ABSTRACT
The pervasive presence of Staphylococcus epidermidis and other coagulase-negative staphylococci on the skin and mucous membranes has long underpinned a casual disregard for the infection risk that these organisms pose to vulnerable patients in healthcare settings. Prior to the recognition of biofilm as an important virulence determinant in S. epidermidis, isolation of this microorganism in diagnostic specimens was often overlooked as clinically insignificant with potential delays in diagnosis and onset of appropriate treatment, contributing to the establishment of chronic infection and increased morbidity or mortality. While impressive progress has been made in our understanding of biofilm mechanisms in this important opportunistic pathogen, research into other virulence determinants has lagged S. aureus. In this review, the broader virulence potential of S. epidermidis including biofilm, toxins, proteases, immune evasion strategies and antibiotic resistance mechanisms is surveyed, together with current and future approaches for improved therapeutic interventions.
Introduction
Colonizing up to 80% of healthy humans,Citation1 the frequent presence of Staphylococcus epidermidis and other coagulase-negative staphylococci (CoNS) on human skin demonstrates both its resilience to environmental extremes and capacity for host immune modulation,Citation2,Citation3 allowing it to evade detection by host defences. The skin can be a harsh environment for microorganisms, due to environmental factors such as variations in pH, temperature, and low moisture levels. Human epithelial surfaces also have multifaceted defence mechanisms mediated by the innate immune system, including production of a diverse array of antimicrobial peptides, sebum production, and the shedding of keratinocytes, all of which collectively inhibit colonization and infection by pathogenic organisms. Yet paradoxically, the skin still needs bacteria: formation of a healthy skin microflora can help prevent skin dysfunction, particularly well documented in the case of atopic dermatitis but also playing a significant role in stabilizing the microbial communities of healthy skin.Citation4,Citation5 Commensal inhabitants must balance strategies to survive desiccation, acid stress, and temperature fluctuations against the risks of activating the host immune system.
Due to its ubiquity, isolation of S. epidermidis in diagnostic samples taken during clinical infections was often dismissed as accidental contamination,Citation6 before its emergence as an important pathogen in infections associated with implanted medical devices (). Moreover, this pathogen is increasingly recognized as the sole causative factor in serious infections ranging from bacteraemia,Citation7 sepsis,Citation8,Citation9 endocarditis, meningitis, and toxic shock syndrome,Citation10 as well as superficial skin infections, ocular infectionsCitation11,Citation12 and the aforementioned infections associated with indwelling medical devices including shunts,Citation13 cathetersCitation14 and prosthetic joints.Citation15
Figure 1. Medical interventions including surgical wounds and various implanted medical devices associated with increased risk of S. epidermidis and other CoNS opportunistic infections. Created with Biorender.com.
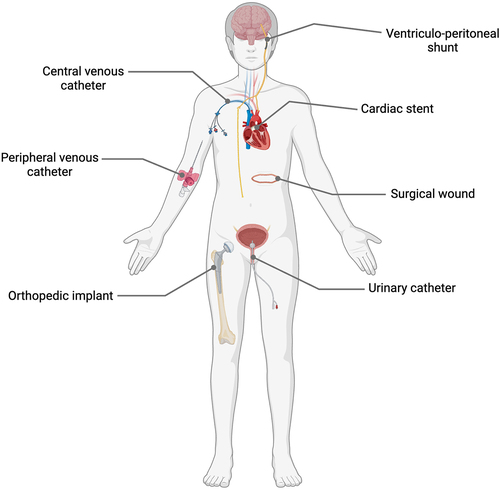
The diverse array of infection vectors, including medical interventions and the patient’s own microflora, serve to emphasize the importance of S. epidermidis, through its ubiquity, as a challenging pathogen to eradicate.Citation16 Moreover, to eliminate this commensal from epithelial surfaces may do more harm than good in the majority of cases, and this opportunistic pathogen continues to pose a significant health risk to vulnerable patients.Citation17
Any discussion about S. epidermidis as a pathogen is invariably qualified by the comment that it lacks the diverse array of virulence factors found in S. aureus. Nevertheless, although this is true, we argue that it is important to consider what aspects of S. epidermidis pathogenicity are being overlooked when this microorganism is primarily categorized as a “common commensal” and not a ubiquitous opportunistic pathogen.
This review summarizes the pathogenicity of S. epidermidis infections including the molecular basis of biofilm-related and non-biofilm virulence determinants, as well as current treatment strategies and future directions for research on this remarkably persistent pathogen.
Establishment of infection
As a frequent constituent of the human microbiome, S. epidermidis is generally considered to lead a commensal lifestyle on epithelial surfaces, such as the skin, nares, and the mucosa. However, S. epidermidis exploits breaches in the host’s cutaneous barriers, both through unintentional injury or surgical procedures in order to gain access to deeper tissues. Normally, the immune system is able to clear low-level infiltration by S. epidermidis cells, but immunocompromised individuals are susceptible to a spectrum of infections, from localized tissue inflammation to severe, systemic disease. Early establishment of infection involves bacterial adherence to surfaces, facilitated by microbial adhesins. This initial adhesion is a critical step, allowing the bacterium to evade clearance and establish a foothold within the host. Following successful adhesion, biofilm formation can begin ().
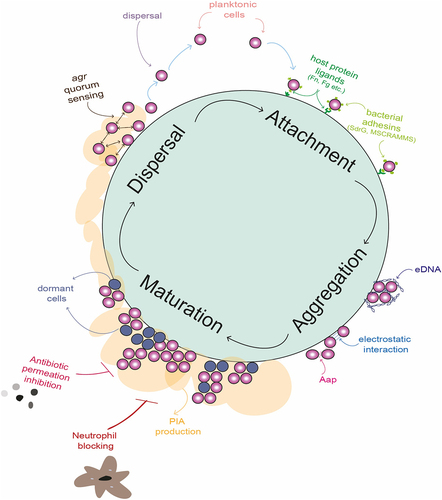
Figure 2. Graphic illustration of the biofilm life cycle in Staphylococcus epidermidis including depictions of important mediators of attachment aggregation, maturation and dispersal stages of biofilm are depicted. The attachment phase is characterised by the deposition of planktonic cells onto a biotic or abiotic surface. This phase is mediated by interactions between host ligands present on the surface, including fibrinogen (Fg) and fibronectin (Fn). Host proteins are then bound by bacterial surface adhesins, such as SdrG and microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), which allow the bacterial cells to remain attached. The accumulation phase results in microcolonies of bacterial cells that self-associate using electrostatic forces and protein adhesins such as accumulation associated protein (Aap). Negatively charged extracellular DNA (eDNA) also surrounds the cells, attracted to the positively charged cell surface and resulting in a “sticky” mesh. A mature biofilm forms when polysaccharide intracellular adhesin (PIA) is excreted by S. epidermidis, forming profuse multi-layered structures. The biofilm matrix shields the bacteria from immune system cells, such as neutrophils, and provides protection from harsh environmental conditions such as desiccation, pH stress and the presence of antibiotics. Channels in the biofilm allow perfusion of nutrients. Within the mature biofilm, a significant proportion of cells remain metabolically dormant, rendering them tolerant to antibiotics that target metabolic processes. The dispersal of biofilm in S. epidermidis is co-ordinated by the action of the agr quorum sensing system, which secretes an autoinducing peptide when cell numbers increase to a critical level. In response to this, transcription of the ica operon, which governs the production of PIA, is reduced and individual cells are released from the biofilm structure. These planktonic cells are then free to disseminate in the host again.
Common vectors of infection
Intravascular catheters, particularly central venous catheters and peripherally inserted central catheters, stand out as major sources of S. epidermidis infections. These devices provide an ideal substrate for bacterial adhesion and biofilm formation, leading to catheter-related bloodstream infections. More generally, CoNS are leading causative agents of bloodstream infections, representing up to 31% of reported cases.Citation18–21 Prosthetic devices, including joint implants and cardiac devices, also represent significant sources of infection, causing chronic and difficult-to-treat biofilm-related infections. Periprosthetic joint infection (PJI) isolates are significantly more likely to be resistant to one or more antibiotics when compared to commensal isolates, and 44% of PJI isolates are from ST2, a sequence type associated with hospital-acquired highly resistant strains.Citation22 Infection of cardiac devices such as pacemakers,Citation23 as well as prosthetic heart valvesCitation24, Citation25 can necessitate removal of the device, or in refractive cases due to highly resistant strains, heart transplantation as salvage therapy.Citation26, Citation26 In one study of bloodstream infections originating from cardiac assist devices, S. epidermidis was identified as the most common causative pathogen.Citation27 In orthopedic S. epidermidis infections, which represent up to 43% of orthopedic device-related infections, aminoglycoside resistance, and strong biofilm-forming capabilities are associated with worsened patient outcomes.Citation28
S. epidermidis is the causative agent in up to 60% of ventriculoperitoneal (VP) shunt infections,Citation29 which are particularly challenging to treat due to poor antimicrobial drug penetration into cerebrospinal fluid (CSF). Post-operative infections of implanted VP shunts, which can affect up to 16% of patients, higher in neonatesCitation29–32 is accompanied by a mortality rate at 10%.Citation29
Up to 31% of surgical site infections following spinal surgery are caused by S. epidermidis, and whilst S. aureus is a more frequent cause of these spinal surgery infections, methicillin resistant S. epidermidis (MRSE) was significantly more abundant than methicillin resistant S. aureus (MRSA) in spinal infections, which can limit treatment options.Citation33 S. epidermidis was identified as the most common cause of latent surgical site infections after spinal fusion surgery, characterized by the slow development of clinical symptoms often mistaken for post-surgical back pain, which allowed the bacteria to become established at the surgical site and cause deeper infection, particularly inflammation of the vertebrae (spondylitis).Citation34 Intracranial infection with MRSE may be associated with poor prognosis, even following vancomycin treatment.Citation35 Isolates collected from infected sutures from a variety of surgical cases also implicated S. epidermidis as the most common pathogen.Citation36 Surgical tools can also become contaminated with skin flora from either the patient or from surgical personnel during surgery, with S. epidermidis being the most commonly isolated species from laparotomy instruments,Citation37 representing a vector of infection even when strict hygiene and aseptic technique are practiced.
Other vectors of implant-associated S. epidermidis infection include, but are not limited to, intrathecal pumps,Citation38 breast prostheses and implants,Citation39–41 intraocular lenses,Citation42, Citation43 surgical mesh used for hernia repair,Citation44,Citation45 and bone-anchored hearing aids.Citation46 Despite these risks, rigorous infection control measures, such as pre-operative decolonization, surgical site disinfection, and intra-operative infection control procedures can help prevent bacteria from breaching the skin during surgical procedures.Citation47–49
Chronic versus acute infection
S. epidermidis is typically responsible for chronic infections involving biofilm, rather than acute infections. However, examples of acute S. epidermidis infections include those associated with ocular implantsCitation43, Citation50 and knee arthroplasty.Citation51 For the latter, poor response to treatment compared to non-staphylococcal infections can also lead to chronic infections.Citation52–55 Prevention, or timely diagnosis and treatment of S. epidermidis infections is therefore of particular importance. The release of bacterial cells from biofilm into the bloodstream is noted as the primary mechanism of CoNS-related sepsis.Citation56 Sepsis, a serious condition in which host immune response to bacteria in the bloodstream leads to systemic inflammation and potentially escalates to severe sepsis or septic shock, can in the case of S. epidermidis infection be viewed as an acute host response to a chronic bacterial infection.Citation57
Biofilm – the primary virulence factor
In a typical S. epidermidis infection scenario, biofilm formation begins with the transfer of bacteria from the skin onto a medical device during surgical implantation. The individual bacterial cells utilize their own surface protein adhesins to adhere to the implant, including interacting with host matrix proteins that coat the device. After adherent microcolonies are established, biofilm formation enters the accumulation phase, driven by the abundant production of polysaccharide intercellular adhesin (PIA)/poly-N-acetylglucosamine (PNAG), or protein adhesins that are covalently attached to the cell wall or associated with the cell surface. PIA forms a protective layer, enveloping the cells until mature biofilms begin to release planktonic cells, initiating a new cycle of dispersion. The role for different adhesins and biofilm mechanisms indicates that “biofilm” is not a uniform process, but a term encompassing various survival modes employed by S. epidermidis to adapt to dynamic and challenging host niches.
Biofilm formation
Several reviews and articles have comprehensively covered S. epidermidis biofilm formation, structure, regulation, and disassembly in detail.Citation52,Citation58–64 Here, the role of the ica operon and the role of non-PIA biofilm factors such as extracellular DNA (eDNA) and key protein adhesins will be summarized to contextualize the interaction and importance of S. epidermidis virulence determinants with these biofilm constituents and regulators.
Role of ica operon in PIA-dependent biofilm
Any review on S. epidermidis virulence cannot overlook the essential role of the icaADBC operon-encoded PIA in biofilm formation by many S. epidermidis isolates. The capacity of S. epidermidis to form biofilm strongly correlates with the establishment of chronic infections, and the accompanying morbidity and mortality to the host. Whilst S. epidermidis is also capable of ica-independent biofilm formation, PIA-mediated biofilm presents a uniquely profuse and resilient state of existence for staphylococci. Early research on “slime-producing” isolates of S. epidermidis identified the ability of what had previously been considered a harmless skin-dwelling commensal organism to persist within the host, turning simple implantation of medical devices into a procedure carrying significant risk for opportunistic infection by a ubiquitous skin commensal microorganism.Citation65–72
The ica operon encodes a set of proteins responsible for PIA production, export, and function. The icaA and icaD genes encode transmembrane proteins that dimerize to form an N-acetylglucosaminyltransferase, responsible for PIA synthesis.Citation73 The IcaB enzyme deacetylates PIA, introducing a positive charge necessary for its adherence to the cell surface, with icaB mutants markedly impaired in adhesion and colonization in the host, as well as being more vulnerable to phagocytosis.Citation74 IcaC elongates the polysaccharide molecules, rendering them functional, whereas icaC mutants produce short, 20-residue oligomers in comparison to full-length PIA, which normally contains over 130 N-acetylglucosamine (GlcNAc) residues.Citation75 The icaR gene located immediately adjacent to the ica operon encodes a transcriptional repressor centrally involved in the regulatory network that controls icaADBC transcription.Citation76–78 Expression of icaR is in turn controlled by a complex network of proteins and transcription factors, including the alternative sigma factor σB and SarA, that combine to regulate the ica operon in response to metabolic, environmental, and host factors ().Citation79 Furthermore, transcription of the icaADBC operon does not precisely align with PIA production, revealing the importance of post-transcriptional regulation in the biofilm phenotype.
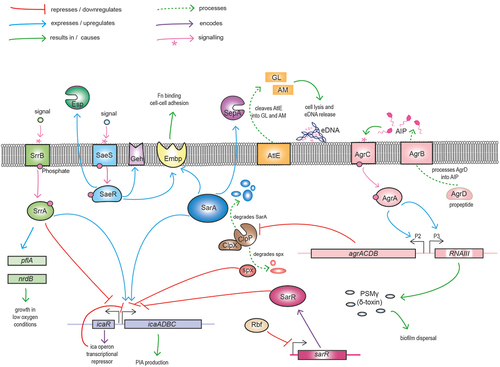
Figure 3. Graphic illustration of major S. epidermidis regulators of growth, biofilm formation and virulence. The icaADBC locus encodes the enzymes responsible for synthesis, export and deacetylation of polysaccharide intercellular adhesin (PIA), which serves as a major mediator of biofilm. The divergently expressed icaR gene encodes the major transcriptional repressor of the ica operon. In response to environmental cues activated SrrB phosphorylates its cognate SrrA effector promoting pflA and ndrB expression and growth under low oxygen conditions. SrrA binds to both the icaA and icaR promoters to differentially regulate biofilm under oxic and microaerobic conditions. Activation of the SaeS kinase leads to phosphorylation of the SaeR response regulator increasing fibronectin (Fn) binding and cell-cell adhesion through upregulation of GehD lipase and the extracellular matrix binding protein (Embp). SaeR also upregulates activity of the serine endopeptidase Esp, negatively impacting S. aureus colonisation and promoting S. epidermidis immune evasion through proteolysis of complement proteins. ClpPX is an ATP-dependent protease and chaperone system that degrades the global regulator SarA, resulting in repression of the repressor icaR and activation of icaADBC and biofilm. Rbf downregulates expression of sarR, which encodes a repressor of the icaADBC operon. The agrD gene from the agrACDB operon encodes a pro-peptide that is exported and processed by AgrB to release the autoinducing peptide (AIP) that constitutes that major quorum sensing system in staphylococci. In response to AIP, the AgrC sensor kinase phosphorylates the AgrA response regulator, thereby leading to activation of the P2 and P3 promoters and increasing expression of the agrACDB and RNAIII loci, respectively. RNAIII is the second effector molecule of the Agr system, functioning as an antisense RNA to control translation of target genes. The cytolysin phenol soluble modulin g (PSMg, δ-haemolysin), encoded by the hld gene located within RNAIII plays a role in biofilm dispersal. The Agr system also negatively regulates expression of the clpP-encoded protease, which degrades Spx, a negative regulator of icaADBC. SarA positively influences Embp expression facilitating cell-cell adhesion. Processing of the major autolysin AtlE by SepA to generate active amidase (AM) and glucosaminidase (GL) autolytic enzymes is important for eDNA release during the early stage of biofilm development.
PIA-independent biofilm
Approximately 30% of S. epidermidis isolates from non-superficial infections lack the ica operon.Citation80 However, the absence of the ica operon does not preclude biofilm formation.Citation81 PIA-independent or protein-mediated biofilm represents a second modality of biofilm formation. Protein-mediated biofilm relies on cell wall proteins that can bind to host ligands, as well as extracellular DNA (eDNA) released by autolysins that also enhances attachment and structure of biofilm. The spatial distribution of adhesins significantly influences biofilm architecture, for example, PIA encapsulates the cell, whereas protein adhesins localize to the membrane, resulting in different overall biofilm architecture which can give advantages in surviving in variant niches. PIA-producing isolates are more commonly isolated from sites exposed to shear force, e.g. the bloodstream and urinary tract, whereas PIA-independent biofilm in S. epidermidis represents the major mode of biofilm production in other prominent infection types, such as ocular infections.Citation80, Citation82 PIA-dependent biofilm-forming strains are approximately twice as likely to cause prosthetic joint infections, compared strains capable of PIA-independent biofilm formation.Citation81
Aap
The cell wall-anchored (CWA) proteins like accumulation-associated protein (Aap) is the best characterized mediator of PIA-independent biofilm in S. epidermidis. Aap has several domains: a signal peptide, an N-terminal A domain, comprising A-repeats and L-type lectin domains, followed by a B-repeat region containing G5-E repeats. The C-terminal end contains an LPXTG motif that is covalently anchored to the cell wall by sortase.Citation83 The A domain can be proteolytically cleaved by the metalloprotease SepA, exposing the B domain, which is then capable of self-association with other Aap B domains, to promote cell aggregation.Citation80
The A-domain of Aap binds to host surfaces through the lectin domain, essential for adherence to host glycans. Heterophilic sugar binding by the lectin region of the A domain is also involved in initial staphylococcal cell–cell contact early in biofilm formation, before homophilic B-repeat bonds allow for tighter binding. Homophilic interactions between adjacent cells involve the G5-E domains binding to one another. Notably, the B-repeat domains exhibit amyloid fibril formation, forming “rope-like” structures within the biofilm matrix, a process dependent on zinc equilibrium.Citation80, Citation84 Single-molecule experiments have revealed the extraordinary mechanical strength of Aap homophilic bonds, with forces reaching up to 1,000 pN – close to the bond strength of covalent chemical bondsCitation85 reinforcing their role in forming highly adhesive and cohesive biofilms (). Intriguingly, the cleavage patterns of Aap vary between strains, with a 2022 study by Wang et al. finding that strain CSF41498 has lower rates of Aap processing resulting in more intact Aap when compared to strain 1457, which has increased cleavage of Aap.Citation86
Extracellular matrix binding protein (EmbP)
Embp is a large, membrane bound protein involved in adhesion. Whilst EmbP plays a moderate role in adhesion to fibronectin (Fn) under low flow conditions, the “Velcro-like” binding that occurs between multiple repeats within EmbP and the repeats exposed in the fibrillated, deposited form of Fn was shown to be crucial to S. epidermidis binding to surfaces under high shear conditions, for example on an implanted intravenous catheter.Citation87 Embp expression is highly induced when adherent cells, such as those in a biofilm, are subjected to osmotic stress indicating that Empb may be more important for survival of S. epidermidis by promoting cell–cell adhesion under the high-salinity conditions found on the skin, rather than as a factor in invasion and adhesion during infection. However, given the role of Embp in binding to Fn, osmotic stress induced Embp expression should enhance adherence to the host extracellular matrix proteins and enhanced biofilm formation in the infection niche.Citation88
Biofilm associated protein (Bap)/bap homolog protein (Bhp)
The first report of a PIA-independent mechanism of biofilm formation in staphylococci identified Bap.Citation89 This 239-kDa sortase-anchored surface protein contributes to the initial attachment and accumulation phases of biofilm.Citation90 Unlike Bap in S. aureus, which is associated primarily with bovine mastitis isolates, Bap in S. epidermidis is commonly found in mastitis isolates from several veterinary species.Citation90 Bap consists of several repeated domains, referred to as Bap repeats, which are responsible for its adhesive properties and the formation of cell-to-cell interactions within the biofilm. Bhp is a protein with significant homology and similarity to Bap,Citation90 which is also found in non-animal derived isolates, including in MRSE isolates from clinical infections.Citation90 Bhp has an N-terminal export signal, followed by an A-domain and a region of tandem repeats that shares 45–57% identity with S. aureus Bap tandem repeats.Citation91 Bhp expression is highest during early exponential phase and decreases in stationary phase.Citation91 Serum reactivity to Bhp was not commonly detected in patients with S. epidermidis infections, suggesting that Bhp is only weakly immunoreactive or not highly expressed during infection, although other cell-wall proteins like SdrF exhibited a similar response.Citation91 Found in both commensal and invasive strains,Citation92 and in up to 15% of blood culture isolates,Citation93 the role of Bhp in S. epidermidis biofilm formation remains unclear, and whilst it shares considerable similarity with Bap, it is important not to over-extrapolate from the small amount of available literature on Bhp.
eDNA and cell lysis
Extracellular DNA (eDNA) is released from lysed bacterial cells, either through breakdown of dead cells or by the activity of autolysins.Citation94 eDNA has multiple properties that make it a valuable biofilm constituent. eDNA is associated with the initial attachment phase to abiotic surfaces.Citation95–97 Strands of eDNA create a scaffold that helps maintain the overall architecture and resilience of the biofilm ().Citation98 eDNA also acts as a barrier against host immune defences by binding and sequestering antimicrobial peptides.Citation99, Citation100
S. epidermidis possesses two autolysins, the major autolysin AtlE and a secondary autolysin Aae, both having dual roles as both autolysins and adhesins. AtlE consists of a signal peptide and a propeptide, followed by amidase (AmiE) domain linked by three direct repeats (R1, R2, R3) to a glucosaminidase domain at the C-terminus. Proteolytic processing occurs by an unidentified protease, resulting in removal of the N-terminal signal peptide and the propeptide after secretion.Citation101,Citation102 After further processing by the metalloprotease SepA,Citation101 the amidase domain retains two of these repeats, and the glucosaminidase domain retains the third. Each repeat contains a glycine-tryptophan (GW) module. The amidase domain is anchored to the cell wall by the R1 and R2 repeats and is required to cleave peptidoglycan during cell division.Citation103,Citation104 Evidence from the functionally interchangeable Atl protein in S. aureus indicates that presence of WTA on the mature cell wall but not the newly created septum discourages AtlE binding during division, encouraging enzymatic separation of daughter cells.Citation104,Citation105
Binding and internalisation by host cells are mediated by the AmiE domain, which interacts with the host heat shock cognate protein Hsc70 directly and with α5β1 integrin via fibronectin bridging.Citation106, Citation107 Vitronectin binding activity is seen with the full-length protein, and to a lesser extent with each of the catalytic domains.Citation102 Deletion mutants of atl are defective in biofilm formationCitation94 and have decreased virulence in a rat catheter model.Citation108 eDNA is found in microcolonies during the initial adherence phase of biofilm accumulation. DNase I is capable of dispersing early-stage biofilms, but ineffective at removing mature biofilm.Citation94, Citation109
Aae is a more recently identified autolysin.Citation110 The N-terminal domain of Aae consists of an LysM domain, implicated in peptidoglycan recognition and binding, made up of three direct repeats. Unlike other bacterial species where it is located at the C-terminal end, the N-terminal position of the LysM domain in staphylococci may allow Aae to associate with the cell wall in the absence of a specific cell wall anchor. Like AtlE, Aae also has adhesive properties and can bind fibrinogen, fibronectin and vitronectin in a dose-dependent manner.Citation110
The link between PIA production and eDNA in biofilm matrix is complex, though the overall trend is positive, with strong PIA production being associated with increased levels of eDNA. PIA may play a role in retaining eDNA within the biofilm structure.Citation98 Thus, eDNA appears to play a role in both PIA-dependent and PIA-independent biofilm development.
Interestingly, several eDNA-binding proteins have been described in the matrix of S. aureus biofilms, many of which were previously not known to bind DNA,Citation96 forming positively charged surface anchors that stabilize the electrostatic net of eDNA in the matrix.Citation96 Several of these proteins have homologues in S. epidermidis, including CopL, which is associated with the COMER-like pathogenicity island found in the hospital-associated MRSE lineage ST2.Citation111 Furthermore, the glucosaminidase region of S. aureus Atl is also known to bind DNA.Citation112 This raises the intriguing possibility that there remain uncharacterized eDNA and protein interactions in S. epidermidis biofilms. Regulation of eDNA release is mediated through SarA, which indirectly negatively regulates AtlE by downregulating SepA, responsible for the proteolytic cleavage required for the bacteriolytic function of AtlE.Citation101
A small regulatory RNA RsaE was recently identified that positively regulates both icaADBC expression and eDNA release. RsaE is expressed differentially throughout S. epidermidis biofilm, and interestingly is not present in all icaADBC-positive strains. Regulation of PIA production is mediated through RsaE interaction icaR mRNA, whilst the effect on autolysis occurs due to negative regulation the lrgA-encoded antiholin protein that prevents cell lysis by the holin CidA.Citation113 In S. aureus, eDNA release by CidA contributes to the function of biofilm,Citation114 suggesting that it may play a similar role in S. epidermidis.Citation113 In S. epidermidis small colony variants (SCVs), imbalance between CidA and LrgA expression results in increased eDNA abundance in biofilm.Citation115
Non-biofilm virulence determinants
Despite being commensals, S. epidermidis strains are capable of expressing several enzymes and toxins, which collectively appear to play a protective role underpinning the characteristic resilience of this organism.
Lipases
Lipase-mediated hydrolysis of lipids can serve as a source of nutrients for S. epidermidis. Interestingly, the glycerol ester hydrolase (geh) lipases of staphylococci have a secondary role as surface-anchored adhesins. S. epidermidis has two known geh lipases, GehD and GehC, under the control of the agr quorum sensing system.Citation116 GehD is a collagen-binding MSCRAMM, which despite lacking an LPXTG motif is localized to the cell wallCitation117 ().
Superantigens and enterotoxins
Staphylococci produce multiple superantigens implicated in toxic shock syndrome (TSS) including TSS toxin 1 (TSST-1), and staphylococcal enterotoxins B and C.Citation118, Citation119 Superantigens bind to Major Histocompatibility Complex class II (MHC II) receptors, precipitating interactions with T-cells and inciting a cascade of events leading to heightened cytokine production, marked inflammation, and the onset of TSS symptoms such as fever, hypotension, vomiting, and confusion.Citation120 Menstrual TSS is caused almost exclusively by TSST-1, whereas staphylococcal enterotoxin B and C as well as superantigens from group B, C, and G streptococci are also known to be involved in non-menstrual TSS.Citation121 CoNS are generally considered to lack superantigens as they lack TSST-1, however, they may possess staphylococcal enterotoxins.Citation10 Up to 95% of S. epidermidis isolates from blood cultures possess at least one staphylococcal enterotoxin.Citation122
Staphylococcal enterotoxins (SEs) exhibit a remarkable tolerance to both heat and pH stress, as well as protease degradation, maintaining their integrity in the harsh environment of the gastrointestinal (GI) tractCitation123. Staphylococcal food poisoning (SFP) from SEs is accompanied by a rapid onset of symptoms, including nausea, emesis, abdominal pain, diarrhea and GI tract inflammation and damage.Citation123 Whilst reports of disease caused by superantigen- and enterotoxin-producing S. epidermidis isolates are limited to sporadic cases in the literature, a small minority of strains do possess and express these virulence factors, which can be clinically important. An early case of SEA-producing S. epidermidis was implicated in a major outbreak of SFP in the 1960s.Citation124 A novel C-type enterotoxin (SEC) from S. epidermidis was shown to induce high levels of cytokine secretion, including IL-2, −4, −6, −8, −10, IFN-γ, TNF-α and GM-CSF.Citation125 This massive release of multiple cytokines in response to MHC II binding is characteristic of superantigenic activity.Citation120 Production of IL-6 by lymphocytes in response to SEC is notable as IL-6 is a “pro-sepsis” cytokine that contributes to the systemic inflammation seen in septic shock.Citation125 Interestingly, the S. epidermidis homologue of C-type enterotoxin induced higher secretion of IL-6 when compared to its S. aureus homologue.Citation125 SEC and another enterotoxin, SEL are often carried on the same S. epidermidis pathogenicity islandCitation126 and oral administration of SEL and SEC in a mouse model was shown to cause oedema and cytotoxicity in the GI tract.Citation127
A recent case of S. epidermidis toxic shock syndrome was reported in which novel PCR assay was used to detect multiple staphylococcal enterotoxins including TSST-1 in blood plasma.Citation10 Interestingly in this patient, S. epidermidis was only isolated from a urinary culture and not blood cultures.Citation10 TSST-1 is also found infrequently in veterinary isolates of S. epidermidis, and its horizontal gene transfer (HGT) from CoNS to S. aureus has been widely studied.Citation128 Notably one paper described likely HGT of a pathogenicity island in the opposite direction: from S. aureus to two virulent strains of S. epidermidis, allowing for expression of an enterotoxin. This was previously considered to be controversial due to the presence of strong restriction barriers in S. epidermidis.Citation128 The presence of superantigens in S. epidermidis is uncommon, and these authors concluded that enterotoxin produced by these strains may have contributed to septic shock in the patients from which they were isolated.Citation128 Overall, while reports of S. epidermidis toxic shock and enterotoxigenicity remain rare in the literature, it nevertheless appears that individual S. epidermidis strains may have the capacity to produce both superantigens and enterotoxins.
Proteases
Serine protease (Esp)/glutamyl endopeptidase (GluSE)/SspA
The serine endopeptidase Esp is a secreted protease that belongs to the glutamyl endopeptidase I family, sharing about 50% identity with the better-characterized S. aureus V8 protease.Citation129 Esp is ubiquitously found in S. epidermidis strains, in which it is also known as SspA and GluSE.Citation17, Citation130 Production of Esp is associated with adherent, biofilm-like growth rather than planktonic growth.Citation130 Esp contributes to the ability of S. epidermidis to compete with S. aureusCitation131 by cleaving the B-domain of the S. aureus Aap protein required for the attachment phase of S. aureus biofilm formation, as well as degrading fibrinogen, fibrin.Citation80, Citation132, Citation133 Elastin, fibronectin, and type I collagen,Citation134 which all play a role in S. aureus interaction with host cells.Citation80, Citation132–134 Esp is also involved in evading host immune defences by degrading complement protein C5.Citation132, Citation134 Finally, Esp is a significant virulence factor in ocular infections,Citation135 which is important given that S. epidermidis is a leading cause of keratitis, blepharitis, conjunctivitis, and endophthalmitis.Citation135
Metalloprotease (SepA)/aureolysin
As noted above SepA processes Aap, cleaving off the A-domain, and sometimes the lectin domain to leave the B-domain.Citation136 The regulator SarA controls expression of sepA, which is not observed for the other proteases ().Citation80 A notable role for SepA is in promoting bacterial survival following neutrophil phagocytosis, contributing to immune evasion and maintenance of infection.Citation137 SepA has also been reported to degrade AMPs and process AtlECitation132 (). SepA-mediated degradation of the anionic human AMP dermcidin is important for survival on the skin and also notable given that the standard staphylococcal defence mechanisms against CAMPs, such as altering the bacterial cell wall charge via D-alanylation of wall teichoic acids, are ineffective against anionic dermicidin.Citation138
Extracellular cysteine protease (EcpA)
EcpA is a secreted cysteine protease with gelatinase and collagenase activity.Citation35 EcpA is negatively regulated by the protease inhibitor EcpB, also known as staphostatin A, and positively regulated by agr system.Citation132, Citation139 Secretion of EcpA damages the epithelial barrier by breaking down host barrier proteins, primarily desmoglein-1.Citation35 This breakdown in dermal integrity increases disease severity in atopic dermatitis.Citation35 EcpA is homologous to SspB in S. aureus which is known to degrade the LL-37 epithelial antimicrobial peptide.Citation132 Cau et al. recently showed that S. epidermidis EcpA also degrades LL-37, and increased expression of IL-6, IL-8, TLSP, IL1-α and IL-1β in host keratinocytes, promoting inflammation.Citation140 Autoinducing peptides (AIPs) from other CoNS species, such as S. hominis, can decrease expression of EcpA and the presence of a healthy skin flora may therefore inhibit S. epidermidis-mediated skin damage in patients with AD.Citation140
Epidermidin leader peptide processing serine protease
The extracellular epidermin leader peptide processing serine protease (EpiP) cleaves the propeptide EpiA, generating the functional lantibiotic form of epidermin.Citation141 Lantibiotics such as epidermin have broad antibacterial activity microorganisms, and consequently self-immunity proteins are required to prevent ill effects from the production of these molecules.Citation142 Whilst the primary function of epidermin is in defence, EpiP is also involved in cleavage of host proteins such as collagen and casein,Citation132, Citation143 resulting in breakdown of connective tissue. Furthermore, the Streptococcus pyogenes homolog of EpiP impairs neutrophil recruitment, raising the possibility that S. epidermidis EpiP has a similar function.Citation143
Cytolysins
The phenol soluble modulins (PSMs) are the major cytolysins in S. epidermidisCitation144, Citation145 and comprise a family of small amphipathic, α-helical peptides that also serve as effectors of staphylococcal biofilm structuring and dispersal both in vitro and in vivo.Citation144, Citation146, Citation147 Clinical PJI-associated biofilm forming strains are less likely to produce PSMs, potentially due to the dispersal effect PSMs have on established biofilm.Citation148
S. epidermidis produces 6 types of PSMs: PSMα, PSMβ1, PSMβ2, PSMγ (also known as δ-toxin), PSMδ, and PSMε, and may additionally possess PSM-mec.Citation149, Citation150 PSMγ also known as δ-haemolysin is encoded by the hld gene located in the RNAIII locus of the agr operon.Citation122, Citation148 α-PSMs are small, approximately 20–25 amino acids in length and are associated with cytolysis of immune cells and induction of neutrophil chemotaxis.Citation151 β-PSMs, characterised by their larger (45 amino acid) size, disrupt the biofilm matrix by interfering with the non-covalent forces involved in cell-cell interactions.Citation152 At lower concentrations of β-PSMs, this allows for the formation of channels, a process essential for the delivery of nutrients to deeper biofilm layers, whereas higher levels of the PSM result in dispersal of biofilm, allowing planktonic cells to be released.Citation152, Citation153 The chromosomal locations for the genes for α and β PSMs are found separate from PSMγ/agr.Citation148 β-PSMs genes occur in tandem on the chromosome, with two identical genes next to each other.Citation148 The psm-mec gene is located in the SCCmec mobile genetic element.Citation154 Independent of the PSM-mec peptide, the psm-mec RNA acts as an independent regulator of RNAIII.Citation155 PSM-mec influences the virulence of S. epidermidis in sepsis and is highly inflammatory and induces neutrophil chemotaxis.Citation154
Beyond the PSMs, β-toxin, encoded by the hlb gene, is a sphingomyelinase, which in S. aureus can lyse erythrocytesCitation156 and kill proliferating human T-lymphocytes,Citation157 but in S. epidermidis has a protective role in promoting production of ceramides important for skin integrity.Citation158
Regulation of virulence
Quorum sensing
Staphylococcal quorum sensing is primarily mediated by autoinducing peptides (AIPs) that are secreted from the cell and recognised by their cognate receptors.Citation159 Quorum sensing regulates S. epidermidis virulence in a cell-density dependent manner, triggering changes in exotoxin secretion and adherence factors as well as regulating formation and dispersal of biofilm.Citation160–163
Accessory gene regulator (Agr) system
The Agr system, encoded by the agrABDC-RNAIII locus, is a quorum sensing system that plays a key role in regulating virulence in S. epidermidis and is regulated in a density-dependent mannerCitation164 The agr system produces two effector molecules. The first, AgrA, is a cytoplasmic response regulator that is phosphorylated by AgrC, a membrane-bound histidine kinase, in response to the agrD-encoded autoinducing peptide (AIP) after a threshold concentration is reached.Citation165, Citation166 The AgrD pro-peptide is processed and exported by AgrB, in tandem with the signal peptidase SpsB.Citation116, Citation167 AgrA also controls expression of a secondary effector molecule, RNAIII.Citation163 RNAIII is a 510 nucleotide non-coding RNA molecule that controls protein production by acting as an antisense RNA, with the long 5’ untranslated region (UTR) of RNAIII when binding to the mRNA product of target genes, impairing translation.Citation168–171
In S. epidermidis, three different agr groups have been described, (I, II, III) based on polymorphisms identified within the agrC, agrB and agrD genes that result in different types of AIP production.Citation116, Citation172, Citation173
AgrA autoregulates its own expression, and also regulates RNAIII transcriptionCitation166 (). Agr controls expression of many S. epidermidis virulence genes, including geh lipase and ecp cysteine protease.Citation160 An active agr system is strictly required for the expression of PSMs.Citation144, Citation148, Citation174 PSM production regulated by the AgrA/AIP circuit occurs primarily in the late-log phase of growth, which coincides with maximal agr activity.Citation160,Citation166
As noted above, the hld gene encoding for production of PSMγ (δ-haemolysin) is encoded within the transcript for RNAIII.Citation170
The cysteine protease EcpA, which triggers production of pro-inflammatory cytokines and disrupts the epithelial membrane is positively regulated by the agr system.Citation140 In the absence of a functional agr system, no activation of host TNF-α and HIV1-LTR is observed, and neutrophil chemotaxis was reduced.Citation175 The presence of the agr system is also associated with resistance to cathelicidin and β-defensin AMPs.Citation176
The agr system plays a central role in controlling the virulence of S. epidermidis under both biofilm and planktonic conditions. A biofilm contains a variety of niches with cells in different metabolic and physiological states, and so the ability of S. epideridis to tightly control the regulation of biofilm dispersal as well as the induction of inflammation in a host is critical for establishment and maintenance of disease ().
LuxS/Autoinducer-2 quorum sensing
Autoinducer-2 (AI-2) is the signal sensed by a proposed cross-species quorum sensing system present in both Gram-positive and Gram-negative bacteria, originally identified in Vibrio harveyii.Citation177 AI-2 is derived from a precursor molecule produced by LuxS. The characterization of AI-2 signalling in staphylococci remains somewhat unresolved, with separate papers describing a role in both positiveCitation178 and negative regulationCitation179 of biofilm. Furthermore, the positive regulation of biofilm was strain dependent with RP62A showing decreased biofilm in a luxS deletion mutant, whereas similar experiments with CSF41498 and clinical isolates revealed no impact on biofilmCitation178 Addition of exogenous AI-2 was shown to differentially regulate genes involved in glycometabolism and amino acid, nitrogen, and nucleotide metabolism.Citation178 PSM production was also increased in the presence of AI-2, independent of agr regulation.Citation178 Whilst its role remains to be fully elucidated, the available evidence suggests that when AI-2 quorum sensing is active in S. epidermidis, it plays a strain-dependent role in modulation of biofilm formation and metabolic regulation.
Global regulators
Alternative sigma factor σB
σB is centrally involved in the environmental regulation of icaADBC expression and biofilm productionCitation180 and controls the transcription of >200 staphylococcal genes.Citation181 Under normal growth conditions σB, which is encoded by the sigB gene in the rsbUVWsigB operon, is bound to the anti-σ factor, RsbW and consequently inactive.Citation79, Citation182 The RsbU phosphatase is activated by environmental stress leading to dephosphorylation of RsbV, which binds to RsbW, thereby releasing σB to engage with the core RNA polymerase and up-regulate transcription of genes with σB-dependent promoters.Citation79, Citation183 However, neither the icaR nor icaADBC promoters are σB-dependent.Citation184 Furthermore, σB controls ica operon expression by indirectly regulating expression of icaR.Citation79, Citation180, Citation183 Thus stress-induced activation of σB leads to repression of icaR transcription, via an unidentified intermediary, and the consequent activation of the ica operon. σB also plays a role in the stability of mature S. epidermidis biofilm, contributing to persistence in a rat catheter infection model.Citation185, Citation186
CodY
The CodY global regulator in S. epidermidis acts as a sensor of nutrient availability to coordinate gene expression in response to nutrient fluctuations. The CodY regulon consists of hundreds of negatively regulated genes that contain CodY boxes, typically located within their promoter regions.Citation187 CodY binding interferes with RNA polymerase interaction with the promoter, downregulating expression. When nutrient levels are high, CodY is activated by its ligands, branched-chain amino acids (BCAAs) or GTP, and binds to the CodY box, resulting in transcriptional repression of target genesCitation160, Citation188 In contrast, under conditions of nutrient limitation, dissociation of CodY from the CodY box leads to de-repression of target gene expression.
In staphylococci, CodY is particularly associated with negative regulation of virulence.Citation187 In S. aureus, CodY negatively regulates the ica operon, leading to reduced production of PIA and impaired biofilm formation.Citation187, Citation189 The role of CodY in S. epidermidis biofilm formation has yet to be clearly elucidated, though a possible role in induction of cell dormancy during stationary phase and in biofilms has been shown, and a role in regulation of agr has been clearly demonstrated.Citation190–192
Other staphylococcal virulence factors regulated by CodY include secreted extracellular proteases like SspA and AtlE, as well as the geh lipase, the hla α-haemolysin and the surface protein SasG.Citation193 CodY also indirectly regulates the global regulator SarA by repressing the expression of rot, which encodes a repressor of sarA. Reduced levels of Rot relieve SarA repression, leading to increased expression of SarA-regulated virulence genesCitation194 (discussed in more detail below). In staphylococci, expression of CodY itself is negatively regulated by the agr systemCitation160 and in turn, strongly represses expression of agr through an unknown intermediate, revealing the complexity of this network of interconnected virulence regulators. Whilst these targets have been primarily identified in S. aureus, S. epidermidis homologues (ica,Citation195 sspA,Citation132 atlE,Citation196 geh,Citation196 sasG/aap,Citation197 sarA,Citation198 agrCitation165) may also be regulated by CodY.
Sar family regulators and Rbf
Sar family regulators are characterized as winged-helix family of transcriptional regulators with conserved DNA binding domains that typically bind to the promoter regions of target genes, up- or down-regulating their expression.Citation199
SarA is particularly associated with the control of biofilm and virulence gene expression including a central role in the positive regulation of PIA-mediated biofilm formation (). IS256 insertional inactivation of sarA impaired biofilm formation in the icaADBC-positive strain RP62A, which was reversed by clean excision of the insertion sequence.Citation180 SarA can bind to the icaA promoter, activating transcription of ica and production of PIA,Citation198 which implied that SarA controls biofilm in an agr-independent manner.Citation198 The S. epidermidis sarA mutant also showed decreased extracellular protease activity.Citation198 SarA may also play a role in switching between polysaccharide- and protein adhesin-mediated biofilm formation.Citation80 A transposon inactivation of sarA increased biofilm production in ica-negative S. epidermidis 1585 due in part to upregulation of the sarA-repressed Embp biofilm protein and SepA metalloprotease (which processes AtlE and Aap)Citation80, Citation101 (). Regulation of sarA itself remains incompletely understood and is driven by three promoters designated SarP1, SarP2, and SarP3, of which SarP1 is σB-dependent. σB regulates ica operon transcription in S. epidermidis via indirect regulation of the icaR repressor.Citation184 SarA positively regulates the ica operon by directly binding to the ica promoter, independently of σBCitation184 ().
SarZ is involved in both the initial attachment and accumulation phases of biofilm formation, as well as regulating the expression of serine protease and geh lipase genes.Citation77 Interestingly, deletion of the sarZ gene resulted in β-haemolysis, a phenotype not typically observed in S. epidermidis.Citation200
SarX also positively regulates the expression of the ica operon though direct interaction with the ica promoter, resulting in increased production of PIA.Citation201, Citation202 SarX also regulates the agr locus, but the effect on biofilm production is predominantly due to ica expression.Citation201
Rbf
The AraC/XylS-family transcriptional regulator rbf (Regulator of biofilm formation) gene is located immediately upstream of sarX and positively influences biofilm in both S. aureus and S. epidermidis.Citation203, Citation204 Rbf does not interact with the ica operon promoter region of S. epidermidis demonstrating that it controls PIA-mediated biofilm indirectly.Citation205 Electrophoretic mobility shift assays also showed that Rbf bound specifically to the sarR promoter but not to the promoters of the sarX, sarA, sarZ, spx, and srrA genes.Citation205 SarR was subsequently shown to be a repressor of ica operon transcription revealing that Rbf controls biofilm by downregulating expression of the sarR repressor.Citation202, Citation205 These findings further reveal the complexity of S. epidermidis PIA regulation as well as the importance and sophisticated fine tuning of this phenotype.
Two-component signal transduction systems
S. aureus exoprotein expression regulator (SaeRS)
SaeRS has been extensively studied in S. aureus,Citation206 but the cognate S. epidermidis system has notable differences, including the absence of the SaeP and SaeQ accessory proteins involved in S. aureus response to neutrophils.Citation207, Citation208 Virulence genes under the control of SaeSR include the geh lipase gene and the esp serine protease gene ().Citation207 In S. aureus, SaeS senses human neutrophil peptide 1 (HNP1), but not in S. epidermidis.Citation206 SaeRS also contributes to inflammation in a mouse catheter infection model, with increased proliferation of polymorphonuclear neutrophils, which occurs independently of PSM production.Citation207 Deletion of SaeR results in impaired nitrate utilization and decreased expression of genes involved in anaerobic growth, implicating the SaeRS system in mediating the switch between aerobic and anaerobic growthCitation207. However, saeR deletion does not impact ica transcription levels, and it is suggested that the differential regulation of virulence genes in a saeR mutant may be indirect, due to the effects on metabolismCitation209. Activation of SaeRS decreases autolysis by downregulating transcription of aae and atlE (), which impacts cell survival and viability during biofilm and planktonic growth.Citation209
Staphylococcal respiratory response protein (srrAB)
Another two-component system that regulates both survival and virulence of S. epidermidis is SrrAB (). Unlike its cognate system in S. aureus, which is only expressed only under microaerobic conditions,Citation210 SrrAB in S. epidermidis is active under aerobic conditions and upregulated under low-oxygen conditions.Citation211 SrrAB regulates both initial adherence and subsequent biofilm matrix production, as well as overall growth and fitness under both aerobic and microaerobic conditions.Citation211 Specifically, under aerobic conditions, the phosphorylated response regulator SrrA, which binds to both the icaA and icaR promoters, positively regulates icaADBC expression while simultaneously downregulating icaR expression, resulting in increased PIA production and biofilm formation.Citation211 Under microaerobic conditions, deletion of srrA results in a significant growth defect, and transcription of both icaR and icaADBC is downregulated.Citation211 SrrA also positively regulates the qoxBACD-encoded respiratory chain terminal oxidase under oxygen-replete conditions. SrrA controls expression of pflBA and nrdD during microaerobic growth, also through direct interaction with the promoter regions of these genes.Citation211 The ability of SrrAB to modulate growth and biofilm production in oxygen-replete and oxygen-limited environments highlights the capacity of S. epidermidis to adapt to a wide range of environmental conditions and is likely to contribute to its pathogenicity.
GraXSR/VraFG
The GraXSR (also referred to as ApsRSX) two-component system and neighboring VraFG efflux system are involved in resistance to cationic AMPs (CAMPs).Citation212 GraS is a membrane-associated histidine kinase which works together with GraX and the response regulator GraR.Citation213 GraXSR is known to modulate the activity of VraFG, a permease and ATPase respectively, and all five proteins function together as a signal transduction complex.Citation213 The function of GraX remains unclear, but it may act to potentiate the signal transduction of the GraXRS/VraFG complex.Citation213 A nine amino acid extracellular loop of the GraS protein senses CAMPs.Citation213 One model suggests that in S. epidermidis, CAMP interaction with the extracellular sensor of GraS dislodges the guard loop of VraG from the membrane, leading to a change in VraG conformation that could positively modulate the activity of GraS.Citation213 Deletion of the guard loop of VraG also leads to knock-on effects such as increased transcription of the MprF gene, which lysinylates phosphatidylglycerol and helps repel CAMPs from the cell membrane.Citation213 The GraXRS system also affects regulation of the dlt operon, which is responsible for D-alanylation of wall teichoic acids, increasing the positive surface charge of the cell envelop and repelling CAMPs.Citation213 CAMPs are currently under investigation as an alternative treatment strategy to traditional antibiotics.Citation214 S. epidermidis, like other species, has intrinsic resistance to some CAMPs, in this case through the activity of GraXSR, and so understanding the mechanisms behind this recognition and repulsion of CAMPs may help develop synthetic CAMPs to overcome resistance.Citation215
Other regulators
ClpP protease and ATPase chaperones (ClpX, ClpC)
ClpP and ClpX constitute an ATP-dependent protease-chaperone system in S. epidermidisCitation216 that orchestrates controlled degradation of specific protein substrates, contributing to cellular homoeostasis.Citation217 Under cellular stress (e.g. antibiotics, temperature, oxidative stress), ClpP expression is increased to manage the accumulation of misfolded proteins, contributing to cellular fitness and survival.Citation217 ClpXP-mediated proteolytic regulation of stress response regulators enhances cellular resilience.Citation218 Paradoxically, deletion of ClpX or ClpP alone improves stationary-phase fitness because the survival of individual cells in stationary phase is enhanced in the absence of ClpXP-dependent degradation of misfolded proteins.Citation219 Deletion of ClpX in S. aureus also enhances survival at high temperatures due to the compensatory role of the heat shock-induced chaperone ClpC,Citation220 whose role in S. epidermidis remains largely unexplored, although it is present in the genomes of many clinical and animal isolates.Citation196, Citation221
ClpP positively regulates biofilm formation, especially primary attachment and PIA production (), impacting virulence in a rat IV-catheter infection model.Citation216 Mutations in spx, encoding an RNA polymerase binding protein conserved in the Bacillota, reversed growth and competence defects caused by ClpX or ClpP mutations in Bacillus.Citation222 Deletion of ClpP appears to have no direct effect on ica operon transcription. However, because ClpP controls the activity of Spx by proteolytic degradation (),Citation223 mutation of clpP is accompanied by downregulation of the ica operon, implicating an unidentified intermediate factor, possibly another ClpP protein target, that neutralizes the negative regulatory effect of Spx on ica transcription.Citation216,Citation224 Downregulation of ClpP by a functional agr system is also consistent with agr-mediated negative regulation of biofilm formation, favoring release of planktonic cell for dissemination.Citation216, Citation224 This flexible regulation of a key virulence factor highlights the capacity of S. epidermidis to respond to environmental and population density cues to ensure persistence within the host.
Phenotypic variation and IS256
IS256 belongs to the IS6 family of insertion sequences and is characterized by its relatively small size (1.2 kb) and the presence of terminal inverted repeats (IRs). These IRs facilitate its transposition within the bacterial genome, allowing IS256 to introduce genetic variability within S. epidermidis populations, including gene inactivation, up- or down-regulation of gene expression and chromosomal rearrangements.Citation225 This genetic diversity can lead to variations in virulence factor expression and pathogenicity amongst isolates.
Compared to icaADBC+ commensal strains, clinical isolates with icaADBC are considerably more likely to carry IS256 (85% vs. 15%).Citation226 IS256 is known to modulate the biofilm phenotype and can cause a switch from PIA-dependent to PIA-independent biofilm when inserted into the icaC locus,Citation227 as well as decreasing ica operon expression and PIA production when inserted into the rsbU and sarA loci.Citation180 An important aspect of IS256 phenotypic switching is that it is reversible, as the insertion sequence can be subsequently excised from the gene, leaving an intact wild-type locus.Citation228 This clean excision is a rare event, however, and appears to be independent of IS256 transposition, which normally leaves 8-bp target site duplications (TSDs).Citation228
The presence of IS256 is considered a virulence marker,Citation229, Citation230 as IS256 is found more frequently in clinical isolates (up to 80%) of S. epidermidis compared to community isolates (13%) from healthy volunteers.Citation231 Isolates from sequence type 2 (ST2) have a high rate of IS256 carriage, up to 100%.Citation232 ST2 is a widely distributed sequence type that is commonly found in clinical infections, especially catheter infectionsCitation231 and is prevalent in hospital environments.Citation233 In prosthetic joint infections, up to 83% of isolates show the presence of IS256, compared to just 4% of commensal isolates.Citation234, Citation235
IS256 is frequently associated with the acquisition and dissemination of antibiotic resistance genes within S. epidermidis populations. Isolates containing IS256 are more likely to exhibit resistance to multiple antibiotics,Citation236 including β-lactams and aminoglycosides.Citation226 IS256 presence is also correlated with carriage of mecA.Citation237 Whilst multiple copies of IS256 are typically distributed throughout the S. epidermidis genome, it is also found as a component of transposon Tn4001, which confers aminoglycoside resistance.Citation238
A striking example of the clinical significance of genomic rearrangements induced by IS256 was reported in a case of recurrent meningitis, in which a switch from a biofilm-positive to biofilm-negative phenotype was associated with IS256 translocation and large chromosomal rearrangements.Citation225 The ability of a single strain to rapidly adapt within the host gives it a clear fitness advantage, particularly when host immune factors increase selective pressure. Furthermore, several studies have suggested that IS256 carriage is a more predictive indicator of S. epidermidis virulence than the presence of icaADBC, supporting the idea that S. epidermidis virulence is also linked to its capacity for adaptation through establishment of genetically diverse subpopulations.Citation226, Citation229, Citation234, Citation239
Host-S. epidermidis interactions
S. epidermidis must modulate the innate immune response to facilitate its persistence. Moreover, the host response to S. epidermidis as a commensal organism and as a pathogen can vary significantly in response to the location and strain, as outlined in several recent reviews.Citation3, Citation8, Citation16, Citation240
Recognition and response
Identifying pathogens involves initial recognition of bacterial components by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors on epithelial cells, macrophages, dendritic cells, and neutrophilsCitation241, Citation242. Following recognition, signalling pathways are triggered, leading to production of pro-inflammatory cytokines, chemokines, and AMPs that induce chemotaxis of immune cells and result in phagocytosis and bacterial killing.Citation243 S. epidermidis activates different PRRs depending on its cell wall components and secreted toxins, such as lipoteichoic acid (TLR2), peptidoglycan (TLR2 and NOD2), lipoproteins (TLR1/TLR2 and TLR2/TLR6), and PSMs (TLR2 and TLR6).Citation244–250
Notably, S. epidermidis can inhibit TLR signalling, as is seen on the skin surface where it dampens TLR2 response in keratinocytes and reduces TLR3-mediated inflammation following skin injury.Citation245 Along with the inhibition of TLR2, S. epidermidis can also induce the expression of anti-inflammatory cytokines, such as IL-10 and TGF-β, and regulatory T cells that suppress the inflammatory response and promote tolerance to the commensal bacteria, discussed in further detail below.Citation251–253 Strains isolated from atopic dermatitis are more likely to cause a pro-inflammatory cytokine response in an in vitro model than strains from the skin of healthy patients, highlighting the complex role of S. epidermidis in epithelial immunity.Citation254
Immune evasion
Neutrophils/polymorphonuclear leukocytes (PMNs) form an important part of host defence against S. epidermidis. Biofilm is particularly associated with downregulation of pro-inflammatory cytokines, allowing infection to persist undetected.Citation255 Production of PIA shields S. epidermidis from PMN phagocytosis, although PIA-independent biofilm can also impede effective opsonization by antibodies and complement components.Citation74, Citation256, Citation257 Evasion of PMNs by S. epidermidis is also associated with repulsion and proteolysis of AMPs by GraRS and SepA respectively, as well as biofilm-mediated shielding of cells from IgG deposition.Citation137, Citation258 Broader evasion of the complement system also contributes to S. epidermidis survival and persistence in the hostCitation259. Phagocytic killing of staphylococci is mediated primarily by the C3b and C5a opsonins,Citation259 and whilst S. epidermidis can activate the complement system, effective opsonization by C3b is impeded in a biofilm.Citation258
S. epidermidis as a beneficial member of the skin microflora
Competitive exclusion
S. epidermidis agr signals can interfere with the S. aureus agr system, resulting in downregulation of virulence factors required for S. aureus to establish infection.Citation165, Citation260 Agr type I and IV S. epidermidis strains from patients with atopic dermatitis exhibited inhibitory effects on the expression of S. aureus virulence factors in vitro, and reduced S. aureus colonization of the skin in a mouse model.Citation261
S. epidermidis produces several antimicrobial peptides that inhibit the growth of S. aureus, including bacteriocins. Bacteriocins are antimicrobial peptides synthesized by the ribosome and typically target species that are closely related to the producer,Citation262 in order to stave off competition for a particular niche. For example, 96% of nasal S. epidermidis isolates produce bacteriocins.Citation263 As a result, many S. epidermidis bacteriocins are active against S. aureus as well as other S. epidermidis and CoNS strains. S. epidermidis is known to produce the bacteriocins pep5,Citation264 epidermin,Citation265 epilancin K7Citation264 epilancin 15×,Citation266 nukacin IVK45Citation263 and epicidin 280.Citation267 A skin isolate of S. epidermidis has been described that produces epidermicin NI01, a plasmid-encoded bacteriocin.Citation268 Plasmid-encoded nukacin IVK45 shows sequence similarity to genes in other staphylococcal species, indicating horizontal gene transfer of bacteriocins between species.Citation263
On healthy skin, S. epidermidis is found to inhibit colonization with harmful Cutibacterium acnes, including selective activity against C. acnes strains associated with acne.Citation269 These strains of S. epidermidis are typically not from the sequence types associated with infectionCitation269. Co-culture experiments with C. acnes and a representative strain of S. epidermidis showed that EpiA (precursor peptide for epidermin, which is known to inhibit survival of C. acnes) and PSMβ expression increased upon co-culture with C. acnes.Citation269
S. epidermidis can also interfere with the virulence of other staphylococcal species. Co-culture of S. epidermidis with S. aureus results in SaeRS-dependent downregulation of the S. aureus hla-encoded haemolysin,Citation270 via a mechanism involving an unidentified non-proteinaceous factor present in culture supernatants.Citation270 The secreted serine protease Esp, which as noted above processes Aap and degrades complement protein C5, also impairs S. aureus biofilm productionCitation271 and can decolonise S. aureus from the nasal passages.Citation131 Furthermore, Esp is also involved in nutrient acquisition via the breakdown of proteins into glutamate, which can be used by S. epidermidis as an energy source.Citation132 Esp highlights the overlapping functions of S. epidermidis persistence/colonisation and virulence factors.Citation80
Immunomodulation
Gamma delta T-cells (GD T-cells) found in the epithelium are activated by the presence of S. epidermidis, leading to increased expression of p-2, an antimicrobial effector protein that kills S. aureus by forming pores in the bacterial membrane of internalised cells, as well as the TNF family transmembrane protein Fas Ligand and the pore-forming toxin granulysin.Citation272 S. epidermidis LTA can activate Toll-like receptor 2 (TLR2) on keratinocytes that have been triggered to induce inflammation via Toll-like receptor 3 (TLR3) in response to skin injury.Citation272 This interaction triggers an anti-inflammatory response through the NF-κB signalling pathway,Citation273 reducing proinflammatory cytokines like TNF and IL-6 production, as well as inducing the production of AMPs.Citation245, Citation274 Similarly, LTA can induce production of IL-6, IL-1β and TNF and elevate nitric oxide levels in macrophage cells lines while also stimulating an IgG response in patients with S. epidermidis device-associated infections.Citation275 Commensal colonisation with S. epidermidis has been shown to induce a specific and co-ordinated dendritic cell response that enhances IL-17A+ CD8+ T-cell migration to the skin, which is associated with improved barrier function.Citation247 These findings indicate that S. epidermidis-mediated modulation of the immune system is associated with both benefit and harm to patients, in keeping with its ability to switch from a protective member of the skin microflora to a pathogen. Indeed, the beneficial effects of S. epidermidis on keratinocyte and GD T-cell mediated-responses in the outermost epithelial cells of the skin contrasts with the deleterious impact on macrophage-mediated responses, which are more commonly detected in the underlying dermis following skin breach.Citation276
Maintenance of skin barrier function and homeostasis
S. epidermidis plays a role in maintaining the skin’s hydration and preventing skin disorders like atopic dermatitis by regulating skin barrier function.Citation277 Production of the sphingomyelinase Sph, a hydrolase that breaks down sphingolipids onto ceramides and phosphocholine, benefiting the organism, protecting the skin from dehydration and promoting healthy turnover of skin cells.Citation3, Citation158 Using a mouse model, butyrate, which is a fatty acid metabolite produced by S. epidermidis, was shown to protect the epidermis from IL-6 production induced by damaging UVB light.Citation278 S. epidermidis also produces trace amines from aromatic amino acids (AAAs) found in high concentrations in skin.Citation279 Whilst S. epidermidis does not appear to utilise AAAs in its own metabolism, excretion of trace amines by SadA-mediated decarboxylation of AAAs accelerates wound healing in a mouse model, via a mechanism associated with internalisation of the bacteria into host cells (offering protection from the host immune system) and inhibition of epinephrine production by keratinocytes (which negatively impacts wound healing by inhibiting cell migration).Citation279 However, it is important to note that whilst S. epidermidis may provide benefits for the host, the function of these interactions is clearly to ensure the survival of the bacteria. Persistence in the host may be benign until circumstances allow for the establishment of infection.
Diagnostics
Diagnostics for invasive S. epidermidis isolates responsible for infection is complicated by the ubiquitous presence of this organism on skin and mucous membranes. Efforts to evaluate the significance of a single positive blood culture of S. epidermidis as indicative of bacteraemia when concurrent with clinical symptoms have shown that whilst consecutive positive cultures is less prone to false positives, the incubation time between from culture initiation to culture positivity also predictive of bacteraemia and requires a shorter delay before diagnosis and treatment.Citation280–282 For patients with symptoms of catheter-related sepsis, the gold standard is isolation of the same strain from catheter tips and blood cultures.Citation283 In the case of PJIs, such as elbow arthroplasty, up to 78% of joint aspirations fail to yield a positive culture, which may be due to difficulties in culturing bacteria from a biofilm, whilst the current gold standard of biopsy and culture is prone to false positives (7.5%) and may result in overtreatment.Citation284–286 Non-culture-based methods such as Raman spectroscopy are faster than traditional culture-based methods and show promising results for diagnosis of bone graft infections.Citation287 Quantitative PCR methods have thus far been hindered by difficulty in finding suitable markers which are both actively transcribed during infection and conserved amongst isolates, whilst minimising the risks of false positives from non-invasive commensal strains.Citation288 Multiplex real time PCR is capable of distinguishing S. epidermidis, MRSE, S. aureus and MRSA.Citation289
Automated identification systems such as the bioMérieux Vitek 2, BectonDickinson Phoenix and Siemens Microscan assign a species level identification based on the results of biochemical tests.Citation290 These automated systems reduce the time and workload required for bacterial identification, and can show high accuracy for S. epidermidis, though other CoNS species are not so easily identified.Citation291 However, unlike real time PCR methods, these systems still require preculture of the isolates.Citation290 Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) systems facilitate rapid species level identification of CoNS.Citation292–295 Whilst most typically require growth of the isolate on agar plates prior to analysis, MALDI-TOF MS can be carried out directly on the clinical specimen, for example synovial fluid directly from PJIs, with better sensitivity than culture-based methods.Citation296, Citation297 Attempts to use MALDI-TOF MS to differentiate MRSE from MSSE have been only partially successful, as the results are prone to false negatives.Citation298, Citation299 A small study also showed that MALDI-TOF MS could potentially differentiate between biofilm-producing and non-biofilm producing strains.Citation300
Current antimicrobial treatment strategies
In the treatment of S. epidermidis infections, antibiotic selection is predicated upon the location of infection, antibiotic susceptibility profile, and the presence of prosthetic devices.
For bloodstream infections and catheter-related infections, vancomycin remains the gold standard due to its efficacy against MRSE, with β-lactams such as nafcillin and oxacillin also considered based on the resistance profile of the pathogen.Citation17, Citation301 For ocular infections, fluoroquinolones, such as moxifloxacin or levofloxacin, are frequently utilized owing to their good penetration into ocular tissues and broad-spectrum activity.Citation302 In the case of endophthalmitis, which can cause severe visual loss if time to treatment is delayed and of which S. epidermidis is a leading cause of infection, intravitreal injection of vancomycin can be used.Citation303 Device-related infections, particularly those involving biofilm formation, often require a combination of surgical intervention and antibiotic therapy, with rifampicin combined with a glycopeptide (e.g. vancomycin) or a lipopeptide (e.g. daptomycin) recommended to eradicate biofilm-associated bacteria without promoting the emergence of rifampicin-resistant staphylococci.Citation301, Citation304, Citation305
Antimicrobial resistance
Emergence of methicillin-resistant S. epidermidis (MRSE)
The emergence of MRSE mirrors the emergence and spread of MRSA. Acquisition of mecA, reduces susceptibility to methicillin and a broad spectrum of β-lactam antibiotics. The mecA-encoded penicillin-binding protein 2a (PBP2a) is less susceptible to binding by all β-lactams, including β-lactamase-resistant derivatives such as methicillin, cloxacillin, flucloxacillin, and oxacillin.Citation196, Citation306, Citation307 The 13 known variants of SCCmec, which carries the mecA gene, are differentiated based on the composition of their cassette chromosome recombinase (ccr) and mec gene complexes.Citation308–311 Types I-VI, including various subtypes have been described in S. epidermidis, with type IV generally being most frequent.Citation312–319 Through the action of the recombinase genes in SCCmec, the cassette can be excised from the genome and transferred between isolates and between species, and there is evidence that S. epidermidis can function as a reservoir for methicillin resistance that can be passed on to other species, including S. aureus.Citation320–322 The prevalence of MRSE has increased drastically in frequency over the past two decades and is more common in nosocomial isolates.Citation323 In certain populations, such as neonates with bloodstream infections, methicillin resistance rates can reach 100%.Citation324, Citation325
Fluoroquinolone resistance
Infections caused by fluoroquinolone-resistant S. epidermidis, typically associated with point mutations in DNA gyrase (gyrA, gyrB) and topoisomerase IV (parC, parE) genes,Citation326 narrow the range of therapeutic options. Moreover, fluoroquinolone resistance is increasing in prevalence, with 23% to 67% of S. epidermidis isolates being resistant to ciprofloxacin,Citation327–329 and up to 60%, 55%, and 27% of MRSE and MSSE intravitreal isolates exhibiting resistance to levofloxacin, moxifloxacin, and delafloxacin, respectively.Citation330 Topical fluoroquinolone solutions used prophylactically for cataract surgery have been correlated with an increased prevalence of fluoroquinolone-resistant S. epidermidis strains, including potential within-host adaptation during a 14-day treatment timeframe,Citation331 although transmission of resistant strains from outside the host remains more common.Citation332
Glycopeptide resistance
Whilst planktonically-growing strains of S. epidermidis are susceptible to glycopeptides like vancomycin and teicoplanin, biofilms exhibit high tolerance to these antibiotics.Citation333–335 Heteroresistance in subpopulations of laboratory grown strains to vancomycin was first recorded in a S. epidermidis human isolate in 1996.Citation336, Citation337 Distinguishing homogeneous resistance from heteroresistance is challenging, but even heteroresistant strains can cause recurrent infection following treatment with vancomycin.Citation338–341 Heteroresistance in catheter-associated bloodstream infections is associated with poor response to glycopeptide treatment, resulting in persistent infection, prolonged hospitalization and death.Citation342 S. epidermidis is more commonly resistant to teicoplanin than vancomycinCitation323, Citation343, Citation344 and newer generation lipoglycopeptides, including dalbavancin, are currently very effective against S. epidermidis in vivo and against planktonic and biofilm S. epidermidis cells in vitro, extending therapeutic options even after vancomycin treatment failure.Citation345–351 However, mutations in walK, the histidine kinase of the essential two-component signalling system WalKR involved in regulating cell-wall biosynthesis and turnover,Citation352 can lead to dalbavancin heteroresistance in patient isolates during treatment.Citation353
Fusidic acid resistance
Resistance to fusidic acid is mediated by Fus family proteins (FusA, FusB, FusC, FusD, FusF) that interfere with antibiotic binding to the ribosome.Citation354, Citation355 FusA, FusD, and FusF are responsible for intrinsic resistance in species including S. saprophyticus and S. cohnii. In S. epidermidis, the most reported Fus proteins are FusB and FusC.Citation356, Citation357 FusB is often carried on mobile phage-related resistance islands that are inserted at att integrase sites in heterologous strains.Citation358, Citation359 The capacity for inter-strain or inter-species transfer of fus genes raises concern, particularly as their carriage has a low fitness cost for staphylococci.Citation355, Citation360
Linezolid resistance
Resistance to the oxazolidinone drug linezolid can arise due to mutations in the 23S rRNA gene.Citation361 Furthermore, methylation of the 23s rRNA A2503 residue by the cfr-encoded methyltransferase leads to resistance to oxazolidinones, phenicols, lincosamides, pleuromutilins, and streptogramin A via decreased antibiotic binding.Citation361 Linezolid-resistant S. epidermidis has been implicated in several endemic outbreaks in clinical settings.Citation362–365 Unlike 23S rRNA mutations, cfr-mediated resistance is carried on a plasmid with a low fitness cost to the host cell,Citation366–370 although linezolid resistant isolates can possess both cfr plasmids and 23S rRNA mutations.Citation371 First reported in a S. sciuri bovine isolate in 2000 and human staphylococcal strains in 2008, cfr-bearing plasmids may also carry resistance genes for macrolide-lincosamide-streptogramin B antibiotics (MLSB) and spectinomycin.Citation366, Citation372, Citation373 Recent reports of MRSE carrying cfr-containing plasmids are particularly concerning, as all strains investigated belonged to ST2, a clonal lineage that is a frequent cause of nosocomial infections and is notable for its worldwide dissemination and multi-drug resistance characteristics.Citation374 Furthermore, several S. aureus isolates from the same hospital contained highly similar plasmids, potentially because of transfer from S. epidermidis.Citation374
Macrolide resistance
Resistance to macrolides associated with cross-resistance to lincosamides and streptogramin B is primarily associated with ermC.Citation375 Erythromycin resistance can be found in approximately 60–65% of S. epidermidis isolates.Citation376, Citation377 ermC is found in up to 85% of constitutively-MLSB resistant and 100% of inducible – MLSB resistant S. epidermidis strains.Citation378 Whilst ErmA, ErmB, and ErmC mediate resistance by methylation of 23S rRNA, other mechanisms of resistance to macrolides include drug efflux (associated with the msr genes) and phosphorylation of the antibiotics (associated with the mph genes).Citation378
Multi-drug resistance (MDR)
The prevalence of multi-resistant strains is increasing in clinical settings.Citation379–381 MRSE already precludes the use of one of the best-tolerated classes of antibiotics, but highly-methicillin resistant strains can also exhibit high rates of resistance to erythromycin (up to 80%), tetracycline (33%), clindamycin (33%), and gentamicin (27%).Citation380 Up to 90% of MRSE isolates can be multi-drug resistant (MDR), posing a significant challenge to treatment.Citation55, Citation382 Amongst ocular isolates, MDR was detected more frequently in infection-related isolates than amongst non-infection-causing isolates, and MDR isolates were significantly more likely to be biofilm producers.Citation383
S. epidermidis as a reservoir of resistance genes
Horizontal gene transfer of resistance cassettes such as SCCmec highlights the adaptability of S. epidermidis in both acquiring and disseminating resistance determinants.Citation360, Citation384–386 Whilst the precise mechanism of SCCmec transfer in vivo remains unclear, in vitro experiments indicate that of S. aureus can be induced to take up SCCmec by transformation via upregulation of competence genes by two-component systems such as SrrBA.Citation387 In a particularly striking illustration of inter-species transfer of resistance, sequence analysis of three staphylococcal strains (MRSA, MSSA, and MRSE) isolated from the same patient indicated that in-patient transfer of SCCmec from the MRSE strain to MSSA had occurred.Citation321 Plasmid-mediated resistance to tetracycline, streptomycin, and erythromycin can also drive dissemination from S. epidermidis to other species.Citation360, Citation386
Prevention and treatment strategies
Prevention
Prophylactic measures used as part of medical procedures include fluoroquinolones, known for their efficacy against MRSE, during ocular surgery,Citation388 vancomycin in prosthetic joint revision surgery,Citation389 and clindamycin, vancomycin (teicoplanin in Europe), cephalosporins or vancomycin in combination with rifampicin to prevent PJIs.Citation47, Citation390 Cephalosporins and vancomycin are routinely used for cardiac device implantation, with in vitro data supporting their use in combination with rifampicin.Citation391 The FDA has also approved the use of rifampicin/minocycline-impregnated mesh on cardiac implants as an additional preventive measure.Citation392 Notably, antimicrobial catheter lock solutions (CLSs), which are traditionally used post-infection, also have prophylactic potential.Citation393 Anticoagulants such as sodium citrate used in CLSs can be antimicrobial, although their use as prophylactics to prevent infection have not been standardized.Citation393 Nevertheless, sodium citrate was shown to be the most effective CLS against S. epidermidis, while taurolidine demonstrated efficacy against E. coli.Citation392, Citation393 However, the potential benefit of using antibiotics in CLSs must be weighed against the risk of promoting resistance, which has been observed with fluoroquinolones in ocular procedures.Citation394 Furthermore, the choice of antibiotic may change over time, as is seen with low susceptibility to clindamycin among S. epidermidis and MSSA PJI isolates when this antibiotic is used prophylactically.Citation390 In this regard, antimicrobial stewardship and susceptibility testing of the frequently isolated strains that cause infection are imperative for responsible use of prophylactic antibiotics.
Antimicrobial coating of medical devices
An attractive approach to combatting infection of biomaterials exploits novel antimicrobial coatings to limit S. epidermidis colonization. Osteopontin, a bovine milk protein, dose-dependently blocks bacterial adhesion under shear flow, potentially targeting early biofilm formation adhesins.Citation395 Polymer blends of polyvinyl chloride (PVC) and polystyrene-ethylene-butylene-styrene (SEBS), infused with the ionic liquid HdmimDMSIP, have activity against S. epidermidis and can be incorporated into devices such as catheter tips.Citation396 Micro-fibrillated cellulose-based antimicrobial films applied to biomaterials are also efficacious against S. epidermidis.Citation397 Cellulose-based hydrogels, incorporating crosslinking agents or ε-poly-L-lysine, also have potential as part of antimicrobial wound dressings.Citation398, Citation399
Colloid materials, characterized by their small particle size and large surface area, offer promising avenues for designing surfaces that incorporate antimicrobial agents. Silver nanoparticles in chitosan nanogel, combined with ampicillin, were effective against oxacillin-resistant S. epidermidis.Citation400 Colloidal suspensions with titanium-peroxy gel layers provide non-antibiotic solutions for combating implant infections.Citation401
In dual-purpose biomaterial modification, coupling titanium with a cationic antimicrobial peptide exhibited significant bactericidal activity against S. epidermidis and improved osteoblast adherence.Citation402 Coating titanium with carboxymethyl chitosan and bone morphogenic protein 2 also inhibited S. epidermidis colonization.Citation403 Surface treatments must be carefully chosen due to varying efficacy against bacterial species and potential cytotoxicity to host cells.Citation400, Citation404–408 For titanium implants, hydrophilicity plays a crucial role in minimizing S. epidermidis attachment, challenging the assumption that hydrophilicity alone predicts implant material efficacy.Citation409 In vivo studies showed that hydrothermally and electrophoretically modified titanium surfaces had superior bacterial repulsion properties.Citation410
Quorum sensing inhibition
The therapeutic potential of quorum sensing inhibition, or “quorum quenching,” which generally centers around competitive inhibition using biosimilar molecules that interfere with autoinduction, the use of enzymes that degrade autoinducing peptides (AIPs), or interference with AIP synthesis was the subject of a recent commentary.Citation163
Vaccines and antibody therapy
Vaccination against an important commensal like S. epidermidis presents significant challenges and even if achieved may be potentially detrimental. However, anti-biofilm vaccines are an interesting possibility. Here, the choice of biofilm antigen will be critical key given that staphylococci express different surface epitopes under varying environmental and physiological conditions.Citation411, Citation412 The antigen will need to be highly conserved, if not amongst all strains, then at least amongst strains associated with the target infection type, and the antigenicity of the bacterial epitope also needs to be considered.Citation411
Early work using whole chloroform-inactivated S. epidermidis cells and cell-free filtrate confirmed the immunogenicity of these vaccines in mice, where both vaccines provided protection from S. epidermidis intraperitoneal challenge but did not offer significant protection from S. aureus mortality.Citation413 Targeting SdrG, a fibrinogen-binding protein, with antibodies impaired S. epidermidis attachment to Fn-coated catheters and increased phagocytosis by macrophages.Citation414, Citation415 Immunization with a subdomain of SdrG was also shown to induce antibody production and impaired infection by S. epidermidis O-47 in a mouse model.Citation416 Another cell wall protein, SesC, showed promise as a vaccine candidate in mouse and rat models of infection.Citation417 Other Ses proteins, including SesB, were also found to exhibit vaccine potentialCitation418 and immunization with the B-domain repeat of Aap resulted in decreased inflammation, impaired bacterial colonization and a significant IgG antibody response.Citation419 The iron transport protein SitC has also been investigated as a vaccine epitope and more recently its S. aureus ortholog MntC, a manganese transporter, was shown to provide protection from both S. aureus and S. epidermidis infection in a murine infection model.Citation420–422
Pagibaximab, a monoclonal antibody therapy that targets LTA, showed promise in preventing neonatal sepsis in phase II clinical trials.Citation423 However, phase III trials showed that whilst the treatment was safe and well-tolerated, prevention of sepsis was unsuccessful.Citation424
Unfortunately to date vaccines and antibody therapies to combat S. epidermidis infection and biofilm formation have not progressed beyond animal trials. Vaccination against more serious S. epidermidis infections, such as neonatal sepsis, is complicated by the immature immune response of neonates and infants that can fail to provide long-lasting memory T- and B-cell responses, as well as poor activity of antigen-presenting cells and lower IgG response compared to adults.Citation425 Nevertheless, vaccine research will continue to be an important part of efforts to find improved ways to control S. epidermidis and other infectionsCitation426–429
Concluding remarks
The pathogenic success of S. epidermidis is deeply rooted not only in its biofilm-forming capacity but also in its resilience and remarkable genomic plasticity, which facilitates the acquisition of virulence determinants and rapid adaption to shifting environmental conditions. Research into CoNS infections lags S. aureus, and whilst S. epidermidis is the most studied of the CoNS species, there are still substantial gaps in our understanding of its virulence determinants, resistance modalities, and host–pathogen interactions. Recognition of the importance of CoNS as pathogens is imperative to maintain and expand the global research base in this field. Furthermore, our understanding of CoNS virulence would be improved if S. aureus investigators extended their research, where appropriate, to include comparative studies with S. epidermidis or other CoNS.
The pathogenicity of S. epidermidis is unique in several ways: i. the persistence factors that make S. epidermidis such an effective colonizer of the harsh epithelial environment and how these are redeployed to cause disease, ii. the role of genomic flexibility in the success of S. epidermidis as a pathogen, and iii. the ever-increasing emergence of multidrug resistant strains. Regulation of the major virulence determinant in S. epidermidis, biofilm, is significantly different to S. aureus, and further research is needed to fully understand this in the context of the distinct lifestyle of this pathogen and its interactions with the host including opportunistic infections. To revisit the central question of this review: are we overlooking important disease determinants when S. epidermidis is overlooked as a “common commensal” and not a ubiquitous opportunistic pathogen? The research literature focused on S. epidermidis in recent decades reflects a gradual shift in how this organism is viewed, both as an important commensal for cutaneous health and a complex pathogen capable of causing significant harm to vulnerable individuals. Taking a holistic view continues to provide a more complete understanding of the lifestyle, host–microbe interactions and virulence of S. epidermidis.
All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The figures in this review are openly available in Figshare (DOIs: 10.6084/m9.figshare.25663989 (); 10.6084/m9.figshare.25092626 () and 10.6084/m9.figshare.25092623 ().
Additional information
Funding
Notes on contributors
Órla Burke
Órla Burke Conception and design, writing – original draft, writing – review, editing, and critical revision for intellectual content; writing – approval of the final version for publication.
Merve S. Zeden
Merve S. Zeden Conception and design, writing – original draft, writing – review, editing, and critical revision for intellectual content; writing – approval of the final version for publication.
James P. O’Gara
James P. O’Gara Conception and design, writing – original draft, writing – review, editing, and critical revision for intellectual content; writing – approval of the final version for publication.
References
- Nagase N, Sasaki A, Yamashita K. Isolation and species distribution of staphylococci from animal and human skin. J Vet Med Sci. 2002;64(3):245–37. doi: 10.1292/jvms.64.245
- Nakamizo S, Egawa G, Honda T. Commensal bacteria and cutaneous immunity. Semin Immunopathol. 2015;37(1):73–80. doi: 10.1007/s00281-014-0452-6
- Severn MM, Horswill AR. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat Rev Microbiol. 2023;21(2):97–111. doi: 10.1038/s41579-022-00780-3
- Bjerre RD, Holm JB, Palleja A. Skin dysbiosis in the microbiome in atopic dermatitis is site-specific and involves bacteria, fungus and virus. BMC Microbiol. 2021;21(1):256. doi: 10.1186/s12866-021-02302-2
- Fourniere M, Latire T, Souak D. Staphylococcus epidermidis and Cutibacterium acnes: two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms. 2020;8(11):8. doi: 10.3390/microorganisms8111752
- Abraham B, Gokhale AU, Mohsin J. Staphylococcous epidermidis, staphylococcous schleiferi infections: are CoNS cons? Indian J Crit Care Med. 2020; 24(8):716–8. doi: 10.5005/jp-journals-10071-23523
- Bellou V, Gkentzi D, Giormezis N. Persistent coagulase-negative staphylococcal bacteremia in neonates: clinical, microbiological characteristics and changes within a decade. Antibiotics. 2022;11(6):11. doi: 10.3390/antibiotics11060765
- Nguyen TH, Park MD, Otto M. Host response to Staphylococcus epidermidis colonization and infections. Front Cell Infect Microbiol. 2017; 7:90. 10.3389/fcimb.2017.00090
- Qin L, Da F, Fisher EL. Toxin mediates sepsis caused by methicillin-resistant staphylococcus epidermidis. PLOS Pathog. 2017; 13(2):e1006153. doi: 10.1371/journal.ppat.1006153
- Pomputius WF, Kilgore SH, Schlievert PM. Probable enterotoxin-associated toxic shock syndrome caused by staphylococcus epidermidis. BMC Pediatr. 2023;23(1):108. doi: 10.1186/s12887-023-03914-5
- Flores-Paez LA, Zenteno JC, Alcantar-Curiel MD. Molecular and phenotypic characterization of staphylococcus epidermidis isolates from healthy conjunctiva and a comparative analysis with isolates from ocular infection. PLOS ONE. 2015;10(8):e0135964. doi: 10.1371/journal.pone.0135964
- DeCory HH, Sanfilippo CM, Proskin HM. Characterization of baseline polybacterial versus monobacterial infections in three randomized controlled bacterial conjunctivitis trials and microbial outcomes with besifloxacin ophthalmic suspension 0.6. PLOS ONE. 2020; 15(8):e0237603. doi: 10.1371/journal.pone.0237603
- Amelot A, Bouazza S, George B. Causative role of infection in chronic non-thromboembolic pulmonary hypertension following ventriculo-atrial shunt. Br J Neurosurg 2014; 28(4):559–61. doi: 10.3109/02688697.2013.854311
- Pouget C, Chatre C, Lavigne JP. Effect of antibiotic exposure on staphylococcus epidermidis responsible for catheter-related bacteremia. Int J Mol Sci. 2023;24(2):24. doi: 10.3390/ijms24021547
- Clemente A, Cavagnaro L, Russo A. Spacer exchange in persistent periprosthetic joint infection: microbiological evaluation and survivorship analysis. Arch Orthop Trauma Surg. 2023; 143(3):1361–70. doi: 10.1007/s00402-021-04300-5
- Brown MM, Horswill AR, Kline KA. Staphylococcus epidermidis—skin friend or foe? PLOS Pathog. 2020; 16(11):e1009026. doi: 10.1371/journal.ppat.1009026
- Otto M Staphylococcus epidermidis — the ’accidental’ pathogen. Nat Rev Microbiol. 2009; 7(8):555–67. doi: 10.1038/nrmicro2182
- Edmond MB, Wallace SE, Dk M. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clinical Infectious Diseases. 1999; 29(2):239–44. doi: 10.1086/520192
- Rosenthal VD, Chaparro GJ, Servolo-Medeiros EA. An eight-year multicenter study on short-term peripheral intravenous catheter–related bloodstream infection rates in 100 intensive care units of 9 countries in Latin America: Argentina, Brazil, Colombia, Costa Rica, Dominican Republic, Ecuador, Mexico, Panama and Venezuela. Findings of the International Nosocomial Infection Control Consortium (INICC). Infect Control Hosp Epidemiol. 2021; 42:1098–104.
- Wisplinghoff H, Bischoff T, Tallent SM. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946
- Ott E, Saathoff S, Graf K. The prevalence of nosocomial and community acquired infections in a University hospital. Dtsch Arztebl Int. 2013; 110:533–540. doi: 10.3238/arztebl.2013.0533
- Hellmark B, Unemo M, Nilsdotter-Augustinsson A. Antibiotic susceptibility among staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin Microbiol Infect. 2009; 15(3):238–44. doi: 10.1111/j.1469-0691.2008.02663.x
- Kokotsakis J, Chaudhry UA, Tassopoulos D. Surgical management of superior vena cava syndrome following pacemaker lead infection: a case report and review of the literature. J Cardiothorac Surg. 2014;9(1):107. doi: 10.1186/1749-8090-9-107
- Harinstein ME, Marroquin OC. External coronary artery compression due to prosthetic valve bacterial endocarditis. Catheter Cardiovasc Interv. 2014;83(3):E168–70. doi: 10.1002/ccd.24578
- Teoh EJ, Backhouse L, Chandrasekaran B. Mycotic aneurysm of the superior mesenteric artery and other sequelae of prosthetic valve endocarditis on (1)(8)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2014; 41(10):1993–4. doi: 10.1007/s00259-014-2812-9
- Borde JP, Sitaru G, Kopp WH. Heart transplantation as salvage therapy for progressive prosthetic valve endocarditis due to methicillin-resistant Staphylococcus epidermidis (MRSE). J Cardiothorac Surg. 2016;11(1):100. doi: 10.1186/s13019-016-0505-0
- Simon D, Fischer S, Grossman A. Left ventricular assist device–related infection: treatment and outcome. Clin Infect Dis. 2005; 40(8):1108–15. doi: 10.1086/428728
- Post V, Harris LG, Morgenstern M. Comparative genomics study of staphylococcus epidermidis isolates from orthopedic-device-related infections correlated with patient outcome. J Clin Microbiol. 2017;55(10):3089–3103. doi: 10.1128/JCM.00881-17
- Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22(7):692–697. doi: 10.1007/s00381-005-0037-8
- Whitehead WE, Kestle JR The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American society of pediatric neurosurgeons. Pediatr Neurosurg. 2001; 35(4):205–10. doi: 10.1159/000050422
- Rehman AU, Rehman TU, Bashir HH. A simple method to reduce infection of ventriculoperitoneal shunts. J Neurosurg Pediatr. 2010;5(6):569–572. doi: 10.3171/2010.2.PEDS09151
- Reddy GK, Bollam P, Caldito G. Ventriculoperitoneal shunt surgery and the risk of shunt infection in patients with hydrocephalus: long-term single institution experience. World Neurosurg. 2012;78(1–2):155–163. doi: 10.1016/j.wneu.2011.10.034
- Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine. 2013;381:E1425–31. doi: 10.1097/BRS.0b013e3182a42a68
- Kim JH, Ahn DK, Kim JW. Particular features of surgical site infection in posterior lumbar interbody fusion. Clin Orthop Surg. 2015;7(3):337–343. doi: 10.4055/cios.2015.7.3.337
- Fu X, Lin Z, Chen S. Treatment of intracranial infection caused by methicillin-resistant staphylococcus epidermidis with linezolid following poor outcome of vancomycin therapy: a case report and literature review. Infect Drug Resist. 2021;14:2533–2542. doi: 10.2147/IDR.S319013
- Edmiston CE Jr., Krepel CJ, Marks RM. Microbiology of explanted suture segments from infected and noninfected surgical patients. J Clin Microbiol. 2013;51(2):417–421. doi: 10.1128/JCM.02442-12
- Saito Y, Kobayashi H, Uetera Y. Microbial contamination of surgical instruments used for laparotomy. Am J Infect Control. 2014;42(1):43–47. doi: 10.1016/j.ajic.2013.06.022
- Aristedis R, Dimitrios P, Nikolaos P. Intrathecal baclofen pump infection treated by adjunct intrareservoir teicoplanin instillation. Surg Neurol Int. 2017;8(1):38. doi: 10.4103/sni.sni_418_16
- Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5(2):94–106. doi: 10.1016/S1473-3099(05)70084-0
- Michalopoulos NV, Frountzas M, Karathanasis P. Implant infections after breast reconstruction surgery following mastectomy: experience from a Greek breast unit. Breast Dis 2022; 41(1):37–44. doi: 10.3233/BD-201077
- Tobias T, Kruchevsky D, Ullmann Y. Implant-based breast reconstruction infections: the importance of recognizing local Pathogens. Isr Med Assoc J 2023; 25:96–100.
- Das D, Bhattacharjee H, Gogoi K. Intraocular lens biofilm formation supported by scanning electron microscopy imaging. Indian J Ophthalmol. 2019;67(10):1708–1709. doi: 10.4103/ijo.IJO_467_19
- Guo HX, Xie RT, Wang Y. Timely vitrectomy without intraocular lens removal for acute endophthalmitis after cataract surgery. Int J Ophthalmol 2022; 15(6):1011–4. doi: 10.18240/ijo.2022.06.21
- Brown RH, Subramanian A, Hwang CS. Comparison of infectious complications with synthetic mesh in ventral hernia repair. Am J Surg. 2013;205(2):182–187. doi: 10.1016/j.amjsurg.2012.02.023
- Patiniott P, Jacombs A, Kaul L. Are late hernia mesh complications linked to staphylococci biofilms? Hernia. 2022;26(5):1293–1299. doi: 10.1007/s10029-022-02583-0
- Monksfield P, Chapple IL, Matthews JB. Biofilm formation on bone-anchored hearing aids. J Laryngol Otol. 2011;125(11):1125–1130. doi: 10.1017/S0022215111002143
- Parvizi J, Shohat N, Gehrke T. Prevention of periprosthetic joint infection: new guidelines. Bone Joint J 2017;99-B(4_Supple_B):3–10. doi: 10.1302/0301-620X.99B4.BJJ-2016-1212.R1
- Papan C, Schroder M, Hoffmann M. Combined antibiotic stewardship and infection control measures to contain the spread of linezolid-resistant Staphylococcus epidermidis in an intensive care unit. Antimicrob Resist Infect Control. 2021;10(1):99. doi: 10.1186/s13756-021-00970-3
- Hickmann AK, Bratelj D, Pirvu T. Management and outcome of spinal implant-associated surgical site infections in patients with posterior instrumentation: analysis of 176 cases. Eur Spine J. 2022;31(2):489–499. doi: 10.1007/s00586-021-06978-y
- Zhu Y, Chen X, Chen P. The occurrence rate of acute-onset postoperative endophthalmitis after cataract surgery in Chinese small- and medium-scale departments of ophthalmology. Sci Rep. 2017;7(1):40776. doi: 10.1038/srep40776
- He R, Yang L, Guo L. Management of acute hematogenous infection following total knee arthroplasty: a case series of 11 patients. Orthop Surg. 2016; 8(4):475–82. doi: 10.1111/os.12297
- Staphylococcal Biofilms. n. null; null:null.
- Saising J, Dube L, Ziebandt AK. Activity of gallidermin on staphylococcus aureus and staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2012;56(11):5804–5810. doi: 10.1128/AAC.01296-12
- Sanchez CJ Jr., Mende K, Beckius ML. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13(1):47. doi: 10.1186/1471-2334-13-47
- Soroush S, Jabalameli F, Taherikalani M. Investigation of biofilm formation ability, antimicrobial resistance and the staphylococcal cassette chromosome mec patterns of methicillin resistant staphylococcus epidermidis with different sequence types isolated from children. Microb Pathog. 2016; 93:126–130. doi: 10.1016/j.micpath.2016.01.018
- Otto M. Staphylococcus epidermidis: a major player in bacterial sepsis? Future Microbiol. 2017; 12(12):1031–3. doi: 10.2217/fmb-2017-0143
- Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10(6):701–706. doi: 10.1586/eri.12.50
- Cue D, Lei MG, Lee CY. Genetic regulation of the intercellular adhesion locus in staphylococci. Front Cell Infect Microbiol 2012; 2:38. doi: 10.3389/fcimb.2012.00038
- Laverty G, Gorman SP, Gilmore BF. Biomolecular mechanisms of staphylococcal biofilm formation. Future Microbiol 2013; 8(4):509–24. doi: 10.2217/fmb.13.7
- Gonzalez T, Biagini Myers JM, Herr AB. Staphylococcal biofilms in atopic dermatitis. Curr Allergy Asthma Rep. 2017;17(12):81. doi: 10.1007/s11882-017-0750-x
- Schilcher K, Horswill AR. Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol Mol Biol Rev. 2020;84(3):e00026–19. doi: 10.1128/MMBR.00026-19
- Francois P, Schrenzel J, Gotz F. Biology and regulation of staphylococcal biofilm. Int J Mol Sci. 2023;24(6):5218. doi: 10.3390/ijms24065218
- Boles BR, Horswill AR. Staphylococcal biofilm disassembly. Trends Microbiol. 2011;19(9):449–455. doi: 10.1016/j.tim.2011.06.004
- Le KY, Villaruz AE, Zheng Y. Role of phenol-soluble modulins in Staphylococcus epidermidis biofilm formation and infection of indwelling medical devices. J Mol Biol. 2019;431(16):3015–3027. doi: 10.1016/j.jmb.2019.03.030
- Christensen GD, Baddour LM, Simpson WA. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987
- Deighton M, Borland R. Regulation of slime production in staphylococcus epidermidis by iron limitation. Infect Immun. 1993;61(10):4473–4479. doi: 10.1128/iai.61.10.4473-4479.1993
- Hussain M, Wilcox MH, White PJ. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol Rev 1993;10(3–4):191–207. doi: 10.1111/j.1574-6968.1993.tb05867.x
- Deighton MA, Borland R, Capstick JA. Virulence of Staphylococcus epidermidis in a mouse model: significance of extracellular slime. Epidemiol Infect. 1996;117(2):267–280. doi: 10.1017/S0950268800001448
- Shiau AL, Wu CL. The inhibitory effect of Staphylococcus epidermidis slime on the phagocytosis of murine peritoneal macrophages is interferon-independent. Microbiol Immunol. 1998;42(1):33–40. doi: 10.1111/j.1348-0421.1998.tb01966.x
- Ammendolia MG, Di Rosa R, Montanaro L. Slime production and expression of the slime-associated antigen by staphylococcal clinical isolates. J Clin Microbiol. 1999;37(10):3235–3238. doi: 10.1128/JCM.37.10.3235-3238.1999
- Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39(6):2151–2156. doi: 10.1128/JCM.39.6.2151-2156.2001
- Foka A, Chini V, Petinaki E. Clonality of slime-producing methicillin-resistant coagulase-negative staphylococci disseminated in the neonatal intensive care unit of a university hospital. Clin Microbiol Infect. 2006;12(12):1230–1233. doi: 10.1111/j.1469-0691.2006.01565.x
- Heilmann C, Schweitzer O, Gerke C. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20(5):1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x
- Vuong C, Kocianova S, Voyich JM. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004;279(52):54881–54886. doi: 10.1074/jbc.M411374200
- Gerke C, Kraft A, Sussmuth R. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273(29):18586–18593. doi: 10.1074/jbc.273.29.18586
- Conlon KM, Humphreys H, O’Gara JP. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in staphylococcus epidermidis. J Bacteriol. 2002;184(16):4400–4408. doi: 10.1128/JB.184.16.4400-4408.2002
- Jeng WY, Ko TP, Liu CI. Crystal structure of IcaR, a repressor of the TetR family implicated in biofilm formation in Staphylococcus epidermidis. Nucleic Acids Res. 2008;36(5):1567–1577. doi: 10.1093/nar/gkm1176
- Hoang TM, Zhou C, Lindgren JK. Transcriptional Regulation of icaADBC by both IcaR and TcaR in Staphylococcus epidermidis. J Bacteriol. 2019;201(6):201. doi: 10.1128/JB.00524-18
- Knobloch JK, Jager S, Horstkotte MA. RsbU-dependent regulation of Staphylococcus epidermidis biofilm formation is mediated via the alternative sigma factor σ b by repression of the negative regulator gene icaR. Infect Immun 2004;72(7):3838–3848. doi: 10.1128/IAI.72.7.3838-3848.2004
- Paharik AE, Kotasinska M, Both A. The metalloprotease SepA governs processing of accumulation-associated protein and shapes intercellular adhesive surface properties in Staphylococcus epidermidis. Mol Microbiol 2017;103(5):860–874. doi: 10.1111/mmi.13594
- Rohde H, Burandt EC, Siemssen N. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials. 2007;28(9):1711–1720. doi: 10.1016/j.biomaterials.2006.11.046
- Juarez-Verdayes MA, Ramon-Perez ML, Flores-Paez LA. Staphylococcus epidermidis with the icaA− /icaD− /IS256 − genotype and protein or protein/extracellular-DNA biofilm is frequent in ocular infections. J Med Microbiol. 2013;62:1579–87. doi: 10.1099/jmm.0.055210-0
- Rahmdel S, Gotz F. The multitasking surface protein of Staphylococcus epidermidis: accumulation-associated protein (Aap). MBio. 2021;12:e0198921. doi: 10.1128/mBio.01989-21
- Yarawsky AE, English LR, Whitten ST. The Proline/Glycine-rich region of the Biofilm Adhesion Protein Aap forms an extended stalk that resists compaction. J Mol Biol. 2017;429(2):261–279. doi: 10.1016/j.jmb.2016.11.017
- Grandbois M, Beyer M, Rief M. How strong is a covalent bond? Science. 1999;283(5408):1727–1730. doi: 10.1126/science.283.5408.1727
- Wang C, Chantraine C, Viljoen A. The staphylococcal biofilm protein Aap mediates cell–cell adhesion through mechanically distinct homophilic and lectin interactions. PNAS Nexus. 2022;1:pgac278. doi: 10.1093/pnasnexus/pgac278
- Khan N, Aslan H, Buttner H. The giant staphylococcal protein Embp facilitates colonization of surfaces through Velcro-like attachment to fibrillated fibronectin. Elife. 2022;11: 11. doi: 10.7554/eLife.76164
- Linnes JC, Ma H, Bryers JD. Giant extracellular matrix binding protein expression in staphylococcus epidermidis is regulated by biofilm formation and osmotic pressure. Curr Microbiol. 2013;66(6):627–633. doi: 10.1007/s00284-013-0316-7
- Lasa I, Penades JR. Bap: a family of surface proteins involved in biofilm formation. Res Microbiol. 2006;157(2):99–107. doi: 10.1016/j.resmic.2005.11.003
- Tormo MA, Knecht E, Gotz F. Bap-dependent biofilm formation by pathogenic species of staphylococcus: evidence of horizontal gene transfer? Microbiology (Reading). 2005;151(7):2465–75. doi: 10.1099/mic.0.27865-0
- Bowden MG, Chen W, Singvall J. Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology (Reading). 2005;151(5):1453–1464. doi: 10.1099/mic.0.27534-0
- Rohde H, Kalitzky M, Kroger N. Detection of virulence-associated genes not useful for discriminating between invasive and commensal Staphylococcus epidermidis strains from a bone marrow transplant unit. J Clin Microbiol 2004;42(12):5614–9. doi: 10.1128/JCM.42.12.5614-5619.2004
- Pinheiro L, Brito CI, Oliveira A. Staphylococcus epidermidis and Staphylococcus haemolyticus: detection of biofilm genes and biofilm formation in blood culture isolates from patients in a Brazilian teaching hospital. Diagn Microbiol Infect Dis. 2016;86(1):11–4. doi: 10.1016/j.diagmicrobio.2016.06.006
- Qin Z, Ou Y, Yang L. Role of autolysin-mediated DNA release in biofilm formation of staphylococcus epidermidis. Microbiology (Reading). 2007;153(7):2083–2092. doi: 10.1099/mic.0.2007/006031-0
- Jakubovics NS, Burgess JG. Extracellular DNA in oral microbial biofilms. Microbes Infect. 2015;17(7):531–537. doi: 10.1016/j.micinf.2015.03.015
- Kavanaugh JS, Flack CE, Lister J. Identification of extracellular DNA-binding proteins in the biofilm matrix. MBio. 2019; 10(3):10. doi: 10.1128/mBio.01137-19
- Fu J, Zhang Y, Lin S. Strategies for interfering with bacterial early stage biofilms. Front Microbiol. 2021; 12:675843. doi: 10.3389/fmicb.2021.675843
- Campoccia D, Montanaro L, Arciola CR Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int J Mol Sci 2021;22(16):9100. doi: 10.3390/ijms22169100
- Doroshenko N, Tseng BS, Howlin RP. Extracellular DNA impedes the transport of vancomycin in staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob Agents Chemother. 2014;58(12):7273–7282. doi: 10.1128/AAC.03132-14
- Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol. 2015;41(3):341–352. doi: 10.3109/1040841X.2013.841639
- Christner M, Heinze C, Busch M. sarA negatively regulates Staphylococcus epidermidis biofilm formation by modulating expression of 1 MDa extracellular matrix binding protein and autolysis-dependent release of eDNA. Mol Microbiol. 2012;86(2):394–410. doi: 10.1111/j.1365-2958.2012.08203.x
- Heilmann C, Hussain M, Peters G. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24(5):1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x
- Zoll S, Patzold B, Schlag M. Structural basis of cell wall cleavage by a staphylococcal autolysin. PLOS Pathog. 2010;6(3):e1000807. doi: 10.1371/journal.ppat.1000807
- Biswas R, Voggu L, Simon UK. Activity of the major staphylococcal autolysin atl. FEMS Microbiol Lett. 2006; 259(2):260–8. doi: 10.1111/j.1574-6968.2006.00281.x
- Schlag M, Biswas R, Krismer B. Role of staphylococcal wall teichoic acid in targeting the major autolysin atl. Mol Microbiol. 2010;75(4):864–873. doi: 10.1111/j.1365-2958.2009.07007.x
- Hirschhausen N, Schlesier T, Schmidt MA. A novel staphylococcal internalization mechanism involves the major autolysin atl and heat shock cognate protein Hsc70 as host cell receptor. Cell Microbiol. 2010;12(12):1746–1764. doi: 10.1111/j.1462-5822.2010.01506.x
- Schlesier T, Siegmund A, Rescher U. Characterization of the atl-mediated staphylococcal internalization mechanism. Int J Med Microbiol. 2020;310(8):151463. doi: 10.1016/j.ijmm.2020.151463
- Rupp ME, Fey PD, Heilmann C. Characterization of the importance of staphylococcus epidermidis autolysin and polysaccharide intercellular Adhesin in the pathogenesis of intravascular catheter–associated infection in a rat model. J Infect Dis. 2001;183(7):1038–42. doi: 10.1086/319279
- Izano EA, Amarante MA, Kher WB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74(2):470–476. doi: 10.1128/AEM.02073-07
- Heilmann C, Thumm G, Chhatwal GS. Identification and characterization of a novel autolysin (aae) with adhesive properties from Staphylococcus epidermidis. Microbiology (Reading). 2003;149(10):2769–2778. doi: 10.1099/mic.0.26527-0
- Almebairik N, Zamudio R, Ironside C. Genomic stability of composite SCCmec ACME and COMER-Like genetic elements in Staphylococcus epidermidis correlates with rate of excision. Front Microbiol 2020; 11:166. doi: 10.3389/fmicb.2020.00166
- Grilo IR, Ludovice AM, Tomasz A. The glucosaminidase domain of Atl – the major Staphylococcus aureus autolysin – has DNA -binding activity. Microbiologyopen. 2014;3(2):247–56. doi: 10.1002/mbo3.165
- Schoenfelder SMK, Lange C, Prakash SA. The small non-coding RNA RsaE influences extracellular matrix composition in Staphylococcus epidermidis biofilm communities. PLOS Pathog. 2019;15(3):e1007618. doi: 10.1371/journal.ppat.1007618
- Fischer A, Kambara K, Meyer H. GdpS contributes to staphylococcus aureus biofilm formation by regulation of eDNA release. Int J Med Microbiol. 2014;304(3–4):284–299. doi: 10.1016/j.ijmm.2013.10.010
- Liu J, Shen Z, Tang J. Extracellular DNA released by glycine-auxotrophic staphylococcus epidermidis small colony variant facilitates catheter-related infections. Commun Biol. 2021;4(1):904. doi: 10.1038/s42003-021-02423-4
- Olson ME, Todd DA, Schaeffer CR. Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross talk, and importance in colonization. J Bacteriol. 2014;196(19):3482–3493. doi: 10.1128/JB.01882-14
- Bowden MG, Visai L, Longshaw CM. Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin? J Biol Chem. 2002;277(45):43017–43023. doi: 10.1074/jbc.M207921200
- Pinchuk IV, Beswick EJ, Reyes VE Staphylococcal enterotoxins. Toxins (Basel). 2010; 2:2177–97. doi: 10.3390/toxins2082177
- Silversides JA, Lappin E, Ferguson AJ. Staphylococcal toxic shock syndrome: mechanisms and management. Curr Infect Dis Rep. 2010;12(5):392–400. doi: 10.1007/s11908-010-0119-y
- Alouf JE, Muller-Alouf H. Staphylococcal and streptococcal superantigens: molecular, biological and clinical aspects. Int J Med Microbiol. 2003;292(7–8):429–440. doi: 10.1078/1438-4221-00232
- Descloux E, Perpoint T, Ferry T. One in five mortality in non-menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbiol Infect Dis 2008;27(1):37–43. doi: 10.1007/s10096-007-0405-2
- Pinheiro L, Brito CI, de Oliveira A. Staphylococcus epidermidis and staphylococcus haemolyticus: molecular detection of cytotoxin and enterotoxin genes. Toxins (Basel) 2015;7(9):3688–99. doi: 10.3390/toxins7093688
- Thomas D, Chou S, Dauwalder O. Diversity in staphylococcus aureus enterotoxins. Chem Immunol Allergy. 2007;93:24–41.
- Breckinridge JC, Bergdoll MS. Outbreak of food-borne gastroenteritis due to a coagulase-negative enterotoxin-producing staphylococcus. N Engl J Med. 1971;284(10):541–543. doi: 10.1056/NEJM197103112841010
- Nanoukon C, Affolabi D, Keller D. Characterization of human type C enterotoxin produced by clinical S. epidermidis isolates. Toxins (Basel). 2018;10(4):10. doi: 10.3390/toxins10040139
- Banaszkiewicz S, Calland JK, Mourkas E. Genetic diversity of composite enterotoxigenic Staphylococcus epidermidis pathogenicity islands. Genome Biol Evol. 2019;11(12):3498–3509. doi: 10.1093/gbe/evz259
- Tabis A, Gonet M, Schubert J, et al. Analysis of enterotoxigenic effect of staphylococcus aureus and staphylococcus epidermidis enterotoxins C and L on mice. Microbiol Res 2022;258:126979. doi: 10.1016/j.micres.2022.126979
- Argemi X, Nanoukon C, Affolabi D. Comparative genomics and identification of an enterotoxin-bearing pathogenicity island, SEPI-1/SECI-1, in Staphylococcus epidermidis pathogenic strains. Toxins (Basel). 2018;10(3):10. doi: 10.3390/toxins10030093
- Vengadesan K, Macon K, Sugumoto S. Purification, crystallization and preliminary X-ray diffraction analysis of the staphylococcus epidermidis extracellular serine protease Esp. Acta Crystallogr, Sect F: struct Biol Cryst Commun. 2013;69(1):49–52. doi: 10.1107/S1744309112047124
- Ohara-Nemoto Y, Ikeda Y, Kobayashi M. Characterization and molecular cloning of a glutamyl endopeptidase from Staphylococcus epidermidis. Microb Pathog. 2002;33(1):33–41. doi: 10.1006/mpat.2002.0515
- Iwase T, Uehara Y, Shinji H. Staphylococcus epidermidis Esp inhibits staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465(7296):346–349. doi: 10.1038/nature09074
- Martinez-Garcia S, Rodriguez-Martinez S, Cancino-Diaz ME. Extracellular proteases of staphylococcus epidermidis: roles as virulence factors and their participation in biofilm. APMIS. 2018;126(3):177–185. doi: 10.1111/apm.12805
- Manne K, Narayana SVL. Structural insights into the role of the N-terminus in the activation and function of extracellular serine protease from staphylococcus epidermidis. Acta Crystallogr D Struct Biol. 2020;76(1):28–40. doi: 10.1107/S2059798319015055
- Ohara-Nemoto Y, Ono T, Shimoyama Y. Homologous and heterologous expression and maturation processing of extracellular glutamyl endopeptidase of Staphylococcus epidermidis. Biol Chem. 2008;389(9):1209–1217. doi: 10.1515/BC.2008.137
- Caballero AR, Tang A, Bierdeman M. Correlation of staphylococcus Epidermidis phenotype and its corneal virulence. Curr Eye Res. 2021;46(5):638–647. doi: 10.1080/02713683.2020.1825748
- Roy P, Horswill AR, Fey PD. Glycan-dependent corneocyte adherence of staphylococcus epidermidis mediated by the lectin subdomain of Aap. MBio 2021;12(4):e0290820. doi: 10.1128/mBio.02908-20
- Cheung GY, Rigby K, Wang R. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLOS Pathog 2010;6(10):e1001133. doi: 10.1371/journal.ppat.1001133
- Lai Y, Villaruz AE, Li M. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol Microbiol. 2007;63(2):497–506. doi: 10.1111/j.1365-2958.2006.05540.x
- Dubin G, Stec-Niemczyk J, Dylag T. Characterisation of a highly specific, endogenous inhibitor of cysteine protease from Staphylococcus epidermidis, a new member of the staphostatin family. Biol Chem. 2004;385(6):543–546. doi: 10.1515/BC.2004.064
- Cau L, Williams MR, Butcher AM. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J Allergy Clin Immunol 2021;147(3):955–66 e16. doi: 10.1016/j.jaci.2020.06.024
- Geissler S, Gotz F, Kupke T. Serine protease EpiP from Staphylococcus epidermidis catalyzes the processing of the epidermin precursor peptide. J Bacteriol. 1996;178(1):284–288. doi: 10.1128/jb.178.1.284-288.1996
- Schnell N, Engelke G, Augustin J. Analysis of genes involved in the biosynthesis of lantibiotic epidermin. Eur J Biochem. 1992;204(1):57–68. doi: 10.1111/j.1432-1033.1992.tb16605.x
- Kuhn ML, Prachi P, Minasov G. Structure and protective efficacy of the staphylococcus aureus autocleaving protease EpiP. Faseb J. 2014;28(4):1780–1793. doi: 10.1096/fj.13-241737
- Cheung GY, Joo HS, Chatterjee SS. Phenol-soluble modulins–critical determinants of staphylococcal virulence. FEMS Microbiol Rev. 2014;38(4):698–719. doi: 10.1111/1574-6976.12057
- Cheung GY, Duong AC, Otto M. Direct and synergistic hemolysis caused by staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect 2012;14(4):380–6. doi: 10.1016/j.micinf.2011.11.013
- Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 2013;64(1):175–188. doi: 10.1146/annurev-med-042711-140023
- Jin Y, Wang Q, Zhang H. Phenol-soluble modulin contributes to the dispersal of staphylococcus epidermidis isolates from catheters. Front Microbiol 2022; 13:934358. doi: 10.3389/fmicb.2022.934358
- Vuong C, Durr M, Carmody AB. Regulated expression of pathogen-associated molecular pattern molecules in staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 2004;6(8):753–759. doi: 10.1111/j.1462-5822.2004.00401.x
- Joo H-S, Otto M. The isolation and analysis of phenol-soluble modulins of staphylococcus epidermidis. In: Fey P, editor. Staphylococcus epidermidis. Totowa (NJ): Humana Press; 2014. p 93–100.
- Qin L, Jw M, Cheung GY. PSM-Mec-A virulence determinant that connects transcriptional regulation, virulence, and antibiotic resistance in staphylococci. Front Microbiol. 2016; 7:1293. doi: 10.3389/fmicb.2016.01293
- Wang R, Braughton KR, Kretschmer D. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 2007; 13(12):1510–4. doi: 10.1038/nm1656
- Le KY, Dastgheyb S, Ho TV. Molecular determinants of staphylococcal biofilm dispersal and structuring. Front Cell Infect Microbiol. 2014; 4:167. doi: 10.3389/fcimb.2014.00167
- Wang R, Khan BA, Cheung GY. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J Clin Invest. 2011; 121(1):238–48. doi: 10.1172/JCI42520
- Queck SY, Khan BA, Wang R. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLOS Pathog. 2009; 5(7):e1000533. doi: 10.1371/journal.ppat.1000533
- Chatterjee SS, Chen L, Joo HS. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant staphylococcus aureus. PLOS ONE 2011; 6(12):e28781. doi: 10.1371/journal.pone.0028781
- Doery HM, Magnusson BJ, Cheyne IM. A phospholipase in staphylococcal toxin which hydrolyses sphingomyelin. Nature 1963; 198(4885):1091–2. doi: 10.1038/1981091a0
- Huseby M, Shi K, Brown CK. Structure and biological activities of beta toxin from staphylococcus aureus. J Bacteriol. 2007;189(23):8719–8726. doi: 10.1128/JB.00741-07
- Zheng Y, Hunt RL, Villaruz AE. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe 2022; 30(3):301–13 e9. doi: 10.1016/j.chom.2022.01.004
- Otto M. Quorum-sensing control in staphylococci â?? a target for antimicrobial drug therapy? FEMS Microbiol Lett. 2004; 241(2):135–41. doi: 10.1016/j.femsle.2004.11.016
- Batzilla CF, Rachid S, Engelmann S. Impact of the accessory gene regulatory system (Agr) on extracellular proteins, codY expression and amino acid metabolism in Staphylococcus epidermidis. Proteomics. 2006;6(12):3602–3613. doi: 10.1002/pmic.200500732
- Horswill AR, Stoodley P, Stewart PS. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal Bioanal Chem. 2007;387(2):371–380. doi: 10.1007/s00216-006-0720-y
- Singh R, Ray P. Quorum sensing-mediated regulation of staphylococcal virulence and antibiotic resistance. Future Microbiol 2014; 9(5):669–81. doi: 10.2217/fmb.14.31
- Otto M, Dickey SW, Wolz C. Editorial: quorum-sensing in gram-positive pathogens – mechanisms, role in infection, and potential as a therapeutic target. Front Cell Infect Microbiol. 2023; 13. doi: 10.3389/fcimb.2023.1236705
- Kies S, Vuong C, Hille M. Control of antimicrobial peptide synthesis by the agr quorum sensing system in Staphylococcus epidermidis: activity of the lantibiotic epidermin is regulated at the level of precursor peptide processing. Peptides. 2003;24(3):329–338. doi: 10.1016/S0196-9781(03)00046-9
- Otto M. Staphylococcus aureus and Staphylococcus epidermidis peptide pheromones produced by the accessory gene regulator agr system. Peptides. 2001;22(10):1603–1608. doi: 10.1016/S0196-9781(01)00495-8
- Bronesky D, Wu Z, Marzi S. Staphylococcus aureus RNAIII and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu Rev Microbiol. 2016;70(1):299–316. doi: 10.1146/annurev-micro-102215-095708
- Canovas J, Baldry M, Bojer MS. Cross-talk between staphylococcus aureus and other staphylococcal species via the agr quorum sensing system. Front Microbiol 2016; 7. doi: 10.3389/fmicb.2016.01733
- Tan L, Huang Y, Shang W. Accessory gene regulator (agr) Allelic variants in cognate staphylococcus aureus strain display similar phenotypes. Front Microbiol 2022; 13:700894. doi: 10.3389/fmicb.2022.700894
- Minich A, Liskova V, Kormanova L. Role of RNAIII in resistance to antibiotics and antimicrobial agents in staphylococcus epidermidis Biofilms. Int J Mol Sci 2022; 23(19):23. doi: 10.3390/ijms231911094
- Boisset S, Geissmann T, Huntzinger E. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator rot by an antisense mechanism. Genes Dev. 2007;21(11):1353–1366. doi: 10.1101/gad.423507
- Le KY, Otto M. Quorum-sensing regulation in staphylococci-an overview. Front Microbiol 2015; 6:1174. doi: 10.3389/fmicb.2015.01174
- Li M, Guan M, Jiang XF. Genetic polymorphism of the accessory gene regulator (agr) locus in Staphylococcus epidermidis and its association with pathogenicity. J Med Microbiol. 2004;53(6):545–549. doi: 10.1099/jmm.0.05406-0
- Martinez-Garcia S, Ortiz-Garcia CI, Cruz-Aguilar M. Competition/antagonism associations of biofilm formation among Staphylococcus epidermidis agr groups I, II, and III. J Microbiol. 2019;57(2):143–153. doi: 10.1007/s12275-019-8322-5
- Tan L, Li SR, Jiang B. Therapeutic targeting of the staphylococcus aureus accessory gene regulator (agr) system. Front Microbiol. 2018; 9:55. doi: 10.3389/fmicb.2018.00055
- Vuong C, Kocianova S, Yao Y. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis. 2004;190(8):1498–1505. doi: 10.1086/424487
- Yao Y, Vuong C, Kocianova S. Characterization of the Staphylococcus epidermidis accessory-gene regulator response: quorum-sensing regulation of resistance to human innate host defense. J Infect Dis. 2006;193(6):841–848. doi: 10.1086/500246
- Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, salmonella typhimurium, and vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96(4):1639–1644. doi: 10.1073/pnas.96.4.1639
- Xue T, Ni J, Shang F. Autoinducer-2 increases biofilm formation via an ica- and bhp-dependent manner in staphylococcus epidermidis RP62A. Microbes Infect. 2015;17(5):345–352. doi: 10.1016/j.micinf.2015.01.003
- Xu L, Li H, Vuong C. Role of the luxS quorum-sensing system in biofilm formation and virulence of staphylococcus epidermidis. Infect Immun. 2006;74(1):488–496. doi: 10.1128/IAI.74.1.488-496.2006
- Conlon KM, Humphreys H, O’Gara JP. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J Bacteriol. 2004;186(18):6208–6219. doi: 10.1128/JB.186.18.6208-6219.2004
- Bischoff M, Dunman P, Kormanec J. Microarray-based analysis of the staphylococcus aureus σ B regulon. J Bacteriol. 2004; 186(13):4085–99. doi: 10.1128/JB.186.13.4085-4099.2004
- Knobloch JK, Bartscht K, Sabottke A. Biofilm formation by staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J Bacteriol 2001; 183(8):2624–33. doi: 10.1128/JB.183.8.2624-2633.2001
- Cotter JJ, Jp O, Mack D. Oxygen-mediated regulation of biofilm development Is controlled by the alternative sigma factor σ B in staphylococcus epidermidis. Appl Environ Microbiol 2009; 75(1):261–4. doi: 10.1128/AEM.00261-08
- Handke LD, Slater SR, Conlon KM. σ B and SarA independently regulate polysaccharide intercellular adhesin production in Staphylococcus epidermidis. Can J Microbiol 2007; 53(1):82–91. doi: 10.1139/w06-108
- Jager S, Mack D, Rohde H. Disintegration of staphylococcus epidermidis biofilms under glucose-limiting conditions depends on the activity of the alternative sigma factor σ B. Appl Environ Microbiol 2005; 71(9):5577–81. doi: 10.1128/AEM.71.9.5577-5581.2005
- Pintens V, Massonet C, Merckx R. The role of σ B in persistence of Staphylococcus epidermidis foreign body infection. Microbiology (Reading) 2008; 154(9):2827–36. doi: 10.1099/mic.0.2007/015768-0
- Majerczyk CD, Sadykov MR, Luong TT. Staphylococcus aureus CodY negatively regulates virulence gene expression. J Bacteriol. 2008;190(7):2257–2265. doi: 10.1128/JB.01545-07
- Waters NR, Samuels DJ, Behera RK. A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in staphylococcus aureus. Mol Microbiol. 2016;101(3):495–514. doi: 10.1111/mmi.13404
- Mlynek KD, Bulock LL, Stone CJ. Genetic and biochemical analysis of CodY-mediated cell aggregation in staphylococcus aureus reveals an interaction between extracellular DNA and polysaccharide in the extracellular matrix. J Bacteriol. 2020;202(8): doi: 10.1128/JB.00593-19
- Roux A, Todd DA, Velazquez JV. CodY-mediated regulation of the staphylococcus aureus agr system integrates nutritional and population density signals. J Bacteriol. 2014;196(6):1184–1196. doi: 10.1128/JB.00128-13
- Carvalhais V, Amado F, Cerveira F. Immunoreactive pattern of Staphylococcus epidermidis biofilm against human whole saliva. Electrophoresis. 2015;36(9–10):1228–1233. doi: 10.1002/elps.201500043
- Gaio V, Lopes N, Cerca N. codY and pdhA expression is induced in staphylococcus epidermidis biofilm and planktonic populations with higher proportions of viable but non-culturable cells. Front Cell Infect Microbiol. 2021; 11:771666. doi: 10.3389/fcimb.2021.771666
- Majerczyk CD, Dunman PM, Luong TT. Direct targets of CodY in staphylococcus aureus. J Bacteriol. 2010;192(11):2861–2877. doi: 10.1128/JB.00220-10
- Sadykov MR, Olson ME, Halouska S. Tricarboxylic acid cycle-dependent regulation of saphylococcus epidermidis polysaccharide intercellular adhesin synthesis. J Bacteriol. 2008;190(23):7621–7632. doi: 10.1128/JB.00806-08
- Cramton SE, Gerke C, Schnell NF. The intercellular adhesion (ica) locus is present in staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999; 67(10):5427–33. doi: 10.1128/IAI.67.10.5427-5433.1999
- Gill SR, Fouts DE, Archer GL. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187(7):2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005
- Roche FM, Meehan M, Foster TJ. The staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology (Reading). 2003;149(10):2759–2767. doi: 10.1099/mic.0.26412-0
- Tormo MA, Marti M, Valle J. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J Bacteriol. 2005;187(7):2348–2356. doi: 10.1128/JB.187.7.2348-2356.2005
- Lucas AL, Manna AC Phenotypic characterization of sarR mutant in staphylococcus aureus. Microb Pathog. 2013; 57:52–61. doi: 10.1016/j.micpath.2012.11.008
- Wang L, Li M, Dong D. SarZ is a key regulator of biofilm formation and virulence in staphylococcus epidermidis. J Infect Dis. 2008;197(9):1254–1262. doi: 10.1086/586714
- Rowe SE, Mahon V, Smith SG. A novel role for SarX in staphylococcus epidermidis biofilm regulation. Microbiology (Reading). 2011;157(4):1042–1049. doi: 10.1099/mic.0.046581-0
- Cue D, Lei MG, Lee CY. Activation of sarX by Rbf is required for biofilm formation and icaADBC expression in staphylococcus aureus. J Bacteriol. 2013;195(7):1515–1524. doi: 10.1128/JB.00012-13
- Lim Y, Jana M, Luong TT. Control of glucose- and NaCl-induced biofilm formation by rbf in staphylococcus aureus. J Bacteriol. 2004;186(3):722–729. doi: 10.1128/JB.186.3.722-729.2004
- Cue D, Lei MG, Luong TT. Rbf promotes biofilm formation by staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J Bacteriol. 2009;191(20):6363–6373. doi: 10.1128/JB.00913-09
- Rowe SE, Campbell C, Lowry C. AraC-type regulator Rbf controls the staphylococcus epidermidis biofilm phenotype by negatively regulating the icaADBC repressor SarR. J Bacteriol. 2016;198(21):2914–2924. doi: 10.1128/JB.00374-16
- Liu Q, Yeo WS, Bae T. The SaeRS two-component system of staphylococcus aureus. Genes (Basel). 2016;7(10):81. doi: 10.3390/genes7100081
- Handke LD, Rogers KL, Olson ME. Staphylococcus epidermidis saeR is an effector of anaerobic growth and a mediator of acute inflammation. Infect Immun. 2008;76(1):141–152. doi: 10.1128/IAI.00556-07
- Collins MM, Behera RK, Pallister KB. The accessory gene saeP of the SaeR/S two-component gene regulatory system impacts staphylococcus aureus virulence during neutrophil interaction. Front Microbiol 2020; 11:561. doi: 10.3389/fmicb.2020.00561
- Lou Q, Zhu T, Hu J. Role of the SaeRS two-component regulatory system in staphylococcus epidermidis autolysis and biofilm formation. BMC Microbiol. 2011;11(1):146. doi: 10.1186/1471-2180-11-146
- Pragman AA, Yarwood JM, Tripp TJ. Characterization of virulence factor regulation by SrrAB, a two-component system in staphylococcus aureus. J Bacteriol. 2004;186(8):2430–2438. doi: 10.1128/JB.186.8.2430-2438.2004
- Wu Y, Wu Y, Zhu T. Staphylococcus epidermidis SrrAB regulates bacterial growth and biofilm formation differently under oxic and microaerobic conditions. J Bacteriol. 2015;197(3):459–476. doi: 10.1128/JB.02231-14
- Kraus D, Herbert S, Kristian SA. The GraRS regulatory system controls staphylococcus aureus susceptibility to antimicrobial host defenses. BMC Microbiol. 2008;8(1):85. doi: 10.1186/1471-2180-8-85
- Costa SK, Cho J, Cheung AL. GraS sensory activity in staphylococcus epidermidis is modulated by the “Guard loop” of VraG and the ATPase activity of VraF. J Bacteriol. 2021;203(17):e0017821. doi: 10.1128/JB.00178-21
- Huang Y, Huang J, Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell. 2010;1(2):143–152. doi: 10.1007/s13238-010-0004-3
- Liu WP, Chen YH, Ming X. Design and synthesis of a novel cationic peptide with potent and broad-spectrum antimicrobial activity. Biomed Res Int. 2015; 2015:578764. doi: 10.1155/2015/578764
- Wang C, Li M, Dong D. Role of ClpP in biofilm formation and virulence of staphylococcus epidermidis. Microbes Infect. 2007;9(11):1376–1383. doi: 10.1016/j.micinf.2007.06.012
- Frees D, Qazi SN, Hill PJ. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol Microbiol 2003; 48(6):1565–78. doi: 10.1046/j.1365-2958.2003.03524.x
- Frees D, Gerth U, Ingmer H. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of staphylococcus aureus. Int J Med Microbiol. 2014;304(2):142–149. doi: 10.1016/j.ijmm.2013.11.009
- Alqarzaee AA, Chaudhari SS, Islam MM. Staphylococcal ClpXP protease targets the cellular antioxidant system to eliminate fitness-compromised cells in stationary phase. Proc Natl Acad Sci USA. 2021;118(47):e2109671118. doi: 10.1073/pnas.2109671118
- Mack D, Becker P, Chatterjee I. Mechanisms of biofilm formation in staphylococcus epidermidis and staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int J Med Microbiol. 2004; 294(2–3):203–12. doi: 10.1016/j.ijmm.2004.06.015
- Savijoki K, Iivanainen A, Siljamaki P. Genomics and proteomics provide new insight into the commensal and pathogenic lifestyles of bovine- and human-associated staphylococcus epidermidis strains. J Proteome Res. 2014;13(8):3748–3762. doi: 10.1021/pr500322d
- Zuber P. Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J Bacteriol. 2004;186(7):1911–1918. doi: 10.1128/JB.186.7.1911-1918.2004
- Pamp SJ, Frees D, Engelmann S. Spx is a global effector impacting stress tolerance and biofilm formation in staphylococcus aureus. J Bacteriol. 2006;188(13):4861–4870. doi: 10.1128/JB.00194-06
- Wang C, Fan J, Niu C. Role of spx in biofilm formation of Staphylococcus epidermidis. FEMS Immunol Med Microbiol. 2010;59(2):152–160. doi: 10.1111/j.1574-695X.2010.00673.x
- Ziebuhr W, Dietrich K, Trautmann M. Chromosomal rearrangements affecting biofilm production and antibiotic resistance in a Staphylococcus epidermidis strain causing shunt-associated ventriculitis. Int J Med Microbiol. 2000;290(1):115–120. doi: 10.1016/S1438-4221(00)80115-0
- Kozitskaya S, Cho SH, Dietrich K. The bacterial insertion sequence element IS256 occurs preferentially in nosocomial staphylococcus epidermidis isolates: association with biofilm formation and resistance to aminoglycosides. Infect Immun. 2004;72(2):1210–1215. doi: 10.1128/IAI.72.2.1210-1215.2004
- Hennig S, Nyunt Wai S, Ziebuhr W. Spontaneous switch to PIA-independent biofilm formation in an ica-positive staphylococcus epidermidis isolate. Int J Med Microbiol. 2007;297(2):117–122. doi: 10.1016/j.ijmm.2006.12.001
- Hennig S, Ziebuhr W. A transposase-independent mechanism gives rise to precise excision of IS256 from insertion sites in staphylococcus epidermidis. J Bacteriol. 2008;190(4):1488–1490. doi: 10.1128/JB.01290-07
- Gu J, Li H, Li M. Bacterial insertion sequence IS256 as a potential molecular marker to discriminate invasive strains from commensal strains of Staphylococcus epidermidis. J Hosp Infect. 2005;61(4):342–348. doi: 10.1016/j.jhin.2005.04.017
- Murugesan S, Mani S, Kuppusamy I. Role of insertion sequence element is256 as a virulence marker and its association with biofilm formation among methicillin-resistant staphylococcus epidermidis from hospital and community settings in Chennai, South India. Indian J Med Microbiol. 2018;36(1):124–126. doi: 10.4103/ijmm.IJMM_17_276
- Du X, Zhu Y, Song Y. Molecular analysis of Staphylococcus epidermidis strains isolated from community and hospital environments in China. PLOS ONE. 2013;8(5):e62742. doi: 10.1371/journal.pone.0062742
- Li M, Wang X, Gao Q. Molecular characterization of staphylococcus epidermidis strains isolated from a teaching hospital in Shanghai, China. J Med Microbiol. 2009;58(4):456–461. doi: 10.1099/jmm.0.007567-0
- Lee JYH, Monk IR, Goncalves da Silva A. Global spread of three multidrug-resistant lineages of staphylococcus epidermidis. Nat Microbiol. 2018;3(10):1175–1185. doi: 10.1038/s41564-018-0230-7
- Koskela A, Nilsdotter-Augustinsson A, Persson L. Prevalence of the ica operon and insertion sequence IS256 among staphylococcus epidermidis prosthetic joint infection isolates. Eur J Clin Microbiol Infect Dis 2009; 28(6):655–60. doi: 10.1007/s10096-008-0664-6
- Sanchez A, Benito N, Rivera A. Pathogenesis of staphylococcus epidermidis in prosthetic joint infections: can identification of virulence genes differentiate between infecting and commensal strains? J Hosp Infect. 2020;105(3):561–568. doi: 10.1016/j.jhin.2020.04.026
- Montanaro L, Campoccia D, Pirini V. Antibiotic multiresistance strictly associated with IS256 and ica genes in staphylococcus epidermidis strains from implant orthopedic infections. J Biomed Mater Res A 2007; 83(3):813–8. doi: 10.1002/jbm.a.31399
- Petrelli D, Zampaloni C, D’Ercole S. Analysis of different genetic traits and their association with biofilm formation in Staphylococcus epidermidis isolates from central venous catheter infections. Eur J Clin Microbiol Infect Dis. 2006;25(12):773–781. doi: 10.1007/s10096-006-0226-8
- Byrne ME, Rouch DA, Skurray RA. Nucleotide sequence analysis of IS256 from the staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2
- Montanaro L, Speziale P, Campoccia D. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol 2011; 6(11):1329–49. doi: 10.2217/fmb.11.117
- Sabate Bresco M, L O, Zeiter S. Influence of fracture stability on Staphylococcus epidermidis and staphylococcus aureus infection in a murine femoral fracture model. Eur Cell Mater. 2017; 34:321–40. 10.22203/eCM.v034a20
- Areschoug T, Gordon S. Pattern recognition receptors and their role in innate immunity: focus on microbial protein ligands. In: Egesten A, A Schmidt H Herwald, editors. Contributions to microbiology. Basel: KARGER; 2008. p 45–60.
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227(1):221–233. doi: 10.1111/j.1600-065X.2008.00731.x
- Strunk T, Power Coombs MR, Currie AJ. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLOS ONE. 2010;5(4):e10111. doi: 10.1371/journal.pone.0010111
- Hajjar AM, O’Mahony DS, Ozinsky A. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166(1):15–19. doi: 10.4049/jimmunol.166.1.15
- Lai Y, Di Nardo A, Nakatsuji T. Commensal bacteria regulate Toll-like receptor 3–dependent inflammation after skin injury. Nat Med 2009; 15(12):1377–82. doi: 10.1038/nm.2062
- Hruz P, Zinkernagel AS, Jenikova G. NOD2 contributes to cutaneous defense against staphylococcus aureus through α-toxin-dependent innate immune activation. Proc Natl Acad Sci USA 2009; 106(31):12873–8. doi: 10.1073/pnas.0904958106
- Muller-Anstett MA, Muller P, Albrecht T. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLOS ONE. 2010;5(10):e13153. doi: 10.1371/journal.pone.0013153
- Fournier B. The function of TLR2 during staphylococcal diseases. Front Cell Infect Microbiol. 2012; 2:167. 10.3389/fcimb.2012.00167
- Hanzelmann D, Joo HS, Franz-Wachtel M. Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat Commun. 2016;7(1):12304. doi: 10.1038/ncomms12304
- de Vor L, Rooijakkers SHM, van Strijp JAG. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett. 2020; 594(16):2556–69. doi: 10.1002/1873-3468.13767
- Naik S, Bouladoux N, Linehan JL. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015; 520(7545):104–8. doi: 10.1038/nature14052
- Scharschmidt TC, Vasquez KS, Truong HA. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity. 2015;43(5):1011–1021. doi: 10.1016/j.immuni.2015.10.016
- Li D, Wang W, Wu Y. Lipopeptide 78 from staphylococcus epidermidis Activates β-Catenin to inhibit skin inflammation. J Immunol 2019; 202(4):1219–28. doi: 10.4049/jimmunol.1800813
- Ochlich D, Rademacher F, Drerup KA. The influence of the commensal skin bacterium Staphylococcus epidermidis on the epidermal barrier and inflammation: implications for atopic dermatitis. Exp Dermatol. 2023;32(4):555–561. doi: 10.1111/exd.14727
- Spiliopoulou AI, Kolonitsiou F, Krevvata MI. Bacterial adhesion, intracellular survival and cytokine induction upon stimulation of mononuclear cells with planktonic or biofilm phase staphylococcus epidermidis. FEMS Microbiol Lett. 2012;330(1):56–65. doi: 10.1111/j.1574-6968.2012.02533.x
- Cerca N, Jefferson KK, Oliveira R. Comparative antibody-mediated phagocytosis of staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74(8):4849–4855. doi: 10.1128/IAI.00230-06
- Schommer NN, Christner M, Hentschke M. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun. 2011;79(6):2267–2276. doi: 10.1128/IAI.01142-10
- Kristian SA, Birkenstock TA, Sauder U. Biofilm formation induces C3a release and protects staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J Infect Dis. 2008;197(7):1028–1035. doi: 10.1086/528992
- Serruto D, Rappuoli R, Scarselli M. Molecular mechanisms of complement evasion: learning from staphylococci and meningococci. Nat Rev Microbiol. 2010; 8(6):393–9. doi: 10.1038/nrmicro2366
- Peng P, Baldry M, Gless BH. Effect of Co-inhabiting coagulase negative staphylococci on S. aureus agr quorum sensing, host factor binding, and biofilm formation. Front Microbiol. 2019; 10:2212. doi: 10.3389/fmicb.2019.02212
- Zhou Y, Xu X, Liu Y. Heterogeneous regulation of staphylococcusAureus by different staphylococcus Epidermidisagr types in atopic dermatitis. J Invest Dermatol. 2023; 143(12):2484–93 e11. doi: 10.1016/j.jid.2023.05.014
- Cotter PD, Ross RP, Hill C Bacteriocins — a viable alternative to antibiotics? Nat Rev Microbiol 2013; 11(2):95–105. doi: 10.1038/nrmicro2937
- Janek D, Zipperer A, Kulik A. High frequency and diversity of antimicrobial activities produced by Nasal staphylococcus strains against bacterial competitors. PLOS Pathog. 2016;12(8):e1005812. doi: 10.1371/journal.ppat.1005812
- Bierbaum G, Gotz F, Peschel A. The biosynthesis of the lantibiotics epidermin, gallidermin, Pep5 and epilancin K7. Antonie Van Leeuwenhoek. 1996;69(2):119–127. doi: 10.1007/BF00399417
- Allgaier H, Jung G, Werner RG. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur J Biochem 1986; 160(1):9–22. doi: 10.1111/j.1432-1033.1986.tb09933.x
- Ekkelenkamp MB, Hanssen M, Danny Hsu ST. Isolation and structural characterization of epilancin 15X, a novel lantibiotic from a clinical strain of staphylococcus epidermidis. FEBS Lett. 2005;579(9):1917–1922. doi: 10.1016/j.febslet.2005.01.083
- Heidrich C, Hantke K, Bierbaum G. Identification and analysis of a gene encoding a Fur-like protein of staphylococcus epidermidis. FEMS Microbiol Lett. 1996;140(2–3):253–259. doi: 10.1111/j.1574-6968.1996.tb08345.x
- Sandiford S, Upton M. Identification, characterization, and recombinant expression of epidermicin NI01, a novel unmodified bacteriocin produced by staphylococcus epidermidis that displays potent activity against staphylococci. Antimicrob Agents Chemother. 2012;56(3):1539–1547. doi: 10.1128/AAC.05397-11
- Ahle CM, Stodkilde K, Poehlein A. Interference and co-existence of staphylococci and cutibacterium acnes within the healthy human skin microbiome. Commun Biol. 2022;5(1):923. doi: 10.1038/s42003-022-03897-6
- Nurxat N, Wang L, Wang Q. Commensal staphylococcus epidermidis defends against staphylococcus aureus through SaeRS two-component system. ACS Omega. 2023;8(20):17712–17718. doi: 10.1021/acsomega.3c00263
- Sugimoto S, Iwamoto T, Takada K. Staphylococcus epidermidis Esp degrades specific proteins associated with staphylococcus aureus biofilm formation and host-pathogen interaction. J Bacteriol. 2013;195(8):1645–1655. doi: 10.1128/JB.01672-12
- Pastar I, O’Neill K, Padula L. Staphylococcus epidermidis boosts innate immune response by activation of gamma delta T cells and induction of perforin-2 in human skin. Front Immunol 2020; 11:550946. doi: 10.3389/fimmu.2020.550946
- Lai Y, Gallo RL Toll-like receptors in skin infections and inflammatory diseases. Infect Disord Drug Tar 2008; 8(3):144–55. doi: 10.2174/1871526510808030144
- Lai Y, Cogen AL, Radek KA. Activation of TLR2 by a small molecule produced by staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130(9):2211–2221. doi: 10.1038/jid.2010.123
- Jones KJ, Perris AD, Vernallis AB. Induction of inflammatory cytokines and nitric oxide in J774.2 cells and murine macrophages by lipoteichoic acid and related cell wall antigens from staphylococcus epidermidis. J Med Microbiol. 2005;54(4):315–321. doi: 10.1099/jmm.0.45872-0
- Rodrigues M, Black GG, white, and gray: macrophages in skin repair and disease. Curr Pathobiol Rep 2017; 5(4):333–42. doi: 10.1007/s40139-017-0152-8
- Bjerre RD, Bandier J, Skov L. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol. 2017;177(5):1272–1278. doi: 10.1111/bjd.15390
- Keshari S, Balasubramaniam A, Myagmardoloonjin B. Butyric acid from probiotic staphylococcus epidermidis in the skin microbiome down-Regulates the ultraviolet-induced pro-inflammatory IL-6 Cytokine via short-chain fatty acid receptor. Int J Mol Sci. 2019;20(18):20. doi: 10.3390/ijms20184477
- Luqman A, Muttaqin MZ, Yulaipi S. Trace amines produced by skin bacteria accelerate wound healing in mice. Commun Biol. 2020;3(1):277. doi: 10.1038/s42003-020-1000-7
- Garcia P, Benitez R, Lam M. Coagulase-negative staphylococci: clinical, microbiological and molecular features to predict true bacteraemia. J Med Microbiol. 2004;53(1):67–72. doi: 10.1099/jmm.0.04994-0
- Papadimitriou-Olivgeri I, Giormezis N, Papadimitriou-Olivgeris M. Number of positive blood cultures, biofilm formation, and adhesin genes in differentiating true coagulase-negative staphylococci bacteremia from contamination. Eur J Clin Microbiol Infect Dis. 2016;35(1):57–66. doi: 10.1007/s10096-015-2506-7
- Osaki S, Kikuchi K, Moritoki Y. Distinguishing coagulase-negative staphylococcus bacteremia from contamination using blood-culture positive bottle detection pattern and time to positivity. J Infect Chemother. 2020;26(7):672–675. doi: 10.1016/j.jiac.2020.02.004
- Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296(23):1305–1309. doi: 10.1056/NEJM197706092962301
- Neut D, van Horn JR, van Kooten TG. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthop Relat Res 2003;413:261–8. doi: 10.1097/01.blo.0000073345.50837.84
- Gbejuade HO, Lovering AM, Webb JC The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015; 86(2):147–58. doi: 10.3109/17453674.2014.966290
- Egglestone A, Ingoe H, Rees J. Scoping review: diagnosis and management of periprosthetic joint infection in shoulder arthroplasty. Shoulder Elbow. 2019;11(3):167–181. doi: 10.1177/1758573218779076
- Wurm A, Kuhn J, Kugel K. Raman microscopic spectroscopy as a diagnostic tool to detect staphylococcus epidermidis in bone grafts. Spectrochim Acta A Mol Biomol Spectrosc 2022; 280:121570. doi: 10.1016/j.saa.2022.121570
- Bras S, Franca A. Transcriptome mining to identify molecular markers for the diagnosis of Staphylococcus epidermidis bloodstream infections. Antibiotics. 2022;11(11):11. doi: 10.3390/antibiotics11111596
- Jukes L, Mikhail J, Bome-Mannathoko N. Rapid differentiation of staphylococcus aureus, staphylococcus epidermidis and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR. J Med Microbiol. 2010;59(12):1456–1461. doi: 10.1099/jmm.0.023168-0
- Jin WY, Jang SJ, Lee MJ. Evaluation of VITEK 2, MicroScan, and phoenix for identification of clinical isolates and reference strains. Diagn Microbiol Infect Dis. 2011;70(4):442–447. doi: 10.1016/j.diagmicrobio.2011.04.013
- Chatzigeorgiou KS, Siafakas N, Petinaki E. Identification of staphylococci by phoenix: validation of a new protocol and comparison with Vitek 2. Diagn Microbiol Infect Dis. 2010;68(4):375–381. doi: 10.1016/j.diagmicrobio.2010.08.010
- Zhu W, Sieradzki K, Albrecht V. Evaluation of the Biotyper MALDI-TOF MS system for identification of staphylococcus species. J Microbiol Methods. 2015; 117:14–17.10.1016/j.mimet.2015.07.014
- Peel TN, Cole NC, Dylla BL. Matrix-assisted laser desorption ionization time of flight mass spectrometry and diagnostic testing for prosthetic joint infection in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 2015;81(3):163–168. doi: 10.1016/j.diagmicrobio.2014.11.015
- Al-Manei K, Ghorbani M, Naud S. Clinical microbial identification of severe oral infections by MALDI-TOF mass spectrometry in Stockholm County: an 11-Year (2010 to 2020) epidemiological investigation. Microbiol Spectr 2022; 10(6):e0248722. doi: 10.1128/spectrum.02487-22
- Rosa NM, Penati M, Fusar-Poli S. Species identification by MALDI-TOF MS and gap PCR–RFLP of non-aureus staphylococcus, mammaliicoccus, and streptococcus spp. associated with sheep and goat mastitis. Vet Res. 2022; 53(1):84. doi: 10.1186/s13567-022-01102-4
- Kleinschmidt S, Huygens F, Faoagali J. Staphylococcus epidermidis as a cause of bacteremia. Future Microbiol 2015; 10(11):1859–79. doi: 10.2217/fmb.15.98
- Kuo FC, Chien CC, Lee MS. Rapid diagnosis of periprosthetic joint infection from synovial fluid in blood culture bottles by direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLOS ONE. 2020;15(9):e0239290. doi: 10.1371/journal.pone.0239290
- Schuster D, Josten M, Janssen K. Detection of methicillin-resistant coagulase-negative staphylococci harboring the class a mec complex by MALDI-TOF mass spectrometry. Int J Med Microbiol. 2018;308(5):522–526. doi: 10.1016/j.ijmm.2018.05.001
- Alksne L, Makarova S, Avsejenko J. Determination of methicillin-resistant staphylococcus aureus and staphylococcus epidermidis by MALDI-TOF MS in clinical isolates from Latvia. Clin Mass Spectrom 2020; 16:33–39. doi: 10.1016/j.clinms.2020.03.001
- Caputo P, Di Martino MC, Perfetto B. Use of MALDI-TOF MS to discriminate between biofilm-producer and non-producer strains of Staphylococcus epidermidis. Int J Environ Res Public Health. 2018;15(8):15. doi: 10.3390/ijerph15081695
- Skovdal SM, Jorgensen NP, Meyer RL. JMM profile: staphylococcus epidermidis. J Med Microbiol. 2022;71(10):71. doi: 10.1099/jmm.0.001597
- Wozniak RAF, Aquavella JV. Antibiotics in ophthalmology practice. Expert Rev Ophthalmol. 2017;12(3):243–250. doi: 10.1080/17469899.2017.1318065
- Michael E, Welch S, Niederer RL. Rapid treatment of endophthalmitis with intravitreal antibiotics is associated with better vision outcomes. Clin Exp Ophthalmol. 2023;51(2):137–143. doi: 10.1111/ceo.14186
- Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125(4):353–364. doi: 10.1111/apm.12687
- Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158–168. doi: 10.1111/j.1574-695X.2012.00938.x
- Tesch W, Ryffel C, Strassle A. Evidence of a novel staphylococcal mec-encoded element (mecR) controlling expression of penicillin-binding protein 2’. Antimicrob Agents Chemother. 1990;34(9):1703–1706. doi: 10.1128/AAC.34.9.1703
- Archer GL, Niemeyer DM. Origin and evolution of DNA associated with resistance to methicillin in staphylococci. Trends Microbiol. 1994;2(10):343–347. doi: 10.1016/0966-842X(94)90608-4
- Wu Z, Li F, Liu D. Novel type XII staphylococcal cassette chromosome mec harboring a new cassette chromosome Recombinase, CcrC2. Antimicrob Agents Chemother. 2015;59(12):7597–7601. doi: 10.1128/AAC.01692-15
- Lee AS, de Lencastre H, Garau J. Methicillin-resistant staphylococcus aureus. Nat Rev Dis Primers. 2018;4(1):18033. doi: 10.1038/nrdp.2018.33
- Lakhundi S, Zhang K. Methicillin-resistant staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31(4): doi: 10.1128/CMR.00020-18
- Zhu T, Zhao Y Correlation between type IIIA CRISPR–cas system and SCCmec in Staphylococcus epidermidis. Arch Microbiol 2021; 203(10):6275–86. doi: 10.1007/s00203-021-02595-x
- Wisplinghoff H, Rosato AE, Enright MC. Related clones containing SCCmec type IV predominate among clinically significant staphylococcus epidermidis isolates. Antimicrob Agents Chemother. 2003;47(11):3574–3579. doi: 10.1128/AAC.47.11.3574-3579.2003
- Malik S, Coombs GW, Fg O. Molecular typing of methicillin-resistant staphylococci isolated from cats and dogs. J Antimicrob Chemother 2006; 58(2):428–31. doi: 10.1093/jac/dkl253
- Jamaluddin TZ, Kuwahara-Arai K, Hisata K. Extreme genetic diversity of methicillin-resistant Staphylococcus epidermidis strains disseminated among healthy Japanese children. J Clin Microbiol 2008; 46(11):3778–83. doi: 10.1128/JCM.02262-07
- Ruppe E, Barbier F, Mesli Y. Diversity of staphylococcal cassette chromosome mec structures in methicillin-resistant staphylococcus epidermidis and staphylococcus haemolyticus strains among outpatients from four countries. Antimicrob Agents Chemother. 2009;53(2):442–449. doi: 10.1128/AAC.00724-08
- Garza-Gonzalez E, Morfin-Otero R, Llaca-Diaz JM. Staphylococcal cassette chromosome mec (SCC mec) in methicillin-resistant coagulase-negative staphylococci. A review and the experience in a tertiary-care setting. Epidemiol Infect. 2010;138(5):645–654. doi: 10.1017/S0950268809991361
- Zong Z, Peng C, Lu X. Diversity of SCCmec elements in methicillin-resistant coagulase-negative staphylococci clinical isolates. PLOS ONE. 2011;6(5):e20191. doi: 10.1371/journal.pone.0020191
- Saber H, Jasni AS, Jamaluddin T. A review of staphylococcal cassette chromosome mec (SCCmec) types in coagulase-negative staphylococci (CoNS) species. Malays J Med Sci. 2017;24(5):7–18. doi: 10.21315/mjms2017.24.5.2
- Abbasi Montazeri E, Seyed-Mohammadi S, Asarehzadegan Dezfuli A. Investigation of SCC mec types I–IV in clinical isolates of methicillin-resistant coagulase-negative staphylococci in Ahvaz, Southwest Iran. Biosci Rep. 2020;40(5):40. doi: 10.1042/BSR20200847
- Barbier F, Ruppe E, Hernandez D. Methicillin-resistant coagulase-negative staphylococci in the community: high homology of SCCmec IVa between staphylococcus epidermidis and major clones of methicillin-resistant staphylococcus aureus. J Infect Dis. 2010;202(2):270–281. doi: 10.1086/653483
- Bloemendaal AL, Brouwer EC, Fluit AC. Methicillin resistance transfer from staphylocccus epidermidis to methicillin-susceptible Staphylococcus aureus in a patient during antibiotic therapy. PLOS ONE 2010; 5:e11841. 7 10.1371/journal.pone.0011841
- Stojanov M, Sakwinska O, Moreillon P. Expression of SCCmec cassette chromosome recombinases in methicillin-resistant staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 2013;68(4):749–757. doi: 10.1093/jac/dks494
- Berends MS, Luz CF, Ott A. Trends in occurrence and phenotypic resistance of coagulase-negative staphylococci (CoNS) found in human blood in the Northern Netherlands between 2013 and 2019. Microorganisms. 2022;10(9):1801. doi: 10.3390/microorganisms10091801
- Salgueiro VC, Iorio NL, Ferreira MC. Methicillin resistance and virulence genes in invasive and nasal staphylococcus epidermidis isolates from neonates. BMC Microbiol 2017; 17(1):15. doi: 10.1186/s12866-017-0930-9
- Peixoto PB, Massinhani FH, Netto Dos Santos KR. Methicillin-resistant Staphylococcus epidermidis isolates with reduced vancomycin susceptibility from bloodstream infections in a neonatal intensive care unit. J Med Microbiol. 2020;69(1):41–45. doi: 10.1099/jmm.0.001117
- Hooper DC. Fluoroquinolone resistance among gram-positive cocci. Lancet Infect Dis. 2002;2(9):530–538. doi: 10.1016/S1473-3099(02)00369-9
- Bispo PJ, Alfonso EC, Flynn HW. Emerging 8-methoxyfluoroquinolone resistance among methicillin-susceptible Staphylococcus epidermidis isolates recovered from patients with endophthalmitis. J Clin Microbiol 2013; 51(9):2959–63. doi: 10.1128/JCM.00846-13
- Kang JY, Lee W, Noh GM. Fluoroquinolone resistance of Staphylococcus epidermidis isolated from healthy conjunctiva and analysis of their mutations in quinolone-resistance determining region. Antimicrob Resist Infect Control. 2020;9(1):177. doi: 10.1186/s13756-020-00841-3
- Darboe KS, Oh TH, Choi SM. Antimicrobial susceptibility of staphylococcus species isolated from prosthetic joints with a focus on fluoroquinolone-resistance mechanisms. Diagn Microbiol Infect Dis. 2021;99(1):115173. doi: 10.1016/j.diagmicrobio.2020.115173
- Fan KC, Lin J, Yannuzzi NA. In vitro susceptibilities of methicillin-susceptible and resistant staphylococci to traditional antibiotics compared to a novel fluoroquinolone. J Ophthalmic Inflamm Infect. 2020;10(1):9. doi: 10.1186/s12348-020-0200-0
- Miyanaga M, Nejima R, Miyai T. Changes in drug susceptibility and the quinolone-resistance determining region of Staphylococcus epidermidis after administration of fluoroquinolones. J Cataract Refract Surg. 2009;35(11):1970–1978. doi: 10.1016/j.jcrs.2009.05.049
- Munier AL, de Lastours V, Barbier F. Comparative dynamics of the emergence of fluoroquinolone resistance in staphylococci from the nasal microbiota of patients treated with fluoroquinolones according to their environment. Int J Antimicrob Agents. 2015;46(6):653–659. doi: 10.1016/j.ijantimicag.2015.09.004
- Cerca F, Franca A, Perez-Cabezas B. Dormant bacteria within Staphylococcus epidermidis biofilms have low inflammatory properties and maintain tolerance to vancomycin and penicillin after entering planktonic growth. J Med Microbiol 2014; 63(10):1274–83. doi: 10.1099/jmm.0.073163-0
- Claessens J, Roriz M, Merckx R. Inefficacy of vancomycin and teicoplanin in eradicating and killing staphylococcus epidermidis biofilms in vitro. Int J Antimicrob Agents. 2015;45(4):368–375. doi: 10.1016/j.ijantimicag.2014.11.011
- Sakimura T, Kajiyama S, Adachi S. Biofilm-forming Staphylococcus epidermidis expressing vancomycin resistance early after adhesion to a metal surface. Biomed Res Int 2015; 2015:943056. doi: 10.1155/2015/943056
- Krcmery V Jr., Trupl J, Drgona L. Nosocomial bacteremia due to vancomycin-resistant Staphylococcus epidermidis in four patients with cancer, neutropenia, and previous treatment with vancomycin. Eur J Clin Microbiol Infect Dis. 1996;15(3):259–261. doi: 10.1007/BF01591369
- Del’ Alamo L, Cereda RF, Tosin I. Antimicrobial susceptibility of coagulase-negative staphylococci and characterization of isolates with reduced susceptibility to glycopeptides. Diagn Microbiol Infect Dis. 1999;34(3):185–191. doi: 10.1016/S0732-8893(99)00034-6
- Dunne WM Jr., Qureshi H, Pervez H. Staphylococcus epidermidis with intermediate resistance to vancomycin: elusive phenotype or laboratory artifact? Clin Infect Dis. 2001;33(1):135–137. doi: 10.1086/320890
- Nakajima J, Hitomi S, Koganemaru H. Isolation of Staphylococcus epidermidis intermediately resistant to vancomycin in a case of central venous catheter-associated bloodstream infection. J Infect Chemother. 2013;19(5):983–986. doi: 10.1007/s10156-013-0562-4
- Tevell S, Claesson C, Hellmark B. Heterogeneous glycopeptide intermediate staphylococcus epidermidis isolated from prosthetic joint infections. Eur J Clin Microbiol Infect Dis 2014; 33(6):911–7. doi: 10.1007/s10096-013-2025-3
- Banousi A, Evangelopoulos DS, Stylianakis A. A comparative study of heterogeneous antibiotic resistance of microbial populations in conventional periprosthetic tissue cultures and sonication fluid cultures of orthopaedics explanted prostheses. Eur J Orthop Surg Traumatol. 2020;30(7):1307–1318. doi: 10.1007/s00590-020-02704-4
- Dao TH, Alsallaq R, Parsons JB. Vancomycin heteroresistance and clinical outcomes in bloodstream infections caused by Coagulase-Negative Staphylococci. Antimicrob Agents Chemother. 2020;64(11):64. doi: 10.1128/AAC.00944-20
- Biavasco F, Vignaroli C, Varaldo PE. Glycopeptide resistance in coagulase-negative staphylococci. Eur J Clin Microbiol Infect Dis. 2000;19(6):403–417. doi: 10.1007/s100960000299
- Kresken M, Klare I, Wichelhaus TA. Glycopeptide resistance in Enterococcus spp. And coagulase-negative staphylococci from hospitalised patients in Germany: occurrence, characteristics and dalbavancin susceptibility. J Glob Antimicrob Resist. 2022; 28:102–107. 10.1016/j.jgar.2021.12.016
- Knafl D, Tobudic S, Cheng SC. Dalbavancin reduces biofilms of methicillin-resistant staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). Eur J Clin Microbiol Infect Dis. 2017;36(4):677–680. doi: 10.1007/s10096-016-2845-z
- Bartoletti M, Mikus E, Pascale R. Clinical experience with dalbavancin for the treatment of deep sternal wound infection. J Glob Antimicrob Resist. 2019; 18:195–198. doi: 10.1016/j.jgar.2019.03.015
- Azamgarhi T, Donaldson J, Shah A. Dalbavancin to treat infected massive endoprostheses: a case report and cost comparison analysis. J Bone Jt Infect 2019; 4(5):234–7. doi: 10.7150/jbji.37980
- Ziemyte M, Rodriguez-Diaz JC, Ventero MP. Effect of dalbavancin on staphylococcal biofilms when administered alone or in combination with biofilm-detaching compounds. Front Microbiol. 2020; 11:553. doi: 10.3389/fmicb.2020.00553
- Di Pilato V, Ceccherini F, Sennati S. In vitro time-kill kinetics of dalbavancin against staphylococcus spp. biofilms over prolonged exposure times. Diagn Microbiol Infect Dis 2020; 96(2):114901. doi: 10.1016/j.diagmicrobio.2019.114901
- Pantel A, Nachar O, Boudet A. In vitro activity of dalbavancin against Gram-positive bacteria isolated from diabetic foot osteomyelitis. J Antimicrob Chemother. 2021;76(8):2057–2060. doi: 10.1093/jac/dkab117
- Simon S, Frank BJH, Hartmann S. Dalbavancin in gram-positive periprosthetic joint infections. J Antimicrob Chemother. 2022;77(8):2274–2277. doi: 10.1093/jac/dkac178
- Jiang J-H, Dexter C, Cameron DR. Evolution of daptomycin resistance in coagulase-negative staphylococci involves mutations of the essential two-component regulator WalKR. Antimicrob Agents Chemother. 2019;63(3):63. doi: 10.1128/AAC.01926-18
- Al Janabi J, Tevell S, Sieber RN. Emerging resistance in Staphylococcus epidermidis during dalbavancin exposure: a case report and in vitro analysis of isolates from prosthetic joint infections. J Antimicrob Chemother. 2023;78(3):669–677. doi: 10.1093/jac/dkac434
- O’Neill AJ, Chopra I. Molecular basis of fusB-mediated resistance to fusidic acid in staphylococcus aureus. Mol Microbiol. 2006;59(2):664–676. doi: 10.1111/j.1365-2958.2005.04971.x
- O’Neill AJ, McLaws F, Kahlmeter G. Genetic basis of resistance to fusidic acid in staphylococci. Antimicrob Agents Chemother. 2007;51(5):1737–1740. doi: 10.1128/AAC.01542-06
- Lin YT, Hung WC, Tsai JC. Wide dissemination of SCCfusC in fusidic acid-resistant coagulase-negative staphylococci and implication for its spread to methicillin-resistant staphylococcus aureus in Taiwan. Int J Antimicrob Agents. 2018;51(6):875–880. doi: 10.1016/j.ijantimicag.2018.01.020
- Chen S, Rao L, Lin C. The dissemination of fusidic acid resistance among staphylococcus epidermidis clinical isolates in Wenzhou, China. Infect Drug Resist. 2022; 15:2537–2544. 10.2147/IDR.S365071
- Chen HJ, Tsai JC, Hung WC. Identification of fusB-mediated fusidic acid resistance islands in staphylococcus epidermidis isolates. Antimicrob Agents Chemother. 2011;55(12):5842–5849. doi: 10.1128/AAC.00592-11
- Chen C, Krishnan V, Macon K. Secreted proteases control autolysin-mediated biofilm growth of staphylococcus aureus. J Biol Chem 2013; 288(41):29440–52. doi: 10.1074/jbc.M113.502039
- Hung WC, Chen HJ, Lin YT. Skin commensal staphylococci may act as reservoir for fusidic acid resistance genes. PLOS ONE. 2015;10(11):e0143106. doi: 10.1371/journal.pone.0143106
- Long KS, Poehlsgaard J, Kehrenberg C. The cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin a antibiotics. Antimicrob Agents Chemother. 2006;50(7):2500–2505. doi: 10.1128/AAC.00131-06
- Bonilla H, Huband MD, Seidel J. Multicity outbreak of linezolid-resistant staphylococcus epidermidis associated with clonal spread of a cfr-containing strain. Clin Infect Dis 2010; 51(7):796–800. doi: 10.1086/656281
- Barros M, Branquinho R, Grosso F. Linezolid-resistant Staphylococcus epidermidis, Portugal, 2012. Emerg Infect Dis. 2014;20(5):903–905. doi: 10.3201/eid2005.130783
- Decousser JW, Desroches M, Bourgeois-Nicolaos N. Susceptibility trends including emergence of linezolid resistance among coagulase-negative staphylococci and meticillin-resistant staphylococcus aureus from invasive infections. Int J Antimicrob Agents. 2015;46(6):622–630. doi: 10.1016/j.ijantimicag.2015.07.022
- Bemer P, Aubry A, Tessier E Emergence of methicillin-resistant staphylococcus epidermidis resistant to linezolid: activity of ceftaroline versus ceftobiprole in a French University Hospital. Int J Antimicrob Agents. 2022; 60(3):106613. doi: 10.1016/j.ijantimicag.2022.106613
- LaMarre JM, Locke JB, Shaw KJ. Low fitness cost of the multidrug resistance gene cfr. Antimicrob Agents Chemother. 2011;55(8):3714–3719. doi: 10.1128/AAC.00153-11
- Mendes RE, Deshpande LM, Bonilla HF. Dissemination of a pSCFS3-like cfr-carrying plasmid in staphylococcus aureus and Staphylococcus epidermidis clinical isolates recovered from hospitals in Ohio. Antimicrob Agents Chemother. 2013;57(7):2923–2928. doi: 10.1128/AAC.00071-13
- Brenciani A, Morroni G, Pollini S. Characterization of novel conjugative multiresistance plasmids carrying cfr from linezolid-resistant Staphylococcus epidermidis clinical isolates from Italy. J Antimicrob Chemother. 2016;71(2):307–313. doi: 10.1093/jac/dkv341
- Brenciani A, Morroni G, Mingoia M. Stability of the cargo regions of the cfr-carrying, multiresistance plasmid pSP01 from Staphylococcus epidermidis. Int J Med Microbiol. 2016;306(8):717–721. doi: 10.1016/j.ijmm.2016.08.002
- Lazaris A, Coleman DC, Kearns AM. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant staphylococcus epidermidis and vancomycin-resistant enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J Antimicrob Chemother. 2017;72(12):3252–3257. doi: 10.1093/jac/dkx292
- LaMarre J, Mendes RE, Szal T. The genetic environment of the cfr gene and the presence of other mechanisms account for the very high linezolid resistance of Staphylococcus epidermidis isolate 426-3147L. Antimicrob Agents Chemother. 2013;57(3):1173–1179. doi: 10.1128/AAC.02047-12
- Schwarz S, Werckenthin C, Kehrenberg C. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in staphylococcus sciuri. Antimicrob Agents Chemother. 2000;44(9):2530–2533. doi: 10.1128/AAC.44.9.2530-2533.2000
- Mendes RE, Deshpande LM, Castanheira M. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Chemother. 2008;52(6):2244–2246. doi: 10.1128/AAC.00231-08
- Cortes MF, Andre C, Martins Simoes P. Persistence of a multidrug-resistant worldwide-disseminated methicillin-resistant Staphylococcus epidermidis clone harbouring the cfr linezolid resistance gene in a French hospital with evidence of interspecies transfer to several staphylococcus aureus lineages. J Antimicrob Chemother. 2022;77(7):1838–1846. doi: 10.1093/jac/dkac119
- He HJ, Sun FJ, Wang Q. Erythromycin resistance features and biofilm formation affected by subinhibitory erythromycin in clinical isolates of Staphylococcus epidermidis. J Microbiol Immunol Infect. 2016;49(1):33–40. doi: 10.1016/j.jmii.2014.03.001
- Bouchami O, Achour W, Ben Hassen A. Prevalence and mechanisms of macrolide resistance among Staphylococcus epidermidis isolates from neutropenic patients in Tunisia. Clin Microbiol Infect. 2007;13(1):103–106. doi: 10.1111/j.1469-0691.2006.01567.x
- Gatermann SG, Koschinski T, Friedrich S. Distribution and expression of macrolide resistance genes in coagulase-negative staphylococci. Clin Microbiol Infect. 2007;13(8):777–781. doi: 10.1111/j.1469-0691.2007.01749.x
- Juda M, Chudzik-Rzad B, Malm A. The prevalence of genotypes that determine resistance to macrolides, lincosamides, and streptogramins B compared with spiramycin susceptibility among erythromycin-resistant Staphylococcus epidermidis. Mem Inst Oswaldo Cruz. 2016;111(3):155–160. doi: 10.1590/0074-02760150356
- Xu Z, Misra R, Jamrozy D. Whole genome sequence and comparative genomics analysis of multi-drug resistant environmental staphylococcus epidermidis ST59. G3 (Bethesda). 2018; 8(7):2225–30. doi: 10.1534/g3.118.200314
- Mirzaei R, Yousefimashouf R, Arabestani MR. The issue beyond resistance: Methicillin-resistant Staphylococcus epidermidis biofilm formation is induced by subinhibitory concentrations of cloxacillin, cefazolin, and clindamycin. PLOS ONE. 2022;17(11):e0277287. doi: 10.1371/journal.pone.0277287
- Aliyu S, McGowan K, Hussain D. Prevalence and outcomes of multi-drug resistant blood stream infections among nursing home residents admitted to an acute care hospital. J Intensive Care Med. 2022;37(4):565–571. doi: 10.1177/08850666211014450
- Miragaia M, Couto I, Pereira SF. Molecular characterization of methicillin-resistant Staphylococcus epidermidis clones: evidence of geographic dissemination. J Clin Microbiol 2002; 40(2):430–8. doi: 10.1128/JCM.40.2.430-438.2002
- Farina N, Samudio M, Carpinelli L. Methicillin resistance and biofilm production of staphylococcus epidermidis isolates from infectious and normal flora conjunctiva. Int Ophthalmol. 2017;37(4):819–825. doi: 10.1007/s10792-016-0339-8
- Hanssen AM, Kjeldsen G, Sollid JU Local variants of staphylococcal cassette chromosome mec in sporadic methicillin-resistant staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci: evidence of horizontal gene transfer? Antimicrob Agents Chemother 2004; 48(1):285–96. doi: 10.1128/AAC.48.1.285-296.2004
- Mongkolrattanothai K, Boyle S, Murphy TV. Novel non-mecA-containing staphylococcal chromosomal cassette composite island containing pbp4 and tagF genes in a commensal staphylococcal species: a possible reservoir for antibiotic resistance islands in staphylococcus aureus. Antimicrob Agents Chemother. 2004;48(5):1823–1836. doi: 10.1128/AAC.48.5.1823-1836.2004
- Fisarova L, Pantucek R, Botka T. Variability of resistance plasmids in coagulase-negative staphylococci and their importance as a reservoir of antimicrobial resistance. Res Microbiol. 2019;170(2):105–111. doi: 10.1016/j.resmic.2018.11.004
- Maree M, Thi Nguyen LT, Ohniwa RL. Natural transformation allows transfer of SCCmec-mediated methicillin resistance in staphylococcus aureus biofilms. Nat Commun. 2022;13(1):2477. doi: 10.1038/s41467-022-29877-2
- Bucci FA Jr., Evans RE, Amico LM. Antibacterial efficacy of prophylactic besifloxacin 0.6% and moxifloxacin 0.5% in patients undergoing cataract surgery. Clin Ophthalmol. 2015; 9:843–52. doi: 10.2147/OPTH.S83162
- Liu C, Kakis A, Nichols A. Targeted use of vancomycin as perioperative prophylaxis reduces periprosthetic joint infection in revision TKA. Clin Orthop Relat Res. 2014;472(1):227–231. doi: 10.1007/s11999-013-3029-0
- Nodzo SR, Boyle KK, Frisch NB Nationwide organism susceptibility patterns to common preoperative prophylactic antibiotics: what are we covering? J Arthroplasty. 2019; 34(7):S302–S6. doi: 10.1016/j.arth.2019.01.017
- Stavrakis AI, Niska JA, Shahbazian JH. Combination prophylactic therapy with rifampin increases efficacy against an experimental Staphylococcus epidermidis subcutaneous implant-related infection. Antimicrob Agents Chemother. 2014;58(4):2377–2386. doi: 10.1128/AAC.01943-13
- Bloom HL, Constantin L, Dan D. Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope. Pacing Clin Electrophysiol. 2011;34(2):133–142. doi: 10.1111/j.1540-8159.2010.02931.x
- Austin PD, Stapleton P, Elia M. Comparative effect of seven prophylactic locks to prevent biofilm biomass and viability in intravenous catheters. J Antimicrob Chemother. 2022;77(8):2191–2198. doi: 10.1093/jac/dkac181
- Kaldirim H, Yazgan S, Kirgiz A. Effect of topical antibiotic prophylaxis on conjunctival flora and antibiotic resistance following intravitreal injections in patients with type 2 diabetes. Korean J Ophthalmol. 2020;34(4):265–273. doi: 10.3341/kjo.2019.0144
- Kristensen MF, Zeng G, Neu TR. Osteopontin adsorption to Gram-positive cells reduces adhesion forces and attachment to surfaces under flow. J Oral Microbiol. 2017;9(1):1379826. doi: 10.1080/20002297.2017.1379826
- Zampino DC, Samperi F, Mancuso M. Polymer blends based on 1-hexadecyl-3-methyl imidazolium 1,3-dimethyl 5-sulfoisophthalate ionic liquid: thermo-mechanical, surface morphology and antibacterial properties. Polymers. 2023;15(4):15. doi: 10.3390/polym15040970
- Qi S, Kiratzis I, Adoni P. Porous cellulose thin films as sustainable and effective antimicrobial surface coatings. ACS Appl Mater Interfaces. 2023;15(17):20638–20648. doi: 10.1021/acsami.2c23251
- Basu A, Heitz K, Stromme M. Ion-crosslinked wood-derived nanocellulose hydrogels with tunable antibacterial properties: Candidate materials for advanced wound care applications. Carbohydr Polym. 2018; 181:345–350. 10.1016/j.carbpol.2017.10.085
- Fursatz M, Skog M, Sivler P. Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide ε -poly-L-Lysine. Biomed Mater. 2018; 13(2):025014. doi: 10.1088/1748-605X/aa9486
- Lopez-Carrizales M, Mendoza-Mendoza E, Peralta-Rodriguez RD. Characterization, antibiofilm and biocompatibility properties of chitosan hydrogels loaded with silver nanoparticles and ampicillin: an alternative protection to central venous catheters. Colloids Surf B Biointerfaces. 2020; 196:111292. doi: 10.1016/j.colsurfb.2020.111292
- Janson O, Gururaj S, Pujari-Palmer S. Titanium surface modification to enhance antibacterial and bioactive properties while retaining biocompatibility. Mater Sci Eng C Mater Biol Appl. 2019; 96:272–279. doi: 10.1016/j.msec.2018.11.021
- Boix-Lemonche G, Guillem-Marti J, D’Este F. Covalent grafting of titanium with a cathelicidin peptide produces an osteoblast compatible surface with antistaphylococcal activity. Colloids Surf B Biointerfaces 2020; 185:110586. doi: 10.1016/j.colsurfb.2019.110586
- Shi Z, Neoh KG, Kang ET. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules. 2009;10(6):1603–1611. doi: 10.1021/bm900203w
- Xie CM, Lu X, Wang KF. Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl Mater Interfaces. 2014;6(11):8580–8589. doi: 10.1021/am501428e
- Ren X, van der Mei HC, Ren Y. Antimicrobial loading of nanotubular titanium surfaces favoring surface coverage by mammalian cells over bacterial colonization. Mater Sci Eng C Mater Biol Appl. 2021; 123:112021. doi: 10.1016/j.msec.2021.112021
- Os C, On L, Ol ND. Osteointegration, antimicrobial and antibiofilm activity of orthopaedic titanium surfaces coated with silver and strontium-doped hydroxyapatite using a novel blasting process. Drug Deliv Transl Res. 2021;11(2):702–716. doi: 10.1007/s13346-021-00946-1
- Joo H, Park J, Sutthiwanjampa C. Surface coating with hyaluronic acid-gelatin-crosslinked hydrogel on gelatin-conjugated poly(dimethylsiloxane) for implantable medical device-induced fibrosis. Pharmaceutics. 2021;13(2):269. doi: 10.3390/pharmaceutics13020269
- Chen J, Xu M, Wang L. Converting lysozyme to hydrogel: A multifunctional wound dressing that is more than antibacterial. Colloids Surf B Biointerfaces. 2022; 219:112854. doi: 10.1016/j.colsurfb.2022.112854
- Wassmann T, Kreis S, Behr M. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int J Implant Dent. 2017;3(1):32. doi: 10.1186/s40729-017-0093-3
- Gasik M, Braem A, Chaudhari A. Titanium implants with modified surfaces: meta-analysis of in vivo osteointegration. Mater Sci Eng C Mater Biol Appl 2015; 49:152–158. doi: 10.1016/j.msec.2014.12.074
- Harro JM, Peters BM, O’May GA. Vaccine development in staphylococcus aureus: taking the biofilm phenotype into consideration. FEMS Immunol Med Microbiol. 2010;59(3):306–323. doi: 10.1111/j.1574-695X.2010.00708.x
- Mirzaei B, Babaei R, Valinejad S. Staphylococcal vaccine antigens related to biofilm formation. Hum Vaccin Immunother. 2021;17(1):293–303. doi: 10.1080/21645515.2020.1767449
- McKenney D, Pouliot K, Wang Y. Vaccine potential of poly-1-6 β-d-N-succinylglucosamine, an immunoprotective surface polysaccharide of staphylococcus aureus and staphylococcus epidermidis. J Biotechnol. 2000; 83(1–2):37–44. doi: 10.1016/S0168-1656(00)00296-0
- Rennermalm A, Nilsson M, Flock JI. The fibrinogen binding protein of staphylococcus epidermidis is a target for opsonic antibodies. Infect Immun. 2004;72(5):3081–3083. doi: 10.1128/IAI.72.5.3081-3083.2004
- Mack D, Davies AP, Harris LG, et al. Staphylococcus epidermidis biofilms: functional molecules, relation to virulence, and vaccine potential. In: Lindhorst T, Oscarson S, editors. GLycoscience and microbial adhesion. Topics in current chemistry. Vol. 288. Berlin, Heidelberg: Springer; 2009. p. 157–82. doi: 10.1007/128_2008_19
- Sellman BR, Timofeyeva Y, Nanra J. Expression of Staphylococcus epidermidis SdrG increases following exposure to an in vivo environment. Infect Immun. 2008;76(7):2950–2957. doi: 10.1128/IAI.00055-08
- Shahrooei M, Hira V, Khodaparast L. Vaccination with SesC decreases staphylococcus epidermidis biofilm formation. Infect Immun. 2012;80(10):3660–3668. doi: 10.1128/IAI.00104-12
- Hofmans D, Khodaparast L, Khodaparast L. Ses proteins as possible targets for vaccine development against staphylococcus epidermidis infections. J Infect. 2018;77(2):119–130. doi: 10.1016/j.jinf.2018.03.013
- Yan L, Zhang L, Ma H. A single B-repeat of Staphylococcus epidermidis accumulation-associated protein induces protective immune responses in an experimental biomaterial-associated infection mouse model. Clin Vaccine Immunol. 2014;21(9):1206–1214. doi: 10.1128/CVI.00306-14
- Sellman BR, Howell AP, Kelly-Boyd C. Identification of immunogenic and serum binding proteins of staphylococcus epidermidis. Infect Immun. 2005;73(10):6591–6600. doi: 10.1128/IAI.73.10.6591-6600.2005
- Anderson AS, Scully IL, Timofeyeva Y. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis. 2012;205(11):1688–1696. doi: 10.1093/infdis/jis272
- Cole LE, Zhang J, Kesselly A. Limitations of murine models for assessment of antibody-mediated therapies or vaccine candidates against staphylococcus epidermidis bloodstream infection. Infect Immun. 2016;84(4):1143–1149. doi: 10.1128/IAI.01472-15
- Weisman LE, Thackray HM, Steinhorn RH. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128(2):271–279. doi: 10.1542/peds.2010-3081
- Patel M, Kaufman DA. Anti-lipoteichoic acid monoclonal antibody (pagibaximab) studies for the prevention of staphylococcal bloodstream infections in preterm infants. Expert Opin Biol Ther. 2015;15(4):595–600. doi: 10.1517/14712598.2015.1019857
- Pichichero ME. Challenges in vaccination of neonates, infants and young children. Vaccine. 2014;32(31):3886–3894. doi: 10.1016/j.vaccine.2014.05.008
- Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36(6):697–705. doi: 10.1016/j.arcmed.2005.06.009
- Ozawa S, Mirelman A, Stack ML. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine. 2012;31(1):96–108. doi: 10.1016/j.vaccine.2012.10.103
- Wang CH, Hsieh YH, Powers ZM. Defeating antibiotic-resistant bacteria: exploring alternative therapies for a post-antibiotic era. Int J Mol Sci. 2020;21(3):1061. doi: 10.3390/ijms21031061
- Kwon JH, Powderly WG. The post-antibiotic era is here. Science. 2021;373(6554):471. doi: 10.1126/science.abl5997
