.1574-6968.2003.tb11501.x
- Trieu-Cuot P, Carlier C, Poyart-Salmeron C, et al. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene. 1991;102(1):99–104. doi: 10.1016/0378-1119(91)90546-n
- Takamatsu D, Osaki M, Sekizaki T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid. 2001;46(2):140–148. doi: 10.1006/plas.2001.1532
- Takamatsu D, Osaki M, Sekizaki T. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid. 2001;45(2):101–113. doi: 10.1006/plas.2000.1510
- Xu Y, Zhong LL, Srinivas S, et al. Spread of MCR-3 Colistin resistance in China: an epidemiological, genomic and mechanistic study. EBioMedicine. 2018;34:139–157. doi: 10.1016/j.ebiom.2018.07.027
- Donahoe SL, Phalen DN, McAllan BM, et al. Differential gamma interferon- and tumor necrosis factor alpha-driven cytokine response distinguishes acute infection of a metatherian host with Toxoplasma gondii and Neospora caninum. Infect Immun. 2017;85(6):e00173–17. doi: 10.1128/IAI.00173-17
- Lei R, Hou J, Chen Q, et al. Self-assembling myristoylated human alpha-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano. 2018;12(6):5284–5296. doi: 10.1021/acsnano.7b09109
- van Harten RM, van Woudenbergh E, van Dijk A, et al. Cathelicidins: Immunomodulatory Antimicrobials. Vaccines (Basel). 2018;6(3):63. doi: 10.3390/vaccines6030063
- Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176(5):3044–3052. doi: 10.4049/jimmunol.176.5.3044
- Tang X, Basavarajappa D, Haeggstrom JZ, et al. P2X7 receptor regulates internalization of antimicrobial peptide LL-37 by human macrophages that promotes intracellular pathogen clearance. J Immunol. 2015;195(3):1191–1201. doi: 10.4049/jimmunol.1402845
- Duarte-Mata DI, Salinas-Carmona MC. Antimicrobial peptides immune modulation role in intracellular bacterial infection. Front Immunol. 2023;14:1119574. doi: 10.3389/fimmu.2023.1119574
- Agarwal V, Sroka M, Fulde M, et al. Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J Biol Chem. 2014;289(22):15833–15844. doi: 10.1074/jbc.M113.530212
- Agarwal V, Kuchipudi A, Fulde M, et al. Streptococcus pneumoniae endopeptidase O (PepO) is a multifunctional plasminogen- and fibronectin-binding protein, facilitating evasion of innate immunity and invasion of host cells. J Biol Chem. 2013;288(10):6849–6863. doi: 10.1074/jbc.M112.405530
- Chen C, Liu H, Zabad S, et al. MoonProt 3.0: an update of the moonlighting proteins database. Nucleic Acids Res. 2021;49(D1):D368–D372. doi: 10.1093/nar/gkaa1101
- Cole JN, Nizet V, Kudva IT, et al. Bacterial Evasion of Host Antimicrobial Peptide Defenses. Microbiol Spectr. 2016;4(1):10. doi: 10.1128/microbiolspec.VMBF-0006-2015
- Xie F, Zan Y, Zhang Y, et al. The cysteine protease ApdS from Streptococcus suis promotes evasion of innate immune defenses by cleaving the antimicrobial peptide cathelicidin LL-37. J Biol Chem. 2019;294(47):17962–17977. doi: 10.1074/jbc.RA119.009441
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38(Database issue):D227–D233. doi: 10.1093/nar/gkp971
- Schmidtchen A, Frick IM, Andersson E, et al. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol. 2002;46(1):157–168. doi: 10.1046/j.1365-2958.2002.03146.x
- Sieprawska-Lupa M, Mydel P, Krawczyk K, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother. 2004;48(12):4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004
- Vandamme D, Landuyt B, Luyten W, et al. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012;280(1):22–35. doi: 10.1016/j.cellimm.2012.11.009
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399
- Sigurdardottir T, Andersson P, Davoudi M, et al. In silico identification and biological evaluation of antimicrobial peptides based on human cathelicidin LL-37. Antimicrob Agents Chemother. 2006;50(9):2983–2989. doi: 10.1128/AAC.01583-05
- Bei Y, Pan LL, Zhou Q, et al. Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Med. 2019;17(1):42. doi: 10.1186/s12916-019-1268-y
